Abstract
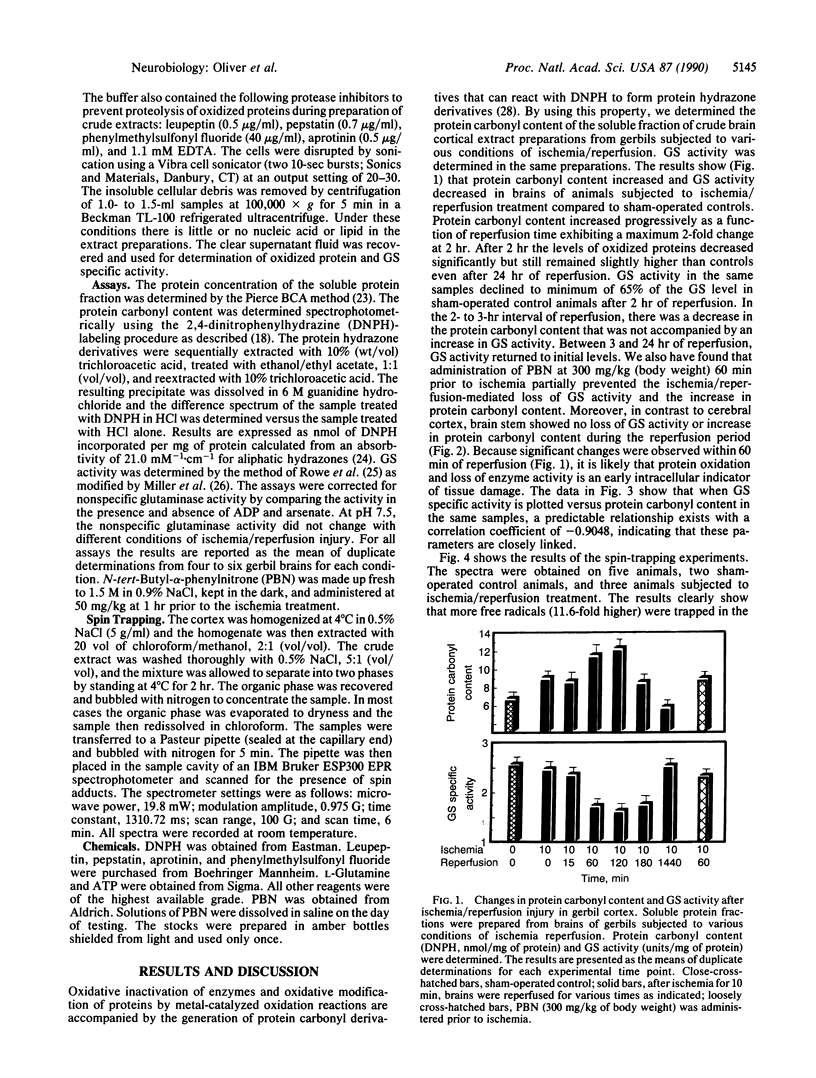
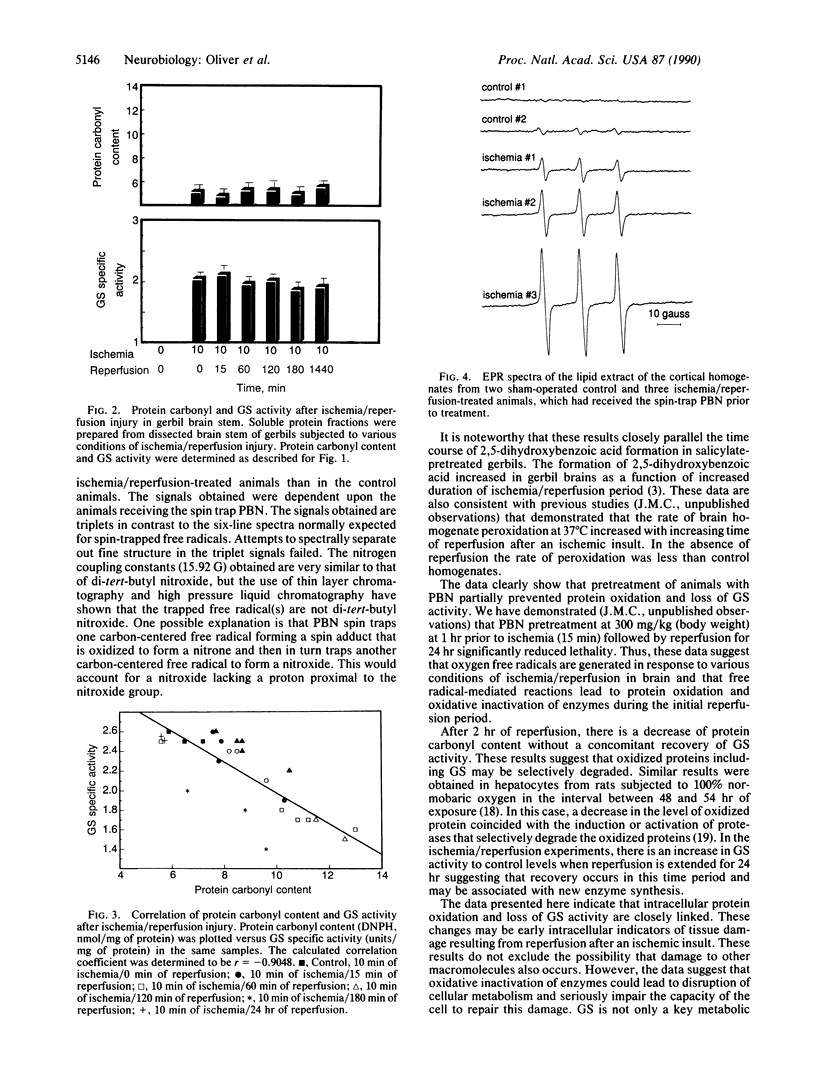
Free radical-mediated oxidative damage has been implicated in tissue injury resulting from ischemia/reperfusion events. Global cortical ischemia/reperfusion injury to Mongolian gerbil brains was produced by transient occlusion of both common carotid arteries. Protein oxidation, as measured by protein carbonyl content, increased significantly during the reperfusion phase that followed 10 min of ischemia. The activity of glutamine synthetase, an enzyme known to be inactivated by metal-catalyzed oxidation reactions, decreased to 65% of control levels after 2 hr of reperfusion that followed 10 min of ischemia. We also report that the free radical spin trap N-tert-butyl-alpha-phenylnitrone [300 mg/kg (body weight)] administered 60 min before ischemia/reperfusion is initiated, partially prevents protein oxidation and protects from loss of glutamine synthetase activity. In addition, we report a N-tert-butyl-alpha-phenylnitrone-dependent nitroxide radical obtained in the lipid fraction of the ischemia/reperfusion-lesioned brains, but there was very little radical present in the comparable sham-operated control brains. These data strengthen the previous observation utilizing in vivo-trapping methods, that free radical flux is increased during the reperfusion phase of the ischemia-lesioned gerbil brain. The loss of glutamine synthetase would be expected to increase the levels of brain L-glutamate. Thus, the oxidative inactivation of glutamine synthetase may be a critical factor in the neurotoxicity produced after cerebral ischemia/reperfusion injury.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almassy R. J., Janson C. A., Hamlin R., Xuong N. H., Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. 1986 Sep 25-Oct 1Nature. 323(6086):304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- Bolli R., Patel B. S., Jeroudi M. O., Lai E. K., McCay P. B. Demonstration of free radical generation in "stunned" myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 1988 Aug;82(2):476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Carney J. M., Duchon A., Floyd R. A., Chevion M. Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci Lett. 1988 May 26;88(2):233–238. doi: 10.1016/0304-3940(88)90132-2. [DOI] [PubMed] [Google Scholar]
- Chapman M. L., Rubin B. R., Gracy R. W. Increased carbonyl content of proteins in synovial fluid from patients with rheumatoid arthritis. J Rheumatol. 1989 Jan;16(1):15–18. [PubMed] [Google Scholar]
- Farber J. M., Levine R. L. Sequence of a peptide susceptible to mixed-function oxidation. Probable cation binding site in glutamine synthetase. J Biol Chem. 1986 Apr 5;261(10):4574–4578. [PubMed] [Google Scholar]
- Fucci L., Oliver C. N., Coon M. J., Stadtman E. R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L. Mixed-function oxidation of histidine residues. Methods Enzymol. 1984;107:370–376. doi: 10.1016/0076-6879(84)07025-7. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L. Oxidative modification of glutamine synthetase. I. Inactivation is due to loss of one histidine residue. J Biol Chem. 1983 Oct 10;258(19):11823–11827. [PubMed] [Google Scholar]
- Levine R. L. Oxidative modification of glutamine synthetase. II. Characterization of the ascorbate model system. J Biol Chem. 1983 Oct 10;258(19):11828–11833. [PubMed] [Google Scholar]
- Miller R. E., Hackenberg R., Gershman H. Regulation of glutamine synthetase in cultured 3T3-L1 cells by insulin, hydrocortisone, and dibutyryl cyclic AMP. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1418–1422. doi: 10.1073/pnas.75.3.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C. N., Ahn B. W., Moerman E. J., Goldstein S., Stadtman E. R. Age-related changes in oxidized proteins. J Biol Chem. 1987 Apr 25;262(12):5488–5491. [PubMed] [Google Scholar]
- Oliver C. N. Inactivation of enzymes and oxidative modification of proteins by stimulated neutrophils. Arch Biochem Biophys. 1987 Feb 15;253(1):62–72. doi: 10.1016/0003-9861(87)90637-0. [DOI] [PubMed] [Google Scholar]
- Park J. H., Lee Y. S., Chung C. H., Goldberg A. L. Purification and characterization of protease Re, a cytoplasmic endoprotease in Escherichia coli. J Bacteriol. 1988 Feb;170(2):921–926. doi: 10.1128/jb.170.2.921-926.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Rivett A. J. Preferential degradation of the oxidatively modified form of glutamine synthetase by intracellular mammalian proteases. J Biol Chem. 1985 Jan 10;260(1):300–305. [PubMed] [Google Scholar]
- Rivett A. J. Purification of a liver alkaline protease which degrades oxidatively modified glutamine synthetase. Characterization as a high molecular weight cysteine proteinase. J Biol Chem. 1985 Oct 15;260(23):12600–12606. [PubMed] [Google Scholar]
- Rivett A. J., Roseman J. E., Oliver C. N., Levine R. L., Stadtman E. R. Covalent modification of proteins by mixed-function oxidation: recognition by intracellular proteases. Prog Clin Biol Res. 1985;180:317–328. [PubMed] [Google Scholar]
- Roseman J. E., Levine R. L. Purification of a protease from Escherichia coli with specificity for oxidized glutamine synthetase. J Biol Chem. 1987 Feb 15;262(5):2101–2110. [PubMed] [Google Scholar]
- Siesjö B. K. Mechanisms of ischemic brain damage. Crit Care Med. 1988 Oct;16(10):954–963. doi: 10.1097/00003246-198810000-00006. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Starke-Reed P. E., Oliver C. N. Protein oxidation and proteolysis during aging and oxidative stress. Arch Biochem Biophys. 1989 Dec;275(2):559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]


