Abstract
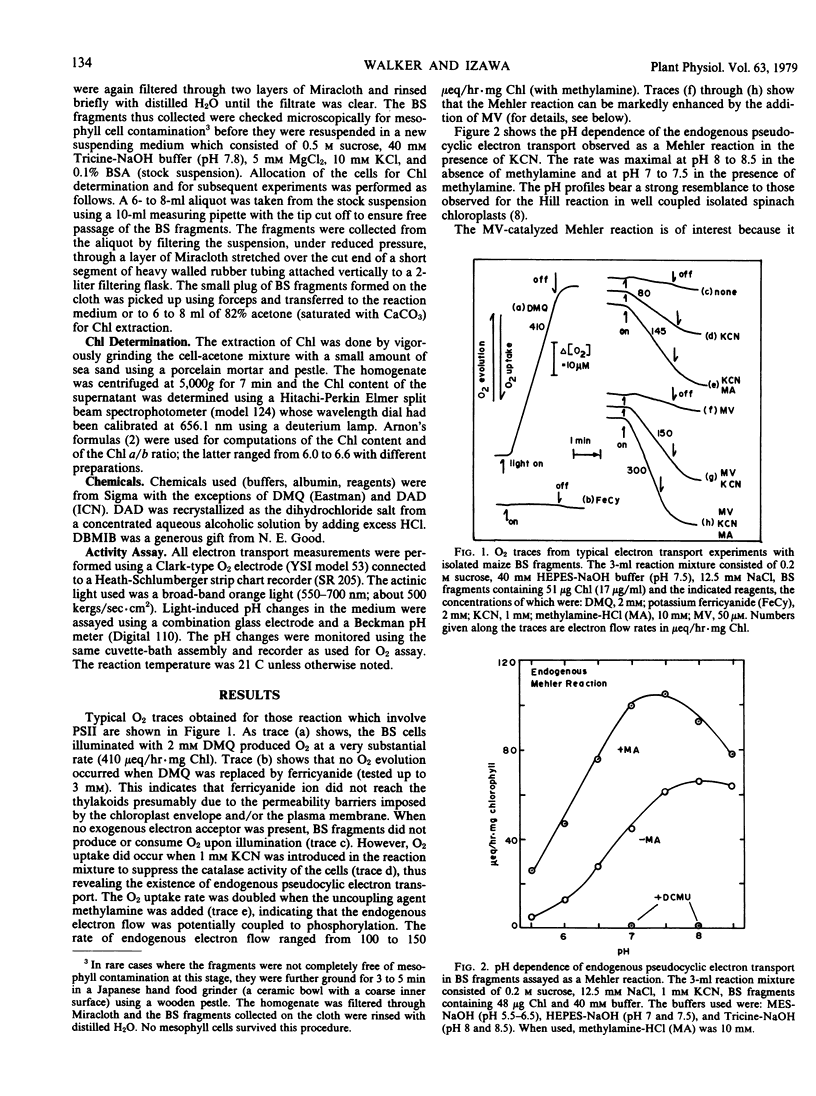
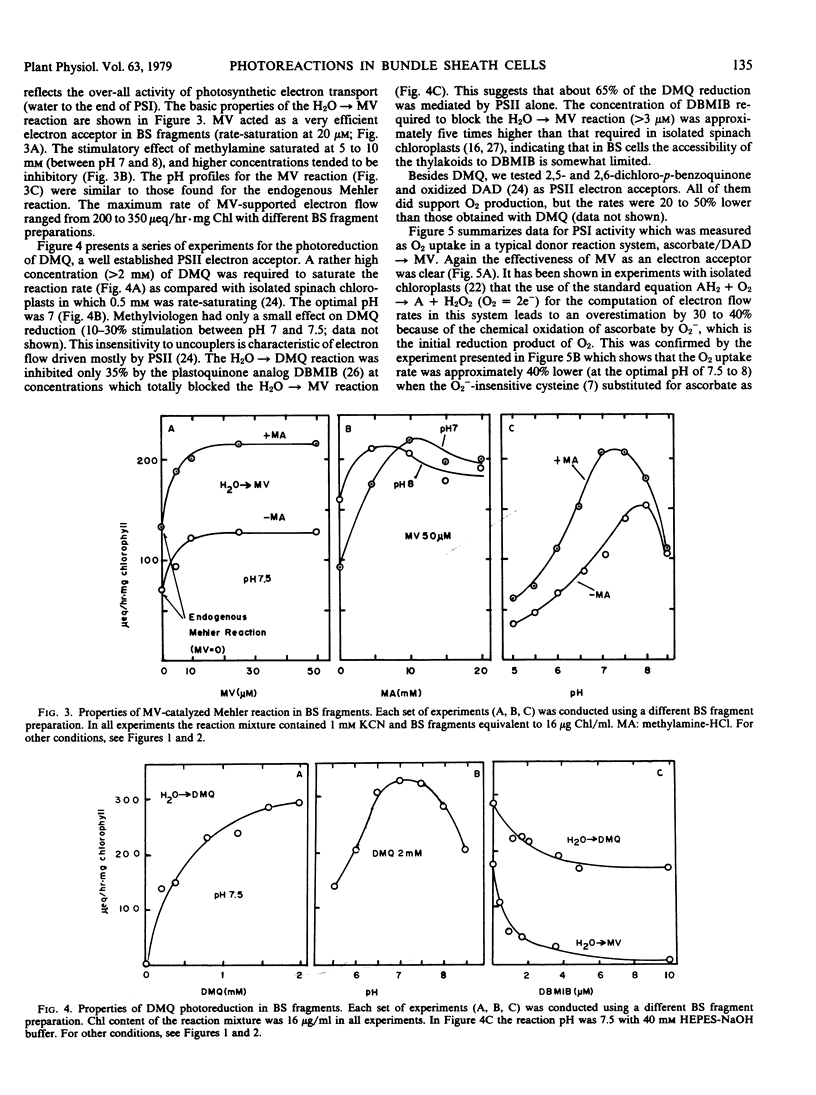
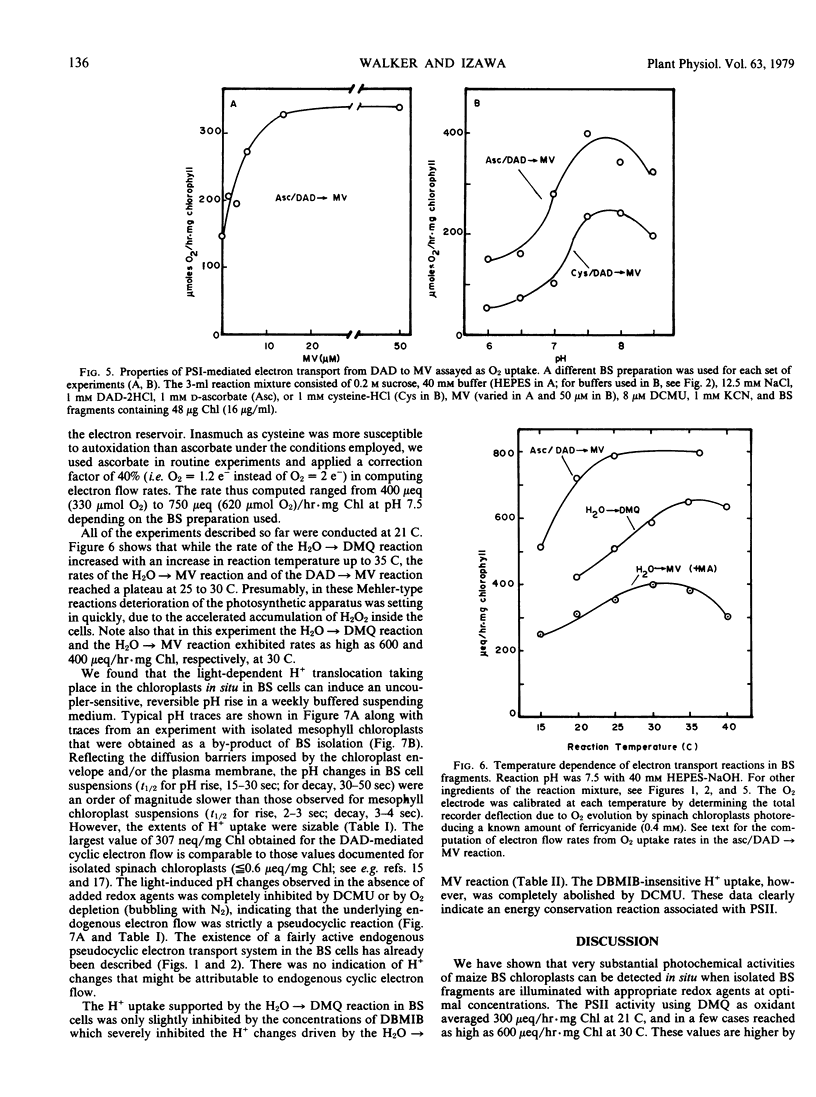
Fragments of bundle sheath strands, free of mesophyll cells and showing a chlorophyll a/b ratio of 6.0 to 6.6 were prepared from Zea mays by a mechanical method. They were unable to photoreduce ferricyanide but were able to photoreduce the membrane-permeant 2,5-dimethylquinone at a rate of 250 to 420 microequivalents per hour per mg chlorophyll (μeq/hr · mg Chl) at 21 C. In the presence of the catalase inhibitor KCN, methylviologen catalyzed a Mehler reaction at a rate of 120 to 180 μeq/hr · mg Chl. This was increased to 200 to 350 μeq/hr · mg Chl when the uncoupler methylamine was added. The rate of endogenous pseudocyclic electron flow, detected as a Mehler reaction, was also considerable (100 to 150 μeq/hr · mg Chl with methylamine). Diaminodurene supported a high rate of photosystem I-mediated electron flow to methylviologen (400 to 750 μeq/hr · mg Chl).
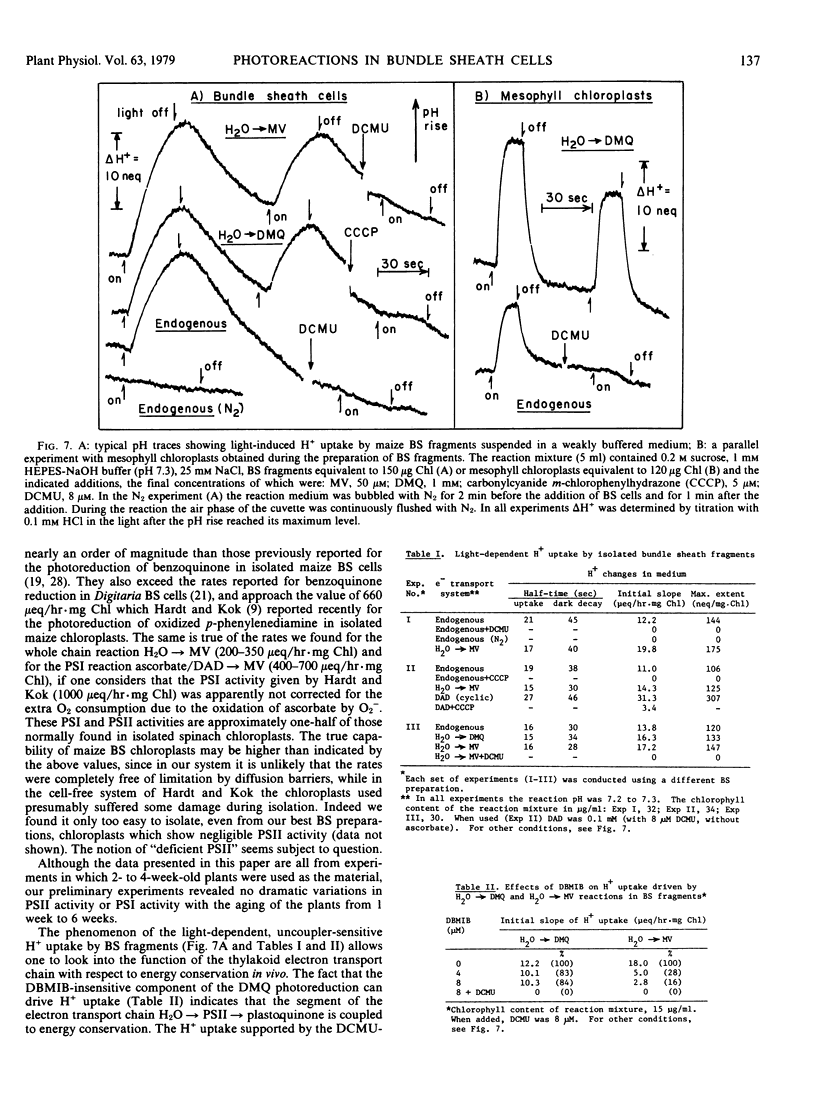
When the tissue fragments were illuminated in a weakly buffered suspension, a reversible rise in the medium pH was observed which apparently originated from H+ translocation in the thylakoids. The kinetics of the pH changes was rather slow (t½ > 15 seconds for pH rise; > 30 seconds for dark decay) but the extent of H+ uptake was substantial (0.1 to 0.3 μeq/mg Chl). All of the electron transport reactions tested, including partial reactions which involve only photosystem I or photosystem II, invariably supported H+ uptake. This suggests that two sites of energy conservation are associated with the photosynthetic chain in the bundle sheath chloroplasts (as in spinach chloroplasts) and that both of these sites are functional in vivo. The pH changes observed in the absence of exogenous electron carriers were abolished by 3-(3,4-dichlorophenyl)-1,1-dimethylurea or by anaerobiosis, indicating that the underlying endogenous electron transport was strictly a pseudocyclic reaction. There was no evidence of endogenous cyclic electron flow which might contribute to the energy metabolism of the bundle sheath cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. S., Bain J. M., Bishop D. G., Smillie R. M. Photosystem II Activity in Agranal Bundle Sheath Chloroplasts from Zea mays. Plant Physiol. 1972 Apr;49(4):461–466. doi: 10.1104/pp.49.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzaz M. B., Govindjee Photochemical properties of mesophyll and bundle sheath chloroplasts of maize. Plant Physiol. 1973 Sep;52(3):257–262. doi: 10.1104/pp.52.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. G., Andersen K. S., Smillie R. M. Incomplete membrane-bound photosynthetic electron transfer pathway in agranal chloroplasts. Biochem Biophys Res Commun. 1971 Jan 8;42(1):74–81. doi: 10.1016/0006-291x(71)90364-0. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Andersen K. S., Smillie R. M. pH Dependence and Cofactor Requirements of Photochemical Reactions in Maize Chloroplasts. Plant Physiol. 1972 Dec;50(6):774–777. doi: 10.1104/pp.50.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber W., Trebst A., Harth E. On a new inhibitor of photosynthetic electron-transport in isolated chloroplasts. Z Naturforsch B. 1970 Oct;25(10):1157–1159. doi: 10.1515/znb-1970-1018. [DOI] [PubMed] [Google Scholar]
- Epel B. L., Neumann J. The mechanism of the oxidation of ascorbate and MN2+ by chloroplasts. The role of the radical superoxide. Biochim Biophys Acta. 1973 Dec 14;325(3):520–529. doi: 10.1016/0005-2728(73)90211-9. [DOI] [PubMed] [Google Scholar]
- Hardt H., Kok B. Comparison of photosynthetic activities of spinach chloroplasts with those of corn mesophyll and corn bundle sheath tissue. Plant Physiol. 1978 Jul;62(1):59–63. doi: 10.1104/pp.62.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Kagawa T. Photosynthetic activities of isolated bundle sheath cells in relation to differing mechanisms of C-4 pathway photosynthesis. Arch Biochem Biophys. 1976 Jul;175(1):39–53. doi: 10.1016/0003-9861(76)90483-5. [DOI] [PubMed] [Google Scholar]
- Heber U. Energy coupling in chloroplasts. J Bioenerg Biomembr. 1976 Jun;8(3):157–172. doi: 10.1007/BF00748961. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Izawa S., Gould J. M., Ort D. R., Felker P., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. 3. A dibromothymoquinone-insensitive phosphorylation reaction associated with photosystem II. Biochim Biophys Acta. 1973 Apr 27;305(1):119–128. doi: 10.1016/0005-2728(73)90237-5. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Izawa S. Studies on the Energy-coupling Sites of Photophosphorylation: V. Phosphorylation Efficiencies (P/e(2)) Associated with Aerobic Photooxidation of Artificial Electron Donors. Plant Physiol. 1974 Mar;53(3):370–376. doi: 10.1104/pp.53.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya G. M., Osmond C. B. Photophosphorylation by mesophyll and bundle sheath chloroplasts of c(4) plants. Plant Physiol. 1972 Feb;49(2):267–269. doi: 10.1104/pp.49.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Izawa S., Good N. E. Photophosphorylation as a function of light intensity. Biochim Biophys Acta. 1970 Nov 3;223(1):158–164. doi: 10.1016/0005-2728(70)90140-4. [DOI] [PubMed] [Google Scholar]
- Saha S., Ouitrakul R., Izawa S., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. J Biol Chem. 1971 May 25;246(10):3204–3209. [PubMed] [Google Scholar]
- Smillie R. M., Andersen K. S., Tobin N. F., Entsch B., Bishop D. G. Nicotinamide adenine dinucleotide phosphate photoreduction from water by agranal chloroplasts isolated from bundle sheath cells of maize. Plant Physiol. 1972 Apr;49(4):471–475. doi: 10.1104/pp.49.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst A., Reimer S. Properties of photoreductions by photosystem II in isolated chloroplasts. An energy-conserving step in the photoreduction of benzoquinones by photosystem II in the presence of dibromothymoquinone. Biochim Biophys Acta. 1973 Apr 27;305(1):129–139. doi: 10.1016/0005-2728(73)90238-7. [DOI] [PubMed] [Google Scholar]
- Woo K. C., Anderson J. M., Boardman N. K., Downton W. J., Osmond C. B., Thorne S. W. Deficient Photosystem II in Agranal Bundle Sheath Chloroplasts of C(4) Plants. Proc Natl Acad Sci U S A. 1970 Sep;67(1):18–25. doi: 10.1073/pnas.67.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]


