Abstract
Glycosylation of small biologically active molecules, either of natural or synthetic origin, has a profound impact on their solubility, stability, and bioactivity, making glycoconjugates attractive compounds as therapeutic agents or nutraceuticals. A large proportion of secondary metabolites, including flavonoids, occur in plants as glycosides, which adds to the molecular diversity that is much valued in medicinal chemistry studies. The subsequent growing market demand for glycosidic natural products has fueled the development of various chemical and biotechnological methods of glycosides preparation. The review gives an extensive overview of the processes of the synthesis of isoflavones and discusses recently developed major routes towards isoflavone-sugar formation processes. Special attention is given to the derivatives of genistein, the main isoflavone recognized as a useful lead in several therapeutic categories, with particular focus on anticancer drug design. The utility of chemical glycosylations as well as glycoconjugates preparation is discussed in some theoretical as well as practical aspects. Since novel approaches to chemical glycosylations and glycoconjugations are abundant and many of them proved suitable for derivatization of polyphenols a new body of evidence has emerged, indicating that sugar moiety can play a much more significant role, when attached to a pharmacophore, then being a mere “solubilizer”. In many cases, it has been demonstrated that semisynthetic glycoconjugates are much more potent cytostatic and cytotoxic agents than reference isoflavones. Moreover, the newly designed glycosides or glycoside mimics can act through different mechanisms than the parent active molecule.
Keywords: Chemical glycosylations, isoflavone glycoconjugates, isoflavones
1. Introduction
The crucial role of natural products in drug discovery and development, in a historical perspective as well as for contemporary medicine, has been well documented and remains beyond dispute [1-3]. Recently, a slightly futuristic notion of whole plants as miniaturized pharmaceutical factories is becoming popularized in connection with currently available transgenic biotechnology for biosimilar pharmaceutics, leading to biosynthesis of therapeutic proteins, which are designed to function as orally active therapeutics, antibodies or vaccines [4-6]. Nevertheless, an even more classical approach to plant molecular farming, in which less advanced products - low molecular weight secondary metabolites - are envisaged as chemopreventive agents or active pharmaceutical ingredients, deserves meticulous investigation, since many of the biologically active secondary metabolites are present in fruits and vegetables and therefore may end up in the human diet. The interactions of secondary metabolites with biological systems are inevitably complex, since they act as multitarget ligands exerting a variety of effects and responses [7, 8]. For a reasonably focused discussion on the biological activity of plant secondary metabolites, either macromolecular targets or low molecular weight ligands (or preferably both!) have to be defined. In this short review we concentrate our attention on isoflavones as biologically active plant constituents and some synthetic analogue molecules, which are capable of influencing human physiology and therefore interesting from a general perspective of improving human well-being.
2. Structure and Biosynthesis of Isoflavones
Flavonoids (classified as phenylpropanoids for biogenetic reasons, and/or as an aryl substituted 1-benzopyran-4-ones from the point of view of heterocyclic chemistry nomenclature) constitute one of the largest and most important groups of plant secondary metabolites because of their widespread occurrence in the plant Kingdom, which indicates not only their importance for environmental interactions with the vegetable host but also their inevitable influence on human diet [9-11]. One of the main biogenetic pathways in plant physiology, which starts with the condensation of D-erythrose 4-phosphate and phosphoenolo-pyruvate, leads to such key intermediates as shikimic acid, phenylalanine and p-cumaroylCoA, from which a plethora of flavonoids, anthocyanins, lignans, stilbenes, tannins, coumarins and catechins are formed [12-15]. Isoflavones were always considered a distinct category within flavonoids, both for structural and functional reasons. Firstly, their specific structural feature – a rearranged position in the chromone heterocyclic scaffold of the aromatic ring B results from the branching action of isoflavone synthase – flavones and their analogs are 2-aryl substituted benzopyranes, while isoflavones bear an aromatic substituent at carbon C-3 (Fig. 1).
Fig. (1).

Isoflavone general structure, ring connections, and carbon numbering.
These regioisomeric plant-derived oxygen heterocycles share a considerable number of physicochemical characteristics and types of biological activity, and in many plant families they occur side by side [16, 17]. However, the second functional distinction of isoflavones from other flavonoids, which became evident around 1940, first manifested itself as a serious veterinary problem when Australian sheep grazing on subterranean clover (Trifolium subterraneum L.) became infertile. Subsequent studies on regional cattle forage revealed the estrogenic action of isoflavones, like formononetin, daidzein and its metabolite - equol contained in popular clover varieties (Trifolium pretense L and (Trifolium repens L), which also became associated with reproductive problems in other vertebrates [18, 19]. After this initial period of isoflavones interaction with mammalian physiology from a vetinerary standpoint, these functional analogs of steroid estrogens underwent a considerable change of image. The term “phytoestrogenicity”, coined in response to this, started a new line of research into possible medicinal, prophylactic, and cosmetic applications and before long became a hallmark of isoflavone biological activity [15, 20].
Any historical account of isoflavone research should begin in the mid-19th century, when ononin (formononetin 7-O-β-D-glucopyranoside) was first isolated from the medicinal plant Ononis spinosa, and recognized as a glycoside, although several decades had elapsed before its complete structure was elucidated. In 1983, J.L. Ingham presented the first extensive survey of naturally occurring isoflavones, summarizing over a century of research across 260 printed pages and taking into account results validated with the use of modern analytical techniques and contemporary spectral methods of structural analysis [21]. This study listed 510 isoflavone related structures, supplemented by ca. 130 glycoside derivatives Some 30 years later, book chapters devoted to natural isoflavones refer to circa 1600 structures with this type of flavonoid skeleton, comprising not just simple isoflavones but also isoflavanones, isoflavenes, rotenoids, pterocarpans, coumestans, chalcones and deoxybenzoins, as well as their O- and C- glycosidic derivatives [17, 20, 22]. All these structures are presently available not only through disclosures in original scientific literature but also in dictionaries and databases [23]. Needless to say, many more analogous chemical entities have supplemented relevant collections of natural products as a result of multidirectional synthetic efforts. Since this review is focused on simple isoflavones, which are present in vegetable sources relevant to agriculture and human nutrition, only a brief alphabetical list of representative aglycone chemotypes is presented in the Table 1 below.
Table 1.
Exemplary isoflavone aglycones representing various substitution patterns.
| Isoflavone | 5 | 6 | 7 | 8 | 2’ | 3’ | 4’ | 5’ |
|---|---|---|---|---|---|---|---|---|
| Afromosin | H | OMe | OH | H | H | H | OMe | H |
| Betavulgarin | OMe | Methylendioxy | H | OH | H | H | H | |
| Biochanin A | OH | H | OH | H | H | H | OMe | H |
| Cajanin | OH | H | OMe | H | OH | H | OH | H |
| Daidzein | H | H | OH | H | H | H | OH | H |
| Formononetin | H | H | OH | H | H | H | OMe | H |
| Genistein | OH | H | OH | H | H | H | OH | H |
| Irigenin | OH | OMe | OH | H | H | OH | OMe | OMe |
| Irilone | OH | Methylenedioxy | H | H | H | OH | H | |
| Irisolidone | OH | OMe | OH | H | H | H | OMe | H |
| Luteone | OH | Isoprenyl | OH | H | OH | H | OH | H |
| Orobol | OH | H | OH | H | H | OH | OH | H |
| Pratensin | OH | H | OH | H | H | OH | OMe | H |
| Prunetin | OH | H | OMe | H | H | H | OH | H |
| Pseudobabtigenin | H | H | OH | H | H | methylenedioxy | H | |
| Sayanedine | H | H | OMe | H | H | OMe | OH | H |
| Tectorigenin | OH | OMe | OH | H | H | H | OH | H |
| Texasin | H | OH | OH | H | H | H | OMe | H |
| Wigheteone | OH | Isoprenyl | OH | H | H | H | OH | H |
Trends and advances in isoflavone research can be conveniently traced by a survey of literature on genistein, which being a principal flavonoid constituent of soybean – a global agricultural crop and basic food component - attracted the attention of biochemists, medicinal chemists, physiologists, and nutritionists, resulting in several hundred citations annually in recent scientific literature [24-28]. The main conclusions which can be drawn from the results of recent studies can be summarized as follows: A) basic studies on genistein structure and physicochemical properties, founded on theoretical calculations and experimental measurements continue, generating new data on the molecule geometry and electron distribution in the ground state as well as in ionized species or complexes [29, 30]. These data help to eliminate widespread but superficial opinions concerning the classification of genistein as an antioxidant or an estrogen, based on quoting insufficient evidence, such as its alleged structural similarity to estrogen as demonstrated by superimposing 2D structural formulae. B) Advances in phytochemical, pharmaceutical and clinical analytical techniques and methods [31-33], which made possible the separation and quantification of isoflavones, their complexes, conjugates and metabolites at nanogram concentrations, allowed their biosynthesis, metabolism and distribution in plants to be followed, along with the application of regular pharmaceutical quality requirements to the study of plant constituents as xenobiotics in humans, when applied as foods, nutraceuticals or pharmaceutical preparations [34], and C) Multiple biological roles of isoflavones for plant hosts in allelopathy and environmental interactions for root nodulation, pollinator attraction, herbivore deterrence, UV protection, abiotic stress modulation etc., have been listed in the literature as an ex post explanation (justification) for previously established pathways of constitutive and inducible biosynthesis and metabolism [9, 15, 22, 35]. Despite considerable advances in the genetic engineering of isoflavones’ biosynthetic pathways, chemical synthesis, undertaken as early as the 19th century, remains the main source of desired structures.
3. Molecular targets related to biological activity of isoflavones
It has long been postulated that supplementation with phytoestrogens should be beneficial for cardiovascular health, treatment of osteoporosis and the relief of such symptoms as hot flushes in post-menopausal women. Even more hopeful assumptions concerned the possible role of phytoestrogens in protection against hormone dependent cancers. Consequently, isoflavones became a popular subject of research and the list of their validated macromolecular targets has extended from nuclear receptors and transcription factors, through phosphorylating enzymes, to ABC transporters, and pro-inflammatory agents. Recently, the knowledge on biochemistry and molecular pharmacology of isoflavones accumulated over decades has been collected in a voluminous dedicated monograph [36].
Soy products are the predominant dietary source of isoflavones that have long attracted interest as agents beneficial for human health. Epidemiological studies suggest that in populations where isoflavones consumption is higher than in western countries, the incidence of cardiovascular problems, cancer, and diabetes is significantly reduced as evident from statistics drawn from Asian population studies [37, 38].
One of the most commonly studied isoflavones, genistein, shows weak estrogen-like properties, which has led to the application of soy concentrates for hormonal replacement therapy in the dietary supplements segment of the market [39-41]. However, a critical evaluation of clinical studies has raised some controversy regarding its efficacy in alleviating postmenopausal symptoms, normalizing blood glucose level and insulin sensitivity [42] and also raises some concerns regarding its safety [43]. Isoflavones have mild antiosteoporotic effects [44] and weak beneficial effects on climacteric syndrome [43, 45, 46]; they also show a slight tendency to reduce the risk of type 2 diabetes, especially among post-menopausal women who did not use hormone replacement therapy [47]. Genistein is generally perceived as a safe compound [39, 48], but some authors recommend caution when administering high doses of genistein to menopausal women not exposed in their youth to this compound, because of the possible unknown and unpredictable risk that phytoestrogen may pose on the endometrium and mammary glands [43]. Nevertheless, the positive effects of genistein on human health is well documented, as manifested in the prevention of many other diseases unrelated directly to the estrogen receptor, including cancer, diabetes, inflammatory diseases, and the management of some metabolic disorders [37, 49-51]. These effects are attributed mainly to the interaction of genistein with molecular targets other than estrogen receptors.
The molecules of genistein and daidzein resemble the structure of estradiol and initially, many of the biological effects of isoflavonoids were attributed to their interactions with estrogen receptors. Later, other molecular targets of isoflavonoids were discovered, among them tyrosine kinases [52] and topoisomerase II [53]. Meanwhile, isoflavones in general, and genistein in particular, have been identified as ligands of many more proteins belonging to many different families, including nuclear receptors (estrogen receptor α and β, androgen receptors, estrogen related receptors α, β, ɣ, and LXR α and β) [54-61], tyrosine kinases (v-src, Mek-4, ABL, PKC, Syk, EGFR, FGFR) [52, 62-65], ion channels (CFTR) [66], membrane transporters (Glut1, Glut4, ABCG2) [67-72], and topoisomerases (TopoI, TopoII α and β, bacterial gyrase, and bacterial topoisomerase IV) [53, 73-76]. A list of macromolecular targets able to bind to genistein is growing [77]. More recently added are: DNA polymerase, thymidylate synthetase, farnesyltransferase, guanylyl cyclase, cyclophilin A, inosine dehydrogenase, purine nucleoside phosphorylase, dihydrofolate reductase, phospholipase A2, carbonic anhydrase I, and protein kinase C.
From many studies on the oral bioavailabilities of genistein it is clear that in vivo plasma concentrations of genistein could attain 0.1–8 μM at a dose of 16 mg/kg of body weight [78, 79]. Reasonable doubts have been expressed, as to whether dietary doses are relevant for the biological activities observed in model experiments. Nevertheless, a certain set of genes involved in cell growth, cell cycle, apoptosis, angiogenesis, tumor cell invasion and metastasis, cholesterol synthesis and lipid metabolism are indicated for more detailed investigation [80-86]. Among the genes regulated by genistein there is a group with a mechanism of regulation mediated by nuclear receptors: estrogen receptors [86] and androgen receptor [85]. The expression profiles of cells treated with estradiol and genistein show a considerable overlap in the genes, not only in reproductive organs but also in other tissues influenced by estrogens. The next line of study for genistein exerting an estrogenic action extends to bones [84], liver and adipose tissue [80], the thymus [86], lymphocytes [83], the brain [87], endothelial cells [50], the cardiovascular system and muscles [88]. The regulation of androgen receptor mediated gene expression is potentially relevant to the chemopreventive activity of genistein administered at a physiological level against prostate cancer [85, 89]. The epigenetic influence of genistein on gene expression should also be considerd; in this line of inquiry there is growing evidence for the chemo preventive potential of genistein against the development of prostate and mammary cancer [90, 91]. In the other study, genistein significantly altered the methylation status in mice prenatally exposed to genistein, measured in terms of the hyper methylation of certain repetitive elements, which coincided with a significant down-regulation of estrogen-responsive genes [90]. Other areas where isoflavones may exert some desirable influence are carbohydrate and lipid metabolism [80]. Thus, for example, genistein normalized the expression of 84 genes affected by the high fat diet. Genes connected with oncogenesis, cell proliferation, protein phosphorylation, and transcription were often observed to be down-regulated by genistein. Concomitantly, genistein caused up-regulation of genes which were related to protein dephosphorylation, heat shock response, and inactivation of mitogen-activated protein kinase (MAPK), apoptosis and cell cycle arrest [92, 93].
The study of isoflavones’ biological activity in different molecular and cellular models, as well as after systemic administration to the organism, including in human clinical trials, is being continued for various therapeutic indications, particularly cancer, osteoporosis, cardiovascular ailments, and neurodegeneration [94]. Considering genistein’s role in nutrition and the epidemiological data on cancer incidence in Asian countries, a possible role for this isoflavone in chemoprevention has been a subject of prolonged discussion. After long debate on the safety of soy based infant formula and phytoestrogenic dietary supplements, more detailed discussions have taken place on the possible human health effects of isoflavone metabolism, both reductive and oxidative, particularly in view of the considerable polymorphism of all the enzymes concerned [95]. Another topic which further complicates the discussion is the possible influence of xenobiotics like genistein (and other isoflavones) on other metabolic processes of normal physiological processes as well as on pathologies like tumorigenesis, through competition for receptor sites, signaling pathways and active sites of cytochromes or conjugative enzymes [96]. The success of all these studies is, to a certain extent, dependent on the availability of new chemical entities derived from natural active substances, serving as signaling molecules that are more selective than parent isoflavones and showing improved bioavailability profiles.
The pleiotropic activity of isoflavones, especially genistein, as presented above, along with many possibilities for molecular functionalization, offers numerous opportunities for a remarkable improvement in isoflavones’ pharmacological properties. In the following chapters we will present glycosylation as a means of obtaining new isoflavone-based chemical entities, exhibiting altered biological activity when compared to genistein, and thereby emerging as new drug candidates.
4. Chemical synthesis
At the turn of the 20th century, a group of plant pigments was isolated, chiefly by S. Kostanecki and his co-workers, their structure was studied and determined as containing the chromenone (2-phenyl-4H-1-benzopyran-4-one) ring system as a common structural feature, which eventually led to the collective generic name – flavones, given by association with the yellow color of many individual compounds belonging to this class [97, 98]. Soon after, principal flavones structures (apigenin, chrysin, fisetin, morin, quercetin) were confirmed by syntheses in which a three carbon linker between two phenolic residues underwent cyclization to form the desired condensed heterocyclic chromenone system [99, 100]. The remarkable ease of such cyclizations, leading to flavones, were observed in particular for 1,3-diaryl-1,3-propandiones and corresponding chalcones. On the other hand, isoflavones (the term coined by R. Robinson), which are regioisomeric to flavones (containing 3-phenyl-4H-1-benzopyran-4-one framework), would require an aryl migration or alternative intermediate for successful cyclization [101, 102]. Among many syntheses elaborated for isoflavone preparation, the majority utilize the deoxybenzoin (chain arylated acetophenone) type intermediate with an appropriately situated phenolic group. Such a substrate requires one carbon chain extension in the vicinity of the carbonyl group, preferably in the form of an aldehyde or methylene function. Formylation of an activated carbon atom can be achieved in many ways, but for multifunctional polyphenolics side reactions, particularly O-formylation, has to be taken into account, hence phenolic group protection may become inevitable [101, 102]. Genistein, an isoflavone discovered as a constituent of Dyer’s Broom plants towards the end of the 19th century, was synthesized by 1928 [103, 104] and has remained a principal target for new synthetic pathways ever since. Initial interest in genistein as a biologically active secondary metabolite stemmed from the discovery of its estrogenic (and anti-estrogenic) properties, which exerted detrimental effects on the breeding of domesticated and captive animals [15]. Modern molecular pharmacology identified many targets beside nuclear estrogen receptors for the isoflavone, including enzymes, transcription factors and signaling pathways. Since many of these targets are therapeutically relevant, genistein acquired the status of a molecular probe and even a drug lead (candidate), which enhanced interest in its synthetic availability as well as in obtaining its structural analogs [36]. Examples of methods which proved suitable for preparation of variety of isoflavone structures are presented below, in the following order: a) deoxybenzoin C-formylation, followed by cyclization; b) 3-halogenated chromenone ring formation, followed by Suzuki-type arylation; c) chalcone oxidative rearrangement accompanied cyclizations; d) miscellaneous methods. Scheme 1 illustrates approach a, which has been successfully realized in many variants, in application to syntheses of genistein. Its particular value consists in its applicability to unprotected phenolic substrates (Scheme 1).
Scheme 1.
Synthesis of genistein via deoxybenzoin route. Reagents and conditions: a) ZnCl2, DME/HCl; b) HCl aq; c) Ac2O, HCOOH, Et3N; d) 36% HCl aq.
The first step – deoxybenzoin formation from floroglucinol – is frequently carried out with phenylacetic acid derivatives under Friedel-Crafts acylation conditions [104, 105]. In the case of genistein preparation, 4-hydroxyphenylacetonitrile (2) turned out to be the reagent of choice, with a Houben-Hoesch reaction variant [50] securing formation of intermediate iminohydrochloride (3) in high yield. The deoxybenzoin C-formylation step, which proceeds with a concomitant heterocyclic ring closure, is depicted in Scheme 1 as a reaction with C1 synthon, since it has been carried out with great variety of reagents. Apart from the Vilsmeyer reagent generated from dimethylformamide (DMF) and phosphorous oxychloride (or phthaloyl dichloride), other formamide functional equivalents have been used and activated with acidic reagents as methanesulfonyl chloride or boron trifluoride etherate. Alternatively, C1 synthon can take the form of zinc cyanide, carbon disulfide, formate esters, orthoesters or anhydrides, and also active methylene groups as, for example, in 1,3,5-triazines [106-112]. The latter condensative transformations require basic catalysis, which can be exercised by a variety of organic or inorganic bases. It should be stressed that deoxybenzoin C-acylation is also possible in chain extended versions, leading to C-2 alkylated derivatives of isoflavones [113]. Noteworthy, formylated deoxybenzoins are also involved as intermediates in rearrangement of 2-benzyloxy chalcone epoxides (7), driven by BF3 as depicted in Scheme 2 below [114].
Scheme 2.
Exemplary rearrangement of chalcone epoxide to isoflavone. Reagents and conditions: a) BF3.Et2O; b) HCl, AcOH.
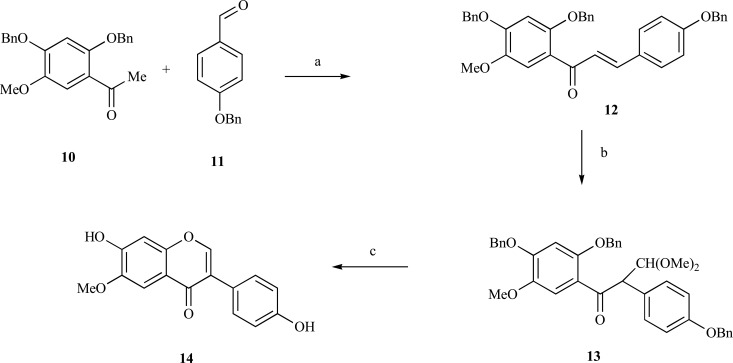
More typically, the chalcone route is applied without exercising intermediate epoxidation. (Scheme 3) [115, 116]. Thalium (III) nitrate in methanolic solution is capable of rearranging chalcones to C-formylated ethanone intermediates (13), which undergo further ring closure as illustrated by the synthesis of glycitein (14).
Scheme 3.
Synthesis of glycitein via chalcone route. Reagents and conditions: a) KOH, EtOH; b) Tl(NO3)2; c) H2/Pd, HCl, MeOH.
Another general approach to isoflavone synthesis consists of Suzuki type coupling of 3-halogenated chromenones with arylboronic acids. In the case of genistein synthesis via 3-iodo-4H-1-benzopyran-4-one (18) (presented in Scheme 4), which starts from (2,4,6-trihydroxyphenyl) ethanone (15), the elaborated procedure requires partial protection of phenolic functions, preferably as methoxymethyl (MOM) ethers (16). The acetophenone chain extension is executed by action of (dimethoxymethyl) dimethylamine (DMF dimethyl acetal), affording intermediate enaminoketone (17), which undergoes smooth cyclization upon treatment with iodine in methanol. The resulting iodinated benzopyranone (18) constitute a favorable substrate for a variety of transition metal catalysed coupling reactions [117, 118]. In the particular coupling reaction used for completion of genistein synthesis, palladium diacetate in the presence of PEG 4000 was used as a catalyst in reaction with (4-hydroxyphenyl)boronic acid, in methanol solution [119, 120].
Scheme 4.
Synthesis of genistein with application of Suzuki coupling. Reagents and conditions: a) MOMCl, DIPEA; b) Me2NCH(OMe)2, DMF; c) I2, MeOH; d) 4- HO-C6H4-B(OH)2, Pd(OAc)2, HCl.
Rearrangement, which parallels biogenetic 2,3-aryl migration catalyzed by isoflavone synthase, is well known in flavonoid chemistry. Thus, flavanones are converted to isoflavones under influence of thallium (III) nitrate in the presence of hyperchloric acid (Scheme 3) [121]. The synthesis of glycytein outlined above (Scheme 3) exemplifies isoflavone synthesis via the chalcone route, which involves a skeletal rearrangement, promoted by thalium (III) salts. This type of transformation was applied repeatedly, despite the obvious inconvenience connected with use of a toxic catalyst and it proved particularly useful for isotopic labeling [122, 123]. Some more elaborate ways for the heterocyclic ring closure have been designed with use of a catalytic olefin metathesis reaction, as illustrated in Scheme 5 [124].
Scheme 5.
Synthesis of formononetin with applications of olefin metathesis. Reagents and conditions: a) POCl3, DMF, MeCN,0-50o C, then H2O, 50o C; b) BnCl, KI, NaHCO3, MeCN, reflux; c) Wittig reaction: methyltriphenylphosphine bromide (MTPPB), t- BuOK, 0oC, 2h; THF, 1 h; 2-bromo-4'-methoxyacetophenone, reflux,1h; d) MTPPB, t- BuOK, 0oC, 2h; e) Grubbs 2nd, CH2Cl2, 40oC, 8 h; f) BH3–SMe2, THF, 0oC, 4 h, then H2O, 10% NaOH, 37% H2O2, 30 min; g) DDQ, 1,4-dioxane, reflux, 8 h; h) Pd(OH)2, EtOH, cyclohexene, reflux, 1 h.
5. Chemical glycosylation of isoflavones
5.1. General Principles
A considerable number of the secondary metabolites found in nature occur as glycosides [125]. Their formation usually follows the main line of biogenetic transformations, leading to a secondary metabolite, and is catalyzed by a separate class of enzymes - glycosyltransferases [126, 127]. Further modifications on thus formed glycosides, due to the inherent multifunctional character of saccharides, serve to expand the structural diversity of the metabolites. The importance of the complex glycosides for molecular recognition, and consequently for modern medicinal chemistry, makes stereoselective formation of a glycosidic bond in the case of complex and multifunctional aglycones one of the most challenging aspects of contemporary carbohydrate chemistry [128]. Although innumerable studies were devoted to stereocontrolled formation of the glycosidic bond in the past, this field still attracts the attention of synthetic chemists and sustains considerable interest [129]. The most commonly used strategies for glycosylation involve coupling by nucleophilic substitution, in which a protected donor, bearing a leaving group (LG) at its anomeric center, reacts with the glycosyl acceptor. The anomeric leaving group displacement can occur by either an SN2 type mechanism, usually under basic conditions, or an SN1 type mechanism under acidic conditions, while additional stereodirecting effects can be exerted by participating C-2 substituents such as the O-acyl group (Scheme 6), which stabilize oxacarbenium ion by formation of a cyclic dioxolenium ion shielded by a protecting group on one side. A nucleophile can attack the anomeric center from only one face to provide a 1,2-trans-glycoside. Consequently, β-D-glucosides, β-D-galactosides, as well as α-D-mannosides are easily synthesized. A list of conditions for activation of different specific donors has been thoroughly discussed [129].
Scheme 6.
Synthesis of glycosides. Reagents: a) BF3.OEt2; TMSOTf; Tf2O.
In contrast to alcohols, phenols exhibit versatile activity, easily undergoing all types of hydrogen atom transfer as well as electron transfer, which requires much more subtle selection of the reaction conditions.
While the list of the principal glycosylating reagents remains the same for all O-nucleophiles, preferences for phenolic substrates may vary considerably [130-132]. Traditionally, glycosyl halides are the most used carbohydrate donors for aromatic O-glycosylation. They are especially valuable for glycosylation under basic conditions and for phenols bearing electron-withdrawing groups. Thioglycosides are also of interest for such reasons as their versatility in donor activation conditions, which extends to higher oxidation states (sulfoxides and sulfones) [133]. The Schmidt imidate glycosylation procedures seem to be particularly popular. They use glycosyl trichloroacetimidates as donors, a Lewis acid (e.g., TMSOTf and BF3 OEt2) as catalyst, and are effective under mild reaction conditions [134, 135]. When the phenols intended for glycosylation are poorly nucleophilic, trichloroacetamide could compete for the C-glycosylation. To avoid this problem, the glycosyl N-phenyltrifluoroacetimidates were introduced as donors [136, 137].
5.2. Exemplary Glycosylations
Glycosylation experiments with genistein were, as in the case of other flavonoids, performed initially with application of acylated halogenose. In the early 1940’s Zemplen et al. published results on the synthesis of flavone glycosides. However, glycosylation of daidzein, genistein, formononetin, and biochanin A with per-O-acetyl glucosyl bromide (28), in the presence of potassium hydroxide aqueous solution, led to the isoflavone-O-glucosides (29-32) in a low yields [138, 139]. The simple, regioselective and stereoselective method for the direct glycosylation of the unprotected isoflavon was proposed by Wähäla et al. [140]. The phase transfer catalyzed synthesis of isoflavone O-glucosides, by treatment of unprotected isoflavone with per-O-acetyl glucosyl bromide, provide the 7-O-glucosides with a moderate yield (Scheme 7). Under proposed conditions, the more acidic 7-OH group was ionized and preferentially glycosylated. As one can expect, the high stereoselectivity of the glycosylation is due to the presence of the neighboring, participating acetyl group of the acetyl bromo sugar.
Scheme 7.
Synthesis of isoflavone O-glycosides in the presence of a base. Reagents and conditions: a) acetone, KOH aq (9%), rt, [138, 139]; b) CH2Cl2, Bu4NBr/ NaOH aq (2.5%), rt [140].
In the next publication, the first stereospecific synthesis of isoflavone 4’-O-β-glucosides from unprotected isoflavone aglycones was proposed [141]. The procedure, involving a solid/liquid crown ether catalyzed phase transfer method has been used for the synthesis of daidzin (29), genistin (30) (Scheme 8).
Scheme 8.
Regioselective glycosylation of isoflavonoids. Reagents and conditions: a) t-BuOK, acetonitrile, 18-crown-6; b) MeONa, MeOH, rt
A number of derivatives of isoflavone glycosides exhibit pleiotropic biological activity when isolated from the biological material, evoking interest from medicinal chemists and pharmacologists alike. These compounds were as a rule obtained only in small amounts, limiting the possibility for enhancing the evaluation of its biological activity. Thus, developing the route for the synthesis of natural compounds and related derivatives is a challenge. It has become increasingly important to develop syntheses yielding glycosylated isoflavonoids possessing improved pharmacological properties, and especially those that are not available in larger quantities from natural sources. An example is the glycosylation of 4’,7,8-trihydroxyisoflavone (A-76202), an inhibitor of the enzymes α-glucosidases I and II (Scheme 9) [142]. The synthesis started with an isoflavone, with selectively protected 4’-OH and 8-OH groups (36). The desired glycoside (39) was prepared in low yield (26%) by treatment of lithium salt with 2,3,5-tri-O-benzoyl-α-D-arabino-furanosyl bromide (37). As one can expect, the main product was the 1,2-trans glycoside (α –D-anomer).
Scheme 9.
Synthesis of A-76202. Reagents and conditions: a) RBr, DBU, MeOH, rt then reflux, 3h, 41%; b) n-BuLi, THF, rt then reflux, 6h, 26%; c) RhCl3 hydrate, EtOH, reflux, 3h; d) NaOH aq, MeOH, rt, 3h, 76%.
It was reported that other variants of glycosylation, coupling with pyranosyl bromide in the presence of silver carbonate and silver triflate [143], or reaction of isoflavone with 2,3,5-tri-O-acetyl-D- arabinofuranosyl fluoride using bis(cyclopentadienyl)haf-nium dichloride and silver trifluoromethanesulfonate according to Suzuki’s method [144] were unsuccessful.
Although A-76202 has strong inhibitory activity for α-glucosidases I and II in vitro, its activity is very weak in vivo. Synthesis of hexopyranosides analogs of A-76202 was performed to overcome this defect (Scheme 10). In a convenient procedure (using 2,3,4,6-tetra-O-acetyl-D-glucopyranosyl bromide (28) and acid acceptor, silver carbonate) β-D-glucoside was obtained in low yield. The reaction is stereoselective and 1,2-trans glucoside is the main product, due to the neighboring acetyl group participation. However, the A-76202 analog (41) was practically inactive in vivo toward rat liver α- glucosidase.
Scheme 10.
Glucosylation of selectively protected isoflavone. Reagents and conditions: a) Ag2CO3, pyridine, rt, 18h; b) RhCl3, EtOH, reflux, 15h; c) KOH, EtOH, rt, 4h.
There are a number of publications describing the comparison of glycosylation methods applied to different acceptors. However, only a few of them are applied in laboratory practice. The Schmidt method, reaction of glycosyl trichloroimidates and glycosyl donors catalyzed with strong acids (e.g. TMSOTf, TMSOH, BF3.Et2O) were found superior for the synthesis of oligosaccharides [134, 135]. However, it was found that due to unfavorable steric interactions that occur between the glycosyl donor and acceptor, or poor nucleophilicity of the hydroxyl group, the Schmidt glycosylation of isoflavones is ineffective. The limitations of the imidoether method were overcome by the application of a more reactive donor, N-4-methoxy-phenyl trifluoroacetimidate (42) [145]. This methodology was used to prepare the 7-O-glycosides (Scheme 11) and 7-O-glucuronides of daidzein, genistein and glycitein. Using lipophilic hexanoyl derivatives of isoflavones that are well soluble in organic solvent, the desired compounds were prepared in high yields. This new synthesis will provide access to large quantities of these compounds and enable further study of their biological properties.
Scheme 11.
Synthesis of genistin. Reagents and conditions: a) hexanoyl chloride, pyridyne, DMAP, CHCl3, 0oC, 91%; b) PhSH, NMP, Im, 78%; c) BF3.Et2O, CH2Cl2, 4A MS, rt, 6h, 78%; d) K2CO3, MeOH, THF, H2O, 40oC, 5h, 94%.
One of the most investigated compounds is genistein. The interest in this molecule as a potential anticancer drug emerged with the discovery of its chemopreventive properties [39-41]. Genistein is a good drug candidate but due to the low bioavailability it is not used as a chemotherapeutic agent. In order to enhance the intrinsic activity of genistein and to explore its pharmacophoric potential, the sugar derivatives were synthesized [146]. The methodology of synthesis of conjugates of unsaturated sugars and genistein was proposed by polish chemists. In our study genistein reacts sluggishly with glycals using a convenient methodology, probably due to the low nucleophilicity of the 7-OH group. An alternative method [147-149] involves the conversion of 1,2-unsaturated sugar to isobutyloxycarbonyl per-O-acetyl-2,3- dideoxy-hex-2-en-pyranoside (45) and then reaction with genistein, catalyzed by tetrakis(triphenyl-phosphine) palladium (Scheme 12). The 7-O-substituted derivatives, genistein per-O-acetyl-2,3-dideoxy-hex-2-en-pyranosides (46,47), were obtained in low yield. The in vitro cytostatic and cytotoxic studies showed that lipophilic glycosides G21 (46) and G30 (47) were significantly more active than the parent isoflavone. The results of in vitro studies indicate that the most active compound of the symbol G21 (46) inhibited tumor cell proliferation at a concentration ten times lower than genistein and is stable in a culture medium [146].
Scheme 12.
Synthesis of genistein 7-O- per-O-acetyl-2,3-dideoxy-hex-2-en-pyranosides. Reagent and conditions: a) Dioxane, H2O (90oC), 45 min; b) THF, Et3N, rt; c) THF, Pd(Ph3P)4, rt, 12h.

These findings led to further extension of genistein glycosidation projects in the direction of structurally diversified glycoconjugates. A simple and efficient method was developed for obtaining the 7-O-, and 4'-O-hydroxyalkyl ether derivatives of genistein acceptors in the reaction of glycosylation [150] (Scheme 13). Substrates, 7-O-ethers (48) were obtained by alkylation of a tetra-n-butyl ammonium salt of genistein. 4’-O-alkylations can be performed selectively but require either temporary protection of phenolic 7-OH or double deprotonation of the isoflavone substrate with a strong base [151]. Products, hydroxyalkyl ethers (48 or 50) react with acylated glycals (hex-1-enitols) under Lewis acid catalysis, undergoing Ferrier rearrangement (Scheme 13). The reaction is highly regio– and stereoselective, affording glycosides (49, 51) with an α–configuration [152].
Scheme 13.
Synthesis of genistein glycoconjugates. Reagents and conditions: a) Bu4NOH, MeOH; b) HO-(CH2)3-Br, DMF; c) t-BuOK, DMF, HO-(CH2)3-Br; d) InCl3,

The antiproliferative potential of “through spacer glycosylated” genistein derivatives, 7-O-Gen and 4’-O-Gen, was assessed in human cell lines of different origin: glioblastoma, breast, colon, prostate, lung and stomach cancers. We found that the most active with respect to tumor cell lines is a compound 49 of the symbol Ram-3. In all the lines tested we observed stronger inhibition of cell growth by 49 than by genistein (6). In vitro, this compound impairs the bipolar structure of the mitotic spindle, resulting in polyploidization of cells and induced apoptosis of tumor cells or aging. The cytotoxic mechanism of action of Ram-3 is related to its interaction with tubulin. Inhibition of tumor cell proliferation by Ram-3 was confirmed in tumor cells of different origin: glioma, breast, colon, prostate, lung and gastric cancer. In all examined lines, we observed a much stronger inhibition of the growth of cells treated with 3-Ram, compared to cells treated with genistein [152]. Unique among derivatives of isoflavones, the ability of G21 and Ram-3 to disrupt microtubules of the mitotic spindle demonstrates that lipophilic sugars (pseudoglycals) are not only carriers of genistein facilitating its transport into cells but also they are important parts of the molecule, necessary for antimitotic activity of a molecule. By studying the biological properties of pseudoglycal genistein glycosides, it was found that the synthesized compounds are inhibitors of tyrosine kinases including EGFR [153]. Due to the key role of kinases in the processes of proliferation, 7-O-glycosidic derivatives of genistein may be useful in therapy. Screening studies of another series of genistein derivatives where the 4'-OH group of isoflavone underwent functionalization allowed to indicate some compounds able to inhibit proliferation of cells as effectively as their 7-OH analogs [154]. It was found, however, that the antiproliferative activity of genistein derivatives modified at the C-4’ is different than the mechanism of action of their regioisomers substituted at C-7. Antiproliferative effect of genistein derivatives substituted at C-4' stems, at least in part, from cell cycle arrest in the G1 phase. The observation described in [154] is also worthy of note, indicating that genistein derivatives, in which the sugar is present on the carbon C-4' are able to induce autophagy.
Isoflavones, likewise flavones, normally accumulate in plants as O-glycosylated derivatives, but C-glycosides are also identified in plant material or may be obtained by synthetic means [155]. Although information on biological activity of synthetic C-glycosides are scarce, basing on the properties and metabolism of natural genistein C-glucoside puerarin, one may expect increased resistance of these compounds to degradation by bacterial flora in the gastrointestinal tract [156]. In general, O-glycosylation reduces flavone bioactivity, while C-glycosylation can enhance some of the benefits of flavones on human health, including their antioxidant and anti-diabetic potential [157].
Genistein is usually metabolized to its aglycone in the lower gut by intestinal glycosidases [158, 159], although its partial absorption without previous hydrolysis in the rat small intestine has also been documented [160]. A glycon moiety can be considered either a protecting group which influences metabolic pathways or a vehicle for an active transport, as demonstrated for the flavonoid quercetin 4-O-β-glycoside, transported by the sodium dependent glucose transporter in Caco-2 and G63 cells [79, 161]. The notion that flavone-C-glycosides are more stable to hydrolysis and they show better activity profile than their O-glycosidic counterparts has initiated an attractive idea of synthesizing semisynthetic glycosides, with the O–glycosidic bond replaced by the C–glycosidic bond, in order to improve biological stability, lipophilicity, and target affinity of isoflavones.
Following this concept, C-glycosidic derivatives of daidzein have been prepared in order to extend a collection of metabolically stable hexenose conjugates [162]. Further structural diversification by exploiting chemical ligation based on 1,3– dipolar cycloaddition was completed, as illustrated in Scheme 14. These new compounds performed (60) considerably better in the cytotoxicity test carried out in the HCT 116 cancer cell lines than genistein [163].
Scheme 14.
Coupling of sugar moiety and isoflavone derivative by C-glycosidic bond. Reagents and conditions: a) BF3. OEt2, CH2Cl2, -30oC overnight; b) CAN (ceric ammonium nitrate), 10 min, 0oC, H2O/MeCN1/4); c) Ph3PBr2, 5 min, CH2Cl 2, rt; d) H2O/CH2Cl2, TBAB, NaN3, rt; e) DMF, propargyl bromide, 45 - 50oC, 1 h; f) Cu(OAc)2, MeOH, overnight, rt.
A synthetic approach allows compounds naturally occurring in plants to be obtained with chemical tools. Recent work shows synthesis and analysis of biological activity of 8-β-D-glucopyranosyl genistein, a promising probe for study of amyloid related events associated with Alzheimer’s disease [164]. Genistein derivatives influence the course of immune processes by modulating IL-12, TNF-a, and NO production by macrophages and lung cancer cell lines [165].
Since the O-glycosides of isoflavones are easily hydrolyzed by glycosidases, the synthesis of glucopyranose and galactopyranose derivatives containing genistein at C-6 sugar was proposed [166]. The treatment of genistein with potassium carbonate, followed by the addition of alkylated agents, 6-deoxy-6-iodo-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose (64), or methyl 2,3,4-tri-O-acetyl-6-deoxy- 6-iodo-α-D-glucopyranoside (61), enabled the mono- and di-ether to be obtained in small yields. The conventional method of removing protective groups resulted in the formation to the conjugates of sugars and genistein (62-67) (Scheme 15).
Scheme 15.
Synthesis of genistein sugar derivatives. Reagents and conditions: a) K2CO3, DMF, rt, 1h; 80oC,48h; b) NaOMe, MeOH, rt, 24h; c) TFA, CH2Cl2, H2O, rt, 24h.
Studies of the biological activity of the compounds prepared indicates that the structure of the sugar affect biological activity. Thus, genistein substituted with galactose (67) inhibits the NO and TNF production, without affecting cell viability [166].
Better bioavailability is a common target of numerous efforts in genistein structure modification, aimed at higher efficiency and better specificity [167-169].
The examples focused on the designed substitution of the hydroxyl group in position 7 demonstrated a significant influence of such modifications of genistein and its analogs on the permeability of lipid bilayers [170], in a study performed by the calcein-leakage method. Interactions of flavonoids with lipids may be important for their stability, as these polyphenols may be involved in the mechanism of prevention of lipid oxidation.
6. Biocatalytic glycosylations
It is generally expected that various new methods of glycosylation of secondary metabolites, including flavonoids, based on enzymatic catalysis, will take over from chemical glycosylations, which in most cases require meticulous elaboration of protecting group application and deliberate choice of an activation method. It is obvious that hypothetical transformation of a secondary metabolite drug lead, aimed at improving its bioavaibility and pharmacodynamic properties and based on the one-step enzymatic reaction, would be a desirable solution. However, relatively few examples of such transformations can be found in literature. One example of the effective use of biotechnology methods in synthetic carbohydrate chemistry is transglucosylation of daidzein (25) using sucrose as a glycosyl donor, catalyzed by Staphylococcus saprophyticus CQ16. [171]. The product (29), 7-O-glucoside of daidzein, was obtained with a very good yield carrying out the reaction in an aqueous medium containing DMSO (Scheme 16). Glucosyltransferase from strain CQ16 is highly regioselective and preferential glycosylation of the flavonoids 7-OH group was found.
Scheme 16.
Synthesis of 7-O-glycosides, derivatives of daidzein and genistein. Reagents and conditions: a) pH 8.3, DMSO (15%), OD660 cells, 30oC, 84h.
Isoflavones are characterized by limited solubility in water, which adversely affects the bioavailability, inter alia, and practical application. These restrictions can be removed by forming chemical conjugates with oligosaccharides. An example of this approach is enzymatic transglycosylation of genistin carried out in the presence of soluble starch, catalyzed by the enzyme Thermus scotoductus 4-R-glucanotransferase (TS4RGTase) [172]. The enzyme transfers a glucosyl unit from starch to genistin (30) and a mixture of glycoconjugates was obtained (Scheme 17). As can be expected, the solubility of the resulting glycoconjugates increases in proportion to the number of sugar units in a molecule.
Scheme 17.
Enzymatic synthesis of maltosides from genistin by TS4RGTase. Reagents and conditions: a) pH 8.3, DMSO (15%),OD660 cells, 30oC, 84h.
7. Conclusion
Isoflavones and their glycosides constitute a group of natural products which became linked to the area of healthcare primarily through incidence of animal breeding problems, coinciding with their presence in human diet, which is quite significant in selected geographical regions, but also through the increasing interest of contemporary medicinal chemistry and experimental pharmacology, which indicated for them many prospectively useful macromolecular targets. Although paradigms of drug discovery seem to have switched in the 21st century from traditional targets like receptors and enzymes to regulation of gene expression and epigenetics, there has been no essential change in the list of molecular descriptors differentiating collections of natural products from libraries of synthetic chemicals, consistently pointing to the advantage of the former. Such evolutionary validated small molecules with proven biocompatibility, as secondary metabolites, will remain valuable also for novel biological activity models, as set by systems biology [173, 174]. Glycoconjugates carrying flavone aglycons are interesting biologically active compounds and therefore key synthetic targets. Unfortunately, owing to the presence of electron-withdrawing groups and the inherently low nucleophilicity of the phenolic hydroxyl groups, isoflavones are difficult to glycosylate by chemical methods. Nevertheless, some successful examples of direct, as well as through spacer, glycosylations were presented. It appears that regioselective hydroxyalkylation of isoflavones followed by glycosylation is the method of choice for synthesis of glycoconjugates. In our opinion, intensified exploration of synthetic chemistry of isoflavone glycosides and glycoconjugates will help to achieve sets of new molecular probes and new drug leads, for the advancement of the ideas and trends described in this review.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
References
- 1.Newman D.J., Cragg G.M., Snader K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000;17:215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 2.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 3.Cragg G.M., Newman D.J. Natural products: A continuing source of novel drug leads. 2013. [DOI] [PMC free article] [PubMed]
- 4.Tremblay R., Wang D., Jevnikar A.M., Ma S. Tobacco, a highly efficient green bioreactor for production of therapeutic proteins. Biotechnol. Adv. 2010;28:214–221. doi: 10.1016/j.biotechadv.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusibov V., Streatfield S.J., Kushnir N. Clinical development of plant-produced recombinant pharmaceuticals: Vaccines, antibodies and beyond. Hum. Vaccin. 2011;7:313–321. doi: 10.4161/hv.7.3.14207. [DOI] [PubMed] [Google Scholar]
- 6.Zeiner M., Cindric J.I., Pozgaj M., Pirkl R., Silic T., Stingeder G. Influence of soil composition on the major, minor and trace metal content of Velebit biomedical plants. J. Pharm. Biomed. Anal. 2015;106:153–158. doi: 10.1016/j.jpba.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Osbourn A.E., Lanzotti V. Plant-derived Natural Products. Synthesis, Function and Application. Dordrecht: Springer-Verlag; 2009. [Google Scholar]
- 8.Lopez Larrea C. Sensing in Nature. New York: Springer-Verlag; 2012. [Google Scholar]
- 9.Moon Y.J., Wang X., Morris M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 10.Korkina L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell Mol. Biol. (Noisy-le-grand) 2007;53:15–25. [PubMed] [Google Scholar]
- 11.Kabera J.N., Semana E., Mussa A.R., He X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014;(2):377–392. [Google Scholar]
- 12.Harborne J.B., Williams C.A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 13.Martens S., Mithofer A. Flavones and flavone synthases. Phytochemistry. 2005;66:2399–2407. doi: 10.1016/j.phytochem.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Vogt T. Phenylpropanoid biosynthesis. Mol. Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- 15.Bucar F. Phytoestrogens in plants: With special reference to isoflavones. In: Preedy V.R., editor. Isoflavones; Chemistry, Analysis, Function, and Effects. Cambridge: RCS Publishing; 2013. pp. 14–27. [Google Scholar]
- 16.Havsteen B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S.K., Kumar S., Chand K., Kathuria A., Gupta A., Jain R. An update on natural occurrence and biological activity of chromones. Curr. Med. Chem. 2011;18:3825–3852. doi: 10.2174/092986711803414359. [DOI] [PubMed] [Google Scholar]
- 18.Hughes C.L., Jr Phytochemical mimicry of reproductive hormones and modulation of herbivore fertility by phytoestrogens. Environ. Health Perspect. 1988;78:171–174. doi: 10.1289/ehp.8878171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams N.R. Detection of the effects of phytoestrogens on sheep and cattle. J. Anim. Sci. 1995;73:1509–1515. doi: 10.2527/1995.7351509x. [DOI] [PubMed] [Google Scholar]
- 20.Knight D.C., Eden J.A. Phytoestrogens-a short review. Maturitas. 1995;22:167–175. doi: 10.1016/0378-5122(95)00937-g. [DOI] [PubMed] [Google Scholar]
- 21.Ingham J.L. Naturally occurring isoflavonoids (1855-1981). Prog. Chem. Org. Nat. Prod. 1983;43:1–266. [Google Scholar]
- 22.Ko K.P. Isoflavones: Chemistry, analysis, functions and effects on health and cancer. Asian Pac. J. Cancer Prev. 2014;15:7001–7010. doi: 10.7314/apjcp.2014.15.17.7001. [DOI] [PubMed] [Google Scholar]
- 23.Bhagwata S., Haytowitza D.B., Wasswa-Kintub S.I., Holdena J.M. USDA develops a database for flavonoids to assess dietary intakes. Procedia Food Sci. 2013;(2):81–86. [Google Scholar]
- 24.Dixon R.A., Ferreira D. Molecules of interest. Genistein. Phytochemistry. 2002;60:205–211. doi: 10.1016/s0031-9422(02)00116-4. [DOI] [PubMed] [Google Scholar]
- 25.Szkudelska K., Nogowski L. Genistein-a dietary compound inducing hormonal and metabolic changes. J. Steroid Biochem. Mol. Biol. 2007;105:37–45. doi: 10.1016/j.jsbmb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee S., Li Y., Wang Z., Sarkar F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman S., Islam R., Swaraz A.M., Ansari A., Parvez A.K., Paul D.K. An insight on genistein as potential pharmacological and therapeutic agent. Asian Pac. J. Trop. Biomed. 2012;2:S1924–S1937. [Google Scholar]
- 28.Spagnuolo C., Russo G.L., Orhan I.E., Habtemariam S., Daglia M., Sureda A., Nabavi S.F., Devi K.P., Loizzo M.R., Tundis R., Nabavi S.M. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015;6:408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yearley E.J., Zhurova E.A., Zhurov V.V., Pinkerton A.A. Binding of genistein to the estrogen receptor based on an experimental electron density study. J. Am. Chem. Soc. 2007;129:15013–15021. doi: 10.1021/ja075211j. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J., Du F., Peng B., Lu R., Gao H., Zhou Z. Structure, electronic properties, and radical scavenging mechanisms of daidzein, genistein, formononetin, and biochanin A: A density functional study. J. Mol. Struct. THEOCHEM. 2010;955:1–6. [Google Scholar]
- 31.Wu Q., Wang M., Simon J.E. Analytical methods to determine phytoestrogenic compounds. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;812:325–355. doi: 10.1016/j.jchromb.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Prokudina E.A., Havlicek L., Al-Maharik N., Lapcik O., Strnad M., Gruz J. Rapid UPLC–ESI–MS/MS method for the analysis of isoflavonoids and other phenylpropanoids. J. Food Compos. Anal. 2012;26:36–42. [Google Scholar]
- 33.Raju K.S., Kadian N., Taneja I., Wahajuddin M. Phytochemical analysis of isoflavonoids using liquid chromatography coupled with tandem mass spectrometry. Phytochem. Rev. 2015;14:469–498. [Google Scholar]
- 34.Balentine D.A., Dwyer J.T., Erdman J.W., Jr, Ferruzzi M.G., Gaine P.C., Harnly J.M., Kwik-Uribe C.L. Recommendations on reporting requirements for flavonoids in research. Am. J. Clin. Nutr. 2015;101:1113–1125. doi: 10.3945/ajcn.113.071274. [DOI] [PubMed] [Google Scholar]
- 35.Wang X. Structure, function, and engineering of enzymes in isoflavonoid biosynthesis. Funct. Integr. Genomics. 2011;11:13–22. doi: 10.1007/s10142-010-0197-9. [DOI] [PubMed] [Google Scholar]
- 36.Preedy V.R. Isoflavones: Chemistry, Analysis, Function and Effects. Cambridge: RCS Publishing; 2013. [Google Scholar]
- 37.Birt D.F., Hendrich S., Wang W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 38.Le Marchand L. Cancer preventive effects of flavonoids-a review. Biomed. Pharmacother. 2002;56:296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 39.Huntley A.L., Ernst E. Soy for the treatment of perimenopausal symptoms-a systematic review. Maturitas. 2004;47:1–9. doi: 10.1016/s0378-5122(03)00221-4. [DOI] [PubMed] [Google Scholar]
- 40.Messina M. Isoflavone intakes by Japanese were overestimated. Am. J. Clin. Nutr. 1995;62:645. doi: 10.1093/ajcn/62.3.645. [DOI] [PubMed] [Google Scholar]
- 41.Knight D.C., Eden J.A. A review of the clinical effects of phytoestrogens. Obstet. Gynecol. 1996;87:897–904. [PubMed] [Google Scholar]
- 42.Ye Y.B., Chen A.L., Lu W., Zhuo S.Y., Liu J., Guan J.H., Deng W.P., Fang S., Li Y.B., Chen Y.M. Daidzein and genistein fail to improve glycemic control and insulin sensitivity in Chinese women with impaired glucose regulation: A double-blind, randomized, placebo-controlled trial. Mol. Nutr. Food Res. 2015;59:240–249. doi: 10.1002/mnfr.201400390. [DOI] [PubMed] [Google Scholar]
- 43.Wuttke W., Jarry H., Seidlova-Wuttke D. Isoflavones-safe food additives or dangerous drugs? Ageing Res. Rev. 2007;6:150–188. doi: 10.1016/j.arr.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Marini H., Minutoli L., Polito F., Bitto A., Altavilla D., Atteritano M., Gaudio A., Mazzaferro S., Frisina A., Frisina N., Lubrano C., Bonaiuto M., D'anna R., Cannata M.L., Corrado F., Adamo E.B., Wilson S., Squadrito F. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann. Intern. Med. 2007;146:839–847. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- 45.D'anna R., Cannata M.L., Atteritano M., Cancellieri F., Corrado F., Baviera G., Triolo O., Antico F., Gaudio A., Frisina N., Bitto A., Polito F., Minutoli L., Altavilla D., Marini H., Squadrito F. Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: A 1-year randomized, double-blind, placebo-controlled study. Menopause. 2007;14:648–655. doi: 10.1097/01.gme.0000248708.60698.98. [DOI] [PubMed] [Google Scholar]
- 46.Atteritano M., Marini H., Minutoli L., Polito F., Bitto A., Altavilla D., Mazzaferro S., D'anna R., Cannata M.L., Gaudio A., Frisina A., Frisina N., Corrado F., Cancellieri F., Lubrano C., Bonaiuto M., Adamo E.B., Squadrito F. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: A two-year randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2007;92:3068–3075. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- 47.Ding M., Franke A.A., Rosner B.A., Giovannucci E., Van Dam R.M., Tworoger S.S., Hu F.B., Sun Q. Urinary isoflavonoids and risk of type 2 diabetes: A prospective investigation in US women. Br. J. Nutr. 2015;114:1694–1701. doi: 10.1017/S0007114515003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaheer K., Akhtar M.H. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. doi: 10.1080/10408398.2014.989958. in press. [DOI] [PubMed] [Google Scholar]
- 49.Kurzer M.S. Hormonal effects of soy in premenopausal women and men. J. Nutr. 2002;132:570S–573S. doi: 10.1093/jn/132.3.570S. [DOI] [PubMed] [Google Scholar]
- 50.Rimbach G., Boesch-Saadatmandi C., Frank J., Fuchs D., Wenzel U., Daniel H., Hall W.L., Weinberg P.D. Dietary isoflavones in the prevention of cardiovascular disease-a molecular perspective. Food Chem. Toxicol. 2008;46:1308–1319. doi: 10.1016/j.fct.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 51.Wegrzyn G., Jakobkiewicz-Banecka J., Gabig-Ciminska M., Piotrowska E., Narajczyk M., Kloska A., Malinowska M., Dziedzic D., Golebiewska I., Moskot M., Wegrzyn A. Genistein: A natural isoflavone with a potential for treatment of genetic diseases. Biochem. Soc. Trans. 2010;38:695–701. doi: 10.1042/BST0380695. [DOI] [PubMed] [Google Scholar]
- 52.Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 53.Markovits J., Linassier C., Fosse P., Couprie J., Pierre J., Jacquemin-Sablon A., Saucier J.M., Le Pecq J.B., Larsen A.K. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- 54.Matsuda H., Shimoda H., Morikawa T., Yoshikawa M. Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): Structure-requirement of hydroxyanthraquinones for estrogenic activity. Bioorg. Med. Chem. Lett. 2001;11:1839–1842. doi: 10.1016/s0960-894x(01)00318-3. [DOI] [PubMed] [Google Scholar]
- 55.Gungor T., Chen Y., Golla R., Ma Z., Corte J.R., Northrop J.P., Bin B., Dickson J.K., Stouch T., Zhou R., Johnson S.E., Seethala R., Feyen J.H. Synthesis and characterization of 3-arylquinazolinone and 3-arylquinazolinethione derivatives as selective estrogen receptor beta modulators. J. Med. Chem. 2006;49:2440–2455. doi: 10.1021/jm0509389. [DOI] [PubMed] [Google Scholar]
- 56.Kostelac D., Rechkemmer G., Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J. Agric. Food Chem. 2003;51:7632–7635. doi: 10.1021/jf034427b. [DOI] [PubMed] [Google Scholar]
- 57.Pike A.C., Brzozowski A.M., Hubbard R.E., Bonn T., Thorsell A.G., Engstrom O., Ljunggren J., Gustafsson J.A., Carlquist M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999;18:4608–4618. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suetsugi M., Su L., Karlsberg K., Yuan Y.C., Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol. Cancer Res. 2003;1:981–991. [PubMed] [Google Scholar]
- 59.Dang Z.C., Audinot V., Papapoulos S.E., Boutin J.A., Lowik C.W. Peroxisome proliferator-activated receptor gamma (PPARgamma) as a molecular target for the soy phytoestrogen genistein. J. Biol. Chem. 2003;278:962–967. doi: 10.1074/jbc.M209483200. [DOI] [PubMed] [Google Scholar]
- 60.Salam N.K., Huang T.H., Kota B.P., Kim M.S., Li Y., Hibbs D.E. Novel PPAR-gamma agonists identified from a natural product library: A virtual screening, induced-fit docking and biological assay study. Chem. Biol. Drug Des. 2008;71:57–70. doi: 10.1111/j.1747-0285.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- 61.Dodo K., Aoyama A., Noguchi-Yachide T., Makishima M., Miyachi H., Hashimoto Y. Co-existence of alpha-glucosidase-inhibitory and liver X receptor-regulatory activities and their separation by structural development. Bioorg. Med. Chem. 2008;16:4272–4285. doi: 10.1016/j.bmc.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 62.Xu L., Ding Y., Catalona W.J., Yang X.J., Anderson W.F., Jovanovic B., Wellman K., Killmer J., Huang X., Scheidt K.A., Montgomery R.B., Bergan R.C. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J. Natl. Cancer Inst. 2009;101:1141–1155. doi: 10.1093/jnci/djp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Traxler P., Trinks U., Buchdunger E., Mett H., Meyer T., Muller M., Regenass U., Rosel J., Lydon N. [(Alkylamino)methyl]acrylophenones: Potent and selective inhibitors of the epidermal growth factor receptor protein tyrosine kinase. J. Med. Chem. 1995;38:2441–2448. doi: 10.1021/jm00013a020. [DOI] [PubMed] [Google Scholar]
- 64.Xie H.Z., Li L.L., Ren J.X., Zou J., Yang L., Wei Y.Q., Yang S.Y. Pharmacophore modeling study based on known spleen tyrosine kinase inhibitors together with virtual screening for identifying novel inhibitors. Bioorg. Med. Chem. Lett. 2009;19:1944–1949. doi: 10.1016/j.bmcl.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 65.Rao G.N. Protein tyrosine kinase activity is required for oxidant-induced extracellular signal-regulated protein kinase activation and c-fos and c-jun expression. Cell. Signal. 1997;9:181–187. doi: 10.1016/s0898-6568(96)00139-8. [DOI] [PubMed] [Google Scholar]
- 66.Melani R., Tomati V., Galietta L.J., Zegarra-Moran O. Modulation of cystic fibrosis transmembrane conductance regulator (CFTR) activity and genistein binding by cytosolic pH. J. Biol. Chem. 2010;285:41591–41596. doi: 10.1074/jbc.M110.166850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Afzal I., Cunningham P., Naftalin R.J. Interactions of ATP, oestradiol, genistein and the anti-oestrogens, faslodex (ICI 182780) and tamoxifen, with the human erythrocyte glucose transporter, GLUT1. Biochem. J. 2002;365:707–719. doi: 10.1042/BJ20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vera J.C., Reyes A.M., Carcamo J.G., Velasquez F.V., Rivas C.I., Zhang R.H., Strobel P., Iribarren R., Scher H.I., Slebe J.C. Genistein is a natural inhibitor of hexose and dehydroascorbic acid transport through the glucose transporter, GLUT1. J. Biol. Chem. 1996;271:8719–8724. doi: 10.1074/jbc.271.15.8719. [DOI] [PubMed] [Google Scholar]
- 69.Bazuine M., Van Den Broek P.J., Maassen J.A. Genistein directly inhibits GLUT4-mediated glucose uptake in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2005;326:511–514. doi: 10.1016/j.bbrc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 70.Imai Y., Tsukahara S., Asada S., Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64:4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 71.Zhang S., Yang X., Morris M.E. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol. Pharmacol. 2004;65:1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 72.Perez M., Real R., Mendoza G., Merino G., Prieto J.G., Alvarez A.I. Milk secretion of nitrofurantoin, as a specific BCRP/ABCG2 substrate, in assaf sheep: Modulation by isoflavones. J. Vet. Pharmacol. Ther. 2009;32:498–502. doi: 10.1111/j.1365-2885.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 73.Okura A., Arakawa H., Oka H., Yoshinari T., Monden Y. Effect of genistein on topoisomerase activity and on the growth of [Val 12]Ha-ras-transformed NIH 3T3 cells. Biochem. Biophys. Res. Commun. 1988;157:183–189. doi: 10.1016/s0006-291x(88)80030-5. [DOI] [PubMed] [Google Scholar]
- 74.Bandele O.J., Osheroff N. Bioflavonoids as poisons of human topoisomerase II alpha and II beta. Biochemistry. 2007;46:6097–6108. doi: 10.1021/bi7000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Constantinou A., Mehta R., Runyan C., Rao K., Vaughan A., Moon R. Flavonoids as DNA topoisomerase antagonists and poisons: Structure-activity relationships. J. Nat. Prod. 1995;58:217–225. doi: 10.1021/np50116a009. [DOI] [PubMed] [Google Scholar]
- 76.Bernard F.X., Sable S., Cameron B., Provost J., Desnottes J.F., Crouzet J., Blanche F. Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob. Agents Chemother. 1997;41:992–998. doi: 10.1128/aac.41.5.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen X., Ung C.Y., Chen Y. Can an in silico drug-target search method be used to probe potential mechanisms of medicinal plant ingredients? Nat. Prod. Rep. 2003;20:432–444. doi: 10.1039/b303745b. [DOI] [PubMed] [Google Scholar]
- 78.Bloedon L.T., Jeffcoat A.R., Lopaczynski W., Schell M.J., Black T.M., Dix K.J., Thomas B.F., Albright C., Busby M.G., Crowell J.A., Zeisel S.H. Safety and pharmacokinetics of purified soy isoflavones: Single-dose administration to postmenopausal women. Am. J. Clin. Nutr. 2002;76:1126–1137. doi: 10.1093/ajcn/76.5.1126. [DOI] [PubMed] [Google Scholar]
- 79.Setchell K.D., Brown N.M., Desai P., Zimmer-Nechemias L., Wolfe B.E., Brashear W.T., Kirschner A.S., Cassidy A., Heubi J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 80.Kim S., Sohn I., Lee Y.S., Lee Y.S. Hepatic gene expression profiles are altered by genistein supplementation in mice with diet-induced obesity. J. Nutr. 2005;135:33–41. doi: 10.1093/jn/135.1.33. [DOI] [PubMed] [Google Scholar]
- 81.Li Y., Che M., Bhagat S., Ellis K.L., Kucuk O., Doerge D.R., Abrams J., Cher M.L., Sarkar F.H. Regulation of gene expression and inhibition of experimental prostate cancer bone metastasis by dietary genistein. Neoplasia. 2004;6:354–363. doi: 10.1593/neo.03478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y., Sarkar F.H. Down-regulation of invasion and angiogenesis-related genes identified by cDNA microarray analysis of PC3 prostate cancer cells treated with genistein. Cancer Lett. 2002;186:157–164. doi: 10.1016/s0304-3835(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 83.Niculescu M.D., Pop E.A., Fischer L.M., Zeisel S.H. Dietary isoflavones differentially induce gene expression changes in lymphocytes from postmenopausal women who form equol as compared with those who do not. J. Nutr. Biochem. 2007;18:380–390. doi: 10.1016/j.jnutbio.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pie J.E., Park J.H., Park Y.H., Ryu Y.M., Kim K.N., Suh S.W., Becker K.G., Cho-Chung Y.S., Kim M.K. Effect of genistein on the expression of bone metabolism genes in ovariectomized mice using a cDNA microarray. J. Nutr. Biochem. 2006;17:157–164. doi: 10.1016/j.jnutbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Rice L., Handayani R., Cui Y., Medrano T., Samedi V., Baker H., Szabo N.J., Rosser C.J., Goodison S., Shiverick K.T. Soy isoflavones exert differential effects on androgen responsive genes in LNCaP human prostate cancer cells. J. Nutr. 2007;137:964–972. doi: 10.1093/jn/137.4.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Selvaraj V., Bunick D., Finnigan-Bunick C., Johnson R.W., Wang H., Liu L., Cooke P.S. Gene expression profiling of 17 beta-estradiol and genistein effects on mouse thymus. Toxicol. Sci. 2005;87:97–112. doi: 10.1093/toxsci/kfi219. [DOI] [PubMed] [Google Scholar]
- 87.Lyou S., Hirano E., Tujioka K., Mawatari Y., Hayase K., Okuyama S., Yokogoshi H. Dietary genistein affects brain protein synthesis rates in ovariectomized female rats. J. Nutr. 2002;132:2055–2058. doi: 10.1093/jn/132.7.2055. [DOI] [PubMed] [Google Scholar]
- 88.Velders M., Solzbacher M., Schleipen B., Laudenbach U., Fritzemeier K.H., Diel P. Estradiol and genistein antagonize the ovariectomy effects on skeletal muscle myosin heavy chain expression via ER-beta mediated pathways. J. Steroid Biochem. Mol. Biol. 2010;120:53–59. doi: 10.1016/j.jsbmb.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi Y., Lavigne J.A., Hursting S.D., Chandramouli G.V., Perkins S.N., Barrett J.C., Wang T.T. Using DNA microarray analyses to elucidate the effects of genistein in androgen-responsive prostate cancer cells: Identification of novel targets. Mol. Carcinog. 2004;41:108–119. doi: 10.1002/mc.20045. [DOI] [PubMed] [Google Scholar]
- 90.Vanhees K., Coort S., Ruijters E.J., Godschalk R.W., Van Schooten F.J., Van Waalwijk Van Doorn-Khosrovani B.S. Epigenetics: Prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J. 2011;25:797–807. doi: 10.1096/fj.10-172155. [DOI] [PubMed] [Google Scholar]
- 91.Day J.K., Bauer A.M., Desbordes C., Zhuang Y., Kim B.E., Newton L.G., Nehra V., Forsee K.M., Macdonald R.S., Besch-Williford C., Huang T.H., Lubahn D.B. Genistein alters methylation patterns in mice. J. Nutr. 2002;132:2419S–2423S. doi: 10.1093/jn/132.8.2419S. [DOI] [PubMed] [Google Scholar]
- 92.Farivar R.S., Gardner-Thorpe J., Ito H., Arshad H., Zinner M.J., Ashley S.W., Whang E.E. The efficacy of tyrosine kinase inhibitors on human pancreatic cancer cell lines. J. Surg. Res. 2003;115:219–225. doi: 10.1016/s0022-4804(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 93.Lavigne J.A., Takahashi Y., Chandramouli G.V., Liu H., Perkins S.N., Hursting S.D., Wang T.T. Concentration-dependent effects of genistein on global gene expression in MCF-7 breast cancer cells: An oligo microarray study. Breast Cancer Res. Treat. 2008;110:85–98. doi: 10.1007/s10549-007-9705-6. [DOI] [PubMed] [Google Scholar]
- 94.Mccue P., Shetty K. Health benefits of soy isoflavonoids and strategies for enhancement: A review. Crit. Rev. Food Sci. Nutr. 2004;44:361–367. doi: 10.1080/10408690490509591. [DOI] [PubMed] [Google Scholar]
- 95.Mccarty M.F. Isoflavones made simple-Genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits. Med. Hypoth. 2006;66:1093–1114. doi: 10.1016/j.mehy.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 96.Masilamani M., Wei J., Sampson H.A. Regulation of the immune response by soybean isoflavones. Immunol. Res. 2012;54:95–110. doi: 10.1007/s12026-012-8331-5. [DOI] [PubMed] [Google Scholar]
- 97.Stanislaus von Kostanecki J.T. Berichte der deutschen chemischen Gesellschaft. 1912.
- 98.Seshadri T.R. Biochemistry of natural pigments; (exclusive of haeme pigments and carotenoids). Annu. Rev. Biochem. 1951;20:487–512. doi: 10.1146/annurev.bi.20.070151.002415. [DOI] [PubMed] [Google Scholar]
- 99.Edwards A.M., Howell J.B. The chromones: History, chemistry and clinical development. A tribute to the work of Dr R.E.C. Altounyan. Clin. Exp. Allergy. 2000;30:756–774. doi: 10.1046/j.1365-2222.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- 100.Gaspar A., Matos M.J., Garrido J., Uriarte E., Borges F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014;114:4960–4992. doi: 10.1021/cr400265z. [DOI] [PubMed] [Google Scholar]
- 101.Levai A. Synthesis of isoflavones. J. Heterocycl. Chem. 2004;41:449–460. [Google Scholar]
- 102.Tawfik H.A., Ewies E.F., El-Hamouly W.S. Synthesis of chromones and their applications during the last ten years. Int. J. Res. Pharm. Chem. 2014;4:1046–1085. [Google Scholar]
- 103.Bradbury R.B., White D.E. The chemistry of subterranean clover. Part I. Isolation of formononetin and genistein. J. Chem. Soc. 1951;(0):3447–3449. [Google Scholar]
- 104.Venkataraman K. Flavones and Isoflavones. Prog. Chem. Org. Nat. Prod. 1959;17:1–69. [Google Scholar]
- 105.Kagal S.A., Nair P.M., Venkataraman K. A Synthesis of isoflavones by a modified Vilsmeier-Haack reaction. Tetrahedron Lett. 1962;3:593–597. [Google Scholar]
- 106.Pelter A., Foot S. A new convenient synthesis of isoflavones. Synthesis. 1976;5:326. [Google Scholar]
- 107.Wähäla K., Hase T.A. Expedient synthesis of polyhydroxyisoflavones. J. Chem. Soc., Perkin Trans. 1. 1991;(12):3005–3008. [Google Scholar]
- 108.Chang Y.C., Nair M.G., Santell R.C., Helferich W.G. Microwave-mediated synthesis of anticarcinogenic isoflavones from soybeans. J. Agric. Food Chem. 1994;42:1869–1871. [Google Scholar]
- 109.Balasubramanian S., Nair M.G. An efficient “one- pot” synthesis of isoflavones. Synth. Commun. 2000;30:469–484. [Google Scholar]
- 110.Singh H., Pratap R. A convenient one-pot synthesis of 7-hydroxy-isoflavones from resorcinol with substituted phenylacetic acids. Tetrahedron Lett. 2006;47:8161–8164. [Google Scholar]
- 111.Yadav S.K. Process for the preparation of chromones, isoflavones and homoisoflavones using Vilsmeier reagent generated from phthaloyl dichloride and DMF. Int. J. Org. Chem. (Irvine) 2014;4:236–246. [Google Scholar]
- 112.Bass R.J. Synthesis of chromones by cyclization of 2-hydroxyphenyl ketones with boron trifluoride-diethyl ether and methanesulphonyl chloride. J. Chem. Soc. Chem. Commun. 1976;(2):78–79. [Google Scholar]
- 113.Kim Y.W., Brueggemeier R.W. A convenient one-pot synthesis of 2-(alkylthio)isoflavones from deoxybenzoins using a phase transfer catalyst. Tetrahedron Lett. 2002;43:6113–6115. [Google Scholar]
- 114.Bhrara S.C., Jain A.C., Seshadri T.R. Scope of isoflavone synthesis using 2'-benzyloxychalcone epoxides. Tetrahedron. 1965;21:963–967. [Google Scholar]
- 115.Farkas L., Gottsegen A., Nogradi M., Antus S. Direct conversion of 2′-hydroxychalcones into isoflavones using thallium(III) nitrate: Synthesis of (±)-sophorol and (±)-mucronulatol. J. Chem. Soc. Chem. Commun. 1972;(14):825–826. [Google Scholar]
- 116.Farkas L., Gottsegen A., Noagradi M., Antus S. Synthesis of sophorol, violanone, lonchocarpan, claussequinone, philenopteran, leiocalycin, and some other natural isoflavonoids by the oxidative rearrangement of chalcones with thallium(III) nitrate. J. Chem. Soc., Perkin Trans. 1. 1974;(0):305–312. [Google Scholar]
- 117.Hoshino Y., Miyaura N., Suzuki A. Novel synthesis of isoflavones by the palladium-catalyzed cross-coupling reaction of 3-bromochromones with arylboronic acids or its esters. Bull. Chem. Soc. Jpn. 1988;61:3008–3010. [Google Scholar]
- 118.Ding K., Wang S. Efficient synthesis of isoflavone analogues via a Suzuki coupling reaction. Tetrahedron Lett. 2005;46:3707–3709. [Google Scholar]
- 119.Denis J.D., Gordon J.S., Carroll V.M., Priefer R. Novel synthesis of the isoflavone genistein. Synthesis. 2010;10:1590–1592. [Google Scholar]
- 120.Selepe M.A., Van Heerden F.R. Application of the Suzuki-Miyaura reaction in the synthesis of flavonoids. Molecules. 2013;18:4739–4765. doi: 10.3390/molecules18044739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kinoshita T., Ichinose K., Sankawa U. One-step conversion of flavanones into isoflavones: A new facile biomimetic synthesis of isoflavones. Tetrahedron Lett. 1990;31:7355–7356. [Google Scholar]
- 122.Oldfield M.F., Chen L., Botting N.P. Synthesis of [3,4,8-13C3]daidzein. Tetrahedron. 2004;60:1887–1893. [Google Scholar]
- 123.Al-Maharik N., Botting N.P. A versatile synthesis of [2,3,4-13C3]isoflavones. J. Labelled Comp. Radiopharm. 2010;53:95–103. [Google Scholar]
- 124.Li S.R., Chen P.Y., Chen L.Y., Lo Y.F., Tsai I.L., Wang E.C. Synthesis of haginin E, equol, daidzein, and formononetin from resorcinol via an isoflavene intermediate. Tetrahedron Lett. 2009;50:2121–2123. [Google Scholar]
- 125.Dembitsky V.M. Chemistry and biodiversity of the biologically active natural glycosides. Chem. Biodivers. 2004;1:673–781. doi: 10.1002/cbdv.200490060. [DOI] [PubMed] [Google Scholar]
- 126.Blanchard S., Thorson J.S. Enzymatic tools for engineering natural product glycosylation. Curr. Opin. Chem. Biol. 2006;10:263–271. doi: 10.1016/j.cbpa.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 127.Gantt R.W., Peltier-Pain P., Thorson J.S. Enzymatic methods for glyco(diversification/randomization) of drugs and small molecules. Nat. Prod. Rep. 2011;28:1811–1853. doi: 10.1039/c1np00045d. [DOI] [PubMed] [Google Scholar]
- 128.Nicolaou K.C., Mitchell H.J. Adventures in carbohydrate chemistry: New synthetic technologies, chemical synthesis, molecular design, and chemical biology. Angew. Chem. Int. Ed. Engl. 2001;40:1576–1624. [PubMed] [Google Scholar]
- 129.Demchenko A.V. Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance. 2008. [Google Scholar]
- 130.Jacobsson M., Malmberg J., Ellervik U. Aromatic O-glycosylation. Carbohydr. Res. 2006;341:1266–1281. doi: 10.1016/j.carres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 131.Jensen K.J. O-glycosylations under neutral or basic conditions. J. Chem. Soc., Perkin Trans. 1. 2002;(20):2219–2233. [Google Scholar]
- 132.Conchie J., Levvy G.A., Marsh C.A. Methyl and phenyl glycosides of the common sugars. Adv. Carbohydr. Chem. Biochem. 1957;12:157–187. doi: 10.1016/s0096-5332(08)60208-8. [DOI] [PubMed] [Google Scholar]
- 133.Zhong W., Boons G.J. Thioglycosides in oligosachcharides sythesis. In: Demchenko A.V., editor. Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance. Weinheim: Wiley-WCH; 2008. pp. 261–303. [Google Scholar]
- 134.Schmidt R.R. New methods for the synthesis of glycosides and oligosachcarides: Are there alternatives to the Koenigs-Knorr method? Angew. Chem. Int. Ed. Engl. 1986;25:212–235. [Google Scholar]
- 135.Zhu X., Schmidt R.R. New principles for glycoside bond formation. Angew. Chem. Int. Ed. Engl. 2009;48:1900–1934. doi: 10.1002/anie.200802036. [DOI] [PubMed] [Google Scholar]
- 136.Yu B., Tao H. Glycosyl trifluoroacatamidates. Part I. preparation and application as new glycosyl donors. Tetrahedron Lett. 2001;42:2405–2407. [Google Scholar]
- 137.Yu B., Sun J. Glycosylation with glycosyl N-phenyltrifluoroacetimidates (PTFAI) and a perspective of the future development of new glycosylation methods. Chem. Commun. (Camb.) 2010;46:4668–4679. doi: 10.1039/c0cc00563k. [DOI] [PubMed] [Google Scholar]
- 138.Zemplen G., Farkas L. Synthese des genistins. Chem. Ber. 1943;76:1110–1112. [Google Scholar]
- 139.Zemplen G., Farkas L., Bien A. Synthese des ononins. Chem. Ber. 1944;77:452–457. [Google Scholar]
- 140.Lewis P.T., Katia S., Wähäla K. The phase-transfer catalyzed synthesis of isoflavone O-glucosides. J. Chem. Soc., Perkin Trans. 1. 1998;(16):2481–2484. [Google Scholar]
- 141.Lewis P.T., Wähäla K. Regiospecific 4'-O- β-glucosidationof isoflavone O-glucosides. Tetrahedron Lett. 1998;39:9559–9562. [Google Scholar]
- 142.Shiozaki M. Synthesis of 4-O-8-dihydroxyisoflavon-7-yl- α-D-arabinofuranoside. Tetrahedron Asymmetry. 1999;10:1477–1482. [Google Scholar]
- 143.Suzuki K., Maeta H., Suzuki T., Matsumoto T. Cp2ZrCl2 AgBF4 in Benzene: A new reagent system for rapid and highly selective α-mannoside synthesis from tetra-O-benzyl-d-mannosyl fluoride. Tetrahedron Lett. 1989;30:6879–6882. [Google Scholar]
- 144.Watanabe Y., Shiozaki M., Kamegai R. Synthesis and biological activity of 4',8-dihydroxyisoflavon-7-yl D-hexopyranosides. Carbohydr. Res. 2001;335:283–289. doi: 10.1016/s0008-6215(01)00246-4. [DOI] [PubMed] [Google Scholar]
- 145.Al-Maharik N., Botting N.P. An efficient method for the glycosylation of isoflavones. Eur. J. Org. Chem. 2008;(33):5622–5629. [Google Scholar]
- 146.Polkowski K., Popiolkiewicz J., Krzeczynski P., Ramza J., Pucko W., Zegrocka-Stendel O., Boryski J., Skierski J.S., Mazurek A.P., Grynkiewicz G. Cytostatic and cytotoxic activity of synthetic genistein glycosides against human cancer cell lines. Cancer Lett. 2004;203:59–69. doi: 10.1016/j.canlet.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 147.Goux C., Lhotse P., Sinou D. Synthesis of allylic aryl ethers via palladium(0)-catalyzed arylation of allylic carbonates. Synlett. 1992;(9):725–727. [Google Scholar]
- 148.Lakhmiri R., Lhoste P., Kryczka B., Sinou D. The use of 1-O-tosyl-glucopyranosie derivatives in glucoside synthesis. J. Carbohydr. Chem. 1993;12:223–235. [Google Scholar]
- 149.Sinou D., Frappa I., Lhoste P., Porwanski S., Kryczka B. Stereoselective palladium(0)-mediated synthesis of 1,4-disaccharides. Tetrahedron Lett. 1995;36:1251–1254. [Google Scholar]
- 150.Rusin A., Gogler A., Glowala-Kosinska M., Bochenek D., Gruca A., Grynkiewicz G., Zawisza J., Szeja W., Krawczyk Z. Unsaturated genistein disaccharide glycoside as a novel agent affecting microtubules. Bioorg. Med. Chem. Lett. 2009;19:4939–4943. doi: 10.1016/j.bmcl.2009.07.089. [DOI] [PubMed] [Google Scholar]
- 151.Szeja W., Puchalka J., Swierk P., Hendrich A.B., Grynkiewicz G. Selective alkylation of genistein and daidzein. Chem. Biol. Interact. 2013;3:95–106. [Google Scholar]
- 152.Rusin A., Zawisza-Puchalka J., Kujawa K., Gogler-Piglowska A., Wietrzyk J., Switalska M., Glowala-Kosinska M., Gruca A., Szeja W., Krawczyk Z., Grynkiewicz G. Synthetic conjugates of genistein affecting proliferation and mitosis of cancer cells. Bioorg. Med. Chem. 2011;19:295–305. doi: 10.1016/j.bmc.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 153.Gruca A., Krawczyk Z., Szeja W., Grynkiewicz G., Rusin A. Synthetic genistein glycosides inhibiting EGFR phosphorylation enhance the effect of radiation in HCT 116 colon cancer cells. Molecules. 2014;19:18558–18573. doi: 10.3390/molecules191118558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Byczek A., Zawisza-Puchalka J., Gruca A., Papaj K., Grynkiewicz G., Rusin M., Szeja W., Rusin A. Genistein derivatives regioisomerically substituted at 7-O- and 4 '-O- have different effect on the cell cycle. . J. Chem., . 2013; 2013 :1–12. [Google Scholar]
- 155.Talhi O., Silva A.M. Advances in C-glycosylflavonoid Research. Curr. Org. Chem. 2012;16:859–896. [Google Scholar]
- 156.Simons A.L., Renouf M., Hendrich S., Murphy P.A. Human gut microbial degradation of flavonoids: Structure-function relationships. J. Agric. Food Chem. 2005;53:4258–4263. doi: 10.1021/jf0500177. [DOI] [PubMed] [Google Scholar]
- 157.Xiao J., Muzashvili T.S., Georgiev M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014;32:1145–1156. doi: 10.1016/j.biotechadv.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 158.Day A.J., Dupont M.S., Ridley S., Rhodes M., Rhodes M.J., Morgan M.R., Williamson G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436:71–75. doi: 10.1016/s0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 159.Piskula M.K., Yamakoshi J., Iwai Y. Daidzein and genistein but not their glucosides are absorbed from the rat stomach. FEBS Lett. 1999;447:287–291. doi: 10.1016/s0014-5793(99)00307-5. [DOI] [PubMed] [Google Scholar]
- 160.Andlauer W., Kolb J., Furst P. Absorption and metabolism of genistin in the isolated rat small intestine. FEBS Lett. 2000;475:127–130. doi: 10.1016/s0014-5793(00)01642-2. [DOI] [PubMed] [Google Scholar]
- 161.Walgren R.A., Lin J.T., Kinne R.K., Walle T. Cellular uptake of dietary flavonoid quercetin 4'-beta-glucoside by sodium-dependent glucose transporter SGLT1. J. Pharmacol. Exp. Ther. 2000;294:837–843. [PubMed] [Google Scholar]
- 162.Szeja W., Swierk P., Rusin A., Grynkiewicz G., Papaj K. An approach to C-glycosidic conjugates of isoflavones. Heterocycl. Commun. 2013;19:133–138. [Google Scholar]
- 163.Szeja W., Grynkiewicz G., Bieg T., Swierk P., Byczek A., Papaj K., Kitel R., Rusin A. Synthesis and cytotoxicity of 2,3-enopyranosyl C-linked conjugates of genistein. Molecules. 2014;19:7072–7093. doi: 10.3390/molecules19067072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jesus A.R., Dias C., Matos A.M., De Almeida R.F., Viana A.S., Marcelo F., Ribeiro R.T., Macedo M.P., Airoldi C., Nicotra F., Martins A., Cabrita E.J., Jimenez-Barbero J., Rauter A.P. Exploiting the therapeutic potential of 8-beta-d-glucopyranosylgenistein: Synthesis, antidiabetic activity, and molecular interaction with islet amyloid polypeptide and amyloid beta-peptide (1-42). J. Med. Chem. 2014;57:9463–9472. doi: 10.1021/jm501069h. [DOI] [PubMed] [Google Scholar]
- 165.Gadgeel S.M., Ali S., Philip P.A., Wozniak A., Sarkar F.H. Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer. 2009;115:2165–2176. doi: 10.1002/cncr.24250. [DOI] [PubMed] [Google Scholar]
- 166.Junior C.O., Castro S.B., Pereira A.A., Alves C.C., Oliveira E.E., Rego R.T., Ferreira A.P., De Almeida M.V. Synthesis of genistein coupled with sugar derivatives and their inhibitory effect on nitric oxide production in macrophages. Eur. J. Med. Chem. 2014;85:615–620. doi: 10.1016/j.ejmech.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 167.Stancanelli R., Mazzaglia A., Tommasini S., Calabro M.L., Villari V., Guardo M., Ficarra P., Ficarra R. The enhancement of isoflavones water solubility by complexation with modified cyclodextrins: A spectroscopic investigation with implications in the pharmaceutical analysis. J. Pharm. Biomed. Anal. 2007;44:980–984. doi: 10.1016/j.jpba.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 168.Li H.Q., Xue J.Y., Shi L., Gui S.Y., Zhu H.L. Synthesis, crystal structure and antimicrobial activity of deoxybenzoin derivatives from genistein. Eur. J. Med. Chem. 2008;43:662–667. doi: 10.1016/j.ejmech.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 169.Zhang L.N., Cao P., Tan S.H., Gu W., Shi L., Zhu H.L. Synthesis and antimicrobial activities of 7-O-modified genistein derivatives. Eur. J. Med. Chem. 2008;43:1543–1551. doi: 10.1016/j.ejmech.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 170.Sroda K., Michalak K., Maniewska J., Grynkiewicz G., Szeja W., Zawisza J., Hendrich A.B. Genistein derivatives decrease liposome membrane integrity--calcein release and molecular modeling study. Biophys. Chem. 2008;138:78–82. doi: 10.1016/j.bpc.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 171.Chu J., Wu X., Li B., He B. Efficient glucosylation of flavonoids by organic solvent-tolerant Staphylococcus saprophyticus CQ16 in aqueous hydrophilic media. J. Mol. Catal., B Enzym. 2014;99:8–13. [Google Scholar]
- 172.Li D., Roh S.A., Shim J.H., Mikami B., Baik M.Y., Park C.S., Park K.H. Glycosylation of genistin into soluble inclusion complex form of cyclic glucans by enzymatic modification. J. Agric. Food Chem. 2005;53:6516–6524. doi: 10.1021/jf050732g. [DOI] [PubMed] [Google Scholar]
- 173.Grynkiewicz G., Szeja W. Synthetic glycosides and glycoconjugates of low molecular weight natural products. Curr. Pharm. Des. 2016;22:1592–1627. doi: 10.2174/1381612822666151211094345. [DOI] [PubMed] [Google Scholar]
- 174.Cherblanc F.L., Davidson R.W., Di Fruscia P., Srimongkolpithak N., Fuchter M.J. Perspectives on natural product epigenetic modulators in chemical biology and medicine. Nat. Prod. Rep. 2013;30:605–624. doi: 10.1039/c3np20097c. [DOI] [PubMed] [Google Scholar]