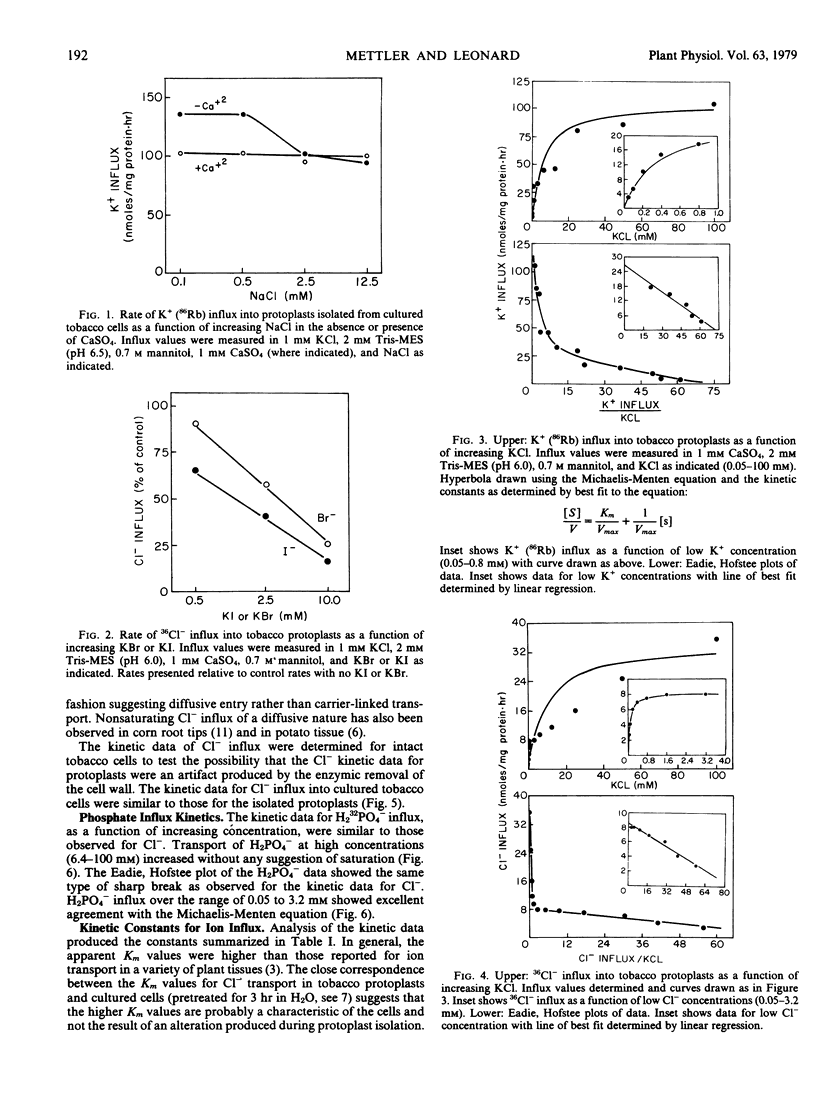
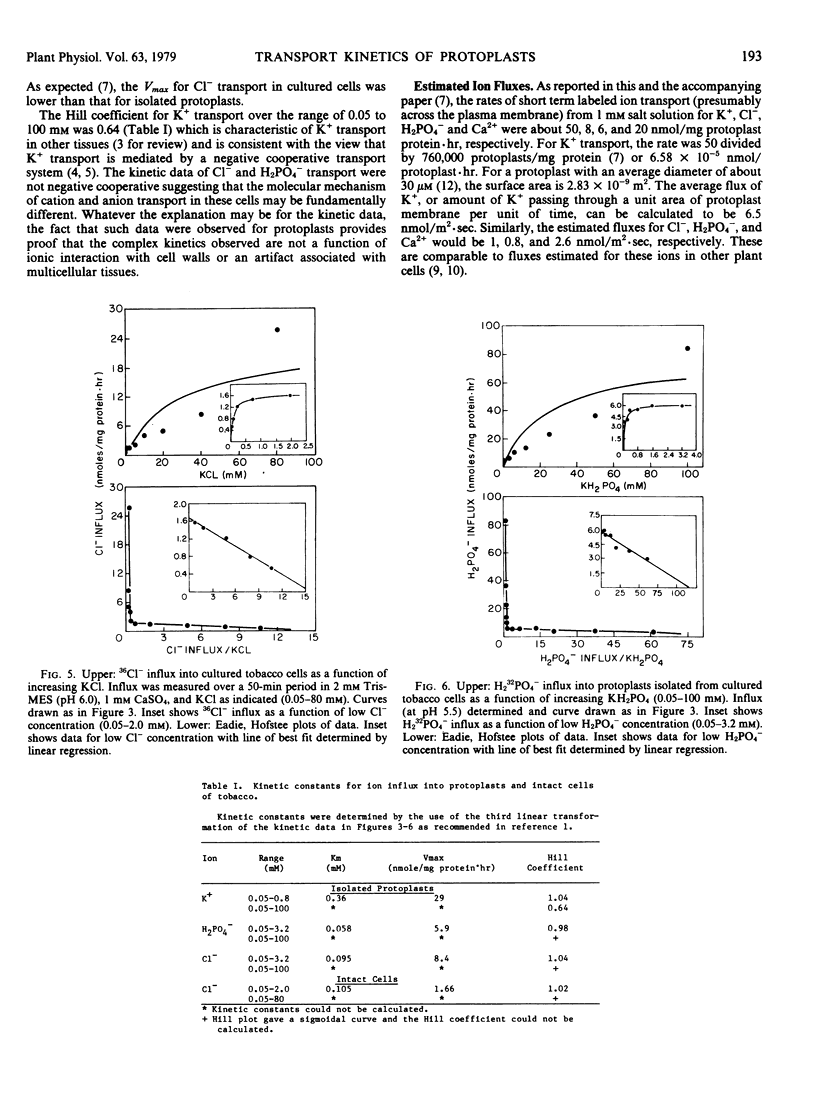
Abstract
Protoplasts were enzymically isolated from suspension cultured cells of Nicotiana glutinosa L. and aspects of transport selectivity and kinetics were studied. In the presence of Ca2+, transport was selective for K+ (86Rb) over Na+. 36Cl− transport was inhibited by Br− or I− but not by H2PO4−. The kinetic data for short term (30 minutes) K+ influx over the range of 0.05 to 100 millimolar KCl were complex but similar to those observed in other plant tissues. In contrast, the kinetic data for Cl− and H232PO4− over the same concentration range were different from those observed for K+, and could be accounted for by a single isotherm in the range of 0.05 to 4 millimolar and by an almost linear increase in influx rate above 4 millimolar. The kinetic data for Cl− transport into intact cultured cells were identical in character to those observed for isolated protoplasts. The results support the view that enzymic removal of the cell wall produced no significant alteration in the transport properties of the protoplast.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Ion transport in isolated protoplasts from tobacco suspension cells: I. General characteristics. Plant Physiol. 1979 Jan;63(1):183–190. doi: 10.1104/pp.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce W. S., Higinbotham N. Compartments and Fluxes of K, NA, and CL in Avena Coleoptile Cells. Plant Physiol. 1970 Nov;46(5):666–673. doi: 10.1104/pp.46.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii K., Laties G. G. Dual mechanisms of ion uptake in relation to vacuolation in corn roots. Plant Physiol. 1966 May;41(5):863–870. doi: 10.1104/pp.41.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimiya H., Murashige T. Evaluation of parameters in the isolation of viable protoplasts from cultured tobacco cells. Plant Physiol. 1974 Dec;54(6):936–944. doi: 10.1104/pp.54.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]


