Abstract
Melanocortin 2 receptor (MC2R) is the only canonical ACTH receptor. Its functional expression requires the presence of an accessory protein, known as melanocortin receptor 2 accessory protein 1 (MRAP1). The vertebrate genome exhibits a paralogue gene called MRAP2, which is duplicated in zebrafish (MRAP2a and MRAP2b), although its function remains unknown. In this paper, we demonstrate that MRAP2a enables MC4R, a canonical MSH receptor, to be activated by ACTH with a similar sensitivity to that exhibited by MC2R. Both proteins physically interact and are coexpressed in the neurons of the preoptic area, a key region in the control of the energy balance and hypophyseal secretion in fish. ACTH injections inhibit food intake in wild-type zebrafish but not in fish lacking functional MC4R. Both MRAP1 and MRAP2a are hormonally regulated, suggesting that these proteins are substrates for feed-back regulatory pathways of melanocortin signaling. Fasting has no effect on the central expression of MRAP2a but stimulates MRAP2b expression. This protein interacts and is colocalized with MC4R in the tuberal hypothalamic neurons but has no effect on the pharmacologic profile of MC4R. However, MRPA2b is able to decrease basal reporter activity in cell lines expressing MC4R. It is plausible that MRAP2b decreases the constitutive activity of the MC4R during fasting periods, driving the animal toward a positive energy balance. Our data indicate that MRAP2s control the activity of MC4R, opening up new pathways for the regulation of melanocortin signaling and, by extension, for the regulation of the energy balance and obesity.
Melanocortins, which are the posttranscriptional products of a complex precursor named proopiomelanocortin (POMC), are mainly composed of ACTH and MSH (α-, β- γ-, and δ-MSH) (1). POMC is mainly produced in the pituitary, and its posttranslational processing occurs in a tissue-specific manner. The proteolytic cleavage of POMC generates ACTH in the corticotrophs of the anterior pituitary, whereas POMC cleavage produces α-MSH and β-endorphin in the melanotrophs of the pars intermedia. POMC is also centrally produced in the arcuate nucleus and the nucleus of the tractus solitarius, where it is mainly processed to α-MSH and β-endorphin (2).
Melanocortin exerts its physiologic role by binding to a family of specific G protein-coupled receptors that positively couple to adenylyl cyclase. Tetrapod species have 5 melanocortin receptors (MC1R-MC5R). MC2R is specific for ACTH, whereas the MSHs bind to the other 4 MCRs, with MC1R and MC3R exhibiting the highest affinity for α-MSH and γ-MSH, respectively (3). Atypically, melanocortin signaling is not exclusively regulated by the binding of endogenous agonists, because naturally occurring antagonists, agouti-signaling protein (ASIP) and agouti-related protein (AGRP), compete with melanocortin peptides by binding to MCRs.
Melanocortin signaling participates in the regulation of multiple physiologic functions (3), but its involvement in the control of corticosteroid synthesis, via MC2R (4), and in the control of energy balance, via MC3R and MC4R (5), are the most studied facets of such signaling. Central activation of MC3R and MC4R is thought to mediate the effects of melanocortin on the energy balance (5) because both MC3R-knockout rat (6) and MC4R-knockout mice (7) display severe alterations in energy homeostasis. Interruption of α-MSH central signaling by the ubiquitous constitutive expression of agouti gene in obese yellow mice (Ay) results in hyperphagia, hyperinsulinemia, increased linear growth, maturity-onset obesity, and yellow fur (8). A similar metabolic syndrome is also observed in transgenic mice ubiquitously overexpressing AGRP (9), and in MC4R-knockout mice (7). The central administration of the C-terminal fragment of AGRP (10) or chemical antagonists for MC3R and MC4R increase food intake in rodents (11), and intracerebroventricular injections of the MCR agonist, MTII, produces a dose-dependent reduction in food intake in mice (11). However, MC4R-deficient mice do not respond to the anorectic effects of MTII, suggesting that α-MSH inhibits feeding primarily by activating MC4R (12).
Of the 5 MCRs, only the activation MC2R fails when expressed in the conventional heterologous cell lines. In nonadrenal cells, the receptor is retarded in the endoplasmic reticulum, and its functional expression requires the presence of an accessory protein, known as melanocortin receptor 2 accessory protein (MRAP), which works as an MC2R-specific transport system to the plasma membrane (13). MRAP is a small protein exhibiting a hydrophobic transmembrane domain mainly expressed in the adrenal cortex. The knockdown of endogenous MRAP in Y1 adrenocortical cells leads to insensitivity to ACTH, demonstrating that MRAP is essential for producing an ACTH-responsive MC2R (14). MRAP interacts with the MC2R to facilitate correct folding, and subsequent glycosylation and receptor cell surface expression (13), but it is also essential for ACTH binding and ACTH-induced cAMP production (15, 16).
Vertebrate genome has an MRAP paralogue that also encodes a small single transmembrane-domain protein (17), now named MRAP2 (13, 16, 18). Most authors continue to call the first characterized protein MRAP rather than MRAP1, but hereinafter we shall use the numerical nomenclature. The function of MRAP2 is controversial (19). Coimmunoprecipitation studies demonstrate that MRAP2 interacts with the MC2R and fully rescues the functional expression of the receptor (18). However, Sebag and Hinkle (16) have reported that MC2R reaches the cell membrane in the presence of MRAP2 but that the increase in ACTH-induced cAMP was much lower (8-fold) than that exhibited in cells expressing both MC2R and MRAP1. Subsequent studies have demonstrated that MRAP2 is an endogenous inhibitor of MC2R activation and competes with MRAP1 to bind to the receptor, thus decreasing the ability of ACTH to stimulate cAMP production (20).
Very recent studies suggest that MRAPs can also modulate the function of other MCRs. Immunoprecipitation studies have demonstrated that both MRAP1 and MRAP2 interact physically with all 5 MCRs (16, 18). Both MRAP1 and MRAP2 reduce the functional expression of MC4R and MC5R but not of MC1R and MC3R in the plasma membrane. Accordingly, both MRAP1 and MRAP2 decrease [Nle4,D-Phe7]-α-MSH-stimulated cAMP production in cells expressing MC3R, MC4R, and MC5R, but only MRAP2 was able to induce a similar effect on MC1R (18). However, the physiologic involvement of these interactions in unknown.
The aim of this study was to investigate the interaction of MRAPs with the MCR system as well as the physiologic involvement of these interactions using zebrafish (zf) as model. We studied the hormonal and physiologic regulation of MRAP expression as a potential pathway for the regulation of melanocortin signaling. Zebrafish genome has 6 MCRs because MC5R is duplicated (MC5Ra and MC5Rb) and 3 different MRAPs. One MRAP sequence groups with tetrapod MRAP1s, and the 2 other MRAP sequences are classified as MRAP2 paralogues (MRAP2a and MRAP2b) (21, 22). We demonstrated that MC4R can be activated by ACTH when the receptor is coexpressed with MRAP2a, exhibiting similar sensitivity to that shown by MC2R. This pharmacologic finding has a clear physiologic significance because both proteins, ie, MC4R and MRAP2a, physically interact and are coexpressed in the same neurons of the preoptic area. ACTH injection inhibits short-term food intake in wild-type zebrafish, as MSH does in other closely related species (23), but not in zebrafish lacking functional MC4R (Sa122). It demonstrates that MC4R is required for the anorexic effects of ACTH. The expression of MRAP2b also colocalizes with MC4R in the tuberal hypothalamus, but it has no effect on agonist binding. Central MRAP2b expression increases during fasting, suggesting that this protein can depress constitutive MC4R signaling during starvation. Finally, we also demonstrate that MRAPs are hormonally regulated, suggesting a new pathway for the fine tuning of melanocortin signaling.
Materials and Methods
Animals, reagents, and primers
Wild-type TU strain zebrafish were raised at 24–28°C, with 14-hour light/12-hour dark cycle. MC4R−/− mutant strain sa122 were obtained from the Sanger Institute Zebrafish Mutation Project and genotyped as previously described (24). Before any manipulation, animals were netted and anesthetized for 1 minute in 2-phenoxy-ethanol (0.05%) in the sampling tank. When required, animals were humanely destroyed by rapid decapitation after anesthesia. All experiments were carried out in accordance with the principles published in the European animal directive (86/609/EEC) for the protection of experimental animals and approved by the Consejo Superior de Investigaciones Científicas (CSIC) ethics committee (project numbers AGL2010-22247-C03-01 and CSD 2007-00002 [to J.M. and C.-R.]). Unless otherwise indicated, all reagents were purchased from Sigma. Primers used in the experiments are summarized in supplemental Table 1.
Cloning procedure
The full coding regions of the zebrafish MCR genes were obtained from public databases (http://www.ensembl.org/index.html), subcloned in pGEM-T easy vector (Promega), and subsequently subcloned directionally into HindIII/XhoI restricted pcDNA5/FRT (Invitrogen). Primers sequences are shown in Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. MRAP constructs were obtained as previously described (21). Briefly different N- or C-terminal epitope tagged proteins (MRAPs and MCR) were made by PCR using Taq DNA Polymerase (Invitrogen) and pcDNA5/zfMRAP1, pcDNA3/zfMRAP2a, pcDNA3/zfMRAP2b, pcDNA5/zfMCRs constructs as templates. Proteins were N- or C-terminally tagged with Flag (DYKDDDDKC) or c-Myc (EQKLISEEDL) epitopes. The expected size products were cloned directionally into HindIII and XhoI restricted pcDNA5/FRT vector and sequenced.
Tissue expression experiments
Total RNA was purified from fresh tissues (testis, ovary, intestine, liver, muscle, spleen, head kidney and post kidney, gills, skin, eyes, heart, brain, and whole fish) with Tri-Reagent (Sigma), and 1 μg was used for cDNA synthesis with Superscript III reverse transcriptase (Invitrogen) primed with random hexamers and oligo(dT)12–18 (Invitrogen). The cDNA of 5 tissues from different animals (n = 5/tissue) was subsequently used as template for quantitative real-time PCR. For MRAP expression quantifications, 1 μL of cDNA was added to 10 μL of 2× Taqman PCR master mix (ABgene, Thermo Scientific), and primers and probes concentrations were 300 nM and 200 nM, respectively. As internal controls, a fragment of β-actin, elongation factor 1 and 18S were amplified. One microliter cDNA (1/100) and 250 nM primers were added to 7.5 μL of 2X Sybrgreen PCR master mix (ABgene, Thermo Scientific). Reactions were carried out in duplicate in a Realplex Mastercycler (Eppendorf). Primer sequences are shown in Supplemental Table 1.
Double in situ hybridization
Animals were anesthetized and humanely destroyed, and tissues carefully dissected. Brains were fixed with paraformaldehyde (PAF, 4%) in phosphate buffer (PB, 0.1 M pH 7.4) overnight, dehydrated, and embedded in Paraplast (Sherwood). Serial 6-μm cross-sections were cut using a rotary microtome. Sections were mounted on 3-aminopropyltriethoxylane-treated slides and then air-dried at room temperature (RT) overnight. Sections were stored at 4°C under dry conditions and used for hybridization within 1 month. The double in situ hybridization procedure was according to (25).
Before hybridization, sections were deparaffinized, rehydrated, and postfixed in 4% PAF for 20 minutes. Slides were then rinsed twice in PB for 7 minutes and treated with a Proteinase-K solution (20 μg/mL in 50 mM Tris-HCl, 5 mM EDTA, pH 8) for 5 minutes at RT. Slides were then washed in PB and postfixed again in PAF for 5 minutes and subsequently rinsed in sterile water and acetylated in a triethanolamine (0.1 M, pH 8)/acetic anhydride solution. Sections were then dehydrated and dried at RT. Nonisotopic riboprobes for full-length zfMC4R and zfMRAP2a were synthesized using a digoxigenin and fluorescein-RNA labeling mix (Roche Diagnostics), respectively, according to the manufacturer's instructions. After 7 minutes incubation at 75°C, riboprobes were diluted simultaneously in hybridization buffer containing 50% formamide, 300 mM NaCl, 20 mM Tris-HCl (pH 8), 5 mM EDTA (pH 8), 10% Dextran sulfate, 1× Denhardt's solution, and 0.5 μg/μL yeast RNA type III. Subsequently, 100 μL of hybridization solution was added to each pretreated slide (see above), which were coverslipped and incubated in a humidified chamber at 55°C overnight. The following day coverslips were removed by incubating slides in a solution containing 5× standard saline citrate (SSC) buffer (1× SSC containing 150 mM NaCl, 15 mM sodium citrate, pH 7), for 30 minutes at 55°C. The slides were then rinsed in 2× SSC, 50% formamide for 30 minutes at 65°C and 3 times immersed in NTE buffer (500 mM NaCl, 10 mM Tris-HCl, 5 mM EDTA, pH 7.5) for 10 minutes at 37°C. After RNAse treatment 2 μg/mL RNAse in NTE) for 30 minutes at 37°C, slides were rinsed once in NTE buffer for 10 minutes at 37°C, once in 2× SSC, 50% formamide, for 30 minutes at 65°C, once in 2× SSC for 10 minutes at RT, and twice in 0.1× SSC for 15 minutes at RT. Then slides were washed twice for 10 minutes at room temperature in buffer A (100 mM Tris-HCl, pH 7.5; 150 mM NaCl) and incubated in blocking solution (2% blocking reagent, Roche Diagnostics in buffer A) for 30 minutes at room temperature. Subsequently, the slides were incubated with anti-digoxigenin alkaline phosphatase-conjugated sheep Fab fragments and anti fluorescein horseradish peroxidase sheep Fab fragments (Roche Diagnostics) diluted in blocking solution. On the next day, sections were washed twice for 10 minutes in buffer B (100 mM Tris, pH 9.5, 100 mM NaCl), incubated for fluorescent detection of horse-radish peroxidase activity with tyramide-biotine amplification diluent (PerkinElmer) for 20 minutes, and subsequently washed in buffer B with 0.1% Triton for 15 minutes and exposed overnight at room temperature to streptavin Alexa 488 diluted 1:300 in blocking buffer. Next day, slides were incubated with HNPP (2-hydroxy-3-naphtoic acid-2′-phenylanilide phosphate) in 2-hydroxy-3-naphtoic acid-2′-phenylanilide phosphate/FastRED solution (Roche Diagnostic) during 3 hours for fluorescence detection of alkaline phosphatase activity. Finally, slides were coverslipped with Vectashield Hard Set mounting medium containing 4′,6-diamidino-2-phenylindole (Invitrogen). Anatomic locations were confirmed by reference to a brain atlas of zebrafish (26).
Cell culture and transfection
Human embryonic kidney (HEK) cells were maintained in DMEM (Gibco) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin mixture (Gibco) in a humidified atmosphere of 5% CO2 at 37°C. Transient transfections were carried out using Lipofectamine LTX (Invitrogen) according to the manufacturer's instructions with 100 ng of each construct, and total amounts of DNA were kept constant in 2 μg with pBSSK plasmid.
Pharmacologic experiments
A HEK-293 cell clone (clone Q), stably expressing β-galactosidase under the control of a vasoactive intestinal peptide promoter placed downstream of tandem repetitions of cAMP responsive elements (CREs) was used to evaluate receptor activation (CRE-galactosidase [GAL]) (27).
ZfMRAP constructs alone or in combination were transiently transfected together with zfMC4R and zfMC5aR constructs in the clon Q. A construct carrying luciferase gene under the control of a constitutive promoter was also transfected to standardize transfection levels. The following day, cells were split up into 96-well plates and stimulated with human α-MSH (Bachem) and ACTH 1–24 (Bachem) ranging from 10−7 to 10−10 M or forskolin 10−6 in assay medium at 48 hours after transfection. After 6 hours, the medium was removed, cells were lysed, and galactosidase activity was measured as previously described (27). The effect of zebrafish AGRP (zfAGRP, kindly donated by Dr. Millhauser from Department of Chemistry, University of California), 10−7 M on ACTH-stimulated MC4R/MRAP2a activity was studied also. Measurements were normalized for the protein content, the luciferase activity, and forskolin-induced galactosidase activity. Protein content was determined using the BCA protein assay kit (Pierce). Luciferase activity was determined using the luciferase assay kit (Promega) following provider instructions.
In order to corroborate the effect of MRAP2b on MC4R basal activity, a cell clone Flp recombinase-mediated homologous recombination system (Flp-lnTM) was used to produce cells lines stably expressing MC4R in HEK-293/FRT cells, a cell line with single genome-integrated FRT (21). The development of isogenic cell lines was carried out according to the manufacturer recommendations. Subsequently, cells were transiently transfected with 500 ng CRE-GAL alone or together with 20 ng of MRAP2b construct. Basal galactosidase levels in unstimulated cells transfected with MC4R or MC4R+MRAP2b were determined as above. Transfection levels were standarized as before.
Western blotting and coimmunoprecipitation
Whole-cell lysates were prepared 24 hours after transfection. Cells were washed once with cold PBS and lysates were generated using lysis buffer, briefly: 50 mM Tris-HCl, 500 mM NaCl, 0,5% TritonX-100, and 1 mM EDTA with protease and phosphatase inhibitors and incubated for 30 minutes on ice. Samples were then spun for 20 minutes at 16 000 × g at 4°C. The supernatant was mixed with Laemmli Sample buffer 2× before use for Western blotting or incubated overnight at 4°C with anti-FLAG magnetic beads (Sigma), or anti-MYC agarose beads (Sigma) for coimmunoprecipitation. After incubation, agarose was washed 4 times in lysis buffer, supernatant was removed, and sodium dodecyl sulfate (SDS) loading buffer was added. Magnetic beads were treated as manufacturer instructions and also resuspended in SDS loading buffer. After boiling for 3 minutes, samples were run in SDS-polyacrylamide gel. Western blotting was performed with anti-FLAG (Sigma), or anti-MYC (Abcam) antibodies used at dilutions of 1:1000 and 1:5000, respectively, and detected by horseradish peroxidase chemiluminiscence reaction of secondary antibody (SuperSignal West Femto, Pierce).
Immunofluorescence microscopy
HEK cells grown onto poly-L-lysine-coated coverslips were transiently transfected with 0.2 μg/well of Myc-MC4R and 0.2 μg/well Flag-MRAP2a, or Flag-MRAP2b constructs. Twenty four hours later, cells were fixed and permeabilized by incubation in methanol for 5 minutes and subsequently in acetone for 1 minute. Then, cells were rehydrated, washed in PBS, blocked, and incubated with mouse anti-c-Myc and rabbit anti-Flag antibodies. Primary antibodies were detected with goat antimouse or antirabbit secondary antibodies coupled to Alexa-Fluor 488 or Alexa-Fluor 594 (Invitrogen) as required. 4′,6-diamidino-2-phenylindole (2 μM) was used to stain nuclei. Coverslips were mounted in Prolong mounting medium for fluorescence (Invitrogen). Cells were also examined with a laser-scanning confocal microscope (Olympus FV1000).
Cell surface ELISA
To measure cell surface receptor expression, 293/FRT/Myc-zfMC4R cells were seeded in poly-L-lysine-coated 24-well plate (1 × 105 per well) and transfected independently with pcDNA3/zfMRAP2a or pcDNA5/zfMRAP2b. Twenty four hours after transfection, cells were washed with PBS, fixed on ice for 15 minutes with 1.85% formaldehyde to evaluate the presence of the receptor in the plasma membrane, or for 5 minutes with methanol for total receptor measurements. Cells were then processed for ELISA as previously described (21). Nonspecific OD492 values were determined by transfecting the untagged versions of each construct when possible or with enhanced green fluorescent protein. Experiments were repeated 3 independent times in triplicate.
In silico analysis of the MRAP1 5′-flanking region
As a first approach to understand the hormonal regulation of the MRAPs, the first 5 kb of the 5′-flanking region of the zfMRAP1 were obtained from Ensembl database (http://www.ensembl.org/index.html) and analyzed for the presence of putative cis-acting elements using MathInspector (Genomatix, http://www.genomatix.de/) and Transcription Element Search System (Tess, http://www.cbil.upenn.edu/cgi-bin/tess/tess) software.
Hormonal and physiologic regulation of MRAP expression
Twenty fish per treatment were reared in individual aquariums and fed twice a day during 1 week at 4% of body weight with control food or the same diet containing 500 μg/g of T3 (Sigma), cortisol (hydrocortisone, Sigma), or bezafibrate (Sigma), an agonist of the peroxisome proliferator-activated receptor α. After 7 days, fish were humanely destroyed and whole body was quickly frozen in dry ice. Total RNA of the whole fish was purified with Maxwell 16LEV Simply RNA Tissue Kit (Promega) as described by the manufacturer. Quantitative PCRs and data analysis were as before but 3 μg of RNA were used as template for cDNA synthesis. For fasting experiments, 30 animals were reared in 2 tanks (n = 15/tank) and fed for 14 days at 4% of the body weight. Thirty additional animals, split up into 2 individual aquaria, were fasted and sampled at 7 and 14 days. For stress experiments 30 animals were maintained during 7 and 14 days in 1/20 of water volume when compared with the control group. After the experimental period, brains were removed carefully, and total RNA was extracted using Tri-Reagent. cDNA synthesis, PCR quantification of MRAP expression, and data analysis were done as before.
Food intake experiments
Adult female and male zebrafish were placed individually in 2-L tanks for 4 consecutive days and food intake level was daily recorded. An excess of quantified food pellets (Supervit granulat, Tropical) were added to the tank at 10 am and the number of pellets was quantified after 2 and 4 hours. These measurements provided a baseline of food intake levels for each individual fish. The fifth day animals were injected ip with saline, 0.1, 2, or 10 μg of hACTH(1–24). A minimal of 10 fish was injected for each treatment. After 15 minutes, food intake levels were recorded in the same manner. Food intake levels of each treated fish were expressed as the percentage of the base line (average of the food intake levels during the previous 4 days). The same protocol was used with the zebrafish strain Sa122, obtained from the Zebrafish Mutation Project (Welcome Trust Sanger Institute), which lacks a functional MC4R. This zebrafish mutant has a different genetic background; therefore we injected both wild-type and mutant fish with saline or 10 μg hACTH(1–24)/fish (the effective doses obtained in the previous experiments). Total food intake was recorded after 4 hours.
Data analysis and statistics
Receptor activation data were fitted to logistic curves using QtiPlot free-software for LINUX (http://soft.proindependent.com/qtiplot.html). For graphic representation the response average for each dose from 3 independent experiments was calculated and data were fitted to logistic and ED50 values were resumed in Tables 1 and 2. For statistical comparisons, data from each independent experiment (n = 3) were fitted to dose-response curves, ED50 average values were compared, and significant differences were indicated by asterisk in Tables 1 and 2. Quantitative real-time PCR data were analyzed with the ΔΔCt (cycle threshold) method. Statistical analysis was conducted by one-way ANOVA followed by Tukey's multiple range test (P < .05).
Table 1.
MC4R or MC5R Were Expressed Alone or in Combination With One of the Different MRAPs in HEK-293 Cells Expressing a Reporter Gene Under the Control of CREs
| MC4R | MC4R MRAP1 | MC4R MRAP2a | MC4R MRAP2b | MC5R | MC5R MRAP1 | MC5R MRAP2a | MC5R MRAP2b | |
|---|---|---|---|---|---|---|---|---|
| α-MSH | 1.30 × 10−8 | 8.49 × 10−9 | 2.14 × 10−8 | 1.70 × 10−8 | 1.16 × 10−9 | 1.09 × 10−9 | 1.37 × 10−9 | 8.78 × 10−10 |
| [±2.49 × 10−8] | [±3.37 × 10−9] | [±3.92 × 10−8] | [±2.43 × 10−8] | [±3.19 × 10−9] | [±1.07 × 1010] | [±1.78 × 10−9] | [±9.61 × 10−10] | |
| ACTH(1–24) | 1.77 × 10−7 | 6.15 × 10−7 | 8.49 × 10−9 a | 4.9 × 10−8 | 9.73 × 10−10 | 9.01 × 10−10 | 1.41 × 10−9 | 6.80 × 10−10 |
| [±6.26 × 10−7] | [±3.84 × 10−7] | [±3.37 × 10−9] | [±5.1 × 10−8] | [±3.02 × 10−10] | [±1.47 × 10−10] | [±6.68 × 10−9] | [±2.08 × 10−10] | |
| ACTH(1–24) + AGRP | 3.57 × 10−7 b | |||||||
| [±6.77 × 10−7] |
The mean of the reporter activation, expressed as percentage of the basal level, for each concentration of melanocortin agonist (hACTH1–24 or α-MSH), was calculated from 3 independent experiments and the resultant data were fitted to logistic curves using Qtiplot free software. For statistical comparisons, data from each independent experiment (n = 3) were fitted to dose-response curves and ED50 average values (M) compared by one-way ANOVA and significant differences were indicated. Numbers in brackets are with the 95% confidence intervals of nonlinear fittings. ED50 values from gene reporter activation for melanocortin analogues on zebrafish MC4R expressed in HEK 293 cells.
Significant differences (P < .05) from MC4R transfected cells.
Significant differences between cells transfected with MC4R and MRAP2a treated with ACTH or ACTH + AGRP after t test (P < .05).
Table 2.
MC4R Was Expressed Alone or in Combination With One or Several MRAPs in HEK-293 Cells Expressing a Reporter Gene Under The Control of CREs
| MC4R | MC4R MRAP2a | MC4R MRAP2a MRAP1 | MC4R MRAP2a MRAP2b | MC4R MRAP2a MRAP2b MRAP1 | MC4R MRAP2b MRAP1 | MC2R | MC2R MRAP1 | MC2R MRAP2a MRAP1 | MC2R MRAP2a | |
|---|---|---|---|---|---|---|---|---|---|---|
| ACTH (1–24) | 8.6 × 10−7 | 9.59 × 10−9 a | 9.87 × 10−9 a | 4.97 × 10−9 a | 9.64 × 10−8 a | — | — | 1.37 × 10−9 | 1.05 × 10−9 | — |
| [±0.36 × 10−6] | [±6.56 × 10−9] | [±3.39 × 10−9] | [±0.97 × 10−9] | [±1.88 × 10−9] | [±0.21 × 10−9] | [±1.13 × 10−9] |
Data were treated as in Table 1. ED50 values from gene reporter activation for ACTH(1–24) on zebrafish MC4R or MC2R and MRAPs expressed in HEK 293 cells. Dashes indicates non-significant fitting.
Results
MC4R and MRAP expression
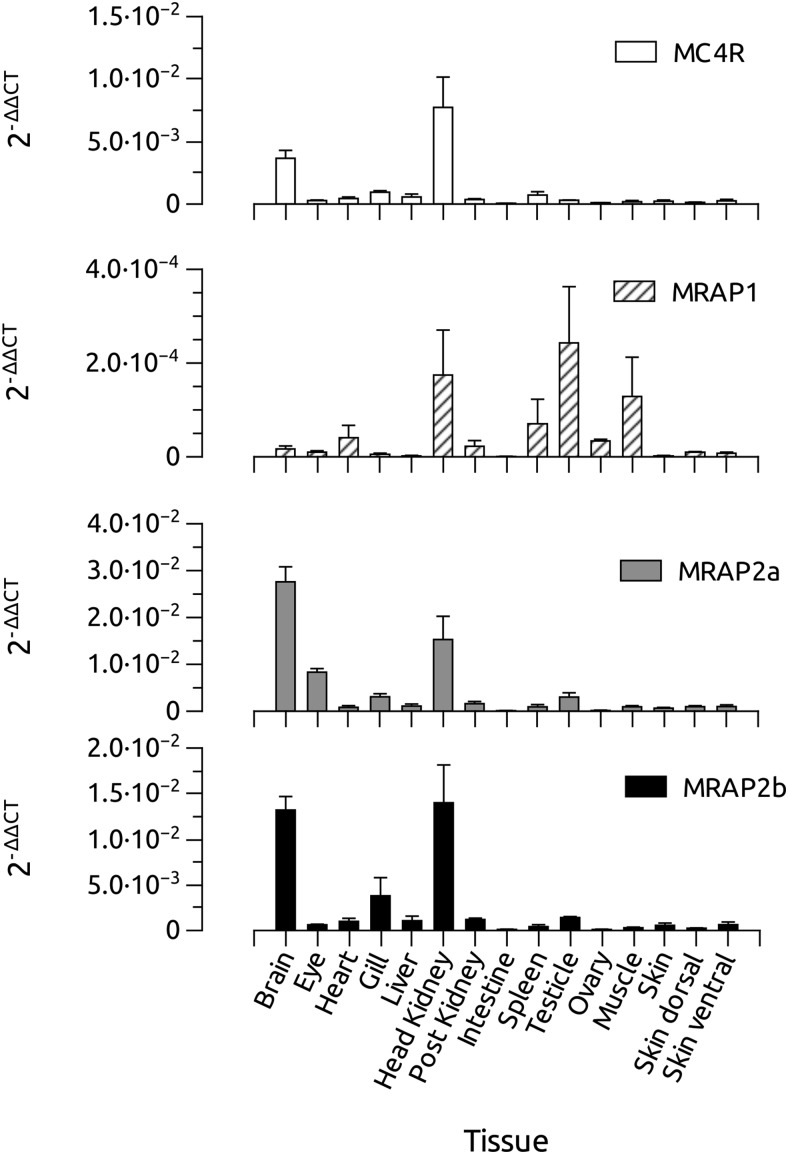
All 4 mRNAs, ie, MC4R, MRAP1, MRAP2a, and MRAP2b, were expressed in the head kidney, but only transcripts of MC4R and MRAP2s were found abundantly in the brain. All 3 MRAPs, but no MC4R, were expressed in the zebrafish testis. In addition, MRAP1 was expressed also in the muscle and spleen whereas MRAP2b was expressed in the eye. Residual levels were also found in some peripheral tissues (Figure 1).
Figure 1.
Distribution of MC4R and MRAPs mRNA expression in different zebrafish tissues, as revealed by quantitative real-time PCR (qPCR). Amplifications of β-actin and 18S and EF1α mRNAs were used as internal control of the reverse transcription.
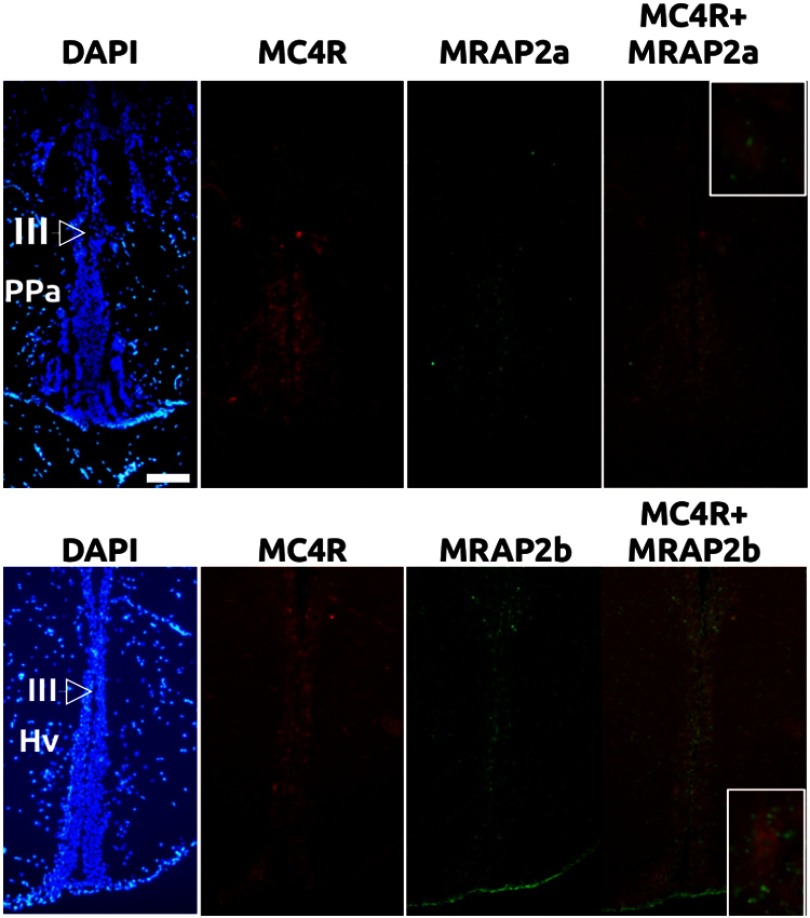
In order to corroborate the expression of MC4R and MRAP2a in the brain but also to demonstrate the coexpression of these genes in the same neurons, double in situ hybridization with nonisotopic probes was done (Figure 2). MC4R and MRAP2a colocalized in the preoptic area, at the level of anterior part of the parvicellular preoptic nucleus and also in the dorsal hypothalamus, particularly in the lateral extension of the third ventricle (data not shown) and the periventricular gray zone of the optic tectum (data not shown). On the contrary, MC4R and MRAP2b colocalized mainly in cells that coat the third ventricle within the medial area of the tuberal hypothalamus (Figure 2).
Figure 2.
Double in situ hybridization of MC4R (red) and MRAP2a (green) at the level of preoptic area (upper panels) or MC4R (red) and MRAP2b (green) at the level of tuberal hypothalamus (lower panels). Samples were aslo stained with 4′6-diamidino-2-phenylindole (DAPI) for morphologic studies. III, third ventricle; HV, ventral hypothalamus; Ppa, anterior preoptic area. Insets in the right panels show magnification of MC4R and MRAP colocalization. Scale bar, 200 μm.
MC4R and MRAP2s physically interact as demonstrated by immunoprecipitation and immunofluorescence studies
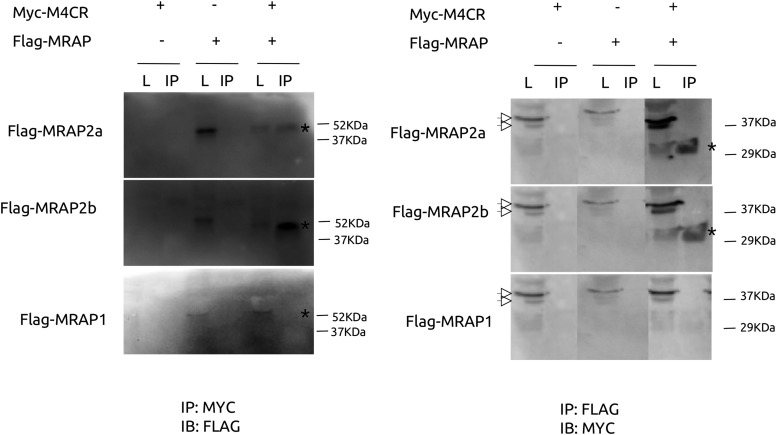
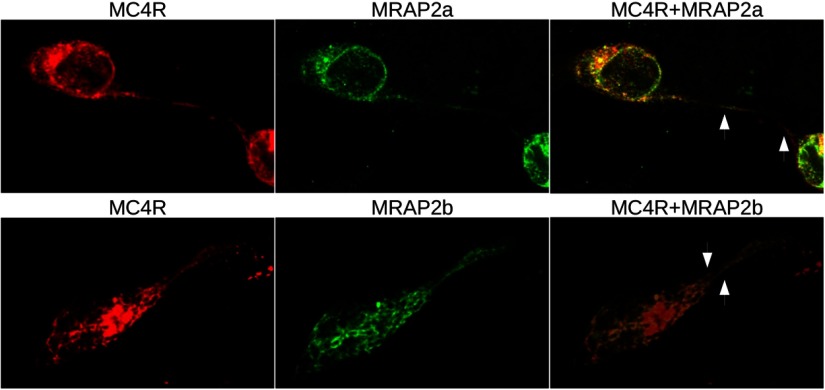
To determine whether MC4R and accessory proteins directly or closely interact, we tested whether receptor coimmunoprecipitated with each MRAP. Both zfMC4 and MC5aR coimmunoprecipitated with MRAP2a and MRAP2b but receptors did not interact with MRAP1 (Figure 3 and Supplemental Figure 1). When coexpressed in vitro, all 3 MC4R, MRAP2a, and MRAP2b proteins were detected by immunocytochemical techniques. High levels of MC4R MRAP2a (Figure 4, upper panels) and MRAP2b (data not shown) were detected in the surrounding area of the cellular nucleus matching the position of the endoplasmic reticulum/Golgi complex. All 3 proteins were detected also in the nuclear membrane (Figure 4; data not shown for MRAP2b). MC4R was targeted partially to the plasma membrane where it was found to colocalize partially with MRAP2a and/or in close apposition/colocalization with MRAP2b (Figure 4).
Figure 3.
MRAP2s and MC4R interactions. HEK cells were transfected with Flag-tagged MRAPs and/or Myc-tagged MC4R. Whole-cell lysates were prepared 24 hours after transfection and used for Western blot or incubated with anti-FLAG magnetic beads or anti-MYC agarose beads for coimmunoprecipitation. Both Flag-MRAP2a and Flag-MRAP2b, but not Flag-MRAP1, interact with Myc-MC4R as seen by immunoblotting (IB) with anti-Flag and anti-Myc, respectively after immunoprecipitation (IP) with anti-Myc. Asterisks indicate positive bands in crude lysates and immunoprecipitated samples whereas white arrowheads show unspecific bands.
Figure 4.
Immunofluorescence assays in live cells showing the expression of MRAPs (green) and/or zfMC4R (red). N-terminally Flag-tagged MRAPs and N-terminally Myc-tagged MC4R were transiently expressed in HEK-293 cells. Photomicrographs are taken with a ×60 objective and are from a single optical section obtained within an acquisition of z stacks (0.10 μm/slice). Arrows indicate regions of the plasma membrane where MRAPs and MC4R are potentially colocalized.
Pharmacologic implication of MC4R/MRAPs interaction
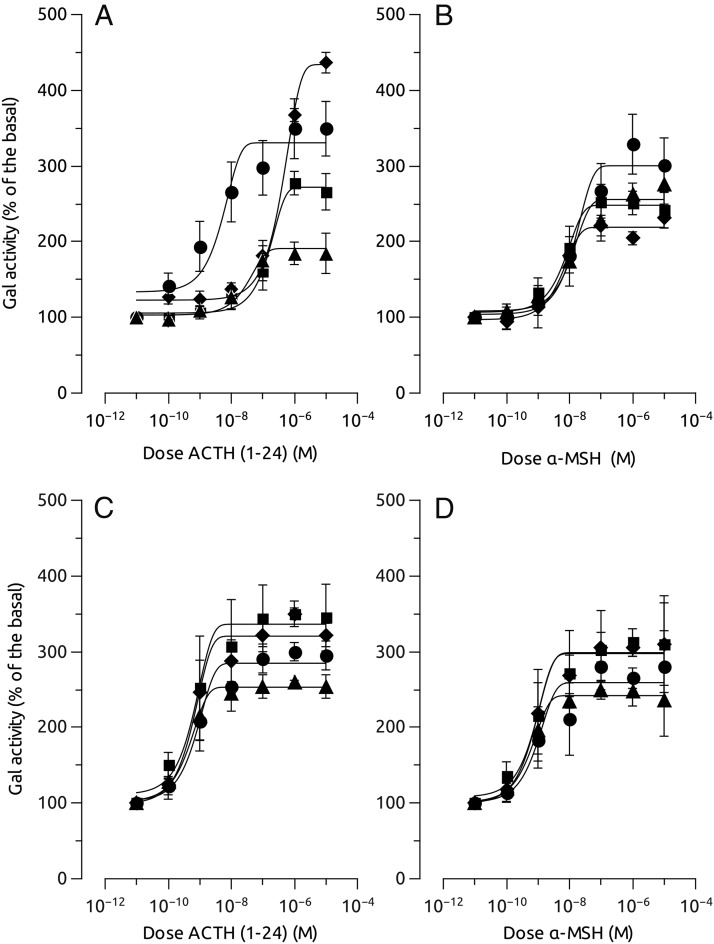
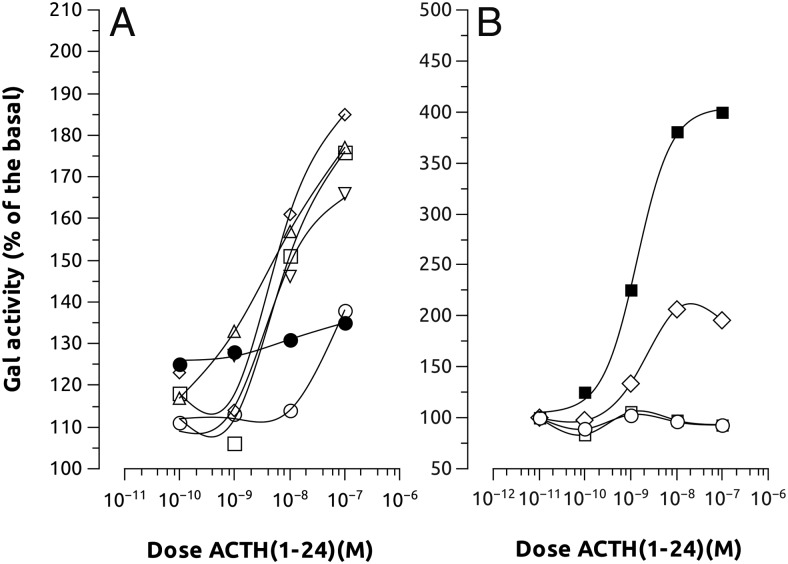
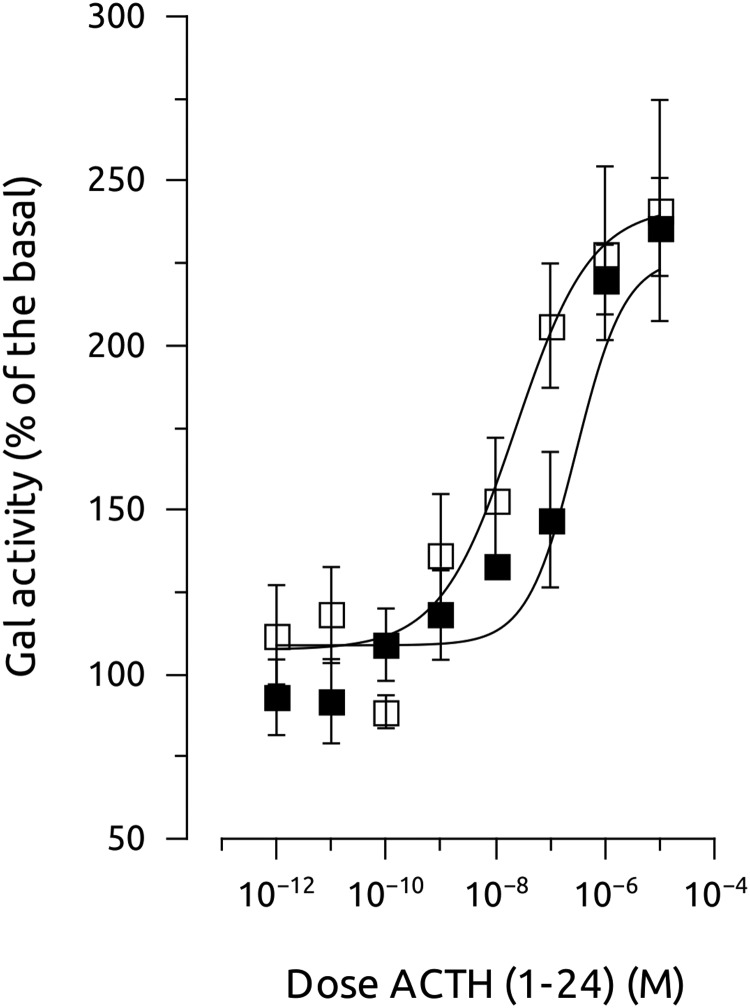
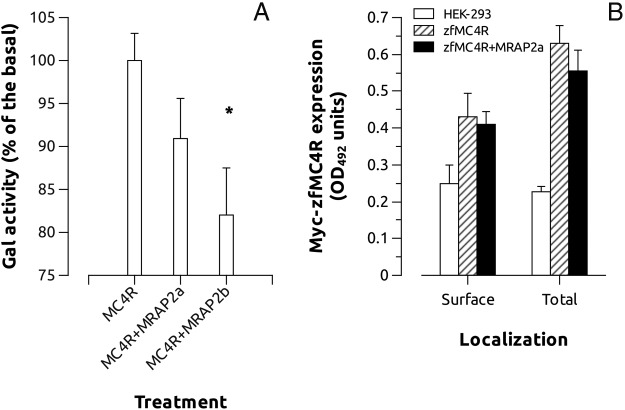
To determine whether the interaction zfMC4R-MRAP has some pharmacologic implication, we coexpressed both the receptor and the accessory proteins in HEK cells and stimulated the cells with increasing α-MSH or ACTH concentrations. Coexpression of MC4R and MRAP1 or MRAP2b, but no MRAP2a, slightly increased the sensitivity of the receptor by α-MSH. However, coexpression of MC4R and MRAP2a significantly increased the sensitivity of the receptor to hACTH(1–24). This effect was not evident when the receptor was expressed together with MRAP1 or MRAP2b (Figure 5 and Table 1). When MC4R was expressed with a combination of MRAPs (MRAP2a+MRAP1, MRAP2a+MRAP2b, MRAP1+MRAP2b, or MRAP2a+MRAP1+MRAP2b) the receptor only showed sensitivity to ACTH when MRAP2a was present in the combination (Figure 6 and Table 2). AGRP worked as a competitive antagonist of ACTH at MC4R when coexpressed with MRAP2a because its presence in the media decreased the ACTH-induced galactosidase activity (Figure 7). MRAPs had no effect on the α-MSH- or ACTH-induced activation of MC5aR, thus supporting previous results on MC4R. When MRAP2b was transfected into HEK-293 FRT cells stably expressing MC4R, basal activity levels decreased about 20%, showing that MRAP2b is able to reduce light but significantly the constitutive activity of the receptor. MRAP2a had no effect on the basal activity of the receptor (Figure 8A).
Figure 5.
Pharmacologic properties of melanocortin agonist, α-MSH and hACTH (1–24) at HEK-293 transiently expressing both MC4R (upper panels) or MC5Ra (lower panels) and different MRAPs (■, MC4R; ♦, MC4R+MRAP1; •, MC4R+MRAP2a; ▴, MC4R+MRAP2b) but stably expressing a cAMP-responsive β-galactosidase reporter gene. Data were normalized to protein levels and expressed as percentage of the basal levels. A construct carrying luciferase gene under the control of a constitutive promoter was also transfected to standardize the transfection levels. Experiments were performed using quadruplicate data points and repeated at least 3 times independently. Data are mean ± SEM of the 3 independent experiments.
Figure 6.
Effects of different MRAP combination on MC4R-induced galactosidase activity. A, Pharmacologic properties of hACTH(1–24) at HEK-293 transiently expressing both MC4Rs with different combinations of MRAPs (○, MC4R; □, MC4R+MRAP2a; ♢, MC4R+MRAP2a+MRAP1; ▵, MC4R+MRAP2a+MRAP2b; ▿, MC4R+MRAP2a+MRAP2b +MRAP1; •, MC4R+MRAP2b+MRAP1, and stably expressing a cAMP-responsive β-galactosidase reporter gene. Only when MRAP2a was present in the combination, the MC4R was able to respond to ACTH stimulation. B, Pharmacologic properties of hACTH(1–24) at HEK-293 transiently expressing both MC2R with different MRAPs (○, MC2R; ■, MC2R+MRAP1; □, MC2R+MRAP2a; ♢, MC2R+MRAP1+MRAP2a). Data were normalized to protein levels and expressed as percentage of the basal levels. Error bars were omitted to facilitate the graph vision. A construct carrying luciferase gene under the control of a constitutive promoter was also transfected to standardize transfection levels. Experiments were performed using quadruplicate data points and repeated 2 times independently.
Figure 7.
Effects of AGRP on hACTH(1–24)-stimulated galactosidase activity in HEK-293 cells transiently expressing both MC4R and MRAP2a but stably expressing a cAMP-responsive β-galactosidase reporter gene (□, MC4R+MRAP2a; ■, MC4R+MRAP2a+AGRP). Data were normalized to protein levels and expressed as percentage of the basal levels. A construct carrying luciferase gene under the control of a constitutive promoter was also transfected to standardize transfection levels. Experiments were performed using quadruplicate data points and repeated at least 2 independent times.
Figure 8.
A, Effects of MRAP2a and MRAP2b on MC4R-induced galactosidase basal activity in HEK-293 cells stably expressing MC4R but transiently expressing galactosidase gene under the control of a constitutive promoter carrying several CRE sites (see Material and Methods for more details). A construct carrying luciferase gene under the control of a constitutive promoter was also transfected to standardize transfection levels. Experiments were performed using quadruplicate data points and repeated 4 independent times. Asterisks show significant differences after Student's t test (P < .05). B, Total and cell surface detection of Myc-zfMC4R using anti-Myc antibodies. Control corresponds to nontransfected HEK-293 cells. Cells were transiently transfected with MC4R or MC4R+MRAP2a and assayed for total and extracellular c-Myc detection by whole-cell ELISA. The results represent the mean ± SEM of 3 independent experiments, each performed in triplicate.
ZfMC4R surface expression
zfMRAP2a expression had no effect on zfMC4R surface or total expression (Figure 8B).
MRAP1 5′-flanking region
In order to obtain information on putative hormonal systems regulating MRAP system, we analyzed the 5′-flanking region of the MRAP1. The proposed promoter sequence contained a number of sites that corresponded to homologs of the consensus sequences of various hormone-responsive elements, in particular, 10 putative glucocorticoid response elements, and 6 potential estrogen response elements. We also highlighted the presence of 9 putative peroxisome proliferative activated receptor homodimers (see Supplemental Figure 2).
Hormonal and physiologic regulation of zfMRAPs
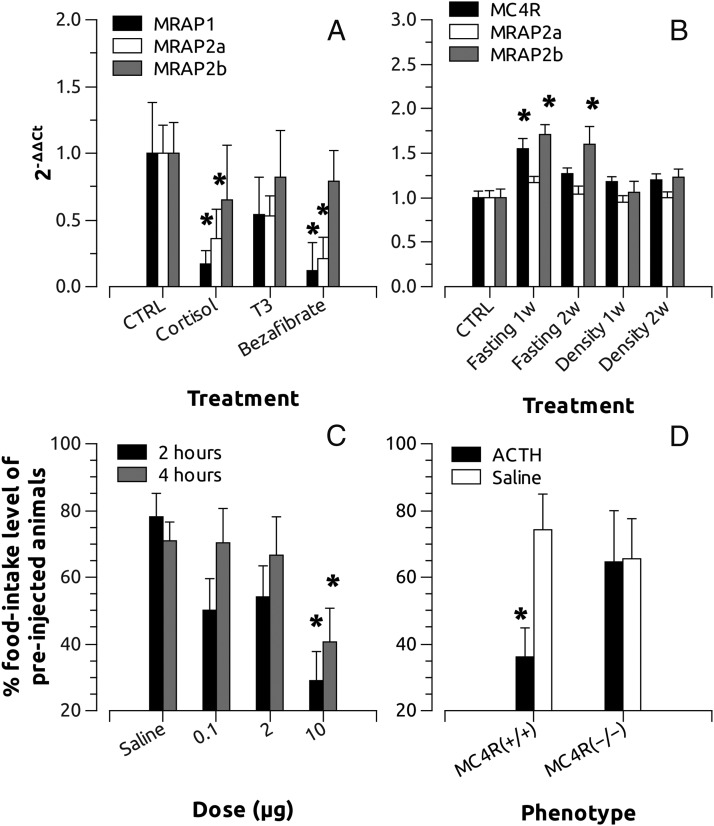
In order to evaluate whether the MRAP system can be a regulatory node of the melanocortin system activity, we studied the expression response of all 3 MRAPs to different hormonal systems. We took advantage of the previous promoter analysis of MRAP1 (see above). Both orally administrated cortisol and bezafibrate for 1 week significantly inhibited the expression of MRAP1 and MRAP2a. A similar effect was also observed on MRAP2b expression but levels did not reach statistical significance. T3 had no effect on MRAP system expression but its oral administration tends to decrease the expression levels of MRAP1 and MRAP2a (Figure 9A).
Figure 9.
A, Effects of cortisol, T3, or bezafibrate, a PPAR agonist, on MRAP expression. Animals were fed with food pellets containing 500 μg/g of the different hormones and humanely destroyed after 7 days. Total RNA of the whole fish was purified and used for cDNA synthesis. MRAP1, MRAP2a, and MRAP2b expression was analyzed with the ΔΔCt (cycle threshold) method. B, Fasting and rearing density effects on brain expression of MRAP2a and MRAP2b. Animals were fed for 14 days at 4% of the body weight and subsequently fasted for 7 and 14 days. For density experiments animals were maintained during 7 and 14 days in 1/20 of water volume when compared with the control (CTRL) group. MRAP2s expression data were treated as before. C, Effects of hACTH(1–24) on zebrafish (wild-type TU strain) food intake levels. Food intake levels were recorded during 4 consecutive days to establish a base line for each intact fish. The fifth day animals were injected ip with hACTH(1–24). After 15 minutes, food intake levels of each treated fish were recorded after 2 and 4 hours and expressed as the percentage of the base line (average of the food intake levels during the previous 4 days). D, Effects of hACTH(1–24) on zebrafish sa122 food intake levels. Food intake levels were recorded and calculated as before (see Figure 12). Asterisks show significant differences after one-way ANOVA and Tukey's method (P < .05).
We also explore the effect of fasting and stress on central expression of the MRAP2s and MC4R. Long-term fasting significantly increased MC4R expression at 1 week but such an increase was abolished at 2 weeks. On the contrary, MRAP2b expression was increased significantly during the whole experimental period. Stress by density had no effects on central MRAP2 expression after 1 or 2 weeks (Figure 9B).
ACTH effects on zebrafish food intake
In order to evaluate the effects of ACTH on food intake levels, we injected ip adult zebrafish (mean body weight, 0.421 ± 0.0013 g) with saline or increasing doses of hACTH(1–24). Animals injected with saline at about 80% of the previous food intake after 2 or 4 hours. However, animals injected with 10 μg of hACTH(1–24) ate only about 30 and 40% after 2 or 4 hours, respectively. No significant differences were observed when fish were injected with lower doses (0.1 or 2 μg) (Figure 9C). When zebrafish lacking a functional MC4R were injected with the effective doses of hACTH(1–24), no effects on food intake were recorded at 4 hours after injection. However, the same doses of hACTH(1–24) injection induced a significant reduction of food intake levels in wild-type animals of the same genetic background (Figure 9D).
Discussion
Genome vertebrate contains from 4 to 6 MCRs but only MC2R is considered the ACTH receptor (22). The functional expression of MC2R requires the coexpression of MRAP1 that promotes receptor traffic to the membrane (13). MRAP1 has an paralogue called MRAP2, which has been duplicated again in zebrafish (MRAP2a and MRPA2b) (21). Although MRAP2 has been shown to interact with other MCRs, its function remains unclear (16, 18). In this paper, we demonstrate that MRAP2a confers to MC4R, a canonical MSH receptor, the capability to be activated by ACTH with a similar sensitivity to that exhibited by MC2R (21). However, the coexpression of MRAP2a has no effect on α-MSH-induced MC4R activation. It means that MRAP2a is able to transform a MSH receptor into an ACTH receptor. This capability is specific for the tandem MRAP2a/MC4R because coexpression of the receptor with MRAP1 or MRAP2b had no effect on ACTH- or α-MSH-induced cAMP production. When several MRAPs were expressed in combination, MC4R was able to respond to ACTH only when MRAP2a was present in the combination, further corroborating this specificity. From the evolutionary point of view, the results reported here provide a new aspects of the functional evolution of the G protein-coupled receptors. Therefore, the coexpression of an accessory protein can supply a new function to the receptor by widening the binding spectrum. These new binding properties allow the receptor to signal new physiologic conditions. The expression of an accessory protein could also expand the tissue response to new hormonal systems or new molecules.
This ability of MRAP2a is restricted to the MC4R because the coexpression of the different MRAPs with the MC5Ra had no effect on the pharmacologic profile of the receptor. We have tested also the interaction of the different MRAPs with the other MCRs, and no effects on ACTH sensitivity were recorded (R. Cortes, M.J. Agulleiro, and J.M. Cerdá-Reverter, unpublished results). In a recent paper, Sebag and Hinkle (20) reported that hMRAP2 competes with hMRAP1 for binding to hMC2R, thereby decreasing the potency of ACTH. Similar to our results, hMRAP1 and hMRAP2 had no effects on NDP-α-MSH-induced activation of hMC4R, but ACTH-induced receptor activation was not tested. Reinick et al (28) reported that dogfish MC5R (Scualus acanthias) responds with higher affinity to ACTH(1–25) than α-MSH, and sensitivity of the receptor to ACTH(1–25) increased by coexpression with zebrafish or mouse MRAP1. However, the coexpression of cartilaginous MRAP2 had no impact on MC5R function. The present experiments cannot discriminate the mechanism by which MRAP2a promotes the ACTH-induced MC4R activation, but immunoprecipitation and immunofluorescence experiments demonstrate that both proteins interact directly or closely by means of an intermediary protein. As expected, this interaction did not modify the surface functional expression of the receptor because α-MSH-induced activation remains unaltered after MRAP2a/MC4R coexpression. This result suggests that MRAP2a modifies the MC4R tertiary structure to increase ACTH-binding affinity.
In contrast to the activation data, MRAP2a interaction is not specific to MC4R because it also immunoprecipitates with MC5Ra. Similarly, MRAP2b binds both receptors, ie, MC4R and MC5Ra but MRAP1 does not. Accordingly, hMRAP2 has been shown to interact with all 5 hMCRs (18). Our results suggest that MRAP2b could have some effect on MC4R function regardless of agonist binding. The MC4R is a constitutive receptor that signals in the absence of ligand. AGRP works as an inverse agonist, decreasing basal activity of the receptor, but also as competitive antagonist, inhibiting MSH-induced activation (27, 29, 30). Cross talk between α-MSH and AGRP regulates the activity of the MC4R, thus controlling output effects on energy balance and growth (5). Decreased MC4R signaling in MC4R knockout mice (7), AGRP overexpressing mice (9), agouti mice (8, 31) or morpholino zebrafish (24) results in hyperphagia, obesity, and increased linear growth. Our results demonstrate that fasting severely increases central MRAP2b expression. Therefore, it is plausible that MRAP2b could decrease constitutive activity of the MC4R during fasting periods, driving the animal toward positive energy balance. Further supporting this idea, we demonstrate that transient transfection of low quantities of MRPA2b is able to decrease basal galactosidase activity in cells lines stably expressing MC4R. In addition, double ISH experiments show that both MC4R and MRAP2b expression colocalize in the neurons of the ventral hypothalamus, a brain area involved in the regulation of pituitary secretion but also in the control of energy balance. In fact, the ventral hypothalamus seems to be the homolog of the mammalian arcuate nucleus (32). Colocalization studies provide an anatomic support to the immunoprecipitation experiments but also a physiologic role to the protein interaction. We anticipate that overexpression of MRAP2b in zebrafish could result in increased linear growth as observed in AGRP transgenic zebrafish (33) or MC4R knockout zebrafish (24).
Both MC4R and MRAP2a mRNAs colocalize also in the preoptic area, tuberal hypothalamus, and optic tectum. The interaction between both proteins in the brain suggests that ACTH could be involved in the regulation of food intake and growth in zebrafish. In support of this, we demonstrate that peripheral administration of ACTH inhibits short-term food intake in wild animals but not in the zebrafish strain sa122 that lacks a functional MC4R. It demonstrates that MC4R, but not MC2R, mediates anorexic effects of ACTH. In mammals, ACTH is synthesized mainly in the pituitary, but central POMC is mainly processed to α-MSH and β-endorphin (2). Therefore, it suggests that peripheral ACTH could reach central structures controlling food intake, particularly brain areas expressing both MC4R and MRAP2a, to inhibit food intake. However, immunohistochemical experiments in carp, a species very closely related to zebrafish, have reported the presence of ACTH in the preoptic area but expression studies demonstrated that POMC is exclusively expressed in the tuberal hypothalamus of goldfish (34). It suggests that hypothalamic POMC can be processed into ACTH and projected to the preoptic area, where MC4R/MRAP2a are expressed, to modulate melanocortin signaling. Alternatively, ACTH could stimulate cortisol secretion, via interrenal MC2R, and inhibits food intake as observed in other fish species (35). However, it is unlikely because sa122 animals exhibit a functional MC2R but food intake levels remain unaltered. In addition, cortisol treatment exhibits long lasting effects on feeding response (35). However, our results show that ACTH-treated animals reduce food intake levels after 2 hours, thus making it improbable that cortisol mediates ACTH effects on food intake. Central effects of ACTH on MC4R are not exempt of AGRP competitive antagonist because the presence of this protein can decrease the ACTH-induced MC4R activation. This result lends weight to the central action of ACTH because AGRP is basically expressed in the zebrafish brain (36).
The MRAP system provides a mechanism for the fine tuning of melanocortin signaling throughout the regulation of the receptor activity and/or response. Supporting this idea, we demonstrate that MRAP1 and MRAP2a expression, both proteins involved in the regulation of ACTH responsiveness via MC2R and MC4R, respectively, are down-regulated by cortisol, T3, and bezafibrate. All 3 compounds could modulate the response to ACTH, regulating the presence of MC2R in the membrane, by decreasing MRAP1 expression, or regulating ACTH binding to MC4R, by down-regulating MRAP2a expression. This fact acquires special relevance in regulatory feedback systems. The MC4R, together with MC2R (24), is also highly expressed in the head-kidney where interrenal tissue, the equivalent of mammalian adrenal tissue, is intermingled. Increased cortisol levels could decrease interrenal MRAP1 and MRAP2a expression, providing a mechanism for a local negative feedback by decreasing the sensitivity to systemic ACTH.
In summary, we demonstrate that MC4R, a canonical MSH receptor involved in the control of energy balance, becomes ACTH receptor-like when coexpressed with MRAP2a. Both proteins, MRAP2a and MC4R, physically interact and are coexpressed at the central nervous system (CNS), in key areas involved in the regulation of energy balance. In addition, ACTH administration inhibits food intake in wild-type animals but not in MC4R-deficient zebrafish, suggesting that MC4R mediates the anorexigenic effects of ACTH. MRAP2a is regulated by several hormonal systems including corticosteroid and thyroid hormones, thus providing an excellent substrate for the fine tuning of melanocortin activity. MRAP2b is also able to interact with MC4R, and both proteins are coexpressed in the brain, in similar areas where MC4R and MRAP2a are coexpressed. MRAP2b cannot modify the receptor response to the agonist but is up-regulated during chronic fasting, suggesting that the protein can decrease the constitutive activity of the receptor during fasting. This result supports a role for MRAP2b in the control of energy balance and suggests that protein dysfunction would result in increased growth and/or obesity.
Acknowledgments
We thank Dr Gregorio Molés for his technical assistance in Western blotting and Jose Monfort and Lucinda Rodríguez for their help with the histologic procedures. We also thank Sebastian Escobar for his support with double in situ hybridization.
This work was supported by grants AGL2010-22247-C03-01 and CSD 2007-00002 from Spanish Science and Education Ministry (MEC) (to J.M.C.-R). Additional funding was obtained from the “Generalitat Valenciana” (PROMETEO 2010/006). M.J.A. and R.C. are recipients of a “Juan de la Cierva” research contract (2009) and FPI fellow from the Spanish Science and Innovation Ministry, respectively.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by grants AGL2010-22247-C03-01 and CSD 2007-00002 from Spanish Science and Education Ministry (MEC) (to J.M.C.-R). Additional funding was obtained from the “Generalitat Valenciana” (PROMETEO 2010/006). M.J.A. and R.C. are recipients of a “Juan de la Cierva” research contract (2009) and FPI fellow from the Spanish Science and Innovation Ministry, respectively.
Footnotes
- AGRP
- agouti-related protein
- CRE-GAL
- cAMP responsive element-galactosidase
- HEK
- human embryonic kidney
- MCR
- melanocortin receptor
- MRAP
- melanocortin receptor accessory protein
- NTE buffer
- 500 mM NaCl, 10 mM Tris-HCl, 5 mM EDTA, pH 7.5
- PAF
- paraformaldehyde
- PB
- phosphate buffer
- POMC
- proopiomelanocortin
- RT
- room temperature
- SDS
- sodium dodecyl sulfate
- SSC
- standard saline citrate.
References
- 1. Nakanishi S, Inoue A, Kita T, et al. Nucleotide sequence of cloned cDNA for bovine corticotropin-β-lipotropin precursor. Nature. 1979;278:423–427. [DOI] [PubMed] [Google Scholar]
- 2. Castro MG, Morrison E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol. 1997;11:35–57. [DOI] [PubMed] [Google Scholar]
- 3. Schiöth HB, Haitina T, Ling MK, et al. Evolutionary conservation of the structural, pharmacological, and genomic characteristics of the melanocortin receptor subtypes. Peptides. 2005;26:1886–1900. [DOI] [PubMed] [Google Scholar]
- 4. Chan LF, Metherell LA, Clark AJ. Effects of melanocortins on adrenal gland physiology. Eur J Pharmacol. 2011;660:171–180. [DOI] [PubMed] [Google Scholar]
- 5. Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. [DOI] [PubMed] [Google Scholar]
- 6. Chen AS, Marsh DJ, Trumbauer ME, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. [DOI] [PubMed] [Google Scholar]
- 7. Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. [DOI] [PubMed] [Google Scholar]
- 8. Lu D, Willard D, Patel IR, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. [DOI] [PubMed] [Google Scholar]
- 9. Ollmann MM, Wilson BD, Yang YK, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. [DOI] [PubMed] [Google Scholar]
- 10. Rossi M, Kim MS, Morgan DG, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of α-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. [DOI] [PubMed] [Google Scholar]
- 11. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. [DOI] [PubMed] [Google Scholar]
- 12. Marsh DJ, Hollopeter G, Huszar D, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–122. [DOI] [PubMed] [Google Scholar]
- 13. Metherell LA, Chapple JP, Cooray S, et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37:166–170. [DOI] [PubMed] [Google Scholar]
- 14. Cooray SN, Almiro Do Vale I, Leung KY, et al. The melanocortin 2 receptor accessory protein exists as a homodimer and is essential for the function of the melanocortin 2 receptor in the mouse y1 cell line. Endocrinology. 2008;149:1935–1941. [DOI] [PubMed] [Google Scholar]
- 15. Roy S, Rached M, Gallo-Payet N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms α and β in isogenic human embryonic kidney 293 cells. Mol Endocrinol. 2007;21:1656–1669. [DOI] [PubMed] [Google Scholar]
- 16. Sebag JA, Hinkle PM. Regions of melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J Biol Chem. 2009;284:610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valsalan R, Krishnan A, Almén MS, Fredrikssson R, Schiöth HB. Early vertebrate origin of melanocortin 2 receptor accessory proteins (MRAPs). Gen Comp Endocrinol. 2013;188:123–132. [DOI] [PubMed] [Google Scholar]
- 18. Chan LF, Webb TR, Chung TT, et al. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci USA. 2009;106:6146–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cerdá-Reverter JM, Agulleiro MJ, Cortés R, et al. 2013 Involvement of melanocortin receptor accessory proteins (MRAPs) in the function of melanocortin receptors. Gen Comp Endocrinol. 2013;188:133–136. [DOI] [PubMed] [Google Scholar]
- 20. Sebag JA, Hinkle PM. Regulation of G protein-coupled receptor signaling: specific dominant-negative effects of melanocortin 2 receptor accessory protein 2. Sci Signal. 2010;3(116):ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agulleiro MJ, Roy S, Sánchez E, Puchol S, Gallo-Payet N, Cerdá-Reverter JM. Role of melanocortin receptor accessory proteins in the function of zebrafish melanocortin receptor type 2. Mol Cell Endocrinol. 2010;320:145–152. [DOI] [PubMed] [Google Scholar]
- 22. Cerdá-Reverter JM, Agulleiro MJ, Guillot R, Sánchez E, Ceinos R, Rotllant J. Fish melanocortin system. Eur J Pharmacol. 2011;660:53–60. [DOI] [PubMed] [Google Scholar]
- 23. Cerdá-Reverter JM, Ringholm A, Schiöth HB, Peter RE. Molecular cloning, pharmacological characterization, and brain mapping of the melanocortin 4 receptor in the goldfish: involvement in the control of food intake. Endocrinology. 2003;144:2336–2349. [DOI] [PubMed] [Google Scholar]
- 24. Zhang C, Forlano PM, Cone RD. AgRP and POMC neurons are hypophysiotropic and coordinately regulate multiple endocrine axes in a larval teleost. Cell Metab. 2012;15:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Escobar S, Felip A, Gueguen MM, et al. Expression of kisspeptins in the brain and pituitary of the European sea bass (Dicentrarchus labrax). J Comp Neurol. 2013;521:933–948. [DOI] [PubMed] [Google Scholar]
- 26. Wullimann MF, Rupp B, Reichert H. 1996 “Neuroanatomy of Zebrafish Brain: A Topological Atlas.” Birkhaeuser Verlag, Switzerland; 1996;144. [Google Scholar]
- 27. Sánchez E, Rubio VC, Thompson D, Metz J, Flik G, Millhauser GL, Cerdá-Reverter JM. Phosphodiesterase inhibitor-dependent inverse agonism of agouti-related protein (AGRP) on melanocortin 4 receptor in sea bass (Dicentrarchus labrax). Am J Physiol. 2009;296:R1293–R1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reinick CL, Liang L, Angleson JK, Dores RM. Functional expression of Squalus acanthias melanocortin-5 receptor in CHO cells: ligand selectivity and interaction with MRAP. Eur J Pharmacol. 2012;680:1–7. [DOI] [PubMed] [Google Scholar]
- 29. Nijenhuis WA, Oosterom J, Adan RA. AgRP(83–132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol. 2001;15:164–171. [DOI] [PubMed] [Google Scholar]
- 30. Ersoy BA, Pardo L, Zhang S, et al. 2012 Mechanism of N-terminal modulation of activity at the melanocortin-4 receptor GPCR. Nat Chem Biol. 2012;8:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proc Natl Acad Sci USA. 1995;92:4728–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cerdá-Reverter JM, Canosa LF. Neuroendocrine systems of the fish brain. In: Bernier NJ, Van Der Kraak G, Farrell AP, Brauner CJ, eds. Fish Neuroendocrinology. Fish Physiology. Vol 28 London, UK: Academic Press;2009:3–74 [Google Scholar]
- 33. Song Y, Cone RD. Creation of a genetic model of obesity in a teleost. FASEB J. 2007;21:2042–2049. [DOI] [PubMed] [Google Scholar]
- 34. Cerdá-Reverter JM, Schiöth HB, Peter RE. The central melanocortin system regulates food intake in goldfish. Regulatory Peptides. 2003;115:101–113. [DOI] [PubMed] [Google Scholar]
- 35. Leal E, Fernández-Durán B, Guillot R, Ríos D, Cerdá-Reverter JM. Stress-induced effects on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): a self-feeding approach. J Comp Physiol B. 2011;181:1035–1044. [DOI] [PubMed] [Google Scholar]
- 36. Song Y, Golling G, Thacker TL, Cone RD. Agouti-related protein (AGRP) is conserved and regulated by metabolic state in the zebrafish, Danio rerio. Endocrine. 2003;22:257–265. [DOI] [PubMed] [Google Scholar]