Abstract
The relationship between ethylene action and metabolism was investigated in the etiolated pea seedling (Pisum sativum L. cv. Alaska) by inhibiting ethylene action with Ag+, high CO2, and low O2 and then determining if ethylene metabolism was inhibited in a similar manner. Ag+ (100 milligrams per liter) was clearly the most potent antiethylene treatment. Ag+ pretreatment inhibited the growth retarding action of 0.2 microliters per liter ethylene by 48% and it also inhibited the incorporation of 0.2 microliters per liter 14C2H4 into pea tips by the same amount. As the ethylene concentration was increased from 0.2 to 30 microliters per liter, the effectiveness of Ag+ in reducing ethylene action and metabolism declined in a similar fashion. Although Ag+ significantly inhibited the incorporation of 14C2H4 into tissue metabolites, the oxidation of 14C2H4 to 14CO2 was unaffected in the same tissue.
CO2 (7%) inhibited ethylene-induced growth retardation but its effectiveness diminished at a greater rate than that of Ag+ with increasing ethylene concentration. High CO2 had just the opposite effect of Ag+ since it inhibited 14C2H4 oxidation to 14CO2 without affecting tissue incorporation. In contrast to Ag+, CO2 did not inhibit ethylene action and metabolism to exactly the same extent, and the inhibition of metabolism did not rapidly decline with increasing 14C2H4 concentration. However, high CO2 did alter the ratio of 14C2H4 tissue incorporation to 14CO2 production in a manner consistent with changes in ethylene effectiveness.
Lowering the O2 concentration to 5% reduced ethylene-induced growth retardation from 70 to 58% at 0.22 microliters per liter and inhibited 14C2H4 (0.25 microliters per liter) tissue incorporation and oxidation to 14CO2 by 26 and 45%, respectively. However, in contrast to Ag+ and high CO2 which slightly promoted growth in ethylene-free air, low O2 reduced pea seedling growth under these conditions thereby severely limiting its usefulness as a specific antiethylene treatment.
Collectively these data suggest that the metabolism of ethylene may be related to its action.
Full text
PDF
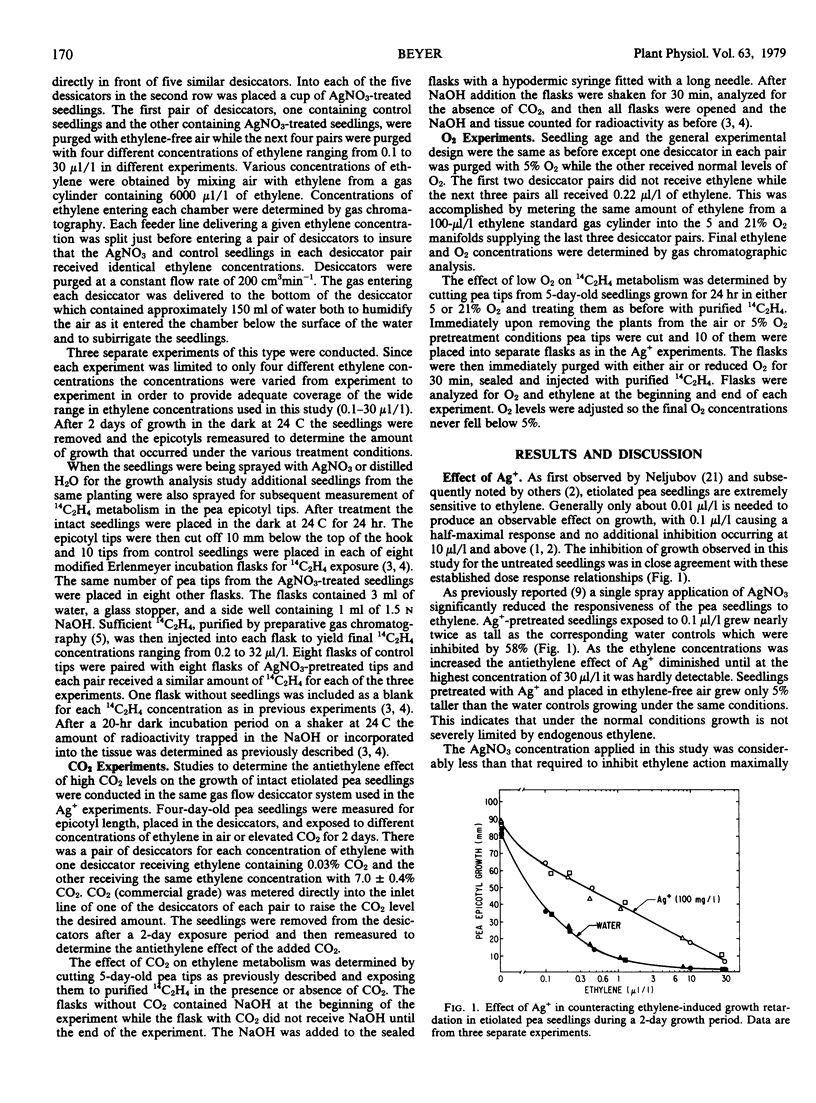
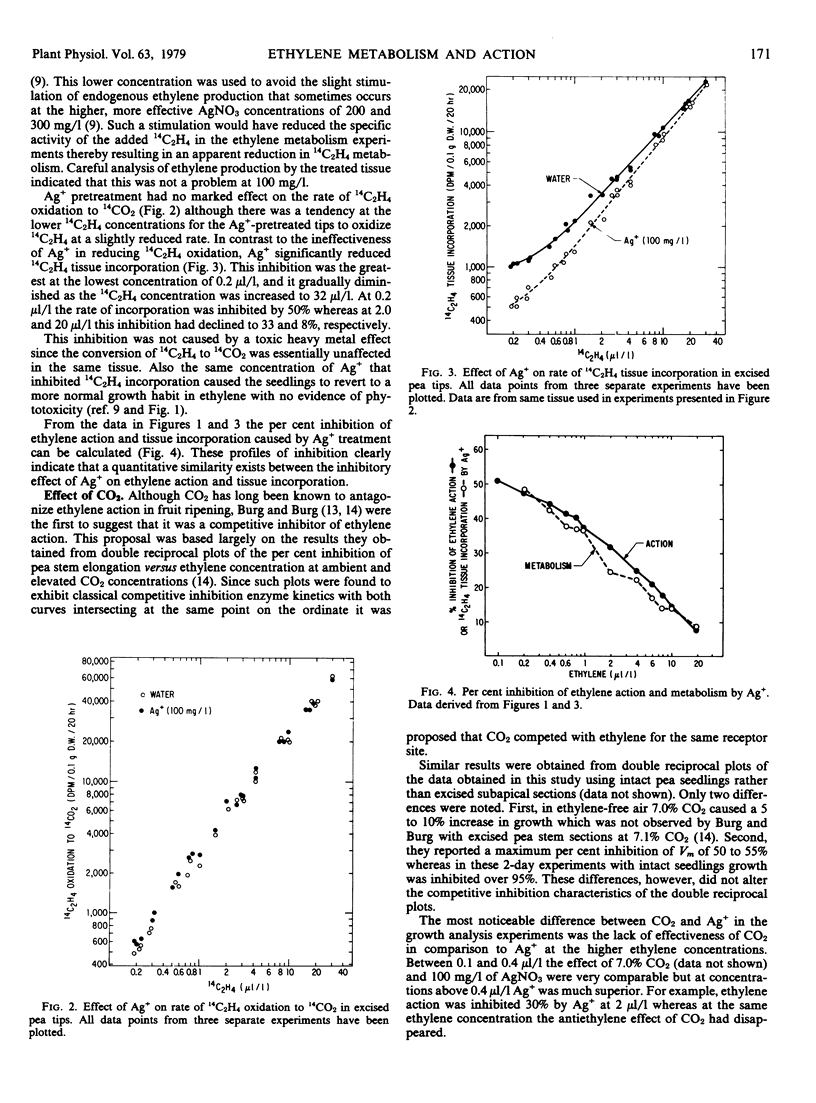
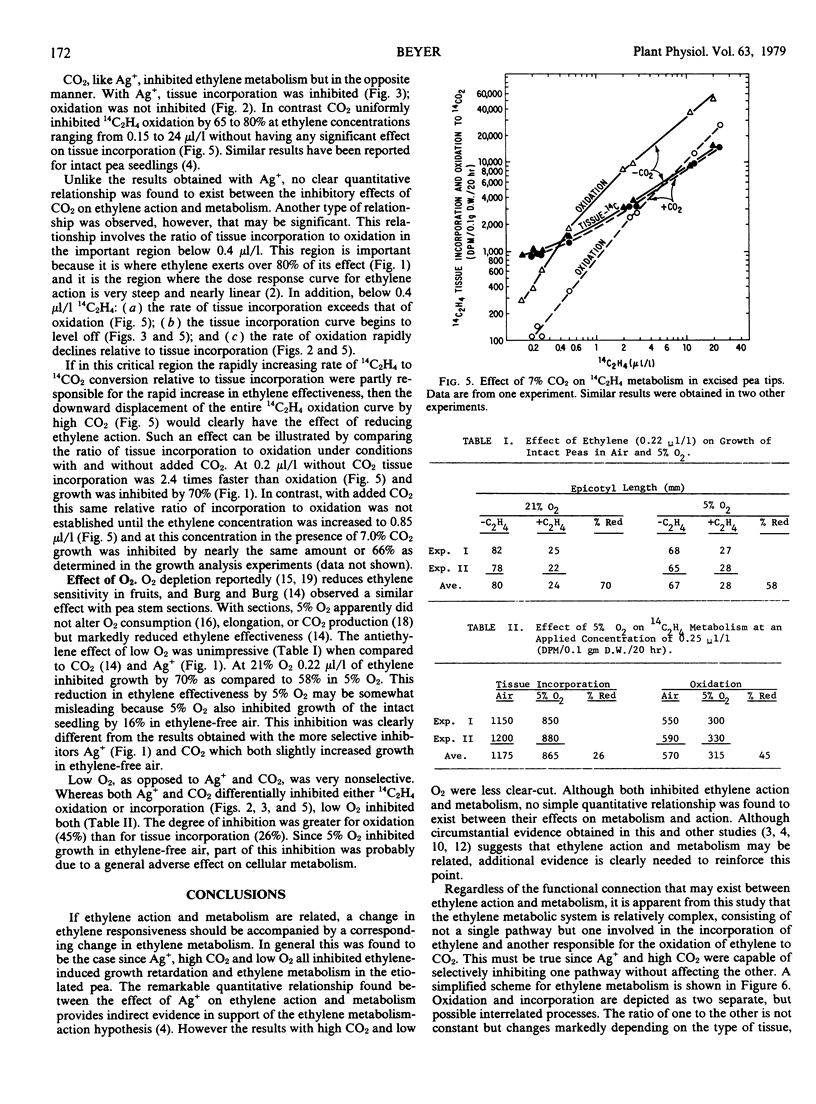
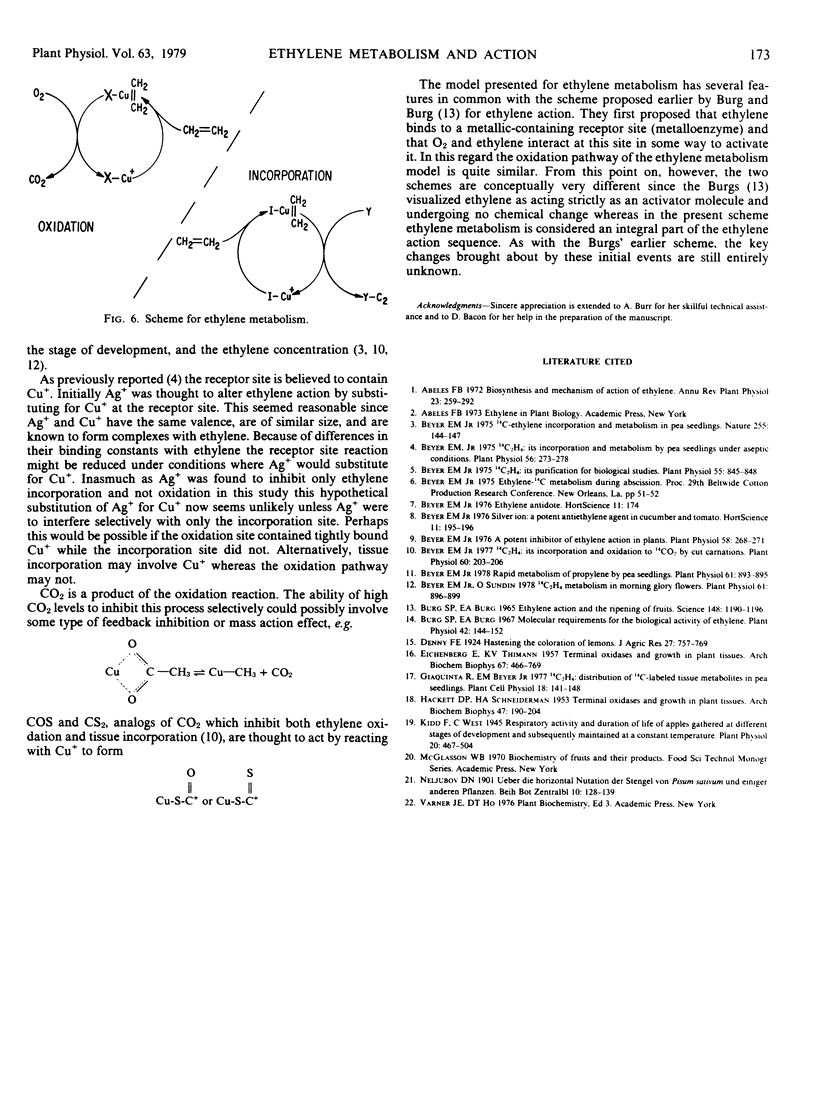
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURG S. P., BURG E. A. ETHYLENE ACTION AND THE RIPENING OF FRUITS. Science. 1965 May 28;148(3674):1190–1196. doi: 10.1126/science.148.3674.1190. [DOI] [PubMed] [Google Scholar]
- Beyer E. M. A potent inhibitor of ethylene action in plants. Plant Physiol. 1976 Sep;58(3):268–271. doi: 10.1104/pp.58.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. C(2)H(4): Its Incorporation and Metabolism by Pea Seedlings under Aseptic Conditions. Plant Physiol. 1975 Aug;56(2):273–278. doi: 10.1104/pp.56.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. C(2)H(4): Its Incorporation and Oxidation to CO(2) by Cut Carnations. Plant Physiol. 1977 Aug;60(2):203–206. doi: 10.1104/pp.60.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. C(2)H(4): its purification for biological studies. Plant Physiol. 1975 May;55(5):845–848. doi: 10.1104/pp.55.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Rapid metabolism of propylene by pea seedlings. Plant Physiol. 1978 Jun;61(6):893–895. doi: 10.1104/pp.61.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Sundin O. C(2)H(4) metabolism in morning glory flowers. Plant Physiol. 1978 Jun;61(6):896–899. doi: 10.1104/pp.61.6.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICHENBERGER E., THIMANN K. V. Terminal oxidases and growth in plant tissues. IV. On the terminal oxidases of etiolated pea internodes. Arch Biochem Biophys. 1957 Apr;67(2):466–478. doi: 10.1016/0003-9861(57)90301-6. [DOI] [PubMed] [Google Scholar]
- HACKETT D. P., SCHNEIDERMAN H. A. Terminal oxidases and growth in plant tissues. I. The terminal oxidase mediating growth of Avena coleoptile and Pisum stem sections. Arch Biochem Biophys. 1953 Nov;47(1):190–204. doi: 10.1016/0003-9861(53)90448-2. [DOI] [PubMed] [Google Scholar]
- Kidd F., West C. RESPIRATORY ACTIVITY AND DURATION OF LIFE OF APPLES GATHERED AT DIFFERENT STAGES OF DEVELOPMENT AND SUBSEQUENTLY MAINTAINED AT A CONSTANT TEMPERATURE. Plant Physiol. 1945 Oct;20(4):467–504. doi: 10.1104/pp.20.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]


