Abstract
Background
Chronic lymphocytic leukemia (CLL) usually expresses CD5 antigen. However, 7–20% of patients are CD5 negative. We report here a series of 19 CD5-negative B-CLL cases.
Material/Methods
We reviewed 19 consecutive CD5-negative B-CLL cases seen in our medical center from 2009 to 2015 and compared them with 105 CD5-positive B-CLL cases. The two groups were compared in terms of clinical parameters, laboratory parameters, and survival characteristics.
Results
Lymphadenopathy was present in 31.5% of the CD5-negative group and 51.4% of the CD5-positive group (p=0.029). Splenomegaly was present in 42.1% of the CD5-negative group and 16.1% of the CD5-positive group (p=0.029). There was no difference between the groups in terms of Binet A, B, and C stages (p=0.118, p=0.051, and p=0.882, respectively). The median thrombocyte count was 144×109/L and 160×109/L in the CD5-negative and CD5-positive groups, respectively (p=0.044). There was no difference between the two groups in terms of median neutrophil count (p=0.169). The mean lymphocyte count was 43.2±4.0×109/L and 36.7±3.2×109/L in the CD5-negative and CD5-positive groups, respectively (p=0.001). There was no difference between the groups in terms of autoimmune hemolytic anemia and autoimmune thrombocytopenia. In five-year follow-up, 84.2% of CD5-negative patients and 90.5% of CD5-positive patients were alive (p=0.393).
Conclusions
We found more isolated splenomegaly, less lymphadenopathy, a higher lymphocyte count, and a lower thrombocyte count in the CD5-negative group. There was no difference between the groups in terms of clinical stage, autoimmune phenomena, hemoglobin and neutrophil count, and survival.
MeSH Keywords: Antigens, CD5; Leukemia, Lymphocytic, Chronic, B-Cell; Survival
Background
Chronic lymphocytic leukemia (CLL) is characterized by the accumulation of mature-appearing lymphocytes in the blood, marrow, lymph nodes, and spleen [1]. In 95% of cases, CLL develops from the malignant transformation of a single B lymphocyte and its clonal expansion; in fewer than 5% of cases, it involves T lymphocytes [2].
In 2008, The International Workshop on Chronic Lymphocytic Leukemia (IWCLL) changed the diagnosis of CLL to require a peripheral blood B-cell count of ≥5×109/L and the presence of monoclonal B-cells that have the typical immunophenotype of CLL cells (CD19+, CD5+, CD23+, and decreased expression of surface Ig, CD20, and CD79b) [3]. CD5 is characteristically only expressed in CLL and mantle cell lymphoma (MCL), thereby distinguishing these entities from other small chronic B-cell lymphoproliferative disorders. However, several recent studies of patients diagnosed with CLL based on clinical presentation and morphologic features have identified a subset of CD5-negative CLLs [4–9].
CLL generally runs an indolent clinical course, with most patients not requiring therapy for a long time [10]. CD5-negative B-CLL is often associated with an aggressive clinical course [11–13]. On the other hand, some CD5-negative B-CLL cases are characterized by stable lymphocytosis and clinical course [14]. Information about biological differences, clinical differences, and the incidence of these two clinical entities classified according to CD5 expression is limited, and the data are contradictory. We report here a series of 19 CD5-negative B-CLL cases and compare their clinical, biological, and prognostic characteristics with a control group of 105 cases of CD5-positive B-CLL.
Material and Methods
Patient selection
The hematology department of The Yuzuncu Yil University, Medical Faculty, Turkey, conducted this study retrospectively. The files and data in hospital record systems on 124 patients who were followed up with the diagnosis of CLL between 2009 and 2015 in our clinic were examined retrospectively. Ethical approval was obtained from the local ethics committee. The procedures followed were in accordance with the Helsinki international ethical standards on human experimentation.
The diagnosis of CLL was made according to the IWCLL criteria [3]. Staging of CLL at diagnosis was established according to the Binet system [3]. The patients were classified into two groups, CD5-negative and CD5-positive B-CLL, based on the data of 124 patients. In our study, lack of CD5 expression was defined as a situation in which fewer than 20% of cells expressed CD5. There were 19 patients in the CD5-negative B-CLL group and 105 patients in the CD5-positive B-CLL group, which was selected as a control group. The two groups were compared in terms of clinical and laboratory parameters and survival.
Laboratory and radiological investigations
Routine hematological, biochemical, and immunological studies were performed for all patients, including hemogram, direct Coombs test, serum protein electrophoresis, peripheral blood smears, serum LDH, and bilirubins. Computerized tomography and ultrasonography were used to detect lymphadenopathy and organomegaly.
Immunophenotyping studies
Immunophenotyping of peripheral blood was performed in patients with B-CLL by four-color multiparameter flow cytometry analysis using a panel of monoclonal antibodies. Antibodies for CD5, CD19, CD23, CD20, CD10, and Sm IgM were used for all cases.
Statistical evaluation
Statistical analysis was performed using the SPSS 19.0 software package. For the studied clinical and hematological parameters, the normality assumption was tested by the Kolmogorov-Smirnov test. After this test, for the parameters that the normality assumption met, the independent samples t-test was performed for the differences between CD5-negative and CD5- positive B-CLL patients. However, the Mann-Whitney U test, which is a non-parametric test, was also performed for the parameters that the normality assumption violated. Survival was analyzed using the Kaplan-Meier method, and the log-rank test was used to compare survival curves. Values are expressed as means ± standard deviation or medians with their range. A p value less than 0.05 was considered statistically significant.
Results
A total of 124 patients (83 males and 41 females) with a diagnosis of B-CLL were included in this study. The mean age of all patients was 65.8±12.3 (34–96) years. There were 19 patients in the CD5-negative B-CLL group. There were 105 patients in the CD5-positive B-CLL group, which served as a control group. There was no difference between groups in terms of gender (p=0.381). The mean ages of CD5-negative and CD5-positive patients were found to be similar (p=0. 339)
CD5-negative and CD5-positive B-CLL patients were evaluated according to the Binet staging system. We did not find a statistically significant difference between the two groups in terms of stage A, B, and C (p=0.118, p=0.051, and p=0.882, respectively).
We found a statistically significant difference between the two groups in terms of lymph node involvement (p=0.029). Patients with CD5-positive B-CLL more frequently had isolated lymphadenopathy at diagnosis (p=0.005). Similarly, extensive lymph node involvement (three or more regions) was found more frequently in the CD5-positive B-CLL group (p=0.030). Patients with CD5-negative B-CLL more frequently had both total and isolated splenomegaly (p=0.029 and p=0.033, respectively). There was no significant difference between the two groups in terms of association of splenomegaly and lymphadenopathy (p=0.860). Hepatic involvement was not found in the two groups. Clinical characteristics of the patients are shown in Table 1.
Table 1.
Clinical characteristics of CD5-negative and CD5-positive B-CLL patients.
| Parameters | CD5-negative patients | CD5-positive patients | p value |
|---|---|---|---|
| Characteristics of patients | |||
|
| |||
| Number of patients | 19 | 105 | |
| Gender | |||
| Male | 11 | 72 | 0.381 |
| Female | 8 | 33 | 0.381 |
| Age (years) (mean ±SD) | 65.8±7.1 | 66.5±7.3 | 0.339 |
|
| |||
| Clinical stage | |||
|
| |||
| Binet stage A | 14 (73.7%) | 59 (56.2%) | 0.118 |
| Binet stage B | 2 (10.5%) | 28 (26.7%) | 0.051 |
| Binet stage C | 3 (15.8%) | 18 (17.1%) | 0.882 |
|
| |||
| Lymphadenopathy | |||
|
| |||
| Total lymphadenopathy | 6 (31.5%) | 54 (51.4%) | 0.029 |
| One region involvement | 3 (15.8%) | 10 (9.5%) | 0.152 |
| Two regions involvement | 1 (5.2%) | 16 (15.2%) | 0.060 |
| Three and more regions involvement | 2 (10.5%) | 28 (26.7%) | 0.030 |
| Isolated lymphadenopathy | 2 (10.5%) | 38 (36.1%) | 0.005 |
| Lymphadenopathy + splenomegaly | 4 (21.0%) | 16 (15.2%) | 0.860 |
|
| |||
| Splenomegaly | |||
|
| |||
| Total splenomegaly | 8 (42.1%) | 17 (16.1%) | 0.029 |
| Isolated splenomegaly | 4 (21.0%) | 1 (0.9%) | 0.033 |
| Splenomegaly + lymphadenopathy | 4 (21.0%) | 16 (15.2%) | 0.860 |
|
| |||
| Hepatomegaly | Absent | Absent | Absent |
The mean hemoglobin level and neutrophil count did not differ between the two groups (p=0.180 and p=0.169, respectively). The median platelet count was higher in the CD5-positive group (p=0.044). Lymphocytosis was more obvious in the CD5-negative patients (p=0.001). Autoimmune hemolytic anemia (AIHA) and autoimmune thrombocytopenia (AITP) were observed similarly in both groups (p=0.790 and p=0.526, respectively). Hematological parameters and autoimmune manifestations of patients are shown in Table 2. In five-year follow-up, 84.2% of CD5-negative patients and 90.5% of CD5-positive patients were alive (Table 3). There was no statistical difference between the groups in terms of survival data at five-year follow-up (p=0.393) (Figure 1).
Table 2.
Hematological characteristics of CD5-negative and CD5-positive B-CLL patients.
| Parameters | CD5-negative patients | CD5-positive patients | p value |
|---|---|---|---|
| Neutrophil count (×109/L) (median and min–max) | 3.5 (1.0–5.4) | 3.3 (0.8–6.9) | 0.169 |
|
| |||
| Lymphocyte count (×109/L) (mean ±SD and min–max) | 43.2±4.0 (34.8–48.0) | 36.7±3.2 (29.3–46.1) | 0.001 |
|
| |||
| Hemoglobin (g/dL) (mean ±SD and min–max) | 13.3±1.6 (8.8–16.9) | 12.7±1.7 (7.6–16.6) | 0.180 |
|
| |||
| Hemoglobin >10 | 17 (89.5%) | 93 (88.6%) | 0.907 |
| Hemoglobin <10 | 2 (10.5%) | 12 (11.4%) | 0.907 |
|
| |||
| Platelet count (×109/L) (median and min–max) | 144 (89–220) | 160 (67–280) | 0.044 |
|
| |||
| Platelet >100 | 18 (94.8%) | 99 (94.3%) | 0.515 |
| Platelet <100 | 1 (5.2%) | 6 (5.7%) | 0.936 |
|
| |||
| Autoimmune events | |||
|
| |||
| Autoimmune hemolytic anemia | 1 (5.2%) | 2 (1.9%) | 0.790 |
| Autoimmune thrombocytopenia | 1 (5.2%) | 4 (3.8%) | 0.526 |
| Total autoimmune events | 2 | 6 | 0.515 |
Table 3.
Five-year survival characteristics of patients.
| Patients | Total (n) | Events (n) | No events (n) |
|---|---|---|---|
| CD5-negative patients | 19 | 3 (15.7%) | 16 (84.2%) |
| CD5-positive patients | 105 | 10 (9.5%) | 95 (90.5%) |
| Overall | 124 | 13 (10.4%) | 111 (89.5%) |
Figure 1.
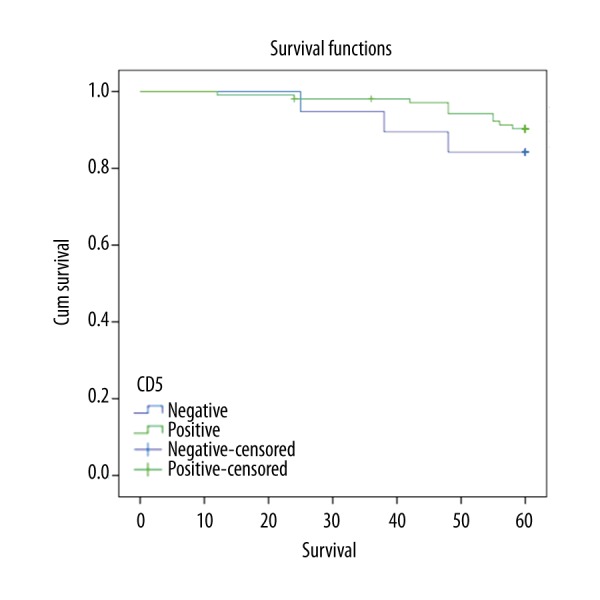
Kaplan-Meier chart of the overall survival in CD5-positive and CD5-negative B-CLL patients (log-rank test p=0.393).
Discussion
CLL is the most common form of leukemia in adults [15]. The defining feature of the B-CLL clone is the co-expression of CD19, CD20, CD5, and CD23. The levels of surface immunoglobulin, CD20, and CD79 are characteristically low compared to those found on normal B cells [16]. Some patients diagnosed with CLL according to clinical and morphological features have CD5-negative clonal B cells in the peripheral blood [11,12,17]. The incidence of CD5-negative B-CLL varies from 7% to 20% [17–19]. The incidence of CD5-negative CLL was 15.3% in our study. There have been many studies on clinical features of CD5-negative CLL. Contradictory results have been found in these studies due to disagreement mainly on the definition of CD5 negativity [12,17,19–21]. Efstathiou et al. defined CD5 negativity as the expression of CD5 by fewer than 5% of cells [22]. Cartron et al. used the same concept, studying a group of 42 CD5-negative B-CLL patients [12]. Some authors accept a cut-off value of 20% to 30% for CD5 positivity [17,19]. In our study, CD5 negativity was defined as a situation where fewer than 20% of cells expressed CD5.
CD5-negative B-CLL usually has been associated with a higher incidence of splenomegaly. For instance, both Cartron et al. [12] and Geisler et al. [19] detected significantly more splenomegaly in CD5-negative cases compared to CD5-positive cases. Similarly, we also found the incidence of splenomegaly to be higher in CD5-negative CLL patients compared to the CD5-positive group. On the other hand, splenomegaly was reported to be more frequent in CD5-positive patients in a study [22]. We found that isolated splenomegaly was also more common in the CD5-negative group, similar to total splenomegaly (spleen + lymph node). Similarly, Cartron et al. [12] also found that isolated splenomegaly was more common in the CD5-negative group. Isolated splenomegaly is seen more often in some types of non-Hodgkin lymphoma (NHL) rather than CLL. Thus, these NHL types were taken into consideration in differential diagnosis, and these entities were eliminated with appropriate tests. Lymphadenopathy was detected to be present less frequently in CD5-negative patients compared to CD5-positive patients in two previously conducted studies [12,22]. We also found a higher rate of lymphadenopathy in CD5-positive patients compared to CD5-negative patients.
The data associated with disease stage have been contradictory in CD5-negative patients. Some authors reported that CD5-negative B-CLL patients had a more advanced stage of disease [13,18,21]. Similarly, it was pointed out in another study that CD5 negativity was associated with shorter survival and a more advanced stage of disease [13]. Unlike these studies, some other studies reported that CD5-negative patients had an earlier stage of disease compared to CD5-positive patients [12,22]. There was no difference between the groups in terms of disease stages in our study. Although there was an accumulation of stage B disease in the CD5-positive group, it was not significant.
Findings presented by the studies regarding hematological parameters have been quite different [23,24]. CD5-negative patients were found to have less hemoglobin than CD5-positive patients in two previously conducted studies [18,21]. On the other hand, frequency of anemia among CD5-positive patients was reported to be higher than that among CD5-negative patients in another study [22]. Unlike these studies, Cartron et al. [12] found no difference between the groups in terms of hemoglobin, neutrophils, lymphocytes, and thrombocyte count. We found no difference between these two entities in terms of hemoglobin and neutrophil count. However, we detected a higher lymphocyte count and a lower thrombocyte count in the CD5-negative group.
The relationship between CLL and autoimmune cytopenias is well established. The most common complication is AIHA (about 7%), whereas the incidence of AITP, and particularly autoimmune neutropenia and pure red cell aplasia, is lower in most studies (<1–2%) [25–29]. In our study, the rate of autoimmune hemolytic anemia was 4% and the rate of immune thrombocytopenia was 2.4% in all CLL patients. There have been a very limited number of studies investigating differences in autoimmune manifestations in CD5-negative and CD5-positive subgroups. Therefore, it is difficult to make general conclusions. There was no difference between the groups in terms of autoimmune manifestations in our study. Similar to our study, there was no difference between CD5-negative and CD5-positive groups in terms of autoimmune manifestations in another study [12]. On the other hand, the incidence of hemolytic anemia was found to be significantly higher in CD5-positive patients, but there was no other immunological difference in the other study [22].
CD5 negativity has been generally reported to be associated with shorter survival [13]. However, data about effect of CD5 negativity on survival have been contradictory. There have been studies reporting that CD5-negative patients have better, worse, or similar survival ratios compared to CD5-positive patients. For instance, Kurec et al. [18] found that 5-year survival was worse in the CD5-negative CLL group. CD5-negative patients survived an average of 13 months longer compared to CD5-positive patients in another study [22]. There was no difference between the groups in terms of survival considering 5-year follow-up data in our study. Similarly, there was no survival difference between the two groups in two previously conducted studies [12,17].
Conclusions
We found more isolated splenomegaly, less lymphadenopathy, a higher lymphocyte count, and a lower thrombocyte count in the CD5-negative group. There was no difference between the groups in terms of clinical stage, autoimmune phenomena, hemoglobin and neutrophil count, and survival. Since there were fewer CD5-negative patients in our study and there have been different results in the literature, it is necessary to conduct an extensive prospective study in order to identify clinical and biological effects of CD5-negative CLL.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: Departmental sources
References
- 1.Landgren O, Albitar M, Ma W, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360:659–67. doi: 10.1056/NEJMoa0806122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lydyard PM, Youinou PY, Cooke A. CD5-positive B cells in rheumatoid arthritis and chronic lymphocytic leukemia. Immunol Today. 1987;8:37–39. doi: 10.1016/0167-5699(87)90235-0. [DOI] [PubMed] [Google Scholar]
- 3.Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang JC, Finn WG, Goolsby CL, et al. CD5- small B-cell leukemias are rarely classifiable as chronic lymphocytic leukemia. Am J Clin Pathol. 1999;111:123–30. doi: 10.1093/ajcp/111.1.123. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro JL, Miller ML, Pohlman B, et al. CD5- B-cell lymphoproliferative disorders presenting in blood and bone marrow. A clinicopathologic study of 40 patients. Am J Clin Pathol. 1999;111:477–87. doi: 10.1093/ajcp/111.4.477. [DOI] [PubMed] [Google Scholar]
- 6.Batata A, Shen B. Immunophenotyping of subtypes of B-chronic (mature) lymphoid leukemia. A study of 242 cases. Cancer. 1992;70:2436–43. doi: 10.1002/1097-0142(19921115)70:10<2436::aid-cncr2820701009>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Maloum K, Davi F, Magnac C, et al. Analysis of VH gene expression in CD5+ and CD5- B-cell chronic lymphocytic leukemia. Blood. 1995;86:3883–90. [PubMed] [Google Scholar]
- 8.Matutes E, Oscier D, Garcia-Marco J, et al. Trisomy 12 defines a group of CLL with atypical morphology: correlation between cytogenetic, clinical and laboratory features in 544 patients. Br J Haematol. 1996;92:382–88. doi: 10.1046/j.1365-2141.1996.d01-1478.x. [DOI] [PubMed] [Google Scholar]
- 9.Matutes E, Owusu-Ankomah K, Morilla R, et al. The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia. 1994;8:1640–45. [PubMed] [Google Scholar]
- 10.Romano C, Sellitto A, Chiurazzi F, et al. Clinical and phenotypic features of CD5-negative B cell chronic lymphoproliferative disease resembling chronic lymphocytic leukemia. Int J Hematol. 2015;101:67–74. doi: 10.1007/s12185-014-1703-y. [DOI] [PubMed] [Google Scholar]
- 11.Maloum K, Pritsch O, Magnac C, et al. VH gene expression in CD5 positive and CD5 negative B cell chronic lymphoid malignancies. Leuk Lymphoma. 1997;24:437–48. doi: 10.3109/10428199709055582. [DOI] [PubMed] [Google Scholar]
- 12.Cartron G, Linassier C, Bremond JL, et al. CD5 negative B-cell chronic lymphocytic leukemia: Clinical and biological features of 42 cases. Leuk Lymphoma. 1998;31:209–16. doi: 10.3109/10428199809057600. [DOI] [PubMed] [Google Scholar]
- 13.Huang JC, Finn WG, Goolsby CL, et al. CD5- small B-cell leukemias are rarely classifiable as chronic lymphocytic leukemia. Am J Clin Pathol. 1999;111:123–30. doi: 10.1093/ajcp/111.1.123. [DOI] [PubMed] [Google Scholar]
- 14.Keung YK, Buss D, Pettenati M, Powell BL. CD5-negative chronic lymphocytic leukemia or monoclonal B-lymphocytosis of undetermined significance? Am J Hematol. 2002;70:334. doi: 10.1002/ajh.10160. [DOI] [PubMed] [Google Scholar]
- 15.García-Muñoz R, Galiacho VR, Llorente L. Immunological aspects in chronic lymphocytic leukemia (CLL) development. Ann Hematol. 2012;91:981–96. doi: 10.1007/s00277-012-1460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginaldi L, De Martinis M, Matutes E, et al. Levels of expression of CD19 and CD20 in chronic B cell leukaemias. J Clin Pathol. 1998;51:364–69. doi: 10.1136/jcp.51.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Rossi G, Mauro FR, Lo Coco F, et al. CD5 negative lymphocytosis mimicking typical B-chronic lymphocytic leukaemia. Description of 26 cases. Nouv Rev Fr Hematol. 1993;35:451–55. [PubMed] [Google Scholar]
- 18.Kurec AS, Threatte GA, Gottlieb AJ, et al. Immunophenotypic subclassification of chronic lymphocytic leukaemia (CLL) Br J Haematol. 1992;81:45–51. doi: 10.1111/j.1365-2141.1992.tb08169.x. [DOI] [PubMed] [Google Scholar]
- 19.Geisler CH, Larsen JK, Hansen NE, et al. Prognostic importance of flow cytometric immunophenotyping of 540 consecutive patients with B-cell chronic lymphocytic leukemia. Blood. 1991;78:1795–802. [PubMed] [Google Scholar]
- 20.Bassan R, Pronesti M, Buzzetti M, et al. Surface immunoglobulin intensivity and p67 (CD5) antigen expression define different forms of B-cell chronic lymphocytic leukaemia. Haematologica. 1987;72:221–25. [PubMed] [Google Scholar]
- 21.Salomon-Nguyen F, Valensi F, Merle-Beral H, Flandrin G. A scoring system for the classification of CD5-B CLL versus CD5+ B CLL and B PLL. Leuk Lymphoma. 1995;16:445–50. doi: 10.3109/10428199509054432. [DOI] [PubMed] [Google Scholar]
- 22.Efstathiou S, Tsioulos D, Zacharos I, et al. The prognostic role of CD5 negativity in B-cell chronic lymphocytic leukaemia: A case-control study. Haematologia (Budap) 2002;32:209–18. doi: 10.1163/15685590260461020. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Amato D, Fernandes B. CD5-negative phenotype of monoclonal B-lymphocytosis of undetermined significance (MLUS) Am J Hematol. 2002;69:147–49. doi: 10.1002/ajh.10044. [DOI] [PubMed] [Google Scholar]
- 24.Sheikh SS, Kallakury BV, Al-Kuraya KA, et al. CD5-negative, CD10-negative small B-cell leukemia: Variant of chronic lymphocytic leukemia or a distinct entity? Am J Hematol. 2002;71:306–10. doi: 10.1002/ajh.10222. [DOI] [PubMed] [Google Scholar]
- 25.Mauro FR, Foa R, Cerretti R, et al. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: clinical, therapeutic, and prognostic features. Blood. 2000;95:2786–92. [PubMed] [Google Scholar]
- 26.Zent CS, Ding W, Schwager SM, et al. The prognostic significance of cytopenia in chronic lymphocytic leukaemia/small lymphocytic lymphoma. Br J Haematol. 2008;141:615–21. doi: 10.1111/j.1365-2141.2008.07086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno C, Hodgson K, Ferrer G, et al. Autoimmune cytopenia in chronic lymphocytic leukemia: Prevalence, clinical associations, and prognostic significance. Blood. 2010;116:4771–76. doi: 10.1182/blood-2010-05-286500. [DOI] [PubMed] [Google Scholar]
- 28.Borthakur G, O’Brien S, Wierda WG, et al. Immune anaemias in patients with chronic lymphocytic leukaemia treated with fludarabine, cyclophosphamide and rituximab--incidence and predictors. Br J Haematol. 2007;136:800–5. doi: 10.1111/j.1365-2141.2007.06513.x. [DOI] [PubMed] [Google Scholar]
- 29.Dearden C, Wade R, Else M, et al. The prognostic significance of a positive direct antiglobulin test in chronic lymphocytic leukemia: A beneficial effect of the combination of fludarabine and cyclophosphamide on the incidence of hemolytic anemia. Blood. 2008;111:1820–26. doi: 10.1182/blood-2007-07-101303. [DOI] [PubMed] [Google Scholar]


