Abstract
GnRH regulates circulating levels of the gonadotropins but has also been implicated in establishing the gonadotrope cell population. Consistent with this, GnRH induces proliferation of partially differentiated gonadotropes, while reducing the numbers of fully differentiated cells. We have previously reported that the proapoptotic protein, prohibitin (PHB) is expressed more abundantly in gonadotrope-derived LβT2 cells than in partially differentiated αT3-1 gonadotrope precursor cells, suggesting a possible role for PHB in GnRH-induced apoptosis. We show here that PHB is required for GnRH-induced apoptosis in mature gonadotropes. PHB expression and activity are regulated by GnRH: its transcription is via c-Jun NH2-terminal kinase, whereas its nuclear export follows activation of ERK. Moreover, PHB levels are down-regulated by microRNA27, which is expressed at lower levels in mature gonadotropes, possibly explaining the switch to an apoptotic response with development. PHB is required for mitochondrial import of the proapoptotic BAX, whose expression is also induced by GnRH-activated c-Jun NH2-terminal kinase, as is expression of the BH3-only protein, HRK, and this too plays a role in GnRH-induced apoptosis. Finally, we show that gonadotrope-specific PHB-knockout mice display reproductive abnormalities, including a larger gonadotrope population, increased LH levels, reduced fertility, and altered gonad development. We thus demonstrate a role for PHB in GnRH-induced cell death in mature gonadotropes, which is crucial for the normal development and function of the reproductive axis.
The gonadotropin hormones, LH and FSH, that regulate reproductive function are produced in the pituitary gonadotropes under the regulation of the hypothalamic GnRH. GnRH first arrives at the developing pituitary gland before final differentiation of the gonadotrope and is essential for development of this population of cells, as indicated in hpg mutant mice lacking GnRH, in which the gonadotrope population is severely depleted (1). Moreover, the GnRH-induced production of LH is required to establish the initial population of FSH gonadotropes early in embryonic development (2). Despite this, the gonadotropin hormones are not required for early gonadal development (3). It is likely, therefore, that GnRH is delivered to the pituitary at this stage for it to elicit stimulatory effects on the development and maturation of this population of cells. GnRH increases the proliferation of immature, partially differentiated cell lines, but not of mature gonadotrope cell lines, in which it appears to induce cell cycle arrest (4, 5). Thus, after the gonadotrope becomes fully differentiated, the proliferative response is constrained, and the role of GnRH maybe largely restricted to increasing hormone production and release.
The involvement of GnRH in regulating gonadotrope cell number has been shown in several studies (4–7). In immature αT3-1 gonadotrope-like cells, it promotes an increase in cell numbers which is at least partly due to stimulating cell proliferation; this is dependent on both calcineurin-mediated signaling and β-catenin (5). Conversely, GnRH was shown to suppress proliferation of mature fully differentiated LβT2 gonadotrope-like cells, as well as human embryonic kidney 293 cells stably transfected with rat or human GnRH receptor (7). Similar findings have been demonstrated in other GnRH receptor–expressing nongonadotrope cell types and particularly in various cancer cells (for reviews, see Refs. 8–12).
We previously suggested that the loss of the GnRH-proliferative effect with gonadotrope development might be due to the increased levels of prohibitin (PHB) (4). In that study, a reduction in PHB expression levels in LβT2 cells was sufficient to endow a GnRH-induced increase in cell proliferation similar to that seen in the αT3-1 cells. Conversely, after overexpression of PHB or its 3′-untranslated region (UTR) in αT3-1 cells, the GnRH-induced increase in cell number was reduced or abolished (4).
PHB is a highly conserved protein reported to have a diverse range of functions, including both antiproliferative and proapoptotic effects (for reviews, see Refs. 13,14). It was shown in several cancer cell lines to repress E2F transcriptional activity and block the G (1) to S transition, causing cell cycle arrest (15–18). However, PHB has also been shown to play a role in apoptosis of several cell types and was found to be exported from the nucleus to the cytoplasm specifically in response to various apoptotic stimuli (19–21).
In the current study, we hypothesized that the increase in PHB levels that occurs with development might be responsible for a switch in the GnRH effect away from proliferation to a reduction in gonadotrope cell number and that this involves apoptosis.
Materials and Methods
Cell culture and transfections
Murine gonadotrope-derived αT3-1 and LβT2 cell lines (gifts from Pamela Mellon) were cultured as reported previously (22), and some were treated with GnRH (10–100 nM; Sigma-Aldrich). As required, chemicals were added 30 minutes before GnRH treatment, with the following final concentrations: 10 μM SP600125 (c-Jun NH2-terminal kinase [JNK] inhibitor; Sigma-Aldrich), 10 μM SB202190 (p38 MAPK inhibitor; Sigma-Aldrich), 10 μM U0126 (MAPK kinase [MEK] 1/2 inhibitor, Calbiochem; Merck Millipore), and 20 nM leptomycin (Cell Signaling).
The transient transfection of expression vectors and small interfering (si) RNA constructs was performed at 50% cell confluence, using a GenePORTER 2 Transfection Reagent Kit (Genlantis, Inc), according to the manufacturer's instructions, as described previously (22).
The Phb expression vector was created by amplifying the coding sequence from LβT2 cells, using the primers (5′) AGATCTGGGGAATTCATGTGGAGGTC (3′) and (5′) AAGCTTCTGGGGAAGCTGGAGAAGCA (3′). The amplified fragment was inserted into the pGEM-T easy vector (Promega), digested with the BglII and HindIII restriction enzymes (New England Biolabs Inc) and then cloned into the mammalian expression vector pEGFP (Clontech Laboratories Inc). The dominant negative (dn) CDC42 expression construct was purchased (Addgene) and the microRNA27b (miR27b) expression construct was a gift from Eran Hornstein (Weizmann Institute of Science, Rehovot, Israel). Constitutively active (ca) MEK and dnMEK expression constructs were gifts from Boon-Chuan Low (National University of Singapore, Singapore) and Zvi Naor (Tel Aviv University, Tel Aviv, Israel), respectively.
The siPHB construct in pSUPER has been reported (4). Knock down of HRK used two siRNA constructs (both in pSUPER) targeting different Hrk fragments, containing: (5′) TAAAGAGCTGATGGTGGATTCAAGAGAATCCACCATCAGCTCTTTA (3′) and (5′) ATGTGAACTCTGAGACTTTTCAAGAGAAAAGTCTCAGAGTTCACAT (3′) and their complimentary sequences. All constructs were verified by sequencing.
Cell quantification assays
To quantitate cell number, approximately 1 × 104 cells were plated on 96-well plates. The αT3-1 cells but not LβT2 cells were serum-starved (0.1%) before treatment, and subsequently cells were subjected to the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (Beit HaEmek), according to the manufacturer's instructions, or the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay (4). The optical density was read at an absorbance wavelength of 450 nm for the XTT assay and 560 nm for the MTT assay, with reference wavelengths of 690 and 670 nm, respectively.
Quantitative PCR (qPCR)
Total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies), and cDNA was synthesized using oligo(dT) primers and the ImProm-II Reverse Transcriptase Kit (Promega), both according to the manufacturers' instructions. The cDNA was diluted 1:1 with RNase-free water and then either used immediately or stored at −20°C. Real-time PCR was performed using an ABsolute Blue SYBR Green ROX Mix Kit (Thermo Fisher Scientific), with gene-specific primers (Phb, TGTCATCTTTGACCGATTCC and TTCGCAGTGTGATATTGACG; Bax, ACAGATCATGAAGACAGGGG and GGTTGCCCTCTTCTACTTTG; Hrk, ATTCCGTACCTGTGCATGCCTG and TGTGCTGAACAGTTGGTCCACG; and Sf-1, AAATTCCTGAACAACCACAGC and GCATCTCAATGAGAAGGTTG [all listed 5′ to 3′]), and the Eco Real-Time PCR System (Illumina Inc).
The miRs were purified using the miRNeasy kit (QIAGEN). The samples were quantified by spectrophotometric readings at 260 and 280 nm and stored at −20°C until use. Subsequently, to quantify the amount of the specific miR, the small RNA molecules were polyadenylated to allow for reverse transcription. Both these steps, as well as the qPCR reaction, were performed with the miScript PCR Starter Kit (QIAGEN), according to the instructions given. The oligo(dT) primers used in the reaction have a 3′ degenerate anchor and a universal tag sequence on the 5′ end, allowing amplification of mature miRs in the qPCR step. The resulting cDNA was diluted 1:256 with RNase-free water and then used for qPCR with the same kit. For detection of miR27 a forward primer for miR27b ([5′] UUCACAGUGGCUAAGUUCUGC [3′]) and a reverse primer for the universal tag sequence on the 5′ end of the oligo(dT) were used. A forward primer for the human RNU6B small noncoding RNA contained in the kit, with the same reverse primer, served as the positive control.
Protein analysis
Cells were collected into ice-cold PBS, pelleted (5000 × g; 3 minutes at 4°C), and lysed using RIPA buffer with phosphatase and protease inhibitors, after which the proteins were extracted by centrifugation (20,000 × g; 15 minutes at 4°C). For separation of nuclear and cytoplasmic proteins, the NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit (Pierce; Thermo Fisher Scientific) was used as described previously (4). Proteins were quantified using the Bradford reagent (Bio-Rad Laboratories) and denatured for SDS-PAGE at 95°C for 5 minutes with β-mercaptoethanol.
The protein samples were loaded onto 8% to 12.5% SDS denaturing gel and run at 100 V for stacking and 120 V for resolving. Transfer of the proteins to a nitrocellulose blot was performed at 105 V for 1.5 hours. The blot was then dyed with Indian ink for 15 minutes, before blocking with 5% bovine serum albumin in 0.1% Tween in PBS (PBS-T) for 1.5 hours at room temperature (RT). Thereafter, the blot was incubated overnight at 4°C with primary antibodies (Abs) diluted in 0.1% PBS-T. The blot was then washed three times with PBS-T and incubated with secondary Ab (RT, 1 hour), also in PBS-T (at 1:5000), after which it was washed three times more and incubated with a SuperSignal WestPico Chemiluminescent Substrate Kit (Pierce; Thermo Fisher Scientific) for 5 minutes at RT. Analysis was performed using the ImageQuant LAS 4000 imaging system (GE Healthcare Bio-Sciences AB).
The Abs used were as follows: PHB (sc-28259), actin (sc-81178), HRK (sc-26828), GAPDH (sc-25778), goat antirabbit and antimouse horseradish peroxidase–conjugated secondary Abs (sc-2004 and sc-2005), pERK (sc-7383 or sc-7976), ERK2 (sc-1647); pJNK (sc-6254), and pp38 MAPK (all from Santa Cruz Biotechnology); BCL2 (2870) and cleaved PARP (9544) (both from Cell Signaling Technology); and BAX (ab-7977–1; Abcam). The secondary Abs and Abs against GAPDH and actin were diluted 1:5000, the Ab to PHB was diluted 1:2000, the Abs to ERK2, pERK, pJNK, BAX, BCL2, and HRK were diluted 1:1000, and the Ab to cleaved poly(ADP-ribose) polymerase (PARP) was used at 1:500.
Indirect immunofluorescence
Indirect immunofluorescence was performed essentially as described previously (23) with Alexa Fluor 568-goat antirabbit IgG (Invitrogen Life Technologies) as the secondary Ab at 1:250 dilution, and the following primary Abs at 1:50 dilution: BAX (sc-6236), PHB (sc-28259), and β-actin (sc-1615-r) for control (all from Santa Cruz Biotechnology). All Ab dilutions were made in PBS-T. After immunostaining, the samples were washed with PBS-T and then incubated for 20 minutes at 37°C with either the nuclei dye Hoechst 33258 (Invitrogen Life Technologies; 1 μg/mL in PBS-T) or the mitochondrial dye MitoTracker Green (Invitrogen Life Technologies; 20 nM in PBS-T). The cells were then washed with PBS-T, mounted on slides (using Fluoromount solution; Southern Biotechnology Associates), closed hermetically, and stored in the dark at RT until microscopic analysis. Controls included omitting the following: secondary Ab, for sample autofluorescence in the red channel; primary Ab, for background staining caused by secondary Ab recognition of nonspecific targets; MitoTracker Green, for sample autofluorescence in the green channel; and Hoechst stain, for sample autofluorescence in the blue channel. In addition, Ab specificity was verified by comparing the staining pattern with that of the primary Ab against actin instead of against BAX/PHB. The slides were visualized with a Zeiss LSM 700 confocal microscope (Carl Zeiss Microscopy GmbH) and analyzed with Zeiss Zen LSM Axiovision 2009 software (Carl Zeiss Microscopy GmbH). The colocalization factor was calculated for each sample (13–25 samples per treatment) as the percentage of pixels with both green and red staining from the total pixels with each color, and the mean colocalization factor was calculated for each treatment.
Preparation of primary gonadotrope culture from GRIC/R26-YFP mice
Primary gonadotropes were extracted from genetically modified mice expressing yellow fluorescent protein (YFP) in their gonadotropes. These mice are generated by breeding female GnRH-receptor-IRES-Cre (GRIC) mice (24) and male ROSA26-YFP (R26-YFP) mice (25). Sexually mature GRIC/R26-YFP mice were killed, pituitary glands were removed, and primary cultures were prepared as described previously (24, 26). In brief, after their collagenase-trypsin dispersal (in 0.25% collagenase, 0.25% trypsin-EDTA, and 25 mM HEPES, pH 7.2, in PBS; 40 minutes at 37°C), the cells were pelleted (1000 rpm, 5 minutes), resuspended in phenol red–free DMEM and placed on ice for flow cytometry (fluorescence-activated cell sorting) sorting. The gonadotropes were collected based on their YFP fluorescence, using a FACSAria 2 sorter (BD Biosciences) and then were cultured in 96-well plates in the same phenol-red free DMEM with addition of 10% certified fetal bovine serum, 10 mM HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin. After 24 hours, they were exposed to GnRH.
Generation and analysis of gonadotrope-specific PHB-knockout mice
To generate a gonadotrope-specific knockout of PHB (PHB conditional knockout [cKO]), GRIC mice were crossed to a mouse strain harboring a conditional Phb null allele (both C57BL/6). The latter Phbfl mice carry two loxP sites flanking part of the Phb gene that is required for PHB activity (27), such that functional PHB is not expressed from this allele. These PHB cKO mice were killed using CO2, and thoracotomy was performed immediately before blood sampling from the heart. The circulating LH and FSH levels were measured using rodent LH and FSH ELISA test kits (Endocrine Technologies Inc), according to the manufacturer's instructions. The hormone levels were quantified using LH and FSH standard curves contained in the kit, which were assayed in the same way. The pituitaries were collected into TRIzol reagent and stored at −80°C until RNA extraction. First, they were homogenized in the TRIzol using a manual homogenizer and then passed through a 1-mL syringe with 21-gauge needle. Chloroform (0.2 mL) was added, and the samples were shaken vigorously for 15 seconds before incubation at RT for 15 minutes, followed by centrifugation at 12,000 × g for 15 minutes at 4°C. The aqueous phase was transferred to a collection tube from a RNeasy kit (QIAGEN) and the rest of the procedure was performed out using this kit, according to the instructions. The RNA was eluted in 15 μL of RNase-free water and quantified using spectrophotometric readings at 260 and 280 nm. The gonads were also removed, weighed, and fixed in Bouin solution, before embedding in paraffin, sectioning, and hematoxylin and eosin (H&E) staining, with the help of Patho-Lab Diagnostics Ltd (Ness-Ziona, Israel) and Dr Itzchak Brickner (Tel Aviv University, Tel Aviv, Israel).
All mice were held and bred at the Technion Animal Facility and handled humanely, in accordance with Institutional Animal Care and Use Committee guidelines.
Statistical analysis
All data are from at least 3 independent experiments, which were either combined or are shown as a representative figure. Statistical analysis comprising the t test to compare two groups or ANOVA followed by the Tukey-Kramer test for multiple comparisons were used to confirm differences between means of independently treated cells, and differences were considered significant at P < .05.
Results
GnRH induces apoptosis in LβT2 cells via PHB
To corroborate previous findings (4), an MTT assay was performed to measure the changes in immature and mature gonadotrope-like cell lines in response to GnRH. In the partially differentiated αT3-1 cells, GnRH exposure for 24 or 48 hours induced a 20% to 30% increase in cell numbers, whereas in the fully differentiated LβT2 cells, cell numbers dropped to less than 60% by 48 hours (Figure 1A).
Figure 1.
GnRH-induced apoptosis in mature gonadotropes requires PHB. A, Immature αT3-1 and mature LβT2 cells were cultured with or without GnRH treatment (10 nM) for 24 or 48 hours, before the MTT assay to assess the effect on cell numbers. Data are means ± SEM; n = 6. ANOVA followed by the Tukey ad hoc test was performed to compare all mean values in each of the cell lines separately; the same letters (capital for αT3-1 and lower case for LβT2 cells) were designated to values that are statistically similar (P > .05) to each other within the same group. B, αT3-1 and LβT2 cells were treated similarly with GnRH (100 nM, 24 hours), before whole-cell lysis and Western blot analysis of cleaved PARP, with GAPDH as the loading control. C, Western blot analysis of cleaved PARP was performed similarly in control LβT2 cells and after PHB knockdown (siPHB), with or without GnRH treatment (100 nM, 24 hours); PHB levels are also shown. D, XTT assays were performed in control and siPHB LβT2 cells, with and without GnRH treatment (100 nM, 24 hours), as before. Data are means ± SEM; n = 11 to 14. Statistical analysis was performed to assess the effect of GnRH treatment for control or siPHB cells: **, P < .01; NS, P > .05. E, PHB was overexpressed in αT3-1 cells, and the effect of GnRH on cleaved PARP levels was assessed as for C. F, Similarly, XTT assays were carried out after overexpression of PHB in αT3-1 cells, with or without GnRH treatment, and results are presented as for D; n = 12.
Although GnRH has previously been shown to cause arrest of cell proliferation (4, 7), we considered that the GnRH effect in reducing the number of LβT2 cells is probably due, at least in part, to apoptosis. To confirm this, we measured levels of cleaved PARP, as an indicator of the DNA damage that occurs during apoptosis. Cleaved PARP levels were increased in LβT2 cells treated with GnRH for 24 hours but remained at basal, barely detectable, levels in GnRH-treated αT3-1 cells (Figure 1B).
We hypothesized that PHB might be a major determinant in the effect of GnRH in reducing LβT2 cell numbers through apoptosis, so we tested whether PHB knockdown, using siRNA (siPHB), affects the GnRH-induced PARP cleavage. The siPHB clearly reduced the protein levels of PHB in these cells and also attenuated the GnRH-induced increase in cleaved PARP (Figure 1C). Moreover, an XTT assay confirmed that the GnRH-induced decrease in cell numbers was also abolished by the knockdown of PHB (Figure 1D).
To further substantiate the role of PHB in the GnRH-induced apoptosis, we overexpressed PHB in αT3-1 cells, in which it is normally found only at low levels. Although the PHB overexpression alone may have had a minor stimulatory effect on levels of cleaved PARP, their levels increased strongly after GnRH treatment in these cells, whereas in the control cells, the GnRH had no effect on cleaved PARP (Figure 1E). Overexpression of PHB in the αT3-1 cells also abolished the GnRH-induced increase in their numbers, as seen in the XTT assay (Figure 1F).
GnRH elevates levels of Phb steady-state mRNA and induces apoptosis through a JNK-dependent pathway
Given that PHB was seen to mediate GnRH effects on apoptosis, we went on to test how it might be regulated by GnRH. In both LβT2 cells and FACS-purified primary gonadotrope cells from adult GRIC/R26-YFP mice, GnRH exposure led to an elevation in Phb mRNA steady-state levels, which increased 3- to 4.5-fold after 8 to 12 hours of GnRH (Figure 2, A and B). Preincubation of the cells with inhibitors to ERK1/2, JNK, or p38 MAPK, which mediate many of the GnRH effects in these cells (28), revealed that most of this stimulatory effect of GnRH on the Phb mRNA levels is via the JNK pathway, with little apparent effect of the other inhibitors on this GnRH-induced effect (Figure 2C).
Figure 2.
GnRH elevates levels of PHB steady-state mRNA and induces apoptosis through a JNK-dependent pathway. A and B, LβT2 (A) or primary gonadotropes (B) from mature mice were cultured with or without GnRH for 8 or 12 hours, respectively, before extraction of RNA and real-time qPCR to measure Phb transcript levels, with RPLO as the internal control. Data are means ± SEM; n = 3 to 4. *, P < .05. C, LβT2 cells were treated with GnRH (100 nM for 6 hours) and/or inhibitors (10 μM added 30 minutes before) to JNK (SP600125), ERK1/2 (U0126), or p38 MAPK (SB202190), before RNA extraction and qPCR to measure the effects on Phb mRNA levels, expressed as fold over control after normalization to levels of GAPDH. Data are means ± SEM; n = 4. Statistical analysis was performed to compare cells treated with or without GnRH and the same inhibitor: **, P < .01; *, P < .05; NS, P > .05. D, LβT2 cells were transfected with dnCDC42 and/or exposed to GnRH (10 nM, 12 hours) before Western blotting to assess changes in PARP cleavage, with GAPDH as the loading control. The specific effect of this construct on pJNK and not on the other MAPKs is shown below in the left panel of E. E, MTT assays were performed in LβT2 cells, some of which were transfected with the dnCDC4 construct, with or without subsequent GnRH treatment (10 nM, 48 hours). Statistical analysis with ANOVA followed by the Tukey ad hoc test to compare all mean values and those sharing the same letter are statistically similar (P > .05); n = 4.
To verify that the JNK pathway also plays a role in GnRH-induced apoptosis, we blocked JNK activity by overexpressing dnCDC42 and tested the effects on the GnRH-induced PARP cleavage and on the cell numbers. Both the apoptotic effect of GnRH, as measured by cleaved PARP, as well as its effect on cell numbers were clearly reduced when the activation of JNK was prevented in this way (Figure 2, D and E).
miR27 regulates PHB expression and is responsible for its diverse protein levels in the partially and fully differentiated gonadotrope cell lines
Despite the clear increase in Phb mRNA levels after GnRH treatment, we were not able to see any consistent increase in PHB protein levels (Figure 1C), suggesting the involvement of posttranscriptional regulation of PHB expression. To understand further the regulation of PHB expression, the potential disconnect with its mRNA levels, and its lower levels in the partially differentiated cells (4), we examined a possible role for posttranscriptional regulation by miRs. The likelihood that the 3′-UTR of Phb acts to bind repressive miRs seemed reasonable, given previous findings that overexpression of the 3′-UTR acts similarly to overexpression of the coding region itself (4, 29, 30), which could be explained if the overexpressed 3′-UTR is titrating away a negative regulator.
Three different bioinformatic engines were used to predict potential miR binding sites on the 3′-UTR of the mouse Phb transcript: DIANA-microT v3.0, miRBase, and TargetScanMouse. All three engines predicted a high (85%–95%) probability of miR27 (miR27a and miR27b) binding to the 3′-UTR of Phb, and the TargetScanMouse engine revealed that the miR27 binding site (binding both miR27a and miR27b) is well conserved among species, suggesting its possible important function and a regulatory role (Figure 3A). To test the effect of miR27 on PHB levels, we overexpressed this miR in LβT2 cells, which led to a significant reduction in PHB protein levels (Figure 3B). Moreover, XTT assays performed after miR27 overexpression, with or without GnRH, revealed that miR27 overexpression attenuated the reduction in cell numbers in response to GnRH (Figure 3C). We reasoned that if this reduction is even partially caused by negative control of miR27b on PHB expression, overexpression of PHB should restore the apoptotic response. Indeed, in cells in which PHB was also overexpressed, the effect of GnRH was evident once more (Figure 3C).
Figure 3.
miR27 regulates PHB expression and is responsible for its diverse protein levels in the partially and fully differentiated gonadotrope cell lines. A, TargetScanMouse predicted miR27b binding to a sequence (boxed) in the Phb 3′-UTR, which is conserved across species. B, miR27 was overexpressed in LβT2 cells, and the effect on PHB expression was tested by Western blotting, with GAPDH as loading control. C, LβT2 cells were transfected with expression vectors for miR27 and/or Phb, and some of these cells were exposed to GnRH (100 nM, 24 hours); an XTT assay was then used to measure cell numbers. Data are means ± SEM; n = 4 to 6. The t test was used to assess the effect of GnRH treatment within each transfection group, and differences are presented as in Figure 2. D, RNA was extracted from LβT2 and αT3-1 cells for measurement of miR27b by real-time PCR; levels were normalized to those of U6. Data are means ± SEM; n = 4. **, P < .01.
We hypothesized that dissimilar miR27 expression levels might be responsible for the diverse levels of PHB expression in the αT3-1 and LβT2 cells, and so we compared the miR27 levels in these cells. The miR27 was seen to be expressed in αT3-1 cells at >3-fold the levels in LβT2 cells, suggesting that in these cells, miR27 probably reduces PHB protein expression levels by negatively regulating its translation (Figure 3D).
GnRH also regulates PHB nuclear export, which is essential for the apoptotic effect, and this involves ERK
A number of different functions have been ascribed to PHB (for reviews, see Refs. 14, 31, 32) and it is known to regulate expression of various genes by acting as a transcriptional corepressor (14, 15, 17, 33); however, its effects on apoptosis most likely occur in the cytoplasm and/or mitochondria. To understand further how GnRH regulates PHB activity, we looked to see whether its cellular localization is altered by GnRH. Cellular fractionation of αT3-1 and LβT2 cells treated with GnRH for 0.25 to 24 hours revealed that GnRH induces a rapid reduction in PHB levels in the nuclear fraction of the LβT2 cells, which does not occur in αT3-1 cells (Figure 4A). This drop in nuclear levels was concomitant with an increase in PHB levels in the cytoplasm (Figure 4A).
Figure 4.
GnRH also regulates PHB nuclear export, which is essential for the apoptotic effect, and this involves ERK. A, LβT2 and αT3-1 cells were exposed to GnRH for 0.25 to 24 hours, and samples separated into nuclear and cytoplasmic fractions for Western blot analysis for PHB, with actin as loading control. B, Indirect immunofluorescence was carried out in LβT2 cells treated with GnRH (100 nM, 6 hours), using a primary Ab to PHB and Alexa Fluor 568-goat antirabbit IgG; Hoechst 33342 was used for nuclei staining. Scale bar corresponds to 20 μm. C, LβT2 cells were treated with leptomycin (20 nM, 30 minutes before GnRH) and/or GnRH (100 nM, 24 hours) before Western blot analysis of cleaved PARP levels, with GAPDH as the loading control. D, caMEK was overexpressed, and the levels of nuclear PHB were measured, as well as the levels of total and phosphorylated ERK (ERK2 and pERK, respectively). E, dnMEK was overexpressed, and some of the cells treated with GnRH, before Western blot analysis on the same nuclear proteins, as before. F, Levels of cleaved PARP were measured after treatment with GnRH (100 nM, 24 hours) and/or the ERK inhibitor, U0126 (10 μM, 30 minutes before GnRH); actin served as the loading control.
To verify whether this drop in nuclear PHB levels is indeed due to its translocation, indirect immunofluorescence was performed, using a fluorophore-conjugated secondary Ab against a primary PHB Ab. In most of the untreated LβT2 cells, PHB was seen in both the nucleus and cytoplasm, whereas in 40% of these cells, PHB was found exclusively in the cytoplasm. After GnRH treatment, the number of cells containing PHB exclusively in the cytoplasm had risen to 65%, with the remainder of the cells showing PHB in both cellular compartments (n = 25–30) (Figure 4B), thus confirming that GnRH treatment induces PHB nuclear export. To assess whether the translocation out of the nucleus plays a role in the GnRH-induced apoptosis, the cells were treated with the nuclear export inhibitor, leptomycin, for 30 minutes before addition of GnRH for another 24 hours. The subsequent measurement of cleaved PARP indicated that nuclear export is required for the apoptotic response to GnRH (Figure 4C).
We hypothesized that GnRH-induced PHB nuclear export is probably driven by one of the activated kinases in the GnRH signaling pathways. We therefore transfected caMEK and measured changes in nuclear PHB levels. ERK1/2 activation caused a clear drop in nuclear PHB levels, which was concomitant with the phosphorylation of ERK1/2 (Figure 4D). Conversely, overexpression of dnMEK, with or without GnRH treatment, abated the GnRH effect on both PHB and pERK levels (Figure 4E). Finally, the requirement for ERK1/2 activation in GnRH-induced apoptosis was seen through use of an ERK1/2 inhibitor, U0126, which virtually abolished the GnRH-induced elevation in cleaved PARP (Figure 4F). Thus, GnRH-induced nuclear export of PHB, required for the ensuing apoptosis, is likely via ERK activation.
GnRH-induces PHB-dependent BAX localization to the mitochondria, while also increasing expression of proapoptotic factors BAX and HRK, which are required for the apoptotic effect
We reasoned that GnRH-induced apoptosis probably involves the mitochondrial import of the proapoptotic protein, BAX, which might be mediated by PHB. Initially we performed indirect immunofluorescence in LβT2 cells, some of which were treated with GnRH, using a fluorophore-conjugated secondary Ab against a primary Ab for BAX. After GnRH treatment, the BAX signal was stronger and also more concentrated in the mitochondria, indicating its translocation in response to GnRH-mediated signals (Figure 5A). A similar study was performed in cells in which PHB was knocked down (siPHB), and the localization of BAX was quantitated. In contrast to the control cells, GnRH-induced BAX localization to the mitochondria was not apparent in the siPHB cells, suggesting that BAX translocation to the mitochondria in response to GnRH requires PHB (Figure 5B).
Figure 5.
PHB is required for GnRH-induced BAX localization to the mitochondria, and GnRH induces BAX expression via JNK. A, Indirect immunofluorescence for BAX was performed in LβT2 cells, some of which were exposed to GnRH (100 nM, 6 hours). Mitochondria were stained using MitoTracker Green, and representative cells are shown. Scale bars correspond to 5 μm. B, GnRH-induced localization of BAX in the mitochondria was measured in control and PHB knockdown cells, and the relative levels of BAX (of total) in the mitochondria were quantitated. Data are means ± SEM; n = 6 to 18. Statistical analysis was performed as for Figure 2E. C, Western blot analysis was used to determine the effect of GnRH (100 nM, 12–48 hours) on levels of BAX in LβT2 cells, with GAPDH as the loading control. D, Cells were exposed to GnRH (100 nM, 6 hours) with and without the JNK inhibitor, SP600125 (added 30 minutes before GnRH), and levels of Bax mRNA were measured by qPCR. Data are presented as for Figure 2C; n = 4. Statistical analysis was performed as in for Figure 2E.
Notably, these studies also revealed a stronger BAX signal after the GnRH treatment compared with that in the untreated controls (Figure 5A). Accordingly, we set out to check whether GnRH stimulates an increase in BAX expression levels. Indeed, Western blot analysis showed that there was a clear increase in the BAX protein levels after 24 to 48 hours of GnRH treatment (Figure 5C). Given that we had already found that the JNK pathway is involved in the GnRH-induced apoptosis in these cells, we sought to check whether JNK also mediates the GnRH-induced increase in BAX expression. LβT2 cells were treated with the JNK inhibitor, SP600125, for 30 minutes, followed by GnRH treatment for 6 hours before RNA extraction and qPCR analysis. The levels of Bax mRNA were clearly increased by GnRH, and this increase was abolished by the SP600125, indicating that the effect occurs via a JNK-dependent pathway (Figure 5D).
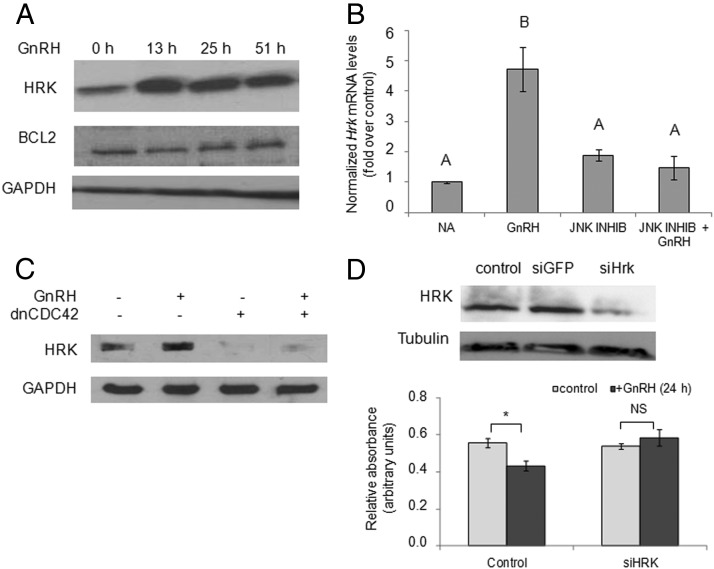
BAX is normally prevented from entering the mitochondria but upon apoptotic stimulus, BH3-only proteins are activated, and these release BAX from the BCL2 protein that held it in the cytoplasm. Given that one such BH3-only protein, HRK, is also reportedly regulated by JNK (34), we tested whether GnRH alters HRK expression and whether HRK is also involved in GnRH-induced apoptosis. After exposure of LβT2 cells to GnRH for 13 to 51 hours, HRK protein levels were markedly elevated, whereas levels of BCL2 appeared unaltered (Figure 6A), revealing a clear shift in the ratio of these pro- and antiapoptotic proteins. The stimulatory effect of GnRH on Hrk mRNA levels was confirmed by real-time PCR, and in cells preincubated with SP600125 before addition of GnRH, this GnRH effect was abolished, indicating that it indeed occurs via a JNK-dependent pathway (Figure 6B). This finding was further confirmed in cells transfected with a dnCDC42, which attenuated the GnRH elevation in HRK protein levels (Figure 6C).
Figure 6.
GnRH also induces expression of HRK, and this too plays a role in the GnRH-induced cell death. A, LβT2 cells were treated with GnRH (10 nM; 13–51 hours) before Western blot analysis of HRK protein; also shown is BCL2, and GAPDH served as the loading control. B, Cells were exposed to GnRH with and without the JNK inhibitor, SP600125, as in Figure 5D, and levels of Hrk mRNA were measured by qPCR. NA, no addition (control). Data are presented and analyzed as for Figure 5D; n = 4. C, LβT2 cells were treated with GnRH (10 nM, 15 hours) after overexpression of dnCDC42 in some of the cells. Western analysis for HRK was then performed as before. D, Cells were transfected with siRNA targeting HRK, and the effective knockdown is shown (upper panel). An XTT assay was then carried out in control and siHrk cells, with and without GnRH treatment (100 nM, 24 hours). Data are presented as for Figure 2E; n = 11 to 13. *, P < .05; NS, P > .05.
We went on to investigate whether HRK does indeed play a role in the GnRH-induced gonadotrope cell death. HRK expression was knocked down in LβT2 cells, using siRNA (siHrk) before GnRH treatment and the XTT assay. The reduction in HRK levels clearly abrogated the GnRH-activated decrease in cell number, suggesting that HRK does play a role in the apoptotic process in these mature gonadotrope-like cells (Figure 6D).
Gonadotrope-specific PHB-knockout mice show abnormal reproduction
Our findings using the cell lines clearly indicate a role for PHB in mediating the effects of GnRH on the size of the gonadotrope population. To verify that this regulation plays a physiological role in reproductive development in vivo, we generated mice in which PHB expression is knocked out specifically in the gonadotropes and assessed various reproductive parameters. We first evaluated the effect of the gonadotrope-specific PHB cKO on the gonadotrope population size, as indicated by levels of the gonadotrope-specific marker, Sf1. Sf1 mRNA levels are not affected by modification of PHB levels (data not shown), so any changes in levels should reflect alterations in the size of the gonadotrope population. The Sf1 mRNA levels in pituitaries of adult PHB cKO mice of both sexes were nearly 2-fold higher than those of control wild-type (WT) mice of the same strain (Figure 7A), indicating a larger gonadotrope population in the PHB cKO mice and supporting a role for PHB in limiting the size of this cell population. To assess their fertility, we crossed both sexes of these mice with WT control mice, and the PHB cKO mice litter size was consistently almost half that of the WT-crossed mice (Figure 7B), confirming that the fertility is adversely affected.
Figure 7.
PHB knockdown in the gonadotropes alters reproductive function. A, Pituitaries were excised from control and PHB cKO mice, and the mRNA levels of the gonadotropin-specific marker, Sf1, were measured by qPCR. Sf-1 mRNA levels are shown as fold change over the levels in WT control mice, after normalization to levels of RPLO. Data are means ± SEM; n = 5 to 8. *, P < .05. B, The mean number of offspring per litter of WT control and PHB cKO mice. Data are means ± SEM; n = 7 to 14. **, P < .01. C and D, LH (C) and FSH (D) circulating levels in adult WT control and PHB cKO mice. Data are means ± SEM; n = 12 to 13. *, P < .05; NS, P > .05. In A to D, values are combined for both sexes, because they shared similar values and trends. E and F, GSI of mature (E) and immature (F) WT control and PHB cKO mice. Data are means ± SEM; n = 4 to 8 or 6 to 10. The t test was used to compare values in PHB cKO vs WT control mice separately for each sex.
Circulating levels of LH and FSH were also measured in adult mice, but only the LH levels were altered, being elevated significantly in PHB cKO mice compared with levels in the WT mice (Figure 7, C and D). We expected that these differences would give rise to other adverse effects on gonadal development, so also measured the gonadal weights in these mice and found that the gonadosomatic index (GSI) (percentage of gonadal weight per body weight) was significantly higher in adult PHB cKO mice than in the WT mice in both sexes (Figure 7E). Interestingly, however, in immature mice the GSI values were significantly lower in the PHB cKO mice than in the WT controls (Figure 7F).
Histological analysis of the ovaries of adult mice (at 5 months) revealed the presence of numerous large atretic follicles in the PHB cKO mice, which were not seen in the ovaries of the age-matched WT control mice (Figure 8, A–C). These probably represent follicles that did not complete final maturation, possibly due to higher levels of circulating LH and would explain both the greater GSIs in these animals and possibly also the smaller litter sizes. The ovaries of immature (3 weeks old) PHB cKO mice, like those of WT controls, contained follicles at all stages of development, and we were not able to observe any major differences (Figure 8, D–F). In the males, there were no obvious differences between testes of the mature WT mice and those of the PHB cKO mice, with both groups showing seminiferous tubules with all stages of spermatogenesis (Figure 8, G–I). However, the testes of the immature mice did look different, with the lumens of the tubules of the PHB cKO mice appearing smaller than those in the WT controls, and many of them contained cellular masses (Figure 8, J–M).
Figure 8.
Gonadal histology of female and male PHB cKO mice. A–F, Ovarian sections from representative WT control (A and D) and PHB cKO mice (B, C, E, and F), stained with H&E. The upper panel shows the ovaries from mature (5 months old) mice, and the lower panel shows those from immature (3–4 weeks old) mice. A, atretic follicle; An, antral follicle; P, primary follicle; ZP, zona pellucida of atretic follicle; *, primordial follicle. Scale bars correspond to 400 μm. G–M, Testes sections from representative (G, J, and K) WT control and (H, I, L, and M) PHB cKO mice, stained with H&E. The upper panel shows testes from mature (2 months old) mice, and the lower panel shows those from immature (3–4 weeks old) mice. Scale bars correspond to 60 μm (G, H, I, K, and M) or 400 μm (J and L).
Discussion
We have demonstrated a central role for PHB in GnRH-induced apoptosis in the mature fully differentiated gonadotrope, in which it is expressed at higher levels than in immature cells and confirm that PHB expression levels determine the nature of the apoptotic response. We also show that PHB expression and activity are regulated by GnRH: its steady-state mRNA levels are elevated via the JNK pathway, whereas it is exported from the nucleus in response to GnRH-activated ERK (Figure 9).
Figure 9.
Working model of the role and regulation of PHB in GnRH-induced apoptosis in the mature gonadotrope. As described in the text, the working model shows that GnRH promotes apoptosis via JNK-mediated induction of expression of the three proapoptotic factors, PHB, BAX, and HRK, whereas PHB is exported from the nucleus specifically in response to activation of ERK1/2. The combination of increased BAX, as well as its HRK-mediated release from BCL2, allows BAX import into the mitochondria, which is facilitated by PHB through a mechanism that has yet to be fully elucidated. Inside the mitochondria, BAX activates release of cytochrome c (CYTC), which activates the cascade of proteolytic cleavages leading to apoptosis. PKC, protein kinase C; PLC, phospholipase C.
Even though PHB is already found at high levels in the cytosolic fraction, its nuclear export appears to be required for the apoptotic effect. The reason for this is not clear, but a similar observation was made in breast cancer cells, in which PHB is transported out of the nucleus in response to apoptotic stimuli (19, 35). It is possible that the modification of PHB that signals its exit, which includes exposing its nuclear export signal, might also direct its apoptotic function in the cytoplasm. We were not able to see PHB phosphorylation in response to GnRH, but PHB has been shown to be phosphorylated in other contexts, such as during activation of T cells, as well as in myoblasts and breast cancer cell lines in response to insulin stimulation (36, 37). The functional significance of PHB phosphorylation is not yet known, although in plants PHB phosphorylation was suggested to be involved in activation of cell death (38).
We considered that in the cytoplasm, the activated PHB might promote apoptosis by targeting the activity of the BCL2, which normally binds BAX to prevent its translocation to the mitochondria and the subsequent initiation of apoptosis. Upon apoptotic stimuli, various proteins, including the BH3-only proteins, neutralize the action of BCL2, thus allowing BAX to enter the mitochondria. Indeed, we found that GnRH induces BAX translocation to the mitochondria, and this was abated after PHB knockdown. The exact role of PHB in this process is not yet clear, but in prostate cancer cells, PHB was shown to interact directly with BCL2 during TGF-β-induced apoptosis (21), although in LβT2 cells, we could not detect any interaction between PHB and BCL2 (data not shown). Another possibility is that the interaction between BCL2 and BAX is disrupted by BH3-only family members such as HRK (39, 40). This appears likely because we saw that HRK also plays a major role in GnRH-induced cell death.
Expression of HRK and BAX is increased in response to the GnRH-activated JNK pathway. Several studies reported previously that BAX levels increase in response to GnRH analogs in endometrial epithelial cell culture, in corpora lutea, and in prostate cell lines (41–43). Moreover, JNK-activated pathways promote Hrk transcription in neurons after various apoptotic stimuli via an increase in the transcription factor, c-JUN (44–47). c-JUN is a direct target of JNK, and a number of studies have suggested that activation of the JNK pathway and subsequent phosphorylation of c-JUN may be a fundamental step in the induction of neuronal apoptosis (48–50). GnRH markedly up-regulates c-JUN in the gonadotrope, indicating that a similar pathway is probably involved in HRK activation in these cells (51, 52). BH3-only proteins such as HRK are stringently regulated at the transcriptional and posttranslational levels during apoptosis, and induction of apoptosis is frequently caused by the change in the ratio of the proapoptotic and antiapoptotic proteins (39, 53). The fact that proapoptotic HRK is being up-regulated by GnRH while the antiapoptotic BCL2 levels remained unaltered suggests that the shift in the ratio of these two proteins in favor of HRK promotes apoptosis.
In contrast with its proapoptotic effects in fully differentiated cells, early in pituitary development GnRH is involved in establishing the gonadotrope population of cells and stimulates their proliferation (4, 5). This presumably requires a mechanism to repress activation of PHB expression. We show here that miR27 probably fulfills this role, as its levels in immature gonadotropes are considerably higher than in mature gonadotropes, and its overexpression reduced both PHB protein levels and the GnRH-induced reduction in cell number, indicating its ability to regulate PHB levels in the gonadotrope and thus prevent apoptosis. Moreover, miR27 was recently suggested to target PHB in prostate cancer cells and in gastric adenocarcinoma (54, 55). Thus, PHB activity may be tightly regulated by miR27, allowing GnRH-induced apoptosis to occur only in mature gonadotropes, in which this negative regulation is relieved. Such a mechanism of regulating PHB expression and activity is in accordance with previous reports for a regulatory role of its 3′-UTR (4, 29, 30).
Verification of the physiological importance of PHB in governing the gonadotrope population size was confirmed by examining the effects of gonadotrope-specific PHB cKO on reproductive function. Pituitaries of PHB cKO mice express higher levels of Sf1, indicating that their gonadotrope population is larger, whereas their fertility was clearly reduced, with both sexes siring smaller litter sizes. Moreover, the PHB cKO mice also had higher levels of circulating LH than the controls, and, accordingly, the gonad development appeared also to be affected, although the trend was very different between immature and mature animals.
In the ovary, LH promotes steroid synthesis and growth of small antral follicles to the preovulatory stage, presumably explaining the apparent increases in the numbers of large and large atretic follicles in the adult PHB cKO mice. Moreover, chronic elevation of LH is often associated with abnormal maturation of follicles and a lack of ovulation, characterized by depletion in primordial follicles, which were not seen in any of the adult PHB cKO ovaries, as well as underdeveloped granulosa cells and hyperplastic luteinized theca cells (56–60). The increased LH levels in the PHB cKO mice are thus probably responsible for abnormal follicle development, which would explain the reduced fertility of these mice.
In the immature mice, in which the gonads are generally less gonadotropin-dependent than in the adult mice, the lower ovarian weights in the PHB cKO mice most likely also reflect abnormal regulation by LH, probably during the development of primary or secondary follicles, although there was no evidence of depletion of the primordial follicles. A previous study showed that induced activation of the LH receptor in transgenic female (YHR+) mice led to abnormal folliculogenesis, such that at 5 weeks, the mice already contained a large number of developed follicles as well as corpora lutea; however, the adults, as in our study, were subfertile (60).
In males, LH is required for spermatogenesis and steroidogenesis, but the likely effects of elevated LH levels on male fertility are less clear than for female fertility. In various LH-overexpressing mouse models, stronger LH signaling was associated with more severe consequences on fertility, size of testes, seminal vesicles and prostate, and also pubertal maturation (58–63). In YHR+ mice, the induced activation of the LH receptor produced lower testes weights in mice aged 3 to 12 weeks, which was most pronounced at 5 weeks and significantly less so by 12 weeks; the differences were mostly due to a reduction in tubule size, without other notable histological differences (61). This appears similar to the changes in testes size and histology in the PHB cKO mice in this study, some of which might stem from increased androgen synthesis.
The reason for the lack of significant changes in circulating FSH levels in the PHB cKO mice could be the strong negative feedback by inhibin, which plays a dominant role in repressing FSH levels, while barely affecting LH levels (64). Both hormones might also be subject to increased estrogen-negative feedback acting on the hypothalamic GnRH. However, in the pituitary, the lack of PHB would also relieve its effect as a corepressor of estrogen receptor-α, which regulates the α and LHβ, but not the FSHβ subunit genes (J. Feng and P. Melamed, unpublished data and Ref. 65). Hence, it is quite possible that in PHB cKO mice, LH synthesis and secretion are also increased in response to estrogen signaling, whereas FSH is not affected in this way.
We have shown that PHB plays an important role in the normal function of the reproductive axis in vivo. It is, however, feasible that PHB cKO in extrapituitary tissues also contributes to the reduced fertility of these mice, because GnRHR is expressed at low levels in other tissues, including the testes and, to a lesser degree, the ovaries (2, 9). Moreover, PHB is also expressed in the seminiferous tubules and ovarian follicles and was implicated in the modulation of response by granulosa cells to FSH (66–68).
The question remains as to why there exists a mechanism that allows for GnRH-induced apoptosis in the gonadotropes. During early embryonic development, this population of cells must expand to reach its final size, and GnRH clearly plays a role through stimulating cell proliferation and blocking apoptosis (1, 2, 4, 5). However, later in development when the gonadotropes are fully differentiated, their population size remains relatively constant. Because the mature cells continue to be exposed to GnRH, an opposing mechanism is required to counterbalance the proliferation induced by GnRH. We have demonstrated in this study that one such mechanism is the activation of apoptosis, which is mediated, at least in part, by PHB, whose expression in immature cells is restrained by miR27.
Acknowledgments
This work was supported by the Technion V.P.R. Fund-Mallat Family Research Fund, J. and A. Taub Biological Research Fund, and The Russell Berrie Nanotechnology Institute, Technion.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by the Technion V.P.R. Fund-Mallat Family Research Fund, J. and A. Taub Biological Research Fund, and The Russell Berrie Nanotechnology Institute, Technion.
Footnotes
- Ab
- antibody
- ca
- constitutively active
- cKO
- conditional knockout
- dn
- dominant negative
- GSI
- gonadosomatic index
- H&E
- hematoxylin and eosin
- JNK
- c-Jun NH2-terminal kinase
- MEK
- MAPK kinase
- miR
- microRNA
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
- PARP
- poly(ADP-ribose) polymerase
- PHB
- prohibitin
- qPCR
- quantitative PCR
- si
- small interfering
- RT
- room temperature
- UTR
- untranslated region
- WT
- wild-type
- XTT
- 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
- YFP
- yellow fluorescent protein.
References
- 1. McDowell IF, Morris JF, Charlton HM, Fink G. Effects of luteinizing hormone releasing hormone on the gonadotrophs of hypogonadal (hpg) mice. J Endocrinol. 1982;95:331–340. [DOI] [PubMed] [Google Scholar]
- 2. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci USA. 2010;107:16372–16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grumbach MM, Styne D. Puberty: ontogeny, neuroendocrinology, physiology, and disorders. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, eds. Williams Textbook of Endocrinology. 10th ed Philadelphia, PA: Saunders; 2003:1115–1286. [Google Scholar]
- 4. Feng J, Lawson MA, Melamed P. A proteomic comparison of immature and mature mouse gonadotrophs reveals novel differentially expressed nuclear proteins that regulate gonadotropin gene transcription and RNA splicing. Biol Reprod. 2008;79:546–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Melamed P, Savulescu D, Lim S, et al. Gonadotrophin-releasing hormone signalling downstream of calmodulin. J Neuroendocrinol. 2012;24:1463–1475. [DOI] [PubMed] [Google Scholar]
- 6. Childs GV, Unabia G. Epidermal growth factor and gonadotropin-releasing hormone stimulate proliferation of enriched population of gonadotropes. Endocrinology. 2001;142:847–853. [DOI] [PubMed] [Google Scholar]
- 7. Miles LE, Hanyaloglu AC, Dromey JR, Pfleger KD, Eidne KA. Gonadotropin-releasing hormone receptor-mediated growth suppression of immortalized LβT2 gonadotrope and stable HEK293 cell lines. Endocrinology. 2004;145:194–204. [DOI] [PubMed] [Google Scholar]
- 8. Kraus S, Naor Z, Seger R. Gonadotropin-releasing hormone in apoptosis of prostate cancer cells. Cancer Lett. 2006;234:109–123. [DOI] [PubMed] [Google Scholar]
- 9. Cheung LW, Wong AS. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 2008;275:5479–5495. [DOI] [PubMed] [Google Scholar]
- 10. So WK, Cheng JC, Poon SL, Leung PC. Gonadotropin-releasing hormone and ovarian cancer: a functional and mechanistic overview. FEBS J. 2008;275:5496–5511. [DOI] [PubMed] [Google Scholar]
- 11. White CD, Stewart AJ, Lu ZL, Millar RP, Morgan K. Antiproliferative effects of GnRH agonists: prospects and problems for cancer therapy. Neuroendocrinology. 2008;88:67–79. [DOI] [PubMed] [Google Scholar]
- 12. Park MK, Kanaho Y, Enomoto M. Regulation of the cell proliferation and migration as extra-pituitary functions of GnRH. Gen Comp Endocrinol. 2013;181:259–264. [DOI] [PubMed] [Google Scholar]
- 13. Mishra S, Murphy LC, Murphy LJ. The prohibitins: emerging roles in diverse functions. J Cell Mol Med. 2006;10:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thuaud F, Ribeiro N, Nebigil CG, Désaubry L. Prohibitin ligands in cell death and survival: mode of action and therapeutic potential. Chem Biol. 2013;20:316–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rastogi S, Joshi B, Dasgupta P, Morris M, Wright K, Chellappan S. Prohibitin facilitates cellular senescence by recruiting specific corepressors to inhibit E2F target genes. Mol Cell Biol. 2006;26:4161–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi D, Lee SJ, Hong S, Kim IH, Kang S. Prohibitin interacts with RNF2 and regulates E2F1 function via dual pathways. Oncogene. 2008;27:1716–1725. [DOI] [PubMed] [Google Scholar]
- 17. Wang S, Fusaro G, Padmanabhan J, Chellappan SP. Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene. 2002;21:8388–8396. [DOI] [PubMed] [Google Scholar]
- 18. Wang S, Nath N, Adlam M, Chellappan S. Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene. 1999;18:3501–3510. [DOI] [PubMed] [Google Scholar]
- 19. Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem. 2003;278:47853–47861. [DOI] [PubMed] [Google Scholar]
- 20. Joshi B, Ko D, Ordonez-Ercan D, Chellappan SP. A putative coiled-coil domain of prohibitin is sufficient to repress E2F1-mediated transcription and induce apoptosis. Biochem Biophys Res Commun. 2003;312:459–466. [DOI] [PubMed] [Google Scholar]
- 21. Zhu B, Fukada K, Zhu H, Kyprianou N. Prohibitin and cofilin are intracellular effectors of transforming growth factor beta signaling in human prostate cancer cells. Cancer Res. 2006;66:8640–8647. [DOI] [PubMed] [Google Scholar]
- 22. Pnueli L, Luo M, Wang S, Naor Z, Melamed P. Calcineurin mediates the gonadotropin-releasing hormone effect on expression of both subunits of the follicle-stimulating hormone through distinct mechanisms. Mol Cell Biol. 2011;31:5023–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim S, Luo M, Koh M, et al. Distinct mechanisms involving diverse histone deacetylases repress expression of the two gonadotropin beta-subunit genes in immature gonadotropes, and their actions are overcome by gonadotropin-releasing hormone. Mol Cell Biol. 2007;27:4105–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wen S, Schwarz JR, Niculescu D, et al. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149:2701–2711. [DOI] [PubMed] [Google Scholar]
- 25. Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoivik EA, Bjanesoy TE, Mai O, et al. DNA methylation of intronic enhancers directs tissue-specific expression of steroidogenic factor 1/adrenal 4 binding protein (SF-1/Ad4BP). Endocrinology. 2011;152:2100–2112. [DOI] [PubMed] [Google Scholar]
- 27. He B, Kim TH, Kommagani R, et al. Estrogen-regulated prohibitin is required for mouse uterine development and adult function. Endocrinology. 2011;152:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30:10–29. [DOI] [PubMed] [Google Scholar]
- 29. Manjeshwar S, Branam DE, Lerner MR, Brackett DJ, Jupe ER. Tumor suppression by the prohibitin gene 3′ untranslated region RNA in human breast cancer. Cancer Res. 2003;63:5251–5256. [PubMed] [Google Scholar]
- 30. Manjeshwar S, Lerner MR, Zang XP, et al. Expression of prohibitin 3′ untranslated region suppressor RNA alters morphology and inhibits motility of breast cancer cells. J Mol Histol. 2004;35:639–646. [DOI] [PubMed] [Google Scholar]
- 31. Wang S, Faller DV. Roles of prohibitin in growth control and tumor suppression in human cancers. Transl Oncogenomics. 2008;3:23–37. [PMC free article] [PubMed] [Google Scholar]
- 32. Mishra S, Ande SR, Nyomba BL. The role of prohibitin in cell signaling. FEBS J. 2010;277:3937–3946. [DOI] [PubMed] [Google Scholar]
- 33. He B, Feng Q, Mukherjee A, et al. A repressive role for prohibitin in estrogen signaling. Mol Endocrinol. 2008;22:344–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guan QH, Pei DS, Xu TL, Zhang GY. Brain ischemia/reperfusion-induced expression of DP5 and its interaction with Bcl-2, thus freeing Bax from Bcl-2/Bax dimmers are mediated by c-Jun N-terminal kinase (JNK) pathway. Neurosci Lett. 2006;393:226–230. [DOI] [PubMed] [Google Scholar]
- 35. Rastogi S, Joshi B, Fusaro G, Chellappan S. Camptothecin induces nuclear export of prohibitin preferentially in transformed cells through a CRM-1-dependent mechanism. J Biol Chem. 2006;281:2951–2959. [DOI] [PubMed] [Google Scholar]
- 36. Ross JA, Nagy ZS, Kirken RA. The PHB1/2 phosphocomplex is required for mitochondrial homeostasis and survival of human T cells. J Biol Chem. 2008;283:4699–4713. [DOI] [PubMed] [Google Scholar]
- 37. Ande SR, Gu Y, Nyomba BL, Mishra S. Insulin induced phosphorylation of prohibitin at tyrosine 114 recruits Shp1. Biochim Biophys Acta. 2009;1793:1372–1378. [DOI] [PubMed] [Google Scholar]
- 38. Takahashi A, Kawasaki T, Wong HL, Suharsono U, Hirano H, Shimamoto K. Hyperphosphorylation of a mitochondrial protein, prohibitin, is induced by calyculin A in a rice lesion-mimic mutant cdr1. Plant Physiol. 2003;132:1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inohara N, Ding L, Chen S, Núñez G. harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-XL. EMBO J. 1997;16:1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Youle RJ. Cell biology. Cellular demolition and the rules of engagement. Science. 2007;315:776–777. [DOI] [PubMed] [Google Scholar]
- 41. Angelucci C, Iacopino F, Lama G, et al. Apoptosis-related gene expression affected by a GnRH analogue without induction of programmed cell death in LNCaP cells. Anticancer Res. 2004;24:2729–2738. [PubMed] [Google Scholar]
- 42. Endo T, Kiya T, Kitajima Y, et al. Identical changes in Bax expression, but not Fas ligand expression, occur in structural luteolysis in gonadotropin releasing hormone agonist- and prolactin-treated superovulated rats. Life Sci. 2005;76:2159–2169. [DOI] [PubMed] [Google Scholar]
- 43. Bilotas M, Barañao RI, Buquet R, Sueldo C, Tesone M, Meresman G. Effect of GnRH analogues on apoptosis and expression of Bcl-2, Bax, Fas and FasL proteins in endometrial epithelial cell cultures from patients with endometriosis and controls. Hum Reprod. 2007;22:644–653. [DOI] [PubMed] [Google Scholar]
- 44. Harris CA, Johnson EM Jr. BH3-only Bcl-2 family members are coordinately regulated by the JNK pathway and require Bax to induce apoptosis in neurons. J Biol Chem. 2001;276:37754–37760. [DOI] [PubMed] [Google Scholar]
- 45. Yin KJ, Kim GM, Lee JM, He YY, Xu J, Hsu CY. JNK activation contributes to DP5 induction and apoptosis following traumatic spinal cord injury. Neurobiol Dis. 2005;20:881–889. [DOI] [PubMed] [Google Scholar]
- 46. Chen S, Lee JM, Zeng C, Chen H, Hsu CY, Xu J. Amyloid beta peptide increases DP5 expression via activation of neutral sphingomyelinase and JNK in oligodendrocytes. J Neurochem. 2006;97:631–640. [DOI] [PubMed] [Google Scholar]
- 47. Ma C, Ying C, Yuan Z, et al. dp5/HRK is a c-Jun target gene and required for apoptosis induced by potassium deprivation in cerebellar granule neurons. J Biol Chem. 2007;282:30901–30909. [DOI] [PubMed] [Google Scholar]
- 48. Watson A, Eilers A, Lallemand D, Kyriakis J, Rubin LL, Ham J. Phosphorylation of c-Jun is necessary for apoptosis induced by survival signal withdrawal in cerebellar granule neurons. J Neurosci. 1998;18:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ham J, Eilers A, Whitfield J, Neame SJ, Shah B. c-Jun and the transcriptional control of neuronal apoptosis. Biochem Pharmacol. 2000;60:1015–1021. [DOI] [PubMed] [Google Scholar]
- 50. Young JE, Garden GA, Martinez RA, et al. Polyglutamine-expanded androgen receptor truncation fragments activate a Bax-dependent apoptotic cascade mediated by DP5/Hrk. J Neurosci. 2009;29:1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Melamed P, Zhu Y, Tan SH, Xie M, Koh M. Gonadotropin-releasing hormone activation of c-jun, but not early growth response factor-1, stimulates transcription of a luteinizing hormone beta-subunit gene. Endocrinology. 2006;147:3598–3605. [DOI] [PubMed] [Google Scholar]
- 52. Binder AK, Grammer JC, Herndon MK, Stanton JD, Nilson JH. GnRH regulation of Jun and Atf3 requires calcium, calcineurin, and NFAT. Mol Endocrinol. 2012;26:873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gross A, McDonnell JM, Korsmeyer SJ. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. [DOI] [PubMed] [Google Scholar]
- 54. Liu T, Tang H, Lang Y, Liu M, Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 2009;273:233–242. [DOI] [PubMed] [Google Scholar]
- 55. Fletcher CE, Dart DA, Sita-Lumsden A, Cheng H, Rennie PS, Bevan CL. Androgen-regulated processing of the oncomir miR-27a, which targets prohibitin in prostate cancer. Hum Mol Genet. 2012;21:3112–3127. [DOI] [PubMed] [Google Scholar]
- 56. Bulun SE, Adashi EY. The physiology and pathology of the female reproductive axis. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, eds. Williams Textbook of Endocrinology. 10th ed Philadelphia, PA: Saunders; 2003:587–665. [Google Scholar]
- 57. Mann RJ, Keri RA, Nilson JH. Consequences of elevated luteinizing hormone on diverse physiological systems: use of the LHβCTP transgenic mouse as a model of ovarian hyperstimulation-induced pathophysiology. Recent Prog Horm Res. 2003;58:343–375. [DOI] [PubMed] [Google Scholar]
- 58. Peltoketo H, Zhang FP, Rulli SB. Animal models for aberrations of gonadotropin action. Rev Endocr Metab Disord. 2011;12:245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Risma KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci USA. 1995;92:1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Flaws JA, Abbud R, Mann RJ, Nilson JH, Hirshfield AN. Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biol Reprod. 1997;57:1233–1237. [DOI] [PubMed] [Google Scholar]
- 61. Meehan TP, Harmon BG, Overcast ME, et al. Gonadal defects and hormonal alterations in transgenic mice expressing a single chain human chorionic gonadotropin-lutropin receptor complex. J Mol Endocrinol. 2005;34:489–503. [DOI] [PubMed] [Google Scholar]
- 62. Rulli SB, Ahtiainen P, Mäkelä S, Toppari J, Poutanen M, Huhtaniemi I. Elevated steroidogenesis, defective reproductive organs, and infertility in transgenic male mice overexpressing human chorionic gonadotropin. Endocrinology. 2003;144:4980–4990. [DOI] [PubMed] [Google Scholar]
- 63. Ahtiainen P, Rulli SB, Shariatmadari R, et al. Fetal but not adult Leydig cells are susceptible to adenoma formation in response to persistently high hCG level: a study on hCG overexpressing transgenic mice. Oncogene. 2005;24:7301–7309. [DOI] [PubMed] [Google Scholar]
- 64. Winters SJ, Moore JP. Intra-pituitary regulation of gonadotrophs in male rodents and primates. Reproduction. 2004;128:13–23. [DOI] [PubMed] [Google Scholar]
- 65. Luo M, Koh M, Feng J, Wu Q, Melamed P. Cross talk in hormonally regulated gene transcription through induction of estrogen receptor ubiquitylation. Mol Cell Biol. 2005;25:7386–7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Choongkittaworn NM, Kim KH, Danner DB, Griswold MD. Expression of prohibitin in rat seminiferous epithelium. Biol Reprod. 1993;49:300–310. [DOI] [PubMed] [Google Scholar]
- 67. Thompson WE, Ramalho-Santos J, Sutovsky P. Ubiquitination of prohibitin in mammalian sperm mitochondria: possible roles in the regulation of mitochondrial inheritance and sperm quality control. Biol Reprod. 2003;69:254–260. [DOI] [PubMed] [Google Scholar]
- 68. Chowdhury I, Garcia-Barrio M, Harp D, Thomas K, Matthews R, Thompson WE. The emerging roles of prohibitins in folliculogenesis. Front Biosci (Elite Ed). 2012;4:690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]