Abstract
Classical protein kinase C (PKC) enzymes are known to be important factors in cell physiology both in terms of health and disease. They are activated by triggering signals that induce their translocation to membranes. The consensus view is that several secondary messengers are involved in this activation, such as cytosolic Ca2+ and diacylglycerol. Cytosolic Ca2+ bridges the C2 domain to anionic phospholipids as phosphatidylserine in the membrane, and diacylglycerol binds to the C1 domain. Both diacylglycerol and the increase in Ca2+ concentration are assumed to arise from the extracellular signal that triggers the hydrolysis of phosphatidylinositol-4,5-bisphosphate. However, results obtained during the last decade indicate that this phosphoinositide itself is also responsible for modulating classical PKC activity and its localization in the plasma membrane.
Keywords: Protein kinase C; Phosphatidylinositol-4,5-bisphosphate; C2 domain; Diacylglycerol; C1 domain
Introduction
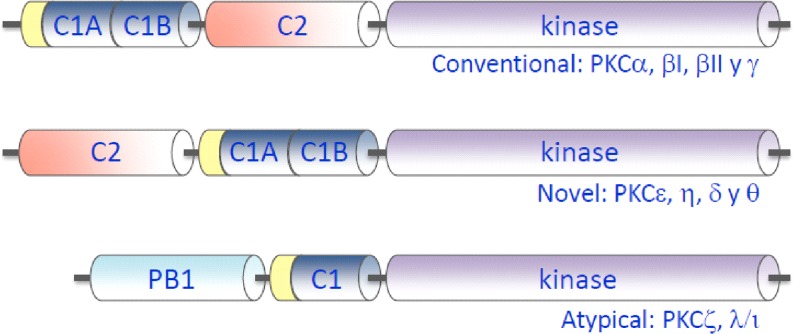
Protein kinase C (PKC) is a family of related protein kinases. In pioneering studies, Nishizuka and co-workers were the first to identify this novel type of protein kinase (Inoue et al. 1977) and to show that they are activated by calcium and phospholipids (Takai et al. 1979a) and also by diacylglycerols in direct relation to the turnover of phosphatidylinositol (Takai et al. 1979b). These protein kinases became the focus of much attention when it was also observed that they are activated by phorbol esters, which are tumor promoters (Castagna et al. 1982), suggesting that they might therefore be involved in cell regulation and in transforming normal cells into tumoral ones. It was subsequently discovered later that phorbol esters function as analogs of diacylglycerols, binding to sites for which the physiological ligand is diacylglycerol (Sharkey et al. 1984; Tanaka et al. 1986). The PKC family consists at least ten different isoforms in mammalian cells, where they play important roles in the transduction of signals coupled to receptor-mediated hydrolysis of membrane phospholipids and increases in Ca2+ concentration in the cell cytoplasm (Nishizuka 1992). PKC isoenzymes are highly homologous with respect to their catalytic domains, and their regulatory domains determine the response of individual members to activators. The mammalian isoenzymes can be classified into three groups according to their structure and cofactor regulation (Fig. 1). The first group includes the classical (or conventional) isoforms (α, βI, βII and γ), which are regulated by cytosolic calcium concentration, acidic phospholipids and diacylglycerol. The second group corresponds to the novel PKCs (δ, ε, η and θ), which are activated by acidic phospholipids and diacylglycerol in a calcium-independent manner. These two groups contain in their regulatory regions both conserved C1 domains that are responsible for sensing diacylglycerol or phorbol esters and C2 domains that are responsible for sensing Ca2+and/or acidic phospholipids in different subcellular compartments (Fig. 1). The activating effect of diacylglycerol on PKC activity may also be partially determined by the modulation of the membrane structure exerted by this lipid (Jimenez-Monreal et al. 1999; Micol et al. 1999; Sanchez-Pinera et al. 1999; Gomez-Fernandez and Corbalan-Garcia 2007). The third group comprises the atypical PKC isoforms (ξ, τ/λ), which are not regulated by diacylglycerol or calcium (Fig. 1). An abundance of experimental data has been compiled on the key role of PKCs in the regulation of cell physiology (Zeng et al. 2012) and their involvement in many pathological processes. These data indicate that PKCs are a possible pharmacological target (Mochly-Rosen et al. 2012; Wu-Zhang and Newton 2013).
Fig. 1.
Domain composition of the primary structures of members of the protein kinase C (PKC) family. The regulatory domain is located in the amino terminal region which contains different types of domains, depending on the isoenzyme, namely, conventional (C1A, C1B, C2 and kinase), novel (C2, C1A, C1B and kinase) and atypical (PB1, pseudoC1 and kinase)
In this review we focus on the activation of the classical PKCs in which the C2 domain plays an important role (Corbalan-Garcia and Gomez-Fernandez 2006; Farah and Sossin 2012) and especially on the importance of phosphatidylinositol-4,5-bisphosphate in its role as PKC activator. The classical scheme of activation of classical PKCs assumes that the extracellular signals acting through different types of receptors induce the hydrolysis of phosphatidylinositol-4,5-bisphosphate, producing diacylglycerol and inositol 1,4,5-trisphosphate, which, in turn, releases Ca2+ from intracellular stores. Ca2+ and diacylglycerol will then facilitate enzyme translocation to the membrane. In this review, the role of phosphatidylinositol-4,5-bisphosphate in the modulation of the activity and the location of classical PKCs will be reviewed, while the evidence accumulated in the last decade showing the key role played by this phosphoinositide will be examined.
The C2 domain binds simultaneously to two different activating phospholipids
The C2 domain of classical PKCs is an interesting protein module since it may translocate to membranes by binding lipids through two different sites. Co-crystallization of the C2 domain of PKCα with phosphatidylserine and Ca2+ revealed that this cation forms a bridge between the phospholipid and the protein (Verdaguer et al. 1999). The Ca2+-binding region is located in the flexible top loops that bind two or three Ca2+ ions, depending on the isoenzyme, and also interact with anionic phospholipids, but with a certain specificity for phosphatidylserine (Verdaguer et al. 1999; Conesa-Zamora et al. 2000; Conesa-Zamora et al. 2001; Ochoa et al. 2002). Interestingly, phosphatidylserine also binds directly to residues N189, T251, R216, and R249 of PKCα (Verdaguer et al. 1999; Conesa-Zamora et al. 2001).
The binding of short-chain phosphatidylserine and phosphatidic acid to a site other than the calcium-binding site was observed in early co-crystallizing experiments, and this second site was located in a groove formed by β3, β4 and β6 strands (Verdaguer et al. 1999; Ochoa et al. 2002).
Several lysine residues (K197, K199, K209 and K211) located in this second site were found to interact with the negative phospholipid. However, a mutational binding screening demonstrated that this site was not important to bind phosphatidylserine-containing vesicles (Ochoa et al. 2002; Sanchez-Bautista et al. 2006). On the contrary, these mutants were essential to bind phosphatidylinositol-4,5-bisphosphate-containing vesicles, suggesting the existence of a dual interaction of the C2 domain with two different phospholipids through two different motifs (Corbalan-Garcia et al. 2003; Marin-Vicente et al. 2005; Sanchez-Bautista et al. 2006; Guerrero-Valero et al. 2007; Marin-Vicente et al. 2008; Guerrero-Valero et al. 2009).
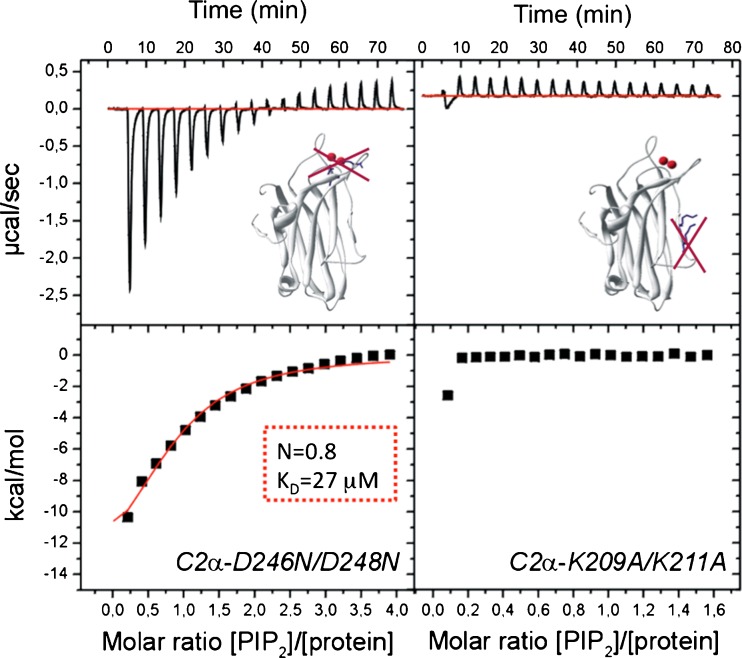
Isothermal titration calorimetry (ITC) studies have demonstrated that in the presence of Ca2+, the C2 domain has an equilibrium dissociation constant (K d) of 20 μM for IP3 and 18 μM for phosphatidylcholine/phosphatidylserine vesicles. In these studies, omitting Ca2+ led to a K d of 430 μM, clearly showing that the membrane interaction of this domain is a Ca2+-dependent process. However, when phosphatidylinositol-4,5-bisphosphate was included in the vesicles, the K d decreased by tenfold (1.85 μM) (Table 1) with one bound lipid molecule, clearly demonstrating a higher affinity to bind to this phosphoinositide than to the classical activator phosphatidylserine. Even in the absence of Ca2+, the K d for phosphatidylcholine/phosphatidylinositol-4,5-bisphosphate (95:5 molar ratio) was still 48 μM (Fig. 2). Interestingly, after target mutagenesis of the calcium-binding site (D246/248 N) in the C2 domain, the K d increased (in the presence of Ca2+) to 26.5 μM, but still maintained one phosphatidylinositol-4,5-bisphosphate molecule bound to the domain. When the mutation affected the polybasic cluster (K209/211A) no binding was detected to phosphatidylcholine/phosphatidylinositol-4,5-bisphosphate vesicles (95:5 molar ratio). The higher affinity of the domain for phosphatidylinositol-4,5-bisphosphate than for phosphatidylserine was evident when the binding to phosphatidylcholine/phosphatidylserine/phosphatidylinositol-4,5-bisphosphate (70:25:5 molar ratio) was measured. In this case when binding data were fitted to a two sets of site models, the K d values were 1.6 and 1.7 μM, and when the data were fitted to a one-site model, the K d was 1.9 μM (Table 1). This value is very similar to the K d observed for the binding to phosphatidylcholine/phosphatidylinositol-4,5-bisphosphate (1.85 μM), indicating that the affinity for phosphatidylinositol-4,5-bisphosphate is much higher than that for phosphatidylserine. The affinity was also found to be higher for phosphatidylinositol-4,5-bisphosphate than for other phosphoinositides, such as phosphatidylinositol-3,4,5-trisphosphate, phosphatidylinositol-4-phosphate, phosphatidylinositol-3,5-bisphosphate, phosphatidylinositol-3-phosphate and phosphatidylinositol (Sanchez-Bautista et al. 2006).
Table 1.
Determination of the dissociation constant values of lipid binding to the protein kinase Cα C2 domain using isothermal titration calorimetry measurement and comparison of the wild-type C2 domain to other forms that have been the subject of target-site mutations
| Protein | Lipida | Presence of Ca2+ | K d (μM) | Reference |
|---|---|---|---|---|
| WT-C2 | Soluble IP3 | + | 19.8 ± 1.7 | Guerrero-Valero et al. (2009) |
| WT-C2 | POPC/POPS (2:3 molar ratio) | + | 18.0 ± 1.2 | Torrecillas et al. (2004) |
| WT-C2 | POPC/POPS (2:3 molar ratio) | - | 430 ± 23 | Torrecillas et al. (2004) |
| WT-C2 | POPC/PIP2 (95:5 molar ratio) | + | 1.9 ± 0.4 | Sanchez-Bautista et al. (2006) |
| WT-C2 | POPC/PIP2 (95:5 molar ratio) | - | 48.3 ± 9.7 | Sanchez-Bautista et al. (2006) |
| C2 D246/248N | POPC/PIP2 (95:5 molar ratio) | + | 26.5 ± 5.0 | Sanchez-Bautista et al. (2006) |
| C2 K209/211A | POPC/PIP2 (95:5 molar ratio) | + | No binding detected | Sanchez-Bautista et al. (2006) |
| WT-C2 (two sets of sites) | POPC/POPS/PIP2 (70:25:5 molar ratio) | + | 1.6 ± 0.2 1.7 ± 0.4 |
Guerrero-Valero et al. (2007) |
| WT-C2 (one set of sites) | POPC/POPS/PIP2 (70:25:5 molar ratio) | + | 1.9 ± 0.1 | Guerrero-Valero et al. (2007) |
K d,Equilibrium dissociation constant; WT-C2, wild-type protein kinase C (PKC) domain;
When phospholipids were used, they were in the form of sonicated vesicles. When Ca2+ was present, it was added to reach saturating concentrations. In the last two cases, the origin for isothermal titration calorimetry (ITC) allowed fitting the results to two sets of binding sites model or to a one-site model
aIP3, Inositol 1,4,5-trisphosphate; POPC, phosphatidylcholine; PIP2, phosphatidylinositol-4,5-bisphosphate; POPS, phosphatidylserine
Fig. 2.
Phosphatidylinositol-4,5-bisphosphate binds to the lysine-rich cluster of the PKCα-C2 domain. a Binding isotherm obtained by using isothermal titration calorimetry (ITC) to measure the titration of 30 mM vesicles containing a mixture of phosphatidylcholine/phosphatidylinositol-4,5-bisphosphate (PIP 2) (molar ratio 95:5) into a cell containing 60 μM of the C2α-D246N/D248N mutant in the presence of 1 mM CaCl2. b Binding isotherm obtained by using ITC to measure the titration of 10 mM vesicles containing a mixture of phosphatidylcholine/ phosphatidylinositol-4,5-bisphosphate (molar ratio 95:5) into a cell containing 60 μM of the C2α-K209A/K211A mutant in the presence of 1 mM CaCl2
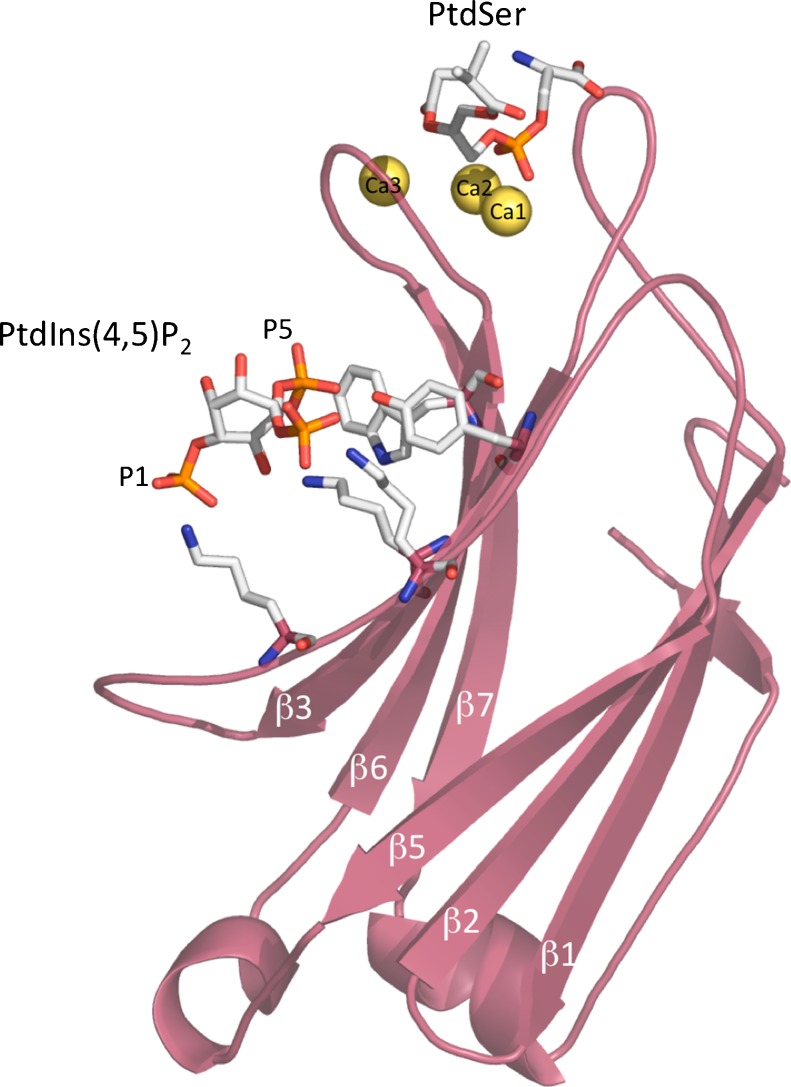
A full picture of the mode of interaction of phosphatidylinositol-4,5-bisphosphate with the C2 domain of PKCα was obtained from the crystal structure of this domain in a complex with Ca2+, a phosphatidylserine and a soluble form of phosphatidylinositol-4,5-bisphosphate with short acyl chains (Fig. 3) (Guerrero-Valero et al. 2009). Phosphatidylserine was observed to bind to the calcium-binding region, as reported by Verdaguer et al. (1999), whereas phosphatidylinositol-4,5-bisphosphate occupied the concave β3 and β4. Strikingly, the structure revealed a phosphatidylinositol-4,5-bisphosphate-C2 domain-binding mode in which the aromatic residues Tyr-195 and Trp-245 established direct interactions with the phosphate moieties of the inositol ring. Mutations that abolished Tyr-195 and Trp-245 recognition of phosphatidylinositol-4,5-bisphosphate severely impaired the ability of PKCα to localize in the plasma membrane. These authors also reported that these residues were highly conserved among C2 domains of topology type I (Guerrero-Valero et al. 2009).
Fig. 3.
Structure of PKCα-C2 domain bound to Ca2+-phosphatidylserine–phosphatidylinositol-4,5-bisphosphate in a quaternary complex. The C2 molecule is shown in magenta. The three calcium ions are shown in yellow spheres, with one of these spheres bridging the protein with PtdSer at the tip of the domain. The PIP2 molecule is bound to the β3–β4 chains (Guerrero-Valero et al. 2009). Protein Data Bank (PDB) accession number 3GPE
Rapid-binding kinetic studies of classical PKCα-C2 domains to lipid vesicles: the effect of phosphatidylinositol-4,5-bisphosphate
The mechanism of the Ca2+-induced isolated binding of the C2 domain of PKCβII to anionic membranes was studied by stopped-flow fluorescence spectroscopy (Nalefski and Newton 2001) and found to proceed via at least two steps: (1) rapid binding of two or more Ca2+ ions to the free domain with relatively low affinity and (2) diffusion-controlled association of the Ca2+-occupied domain with vesicles. The presence of Ca2+ increases the affinity of the C2 domain for anionic membranes by both decreasing the dissociation rate constant (k off) and increasing the association rate constant (k on) for membrane binding. Experiments addressing the role of electrostatic interactions have revealed that these interactions stabilize the initial C2 domain–membrane encounter complex or the high-affinity membrane-bound complex. Studies with the full-length PKC βII indicated that Ca2+-induced binding to membranes by the full-length protein proceeds minimally via two kinetically resolvable steps: (1) a rapid bimolecular association of the enzyme with vesicles near the diffusion-controlled limit and, more likely, (2) subsequent conformational changes of the membrane-bound enzyme. As is the case for the C2 domain, the k off for full-length PKC βII increases when Ca2+ is rapidly removed (Nalefski and Newton 2001). Thus, both the C2 domain and the slow conformational change prolong the lifetime of the PKC βII–membrane ternary complex in the presence of Ca2+, with rapid membrane release triggered by the removal of Ca2+. These results provide a molecular explanation for cofactor regulation of PKC, whereby the C2 domain searches three-dimensional space at the diffusion-controlled limit to direct PKC towards relatively common anionic phospholipids.
Corbin et al. (2007) used stopped-flow technology to study the binding of a C2 domain, in this case that of PKCα, to model membranes. They also investigated how the binding of isolated C2 domain to model membranes depends not only on Ca2+ concentration, but also on the interplay between Ca2+ concentration and the phospholipid targets located in the membrane, namely phosphatidylserine and phosphatidylinositol-4,5-bisphosphate. These authors proposed that the C2 domain has a very low Ca2+ affinity to be activated by physiological Ca2+ signals in most regions of the cell and that only when the C2 domain nears its target membrane, which provides a high local concentration of target lipid, is the effective Ca2+ affinity actually increased by the coupled binding equilibrium to a level that enables substantial Ca2+ activation and target docking. They also suggested that for the isolated PKCα C2 domain in the presence of physiological Ca2+ levels, the target lipids phosphatidylserine and phosphatidylinositol-4,5-bisphosphate are together sufficient to recruit the PKCα C2 domain to a lipid mixture mimicking the plasma membrane inner leaflet.
Scott et al. (2013) explored the molecular mechanisms by which the C2 domain controls the initial step in the activation of PKC using stopped-flow fluorescence spectroscopy to measure the k on and k off. These authors observed that hydrophobic interactions are the major driving force in the binding of the C2 domain to anionic membranes, whereas electrostatic interactions are dominant in membrane retention. More specifically, the mutation of given hydrophobic or certain basic residues in the Ca2+-binding loops reduces membrane affinity in a variety of ways, with the mutation of hydrophobic residues primarily altering the k on, whereas the mutation of charged residues primarily affect the k off. Live cell imaging revealed that the introduction of these mutations into full-length PKCα not only reduced the Ca2+-dependent translocation to plasma membrane but, by impairing the plasma membrane-sensing role of the C2 domain, caused phorbol ester-triggered redistribution of PKCα to other membranes, such as the Golgi. These data revealed that not only the amplitude but also the subcellular location of conventional PKC signaling can be tuned by altering the affinity of this module for membranes.
In another study, the membrane-binding kinetics of full-length PKCα were measured as a function of membrane lipid composition by Förster resonance energy transfer (FRET) in a stopped flow fluorescence study (Perez-Lara et al. 2012). The results best fitted a binding mechanism in which protein bound to the membrane following a two-phase mechanism in which a first bimolecular reaction was followed by a slow unimolecular reaction. In the absence of phosphatidylinositol-4,5-bisphosphate, the rapidity of the protein binding rate was especially dependent on phosphatidylserine concentration. Formation of the slow high-affinity complex during the second phase seems to involve specific interactions with phosphatidylserine and diacylglycerol since it is only sensitive to changes within relatively low concentration ranges of these lipids. Both the k on and the k off fell in the presence of phosphatidylinositol-4,5-bisphosphate. Based on these results, the authors proposed a model in which the interactions between full-length PKCα and the lipid membrane are mediated by a balance between nonspecific electrostatic interactions with anionic lipids (namely phosphatidylserine) and specific interactions with membrane-bound ligands; in contrast, PKCα binds to the membranes via a two-step mechanism consisting of the rapid initial recruitment of PKCα by membranes driven by interactions with phosphatidylserine and/or phosphatidylinositol-4,5-bisphosphate, although interactions with diacylglycerol may also be involved through the binding of the C1 domain.
Docked orientation of the PKCα-C2 domain to the membrane: modulation by phosphatidylinositol-4,5-bisphosphate
To obtain a comprehensive picture of how the interaction of the C2 domain with the membrane may modulate the activation of PKC, it is important to understand the docking mode. The membrane docking of C2 domains has been investigated using different biophysical techniques, including site-directed spin labeling, X-ray reflectivity and attenuated total reflectance (ATR)-infrared spectroscopy (IR).
Landgraf et al. (2008) used electron paramagnetic resonance (EPR) site-directed spin labeling and relaxation methods to generate a medium–resolution model of the PKCα C2 domain docking to a membrane of lipid. The results were used to define two membrane docking geometries for the C2 domain bound to physiological membranes lacking or containing phosphatidylinositol-4,5-bisphosphate, respectively. In the proposed model, both in the absence and in the presence of phosphatidylinositol-4,5-bisphosphate, two Ca2+ ions bound to the C2 domain lie near the anionic phosphate plane in the headgroup region, which is consistent with the known ability of the Ca2+ and membrane-binding loops (CBRs) to bind to the headgroup of the phosphatidylserine target lipid. These authors proposed that when phosphatidylinositol-4,5-bisphosphate is present, this phospholipid binds to the polybasic lipid binding site on the β3-β4 hairpin and that this large lipid would cause the long axis of the domain to tilt 40 ± 10° degrees toward the bilayer normal.
A different approach was based on X-ray reflectivity measurements was adopted by Chen et al. (2009). This technique was used to determine the configuration of the PKCα-C2 domain bound to a lipid monolayer of a 7:3 mixture of 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine and 1-stearoyl-2-oleoyl-sn-glycero-3-phosphoserine supported on a buffered aqueous solution. The reflectivity was analyzed in terms of the known crystallographic structure of PKCα-C2 and a slab model representation of the lipid layer. The configuration of lipid-bound PKCα-C2 was described by two angles that define its orientation, namely, θ = 35° ± 10° and ϕ =210° ± 30°, and a penetration depth (=7.5 ± 2 Å) into the lipid layer. In this structure, the β-sheets of PKCα-C2 are nearly perpendicular to the lipid layer and the domain penetrates into the headgroup region of the lipid layer, but not into the tail group region. The configuration of PKCα-C2, as determined by X-ray reflectivity, was analyzed to test all possible protein orientations, and the authors concluded that the obtained data cannot be explained by a protein that is orientated parallel to the membrane—rather they concluded that it is oriented with its main axes nearly perpendicular to the plane of the membrane (Chen et al. 2009).
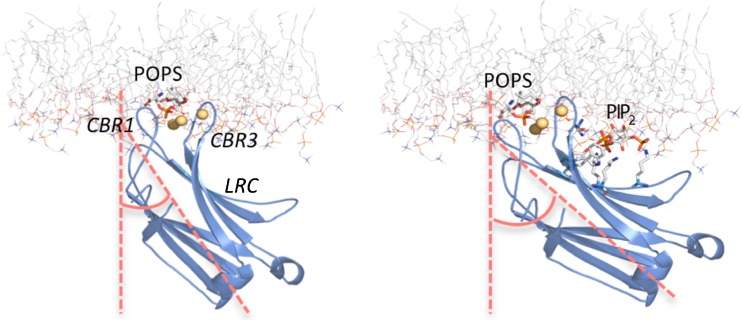
Different results were obtained when attenuated total ATR-IR spectroscopy was used to study the association of the PKCα-C2 domain with different phospholipid membranes (Ausili et al. 2011). Interestingly, when associated with phosphatidylcholine/phosphatidylserine membranes, the β-sandwich of the PKCα-C2 domain was found to be inclined at an angle α = 35° to the membrane normal, i.e. oriented more perpendicularly than parallel to the membrane. However, in the process of membrane docking, the tilt angle increased to α = 44° in the presence of phosphatidylinositol-4,5-bisphosphate, indicating that the β-sandwich comes closer to the membrane surface (Fig. 4) and thereby confirming the importance of this lipid in determining C2 domain docking and the consequent activation of PKCα (Ausili et al. 2011).
Fig. 4.
C2 domain of PKCα docked onto the surface of membranes without (left) and with (right) PIP2. The C2 domain of PKCα is represented as a ribbon model in blue by using the PDB codes 1DSY(left) (Verdaguer et al. 1999) and 3GPE (right) (Guerrero-Valero et al. 2009). The lipid membrane corresponds to a phosphatidylcholine (POPC) molecular dynamics simulation (POPC128A) (Hoff et al. 2005) and is represented as a stick model with carbon in gray, nitrogen in blue and oxygen in red. The headgroups of the two anionic phospholipids, phosphatidylserine (POPS) and PIP2 led us to dock the domain in the hydrophilic region of the membrane bilayer. It can be observed that in the presence of PIP2, the angle formed by the axes of the protein with the normal to the bilayer increases. Figure prepared with PyMOL (DeLano Scientific LLC, San Francisco, CA)
The results obtained by means of these three different biophysical approaches, namely EPR-relaxation (Landgraf et al. 2008), X-ray reflectivity (Chen et al. 2009) and ATR-IR spectroscopy, were compared after transforming the data as previously described (Ausili et al. 2011). Whereas EPR-relaxation enhancements within the membrane are well established in site-directed EPR by calibration with spin-labeled lipid chains (Marsh 1999; Kohout et al. 2002), those outside the membrane rely on uncertain extrapolations that rapidly become insensitive to position in the aqueous phase. This accounts for the lack of agreement between the orientation of the PKCα-C2 domain deduced from site-directed spin-labeling and that deduced from IR-dichroism (and X-ray reflectivity). Supporting this interpretation is the observation that the orientation deduced from site-directed EPR for the cytosolic phospholipase A2 (cPLA2) C2 domain, which penetrates the hydrophobic region of the membrane, agrees far more with the direct determination from IR-dichroism than from the non-penetrant PKCα-C2 domain (Ausili et al. 2011).
The three techniques so far employed in the study of C2 membrane docking supply useful information and ideally could be used in combination.
Subcellular studies of PKCα translocation to the membranes and the role of phosphatidylinositol-4,5-bisphosphate
One of the most productive ways of studying the activation of PKCs in vivo has been to study them fused to fluorescent proteins and subsequently overexpressed in a variety of cells so that their subcellular location can be followed by confocal microscopy (Sakai et al. 1997; Oancea and Meyer 1998; Wang et al. 1999; Bolsover et al. 2003). While this approach enables the translocation of classical PKCs to the membrane to be detected, with the limitation that binding to the membrane is necessary for activation, enzymatic activity cannot be measured. However, this inconvenience may be overcome in some cases by using systems that allow the phosphorylation activity of PKCs within the cell to be measured (Violin and Newton 2003; Violin et al. 2003).
Many cell systems have been used as a model of the in vivo behavior of classical PKCs, but in each type of cell different signals have been seen to be involved in the translocation of these enzymes to the membrane. Oancea and Meyer (1998) reported that the stimulation of receptors in RBL-2H3 cells both triggered repetitive calcium spikes and induced a repetitive translocation of green fluorescent protein (GFP)-tagged PKCγ to the plasma membrane. These authors observed that while calcium acted rapidly, diacylglycerol binding to PKCγ was initially prevented by a pseudosubstrate clamp, which kept the diacylglycerol-binding site inaccessible and delayed calcium- and diacylglycerol-mediated kinase activation. After termination of the calcium signals, bound diacylglycerol prolonged the kinase activity (Oancea and Meyer 1998). Using the same RBL-2H3 cell system, Bolsover et al. (2003) demonstrated that full-length PKCα tagged with a GFP behaved in a similar way to PKCγ. Further substitution of several aspartate residues by asparagine in the Ca2+phosphatidylserine-binding site of PKCγ completely abolished its Ca2+-dependent membrane targeting. Strikingly, these mutations enabled the mutant proteins to translocate in a diacylglycerol-dependent manner, indicating that once the calcium-binding region in the C2 domain is neutralized, the C1 domain is more accessible to diacylglycerol.
Evans et al. (2006) used intracellular co-imaging of fluorescent fusion proteins and an in vitro FRET membrane-binding assay to further investigate the nature of the translocation of the C2 domain to the membrane. These authors found that Ca2+-activated PKCα and its isolated C2 domain localize exclusively in the plasma membrane in vivo and that phosphatidylinositol-4,5-bisphosphate, located in the plasma membrane, dramatically enhances the Ca2+-triggered binding of the C2 domain to membranes in vitro. Similarly, a hybrid construct substituting the PKCα Ca2+-binding loops and phosphatidylinositol-4,5-bisphosphate binding site (β-strands 3–4) into a different C2 domain exhibited a native Ca2+-triggered targeting to the plasma membrane and recognizing phosphatidylinositol-4,5-bisphosphate. Conversely, a hybrid containing the Ca2+-binding loops but lacking the phosphatidylinositol-4,5-bisphosphate site translocated primarily to the trans-Golgi network and failed to recognize phosphatidylinositol-4,5-bisphosphate. Similarly, PKCα C2 domains possessing mutations in the phosphatidylinositol-4,5-bisphosphate site primarily targeted the trans-Golgi network and failed to recognize phosphatidylinositol-4,5-bisphosphate. Overall, these findings suggest that Ca2+-binding loops are essential for Ca2+-triggered membrane binding but that they are not sufficient for specific plasma membrane targeting. Instead, targeting specificity is provided by basic residues on β-strands 3–4, which bind to plasma membrane phosphatidylinositol-4,5-bisphosphate. The specificity for different subcellular compartments was confirmed by in vitro experiments in which the C2 domain of PKCα was compared with the C2 domain of cytosolic phospholipase A2α (Corbin et al. 2007). Based on this comparison, the authors suggested that at sufficiently high Ca2+ concentrations, binding of the C2 domain to the target lipid phosphatidylserine would be sufficient to drive membrane association; however, at typical physiological Ca2+ concentrations, binding to both phosphatidylserine and phosphatidylinositol-4,5-bisphosphate would be required for specific plasma membrane targeting (Lai et al. 2010).
Another cell system that has been studied involved nerve growth factor (NFG)-differentiated pheochromocytoma PC12 cells, which are commonly used as a neural differentiation model and which are also differentiated by trinucleotide-stimulation of cell receptors (Marin-Vicente et al. 2005). Surprisingly, these authors observed that the Ca2+ influx initiated by the ATP stimulation of P2X receptors, rather than the Ca2+ released from the intracellular stores, was the driving force behind the translocation of PKCα to the plasma membrane. They also showed that the localization process depended on two regions of the C2 domain: the Ca2+-binding region and the lysine-rich cluster, with the former binding bind Ca2+and the latter binding phosphatidylinositol 4,5-bisphosphate. It was demonstrated that diacylglycerol was not involved in the localization of PKCα through its C1 domain, while the presence of phosphatidylinositol-4,5-bisphosphate increased the permanence of PKCα in the plasma membrane. Finally, the results showed that ATP cooperated with NGF during the differentiation process of PC12 cells by increasing the length of the neurites, an effect that was inhibited when the cells were incubated in the presence of a specific inhibitor of PKCα, suggesting a role for this isoenzyme in the neural differentiation process (Marin-Vicente et al. 2005). Therefore, unlike other cell systems studied, such as the RBL-2H3 system (Bolsover et al. 2003) or NIH3T3 cells (Rodriguez-Alfaro et al. 2004), the main driving force that triggers membrane translocation of PKCα is not the hydrolysis product of PIP2 but the Ca2+ ions entering the cell from the outside (Marin-Vicente et al. 2005). Overall, these results suggest a novel mechanism of PKCα activation in differentiated PC12 cells, whereby Ca2 influx, together with phosphatidylinositol-4,5-bisphosphate, anchors the PKCα to the plasma membrane through two distinct motifs of its C2 domain, leading to enzyme activation.
The importance of phosphatidylinositol-4,5-bisphosphate for the membrane translocation of PKCα in PC12 cells was demonstrated in one study in which the concentration of this phosphoinositide was altered in the cell membranes using a rapamycin-triggered heterodimerization strategy (Banaszynski and Wandless 2006; Heo et al. 2006). This tool allows the rapid modification of phosphatidylinositol-4,5-bisphosphate levels in the plasma membrane and, thereby, the study of its influence on different processes (Marin-Vicente et al. 2008). In their study, Marin-Vicente et al. (2008) showed that an increase in the phosphatidylinositol-4,5-bisphosphate concentration prolongs the permanence of PKCα in the plasma membrane when PC12 cells are stimulated with ATP, independently of the diacylglycerol generated. The depletion of this phosphoinositide decreases both the percentage of protein able to translocate to the plasma membrane and its permanence there. It has been demonstrated that the Lys residues located in the polybasic cluster located in the C2 domain of PKCα (Marin-Vicente et al. 2008) and also other residues located in this groove (as Tyr-195 and Trp-245) (Guerrero-Valero et al. 2009) are responsible for this phosphoinositide–protein interaction.
Furthermore, the C2 domain acts as a dominant interfering module in the neural differentiation process of PC12 cells (Marin-Vicente et al. 2008; Guerrero-Valero et al. 2009). Additionally, an inhibitory effect was obtained by knocking down PKCα with small interfering RNA duplexes. Taken together, these data demonstrate that phosphatidylinositol-4,5-bisphosphate itself targets PKCα to the plasma membrane through the polybasic cluster located in the C2 domain, an interaction that is critical for the signaling network involved in neural differentiation triggered by NGF and ATP. Of great interest in this respect is the study of downstream signaling partners of PKCα by Marin-Vicente et al. (2011); these authors approached the problem by analyzing the proteins co-immunoprecipitated with this enzyme in PC12 cells differentiated with NGF and ATP. Liquid chromatography-tandem mass spectrometry analysis (LC-MS/MS) identified plectin, peripherin, filamin A, fascin and β-actin as potential interacting proteins. The colocalization of PKCα and its interacting proteins increased when PC12 cells were differentiated with NGF and ATP. This analysis detected cytoskeletal components acting at two levels, i.e. the assembly of the intermediate filament peripherin and the organization of cortical actin. Peripherin and plectin organization and the cortical remodeling of β-actin were dramatically affected when PKCα was downregulated, suggesting that all three proteins might be functional targets of ATP-dependent PKCα signaling.
Role of phosphatidylinositol 4,5-bisphosphate in the catalytic activation of classical PKCs
The classical model for the activation of conventional PKCs always considers the need of Ca2+/phosphatidylserine and diacylglycerol as allosteric regulators (Newton 2010). It has been extensively demonstrated that these regulators need a coordinated binding to the C2 and C1 domains, respectively, to allow high-affinity membrane recruitment and activation since the elevation of only one of these second messengers is not sufficient to allow efficient membrane recruitment (Oancea and Meyer 1998; Bolsover et al. 2003; Corbalan-Garcia and Gomez-Fernandez 2006; Newton 2010).
Site-directed mutagenesis of the residues located in the calcium-binding region (D187, D246 and D248) of the C2 domain has been observed to substantially inhibit the enzymatic activity of PKCα (Medkova and Cho 1998a; Conesa-Zamora et al. 2000). It was also demonstrated that C2 domain functionality cannot be overcome by an excess of the physiological activator diacylglycerol, suggesting that both domains act in a concerted and sequential order (Medkova and Cho 1998b; Conesa-Zamora et al. 2000). The latest model for conventional PKC activation implies that once the enzyme is in a catalytically competent but inactive conformation, Ca2+-binding to the C2 domain has to occur to pretarget PKC to the plasma membrane, where it binds anionic phospholipids (Medkova and Cho 1999; Conesa-Zamora et al. 2001; Corbalan-Garcia et al. 2003; Newton 2010). This first interaction facilitates the encounter of the second ligand diacylglycerol that binds to the C1 domain and fully activates the enzyme (Newton 1995).
The finding that the phosphatidylinositol-4,5-bisphosphate molecule itself specifically interacts with the polybasic cluster of the C2 domain of classical PKCs (Corbalan-Garcia et al. 2003; Marin-Vicente et al. 2005; Sanchez-Bautista et al. 2006; Corbin et al. 2007; Guerrero-Valero et al. 2007, 2009; Marin-Vicente et al. 2008; Lai et al. 2010) has changed the classical view of the localization/activation process for these enzymes since the main source of second messengers now converts into a messenger itself. As mentioned above, increasing the concentration of phosphatidylinositol-4,5-bisphosphate both in model lipid vesicles and at the plasma membrane (Corbalan-Garcia et al. 2003; Sanchez-Bautista et al. 2006; Marin-Vicente et al. 2008) leads to an increase in the affinity of the domain to bind to the membrane based on the longer residence time of the protein (Corbin et al. 2007; Perez-Lara et al. 2012). In addition, the inclusion of phosphatidylinositol-4,5-bisphosphate in the lipid vesicles induces a several-fold increase in the catalytic activity of the enzyme that is not improved by the addition of phorbol ester or diacylglycerol (Corbalan-Garcia et al. 2003; Egea-Jimenez et al. 2013). The use of selective mutants that block either the calcium binding region (D246/248 N) or the lysine-rich cluster (K209/K211A) has been a very powerful and informative tool to unveil the role of each motif in localization and activation of the enzyme (Corbalan-Garcia et al. 2003).
When the enzyme was activated by phosphatidylinositol-4,5-bisphosphate, the catalytic activity of the lysine-rich cluster mutant was completely abolished. Taking into account that the Ca2+-binding region was intact in this case, it is clear that the polybasic cluster is essential in the activation process driven by phosphatidylinositol-4,5-bisphosphate (Corbalan-Garcia et al. 2003). Experiments performed with the calcium-binding region mutant also revealed that some contribution from this site is needed to obtain full activation of the enzyme, strongly suggesting that Ca2+ binding to its own region is a preliminary requirement for the phosphatidylinositol-4,5-bisphosphate interaction with the lysine-rich cluster in the C2 domain. Importantly, when the phorbol ester was added to the lipid vesicles, no relative improvement in the catalytic activity of the C2 domain mutants was observed, further confirming that having a fully functional C1 domain does not overcome the key function accomplished by the C2 domain-Ca2+/phosphatidylinositol-4,5-bisphosphate interactions (Corbalan-Garcia et al. 2003).
Another important finding was the observation that the same mutants of the calcium-binding site and the polybasic cluster behave completely different when the activating lipid vesicles contain phosphatidylserine as the only anionic phospholipid. In this scenario, mutation of the polybasic cluster does not have any effect on catalytic activation and, however, the calcium-binding region mutant is functionally completely inactive. These results indicate that when the activating phospholipid is phosphatidylserine, the C2 domain function is led by the calcium-binding region which in turn requires diacylglycerol to fully activate the enzyme (Bell et al. 1986; Nishizuka 1992; Corbalan-Garcia et al. 2003; Egea-Jimenez et al. 2013). Furthermore, extensive substitutions of the lysine residues in the cluster (K1977A/K199A/K211A) lead to constitutive activation of PKCα (phosphatidylserine-dependent activation), suggesting that these residues are involved in an intramolecular interaction that is disrupted by Ca2+/phosphatidylserine interactions of the wild-type protein.
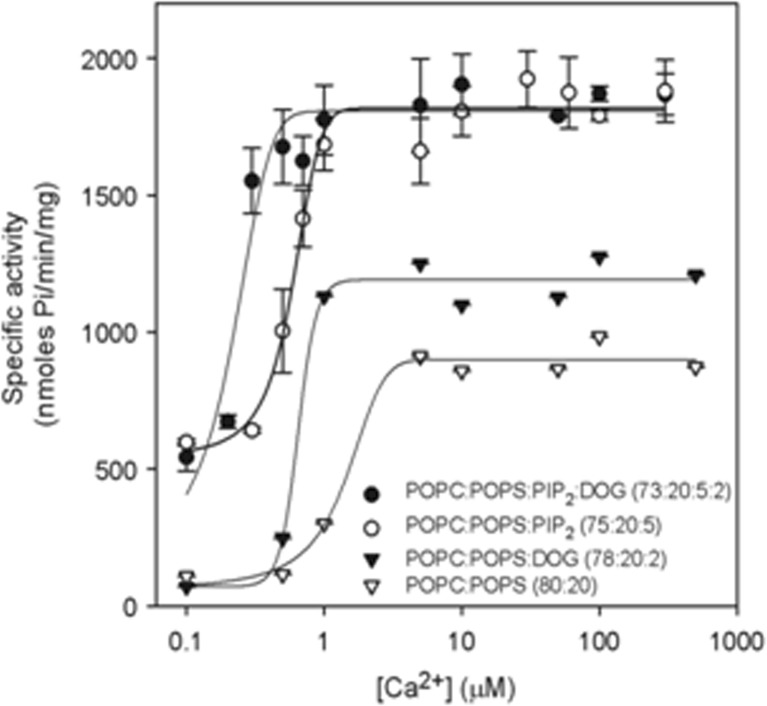
The kinetic modulation of PKCα in the presence of phosphatidylinositol-4,5-bisphosphate has been studied in detail (Egea-Jimenez et al. 2013), revealing that diacylglycerol does play a role, especially in the absence of phosphatidylinositol-4,5-bisphosphate, but that in the presence of the phosphoinositide, this role is reduced. The enzymatic activity of PKCα was studied by activating it with large unilamellar lipid vesicles and varying the concentration of Ca2+ and the contents of diacylglycerol, phosphatidylinositol-4,5-bisphosphate and phosphatidylserine (Fig. 5). The results showed that phosphatidylinositol-4,5-bisphosphate increased the V max (maximum initial velocity under these conditions) of PKCα and that when the phosphatidylinositol-4,5-bisphosphate concentration was 5 mol% of the total lipid in the membrane, the addition of 2 mol% of diacylglycerol did not increase enzyme activity. In addition, phosphatidylinositol-4,5-bisphosphate decreased the K 0.5 of Ca2+ by more than threefold, that of diacylglycerol by almost fivefold and that of phosphatidylserine by almost one-half. TheK 0.5 values of phosphatidylinositol-4,5-bisphosphate amounted to only 0.11 μM in the presence of diacylglycerol and 0.39 in its absence, which is within the expected physiological range for the inner monolayer of a mammalian plasma membrane. As a consequence, PKCα may be expected to operate near its maximum capacity even in the absence of a cell signal-producing diacylglycerol. Nevertheless, we have shown that the presence of diacylglycerol may also help, since the K0.5 for phosphatidylinositol-4,5-bisphosphate notably decreased in its presence. Taken together, these results underline the great importance of phosphatidylinositol-4,5-bisphosphate in the activation of PKCα and demonstrate that in its presence, the most important cell signal for triggering the activity of this enzyme is the increase in the concentration of cytoplasmic Ca2+.
Fig. 5.
The dependence of PKCα activity on Ca2+ concentration. The molar ratios of the lipid components of the vesicles used to activate the enzyme are shown. Ca2+ concentration was 200 μmol. The standard deviation was calculated from three independent experiments. It is shown that the calcium concentration required for maximum activity is decreased in the presence of PIP2. DOG Diacylglycerol. Taken from Egea-Jimenez et al. (2013)
Molecular model for the activation of classical PKCs: role of phosphatidylinositol 4,5-bisphosphate
The recent 3D structure determined for PKCβII (reference molecule) has shed light on the relative orientation of these domains in the full-length context of the enzyme and is providing invaluable data to researchers in this field for use in the interpretation of the myriad of biological and biochemical results obtained by different groups. The combination of X-ray diffraction and small-angle X-ray-scattering has revealed a structural intermediate that immediately suggests that upon Ca2+-binding, the pseudosubstrate liberates from the substrate peptide binding site in the catalytic domain, leading also to a disengagement of the C1A domain independently of phospholipid and diacylglycerol binding (Leonard et al. 2011). Unfortunately, the crystal structure lacked the pseudosustrate sequence and the C1A domain, suggesting that they adopt several conformations that are incompatible with a unique diffraction pattern. Small-angle X-ray scattering analysis further confirmed that the C2 domain projects away from the rest of PKC molecule and that it makes contact with the C1B domain or/and associated linkers but not with the C1A or catalytic domains. In addition, the C1B domain keeps its diacylglycerol-binding buried and occupied in clamping the NFD helix located at the turn motif in the catalytic domain, showing an intermediate state of the protein in which the pseudosustrate has been already liberated from the active site but the enzyme still lacks F629 in a correct position to interact with the adenine ring at the ATP-binding site. The importance of this C1B–NFD helix interaction for phorbol ester-dependent localization of the enzyme has been demonstrated by the substitution of key residues either at the C1B or NFD helix (Leonard et al. 2011). However, due to the double role of these two motifs in the activation of the enzyme, it is difficult to compare the direct effect of these mutations on its catalytic activity. It is intriguing that previous work by Cho and co-workers (Medkova and Cho 1999) demonstrated that mutations at the C1B domain (L125G) did not have any effect on diacylglycerol-dependent membrane binding, activation and monolayer penetration. This demonstrates that unleashing by mutagenesis of the NFD helix does not increase the membrane binding, nor the catalytic activity, and indirectly suggests that the C1A interaction with diacylglycerol might have already induced the conformational change that liberates the C1B-NFD clamp. The apparent discrepancies between these two types of experiments could be explained by the different affinities that independent C1 domains exhibit for diaclyglycerol and phorbol esters (see comparative table in Corbalan-Garcia and Gomez-Fernandez 2006).
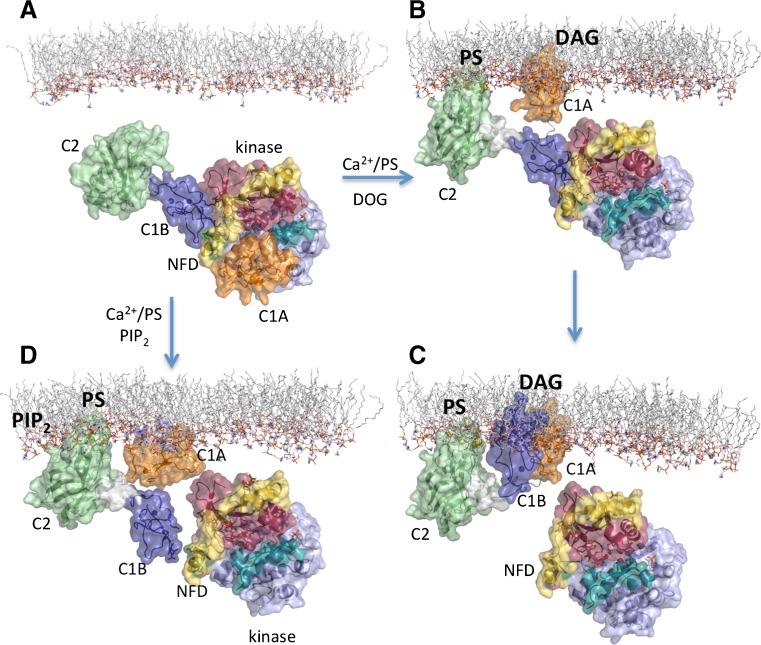
All of this information seems to indicate that classical PKCs might be able to respond to different signaling in the cell by using their C2 and C1 domains (Fig. 6). Basically, increases in intracytosolic Ca2+ trigger a first translocation of PKCα to the plasma membrane. Once there, two situations may arise. The first in that in microdomains enriched only with phosphatidylserine, the docking of the C2 domain is not sufficient to enable full catalytic activity to be achieved and, consequently, the C1A domain is required to locate the target (diacylglycerol) at the membrane. This interaction would further unleash the C1B-NFD clamp to get fully activation of the enzyme (Leonard et al. 2011) (Fig. 6 ). Whether the C1B also anchors at the membrane through diacylglycerol or less specific interactions is something that will have to be further explored. The second possibility is that in microdomains enriched in phosphatidylserine and phosphatidylinositol-4,5-bisphosphate at the plasma membrane (Fig. 5), the C2 domain docks in a different orientation (Ausili et al. 2011) since it has to anchor through two different points, i.e. the calcium-binding region (Ca2+/phosphatidylserine) and the lysine-rich cluster (phosphatidylinositol-4,5-bisphosphate), which might induce a conformational change that already unleashes the C1B domain from the blocking conformation and fully activates the enzyme. Whether in this situation the C1 domains can interact with the membrane independently of diacylglycerol is not known, but there are reports indicating that the C1 domains can interact unspecifically with negatively charged phospholipids (Sanchez-Bautista et al. 2009).
Fig. 6.
Molecular model for the activation of classical PKCs. Two different scenarios are depicted, one is when Ca2+, POPS and DOG act as activators and the other is when PIP2 is additionally present. In the last case, it has been proposed that a change in the tilt of the domain with respect to the membrane facilitates the activation. Structures taken from PDB 3PFQ (Leonard et al. 2011)
Acknowledgments
This work was supported by Ministerio de Economía y Competitividad (Spain), grant [BFU2011-22828], co-financed by the European Regional Development Fund and 08700/PI/08 (Fundación Seneca, Region de Murcia).
Conflict of interest
None.
Footnotes
Special Issue Advances in Biophysics in Latin America
Contributor Information
Senena Corbalán-García, Phone: +34-868-884775, FAX: +34-868-884766, Email: senena@um.es.
Juan C. Gómez-Fernández, Phone: +34-868-884766, FAX: +34-868-884766, Email: jcgomez@um.es
References
- Ausili A, Corbalan-Garcia S, Gomez-Fernandez JC, Marsh D. Membrane docking of the C2 domain from protein kinase Calpha as seen by polarized ATR-IR. The role of PIP. Biochim Biophys Acta. 2011;1808(3):684–695. doi: 10.1016/j.bbamem.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Banaszynski LA, Wandless TJ. Conditional control of protein function. Chem Biol. 2006;13(1):11–21. doi: 10.1016/j.chembiol.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Bell RM, Hannun YA, Loomis CR. Mechanism of regulation of protein kinase C by lipid second messengers. Symp Fundam Cancer Res. 1986;39:145–156. [PubMed] [Google Scholar]
- Bolsover SR, Gomez-Fernandez JC, Corbalan-Garcia S. Role of the Ca2+/phosphatidylserine binding region of the C2 domain in the translocation of protein kinase Calpha to the plasma membrane. J Biol Chem. 2003;278(12):10282–10290. doi: 10.1074/jbc.M212145200. [DOI] [PubMed] [Google Scholar]
- Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257(13):7847–7851. [PubMed] [Google Scholar]
- Conesa-Zamora P, Gomez-Fernandez JC, Corbalan-Garcia S. The C2 domain of protein kinase calpha is directly involved in the diacylglycerol-dependent binding of the C1 domain to the membrane. Biochim Biophys Acta. 2000;1487(2–3):246–254. doi: 10.1016/S1388-1981(00)00099-8. [DOI] [PubMed] [Google Scholar]
- Conesa-Zamora P, Lopez-Andreo MJ, Gomez-Fernandez JC, Corbalan-Garcia S. Identification of the phosphatidylserine binding site in the C2 domain that is important for PKC alpha activation and in vivo cell localization. Biochemistry. 2001;40(46):13898–13905. doi: 10.1021/bi011303o. [DOI] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Garcia-Garcia J, Rodriguez-Alfaro JA, Gomez-Fernandez JC. A new phosphatidylinositol 4,5-bisphosphate-binding site located in the C2 domain of protein kinase Calpha. J Biol Chem. 2003;278(7):4972–4980. doi: 10.1074/jbc.M209385200. [DOI] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Gomez-Fernandez JC. Protein kinase C regulatory domains: the art of decoding many different signals in membranes. Biochim Biophys Acta. 2006;1761(7):633–654. doi: 10.1016/j.bbalip.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Corbin JA, Evans JH, Landgraf KE, Falke JJ. Mechanism of specific membrane targeting by C2 domains: localized pools of target lipids enhance Ca2+ affinity. Biochemistry. 2007;46(14):4322–4336. doi: 10.1021/bi062140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Malkova S, Pingali SV, Long F, Garde S, Cho W, Schlossman ML. Configuration of PKCalpha-C2 domain bound to mixed SOPC/SOPS lipid monolayers. Biophys J. 2009;97(10):2794–2802. doi: 10.1016/j.bpj.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea-Jimenez AL, Perez-Lara A, Corbalan-Garcia S, Gomez-Fernandez JC (2013) Phosphatidylinositol 4,5-bisphosphate decreases the concentration of Ca(2+), phosphatidylserine and diacylglycerol required for protein kinase C alpha to reach maximum activity. PLoS One 8(7):e69041. doi: 10.1371/journal.pone.0069041 [DOI] [PMC free article] [PubMed]
- Evans JH, Murray D, Leslie CC, Falke JJ. Specific translocation of protein kinase Calpha to the plasma membrane requires both Ca2+ and PIP2 recognition by its C2 domain. Mol Biol Cell. 2006;17(1):56–66. doi: 10.1091/mbc.E05-06-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah CA, Sossin WS. The role of C2 domains in PKC signaling. Adv Exp Med Biol. 2012;740:663–683. doi: 10.1007/978-94-007-2888-2_29. [DOI] [PubMed] [Google Scholar]
- Gomez-Fernandez JC, Corbalan-Garcia S. Diacylglycerols, multivalent membrane modulators. Chem Phys Lipids. 2007;148(1):1–25. doi: 10.1016/j.chemphyslip.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Guerrero-Valero M, Ferrer-Orta C, Querol-Audi J, Marin-Vicente C, Fita I, Gomez-Fernandez JC, Verdaguer N, Corbalan-Garcia S. Structural and mechanistic insights into the association of PKCalpha-C2 domain to PtdIns(4,5)P2. Proc Natl Acad Sci USA. 2009;106(16):6603–6607. doi: 10.1073/pnas.0813099106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Valero M, Marin-Vicente C, Gomez-Fernandez JC, Corbalan-Garcia S. The C2 domains of classical PKCs are specific PtdIns(4,5)P2-sensing domains with different affinities for membrane binding. J Mol Biol. 2007;371(3):608–621. doi: 10.1016/j.jmb.2007.05.086. [DOI] [PubMed] [Google Scholar]
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314(5804):1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff B, Strandberg E, Ulrich AS, Tieleman DP, Posten C. 2H-NMR study and molecular dynamics simulation of the location, alignment, and mobility of pyrene in POPC bilayers. Biophys J. 2005;88(3):1818–1827. doi: 10.1529/biophysj.104.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977;252(21):7610–7616. [PubMed] [Google Scholar]
- Jimenez-Monreal AM, Aranda FJ, Micol V, Sanchez-Pinera P, de Godos A, Gomez-Fernandez JC. Influence of the physical state of the membrane on the enzymatic activity and energy of activation of protein kinase C alpha. Biochemistry. 1999;38(24):7747–7754. doi: 10.1021/bi983062z. [DOI] [PubMed] [Google Scholar]
- Kohout SC, Corbalan-Garcia S, Torrecillas A, Gomez-Fernandez JC, Falke JJ. C2 domains of protein kinase C isoforms alpha, beta, and gamma: activation parameters and calcium stoichiometries of the membrane-bound state. Biochemistry. 2002;41(38):11411–11424. doi: 10.1021/bi026041k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CL, Landgraf KE, Voth GA, Falke JJ. Membrane docking geometry and target lipid stoichiometry of membrane-bound PKCalpha C2 domain: a combined molecular dynamics and experimental study. J Mol Biol. 2010;402(2):301–310. doi: 10.1016/j.jmb.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf KE, Malmberg NJ, Falke JJ. Effect of PIP2 binding on the membrane docking geometry of PKC alpha C2 domain: an EPR site-directed spin-labeling and relaxation study. Biochemistry. 2008;47(32):8301–8316. doi: 10.1021/bi800711t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard TA, Rozycki B, Saidi LF, Hummer G, Hurley JH. Crystal structure and allosteric activation of protein kinase C betaII. Cell. 2011;144(1):55–66. doi: 10.1016/j.cell.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Vicente C, Gomez-Fernandez JC, Corbalan-Garcia S. The ATP-dependent membrane localization of protein kinase Calpha is regulated by Ca2+ influx and phosphatidylinositol 4,5-bisphosphate in differentiated PC12 cells. Mol Biol Cell. 2005;16(6):2848–2861. doi: 10.1091/mbc.E05-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Vicente C, Nicolas FE, Gomez-Fernandez JC, Corbalan-Garcia S. The PtdIns(4,5)P2 ligand itself influences the localization of PKCalpha in the plasma membrane of intact living cells. J Mol Biol. 2008;377(4):1038–1052. doi: 10.1016/j.jmb.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Marin-Vicente C, Guerrero-Valero M, Nielsen ML, Savitski MM, Gomez-Fernandez JC, Zubarev RA, Corbalan-Garcia S. ATP enhances neuronal differentiation of PC12 cells by activating PKC alpha interactions with cytoskeletal proteins. J Proteome Res. 2011;10(2):529–540. doi: 10.1021/pr100742r. [DOI] [PubMed] [Google Scholar]
- Marsh D. Quantitation of secondary structure in ATR infrared spectroscopy. Biophys J. 1999;77(5):2630–2637. doi: 10.1016/S0006-3495(99)77096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medkova M, Cho W. Mutagenesis of the C2 domain of protein kinase C-alpha. Differential roles of Ca2+ ligands and membrane binding residues. J Biol Chem. 1998;273(28):17544–17552. doi: 10.1074/jbc.273.28.17544. [DOI] [PubMed] [Google Scholar]
- Medkova M, Cho W. Mutagenesis of the C2 domain of protein kinase Cepsilon. J Biol Chem. 1998;273:17544–17552. doi: 10.1074/jbc.273.28.17544. [DOI] [PubMed] [Google Scholar]
- Medkova M, Cho W. Interplay of C1 and C2 domains of protein kinase C-alpha in its membrane binding and activation. J Biol Chem. 1999;274(28):19852–19861. doi: 10.1074/jbc.274.28.19852. [DOI] [PubMed] [Google Scholar]
- Micol V, Sanchez-Pinera P, Villalain J, de Godos A, Gomez-Fernandez JC. Correlation between protein kinase C alpha activity and membrane phase behavior. Biophys J. 1999;76(2):916–927. doi: 10.1016/S0006-3495(99)77255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 2012;11(12):937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalefski EA, Newton AC. Membrane binding kinetics of protein kinase C betaII mediated by the C2 domain. Biochemistry. 2001;40(44):13216–13229. doi: 10.1021/bi010761u. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270(48):28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298(3):E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95(3):307–318. doi: 10.1016/S0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- Ochoa WF, Corbalan-Garcia S, Eritja R, Rodriguez-Alfaro JA, Gomez-Fernandez JC, Fita I, Verdaguer N. Additional binding sites for anionic phospholipids and calcium ions in the crystal structures of complexes of the C2 domain of protein kinase C alpha. J Mol Biol. 2002;320(2):277–291. doi: 10.1016/S0022-2836(02)00464-3. [DOI] [PubMed] [Google Scholar]
- Perez-Lara A, Egea-Jimenez AL, Ausili A, Corbalan-Garcia S, Gomez-Fernandez JC. The membrane binding kinetics of full-length PKCalpha is determined by membrane lipid composition. Biochim Biophys Acta. 2012;1821(11):1434–1442. doi: 10.1016/j.bbalip.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Alfaro JA, Gomez-Fernandez JC, Corbalan-Garcia S. Role of the lysine-rich cluster of the C2 domain in the phosphatidylserine-dependent activation of PKCalpha. J Mol Biol. 2004;335(4):1117–1129. doi: 10.1016/j.jmb.2003.10.080. [DOI] [PubMed] [Google Scholar]
- Sakai N, Sasaki K, Ikegaki N, Shirai Y, Ono Y, Saito N. Direct visualization of the translocation of the gamma-subspecies of protein kinase C in living cells using fusion proteins with green fluorescent protein. J Cell Biol. 1997;139(6):1465–1476. doi: 10.1083/jcb.139.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Bautista S, Marin-Vicente C, Gomez-Fernandez JC, Corbalan-Garcia S. The C2 domain of PKCalpha is a Ca2+-dependent PtdIns(4,5)P2 sensing domain: a new insight into an old pathway. J Mol Biol. 2006;362(5):901–914. doi: 10.1016/j.jmb.2006.07.093. [DOI] [PubMed] [Google Scholar]
- Sanchez-Bautista S, Corbalan-Garcia S, Perez-Lara A, Gomez-Fernandez JC. A comparison of the membrane binding properties of C1B domains of PKCgamma, PKCdelta, and PKCepsilon. Biophys J. 2009;96(9):3638–3647. doi: 10.1016/j.bpj.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pinera P, Micol V, Corbalan-Garcia S, Gomez-Fernandez JC. A comparative study of the activation of protein kinase C alpha by different diacylglycerol isomers. Biochem J. 1999;337(Pt 3):387–395. doi: 10.1042/0264-6021:3370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AM, Antal CE, Newton AC. Electrostatic and hydrophobic interactions differentially tune membrane binding kinetics of the c2 domain of protein kinase Calpha. J Biol Chem. 2013;288(23):16905–16915. doi: 10.1074/jbc.M113.467456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey NA, Leach KL, Blumberg PM. Competitive inhibition by diacylglycerol of specific phorbol ester binding. Proc Natl Acad Sci USA. 1984;81(2):607–610. doi: 10.1073/pnas.81.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Iwasa Y, Kawahara Y, Mori T, Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979;254(10):3692–3695. [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Kikkawa U, Mori T, Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979;91(4):1218–1224. doi: 10.1016/0006-291X(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Miyake R, Kikkawa U, Nishizuka Y. Rapid assay of binding of tumor-promoting phorbol esters to protein kinase C1. J Biochem. 1986;99(1):257–261. doi: 10.1093/oxfordjournals.jbchem.a135467. [DOI] [PubMed] [Google Scholar]
- Torrecillas A, Laynez J, Menendez M, Corbalan-Garcia S, Gomez-Fernandez JC. Calorimetric study of the interaction of the C2 domains of classical protein kinase C isoenzymes with Ca2+ and phospholipids. Biochemistry. 2004;43(37):11727–11739. doi: 10.1021/bi0489659. [DOI] [PubMed] [Google Scholar]
- Verdaguer N, Corbalan-Garcia S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. EMBO J. 1999;18(22):6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violin JD, Newton AC. Pathway illuminated: visualizing protein kinase C signaling. IUBMB Life. 2003;55(12):653–660. doi: 10.1080/152165401310001642216. [DOI] [PubMed] [Google Scholar]
- Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161(5):899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QJ, Bhattacharyya D, Garfield S, Nacro K, Marquez VE, Blumberg PM. Differential localization of protein kinase C delta by phorbol esters and related compounds using a fusion protein with green fluorescent protein. J Biol Chem. 1999;274(52):37233–37239. doi: 10.1074/jbc.274.52.37233. [DOI] [PubMed] [Google Scholar]
- Wu-Zhang AX, Newton AC. Protein kinase C pharmacology: refining the toolbox. Biochem J. 2013;452(2):195–209. doi: 10.1042/BJ20130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Webster SV, Newton PM. The biology of protein kinase C. Adv Exp Med Biol. 2012;740:639–661. doi: 10.1007/978-94-007-2888-2_28. [DOI] [PubMed] [Google Scholar]