Abstract
How multicellular organisms maintain immune homeostasis across various organs and cell types is an outstanding question in immune biology and cell signaling. In Drosophila, blood cells (hemocytes) respond to local and systemic cues to mount an immune response. While endosomal regulation of Drosophila hematopoiesis is reported, the role of endosomal proteins in cellular and humoral immunity is not well-studied. Here we demonstrate a functional role for endosomal proteins in immune homeostasis. We show that the ubiquitous trafficking protein ADP Ribosylation Factor 1 (ARF1) and the hemocyte-specific endosomal regulator Asrij differentially regulate humoral immunity. Asrij and ARF1 play an important role in regulating the cellular immune response by controlling the crystal cell melanization and phenoloxidase activity. ARF1 and Asrij mutants show reduced survival and lifespan upon infection, indicating perturbed immune homeostasis. The ARF1-Asrij axis suppresses the Toll pathway anti-microbial peptides (AMPs) by regulating ubiquitination of the inhibitor Cactus. The Imd pathway is inversely regulated- while ARF1 suppresses AMPs, Asrij is essential for AMP production. Several immune mutants have reduced Asrij expression, suggesting that Asrij co-ordinates with these pathways to regulate the immune response. Our study highlights the role of endosomal proteins in modulating the immune response by maintaining the balance of AMP production. Similar mechanisms can now be tested in mammalian hematopoiesis and immunity.
Introduction
A cascade of appropriate responses to infection or injury is dynamically regulated to co-ordinate the immune response. However, mechanistic details of how the immune organs and molecules they produce communicate, are poorly understood. In the open circulatory system of Drosophila, hemocytes carry out phagocytosis and melanization whereas the humoral immune response is mediated by the fat body and gut. Plasmatocytes, which form a majority of the hemocyte population, phagocytose invading pathogens, crystal cells melanize and restrict pathogens to the affected area and lamellocytes encapsulate and neutralize large objects such as parasites1. A serine protease cascade in crystal cells activates prophenoloxidase (ProPO), which then catalyzes the conversion of phenols to quinones that then polymerize to form melanin2. A Toll pathway-dependent protease inhibitor Serpin27A produced by the fat body is required to limit melanization to crystal cells3, 4. Thus mechanisms of transport and uptake are essential to regulate systemic communication and melanization. Larvae and adults deficient in Serpin27A or the ProPO mutant Black cells show spontaneous melanization yet are immune compromised5.
Humoral immunity is primarily governed by the Toll and Imd (Immune deficiency) pathways, which regulate anti-microbial peptide (AMP) production. Fungi or Gram positive bacteria induce the Toll pathway, which causes activation of the NF-KB factor Dif or Dorsal and production of AMPs such as Drosomycin, Metchnikowin and Defensin. Infection by Gram negative bacteria causes activation of the Rel homology and IkappaB homology domain protein Relish, through the Imd pathway and leads to the production of Diptericin, Attacin, Cecropin and Drosocin6. Additionally, immune pathways have also been shown in tissue-specific contexts. The JAK/STAT pathway regulates gut- mediated defense mechanisms by controlling intestinal stem cell proliferation7, 8 and is also essential for the production of humoral factors like thio-ester proteins and turandot molecules in response to septic injury9. The receptor tyrosine kinase Pvr also plays an important role in regulating the Imd pathway. Drosophila JNK activates the expression of Pvr ligands, Pvf2 and Pvf3 which bind Pvr and lead to the activation of the Pvr/ERK pathway which negatively regulates the JNK and NF-κB arm of the Imd pathway10.
AMPs are primarily produced by the fat body, the equivalent of the mammalian liver, and secreted into circulation to reach target tissues11. The systemic response by the fat body is mainly governed by the Toll and Imd pathways12. An intriguing question is the mode of communication between the hemocyte and fat body- mediated immune response. Few studies have recently shown that the hemocytes contribute towards fat body mediated immune responses. Psidin is a lysosomal protein required for degradation of bacteria in hemocytes and simultaneously required for Defensin production in the fat body13 and Spaetzle has been shown to have a paracrine effect on the fat body mediated immune response14, 15. Recently, upd3 from hemocytes has been shown to induce Drosomycin production in the gut upon septic injury16. Normal physiological levels of AMPs are altered in response to infections but can also be affected by genetic mutations, thus perturbing homeostasis similar to an infection- induced condition17–20.
Just as in hematopoiesis, a complex network of molecular interactions is essential to regulate immune function and maintain homeostasis. Hence understanding organismal regulation of these processes is a major challenge. Elucidating mechanisms that can integrate the varying inputs received at the cellular level and orchestrate the outcome will be key to generating tools that allow control of the system. Several recent studies highlight the importance of unique signal generation and regulation in cellular organelles, such as endosomes, in a variety of cell types21–27.
Endosomal regulation is a potent mechanism to integrate and modulate signals in Drosophila hematopoiesis28, 29. We recently showed that the GTP bound form of the ubiquitous endosomal protein ADP Ribosylation Factor 1 (ARF1-GTP), interacts with and regulates the hemocyte-specific endosomal protein Asrij to integrate and modulate multiple signalling pathways required to maintain blood cell homeostasis, including the JAK/STAT and Notch pathways and Pvr and Insulin signaling27, 30. Depletion of ARF1 or Asrij leads to increased circulating and differential hemocyte counts. As the primary role of Drosophila hemocytes is to mount an immune response like their mammalian blood cell counterparts, and because both ARF1 and Asrij have conserved functions, we analyzed their requirement in the cellular and humoral immune response of Drosophila. We show that crystal cell number in ARF1 and Asrij mutants correlates with the extent of melanization. Further, perturbation of the ARF1-Asrij axis results in aberrant AMP production and compromises the response to bacterial infection. ARF1 and Asrij have similar effects on the Toll pathway but regulate Imd pathway AMPs inversely. Thus, we demonstrate that regulation by endosomal proteins allows differential response to signals to achieve immune homeostasis.
Results
Depletion of ubiquitous (ARF1) or hemocyte-specific (Asrij) endosomal proteins does not affect phagocytosis
Phagocytosis is a cellular immune response brought about by plasmatocytes, a differentiated hemocyte type. Depletion of either ARF1 or Asrij results in increased plasmatocyte numbers27, 30. To analyze whether there is any alteration in the phagocytic ability of the mutant hemocytes, we incubated the ARF1 knockdown or asrij null mutant larval circulating hemocytes with India ink dye particles31. Our analysis shows that phagocytosis is unaltered in both mutant genotypes as compared to controls (Fig. S1A–D). We also assessed the ability of the adult hemocytes to phagocytose rhodamine conjugated E. coli in Asrij or ARF1 depleted hemocytes and found that there is no difference in their ability to phagocytose the bacteria as compared to the respective controls (Fig. S1E,F). Thus the ARF1-Asrij axis is not essential for phagocytosis.
Increased crystal cell number upon ARF1 or Asrij depletion correlates with increased melanization and phenoloxidase activity
Crystal cells usually attach to the larval cuticle and mechanical injury or infection triggers melanization, leading to blackening of crystal cells31. Asrij and ARF1 were earlier shown to be expressed in crystal cells27, 30. We specifically depleted or overexpressed Asrij or ARF1 in the hemocyte population using a hemocyte specific driver e33cGal4 which is expressed in epidermal tissues but not in immune organs like the fat body and/or the gut32. Larvae depleted of ARF1 using a hemocyte driver (e33cGAL4) as well as asrij null mutant larvae, have increased crystal cells27, 30. To test whether the mutant crystal cells were functional, we subjected them to a melanization assay (see methods). Both mutant genotypes showed increase in melanized cells upon heat activation (about 1.5 fold for ARF1 knockdown and 2 fold for asrij mutant), as compared to controls (Figs 1A–G and 2A–G). The depleted phenotypes could be rescued to near control levels by over-expressing the respective protein in larvae using e33cGal4 (Figs 1F,G and 2F,G). Overexpression of ARF1 or Asrij in wild type animals resulted in reduced crystal cell number as compared to the UAS control corresponding to reduced differentiation seen in these larvae as reported earlier27, 30 (Figs 1D,E,G and 2D,E,G). Notably, the size and intensity of melanized spots was higher in the asrij null mutant compared to controls and Asrij overexpressing larvae (Fig. 2A–G). This suggested increase in crystal cell number may be accompanied by increased function.
Figure 1.
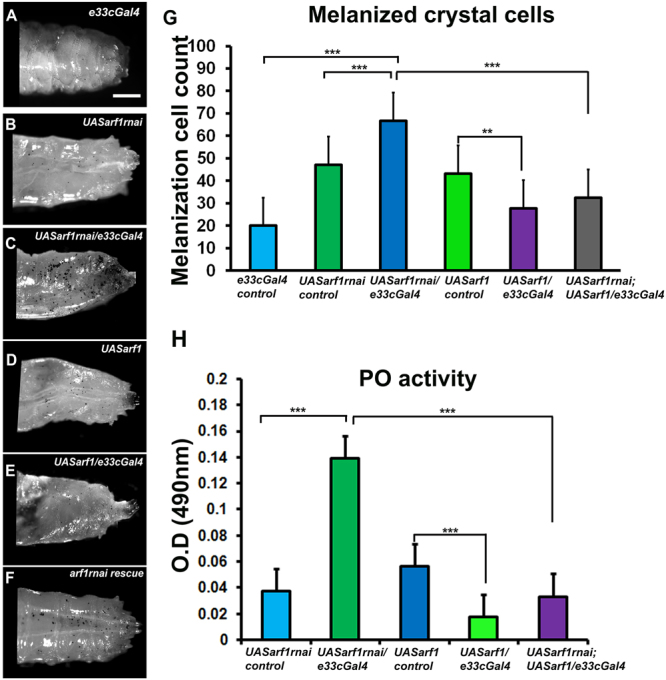
ARF1 regulates crystal cell- mediated melanization and phenoloxidase activity. (A–F) Photomicrographs showing posterior region of third instar larvae of specific genotypes (A) e33cGal4 (B) UAS arf1rnai (C) UAS arf1rnai/e33cGal4 (D) UAS arf1 (E) UAS arf1/e33cGal4 (F)UAS arf1rnai/UASarf1rnai; UAS arf1/e33cGal4 that were heated at 60 °C for 10 min to visualize the melanization response. (G) Melanized crystal cells were quantified and represented graphically. (H) Graph representing phenoloxidase activity in the hemolymph of the indicated genotypes (detected as absorbance at 490 nm) after conversion of L-3, 4-dihydroxyphenylalanine. Scale Bar: (A–F) 100 μm. Number of larvae analyzed per genotype (n = 10). Error bars show standard error of mean. P-value: ** and *** indicate P < 0.01 and P < 0.001 respectively.
Figure 2.
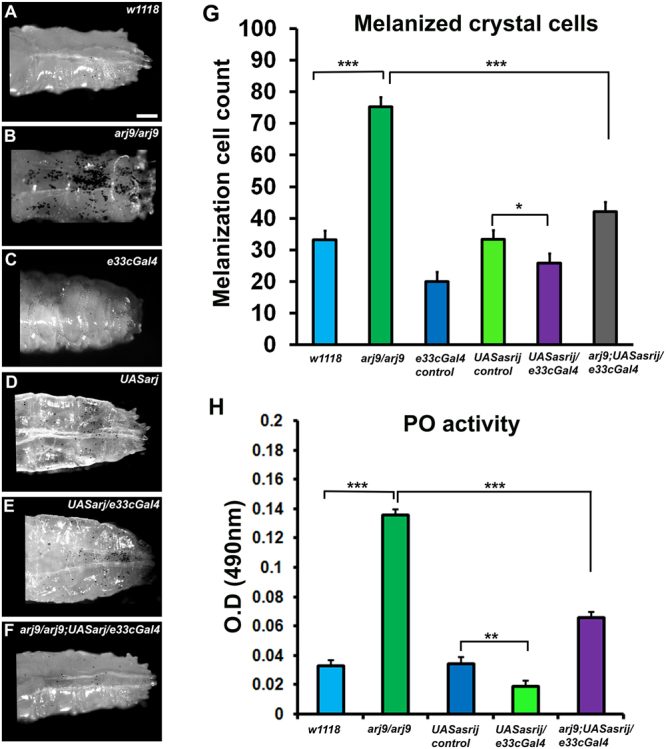
Asrij regulates crystal cell- mediated melanization and phenoloxidase activity. (A–F) Photomicrographs showing posterior region of third instar larvae of specific genotypes (A) w1118 (B) arj9/arj9 (C) e33cGal4 (D) UAS arj (E) UAS arj/e33cGal4 (F) arj9/arj9; UAS arj/e33cGal4 that were heated at 60 °C for 10 min to visualize the melanization response. (G) Melanized crystal cells were quantified and represented graphically. (H) Graph representing phenoloxidase activity in the hemolymph of the indicated genotypes (detected as absorbance at 490 nm) after conversion of L-3, 4-dihydroxyphenylalanine. Scale Bar: (A–F) 100 μm. Number of larvae analyzed per genotype (n = 10). Error bars show standard error of mean. P-value: *, **, *** indicate P < 0.05, P < 0.01 and P < 0.001 respectively.
To test crystal cell function we assayed for phenoloxidase (PO) activity. Melanin biosynthesis is brought about by PO, which catalyzes the oxidation of phenols to quinones, which subsequently polymerize into melanin. A protease cascade cleaves the inactive zymogen ProPO (PPO) to generate active PO. Both ARF1 knockdown and asrij null mutant larvae showed similarly increased PO activity (4 fold above control), which was completely restored by ARF1 overexpression but only partially restored (2 fold above control) upon Asrij over-expression (Figs 1H and 2H). Interestingly, either ARF1 or Asrij over-expression in a wild type background reduced PO activity significantly as compared to the UAS control, as expected from the reduced crystal cell number.
Lamellocytes also have the ability to produce enzymes that can trigger a melanization reaction33, 34. We have earlier shown that there is no difference in the lamellocyte counts in the asrij null mutant whereas lamellocyte counts are increased upon PxnGal4 or e33cGal4 mediated ARF1 knockdown27. We also found that over-expression of Asrij or ARF1 using e33cGal4 in the pan hemocyte population did not change circulating lamellocyte numbers (L1 positive) (Fig. S2E,F). Hence the increased melanization observed in asrij null mutant is exclusively induced by the crystal cell population. However the melanization upon ARF1 knockdown could be due to a contribution of both crystal cells and lamellocytes.
ARF1 and Asrij cooperatively regulate expression of Toll pathway AMPs
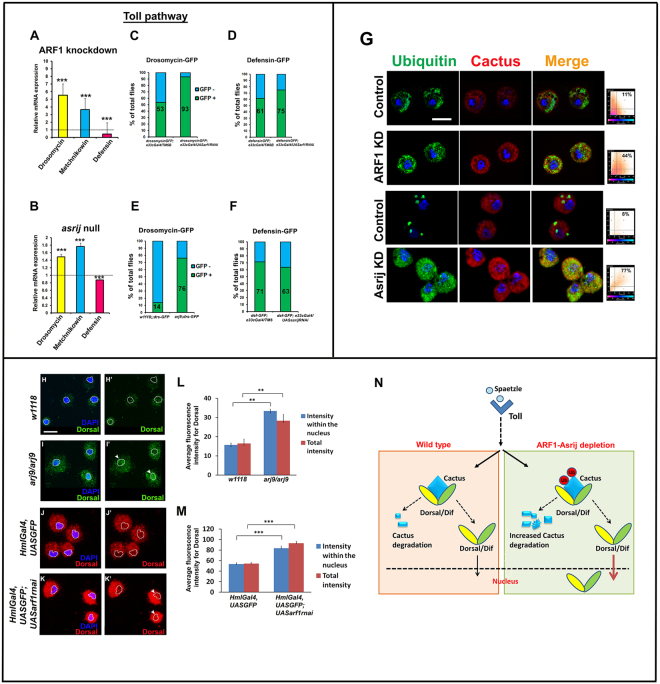
Activation of the Toll pathway results in production of a repertoire of AMPs mainly, Drosomycin in response to fungal infection and Metchnikowin and Defensin in response to infection by Gram positive bacteria35–38. Under normal conditions these peptides are expressed at very low levels. However, by quantitative polymerase chain reaction-based analysis of reverse transcribed RNA (qRT-PCR) from uninfected adult flies, we found that transcript levels of drosomycin and metchnikowin are highly upregulated in ARF1 knockdown flies (e33cGAL4 > UASarf1-RNAi) (5.5 fold and 3.5 fold respectively) compared to the GAL4 control whereas defensin expression is not significantly altered (0.5 fold) (Fig. 3A). These data indicate that ARF1 depletion leads to activation of Toll pathway target genes even in the absence of infection. Asrij mediates ARF1 function by interacting with ARF1-GTP during Drosophila hematopoiesis. Further ARF1 regulates Asrij expression and stability. Hence we also checked the effect of Asrij depletion on AMP transcript levels. In the asrij null mutant we saw modest increase in drosomycin and metchnikowin transcript levels as compared to w1118 control whereas defensin expression was slightly reduced (Fig. 3B). These data indicate that Asrij depletion also leads to differential activation of Toll pathway target genes. However a significant difference was the substantially greater effect of ARF1 depletion on drosomycin and metchnikowin transcript levels than that of Asrij depletion. This suggests that ARF1 has a wider role in regulating AMPs of the Toll pathway.
Figure 3.
ARF1 and Asrij negatively regulate Toll pathway- mediated immune response. (A,B) Quantification of Toll pathway-governed antimicrobial peptide expression by qRT-PCR analysis shows that Drosomycin and Metchnikowin are upregulated and Defensin is downregulated in arf1 knockdown (A) and asrij null mutant (B) larvae. (C–F) Quantification for the total percentile of flies expressing the Toll pathway reporters - Drosomycin-GFP and Defensin-GFP in flies with e33cGal4-mediated arf1 knockdown (C,D) or asrij null (E) or e33cGal4-mediated asrij knockdown (F) respectively. (G) Images showing increased colocalization of Cactus and Ubiquitin in arf1 or asrij knockdown circulating larval hemocytes as compared to respective controls, also indicated by adjacent co-localization plots. (H) Images showing higher Dorsal specific signal in the entire hemocyte as well as in DAPI stained region (nucleus) for the asrij null (arj9/arj9) and arf1 knockdown (HmlGal4, UASGFP; UAS arf1rnai) larvae as compared to the respective controls (w1118 and HmlGal4, UASGFP). White dotted line indicates nuclear area under consideration. Arrowheads mark nuclei with higher Dorsal signal (I,J) Quantification of the fluorescence intensity for Dorsal staining in the entire cell as well as in the DAPI stained area of the cell for asrij null (I) and arf1 knockdown hemocytes (n = 10) (J). (K) Model indicating the suggested role of the ARF1-Asrij endocytic axis in regulating the Toll pathway. Error bars indicate standard error of mean. ** indicates P < 0.01 and *** indicates P-value < 0.001. Scale Bar: (G,H) 10 μm.
We also performed in vivo reporter assays to test the activation status of representative Toll pathway AMPs using transgenic flies that would express GFP under the control of an AMP promoter upon infection. Introduction of Drosomycin-GFP or Defensin-GFP reporter in ARF1 knockdown or in asrij null or asrij knockdown background respectively was assayed after infection with B. subtilis. Quantification of the GFP+ flies showed that ARF1 knockdown, resulted in significantly more GFP positive flies for Drosomycin (93% compared to 53% in GAL4 control) and a smaller increase in Defensin-GFP (75% compared to 61% in GAL4 control) (Fig. 3C,D). Similarly asrij depletion resulted in greatly increased Drosomycin-GFP expressing flies (76% compared to 14% in control) with a small reduction in Defensin-GFP flies (63% compared to 71% in control) (Fig. 3E,F). There was no expression of the target antimicrobial peptide GFP reporters of the Toll pathway in the absence of B. subtilis infection in control as well as mutant/knockdown conditions. Thus our in vivo reporter analysis is in agreement with the mRNA expression analysis.
The ARF1-Asrij axis suppresses Toll pathway AMP production by stabilizing Cactus
Activation of the Toll pathway leads to nuclear translocation of the transcription factors Dorsal and Dif. In the absence of the Toll ligand Spaetzle, Dorsal and Dif are bound by Cactus, a negative regulator of the Toll pathway, inhibiting their activity and nuclear localization39. Receptor activation leads to phosphorylation of Cactus followed by its ubiquitination and degradation, thus releasing Dorsal/Dif to translocate to the nucleus for target AMP gene activation. Since Toll pathway AMPs were upregulated in both Asrij and ARF1 depleted conditions, we probed the status of ubiquitinated Cactus in these conditions. Depletion of ARF1 or Asrij led to increased co-localization of Cactus with Ubiquitin in the circulating hemocytes as well as the fat bodies (Fig. 3G, Fig. S2). ARF1 depletion increased the co-localization in hemocytes to 44% from 11% in control, whereas asrij null hemocytes showed 77% co-localization compared to 8% in control. There was also increased co-localization (22.5%) of Cactus and Ubiquitin in the asrij null mutant fat body cells as compared to the w1118 controls (3%), whereas HmlGal4 mediated ARF1 knockdown larval fat body cells showed (7.5%) co-localization as compared to its respective controls (0.4%) (Fig. S2A,B). Analysis of the adult fat body cells also showed increased cactus and ubiquitin levels in asrij null mutant but not in HmlGal4 mediated ARF1 knockdown adult fat body cells (Fig. S2C,D). This suggests that Cactus may be increasingly targeted for degradation when the ARF1-Asrij axis is perturbed in the larval stage. However additional mechanisms may operate in the adult fat body that compensate the loss of ARF1.
Cactus degradation should lead to increased translocation of the Toll pathway effectors Dorsal/Dif to the nucleus. Since Dorsal translocation is essential for AMP production, we stained for Dorsal in asrij null and ARF1 knockdown hemocytes. There was increased translocation of Dorsal to the nucleus as compared to the control hemocytes (Fig. 3H–M). This indicates increased Toll pathway activity is achieved by promoting nuclear translocation of Dorsal. Thus the ARF1-Asrij axis can maintain homeostatic conditions of Toll signaling by stabilizing Cactus and preventing aberrant AMP production (Fig. 3N).
Differential effect of ARF1 and Asrij on Imd pathway AMPs
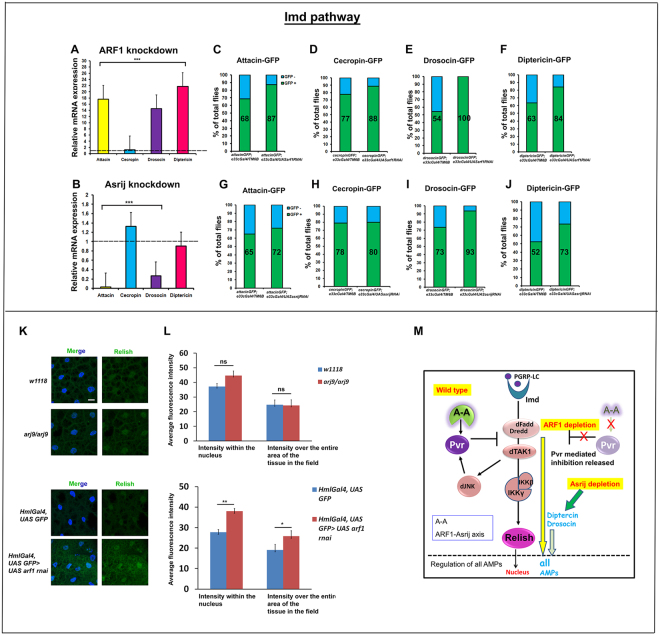
Infection with Gram negative bacteria brings about nuclear localization of the NF-κB transcription factor Relish, thus activating its main target AMP, Diptericin35, 36, 38. The other Imd pathway target genes include attacin A1, cecropin A1 and drosocin. We analyzed the effect of ARF1 depletion on the transcript levels of Imd pathway targets and found that they were constitutively upregulated in ARF1 knockdown flies. Attacin, drosocin and diptericin transcripts were highly upregulated (~18 fold, ~15 fold and ~22 fold) whereas cecropin levels showed modest increase (1.25 fold) as compared to the parental control (Fig. 4A). Upon in vivo AMP-GFP reporter analysis after infection with E. coli, ARF1 knockdown showed a dramatic up-regulation of the percent of GFP positive flies for Attacin, Cecropin, Drosocin and Diptericin (87.5%, 88.8%, 100% and 84.21% respectively) as compared to Gal4 control (68.75%, 77.7%, 54.5% and 63.6% respectively) (Fig. 4C–F).
Figure 4.
ARF1 and Asrij differentially regulate the Imd pathway. (A,B) Quantification of Imd pathway-governed antimicrobial peptide expression by qRT-PCR analysis shows that Attacin, Drosocin and Diptericin are highly up-regulated whereas Cecropin levels are unaffected in e33cGal4-mediated ARF1 knockdown flies (A). Cecropin levels are upregulated and Attacin, Drosocin, Diptericin levels are down-regulated in e33cGal4-mediated Asrij knockdown flies (B). (C–J) Quantification of the total percentile of flies expressing the Imd pathway reporters - Attacin-GFP, Cecropin-GFP, Drosocin-GFP and Diptericin-GFP in flies with e33cGal4 mediated ARF1 knockdown (C–F) or asrij knockdown (G–J) respectively. (K) Images showing unchanged intensity of Relish in the nucleus of arj9/arj9 larval fat body but increase in the intensity in arf1 knockdown larval fat body compared to the respective controls. Scale bar 10 µm. (L) Quantification of Relish intensity over the entire area of the tissue in the field of view as well as in the nucleus (DAPI stained area) in arj9/arj9 and arf1 knockdown fat bodies and in the respective controls (n = 10). Error bar represents standard error of mean. ns indicates statistically non-significant difference. * and ** indicates P-value < 0.05 and <0.01 respectively. (M) Model indicating the suggested role of the ARF1-Asrij endocytic axis in regulating the Imd pathway.
In contrast to the effect of ARF1 depletion, Asrij null mutants showed no significant change in expression of diptericin transcipts, which is a standard indicator of Imd activation (Fig. 4B). However, while levels of attacin and drosocin were reduced, cecropin transcript levels were marginally affected (1.4 fold) and comparable to ARF1 knockdown flies. In vivo AMP-GFP reporter analysis upon E. coli infection for the Imd pathway governed AMPs showed no significant change in GFP+ flies for Attacin and Cecropin (72.22%, 80% in knockdown compared to 65%, 78.94% in controls respectively) whereas Drosocin and Diptericin GFP+ flies were significantly higher (93.75% and 73.68% in knockdown compared to 73.68% and 52.63% in controls respectively) (Fig. 4G–J). There was no expression of the target antimicrobial peptide GFP reporters of the Imd pathway in the absence of E. coli infection in both control or mutant/knockdown conditions. Imd pathway activation results in nuclear localization of Relish and thereby upregulation of AMP genes. Hence, we also looked at Relish localization, as nuclear Relish is indicative of pathway activation. Relish staining has not been reported in hemocytes and we could not detect any specific signal by immunostaining. However fat bodies from infected control flies show nuclear Relish as reported36. Since systemic signals as well as cross talk between the hemocytes and fat body bring about immune regulation, we assayed for Relish nuclear localization in fat bodies of larvae depleted of asrij (null) or ARF1 (HmlGAL4-arf1 RNAi). asrij null fat bodies showed no apparent change in nuclear Relish as compared to the parental control whereas ARF1 depleted fat bodies showed increased nuclear Relish, indicating pathway activation (Fig. 4K,L). This is in agreement with the increase in AMP levels seen in ARF1 depleted flies, whereas Asrij mutants show no significant change for most AMPs. Thus while ARF1 can regulate all Imd pathway AMPs tested, Asrij has a milder differential effect.
These data show that both ARF1 and Asrij have major non-overlapping roles in regulating Imd pathway AMP expression. ARF1 is a generic negative regulator of the Imd pathway, as its depletion leads to heightened levels of all the target AMPs. While there is only a small change in diptericin or cecropin transcript levels upon Asrij depletion, Diptericin peptide levels are higher indicating Asrij normally checks Diptericin levels, possibly post-transcriptionally. While Asrij positively regulates attacin and drosocin transcript expression, the effect on Attacin AMP levels was not significant. However there was a dramatic increase in Drosocin-GFP flies. This indicates that Asrij shows differential/discriminatory effects on AMP production (Fig. 4M). Thus co-ordinated and complementary regulation of Imd targets by the ARF1-Asrij axis is essential to maintain immune homeostasis.
Survival and lifespan of Asrij or ARF1- depleted flies is compromised upon acute bacterial infection
Impaired AMP production upon infection can lead to reduced survival due to an inability to combat the bacterial infection. Loss-of-function mutations in the Toll and Imd pathway effectors such as Dif and Relish40 lead to reduced ability to combat infections. Earlier studies show that Asrij is epistatic to ARF1 and depends on ARF1 for its stability27. While they both similarly regulate the Toll pathway, differential regulation of the Imd pathway suggests complex control on AMP production. Hence we tested the effect of Asrij or ARF1 depletion on the ability of flies to combat infection and survive.
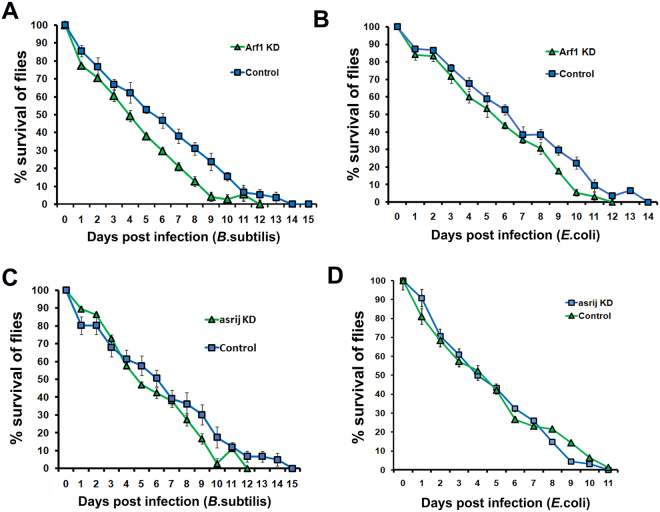
Upon infection with B. subtilis, while 80% control flies continued to survive after 48 hrs with a gradual reduction in number over subsequent days, mutant numbers rapidly declined after 48 hrs and the mutant population perished 3–4 days earlier than controls. This resulted in a steep decrease in % survival upon infection as compared to the Gal4 and w1118 controls respectively (Fig. 5A,C). Thus the increased production of the Toll pathway AMP Drosomycin in the absence of ARF1 or Asrij does not protect lifespan upon Gram positive bacterial infection.
Figure 5.
ARF1 and Asrij knockdown flies show compromised survival upon infection. (A–D) Survival curves showing that e33cGal4-mediated ARF1 or asrij knockdown flies show a reduced survival ability as compared to its control upon infection with either B. subtilis (A,C) or E. coli (B,D). At least 100 flies were tested per genotype over at least three independent experiments.
While ARF1 depletion results in increased production of all Imd pathway AMPs, asrij depletion caused limited and differential activation of Imd pathway AMPs. Upon infection with E. coli, flies depleted of ARF1 or Asrij, both showed compromised survival (Fig. 5B,D). All mutant genotypes perished before controls, indicating that increased AMP production does not confer the ability to combat infection.
In order to know if the reduction in survival of the asrij null flies upon infection is solely due to defect in the hematopoietic system or due to reduction of overall tolerance of the flies, we depleted Asrij specifically in the hemocytes using Hemolectin-Gal4 (HmlGal4) or trachea using breathless-Gal4 (btlGal4) where Asrij was also seen to be expressed in the embryo41. Trachea specific knockdown of Asrij does not reduce the survival significantly as compared to the control upon infection with both B. subtilis or E. coli (Fig. S3A,B). However, hemocyte specific knockdown of Asrij leads to reduction in survival of the flies (Fig. S3C,D). This indicates that the effect of loss of Asrij on the survival is a consequence of malfunctioning of the hematopoietic system and not due to its absence in other organs like trachea.
Further, we also tested whether ARF1 depletion in the hemocyte compartment specifically or in other organs like trachea has any effect on the survival of the flies post infection. Survival of flies depleted of ARF1 in the trachea (Fig. S3E,F) is reduced with or without infection. Also, the flies depleted of ARF1 in the hemocyte compartment have reduced survival post-infection (Fig. S3G,H). This indicates that while Asrij and ARF1 function in the hematopoietic system is essential for regulating the immune response, ARF1 depletion from other organs may deteriorate the overall health of the flies and make them more susceptible to infection. Given its ubiquitous expression, ARF1 may also mediate its effect through other interactors that are involved in responding to infection.
Asrij expression is downregulated upon Gram negative bacterial infection
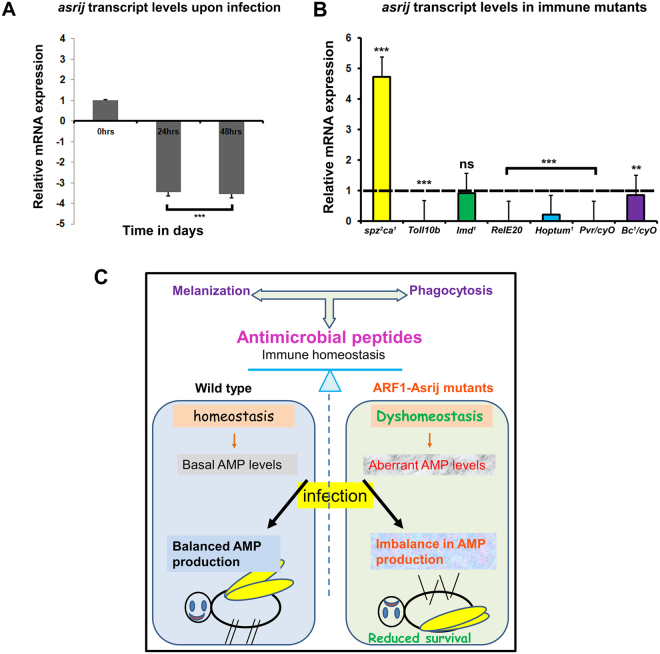
Immune response in normal animals requires AMP upregulation to combat infection6, 8, 42. ARF1 and Asrij have opposite effects on the Imd pathway, suggesting that Asrij acts independent of ARF1 in Imd pathway regulation. However, increased AMP levels in ARF1 knockdown do not provide additional ability to combat infection. Additionally, Asrij is downstream of ARF1 and reduces AMP levels. Therefore Asrij expression levels positively correlate with Imd pathway target AMP transcript levels. Hence we checked the status of Asrij expression upon immune challenge. Wild type flies infected with E. coli showed reduced asrij transcript levels (Fig. 6A). This indicates that asrij levels co-relate with Imd pathway activation.
Figure 6.
Asrij expression is modulated upon infection and in immune mutants. (A,B) qRT-PCR analysis of adult flies showing that asrij mRNA levels are down-regulated 24 and 48 hours post infection (A) and that asrij transcript levels vary among immune mutants (B). (C) Model illustrating loss of immune homeostasis in ARF1-Asrij depleted conditions leading to compromised survival of the flies.
Asrij levels are differentially regulated in immune pathway mutants
Asrij levels are downregulated upon bacterial infection and survival is compromised in Asrij depleted conditions indicating an important role for Asrij in mounting an immune response. Since pathway activation and attenuation are both important for maintaining homeostasis, it is likely that Asrij expression is in turn regulated by components of the Toll or Imd pathways as well as other pathways like Jak/Stat and Pvr. To test this we assayed asrij transcript levels in a range of immune pathway mutants, which either inactivate or activate the pathway. We found that asrij levels are upregulated in Toll pathway mutants like spz rm7 whereas they are downregulated in Toll pathway gain-of-function mutants like Toll 10b (Fig. 6B). This is in agreement with increased ubiquitination of cactus, suggesting a requirement for the ARF1-Asrij axis in regulating Toll pathway activity possibly via a feed-forward loop.
Asrij levels are downregulated in Imd pathway mutants like Rel E20 (Imd effector) suggesting that asrij may be a target of relish. Also mutants of the other pathways like Hop Tum1 (Jak gain of function) and Pvr (Pvr/Cyo) show reduced Asrij levels (Fig. 6B). There was no significant downregulation of asrij levels in Imd and Black cells mutant. This suggests that asrij is differentially regulated by downstream effectors of the Toll and Imd immune pathways. While the Toll pathway suppresses asrij expression, Imd pathway promotes asrij expression. Taken together with our data that Asrij levels are reduced upon E. coli infection this suggests that Asrij expression may be dependent on Imd pathway activation.
Discussion
A balanced cellular and humoral immune response is essential to achieve and maintain immune homeostasis20, 43, 44. In Drosophila, aberrant hematopoiesis and impaired hemocyte function can both affect the ability to fight infection and maintain immune homeostasis. Endosomal proteins are known to regulate Drosophila hematopoiesis27, 30. Here, we show an essential function for endosomal proteins in regulating immunity.
Altered hemocyte number and distribution as a result of defective hematopoiesis, can also lead to immune phenotypes like increased melanization or phagocytosis. We illustrate that perturbation of normal levels of endocytic molecules ARF1 or Asrij leads to aberrant hematopoiesis, affecting the circulating hemocyte number27, 30. We show that this in turn leads to an impaired cellular immune response. The aberrant hematopoietic phenotypes with pan-hemocyte tissue-specific depletion of ARF1 using e33cGal4 or HmlGal4 are comparable to the phenotypes observed in the case of asrij null mutant. Hence we have compared Gal4-mediated ARF1 knockdown to asrij null mutant in this study.
In addition, we also show that ARF1 and Asrij have a direct role in humoral immunity by regulating AMP gene expression. This is likely to be a contribution from the hemocyte compartment which is primarily affected upon perturbation of Asrij or ARF1. It is well established that hemocytes, apart from acting as the cellular arm of the immune response, also act as sentinels and relay signals to the immune organs that mount the humoral immune response. Hemocytes have been shown to produce ligands like Spaetzle and upd3 that activate immune pathways and induce anti-microbial peptide secretion from the fat body or gut16, 45. Asrij or ARF1 could also be affecting the production of such ligand molecules thereby affecting the target immune-activation pathways.
Considering the involvement of Asrij and ARF1 in both the arms of immune response, we propose a model for the role of the ARF1-Asrij axis in maintaining immune homeostasis (Fig. 6C) that can be used for testing additional players in the process.
It is known that ARF1 is involved in clathrin coat assembly and endocytosis46, 47 and has a critical role in membrane bending and scission42. In this context it is also intriguing to note that ARF1, like Asrij, does not seem to have an essential role in phagocytosis. This suggests that hemocytes could be involved in additional mechanisms beyond phagocytosis in order to combat an infection.
Both ARF1 and Asrij control hemocyte proliferation as their individual depletion leads to an increase in the total and differential hemocyte counts. Also, both mutants have higher crystal cell numbers due to over-activation of Notch as a result of endocytic entrapment27, 30. This suggests that increased melanization accompanied by increase in phenoloxidase activity upon ARF1 or Asrij depletion is a consequence of aberrant hematopoiesis and not likely due to a cellular requirement in regulating the melanization response. Constitutive activation of the Toll pathway or impaired Jak/Stat or Imd pathway signaling in various mutants also leads to the formation of melanotic masses36. Thus the phenotypes seen on Asrij or ARF1 depletion could either be due to the defective hematopoiesis which directly affects the cellular immune response or leads to a mis-regulation of the immune regulatory pathways.
Regulation of many signaling pathways, including the immune regulatory pathways takes place at the endosomes48–51. For example, endocytic proteins Mop and Hrs co-localize with the Toll receptor at endosomes and function upstream of MyD88 and Pelle, thus indicating that Toll signalling is regulated by endocytosis50. Our study shows that loss of function of the ARF1-Asrij axis leads to an upregulation of some AMP targets of the Toll pathway. Upon depletion of ARF1-Asrij endosomal axis we find increased ubiquitination of Cactus, a negative regulator of the Toll pathway, in both hemocytes as well as fat bodies. This suggests non-autonomous regulation of signals by the ARF1-Asrij axis, which is in agreement with our earlier model of signalling through this route (Khadilkar et al. 27). Thus the endosomal axis may systemically control the sorting and thereby degradation of Cactus, which in turn promotes the nuclear translocation of Toll effector, Dorsal. This could explain the significant increase in Toll pathway reporter expression such as Drosomycin-GFP. Interestingly the effect of ARF1 depletion on the Toll pathway is more pronounced than that of Asrij depletion. This is not surprising as ARF1 is a ubiquitous and essential trafficking molecule that regulates a variety of signals. This suggests that ARF1 is likely to be involved with additional steps of the Toll pathway and may also interact with multiple regulators of AMP expression.
ARF1 and Asrij show complementary effects on IMD pathway target AMPs. While ARF1 suppresses the production of IMD pathway AMPs, Asrij has a discriminatory role. Asrij seems to promote transcription of AttacinA and Drosocin, whereas it represses Cecropin. However in terms of AMP production only Drosocin and Diptericin are affected, but not to the extent of ARF1. In addition, Relish shows marked nuclear localization in fat body cells of hemocyte-specific arf1 knockdown larvae whereas there is no significant difference in the localization in Asrij depleted larval fat bodies. This indicates that ARF1-Asrij axis exerts differential control over the Imd pathway. Thus ARF1 causes strong generic suppression of the Imd pathway while the role of Asrij could be to fine tune this effect. Mass spectrometric analysis of purified protein complexes indicates that ARF1 and Imd interact52 (Drosophila Protein Interaction Mapping Project, https://interfly.med.harvard.edu). Hence it is very likely that ARF1 regulates Imd pathway activation at the endosomes. Whether this interaction involves Asrij or not remains to be tested and will give insight into modes of differential activation of immune pathways.
Our analysis shows that Asrij is the tuner for endosomal regulation of the humoral immune response by ARF1 and provides specialized tissue- specific and finer control over AMP regulation. This is in agreement with earlier data showing that Asrij acts downstream of ARF127. Since ARF1 is expressed in the fat body27 it could communicate with the hemocyte- specific molecule, Asrij, to mediate immune cross talk.
As we see reduced Asrij expression in Toll and Jak/Stat pathway mutants such as Rel E20 and Hop Tum1, it is likely that these effectors also regulate Asrij, setting up a feedback mechanism to modulate the immune response. We have earlier shown that ARF1-Asrij axis modulates different signalling outputs like Notch by endosomal regulation of NICD (Notch Intracellular Domain) transport and activity and JAK/STAT by endosomal activation of Stat92e. Further, ARF1 along with Asrij regulates Pvr signaling in order to maintain HSC’s27, 30. ARF1 acts downstream of Pvr27. Surprisingly, Asrij levels are downregulated in the Pvr mutant. Hence it is likely that the ARF1-Asrij axis regulates trafficking of the Pvr receptor, which then also regulates Asrij levels thus providing feedback regulation. While active modulation of signal activity and outcome at endosomes could be orchestrated by ARF1 and Asrij, their activities in turn need to be modulated. Our data suggest that targets of Asrij endosomal regulation may in turn regulate Asrij expression at the transcript level. Further, upon Gram positive infection in wild type flies, asrij transcript levels decrease with a concomitant increase in suppressed AMPs such as Cecropin. This indicates additional regulatory loops such as that mediated by the IMD pathway effector NFkB may regulate asrij transcription. Using bioinformatics tools, we do see presence of binding sites for NFκβ and Rel family of transcription factors in the upstream regulatory sequence (1 kb upstream) of asrij and arf1. Hence, we propose feedback regulation of Asrij and ARF1 by the effectors of the Toll and Imd pathway respectively. This is reflected in the regulation of Asrij expression by these pathways. This also implies multiple modes of regulation of asrij and arf1, which are likely important in its role as a tuner of the generic immune response, thereby allowing it to discriminate between AMPs that were thought to be uniformly regulated, such as those downstream of IMD. Thus our analysis gives insight into additional complex regulation of the Drosophila immune response that can now be investigated further.
Asrij and ARF1 being endocytic proteins are likely to interact with a number of molecules that regulate different cell signalling cascades. Due to endosomal localization, molecular interactions may be favored that further translate into signalling output. Hence, it is not surprising that Asrij and ARF1 genetically interact with multiple signalling pathways and can aid crosstalk to regulate important developmental and physiological processes like hematopoiesis or immune response. It is quite likely that Asrij and ARF1 are themselves also part of different feedback loops or feed-forward mechanisms as their levels need to be tightly regulated. We find evidence for this with respect to the Toll, JAK/STAT and Pvr pathway as described earlier. Hence we propose that the Asrij-ARF1 endosomal signalling axis genetically interacts with various signalling components thereby regulating blood cell and immune homeostasis.
AMP transcript level changes upon ARF1 or Asrij depletion also correspond to reporter-AMP levels seen after infection. This suggests that although ARF1 is known to have a role in secretion, mutants do not have an AMP secretion defect. Hence aberrant regulation of immune pathways on perturbation of the ARF1-Asrij axis is most likely due to perturbed endosomal regulation.
ARF1 has a ubiquitous function in the endosomal machinery46 and is well-positioned to regulate the interface between metabolism, hematopoiesis and immunity in order to achieve homeostasis. Along with Asrij and other tissue-specific modulators, it can actively modulate the metabolic and immune status in Drosophila. In this context, it is interesting to note that Asrij is a target of MEF253, which is required for the immune-metabolic switch in vivo 54. Thus Asrij could bring tissue specificity to ARF1 action, for example, by modulating insulin signalling in the hematopoietic system.
It is likely that in Asrij or ARF1 mutants, the differentiated hemocytes mount a cellular immune response and perish as in the case of wild type flies where immunosenescence sets in with age and the ability of hemocytes to combat infection declines55. Since their hematopoietic stem cell pool is exhausted, they may fail to replenish the blood cell population, thus compromising the ability to combat infections. Alternatively, mechanisms that downregulate the inflammatory responses and prevent sustained activation43, 56 may be inefficient when the trafficking machinery is perturbed. This could result in constitutive upregulation thus compromising immune homeostasis56, 57.
In summary, we show that in addition to its requirement in hematopoiesis, the ARF1-Asrij axis can differentially regulate humoral immunity in Drosophila, most likely by virtue of its endosomal function. ARF1 and Asrij bring about differential endocytic modulation of immune pathways and their depletion leads to aberrant pathway activity and an immune imbalance. In humans, loss of function mutations in molecules involved in vesicular machinery like Amphyphysin I in which clathrin coated vesicle formation is affected leads to autoimmune disorders like Paraneoplastic stiff-person syndrome58. Synaptotagmin, involved in vesicle docking and fusion to the plasma membrane acts as an antigenic protein and its mutation leads to an autoimmune disorder called Lambert-Eaton myasthenic syndrome59. Mutations in endosomal molecules like Rab27A, β subunit of AP3, SNARE also lead to immune diseases like Griscelli and Hermansky-Pudlak syndrome60, 61. Mutants of both ARF1 and Asrij are likely to have drastic effects on the immune system. Asrij has been associated with inflammatory conditions such as arthritis, thyroiditis, endothelitis and tonsillitis (http://www.malacards.org/card/tonsillitis?search=OCIAD1), whereas the ARF family is associated with a wide variety of diseases. ARF1 has been shown to be involved in mast cell degranulation and IgE mediated anaphylaxis response62. Generation and analysis of vertebrate models for these genes such as knockout and transgenic mice will provide tools to understand their function in human immunity.
Materials and Methods
Drosophila Stocks
All fly stocks were maintained at standard rearing conditions. Respective UAS or Gal4 parent strains or w1118 (asrij null mutant) were used as controls. Tissue specific Gal4 promoter line was used to drive the expression of UAS responder genes. Following fly lines were used: arj 9/arj 9, UAS-asrij (Kulkarni, Khadilkar et al. 30), Rel E20, Black cells (Bc 1/CyO), Hop Tum1, Imd 1 (NCBS stock centre), Pvr/Cyo (Pernille Rorth, Denmark), Gal4 driver lines e33cGAL4 (Kathryn Anderson, NY, USA), HmlGal4, UASGFP (Tina Mukherjee, inStem), UAS-arf1 (Khadilkar et al.27), btl-Gal4 (Arjun Guha, inStem), UASarf1rnai (VDRC), GFP reporter flies for Toll and Imd pathway (David Ferrandon, France).
Antibodies used
Mouse anti-Dorsal (1:50, 7A4, DSHB), mouse anti-Relish (1:10, 21F3, DSHB), mouse anti-L1 antibody (1:50 gift from Istvan Ando), Mouse anti-Cactus (1:25 3H12, DSHB), Rabbit anti-ubiquitin (1:100 ab7780, Abcam), Rabbit anti-ubiquitin (1:100, ab19247, Abcam).
In vitro Larval Phagocytosis assay
Primary hemocyte cultures were prepared as described earlier63. Briefly, third instar larvae were surface sterilized, and hemolymph was collected by puncturing the integument using dissection forceps into 150 μl of 1X PBS (Phosphate Buffer Saline) in 35-mm coverslip-bottom dishes and incubated with India Ink (HIMEDIA, India) for 10 min followed by 5 washes with PBS. After 1 hour hemocyte preparations were fixed with 2.5% paraformaldehyde for 20 min and imaged. Phagocytosis of India ink particles by primary hemocytes was assessed using Zeiss LSM510 meta.
In-vivo adult phagocytosis assay
20 adult flies of each genotype were injected with Rhodamine conjugated heat-killed E. coli in the abdomen. After 1.5 hours, the flies were bled to collect the hemolymph. The hemocytes were kept for 30 min in sterile Schneider’s media for attachment. After 30 min, the cells were gently washed with 1X PBS to remove extracellular bacteria. Hemocytes were fixed using 4% para-formaldehyde for 20 minutes. Hemocytes were identified by expression of GFP driven by HmlGal4. Bacterial particles detected in the z-sections of the hemocyte images acquired were considered for quantification of phagocytic events. Experiments were repeated at least three times with biological and technical replicates. Images were acquired using Zeiss LSM510 meta confocal microscope.
Circulating hemocyte and fat body staining
Larvae were bled in Schneider’s media and the hemolymph was plated on a cover-slip bottom dish for attachment for 45 minutes. Hemocytes were fixed in 4% para-formaldehyde for 20 minutes followed by wash with PBS. Cells were then permeabilized with 0.4% NP40 and blocked with 20% goat serum for 1 hour. The preps were incubated with primary antibodies at 4 °C overnight. Secondary antibody staining was performed following this and the hemocytes were incubated with DAPI to mark the nuclei. Images were taken in LSM510 Meta Confocal microscope. For hemocyte count, images were taken in Olympus IX81 microscope.
Larvae or adult flies were dissected in 1X PBS to isolate the fat body. The fat body preps were fixed in 4% para-formaldehyde for 20 minutes followed by wash with PBS, permeabilized with 0.1% Triton-X 100 and blocked with 20% normal goat serum for 1 hour. The preps were then incubated with primary antibodies at 4 °C overnight. Secondary antibody staining was then performed using Alexa-488 conjugated anti-mouse IgG and Alexa-568 tagged anti-rabbit IgG antibodies (Life Technologies). The fat body preps were mounted in DAPI. Images were taken in Zeiss LSM510 Meta and LSM880 confocal Microscope. Autofluorescence was taken care of by optimizing the confocal microscope settings using the no primary antibody control.
Crystal cell melanization assay
Crystal cells are characterized by crystalline inclusions that contain the zymogen ProPO and can be visualized due to specific blackening upon heating larvae at 60 °C for 10 min64. Third instar wandering stage larvae were heat treated to visualize crystal cells and imaged using SZX12 stereozoom microscope (Olympus). Melanised crystal cell were counted from three posterior abdominal segments of at least 20 larvae per genotype. Error bars represent the standard deviation. P-values were calculated using one way ANOVA.
Prophenol oxidase activity assay
For the measurement of PO activity by dot blots, 5 µl hemolymph was applied to a filter paper pre-soaked with 20 mM L-DOPA (L-3, 4-dihydroxyphenylalanine- Cat. No. D9628, SIGMA) in phosphate buffer pH 6.6, incubated for 20 minutes at 37 °C and heated in a microwave till the paper was dried completely65. The melanised black hemolymph spots correlate with PO activity in hemolymph and were imaged under an Olympus SZX12 stereozoom microscope.
For photometric measurement of PO activity, 10 µl hemolymph from each strain was pooled on ice by quickly bleeding 3–5 larvae and withdrawing 6 µl hemolymph. Aliquots of mixed hemolymph were activated at 25 °C for 10 minutes, then 40 µl L-DOPA was added and optical density (OD) measured from 0 to 30 minutes at 490 nm with a VmaxTM Kinetic Microplate Reader (BIO-RAD). Activation of PO was measured as the relative change in absorbance (A490). Experiments were repeated at least three times with biological and technical replicates.
RNA extraction and Quantitative Real Time PCR
Drosophila larvae or adults were collected in TRIzol (TRIzol® Reagent, Cat. No. 15596-026, Invitrogen), homogenized for 30–60 seconds and centrifuged. The supernatant was processed for RNA extraction according to the manufacturer’s protocol. RNA was quantified by spectrophotometry and quality was analyzed by agarose gel electrophoresis. Quantitative RT-PCR (qRT-PCR) was performed using SYBR green chemistry in a Rotor Gene 3000 (Corbett Life Science) and analysed with the accompanying software. Primer sequences used for RT-PCR and qRT-PCR are provided in supplementary information (Supplementary Table S1).
Infection and survival assay
Briefly, prior to infection, adult flies of appropriate genotype were starved for 2 hr, then transferred into vials containing filter paper hydrated with 5% sucrose solution mixed with concentrated Ampicillin resistant E. coli (A600 = 1; concentrated to contain ~10 CFU/ml) or Gram positive bacteria (B. subtilis) A600 = 5–10) on cornmeal food. Following incubation at 25 °C for 24 hr, flies were processed for RNA extraction or examined for reporter-GFP expression. For survival assay, flies were challenged with bacteria by oral feeding. Adult flies were starved for 48 hours and then transferred to fresh corn-meal food vials containing fresh filter paper disks inoculated with bacterial cultures. The percentage of survivors was then calculated for each experiment and plotted as a survival curve. (N = 100) for each genotype. Reporter-GFP expressing flies were imaged on an SZX12 stereozoom microscope (Olympus) and processed uniformly for brightness/contrast using Adobe Photoshop CS3.
Statistical analysis
For all the survival assays, 100 flies were used and the assays were repeated thrice. For the melanization experiment, crystal cells were counted from three posterior abdominal segments of at least 20 larvae per genotype. Phenoloxidase assay was repeated thrice with biological and technical replicates. For hemocyte and fat body staining, 10 larvae of each genotype were taken. For hemocyte staining, quantification of fluorescence intensity was done for at least 30 cells per genotype. All the data sets were included for analysis. There was no randomization done. Extreme outliers were excluded for the crystal cell count analysis. For lamellocyte count, L1 staining was done thrice with 10 larvae per genotype each time. Student’s t-test with unequal variances has been used for statistical analysis. P-values are as indicated in the graphs.
Electronic supplementary material
Acknowledgements
This work was funded by a grant to MSI from the Department of Science and Technology, Government of India and by intramural funds from the Jawaharlal Nehru Centre for Advanced Scientific Research.
Author Contributions
R.J.K., A.R., C.D.R., A.R.C.S., S.S.M., V.K. and M.S.I. performed experiments; R.J.K., A.R. and M.S.I. analyzed data and wrote the manuscript; M.S.I. obtained funding and facilities for the work.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Rohan J. Khadilkar and Arindam Ray contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00118-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Evans C, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Developmental Cell. 2003;5:673–690. doi: 10.1016/S1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 2.Cerenius L, Soderhall K. The prophenoloxidase-activating system in invertebrates. Immunol Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 3.De Gregorio E, et al. An Immune-Responsive Serpin Regulates the Melanization Cascade in Drosophila. Developmental Cell. 2002;3:581–592. doi: 10.1016/S1534-5807(02)00267-8. [DOI] [PubMed] [Google Scholar]
- 4.Ligoxygakis P. A serpin mutant links Toll activation to melanization in the host defence of Drosophila. The EMBO Journal. 2002;21:6330–6337. doi: 10.1093/emboj/cdf661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun A, Hoffmann J, Meister M. Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proceedings of the National Academy of Sciences. 1998;95:14337–14342. doi: 10.1073/pnas.95.24.14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulet P. Antimicrobial peptides in insects; structure and function. Developmental & Comparative Immunology. 1999;23:329–344. doi: 10.1016/S0145-305X(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 7.Buchon N, Broderick N, Poidevin M, Pradervand S, Lemaitre B. Drosophila Intestinal Response to Bacterial Infection: Activation of Host Defense and Stem Cell Proliferation. Cell Host & Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Kounatidis I, Ligoxygakis P. Drosophila as a model system to unravel the layers of innate immunity to infection. Open Biology. 2012;2:120075–120075. doi: 10.1098/rsob.120075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- 10.Bond D, Foley E. A Quantitative RNAi Screen for JNK Modifiers Identifies Pvr as a Novel Regulator of Drosophila Immune Signaling. PLoS Pathog. 2009;5:e1000655. doi: 10.1371/journal.ppat.1000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemaitre B, Hoffmann J. The Host Defense of Drosophila melanogaster. Annual Review of Immunology. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal K, Silverman N. Positive and negative regulation of the Drosophila immune response. BMB Reports. 2008;41:267–277. doi: 10.5483/BMBRep.2008.41.4.267. [DOI] [PubMed] [Google Scholar]
- 13.Brennan C, Delaney J, Schneider D, Anderson K. Psidin Is Required in Drosophila Blood Cells for Both Phagocytic Degradation and Immune Activation of the Fat Body. Current Biology. 2007;17:67–72. doi: 10.1016/j.cub.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Shia A, et al. Toll-dependent antimicrobial responses in Drosophila larval fat body require Spatzle secreted by haemocytes. Journal of Cell Science. 2009;122:4505–4515. doi: 10.1242/jcs.049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paddibhatla I, Lee M, Kalamarz M, Ferrarese R, Govind S. Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Drosophila Larvae. PLoS Pathog. 2010;6:e1001234. doi: 10.1371/journal.ppat.1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti S, et al. Remote Control of Intestinal Stem Cell Activity by Haemocytes in Drosophila. PLoS Genet. 2016;12:e1006089. doi: 10.1371/journal.pgen.1006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khush R, Leulier F, Lemaitre B. Drosophila immunity: two paths to NF-κB. Trends in Immunology. 2001;22:260–264. doi: 10.1016/S1471-4906(01)01887-7. [DOI] [PubMed] [Google Scholar]
- 18.Khush R, Cornwell W, Uram J, Lemaitre B. A Ubiquitin-Proteasome Pathway Represses the Drosophila Immune Deficiency Signaling Cascade. Current Biology. 2002;12:1728–1737. doi: 10.1016/S0960-9822(02)01214-9. [DOI] [PubMed] [Google Scholar]
- 19.Kambris Z, et al. Drosophila Immunity: A Large-Scale In Vivo RNAi Screen Identifies Five Serine Proteases Required for Toll Activation. Current Biology. 2006;16:808–813. doi: 10.1016/j.cub.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Becker T, et al. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463:369–373. doi: 10.1038/nature08698. [DOI] [PubMed] [Google Scholar]
- 21.Scita G, Di Fiore P. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 22.Dobrowolski, R. & De Robertis, E. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nature Reviews Molecular Cell Biology doi:10.1038/nrm3244 (2011). [DOI] [PMC free article] [PubMed]
- 23.Sehgal P, Guo G, Shah M, Kumar V, Patel K. Cytokine Signaling: STATS in plasma membrane rafts. Journal of Biological Chemistry. 2002;277:12067–12074. doi: 10.1074/jbc.M200018200. [DOI] [PubMed] [Google Scholar]
- 24.Shah M, et al. Membrane-associated STAT3 and PY-STAT3 in the Cytoplasm. Journal of Biological Chemistry. 2005;281:7302–7308. doi: 10.1074/jbc.M508527200. [DOI] [PubMed] [Google Scholar]
- 25.Sehgal P. Paradigm shifts in the cell biology of STAT signaling. Seminars in Cell & Developmental Biology. 2008;19:329–340. doi: 10.1016/j.semcdb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha A, Khadilkar R, S. V, RoyChowdhury Sinha A, Inamdar M. Conserved Regulation of the JAK/STAT Pathway by the Endosomal Protein Asrij Maintains Stem Cell Potency. Cell Reports. 2013;4:649–658. doi: 10.1016/j.celrep.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khadilkar R, et al. ARF1–GTP regulates Asrij to provide endocytic control ofDrosophilablood cell homeostasis. Proceedings of the National Academy of Sciences. 2014;111:4898–4903. doi: 10.1073/pnas.1303559111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korolchuk V, et al. Drosophila Vps35 function is necessary for normal endocytic trafficking and actin cytoskeleton organisation. Journal of Cell Science. 2007;120:4367–4376. doi: 10.1242/jcs.012336. [DOI] [PubMed] [Google Scholar]
- 29.Shravage B, Hill J, Powers C, Wu L, Baehrecke E. Atg6 is required for multiple vesicle trafficking pathways and hematopoiesis in Drosophila. Development. 2013;140:1321–1329. doi: 10.1242/dev.089490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni V, Khadilkar R, Magadi SS, Inamdar M. Asrij Maintains the Stem Cell Niche and Controls Differentiation during Drosophila Lymph Gland Hematopoiesis. PLoS ONE. 2011;6:e27667. doi: 10.1371/journal.pone.0027667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita, H., Ueda, A., Nishida, T. & Otori, T. Uptake of India ink particles and latex beads by corneal fibroblasts. Cell and Tissue Research 250 (1987). [DOI] [PubMed]
- 32.Matova N, Anderson K. Rel/NF- B double mutants reveal that cellular immunity is central to Drosophila host defense. Proceedings of the National Academy of Sciences. 2006;103:16424–16429. doi: 10.1073/pnas.0605721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nam Hyuck-Jin, et al. Involvement of pro-phenoloxidase 3 in lamellocyte-mediated spontaneous melanization in Drosophila. Mol Cells. 2008;26:606–610. [PubMed] [Google Scholar]
- 34.Cerenius L, Lee B, Söderhäll K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends in Immunology. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Ferrandon D, Imler J, Hetru C, Hoffmann J. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 36.Minakhina S, Steward R. Nuclear factor-kappa B pathways in Drosophila. Oncogene. 2006;25:6749–6757. doi: 10.1038/sj.onc.1209940. [DOI] [PubMed] [Google Scholar]
- 37.Valanne S, Wang J, Ramet M. The Drosophila Toll Signaling Pathway. The Journal of Immunology. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Ligoxygakis P. Pathogen recognition and signalling in the Drosophila innate immune response. Immunobiology. 2006;211:251–261. doi: 10.1016/j.imbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Wu, Louisa P. & Kathryn, V. Anderson Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature392, 93–97 (1998). [DOI] [PubMed]
- 40.Hoffmann J, Reichhart J. Drosophila innate immunity: an evolutionary perspective. Nature Immunology. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 41.Inamdar, Maneesha S. Drosophila asrij is expressed in pole cells, trachea and hemocytes. Development genes and evolution 213.3, 134-137 (2003). [DOI] [PubMed]
- 42.Engström Y, et al. κB-like Motifs Regulate the Induction of Immune Genes in Drosophila. Journal of Molecular Biology. 1993;232:327–333. doi: 10.1006/jmbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- 43.Ryu J, et al. Innate Immune Homeostasis by the Homeobox Gene Caudal and Commensal-Gut Mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 44.Leulier F, Royet J. Maintaining immune homeostasis in fly gut. Nature Immunology. 2009;10:936–938. doi: 10.1038/ni0909-936. [DOI] [PubMed] [Google Scholar]
- 45.Shia A, et al. Toll-dependent antimicrobial responses in Drosophila larval fat body require Spatzle secreted by haemocytes. Journal of Cell Science. 2009;122:4505–4515. doi: 10.1242/jcs.049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nature Reviews Molecular Cell Biology. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 47.Beck R, et al. Coatomer and dimeric ADP ribosylation factor 1 promote distinct steps in membrane scission. J Cell Biol. 2011;194:765–777. doi: 10.1083/jcb.201011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Husebye H, et al. The Rab11a GTPase Controls Toll-like Receptor 4-Induced Activation of Interferon Regulatory Factor-3 on Phagosomes. Immunity. 2010;33:583–596. doi: 10.1016/j.immuni.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devergne O, Ghiglione C, Noselli S. The endocytic control of JAK/STAT signalling in Drosophila. Journal of Cell Science. 2007;120:3457–3464. doi: 10.1242/jcs.005926. [DOI] [PubMed] [Google Scholar]
- 50.Huang H, Chen Z, Kunes S, Chang G, Maniatis T. Endocytic pathway is required for Drosophila Toll innate immune signaling. Proceedings of the National Academy of Sciences. 2010;107:8322–8327. doi: 10.1073/pnas.1004031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lund V, DeLotto Y, DeLotto R. Endocytosis is required for Toll signaling and shaping of the Dorsal/NF- B morphogen gradient during Drosophila embryogenesis. Proceedings of the National Academy of Sciences. 2010;107:18028–18033. doi: 10.1073/pnas.1009157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guruharsha K, et al. A Protein Complex Network of Drosophila melanogaster. Cell. 2011;147:690–703. doi: 10.1016/j.cell.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cunha P, et al. Combinatorial Binding Leads to Diverse Regulatory Responses: Lmd Is a Tissue-Specific Modulator of Mef2 Activity. PLoS Genetics. 2010;6:e1001014. doi: 10.1371/journal.pgen.1001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark R, et al. MEF2 Is an In Vivo Immune-Metabolic Switch. Cell. 2013;155:435–447. doi: 10.1016/j.cell.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horn L, Leips J, Starz-Gaiano M. Phagocytic ability declines with age in adult Drosophila hemocytes. Aging Cell. 2014;13:719–728. doi: 10.1111/acel.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaidman-Rémy A, et al. The Drosophila Amidase PGRP-LB Modulates the Immune Response to Bacterial Infection. Immunity. 2006;24:463–473. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 57.Bischoff V, et al. Downregulation of the Drosophila Immune Response by Peptidoglycan-Recognition Proteins SC1 and SC2. PLoS Pathog. 2006;2:e14. doi: 10.1371/journal.ppat.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Camilli P, et al. The synaptic vesicle-associated protein amphiphysin is the 128-kD autoantigen of Stiff-Man syndrome with breast cancer. Journal of Experimental Medicine. 1993;178:2219–2223. doi: 10.1084/jem.178.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takamori Shigeo, et al. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- 60.Menasche G, et al. Biochemical and functional characterization of Rab27a mutations occurring in Griscelli syndrome patients. Blood. 2002;101:2736–2742. doi: 10.1182/blood-2002-09-2789. [DOI] [PubMed] [Google Scholar]
- 61.Stow J, Manderson A, Murray R. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol. 2006;6:919–929. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 62.Nishida K, et al. Gab2, via PI-3K, Regulates ARF1 in Fc RI-Mediated Granule Translocation and Mast Cell Degranulation. The Journal of Immunology. 2011;187:932–941. doi: 10.4049/jimmunol.1100360. [DOI] [PubMed] [Google Scholar]
- 63.Guha A, et al. shibire mutations reveal distinct dynamin-independent and -dependent endocytic pathways in primary cultures of Drosophila hemocytes. Journal of Cell Science. 2003;116:3373–3386. doi: 10.1242/jcs.00637. [DOI] [PubMed] [Google Scholar]
- 64.Neyen C, et al. The Black cells phenotype is caused by a point mutation in the Drosophila pro-phenoloxidase 1 gene that triggers melanization and hematopoietic defects1Equal contribution.1. Developmental & Comparative Immunology. 2015;50:166–174. doi: 10.1016/j.dci.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Sorrentino RP, Small CN, Govind S. Quantitative analysis of phenol oxidase activity in insect hemolymph. BioTechniques. 2002;32:815. doi: 10.2144/02324st08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.