Abstract
The biogenic amine tyramine (TA) regulates many aspects of invertebrate physiology and development. Although three TA receptor subtypes have been identified (TAR1-3), specific receptors have not been linked to physiological responses in native tissue. In the Malpighian (renal) tubule of Drosophila melanogaster, TA activates a transepithelial chloride conductance, resulting in diuresis and depolarization of the transepithelial potential. In the current work, mutation or RNAi-mediated knockdown in the stellate cells of the tubule of TAR2 (tyrR, CG7431) resulted in a dramatic reduction, but not elimination, of the TA-mediated depolarization. Mutation or knockdown of TAR3 (tyrRII, CG16766) had no effect. However, deletion of both genes, or knockdown of TAR3 on a TAR2 mutant background, eliminated the TA responses. Thus while TAR2 is responsible for the majority of the TA sensitivity of the tubule, TAR3 also contributes to the response. Knockdown or mutation of TAR2 also eliminated the response of tubules to the related amine octopamine (OA), indicating that OA can activate TAR2. This finding contrasts to reports that heterologously expressed TAR2 is highly selective for TA over OA. This is the first report of TA receptor function in a native tissue and indicates unexpected complexity in the physiology of the Malpighian tubule.
Introduction
The biogenic amine tyramine (TA) is an important but relatively understudied modulator of many aspects of invertebrate physiology. Long considered to be simply an intermediate in the synthesis of octopamine (OA) from tyrosine, TA is now recognized to have physiological effects independent of OA1, 2. These include effects on egg melanisation3, sex pheromone production4, olfactory behavior5, locomotion and flight6–9, neuromuscular transmission5, 10, sleep11, appetite12, behavioral responses to cocaine and ethanol13–15, and muscular contractions16–18. Putative tyraminergic neurons, which are immunoreactive against TA but not OA, have been identified in both Drosophila and locust10, 16, 19, 20. In the nematode C. elegans, a clear role for TA in modulating the escape response has been demonstrated21–24. Interestingly, TA is also one of the “trace amines” found in vertebrates and has been linked to human disorders such as migraines and ADHD25–27.
Multiple types of insect G-protein coupled receptors are activated by TA; these receptors have been classified into three groups of OA receptors and three groups of TA receptors according to a system proposed by Evans and Maqueira28 and subsequently modified29–31. TA can act as an agonist of many OA receptor subtypes, but the OA receptors show varying degrees of selectivity for OA over TA31–37. The first subtype of TA receptor, the “Oct-TyrR” receptor class, recently renamed TAR1 receptors29, is activated with a weak selectivity for TA over OA38–44. Receptors in this class inhibit adenylyl cyclase, although in some systems they can also trigger calcium release45. Members of a second class of TA receptors, the TAR2 receptors, are coupled to calcium release and are reported to be extremely selective for TA. When expressed in mammalian 293 or CHO cells, the TAR2 receptors from Drosophila, the silk moth Bombyx mori, and the rice stem borer Chilo suppressalis are activated by low nanomolar concentrations of TA but are completely insensitive to micromolar doses of OA29, 46–48. A third tyramine receptor, TAR3, is also present in the Drosophila genome; expression of Drosophila melanogaster TAR3 in CHO cells results in a receptor that couples both to calcium release and to the inhibition of adenylyl cyclase and that is moderately selective for TA over OA and other amines29.
The Malpighian tubules (MTs) of Drosophila melanogaster are the best characterized system for studying TA signaling in a native insect tissue. These epithelial tubes produce primary urine through the transport of water and solutes from the surrounding hemolymph49. Secretion of primary urine by the MTs is driven by the active transport of cations across the apical membrane of the primary cell type, the principal cells. Upon application of nanomolar concentrations of TA or of the peptide leucokinin, primary urine production is stimulated and the transepithelial potential (TEP) depolarized due to an increase in transepithelial chloride conductance50, 51. This increase in chloride conductance is associated with an increase in calcium levels in a secondary cell type, the stellate cell, and is dependent upon the expression of a specific chloride channel in the stellate cells52–54. MTs also show a depolarizing response to OA and dopamine, but only at concentrations several orders of magnitude higher than TA51. Finally, we have shown that TA can be synthesized from tyrosine by tyrosine decarboxylase expressed by the Tdc1 gene in the principal cells, suggesting that TA acts as an agent of cell-cell communication in the MT55.
Despite the growing body of literature on the identification and pharmacological characterization of insect TA receptors, no studies have examined the function of specific TA receptors in their native cellular environment. In the current work, we aim to bridge the gap between the physiological effects of TA and the function of specific TA receptors. We use the power of Drosophila genetics and the sensitivity of the isolated MT to identify TAR2, and to a lesser extent TAR3, as responsible for the TA response in the MT. Surprisingly, the agonist profile of the Drosophila TAR2 is strikingly different in native tissue than in heterologous cells. Finally, we have generated mutant fly lines that will facilitate the identification of further physiological roles for specific TA receptors in Drosophila.
Results
For consistency with the published classification of TA receptors, we will refer to the gene encoding the Drosophila type 2 TA receptor, also called CG7431 or TyrR, as TAR2, and the gene encoding the Drosophila type 3 TA receptor, also called CG16766 or TyrRII, as TAR3. According to FlyAtlas56, expression of both genes is enriched in the larval MT (10.8-fold for TAR2 and 4.0-fold for TAR3) but not in the adult MT (1.2-fold for TAR2 and 1.3-fold for TAR3). However, an earlier transcriptome analysis by the same investigators57 identified TAR2 as the sixth most highly enriched receptor in the adult MT (8.5-fold enrichment). The reason for this discrepancy is unclear. Additionally, in the beetle Nicrophorus vespilloides, TAR2 is reported to have expression levels in the MT at least 10-fold higher than other TA or OA receptor genes58.
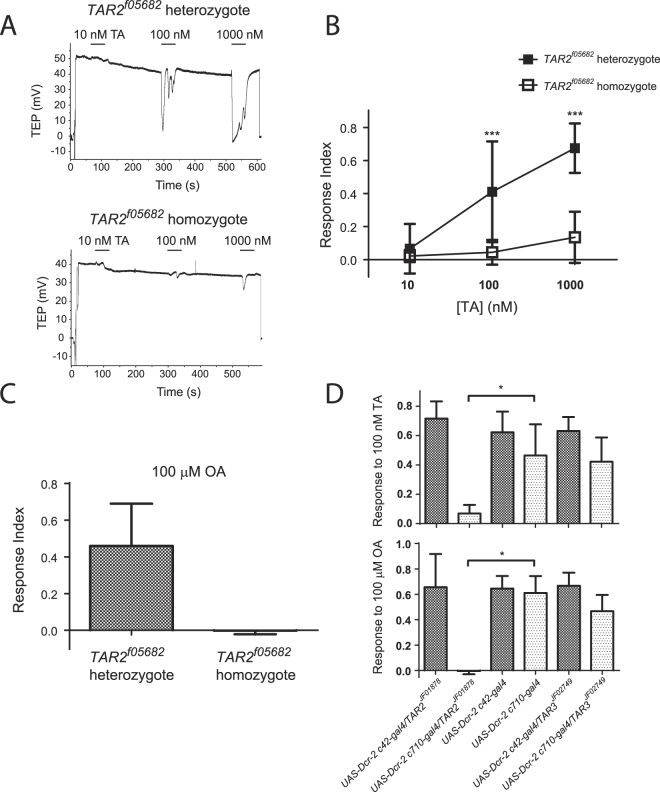
To study the role of the Drosophila TAR2 receptor in the TA response of the MT, we obtained a fly line carrying an allele of TAR2, TAR2 f05682, caused by a transposon insertion in the second intron of the gene (Fig. 1). Quantification of TAR2 expression in the MT by real-time RT-PCR indicated a dramatic reduction in transcript levels compared to heterozygous siblings. Only 1 of 3 homozygous cDNA samples gave a positive qPCR signal for TAR2. Based on the quantification of that signal and comparison to a dilution series of heterozygous cDNA, we estimate that TAR2 transcript abundance was reduced by 200–1000 fold in the homozygous mutants (data not shown). Electrophysiological recording of the TA response in isolated MTs showed a significant reduction, but not an elimination, of TA sensitivity in homozygotes compared to heterozygotes. Figure 2A shows representative responses to 10, 100, and 1000 nM TA; a clear response to the highest dose was observed in the homozygote. A dose-response curve to TA demonstrated significant reductions in the average response to 100 nM and 1000 nM TA in homozygotes (p < 0.001, 2-way ANOVA and Bonferroni posthoc test) but an average response to 1000 nM TA in the homozygotes that differed significantly from zero (p = 0.005, 1-sample t-test) (Fig. 2B). Sensitivity to 100 μM OA was completely abolished in the homozygotes (Fig. 2C). In contrast, there was no difference in the response to the peptide drosokinin (100 pM), the Drosophila ortholog of leucokinin, between heterozygotes and homozygotes (Fig. S1). Flies homozygous for another insertion in the same intron of TAR2, TAR2 LL06812, showed a less dramatic reduction in the TA responses of isolated MTs (data not shown).
Figure 1.
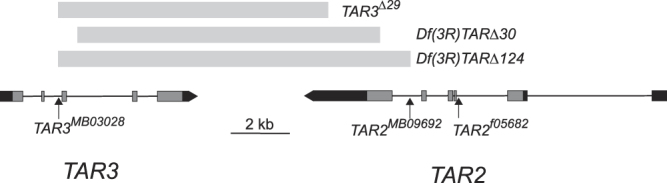
Genomic organization of the TAR2 and TAR3 genes. Boxes indicate exons, with lighter shading indicating coding regions and darker shading indicating untranslated regions. The precise sizes of the deletions created in this study are 12,084 bp (Df(3R)TARΔ124), 10,367 bp (Df(3R)TARΔ30), and 9,294 bp (TAR3 Δ29).
Figure 2.
(A) Representative responses to 10, 100, and 1000 nM TA of a TAR2 f05682 heterozygote (upper trace) and homozygote (lower trace). (B) Dose-response curve for TA in TAR2 f05682 heterozygotes and homozygotes. ***Significant difference between genotypes, p < 0.001, 2-way ANOVA and Bonferroni post-test, n = 8–10 tubules per genotype. (C) Response of TAR2 f05682 heterozygotes and homozygotes to 100 μM OA. The average response amplitude of homozygotes does not differ from zero, 1-sample t-test, p = 0.76. n = 9 tubules per genotype. (D) Effect of TAR2 and TAR3 RNAi on responses to 100 nM TA (upper graph) and 100 μM OA (lower graph). The responses of RNAi tubules were compared to those of the appropriate parental line by Kruskal-Wallis and Dunn’s multiple comparison tests. Asterices indicate that the only difference seen was upon RNAi of TAR2 in the stellate cells. Knockdown of TAR2 in the stellate cells did not eliminate the response to TA (1-sample t-test, p = 0.01) but did eliminate the response to OA (p = 0.82). n = 8–11 tubules per condition.
We used cell-type specific knockdown of TAR2 expression to determine its site of functional expression in the MT. Driving the inducible RNAi transgene TAR2 JF01878 with the principal cell-specific driver c42-gal4 resulted in responses to 100 nM TA and 100 μM OA that were unchanged compared with parental controls. In contrast, expression of the RNAi transgene in the stellate cells with the c710-gal4 driver caused a dramatic reduction in the response to 100 nM TA and an elimination of the response to 100 μM OA (Fig. 2D). To determine the extent of the knockdown in these flies, we measured TAR2 transcript levels in the MT by quantitative RT-PCR. Surprisingly, TAR2 expression in the c710 knockdown flies was not reduced relative to the c42 knockdowns or parental controls (Fig. S2).
Neither knockdown nor mutation of TAR2 resulted in the elimination of responses to high concentrations of TA. To test the possibility that the homologous receptor TAR3 might also function in the MTs, we knocked down TAR3 expression with the RNAi transgene TAR3 JF02749. Tubules in which TAR3 was knocked down in either the principal cells or the stellate cells displayed responses to 100 nM TA and 100 μM OA that were unchanged compared with those of parental controls (Fig. 2D). We were unable to determine the extent of TAR3 knockdown in these MTs by quantitative RT-PCR due to the extremely low levels of TAR3 expression in the MTs (data not shown).
The interpretation of the experiments described above is limited by the fact that neither the available TAR2 mutants nor the RNAi-mediated knockdown of TAR2 or TAR3 completely abolishes expression of the target gene. To generate complete null mutations of both TAR2 and TAR3, we used transposase-mediated recombination between Minos transposon insertions in the two adjacent genes. Three viable deletion lines were generated and the deletions were mapped by PCR and sequencing (see methods). In two of these deletions, Df(3R)TARΔ30 and Df(3R)TARΔ124, significant portions of the coding sequences of both TAR2 and TAR3 are deleted (Fig. 1), and we consider both of these deletions to be null for both TAR genes. In the third line, TAR3 Δ29, the majority of the TAR3 coding sequence is deleted along with the final 758 bp of the predicted 1984 bp TAR2 3′ untranslated region. Quantitative RT-PCR indicated no difference in TAR2 transcript abundance in the MTs of TAR3 Δ29 homozygotes compared with heterozygotes (data not shown), and so we consider this deletion to be a specific null mutation of TAR3. The deletions in the three lines did not knock out any other genes, as the breakpoints of all deletions were contained within the adjacent TAR2 and TAR3 genes.
Tubules isolated from homozygotes of either TAR2-TAR3 deletion line were completely unresponsive to TA concentrations up to 1000 nM and to 100 μM OA (Figs 3, S3). The deletion of these two TAR genes did not abolish the ability of tubules to respond to depolarizing stimuli, as there was no decrement in the response to 100 pM drosokinin (Fig. S3). In contrast, deletion of only TAR3 had no effect on the amplitude of responses to either TA or OA (Figs 3, S3).
Figure 3.
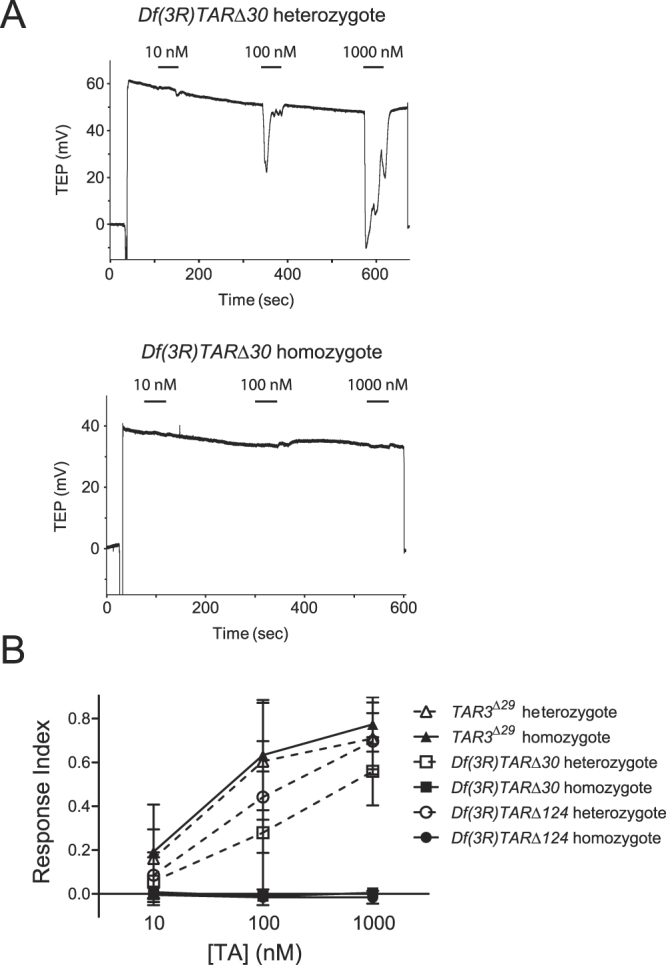
Response of TAR deletion mutants to TA. (A) Representative traces showing the response to 1000 nM TA of a Df(3R)TARΔ30 heterozygote (upper trace) and homozygote (lower trace). (B) Dose-response curve to TA for TAR3 Δ29, Df(3R)TARΔ30, and Df(3R)TARΔ124 heteroyzygotes and homozygotes. The mean responses of Df(3R)TARΔ30, and Df(3R)TARΔ124 homozygotes to each concentration of TA were either not significantly different from zero by a 1-sample test or were negative. There were no differences in the responses of TAR3 Δ29 homozygotes vs heterozygotes, p > 0.05 by 2-way ANOVA and Bonferroni post-test. n = 7–12 tubules per point.
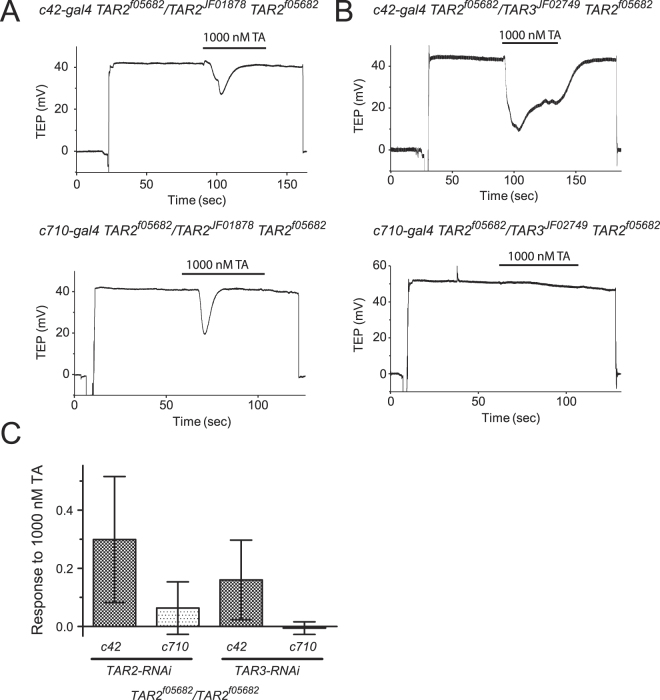
Taken as a whole, the data presented thus far neither confirm nor exclude a functional role for TAR3 in the response to high concentrations of TA. To determine whether the residual response to TA observed in TAR2 f05682 homozygotes was due to functional expression of TAR3, we performed RNAi-mediated knockdown of TAR2 or TAR3 expression on a TAR2 f05682 mutant background. As shown in Fig. 4, additional knockdown of TAR2 in the stellate cells of mutant flies reduced, but did not eliminate, the residual response to 1000 nM TA. In contrast, knockdown of TAR3 in the stellate cells of mutant flies completely eliminated the residual TA response, indicating a minor but measurable function for the TAR3 receptor in the MT.
Figure 4.
Effect of RNAi against TAR2 and TAR3 on a TAR2 mutant background. (A) Representative responses to 1000 nM TA from c42-gal4 TAR2 f05682 /TAR2 JF01878 TAR2 f05682 (upper trace) and c710-gal4 TAR2 f05682 /TAR2 JF01878 TAR2 f05682 (lower trace) tubules. (B) Representative responses to 1000 nM TA from c42-gal4 TAR2 f05682 /TAR3 JF02749 TAR2 f05682 (upper trace) and c710-gal4 TAR2 f05682 /TAR3 JF02749 TAR2 f05682 (lower trace) tubules. (C) Mean responses to 1000 nM of the four genotypes shown in (A and B). Knockdown of both TAR2 and TAR3 in the stellate cells caused a significant reduction in the response compared to knockdown in principal cells, p < 0.05, 1-way ANOVA and Sidak’s multiple comparisons test, but only knockdown of TAR3 in the stellate cells resulted in a mean response that was equal to zero, p = 0.45, 1-sample t-test. n = 8–9 tubules per genotype.
Discussion
In this paper, we report the first functional characterization of individual insect TA receptor subtypes in a native tissue. Our data present clear evidence that the primary receptor for TA in the Malpighian tubules is encoded by TAR2, as both mutation of the gene and RNAi result in a substantial reduction of the depolarizing response to TA. At the same time it is also clear that neither the TAR2 mutation nor RNAi wholly eliminates the TA response. In contrast, chromosomal deletions that remove both TAR2 and TAR3 do eliminate the TA response. There are two interpretations of these data. First, it is possible that the TAR2 receptor solely mediates the TA response, and that the small TA responses in mutants or after RNAi results from residual expression of TAR2. This interpretation is consistent with our detection of very low levels of properly spliced TAR2 transcript in TAR2 f05682 homozygotes. The second interpretation is that both TAR2 and TAR3 contribute to the TA response, and that the residual TA response seen in TAR2 mutants or after TAR2 RNAi is mediated by TAR3. We believe that the results of our final experiment, in which RNAi against TAR3 but not TAR2 eliminates the residual TA response in TAR2 f05682 homozygotes, are consistent only with the second interpretation. For this final result to fit with a single functional TA receptor, one would have to argue that the TAR3 RNAi transgene was more effective at reducing TAR2 expression than the TAR2 RNAi transgene. Such off-target effects are theoretically possible. However, a strong off-target effect of the TAR3 RNAi transgene is not consistent with our observation that only TAR2 RNAi, and not TAR3 RNAi, reduces the TA responses in otherwise wild-type tubules. Thus, we conclude that both TAR2 and TAR3 contribute to the depolarizing response of TA in the Malpighian tubule. As the TA response has been shown to be associated with an increase in intracellular calcium concentrations54, our data are consistent with the finding that both TAR2 and TAR3 receptors can couple to IP3 production and calcium release in heterologous expression systems29, 48.
Our data do reveal one important difference in the agonist profile of the TAR2 receptor in native tissue compared with heterologous cells. When expressed in mammalian cell lines, TAR2 from several different insect species, including Drosophila, forms a receptor that is highly selective for TA over OA by at least five orders of magnitude29, 46–48. While the Bombyx TAR2 receptor is activated by 100 μM OA47, the Drosophila receptor is reported to be insensitive even to this high concentration48. In the MT however, our data show that reduction of TAR2 expression by mutation or RNAi eliminated the depolarizing response to OA, indicating that TAR2 in the tubule can be activated by OA, albeit with a potency approximately 3 orders of magnitude lower than TA. The ability of TAR2 to respond to very high micromolar concentrations of OA is unlikely to be physiologically relevant; it is however, indicative of a significant difference between the agonist selectivity of TAR2 in native tissues compared to mammalian cell lines. The explanation for this difference is unknown but perhaps could be due to abnormal processing or modification of the insect receptor by mammalian cells. We know of no other examples of the agonist profile of a GPCR differing between native tissue and a heterologous expression system.
Expression of the two TA receptors in the stellate cells, but not the principal cells, of the tubules is necessary for depolarizing responses, as evidenced by our cell-type specific RNAi data. This result is consistent with many reports demonstrating that the stellate cells are the site of action of depolarizing diuretic agents such as TA and drosokinin50, 52–54. Our finding that TA receptors are functionally expressed on the stellate cells is especially significant in conjunction with our previous report that TA can be synthesized in situ from tyrosine through the action of tyrosine decarboxylase expressed in the principal cells of the tubule55. Together, these two findings indicate that TA is an agent of cell-cell communication in the Drosophila tubule. This is the first demonstration of cell-cell communication in an insect Malpighian tubule. The functional significance of this communication is still unknown. We have previously hypothesized that significant levels of tyrosine in the hemolymph could result in constitutive activation of TA receptors on the tubule in the intact fly and that TA-mediated diuresis, because it is sensitive to hemolymph osmolality, could contribute to organismal osmoregulation59.
While we found that RNAi-mediated knockdown of TAR2 in the stellate cells eliminated the large majority of the TA response, the same knockdown had no effect on the overall level of TAR2 expression in the tubule. One interpretation of this result is that TAR2 is expressed in another cell type within the MT: either in the principal cells or at high levels in a minor cell type, such as muscle or tracheal cells. However, because we measured the effect of RNAi on transcript abundance only and not on receptor protein levels, our negative result must be interpreted with caution. It is possible that a small reduction in TAR2 transcript abundance resulted in a large decrease in functional receptor expression in the stellate cells.
The TAR3 subtype of TA receptor is reported to have arisen recently in evolution, being only present in members of the Drosophila genus, and to have an expression pattern and agonist specificity distinct from that of TAR2 29. The relatively recent appearance of the TAR3 gene in insect evolution begs the question of whether this receptor subtype has a distinct physiological function. The reported ability of TAR3 to couple to both calcium release and inhibition of adenylyl cyclase, in contrast to TAR229, suggests a possible function of this receptor in fine-tuning the response of the MT to TA. The new TAR mutant lines reported here should prove to be extremely useful in elucidating the function of the TAR2 and TAR3 receptors in many aspects of insect physiology and development.
Methods
Drosophila strains and maintenance
Stocks of Drosophila melanogaster were maintained on cornmeal/yeast/molasses/agar food at 24 °C on a 12 hr:12 hr light-dark cycle. The following stocks were used in these studies: w; TAR3 MB03028 (FBst0023837-BL), w; TAR2 MB09692 /TM3 Ser (FBst0027797-BL), y v; TAR2 JF01878 (FBst0025857-BL), w; TAR2 f05682 /TM3 Ser (FBst1020033-Exelixis), y v; TAR3 JF02749 (FBst0027670-BL), w; P{GawB}c42 (FBst0030835, gift of Prof. Julian Dow), and w; P{GawB}c710 (FBti0009567, gift of Prof. Julian Dow), w; P{UAS-Dcr-2.D}10 P{GawB}c42 and w; P{UAS-Dcr-2.D}10 P{GawB}c710 59, P{FRT} PBac{DsRed}TAR2 LL06812(Drosophila Genetic Resource Center), w 1118 ; sna Sco /SM6a, P{hsILMiT}2.4 (FBst0024613-BL). Genes studied in this work include TAR2 (TyrR) (FBgn0038542), TAR3 (TyrRII) (FBgn0038541), and RpL32 (FBgn0002626).
Creation of recombinants and receptor mutants
Recombinants of TAR2 f05682 with the c42 or c710-gal4 drivers and the TAR2 JF01878 and TAR3 JF02749 RNAi transgenes were created by standard crosses. Transgenes were followed by eye color where possible and confirmed by PCR (see Table S1 for primer sequences). New TA receptor mutants were created with the following crossing scheme:
w; noc Sco /SM6a, hsILMiT x w; TM6b/+ → w; SM6a, hsILMiT/+; TM6b/+
w; SM6a, hsILMiT/+; TM6b/+ x w; TAR3MB03028/TAR3MB03028 → w; SM6a, hsILMiT/+; TAR3 MB03028 /TM6b
-
w; SM6a, hsILMiT/+; TAR3 MB03028 /TM6b x w ; TAR2 MB09692 /TAR2 MB09692 → w; SM6a, hsILMiT/+; TAR3 MB03028 /TAR2 MB09692
Two days after setting up cross 3, flies were transferred to new vials and heat-shocked daily for 1 hr at 37 °C until pupariation to induce transposase expression.
w; SM6a, hsILMiT/+; TAR3 MB03028 /TAR2 MB09692 x w; TM3 Ser/+ → w; Df?/TM3 Ser
Balanced lines were established from individual cross 4 progeny that lacked GFP expression and screened by PCR for deletion of the TAR2 and TAR3 genes (see Table S1).
Quantitative RT-PCR
Posterior MTs were dissected from groups of 7–8 6 to 8 day old adult Drosophila females. For RNAi knockdowns, dissected flies were progeny of crosses between either UAS-Dcr2 c42-gal4 or UAS-Dcr2 c710-gal4 and either y v; TAR2 JF01878 or y v; TAR3 JF02749. Progeny from crosses in both directions were used (i.e. gal4 driver females x RNAi males, and gal4 driver males x RNAi females). MT RNA was isolated and DNAse treated (RNaqueous Micro kit, Thermo Fisher Scientific, Waltham, MA), and 50% of each RNA prep was then used as a template for cDNA synthesis (Qscript, Quansys Biosciences, Logan, UT). Quantitative PCR was performed using Perfecta SYBR mastermix (Quansys), 250 nM primers, 0.1 uL cDNA per reaction in a CFX thermocycler (Bio Rad, Hercules, CA) and analyzed using CFX Manager software (Bio Rad). Primer sequences are shown in Table S1. Each PCR run contained dilution series of one cDNA with all tested primer sets, and the resulting standard curves were fit with straight lines (r2 ≥ 0.98, efficiency 85–101%) and used to quantify unknowns. Levels of TAR2 expression were normalized to those of the housekeeping gene RpL32.
Electrophysiology
MTs were dissected from 6 to 8-day old adult Drosophila females and placed in dishes in which 100 μL of 31 μg/mL poly-L-lysine was allowed to dry and that were rinsed with deionized water briefly before dissection, and the TEP was recorded with a conventional microelectrode as described60. Data acquisition and analysis was performed with pClamp 9 software (Molecular Devices, Sunnyvale, CA). The dissecting and recording saline consisted of the following (in mM): 85 NaCl, 20 KCl, 3 CaCl2, 12 MgCl2, 7.5 NaHCO3, 10 HEPES, 15 glucose, pH 6.8 (260–265 mOsm). Agents added to the recording solution included TA (Sigma-Aldrich, St. Louis, MO), OA (Pfaltz & Bauer, Waterbury, CT), and drosokinin (AnaSpec, Fremont, CA). Recording saline osmolality was measured with a vapor-pressure osmometer (Wescor, Logan, UT).
Response index values for TA and drosokinin responses were calculated as previously described60.
Statistics
For statistical analysis, response index values were transformed by taking the arcsine of the square root of each value. Negative values were transformed by taking the negative arcsine of the square root of the absolute value of the response index. Statistical tests were performed with Graphpad Prism v5.02 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com). Datasets were tested for normality using a D’Agostino-Pearson normality test (this test is only valid for n ≥ 8). Datasets that failed this test were analyzed using non-parametric statistics; see figure legends for details. Error bars in figures indicate standard deviations.
Electronic supplementary material
Acknowledgements
We wish to thank Prof. Julian Dow, The Exelixis Collection at the Harvard Medical School, the Bloomington Drosophila Stock Center (NIH P40OD018537), and the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing fly stocks used in this study. Support was provided by Marquette University and National Science Foundation grant IOS-0744619 to E.M.B.
Author Contributions
Designed experiments: H.Z. and E.M.B., Collected data: H.Z. and E.M.B., Analyzed data: H.Z. and E.M.B., Wrote manuscript: E.M.B.
Competing Interests
The authors declare no competing financial interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00120-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lange AB. Tyramine: from octopamine precursor to neuroactive chemical in insects. Gen Comp Endocrinol. 2008;162:18–26. doi: 10.1016/j.ygcen.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs S, Rende E, Crisanti A, Nolan T. Disruption of aminergic signalling reveals novel compounds with distinct inhibitory effects on mosquito reproduction, locomotor function and survival. Sci Rep. 2014;4:5526. doi: 10.1038/srep05526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirashima A, Yamaji H, Yoshizawa T, Kuwano E, Eto M. Effect of tyramine and stress on sex-pheromone production in the pre- and post-mating silkworm moth, Bombyx mori. J Insect Physiol. 2007;53:1242–1249. doi: 10.1016/j.jinsphys.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Kutsukake M, Komatsu A, Yamamoto D, Ishiwa-Chigusa S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster. Gene. 2000;245:31–42. doi: 10.1016/S0378-1119(99)00569-7. [DOI] [PubMed] [Google Scholar]
- 6.Vierk R, Pflueger HJ, Duch C. Differential effects of octopamine and tyramine on the central pattern generator for Manduca flight. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:265–277. doi: 10.1007/s00359-008-0404-5. [DOI] [PubMed] [Google Scholar]
- 7.Saraswati S, Fox LE, Soll DR, Wu C-F. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J Neurobiol. 2004;58:425–441. doi: 10.1002/neu.10298. [DOI] [PubMed] [Google Scholar]
- 8.Brembs B, Christiansen F, Pflüger HJ, Duch C. Flight initiation and maintenance deficits in flies with genetically altered biogenic amine levels. J Neurosci. 2007;27:11122–11131. doi: 10.1523/JNEUROSCI.2704-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fussnecker BL, Smith BH, Mustard JA. Octopamine and tyramine influence the behavioral profile of locomotor activity in the honey bee (Apis mellifera) J Insect Physiol. 2006;52:1083–1092. doi: 10.1016/j.jinsphys.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci Lett. 2002;329:324–328. doi: 10.1016/S0304-3940(02)00596-7. [DOI] [PubMed] [Google Scholar]
- 11.Crocker A, Sehgal A. Octopamine regulates sleep in Drosophila through protein kinase A-dependent mechanisms. J Neurosci. 2008;28:9377–9385. doi: 10.1523/JNEUROSCI.3072-08a.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nisimura T, et al. Experiential effects of appetitive and nonappetitive odors on feeding behavior in the blowfly, Phormia regina: a putative role for tyramine in appetite regulation. J Neurosci. 2005;25:7507–7516. doi: 10.1523/JNEUROSCI.1862-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie SL, Zhang JX, Hirsh J. Trace amines differentially regulate adult locomotor activity, cocaine sensitivity, and female fertility in Drosophila melanogaster. Dev Neurobiol. 2007;67:1396–1405. doi: 10.1002/dneu.20459. [DOI] [PubMed] [Google Scholar]
- 14.Scholz H. Influence of the biogenic amine tyramine on ethanol-induced behaviors in Drosophila. J Neurobiol. 2005;63:199–214. doi: 10.1002/neu.20127. [DOI] [PubMed] [Google Scholar]
- 15.McClung C, Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr Biol. 1999;9:853. doi: 10.1016/S0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- 16.Donini A, Lange AB. Evidence for a possible neurotransmitter/neuromodulator role of tyramine on the locust oviducts. J Insect Physiol. 2004;50:351–361. doi: 10.1016/j.jinsphys.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Huddart H, Oldfield AC. Spontaneous activity of foregut and hindgut visceral muscle of the locust, Locusta migratoria—II. The effect of biogenic amines. Comp Biochem Physiol C: Comp Pharmacol. 1982;73:303. doi: 10.1016/0306-4492(82)90126-5. [DOI] [Google Scholar]
- 18.da Silva R, Lange AB. Tyramine as a possible neurotransmitter/neuromodulator at the spermatheca of the African migratory locust, Locusta migratoria. J Insect Physiol. 2008;54:1306–1313. doi: 10.1016/j.jinsphys.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Homberg U, Seyfarth J, Binkle U, Monastirioti M, Alkema MJ. Identification of distinct tyraminergic and octopaminergic neurons innervating the central complex of the desert locust, Schistocerca gregaria. J Comp Neurol. 2013;521:2025–41. doi: 10.1002/cne.23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kononenko NL, Wolfenberg H, Pflüger HJ. Tyramine as an independent transmitter and a precursor of octopamine in the locust central nervous system: an immunocytochemical study. J Comp Neurol. 2009;512:433–452. doi: 10.1002/cne.21911. [DOI] [PubMed] [Google Scholar]
- 21.Pirri JK, McPherson AD, Donnelly JL, Francis MM, Alkema MJ. A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron. 2009;62:526–538. doi: 10.1016/j.neuron.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnelly JL, et al. Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol. 2013;11:e1001529. doi: 10.1371/journal.pbio.1001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–60. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Ringstad N, Abe N, Horvitz HR. Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science. 2009;325:96–100. doi: 10.1126/science.1169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Andrea G, et al. The role of tyrosine metabolism in the pathogenesis of chronic migraine. Cephalalgia. 2013;33:932–937. doi: 10.1177/0333102413480755. [DOI] [PubMed] [Google Scholar]
- 26.Branchek TA, Blackburn TP. Trace amine receptors as targets for novel therapeutics: legend, myth and fact. Curr Opin Pharmacol. 2003;3:90–97. doi: 10.1016/S1471-4892(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 27.Berry MD. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials. 2007;2:3–19. doi: 10.2174/157488707779318107. [DOI] [PubMed] [Google Scholar]
- 28.Evans PD, Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005;5:111–8. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- 29.Bayliss A, Roselli G, Evans PD. A comparison of the signalling properties of two tyramine receptors from Drosophila. J Neurochem. 2013;125:37–48. doi: 10.1111/jnc.12158. [DOI] [PubMed] [Google Scholar]
- 30.Farooqui T. Review of octopamine in insect nervous systems. Open Access Insect Physiol. 2012;4:1–17. doi: 10.2147/OAIP.S20911. [DOI] [Google Scholar]
- 31.Wu S-F, et al. Two splicing variants of a novel family of octopamine receptors with different signaling properties. J Neurochem. 2014;129:37–47. doi: 10.1111/jnc.12526. [DOI] [PubMed] [Google Scholar]
- 32.Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophila beta-adrenergic-like octopamine G-protein coupled receptors. J Neurochem. 2005;94:547–560. doi: 10.1111/j.1471-4159.2005.03251.x. [DOI] [PubMed] [Google Scholar]
- 33.Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci. 1998;18:3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balfanz S, Strünker T, Frings S, Baumann A. A family of octopamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J Neurochem. 2005;93:440–451. doi: 10.1111/j.1471-4159.2005.03034.x. [DOI] [PubMed] [Google Scholar]
- 35.Ohtani A, et al. Molecular cloning and heterologous expression of an alpha-adrenergic-like octopamine receptor from the silkworm Bombyx mori. Insect Mol Biol. 2007;15:763–772. doi: 10.1111/j.1365-2583.2006.00676.x. [DOI] [PubMed] [Google Scholar]
- 36.Grohmann L, et al. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J Neurochem. 2003;86:725. doi: 10.1046/j.1471-4159.2003.01876.x. [DOI] [PubMed] [Google Scholar]
- 37.Busch S, Selcho M, Ito K, Tanimoto H. A map of octopaminergic neurons in the Drosophila brain. J Comp Neurol. 2009;513:643–667. doi: 10.1002/cne.21966. [DOI] [PubMed] [Google Scholar]
- 38.Gross AD, et al. Pharmacological characterization of a tyramine receptor from the southern cattle tick, Rhipicephalus (Boophilus) microplus. Insect Biochem Mol Biol. 2015;63:47–53. doi: 10.1016/j.ibmb.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Arakawa S, et al. Cloning, localization, and permanent expression of a Drosophila octopamine receptor. Neuron. 1990;4:343–354. doi: 10.1016/0896-6273(90)90047-J. [DOI] [PubMed] [Google Scholar]
- 40.Broeck JV, Vulsteke V, Huybrechts R, De Loof A. Characterization of a cloned locust tyramine receptor cDNA by functional expression in permanently transformed Drosophila S2 cells. J Neurochem. 1995;64:2387–2395. doi: 10.1046/j.1471-4159.1995.64062387.x. [DOI] [PubMed] [Google Scholar]
- 41.Molaei G, Paluzzi J-P, Bendena WG, Lange AB. Isolation, cloning, and tissue expression of a putative octopamine/tyramine receptor from locust visceral muscle tissues. Arch Insect Biochem Physiol. 2005;59:132–149. doi: 10.1002/arch.20067. [DOI] [PubMed] [Google Scholar]
- 42.Ohta H, Utsumi T, Ozoe Y. B96Bom encodes a Bombyx mori tyramine receptor negatively coupled to adenylate cyclase. Insect Mol Biol. 2003;12:217–223. doi: 10.1046/j.1365-2583.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- 43.Saudou F, Amlaiky N, Plassat JL, Borrelli E, Hen R. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 1990;9:3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blenau W, Balfanz S, Baumann A. Amtyr1: characterization of a gene from honeybee (Apis mellifera) brain encoding a functional tyramine receptor. J Neurochem. 2000;74:900–908. doi: 10.1046/j.1471-4159.2000.0740900.x. [DOI] [PubMed] [Google Scholar]
- 45.Robb S, et al. Agonist-specific coupling of a cloned Drosophila octopamine/tyramine receptor to multiple second messenger systems. EMBO J. 1994;13:1325–1330. doi: 10.1002/j.1460-2075.1994.tb06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu S-F, Xu G, Ye G-Y. Characterization of a tyramine receptor type 2 from hemocytes of rice stem borer, Chilo suppressalis. J Insect Physiol. 2015;75:39–46. doi: 10.1016/j.jinsphys.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Huang J, et al. Molecular cloning and pharmacological characterization of a Bombyx mori tyramine receptor selectively coupled to intracellular calcium mobilization. Insect Biochem Mol Biol. 2009;39:842–849. doi: 10.1016/j.ibmb.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Cazzamali G, Klaerke DA, Grimmelikhuijzen CJP. A new family of insect tyramine receptors. Biochem Biophys Res Comm. 2005;338:1189–1196. doi: 10.1016/j.bbrc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 49.Beyenbach KW, Skaer H, Dow JAT. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol. 2010;55:351–374. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- 50.O’Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in Malpighian tubules of Drosophila melanogaster. J Exp Biol. 1996;199:1163–1175. doi: 10.1242/jeb.199.5.1163. [DOI] [PubMed] [Google Scholar]
- 51.Blumenthal EM. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am J Physiol: Cell Physiol. 2003;284:C718–28. doi: 10.1152/ajpcell.00359.2002. [DOI] [PubMed] [Google Scholar]
- 52.O’Donnell MJ, et al. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol. 1998;274:R1039–49. doi: 10.1152/ajpregu.1998.274.4.R1039. [DOI] [PubMed] [Google Scholar]
- 53.Cabrero P, et al. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci USA. 2014;111:14301–14306. doi: 10.1073/pnas.1412706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cabrero P, Richmond L, Nitabach M, Davies SA, Dow JAT. A biogenic amine and a neuropeptide act identically: tyramine signals through calcium in Drosophila tubule stellate cells. Proc Biol Sci. 2013;280:20122943. doi: 10.1098/rspb.2012.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blumenthal EM. Isoform- and cell-specific function of tyrosine decarboxylase in the Drosophila Malpighian tubule. J Exp Biol. 2009;212:3802–3809. doi: 10.1242/jeb.035782. [DOI] [PubMed] [Google Scholar]
- 56.Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nature Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, et al. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biology. 2004;5:R69. doi: 10.1186/gb-2004-5-9-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cunningham CB, Douthit MK, Moore AJ. Expression of octopaminergic receptor genes in 4 nonneural tissues in female Nicrophorus vespilloides beetles. Insect Sci. 2014;22:495–502. doi: 10.1111/1744-7917.12133. [DOI] [PubMed] [Google Scholar]
- 59.Blumenthal EM. Modulation of tyramine signaling by osmolality in an insect secretory epithelium. Am J Physiol: Cell Physiol. 2005;289:C1261–7. doi: 10.1152/ajpcell.00026.2005. [DOI] [PubMed] [Google Scholar]
- 60.Ruka KA, Miller AP, Blumenthal EM. Inhibition of diuretic stimulation of an insect secretory epithelium by a cGMP-dependent protein kinase. Am J Physiol: Renal Physiol. 2013;304:F1210–6. doi: 10.1152/ajprenal.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.