Abstract
The existence of interindividual variations in G protein-coupled receptor sequences has been recognized early on. Recent advances in large-scale exon sequencing techniques are expected to dramatically increase the number of variants identified in G protein-coupled receptors, giving rise to new challenges regarding their functional characterization. The current minireview will illustrate these challenges based on the MTNR1B gene, which encodes the melatonin MT2 receptor, for which exon sequencing revealed 40 rare nonsynonymous variants in the general population and in type 2 diabetes (T2D) cohorts. Functional characterization of these MT2 mutants revealed 14 mutants with loss of Gi protein activation that associate with increased risk of T2D development. This repertoire of disease-associated mutants is a rich source for structure-activity studies and will help to define the still poorly understood role of melatonin in glucose homeostasis and T2D development in humans. Defining the functional defects in carriers of rare MT2 mutations will help to provide personalized therapies to these patients in the future.
G protein-coupled receptors (GPCRs) constitute the largest family of membrane receptors with approximately 800 members in humans (1, 2). They are composed of 7-transmembrane (TM) spanning domains connected by short intra- and extracellular loops and respond to a large panel of signals such as photons, ions, metabolites, amino acids, lipids, peptides, and proteins. Despite this ligand diversity, the overall architecture and activation mechanism is believed to be highly conserved for these receptors (3). Similarly, many GPCRs share a common gene structure, typically containing no or only a single intron.
GPCRs are expressed at the cell surface where they participate in the transmission of signals from the extracellular to intracellular environment by activating various intracellular signaling pathways. Due to the high number of GPCRs and to their strategic position in cellular homeostasis, GPCRs are involved in most physiologic responses to hormones, neurotransmitters, and environmental stimulants, and GPCR deregulation is associated with multiple diseases, in particular of the endocrine system (4, 5).
After the cloning of the first GPCR genes in the 1980s, the existence of gene variants was rapidly recognized. First, frequent variants were identified (minor allelic frequency > 1%) and with increasing sequencing capacities also rare and very rare variants (minor allelic frequency = 0.1%–1% or <0.1%, respectively), several of which have been shown to be disease related (5, 6).
The functional consequences of a gene variant will depend on its localization. Variants located in the coding region may be silent (synonymous variants) or modify the amino acid sequence of the receptor (nonsynonymous variants).
A study on 64 randomly selected GPCR genes in a small sample of 82 individuals revealed an unexpectedly high prevalence of frequent nonsynonymous variants in the coding region of GPCR genes (7). Interestingly, these variants are not evenly distributed over the coding region, which was particularly true for disease-causing variants (8). Most prominent regions are the TM-spanning domains followed by intracellular loops. Localization of variants in these regions is highly likely to have a major impact on receptor function. Intriguingly, the prevalence of frequent nonsynonymous variants seems to be highest in the most conserved receptor regions (TM-spanning domain) and the lowest in the most variable receptor regions such as the carboxy terminus (7). Variants may also exist outside of the coding region such as in the promoter regions or the 5′-untranslated region or 3′-untranslated region where they may modulate gene transcription or mRNA stability and thus modify receptor expression levels.
Recent genome-wide association studies (GWAS) identified many gene variants located either in introns or in chromosomal regions close to known genes. However, elucidating the functional consequences of such variants proved to be challenging. Variants affecting receptor function or receptor expression levels can lead to gain- or loss-of-function phenotypes. Both scenarios can be associated with disease. Gain of function is typically achieved by enhanced ligand binding or constitutive receptor activity, absence of desensitization, enhanced cell surface expression, or increased receptor expression. Loss of function is obtained by reduced or impaired ligand binding, enhanced desensitization, and diminished expression or cell surface localization.
Rare disease-causing mutations have been identified for several GPCRs. Prominent examples are the vasopressin V2 receptor for which more than 200 different mutations have been identified in patients with nephrogenic diabetes insipidus (9). Another example is the melanocortin MC4 receptor for which more than 50 mutations have been observed in morbidly obese adults and children (10, 11). For more details and further disease-causing mutants identified in other GPCRs, the reader is invited to consult recent expert reviews (5, 12, 13).
In addition to rare, disease-causing variants, multiple other rare and frequent variants in GPCR genes exist with mostly unknown functional effects (14). The corresponding mutations may be either neutral (without any obvious functional phenotype) or may modify the receptor's function and thus have an impact on the risk of disease development. The development of large-scale sequencing methods is likely to drastically increase the number of identified variants. Following the example of the MTNR1B gene and its gene product, the melatonin MT2 receptor, we will illustrate the different steps starting from the identification of rare and frequent gene variants to the functional characterization of the corresponding receptor mutants and genetic association studies to evaluate their impact on disease risk. In the second part of the minireview, we will discuss the consequences of these MT2 mutants on receptor structure and their impact on our understanding of melatonin's role in glucose homeostasis.
MTNR1B Gene Variants: From GWAS to Rare Nonsynonymous Variants
The first hint for an association between the melatonin MT2 receptor and increased fasting plasma glucose (FPG) levels and type 2 diabetes (T2D) risk was suggested by GWAS designed to identify frequent variants associated with increased FPG and T2D risk in an unbiased manner (15, 16). Indeed, variants rs1387153 and rs10830963 strongly associated with both parameters. Both variants are frequent and are located close to the MNTR1B gene, rs1387153 approximately 29 kb upstream of exon 1 and rs10830963 in the middle of the single intron of 11 kb. Follow-up studies and a meta-analysis with more than 170 000 individuals confirmed that the intronic rs10830963 shows the most robust association with T2D risk (17). The effect on FPG was also replicated in children and adolescents, suggesting an early impact of the rs10830963-G risk allele during development (18, 19). A recent study determined the association of the rs10830963-G allele on the risk of normal subjects to become glucose intolerant (prediabetic) and for intolerant subjects to become diabetic (20). Surprisingly, the risk allele seems to promote the progression from the normal to the prediabetic state rather than the progression from the prediabetic to the diabetic state. Other, more detailed, studies on insulin secretion showed an association of the rs10830963-G allele with decreased early-phase insulin secretion, recapitulating the defect observed in T2D patients (21, 22). Gestational diabetes is a particular form of glucose intolerance that occurs during pregnancy. Although the genes involved are still largely unknown, several arguments suggest that some genes might be in common with T2D risk genes. Consistently, several variants close to the MTNR1B gene, including rs10830963, have been associated with gestational diabetes in a cohort of more than 7000 individuals (23, 24).
Although the genetic association of the rs10830963 variant with T2D risk is now extremely well documented, its intronic location in an unconserved region does not provide any hint about its functional relevance. The product of the MTNR1B gene, the melatonin MT2 receptor, is a member of the melatonin receptor family composed of MT1, MT2, and the orphan GPR50 (25). Interestingly, GWAS has identified rs2119882, a variant localized at the promoter region of the MTNR1A gene, to be associated among other endocrine dysfunctions with higher fasting plasma glucose concentrations (26). Although these studies suggest a link between melatonin receptors and glucose homeostasis, the literature remains contradictory and the lack of convincing evidence, particularly in humans, does not allow drawing any definite conclusions (see below).
Functional in Vitro Profiling of Rare MT2 Mutants
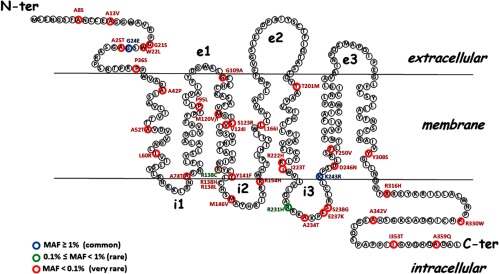
To evaluate the functional significance of the frequent MTNR1B variants in GWAS, we decided together with the team of Philippe Froguel (University of Lille, Lille, France) to search for additional, rare, variants at the MTNR1B locus and more precisely for nonsynonymous variants in the 2 exons of the MTNR1B gene. Sequencing of more than 7600 individuals from the general population and a T2D cohort, revealed 40 nonsynonymous rare variants uniformly distributed over the coding region (27) (Figure 1). To determine the impact of the corresponding mutations on MT2 receptor function, we expressed each mutant individually in HEK293 cells and performed several functional tests (Table 1). We first assessed the ability of the mutants to reach the cell surface. No defects were observed because all mutants were expressed at the cell surface to a similar extent as the wild-type receptor. We subsequently determined the pharmacologic properties of the mutants by determining their affinity for the radiolabeled agonist 2-[125I]iodomelatonin. For most mutants, the affinity was not significantly modified. Eleven mutants had a slightly decreased affinity (up to 2.5-fold lower). Most strikingly, 4 mutants (A42P, L60R, P95L, and Y308S) completely lost their capacity to bind 2-[125I]iodomelatonin with high affinity despite normal cell surface expression.
Figure 1.
Distribution of the 40 Nonsynonymous MT2 Variants Identified by Sequencing of 7632 Individuals, Including 2186 with T2D. Nonsynonymous variants are colored: blue, minor alleic frequency (MAF) ≥ 1%; green, MAF between 0.1% and 1%; red MAF < 0.1%. N-ter, N-terminal; C-ter, C-terminal.
Table 1.
Genetics and Functional Parameters of MT2 Variants
| Localizationa | Amino Acid Positionb | Mutationc | Functional Responsed |
MAF%e | Increased T2D Riskf | ||
|---|---|---|---|---|---|---|---|
| Ligand Binding | Gi Activation | ERK Activation | |||||
| Extracellular N terminus | 8 | Ala → Ser | + | + | + | <0.1 | |
| 13 | Ala → Val | + | + | + | <0.1 | ||
| 21 | Gly → Ser | + | + | + | <0.1 | ||
| 22 | Trp → Leu | + | No response | + | <0.1 | * | |
| 24 | Gly → Glu | + | + | + | >1 | ||
| 25 | Ala → Thr | + | + | + | <0.1 | ||
| 36 | Pro → Ser | + | + | + | <0.1 | ||
| TM1 | 42 | Ala → Pro | No binding | No response | No response | <0.1 | * |
| 52 | Ala → Thr | + | No response | + | <0.1 | * | |
| 60 | Leu → Arg | No binding | No response | No response | <0.1 | * | |
| i1 | |||||||
| TM2 | 74 | Ala → Thr | + | No response | + | <0.1 | * |
| 95 | Pro → Leu | No binding | No response | No response | <0.1 | * | |
| e1 | |||||||
| TM3 | 109 | Gly → Ala | + | + | + | <0.1 | |
| 120 | Met → Val | + | + | + | <0.1 | ||
| 120 | Met → Ile | + | + | + | <0.1 | ||
| 123 | Ser → Arg | + | + | + | <0.1 | ||
| 124 | Val → Ile | + | + | + | <0.1 | ||
| 138 | Arg → Leu | + | No response | + | <0.1 | * | |
| 138 | Arg → His | + | No response | + | <0.1 | * | |
| 138 | Arg → Cys | + | No response | No response | 0.1 < x < 1 | ||
| i2 | 141 | Tyr → Phe | + | + | + | <0.1 | |
| 146 | Met → Val | + | + | + | <0.1 | ||
| 154 | Arg → His | + | + | + | <0.1 | ||
| TM4 | 166 | Leu → Ile | + | No response | + | <0.1 | * |
| e2 | |||||||
| TM5 | 201 | Thr → Met | + | + | + | <0.1 | |
| 222 | Arg → His | + | No response | + | <0.1 | * | |
| 223 | Ile → Thr | + | + | + | <0.1 | ||
| i3 | 231 | Arg → His | + | + | + | 0.1 < x < 1 | |
| 234 | Ala → Thr | + | + | + | <0.1 | ||
| 237 | Glu → Lys | + | + | + | <0.1 | ||
| 238 | Ser → Gly | + | + | + | <0.1 | ||
| TM6 | 243 | Lys → Arg | + | + | + | >1 | |
| 246 | Asp → Asn | + | + | + | <0.1 | ||
| 250 | Phe → Val | + | + | No response | <0.1 | ||
| e3 | |||||||
| TM7 | 308 | Tyr → Ser | No binding | No response | No response | <0.1 | * |
| Intracellular C terminus | 316 | Arg → His | + | + | + | <0.1 | |
| 330 | Arg → Trp | + | No response | + | <0.1 | * | |
| 342 | Ala → Val | + | + | + | <0.1 | ||
| 353 | Ile → Thr | + | No response | + | <0.1 | * | |
| 359 | Ala → Glu | + | + | + | <0.1 | ||
Receptor domain localization of mutated amino acids (TM, transmembrane domain; e, extracellular loop; i, intracellular loop).
Position of mutated amino acid in the receptor protein sequence. Full “loss-of-function” mutants (no ligand binding) are indicated in bold letters.
Amino acid change (3-letter code) introduced by the mutation.
+ Indicates functional characteristics comparable to the wild-type MT2. Receptor surface expression was not affected by the mutation.
Minor allelic frequency of the mutation.
* Indicates increased T2D risk: only rare mutations (minor alleic frequency < 0.1%) leading to Gi activation deficiency are associated with increased T2D risk.
We further tested the signaling properties of the MT2 mutants, namely their ability to activate the ERK1/2 pathway and to inhibit the adenylyl cyclase pathway. As expected, the 4 mutants with impaired ligand binding were also inactive in both signaling assays. Ten additional mutants were also inactive on the Gi/AC pathway (W22L, A52T, A74T, R138C, R138H, R138L, L166I, R222H, R330W, and I353T) and one (F250V) on the ERK1/2 pathway classifying them as loss-of-function mutations in respect to these signaling pathways. Based on this functional characterization, MT2 receptor mutants can be divided into 2 groups: loss-of-function mutants and neutral mutants with respect to their capacity to modulate the Gi/AC pathway. We then asked whether either group is associated with T2D risk. Interestingly, only the loss-of-function group associated with an increased (about 6 times) risk of developing T2D. This established a first functional link between MT2 loss-of-function receptors and T2D risk. This result is unexpected because a previous hypothesis postulated rather a negative relationship between melatonin and glucose homeostasis due to the inhibitory action of melatonin on insulin secretion observed in rodent pancreatic β-cells (see below).
The current in vitro functional study is obviously incomplete because MT2 receptors, like other GPCRs, modulate multiple signaling cascades. Apart from their capacity to inhibit the cAMP pathway, MT2 receptors have been shown to inhibit cGMP levels, to regulate ion channel activity, to recruit ß-arrestins, to promote inositol phosphate production, and to increase intracellular Ca2+ levels under certain circumstances (25, 28). To fully understand melatonin's effect on T2D risk, the full signaling profile must be established in future studies. Such a multiparameter analysis will provide two types of information: 1) the signaling profile most strongly associated with disease risk; and 2) the profile of the functional defect. Once the defective signaling pathway(s) are identified the design of tailored ligands, promoting (potentiating) only the activation of defective signaling components, can be envisioned, to specifically potentiate the response of partial loss-of-function mutants. Pathway-selective ligands with improved selectivity hold great promise for future drug design due to their restricted action profiles.
Impact of Rare MT2 Mutants on Receptor Structure and Activity
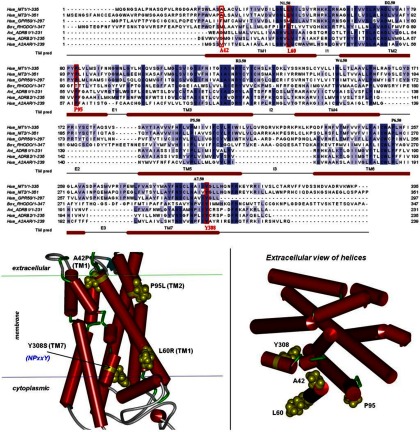
The 4 loss-of-function MT2 mutants, A42P, L60R, P95L, and Y308S, provide a unique opportunity to elucidate the structural basis of the MT2 deficiency associated with T2D. Studies in transfected HEK293 cells suggest that these MT2 mutants do not have major defects in global protein folding and trafficking properties because all mutants were expressed at the cell surface to a similar extent as the wild-type receptor. Consequently, impaired function of MT2 mutants is likely to be due to local structural rearrangements or modifications in receptor dynamics affecting ligand binding, receptor stability, or conformational changes involved in receptor activation.
The 4-point mutations are located in the TM domain of the MT2 receptor (Figure 2). Positions 42 and 60 are located at the 2 extremities of TM1. Position 95 is located at the extracellular site of TM2 whereas position 308 is part of TM7, located close to the cytoplasmic receptor interface. Among the 4 positions, only position 42 is not well conserved in the wild-type MT2 receptor as compared with a set of 260 homologous GPCR sequences (Table 2). The likelihood that an alanine residue occurs at position 42 is only 0.3, suggesting a significant variability at this position. However, the likelihood to see a proline residue at this position (as in the mutant receptor) is null, indicating that it is not tolerated because it may have a significant deleterious impact on the α-helical structure. Proline residues are indeed known to introduce kinks in the helix axis (29) and to reorganize the conformational packing of the helix bundle. Our 3-dimensional model shows that position 42 is located in a buried central region overlapping the TM bundle and extracellular region that could be part of the predicted melatonin-binding site (Figure 2). Thus, the deleterious effect of the proline substitution at position 42 might be due to a direct perturbation of the ligand-binding site. Moreover, additional effects of this proline substitution on the global conformational equilibrium and the destabilization of the active receptor state cannot be fully excluded at the moment.
Figure 2.
3-Dimensional Model of Wild-Type Melatonin MT2 Receptor. 3D homology model were built on the basis of an alignment with sequences of crystallized GPCRs rhodopsin, β-adrenergic receptors, adenosine A2A receptor. TM domains of melatonin receptors were predicted as the maximal common helix core between all template GPCR crystals.
Table 2.
Structural Analysis of the Deficient Mutations
| MT2 Mutants | Pwta | Pmuta | Location in 3-Dimensional Wild-Type Model | Residue Exposure | Local Effect | Putative Global Effect |
|---|---|---|---|---|---|---|
| Ala42Pro | 0.30 | 0 | Very close to extracellular region of TM1 | Protein | Gain of a kink in TM1 axis | Reorganization of helix packing impairing the native flexibility |
| Direct perturbation of the ligand binding site | ||||||
| Leu60Arg | 0.76 | 0 | Close to cytoplasmic region of TM1 | Membrane | Loss of hydrophobic protein-lipid tail contacts | Destabilization of cytoplasmic region for protein G binding |
| Gain of electrostatic protein-lipid head contacts | Stabilization of receptor impairing the native flexibility | |||||
| Pro95Leu | 0.96 | 0 | Close to extracellular region of TM2 | Membrane | Loss of a kink in TM2 axis | Penalizing change of extracellular flexibility/structure for ligand binding |
| Tyr308Ser | 1 | 0 | Very close to cytoplasmic region of TM7 | Protein | Perturbation | |
| Part of the conserved NPXXY TM7 pattern |
Pwt and Pmut are likelihoods at the critical positions of wild-type and mutated residues, respectively. The occurrences are deduced from 260 homologous sequences, not smaller than 75 residues and in the range 30–94% of sequence identity with the human melatonin MT2 receptor, extracted and aligned from a BLAST search (Basic local alignment search tool, [122]) against the UniRef100 database (123).
The leucine residue located at position 60 of the wild-type receptor is conserved in 76% of the 260 aligned sequences. Despite the presence of other amino acids in 24% of all cases, arginine residues or any other basic residues are never found at this position. From a biochemical point of view, the Leu60Arg substitution exhibits a strong change in hydrophobic potential from a hydrophobic leucine residue with a spherical side chain to a positively charged arginine residue with a long flexible side chain. Our 3-dimensional model predicts that position 60 is exposed to the membrane interface. It is therefore conceivable that the arginine mutation destabilizes the receptor by a local perturbation of hydrophobic contacts with membrane lipids. Given the close proximity of position 60 to the cytoplasmic interface of the membrane, the arginine substitution could alternatively maximize the anchoring of the receptor in the membrane by electrostatic interactions between its positive guanidinium side chain and negatively charged phosphate groups of lipid heads and thus stabilize the receptor in an inactive state.
With 96% and 100% sequence identity, proline and tyrosine residues at positions 95 and 308, respectively, in the wild-type receptor can be considered as essential amino acids of class A GPCRs at these positions. Similar to the Ala42Pro substitution, loss of the proline residue at position 95 is highly likely to change the helix axis and helix bundle packing. Interestingly, rhodopsin, an extensively studied class A GPCR member, has a threonine residue at the corresponding position. Because rhodopsin acts mainly as a constitutive rhodopsin-retinal complex, a proline residue at this position might be only essential for receptors with diffusible ligands. Proline residues are known to be hinge residues inducing kink angles in TM helices that modulate structural changes for ligand or G protein binding (30, 31). Based on this assumption, the proline residue at this position might be involved in structural organization of the upper region of TM2, neighboring TMs, and extracellular regions to favor receptor states able to bind melatonin.
The deficiency associated with the Tyr308Ser substitution is the most obvious because it occurs within one of the most conserved motifs of class A GPCRs, the 7.49NPxxY7.53 consensus motif (Ballestos-Wienstein annotation). Located at the heart of a large hydrogen bond network mediated by water molecules, the side chain of Tyr7.53 (corresponding to position 308 in the MT2 receptor) has been shown to undergo a huge 180° switch toward water molecules trapped in an internal cavity delimited by TM2, TM3, TM6, and TM7 and released by the displacement of TM6 during activation of rhodopsin (32–34). The serine substitution observed in the MT2 mutant at this position is thus likely to disrupt this hydrogen network and the subsequent stabilization of the active receptor state. Table 2 summarizes the sequence and structural features of the 4 deleterious mutants and potential local and global effects on receptor structure and function.
From Rare MT2 Mutants to T2D Development?
In mammals glucose homeostasis is crucial for the survival of the organism and is finely tuned by insulin and glucagon secretion from the islets of Langerhans in the pancreas and by the action of insulin in the target tissues (adipose tissue, muscle and liver). The pathology of T2D is generally characterized by hyperglycemia linked to an inability of pancreatic β-cells to produce enough insulin with parallel abnormalities in glucagon secretion by pancreatic α-cells, β-cell apoptosis, peripheral insulin resistance (35), and circadian misalignment (36). The interest of the role of melatonin in glucose homeostasis has been stimulated to a great degree by the identification of several polymorphisms linked to the MTNR1B gene as risk factors for developing T2D (27). In light of these recent findings, the existing literature on the role of melatonin and glucose homeostasis must be revisited.
Melatonin and glucose homeostasis: lessons and limitations of rodent animal models
Melatonin secreted by the pineal gland peaks at night in both nocturnal and diurnal mammals decoding the length of the night. It is important to note that despite the fact that both humans and rodents secrete melatonin during the night, the circadian rhythms of food intake and metabolism are shifted by 12 hours between humans and rodents. In some mammals, such as hamsters, sheep, and goats, melatonin is involved in the regulation of seasonal physiology and behavior (37). Mice are particular in relation to melatonin production, because several inbred strains of laboratory mice (for example, C57BL/6J, 129/Sv) have undetectable or very low plasma melatonin levels due to deficient or absent enzymes involved in melatonin biosynthesis such as aralkylamine N-acetyltransferase (AANAT) and hydroxyindole-O-methyltransferase (38, 39). Unlike rodents, which are active and mainly feed at night, humans are at rest and fasting, and during this postabsorptive period, plasma glucose levels are regulated primarily by gluconeogenesis and reduced glucose utilization (40). Furthermore, there are differences in the way that melatonin production is regulated in rats and humans (41). In general, the nocturnal rise in melatonin synthesis is primarily due to accumulation of active AANAT. In rats, protein synthesis of the enzyme increases at night, delaying melatonin production and increase of blood levels from the onset of darkness. In humans, a continuously high amount of AANAT exists, which is activated posttranslationally during darkness. Thus, humans produce melatonin at night at an earlier time point compared with rats (41). For these reasons, the choice of the correct animal model in relation to melatonin production and the extrapolation of any data from rodents to humans should be carefully considered.
How the absence of melatonin influences metabolic parameters (pinealectomy)
Pinealectomy represents a common intervention for assessing the effect of the lack of pineal melatonin in metabolism of laboratory animals. Although melatonin is the main product of the pineal gland, other, even though minor, molecules are secreted by the pineal gland, a fact that should be taken into account when interpreting results from pinealectomized (Pinx) animals (37). In Pinx rats, under basal conditions, glucose and glucagon levels were higher whereas insulin levels were lower compared with control rats (42, 43). A marked glucose intolerance under fed conditions was also observed, a situation significantly attenuated by treatment with melatonin (42). These characteristics point to a potential direct role of melatonin on pancreatic function in rats. Similar results were obtained in a subsequent study (44), reporting additional decreases in adipose cell responsiveness to insulin, a significant reduction of glucose transporter (Glut)4, the main glucose transporter of insulin-sensitive tissues, and a loss of the daily rhythm in the pancreatic ability to respond to glucose challenge. The same group had previously shown that a 4-hour treatment with melatonin enhances the insulin sensitivity of adipocytes (45). In addition, replacement of melatonin in Pinx rats for 30 days restores Glut4 protein content in isolated adipocytes, as well as the in vivo insulin sensitivity (46). These results, coupled with the findings of MT1 and MT2 receptor expression in rat inguinal and epididymal adipocytes (47), support a direct role of melatonin in rat adipocytes. Similar results were obtained in human white and brown adipose tissues. However, in contrast to rodents, melatonin treatment of human cells decreased Glut4 expression (48).
Not all studies agree on the consequences of pinealectomy on glucose homeostasis. In 2 studies, pinealectomy did not significantly alter basal plasma glucose and insulin levels or hepatic glucose production and whole-body or individual tissue glucose utilization (49, 50). The contradictory results are probably due to different experimental settings that do not allow direct comparisons. However, although basal glucagon and insulin levels were similar in both Pinx and control rats, Pinx rats displayed decreased glucagon secretion under hypoglycemic conditions (50). This reduction is probably related to the lack of melatonin, because melatonin supplements normalized glucagon levels quickly. Therefore, lack of melatonin resulted in decreased pancreatic glucagon secretion during hypoglycemia, suggesting that melatonin may sensitize α-cells to respond to low glucose conditions. A more recent study showed that Pinx rats displayed hepatic insulin resistance and increased gluconeogenesis and phosphoenol pyruvate carboxykinase (PEPCK) expression at the end of the nocturnal feeding period (51). The observed desynchronization of the gluconeogenic state of the liver from the feeding status is also encountered in humans with T2D that exhibit increased gluconeogenesis and hyperglycemia, especially during the first morning hours, in parallel to decreased levels of circulating melatonin (52). Thus, melatonin appears to collaborate to control nighttime gluconeogenesis and keep hepatic levels of PEPCK down-regulated until the end of dark phase in rodents. In addition, expression of melatonin receptors in the rat liver exhibits circadian rhythm, which is blunted by pinealectomy (53). Therefore, it would be interesting to test whether an alteration in the circadian control of hepatic gluconeogenesis and/or PEPCK expression contributes to the increased nighttime hyperglycemia induced by the absence of melatonin. A disrupted rhythm of insulin secretion was reported for islets isolated from Pinx rats compared with control islets pointing to the importance of melatonin as a synchronizer of biological rhythms linked to the development of T2D (54).
Pinealectomy in humans is a rare procedure that patients with pineal tumors are required to undergo and hence, there is no sufficient information on the effects on human physiology apart from a few reports referring to sleep disturbances (55). Lower or modified melatonin secretion patterns are reported for patients with bipolar disorders, due to defects in melatonin biosynthesis linked to mutations in the HIOMT enzyme (56). Interestingly, these populations belong to high-risk groups for developing metabolic syndrome-related diseases (57–60).
Taken together, the available literature on Pinx rats and humans, as well as carriers of mutations linked to melatonin biosynthesis, suggest a possible role of melatonin in the regulation of glucose homeostasis. However, a clear picture is still lacking particularly for humans for which data are scarce.
Models of diabetes: influence on melatonin levels and effect of melatonin treatment
Animal models of diabetes have been used to examine the influence of diabetes on melatonin levels and, in turn, the effect of melatonin treatment on glucose homeostasis and the diabetic status in these models.
Pineal melatonin synthesis has been reported to be decreased in experimentally induced diabetic Syrian hamster (61) and in T2D Goto-Kakizaki (GK) rats (62, 63), and improvement of glucose metabolism after melatonin administration was observed in an insulin-resistant mouse model (64). This beneficial effect of melatonin was due, at least in part, to the restoration of insulin-induced stimulation of blood flow and substrate delivery to skeletal muscle tissue. In agreement with the above studies, in a model of T2D rats, the Otsuka Long Evans Tokushima Fatty, long-term melatonin administration reduced plasma levels of triglycerides, total cholesterols, leptin, and insulin and increased the diminished Δ-5 desaturase activity in the liver, although no reduction of plasma glucose levels were observed in this case (65). Melatonin in that study served as an effective drug for reducing hyperinsulinemia and hypertriglyceridemia via restoring normal lipid metabolism in T2D. In a separate study, long-term nocturnal enteral administration of melatonin in control and GK rats reduced plasma insulin without affecting glucose levels, pointing to an antagonistic role between melatonin and insulin in rats, in agreement with the in vitro results from rodent cell lines (66). The authors also considered the possibility of a protective role of melatonin on pancreatic β-cell overstrain, because the exhaustion of the β-cells is implicated in the genesis of T2D, and, in this sense, melatonin can potentially be important for the prevention of the disease.
Diabetes in rats can also be induced by selective destruction of the insulin-producing β-cells of the pancreas with a single, rapid injection of streptozotocin (STZ), a naturally occurring chemical particularly toxic to β-cells. Indeed, STZ doses of 50–65 mg/kg lead to hyperglycemia, but severe ketosis does not develop even if insulin is not administered. The STZ-diabetic rat mimics many, but not all, the complications observed in diabetic humans and serves as a useful model for diabetes (67). A combined treatment of insulin and melatonin of STZ-induced diabetic rats had beneficial effects on blood glucose levels and body weight. Melatonin, or insulin alone, provided limited protection against hyperglycemia-induced oxidative damage in diabetic rats, whereas combined treatment with insulin and melatonin suppressed hyperglycemia, prevented oxidative damage, and restored endothelium function, indicating beneficial effects of the combined treatment (68). In STZ rats, insulin-positive β-cells appeared degranulated and degenerated or necrotic, explaining the decreased insulin secretion and increased blood glucose levels observed. Melatonin administration however, ameliorated the diabetic phenotype by causing a partial regeneration/proliferation of pancreatic β-cells, a decrease in serum glucose, and an increase in insulin concentrations (69). In a separate study, in pancreatic sections from STZ rats, a marked increase in the number of apoptotic caspase 3-positive cells and only a few antiapoptotic Bcl-xL and insulin-positive cells were observed (70). Treatment with melatonin resulted in the appearance of high-intensity insulin and antiapoptotic Bcl-xL-positive cells in the pancreas whereas the numbers of apoptotic cells were reduced. Collectively, these studies indicate that melatonin may have a positive impact on β-cell neogenesis and proliferation, as well as prevention of apoptosis. Consistently, in isolated rat pancreatic islets, melatonin administration-induced IGF receptor and insulin receptor (IR) tyrosine phosphorylation, and downstream pathways involved in cell survival and growth. Thus, melatonin may regulate growth and differentiation of pancreatic islets by activating IGF receptor and IR signaling pathways (71). The importance of melatonin in the proliferation and secretory capacity of pancreatic cells is supported by the data from morphometric analysis of pancreatic islets, which showed a larger area and a lower pancreatic islet density 70 days after pinealectomy and an increase in degenerative pathologic processes 25 days after pinealectomy (72). In conclusion, the studies on diabetic rats indicate a protective role of melatonin on the preservation of the pancreatic β-cell.
Less information is available about a possible correlation between melatonin levels and T2D in humans. These studies are complicated by high interindividual and age-dependent differences of melatonin secretion in humans (40). Nocturnal melatonin levels were reported to be significantly lower in T2D patients compared with healthy individuals (62). Similar results were obtained in a study of T2D patients with proliferative diabetic retinopathy, allowing the authors to postulate that dysfunctional retinal light perception is possibly linked to reduced melatonin secretion (73). In a recent study, an independent association between melatonin secretion and incidence of T2D was found in a nested, case control study among women participating in the Nurses' Health Study; participants with low melatonin secretion had an odd ratio of 2.17 for developing T2D compared with participants with high melatonin secretion (74). Taken together, the number of association studies on melatonin levels in T2D patients is still limited. Currently available data are consistent with the hypothesis that melatonin levels tend to be lower in T2D patients. Whether melatonin complementation improves metabolic parameters in T2D patients, as observed in several animal studies, remains to be shown.
Overweight/obese/aged models: effects of melatonin treatment
The effect of melatonin has also been extended toward body weight regulation. Daily melatonin administration in male, middle-aged, and older rats suppressed visceral fat, nonfasting plasma insulin, and leptin levels, independent of changes in plasma corticosterone, testosterone, T3, and IGF-1 levels (75). Because increased visceral fat is associated with increased insulin resistance, diabetes, and cardiovascular diseases, melatonin may ameliorate or prevent pathologies linked to metabolic syndrome. In agreement with this study, long-term melatonin treatment in middle-aged male rats decreased body weight, intraabdominal adiposity, plasma insulin, and plasma leptin to levels found in young rats, without altering food intake or total adiposity (76). Thus, we can speculate that the decrease of melatonin levels with aging might be linked to increased body weight, visceral adiposity, and associated metabolic complications present in aged rats. Furthermore, the high-fat diet-induced body weight gain of Sprague Dawley rats was significantly less pronounced when daily injected with melatonin for 3 weeks (77). Treated animals showed no modifications in plasma insulin levels, but decreased plasma glucose, leptin, and triglyceride levels. Consistently, pinealectomy increased body weight gain and feed efficiency in this high-fat diet-induced obesity model (77). A tendency for increased adipose tissue weight, insulinemia, and glycemia was observed, which was prevented by treatment with melatonin. In agreement with these data, in obese rats, daily ip injection for 8 weeks of melatonin, or NEU-P11, a melatonin agonist, inhibited both body weight gain and deposit of abdominal fat with no influence on food intake. An improvement of insulin sensitivity was observed, along with decreased levels of total cholesterol and triglycerides, whereas high-density lipoprotein cholesterol was increased (78). The results from the animal studies indicate that obesity, commonly present in aged but also young individuals, can potentially be prevented, to a certain degree, by melatonin administration.
Impairment of nocturnal melatonin secretion is also observed in aged humans with significant interindividual variability (40). Notably, in several neurodegenerative disorders, the levels of melatonin can be strongly decreased compared with the age-matched controls (40). During the course of aging, the body fat distribution changes (eg, increased visceral fat), among other detrimental changes that contribute to increased risk for metabolic diseases. Whether melatonin has a positive impact in ameliorating metabolic-associated complications by lowering body weight is not known, because, to our knowledge, there are no clinical trials available testing melatonin's potential weight-lowering effects. Positive results from animal studies may encourage the establishment of such clinical trials.
Knockout (KO) mouse models for melatonin
Up to the present time, several general KO mouse models have been produced for both MT1 and MT2 receptors. Both KO mice for melatonin receptors in the melatonin-proficient CH3 background are associated with disturbed circadian rhythms, in relation to phase shifts and amplitude changes of the clock output genes nuclear receptor subfamily 1, group D, member 1 (NR1D1, more commonly known as RevErba) and D site of albumin promoter (albumin D-box) binding protein (DBP) in the pancreas and liver (79). Moreover, an impact of melatonin receptor deficiency on glucagon receptor (GCGR) expression levels in the liver (increased expression of GCGR) (80), insulin transcripts (increased expression of Ins2 transcript), and altered regulation of insulin secretion and glucose homeostasis were monitored in the KO animals (79). Insulin secretion from isolated islets of MT1, MT2, or MT1/MT2 KO animals was found to be increased relative to the wild type when they were stimulated by melatonin (80). In a separate study by Contreras-Alcantara et al. (81) MT1 but not MT2 KO mice exhibited impaired glucose metabolism, most probably due to increased insulin resistance. Collectively, melatonin receptor KO mice show a mild metabolic phenotype with some alteration in circadian rhythms and impaired glucose metabolism. Tissue-specific KO animals might help to better dissect the role of melatonin receptors in specific tissues such as the pancreas.
As reported earlier, T2D risk is increased about 6 times in individuals carrying loss-of-function MT2 mutants (27). It would be particularly interesting if this result could be replicated in appropriate knock-in animal models that would carry the relevant MT2 mutations.
In conclusion, the above studies show that rodent models can provide valuable information about the role of melatonin in glucose homeostasis. These studies suggest that melatonin may act as metabolic regulator by directly acting on pancreas, liver and/or adipose tissue. In cases of disturbed energy balance, such as diabetes or obesity, melatonin improved body weight gain and prevented some of the side effects of altered glucose homeostasis, such as insulin sensitivity. On the other hand, lack of pineal melatonin seems to alter levels of plasma glucagon or insulin and contribute to insulin resistance, effects that in certain cases can be ameliorated by administration of melatonin. However, whether these data can be extrapolated to humans remains to be shown.
Circadian rhythms, diabetes, and melatonin
Growing evidence connects the metabolic syndrome, including diabetes and obesity, with the deregulation of the circadian system (82). Melatonin is an interesting hormone in this respect, because it may play a central role both as an input and output factor of the circadian system. Indeed, melatonin synthesis is regulated by light and the circadian master clock located in the suprachiasmatic nucleus (SCN). Importantly, melatonin can feed back on SCN neurons to regulate the synchronization of its oscillators (83). A set of transcription factors rhythmically expressed comprise the clock regulators including the clock circadian regulator (Clock) and Aryl hydrocarbon receptor nuclear translocator-like (Arntl, also known as Bmal1) that heterodimerize and activate transcription of target genes, including Period (Per1, 2, and 3) and Cryptochrome (Cry 1 and 2) (84). In addition to the SCN, clock activities have been identified in numerous peripheral tissues including the liver, white adipose tissue, and pancreas that control metabolic processes (85, 86). Peripheral clocks are autonomous oscillators synchronized by the master clock and, in some cases, regulated by environmental cues such as feeding. Melatonin production reflects the length of the night, and this information is transmitted to the tissues of the organism that contain melatonin receptors (87). Melatonin has thus the potential to entrain circadian rhythms in peripheral organs. However, this has only been shown in some tissues outside of the SCN, such as the pars tuberalis of the pituitary and the fetal adrenal gland (88). In the pars tuberalis of MT1 but not MT2 KO mice expression of mPer1, mCry1, Clock, and Bmal1 was dramatically reduced, showing that melatonin acting through MT1 receptors is an important regulator of clock gene expression in this tissue (89). With regard to the pancreas, some marginal effects were monitored for MT1 KO including a slight delay (3 h) phase advance for the RevErbα and DBP and an increase in the amplitude of RevErbα. MT2 KO pancreases exhibited strong increases in the amplitude of RevErbα and DBP, indicating a potential regulatory role for MT1 and MT2 on the circadian rhythm of the pancreas by regulating the clock output genes RevErba and DBP (79).
Dysfunction of the circadian rhythms is linked to altered glucose homeostasis and metabolic abnormalities. Experimentally induced desynchronization between endogenous circadian rhythms and sleep-wake cycles has been reported to significantly contribute to higher fasting plasma glucose levels (90). Similar observations were made in shift workers who show an increased risk for T2D (91). In these cases, melatonin dysfunction can either serve as a cause of circadian misalignment (eg, in aged individuals, or individuals carrying mutations in melatonin receptors) or as a consequence of a disease. A genetic association study signified the relevance of a promoter polymorphism in the Bmal1 gene, a key component of the mammalian molecular clock, with T2D and hypertension highlighting the importance of the molecular clock in the prevention of T2D (92). Notably, a meta-analysis study including a total of 122 743 participants for fasting glucose established genome-wide significant associations for 9 new loci for fasting glucose including a variant in the promoter region of CRY2 contributing further evidence to the key role of the circadian machinery in regulating glucose homeostasis (93). Moreover, a nonsynonymous polymorphism (G639V) in the clock gene PER3 was identified by GWAS as a risk variant for T2D in Mexican populations (94). The above studies clearly show an association between the circadian clocks and diabetes that may enable the understanding of the circadian misalignment with diabetes for effective future therapeutics.
In healthy individuals, insulin secretion exhibits a circadian pattern, increasing from a nadir between midnight and 6 am and reaching a peak between noon and 6 pm, following an opposite pattern of secretion to melatonin (36). Plasma glucagon concentration has also been shown to exhibit a daily rhythm, at least in rats, which is governed by the master clock located in the SCN (95, 96). Bmal1 is required for normal insulin secretion, and glucose homeostasis as islets from pancreas-specific Bmal1−/− mice exhibited severe glucose intolerance and defective insulin production (97). Clock and Bmal1 pancreas-specific ablation in mice resulted in functional defects in insulin secretion and decreases in islet size and survival, indicating a key role of peripheral clocks in the regulation of glucose homeostasis (98). Despite normal glucose-stimulated calcium influx in these mice, insulin exocytosis is ablated, allowing the speculation that the defects in insulin secretion are linked to an altered protein-packaging system involved in the fusion of secretory granules (99).
Taken together, it is well established that dysfunction of central and peripheral circadian rhythms leads to metabolic disorders including T2D in mice. Whether a defective melatonin transmission system participates in the deregulation of the circadian system remains an open question. Melatonin could either directly influence the clock machinery in the pancreas or indirectly via the SCN.
Pancreas and melatonin
Apart from regulating circadian rhythms, melatonin might also modulate the physiology of peripheral tissues involved in glucose homeostasis. The pancreas has been the focus of many studies in this respect. β-Cells, the predominant cell type in pancreatic islets (70%), plays an essential role in glucose homeostasis through insulin secretion in response to nutrients (100). α-Cells, which constitute approximately 20% of the islet, release glucagon to stimulate production of glucose from the liver in response to hypoglycemia. α-Cell dysfunctions have been described in T2D, including relative glucagon hypersecretion at normal and elevated glucose levels, as well as an impaired response to hypoglycemia (101). Glucagon secretion is thought to be suppressed, among other factors, by insulin via intra-islet paracrine mechanisms (102). However, evidence suggests that human and rat islets require insulin prior to responding to decreased glucose (103, 104). Because IR in α-cells, and hence insulin, is required for glucagon release during hypoglycemia (105), defects in the insulin/IR-signaling pathway might account for the failure of glucose-stimulated glucagon secretion. Plasticity exists between the 2 cell types: in conditions of β-cell injury, α-cells have been reported to produce glucagon-like peptide 1 (GLP-1) and its receptor, a first step toward the differentiation into β-cells (102), whereas dedifferentiation of β- into α-cell has also been reported to contribute to decreased β-cell mass and hyperglucagonemia (106). Therefore, the importance of the normal function of the different pancreatic cell types is crucial for the organism to achieve normoglycemia.
Pancreatic β-cell proliferation, survival and insulin secretion are thought to be regulated by signaling pathways linked to GPCRs, such as GLP-1R, the pituitary adenylate cyclase-activating polypeptide type I receptor, glucose-dependent insulinotropic polypeptide receptor, and M3 muscarinic receptor (107). Expression of MT1 and MT2 receptors has been shown for human and rodent pancreatic tissues and islets and rodent cell lines (16, 21, 80, 108–113). The presence of melatonin receptors in the pancreas suggests that their activation by melatonin might directly influence insulin or glucagon production and provides a biochemical basis to explain how decreased melatonin levels of diabetic patients could affect the function of the pancreas (62, 114).
Pancreatic β-cells and melatonin receptors
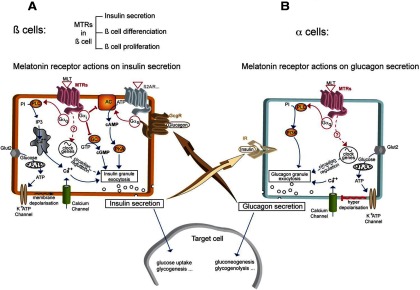
The predominant trigger for insulin secretion is glucose, which is taken up by β-cells via GLUT2 (Figure 3A). Further metabolism of glucose in the mitochondria results in the increase of ATP at the expense of ADP that leads to the closure of KATP channels, thereby initiating electrical activity, calcium channel opening, and calcium influx that triggers exocytosis of insulin from insulin granules (115). The insulinotropic effect is also mediated by stimulation of cAMP levels and activation of PKA-dependent and independent pathways engaged by GPCRs that are coupled to Gs proteins such as the GLP1R, glucose-dependent insulinotropic polypeptide receptor, or pituitary adenylate cyclase-activating polypeptide type I receptor (107). GPCRs coupled to Gq/11 signaling such as M3 muscarinic receptors regulate insulin secretion. Melatonin receptors have been reported to couple to Gi proteins and inhibit cAMP production, hence acting as negative regulators of insulin secretion in rodent insulinoma cell lines (108, 109, 112, 116). However, it appears that prolonged pretreatment with melatonin sensitizes the cAMP pathway of β-cells. O/N treatment of INS-1 cells by melatonin resulted in marked increases in insulin secretion, cAMP response element-mediated gene expression, and insulin promoter-driven luciferase gene expression in response to GLP-1 or forskolin (108). In isolated rat islets, insulin secretion was enhanced following melatonin pretreatment both in the absence and presence of GLP-1 or forskolin. In human subjects, insulin secretory responses to nutritional stimuli are commonly more robust in the morning, compared with late at night.
Figure 3.
Melatonin Receptors in Pancreatic α- and β-Cells. A, β-cells: In β-cells, the main signal for insulin secretion is the extracellular glucose concentration. Transporters (Glut2) transfer glucose to mitochondria, allowing ATP production. ATP causes the closure of K+ATP channels leading to membrane depolarization and activation of voltage-dependent L-type Ca++ channels. The subsequent intracellular calcium increase triggers insulin granule exocytosis and insulin liberation to the extracellular medium. Activated GPCRs coupled to Gαs, such as ß2-adrenergic receptor (β2AR) but also glucagon receptor (GcgR), lead to intracellular cAMP increase and activation of cAMP-dependent protein kinase (protein kinase A [PKA]) which is a secondary signal for insulin liberation. Melatonin receptors (MTRs) can interact with insulin secretion by activating different pathways leading to opposite effects: the predominant signal is given by activated MTRs coupled to Gαi which counteract cAMP production by inhibiting adenylyl cyclase (AC) or cGMP production by inhibiting guanylate cyclase (GC), thus leading to decrease of insulin secretion. In addition, MTRs can couple to Gαq to activate phospholipase C (PLC), increasing [Ca++]i from intracellular stores by stimulation of inositol-1,4,5-triphosphate receptor, and inducing insulin secretion. Other possible means of MTRs to regulate β-cell function are regulation of genes of the pancreatic circadian clock, or β-cell proliferation and differenciation. B, α-cells: In α-cells, a very similar granule exocytosis system to β-cells exists, based on K+ATP channels and L-type Ca++ channels. At low glucose concentrations, the system acts the same way as in β-cells: [Ca++]i increase leads to glucagon granule exocytosis and glucagon liberation. At high-glucose concentrations (high intracellular ATP), the closure of K+ATP channels leads to membrane hyperdepolarization, which triggers the shutting of Ca++ channels and inhibition of glucagon secretion. A cross talk between β- and α-cells is represented by IR activation in α-cells as an additional regulator with positive or negative effect of glucagon release, depending on the context. In addition, secreted glucagon is able to increase insulin secretion by activating the Gαs-coupled glucagon receptor (GcgR) present in ß-cells. Activated MTRs seem to act positively on granule secretion through a PLC/phosphatidyl-3-phosphate kinase (PI3K) pathway triggered by Gαq activation. Regulation of the pancreatic circadian clock is another putative way for MTRs to modulate α-cell function.
MT1 receptor is coupled to parallel signaling pathways, with opposite influences on insulin secretion. In INS1 cells, melatonin, acting on its receptors, activates the inhibitory Gi protein that results in subsequent reduction of cAMP production and insulin release (109) but can also stimulate inositol-1,4,5-triphosphate and Ca2+release (Figure 3A). However, its predominant effect is on cAMP inhibition with negative consequences on insulin release. The inhibitory role of MT1 in insulin release is demonstrated by the significant increase in Ins1 mRNA and basal insulin secretion in INS-1 cells after MT1 knockdown (117). Insulin secretion in isolated islets from the control mouse resulted in a decrease in insulin secretion, an effect that was absent in the islets of MT1 or MT1 /MT2 KO animals. Overall, melatonin inhibits insulin secretion in rodent β-cells primarily through the Gi protein/cAMP pathway.
Insulin release can also be potentiated directly by cGMP analogs (118) (Figure 3A). Melatonin can reduce cGMP levels in INS-1 cells and inhibit insulin secretion via activation of MT2 receptors (119, 120) via coupling to Gi proteins. Contrary to the effects reported for rodent cells, melatonin administration in human islets stimulated insulin secretion, whereas cAMP levels were not affected (112). This is the only study, to our knowledge, that examines effects of melatonin in human islets and reports a direct, stimulatory role of melatonin on insulin secretion. Obviously, the results in human have to be replicated before drawing any conclusion.
Pancreatic α-cells and melatonin receptors
The implication of melatonin in glucose metabolism might be through its action on pancreatic α-cells (Figure 3B). Indeed, MT1 expression has been shown for the glucagons-secreting α-cells in humans (112) and for both MT receptors in the murine pancreatic α-cell line αTC1.9 on the mRNA level (80). Treatment of αTC1.9 cells with melatonin enhanced expression and secretion of glucagon, with more pronounced effects under hyperglycemic conditions (80). In diabetic GK rats melatonin administration resulted only in a small decrease of the elevated glucagon levels observed in this animal model. In contrast, in control rats, melatonin increased glucagon levels, in agreement with the in vitro results (80). Moreover, in MT2 KO and MT1/MT2 KO, but not in MT1 KO mice, basal islet glucagon secretion was significantly reduced, pointing to a predominant role of MT2 receptors in glucagon secretion in mice (121). The mechanism for glucagon stimulation by melatonin seems to involve Gq/11-dependent activation of phospholipase C and phosphatidylinositide 3-kinase, but not Gi or Gs-coupled signaling cascades. In conclusion, in rodents, glucagon release can be directly modulated by MT2 receptors.
In human islets, which express predominantly MT1 receptors at the mRNA level, exogenous melatonin increased intracellular calcium levels and stimulated glucagon secretion (112). Because insulin secretion increased concomitantly upon melatonin treatment of these islets, the authors discussed the possibility of glucagon acting in a paracrine fashion by stimulating β-cells to secrete insulin through Gs-coupled glucagon receptors. However, other possibilities such as a direct effect of melatonin on β-cells or an action of insulin on the IR in α-cells to enable glucagon secretion as described earlier (105) cannot be excluded at the moment. Collectively, studies in rodent and human α-cells indicate that these cells might be a direct target of melatonin. Controversies exist about the precise receptor subtype involved and the possibility of paracrine effects between α- and β-cells. More studies, in particular, on human islets are urgently needed to clarify these issues.
Perspectives and Challenges
The identification of frequent variants located in the noncoding region of the MTNR1B gene that associate with T2D risk was a major breakthrough in the field and provided, for the first time, a solid basis for a possible link between melatonin and glucose homeostasis in humans. Subsequent identification of rare nonsynonymous variants in the same gene established a firm functional link between loss of MT2 function and increased T2D risk. Deciphering of how and where melatonin interferes with glucose homeostasis represents one of the most important and challenging questions to be solved in the domain. The involvement of melatonin's capacity to modulate circadian rhythms as a basis of its role in glucose homeostasis regulation is an attracting hypothesis, for which, however, no experimental evidence exists for the moment, neither at the central nor peripheral level. Effects of melatonin on the signaling pathways and metabolism of glucose-sensitive tissues including the pancreas represents the second, alternative, hypothesis to explain melatonin's effects. Among the different tissues, most of the studies concentrated on pancreatic β-cells so far and more recently extended to pancreatic α-cells; however, other tissues cannot be excluded at the moment. Of note, almost all data gathered in this field are from rats and mice. However, due to the fundamental differences concerning the metabolically active period in rodents and humans (day-active humans vs night-active rodents) and the nocturnal melatonin rhythm observed in both, any conclusion or extrapolation from rodents to humans must be handled with caution. Furthermore, it is extremely important to extend the functional characterization of the MT2 mutants study toward establishing their full functional profile. This will allow definition of the precise repertoire of defects that are associated with T2D risk. To further confirm the importance of the melatonin pathway in glucose homeostasis regulation, genetic association studies on other pathway members (receptors and enzymes of the melatonin synthesis) will be necessary. A concerted action of MTNR1B variants with variants in other genes might also be considered in the future in respect to T2D development. All identified carriers of rare MTNR1B variants are heterozygous. Identification of homozygous individuals is likely to reveal the full spectrum of phenotypes associated with loss of MT2 function. Several animal studies suggest that melatonin supplementation may have beneficial effects on glucose homeostasis and body weight regulation under certain circumstances, which should encourage clinical trials in humans to evaluate the therapeutic potential of this hormone in this respect. It is hoped that the functional defects in those individuals with partial loss of MT2 function will help to provide personalized therapies either by potentiating the defective response by pharmacologic intervention or by providing advice in respect to their chronobiotic sleep and eating behaviors.
Acknowledgments
We thank Drs A. Bonnefond and P. Froguel (Institute Pasteur, Lille, University of Lille, France) for a fruitful collaboration on the identification and characterization of MT2 variants.
This work was supported by grants from the Agence Nationale de la Recherche (ANR 2011 -BSV1–012-01 “MLT2D” and ANR-2011-META “MELA-BETES”), the Fondation Recherche Médicale (Equipe FRM DEQ20130326503, to R.J.), Institut National de la Santé et de la Recherche Médicale and Centre National de la Recherche Scientifique. A.K. holds a postdoctoral fellowship from the Fondation pour la Recherche Médicale.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by grants from the Agence Nationale de la Recherche (ANR 2011 -BSV1–012-01 “MLT2D” and ANR-2011-META “MELA-BETES”), the Fondation Recherche Médicale (Equipe FRM DEQ20130326503, to R.J.), Institut National de la Santé et de la Recherche Médicale and Centre National de la Recherche Scientifique. A.K. holds a postdoctoral fellowship from the Fondation pour la Recherche Médicale.
Footnotes
- AANAT
- aralkylamine N-acetyltransferase
- FPG
- fasting plasma glucose
- GCGR
- glucagon receptor
- GK
- Goto-Kakizaki
- GLP-1
- glucagon-like peptide 1
- GLUT
- glucose transporter
- GPCR
- G protein-coupled receptor
- GWAS
- genome-wide association studies
- IR
- insulin receptor
- KO
- knockout
- PEPCK
- phosphoenol pyruvate carboxykinase
- Pinx
- pinealectomized
- SCN
- suprachiasmatic nucleus
- STZ
- streptozotocin
- T2D
- type 2 diabetes
- TM
- transmembrane.
References
- 1. Vassilatis DK, Hohmann JG, Zeng H, et al. . The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci USA. 2003;100:4903–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fredriksson R, Schiöth HB. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol Pharmacol. 2005;67:1414–1425. [DOI] [PubMed] [Google Scholar]
- 3. Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. [DOI] [PubMed] [Google Scholar]
- 4. Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vassart G, Costagliola S. G protein-coupled receptors: mutations and endocrine diseases. Nat Rev Endocrinol. 2011;7:362–372. [DOI] [PubMed] [Google Scholar]
- 6. Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol. 2003;43:381–411. [DOI] [PubMed] [Google Scholar]
- 7. Small KM, Tanguay DA, Nandabalan K, Zhan P, Stephens JC, Liggett SB. Gene and protein domain-specific patterns of genetic variability within the G-protein coupled receptor superfamily. Am J Pharmacogenomics. 2003;3:65–71. [DOI] [PubMed] [Google Scholar]
- 8. Lee A, Rana BK, Schiffer HH, et al. . Distribution analysis of nonsynonymous polymorphisms within the G-protein-coupled receptor gene family. Genomics. 2003;81:245–248. [DOI] [PubMed] [Google Scholar]
- 9. Spanakis E, Milord E, Gragnoli C. AVPR2 variants and mutations in nephrogenic diabetes insipidus: review and missense mutation significance. J Cell Physiol. 2008;217:605–617. [DOI] [PubMed] [Google Scholar]
- 10. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. [DOI] [PubMed] [Google Scholar]
- 11. Xiang Z, Litherland SA, Sorensen NB, et al. . Pharmacological characterization of 40 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists and the agouti-related protein (AGRP) antagonist. Biochemistry. 2006;45:7277–7288. [DOI] [PubMed] [Google Scholar]
- 12. Thompson MD, Burnham WM, Cole DE. The G protein-coupled receptors: pharmacogenetics and disease. Crit Rev Clin Lab Sci. 2005;42:311–392. [DOI] [PubMed] [Google Scholar]
- 13. Johnson JA, Liggett SB. Cardiovascular pharmacogenomics of adrenergic receptor signaling: clinical implications and future directions. Clin Pharmacol Ther. 2011;89:366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kazius J, Wurdinger K, van Iterson M, Kok J, Bäck T, Ijzerman AP. GPCR NaVa database: natural variants in human G protein-coupled receptors. Hum Mutat. 2008;29:39–44. [DOI] [PubMed] [Google Scholar]
- 15. Prokopenko I, Langenberg C, Florez JC, et al. . Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bouatia-Naji N, Bonnefond A, Cavalcanti-Proença C, et al. . A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. [DOI] [PubMed] [Google Scholar]
- 17. Xia Q, Chen ZX, Wang YC, et al. . Association between the melatonin receptor 1B gene polymorphism on the risk of type 2 diabetes, impaired glucose regulation: a meta-analysis. PLoS One. 2012;7:e50107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelliny C, Ekelund U, Andersen LB, et al. . Common genetic determinants of glucose homeostasis in healthy children: the European Youth Heart Study. Diabetes. 2009;58:2939–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barker A, Sharp SJ, Timpson NJ, et al. . Association of genetic Loci with glucose levels in childhood and adolescence: a meta-analysis of over 6,000 children. Diabetes. 2011;60:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walford GA, Green T, Neale B, et al. . Common genetic variants differentially influence the transition from clinically defined states of fasting glucose metabolism. Diabetologia. 2012;55:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lyssenko V, Nagorny CL, Erdos MR, et al. . Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langenberg C, Pascoe L, Mari A, et al. . Common genetic variation in the melatonin receptor 1B gene (MTNR1B) is associated with decreased early-phase insulin response. Diabetologia. 2009;52:1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao S, Liu Y, Tan Y, et al. . Association of genetic variants of melatonin receptor 1B with gestational plasma glucose level and risk of glucose intolerance in pregnant Chinese women. PLoS One. 2012;7:e40113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mao H, Li Q, Gao S. Meta-analysis of the relationship between common type 2 diabetes risk gene variants with gestational diabetes mellitus. PLoS One. 2012;7:e45882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what's new? Br J Pharmacol. 2008;154:1182–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li C, Shi Y, You L, Wang L, Chen ZJ. Melatonin receptor 1A gene polymorphism associated with polycystic ovary syndrome. Gynecol Obstet Invest. 2011;72:130–134. [DOI] [PubMed] [Google Scholar]
- 27. Bonnefond A, Clément N, Fawcett K, et al. . Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet. 2012;44:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chakrabarti P, Chakrabarti S. C–H… O hydrogen bond involving proline residues in α-helices. J Mol Biol. 1998;284:867–873. [DOI] [PubMed] [Google Scholar]
- 30. Huang YH, Chen CM. Statistical analyses and computational prediction of helical kinks in membrane proteins. J Comput Aided Mol Des. 2012;26:1171–1185. [DOI] [PubMed] [Google Scholar]
- 31. Shi L, Liapakis G, Xu R, Guarnieri F, Ballesteros JA, Javitch JA. β2 Adrenergic receptor activation. Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. J Biol Chem. 2002;277:40989–40996. [DOI] [PubMed] [Google Scholar]
- 32. Palczewski K, Kumasaka T, Hori T, et al. . Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. [DOI] [PubMed] [Google Scholar]
- 33. Pardo L, Deupi X, Dölker N, López-Rodríguez ML, Campillo M. The role of internal water molecules in the structure and function of the rhodopsin family of G protein-coupled receptors. Chembiochem. 2007;8:19–24. [DOI] [PubMed] [Google Scholar]
- 34. Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. [DOI] [PubMed] [Google Scholar]
- 35. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. [DOI] [PubMed] [Google Scholar]
- 36. Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol Endocrinol Metab. 1996;271:E246–E252. [DOI] [PubMed] [Google Scholar]
- 37. Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod. 1998;3:13–22. [DOI] [PubMed] [Google Scholar]
- 38. Kennaway DJ, Voultsios A, Varcoe TJ, Moyer RW. Melatonin in mice: rhythms, response to light, adrenergic stimulation, and metabolism. Am J Physiol Regul Integr Comp Physiol. 2002;282:R358–R365. [DOI] [PubMed] [Google Scholar]
- 39. Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491–493. [DOI] [PubMed] [Google Scholar]
- 40. Hardeland R. Melatonin in aging and disease -multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012;3:194–225. [PMC free article] [PubMed] [Google Scholar]
- 41. Watson RR. Melatonin in the Promotion of Health. 2nd ed: CRC Press; 2012 [Google Scholar]
- 42. Diaz B, Blázquez E. Effect of pinealectomy on plasma glucose, insulin and glucagon levels in the rat. Horm Metab Res. 1986;18:225–229. [DOI] [PubMed] [Google Scholar]
- 43. Rodríguez V, Mellado C, Alvarez E, De Diego JG, Blázquez E. Effect of pinealectomy on liver insulin and glucagon receptor concentrations in the rat. J Pineal Res. 1989;6:77–88. [DOI] [PubMed] [Google Scholar]
- 44. Lima FB, Machado UF, Bartol I, et al. . Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am J Physiol. 1998;275:E934–E941. [DOI] [PubMed] [Google Scholar]
- 45. Lima FB, Matsushita DH, Hell NS, Dolnikoff MS, Okamoto MM, Cipolla Neto J. The regulation of insulin action in isolated adipocytes. Role of the periodicity of food intake, time of day and melatonin. Braz J Med Biol Res. 1994;27:995–1000. [PubMed] [Google Scholar]
- 46. Zanquetta MM, Seraphim PM, Sumida DH, Cipolla-Neto J, Machado UF. Calorie restriction reduces pinealectomy-induced insulin resistance by improving GLUT4 gene expression and its translocation to the plasma membrane. J Pineal Res. 2003;35:141–148. [DOI] [PubMed] [Google Scholar]
- 47. Zalatan F, Krause JA, Blask DE. Inhibition of isoproterenol-induced lipolysis in rat inguinal adipocytes in vitro by physiological melatonin via a receptor-mediated mechanism. Endocrinology. 2001;142:3783–3790. [DOI] [PubMed] [Google Scholar]
- 48. Brydon L, Petit L, Delagrange P, Strosberg AD, Jockers R. Functional expression of mt2 (mel1b) melatonin receptors in human paz6 adipocytes. Endocrinology. 2001;142:4264–4271. [DOI] [PubMed] [Google Scholar]
- 49. BizotEspiard JG, Double A, Cousin B, et al. . Lack of melatonin effects on insulin action in normal rats. Hormone Metab Res. 1998;30:711–716. [DOI] [PubMed] [Google Scholar]
- 50. Kosa E, Maurel D, Siaud P. Effects of pinealectomy on glucagon responsiveness to hypoglycaemia induced by insulin injections in fed rats. Exp Physiol. 2001;86:617–620. [DOI] [PubMed] [Google Scholar]
- 51. Nogueira TC, Lellis-Santos C, Jesus DS, et al. . Absence of melatonin induces night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response. Endocrinology. 2011;152:1253–1263. [DOI] [PubMed] [Google Scholar]
- 52. Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia. 2006;49:1619–1628. [DOI] [PubMed] [Google Scholar]
- 53. Venegas C, García JA, Doerrier C, et al. . Analysis of the daily changes of melatonin receptors in the rat liver. J Pineal Res. 2013;54:313–321. [DOI] [PubMed] [Google Scholar]
- 54. Picinato MC, Haber EP, Carpinelli AR, Cipolla NJ. Daily rhythm of glucose-induced insulin secretion by isolated islets from intact and pinealectomized rat. J Pineal Res. 2002;33:172–177. [DOI] [PubMed] [Google Scholar]
- 55. Kocher L, Brun J, Borson-Chazot F, Gonnaud PM, Claustrat B. Increased REM sleep associated with melatonin deficiency after pinealectomy: a case study. Chronobiol Int. 2006;23:889–901. [DOI] [PubMed] [Google Scholar]
- 56. Etain B, Dumaine A, Bellivier F, et al. . Genetic and functional abnormalities of the melatonin biosynthesis pathway in patients with bipolar disorder. Hum Mol Genet. 2012;21:4030–4037. [DOI] [PubMed] [Google Scholar]
- 57. McIntyre RS, Konarski JZ, Misener VL, Kennedy SH. Bipolar disorder and diabetes mellitus: epidemiology, etiology, and treatment implications. Ann Clin Psychiatry. 2005;17:83–93. [DOI] [PubMed] [Google Scholar]
- 58. Chien IC, Chang KC, Lin CH, Chou YJ, Chou P. Prevalence of diabetes in patients with bipolar disorder in Taiwan: a population-based national health insurance study. Gen Hosp Psychiatry. 2010;32:577–582. [DOI] [PubMed] [Google Scholar]
- 59. Salvi V, D'Ambrosio V, Rosso G, Bogetto F, Maina G. Age-specific prevalence of metabolic syndrome in Italian patients with bipolar disorder. Psychiatry Clin Neurosci. 2011;65:47–54. [DOI] [PubMed] [Google Scholar]
- 60. McIntyre RS, Danilewitz M, Liauw SS, et al. . Bipolar disorder and metabolic syndrome: an international perspective. J Affect Disord. 2010;126:366–387. [DOI] [PubMed] [Google Scholar]
- 61. Champney TH, Brainard GC, Richardson BA, Reiter RJ. Experimentally-induced diabetes reduces nocturnal pineal melatonin content in the Syrian hamster. Comp Biochem Physiol A Comp Physiol. 1983;76:199–201. [DOI] [PubMed] [Google Scholar]
- 62. Peschke E, Frese T, Chankiewitz E, et al. . Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J Pineal Res. 2006;40:135–143. [DOI] [PubMed] [Google Scholar]
- 63. Frese T, Bach AG, Mühlbauer E, et al. . Pineal melatonin synthesis is decreased in type 2 diabetic Goto-Kakizaki rats. Life Sci. 2009;85:526–533. [DOI] [PubMed] [Google Scholar]
- 64. Sartori C, Dessen P, Mathieu C, et al. . Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology. 2009;150:5311–5317. [DOI] [PubMed] [Google Scholar]
- 65. Nishida S, Segawa T, Murai I, Nakagawa S. Long-term melatonin administration reduces hyperinsulinemia and improves the altered fatty-acid compositions in type 2 diabetic rats via the restoration of Δ-5 desaturase activity. J Pineal Res. 2002;32:26–33. [DOI] [PubMed] [Google Scholar]
- 66. Peschke E, Schucht H, Mühlbauer E. Long-term enteral administration of melatonin reduces plasma insulin and increases expression of pineal insulin receptors in both Wistar and type 2-diabetic Goto-Kakizaki rats. J Pineal Res. 2010;49:373–381. [DOI] [PubMed] [Google Scholar]
- 67. Wei M, Ong L, Smith MT, et al. . The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ. 2003;12:44–50. [DOI] [PubMed] [Google Scholar]
- 68. Paskaloglu K, Sener G, Ayanğolu-Dülger G. Melatonin treatment protects against diabetes-induced functional and biochemical changes in rat aorta and corpus cavernosum. Eur J Pharmacol. 2004;499:345–354. [DOI] [PubMed] [Google Scholar]
- 69. Kanter M, Uysal H, Karaca T, Sagmanligil HO. Depression of glucose levels and partial restoration of pancreatic β-cell damage by melatonin in streptozotocin-induced diabetic rats. Arch Toxicol. 2006;80:362–369. [DOI] [PubMed] [Google Scholar]
- 70. Simsek N, Kaya M, Kara A, Can I, Karadeniz A, Kalkan Y. Effects of melatonin on islet neogenesis and β cell apoptosis in streptozotocin-induced diabetic rats: an immunohistochemical study. Domest Anim Endocrinol. 2012;43:47–57. [DOI] [PubMed] [Google Scholar]
- 71. Picinato MC, Hirata AE, Cipolla-Neto J, et al. . Activation of insulin and IGF-1 signaling pathways by melatonin through MT1 receptor in isolated rat pancreatic islets. J Pineal Res. 2008;44:88–94. [DOI] [PubMed] [Google Scholar]
- 72. de Lima LM, dos Reis LC, de Lima MA. Influence of the pineal gland on the physiology, morphometry and morphology of pancreatic islets in rats. Braz J Biol. 2001;61:333–340. [DOI] [PubMed] [Google Scholar]
- 73. Hikichi T, Tateda N, Miura T. Alteration of melatonin secretion in patients with type 2 diabetes and proliferative diabetic retinopathy. Clin Ophthalmol. 2011;5:655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. Jama. 2013;309:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rasmussen DD, Boldt BM, Wilkinson CW, Yellon SM, Matsumoto AM. Daily melatonin administration at middle age suppresses male rat visceral fat, plasma leptin, and plasma insulin to youthful levels. Endocrinology. 1999;140:1009–1012. [DOI] [PubMed] [Google Scholar]
- 76. Wolden-Hanson T, Mitton DR, McCants RL, et al. . Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology. 2000;141:487–497. [DOI] [PubMed] [Google Scholar]
- 77. Prunet-Marcassus B, Desbazeille M, Bros A, et al. . Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology. 2003;144:5347–5352. [DOI] [PubMed] [Google Scholar]
- 78. She M, Deng X, Guo Z, et al. . NEU-P11, a novel melatonin agonist, inhibits weight gain and improves insulin sensitivity in high-fat/high-sucrose-fed rats. Pharmacol Res. 2009;59:248–253. [DOI] [PubMed] [Google Scholar]
- 79. Mühlbauer E, Gross E, Labucay K, Wolgast S, Peschke E. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol. 2009;606:61–71. [DOI] [PubMed] [Google Scholar]
- 80. Bähr I, Mühlbauer E, Schucht H, Peschke E. Melatonin stimulates glucagon secretion in vitro and in vivo. J Pineal Res. 2011;50:336–344. [DOI] [PubMed] [Google Scholar]
- 81. Contreras-Alcantara S, Baba K, Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity (Silver Spring). 2010;18:1861–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pulimeno P, Mannic T, Sage D, et al. . Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia. 2013;56:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gillette MU, McArthur AJ. Circadian actions of melatonin at the suprachiasmatic nucleus. Behav Brain Res. 1996;73:135–139. [DOI] [PubMed] [Google Scholar]
- 84. Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 15 Spec No. 2006;2:R271–R277. [DOI] [PubMed] [Google Scholar]
- 85. Yoshino J, Imai S. A clock ticks in pancreatic β cells. Cell Metab. 2010;12:107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia. 1993;49:654–664. [DOI] [PubMed] [Google Scholar]
- 88. Simonneaux V. Naughty melatonin: how mothers tick off their fetus. Endocrinology. 2011;152:1734–1738. [DOI] [PubMed] [Google Scholar]
- 89. von Gall C, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann NY Acad Sci. 2005;1040:508–511. [DOI] [PubMed] [Google Scholar]
- 90. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165:175–183. [DOI] [PubMed] [Google Scholar]
- 92. Woon PY, Kaisaki PJ, Bragança J, et al. . Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dupuis J, Langenberg C, Prokopenko I, et al. . New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Below JE, Gamazon ER, Morrison JV, et al. . Genome-wide association and meta-analysis in populations from Starr County, Texas, and Mexico City identify type 2 diabetes susceptibility loci and enrichment for expression quantitative trait loci in top signals. Diabetologia. 2011;54:2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yamamoto H, Nagai K, Nakagawa H. Role of SCN in daily rhythms of plasma glucose, FFA, insulin and glucagon. Chronobiol Int. 1987;4:483–491. [DOI] [PubMed] [Google Scholar]
- 96. Ruiter M, La Fleur SE, van Heijningen C, van der Vliet J, Kalsbeek A, Buijs RM. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes. 2003;52:1709–1715. [DOI] [PubMed] [Google Scholar]
- 97. Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Marcheva B, Ramsey KM, Buhr ED, et al. . Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Marcheva B, Ramsey KM, Bass J. Circadian genes and insulin exocytosis. Cell Logist. 2011;1:32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dalle S, Ravier MA, Bertrand G. Emerging roles for β-arrestin-1 in the control of the pancreatic β-cell function and mass: new therapeutic strategies and consequences for drug screening. Cell Signal. 2011;23:522–528. [DOI] [PubMed] [Google Scholar]
- 101. Dunning BE, Foley JE, Ahrén B. α Cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia. 2005;48:1700–1713. [DOI] [PubMed] [Google Scholar]
- 102. Habener JF, Stanojevic V. α-Cell role in β-cell generation and regeneration. Islets. 2012;4:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP. Regulation of α-cell function by the β-cell in isolated human and rat islets deprived of glucose: the “switch-off” hypothesis. Diabetes. 2004;53:1488–1495. [DOI] [PubMed] [Google Scholar]
- 104. Raju B, Cryer PE. Maintenance of the postabsorptive plasma glucose concentration: insulin or insulin plus glucagon? Am J Physiol Endocrinol Metab. 2005;289:E181–186. [DOI] [PubMed] [Google Scholar]
- 105. Diao J, Asghar Z, Chan CB, Wheeler MB. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic α-cells. J Biol Chem. 2005;280:33487–33496. [DOI] [PubMed] [Google Scholar]
- 106. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385. [DOI] [PubMed] [Google Scholar]
- 108. Kemp DM, Ubeda M, Habener JF. Identification and functional characterization of melatonin Mel 1a receptors in pancreatic β cells: potential role in incretin-mediated cell function by sensitization of cAMP signaling. Mol Cell Endocrinol. 2002;191:157–166. [DOI] [PubMed] [Google Scholar]
- 109. Peschke E, Bach AG, Mühlbauer E. Parallel signaling pathways of melatonin in the pancreatic β-cell. J Pineal Res. 2006;40:184–191. [DOI] [PubMed] [Google Scholar]
- 110. Mühlbauer E, Peschke E. Evidence for the expression of both the MT1- and in addition, the MT2-melatonin receptor, in the rat pancreas, islet and β-cell. J Pineal Res. 2007;42:105–106. [DOI] [PubMed] [Google Scholar]
- 111. Peschke E, Stumpf I, Bazwinsky I, Litvak L, Dralle H, Mühlbauer E. Melatonin and type 2 diabetes – a possible link? J Pineal Res. 2007;42:350–358. [DOI] [PubMed] [Google Scholar]
- 112. Ramracheya RD, Muller DS, Squires PE, et al. . Function and expression of melatonin receptors on human pancreatic islets. J Pineal Res. 2008;44:273–279. [DOI] [PubMed] [Google Scholar]
- 113. Nagorny CL, Sathanoori R, Voss U, Mulder H, Wierup N. Distribution of melatonin receptors in murine pancreatic islets. J Pineal Res. 2011;50:412–417. [DOI] [PubMed] [Google Scholar]
- 114. O'Brien IA, Lewin IG, O'Hare JP, Arendt J, Corrall RJ. Abnormal circadian rhythm of melatonin in diabetic autonomic neuropathy. Clin Endocrinol (Oxf). 1986;24:359–364. [DOI] [PubMed] [Google Scholar]
- 115. Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell. 2012;148:1160–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Peschke E, Mühlbauer E, Musshoff U, Csernus VJ, Chankiewitz E, Peschke D. Receptor (MT(1)) mediated influence of melatonin on cAMP concentration and insulin secretion of rat insulinoma cells INS-1. J Pineal Res. 2002;33:63–71. [DOI] [PubMed] [Google Scholar]
- 117. Mühlbauer E, Albrecht E, Bazwinsky-Wutschke I, Peschke E. Melatonin influences insulin secretion primarily via MT(1) receptors in rat insulinoma cells (INS-1) and mouse pancreatic islets. J Pineal Res. 2012;52:446–459. [DOI] [PubMed] [Google Scholar]
- 118. Antoine MH, Hermann M, Herchuelz A, Lebrun P. Sodium nitroprusside inhibits glucose-induced insulin release by activating ATP-sensitive K+ channels. Biochim Biophys Acta. 1993;1175:293–301. [DOI] [PubMed] [Google Scholar]
- 119. Stumpf I, Mühlbauer E, Peschke E. Involvement of the cGMP pathway in mediating the insulin-inhibitory effect of melatonin in pancreatic β-cells. J Pineal Res. 2008;45:318–327. [DOI] [PubMed] [Google Scholar]
- 120. Stumpf I, Bazwinsky I, Peschke E. Modulation of the cGMP signaling pathway by melatonin in pancreatic β-cells. J Pineal Res. 2009;46:140–147. [DOI] [PubMed] [Google Scholar]
- 121. Bähr I, Mühlbauer E, Albrecht E, Peschke E. Evidence of the receptor-mediated influence of melatonin on pancreatic glucagon secretion via the Gαq protein-coupled and PI3K signaling pathways. J Pineal Res. 2012;53:390–398. [DOI] [PubMed] [Google Scholar]
- 122. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990; 215:403–410. [DOI] [PubMed] [Google Scholar]
- 123. Suzek BE, Huang H, McGarvey P, Mazumder R, Wu CH. UniRef: comprehensive and non-redundant UniProt reference clusters. Bioinformatics. 2007;23(10):1282–1288. [DOI] [PubMed] [Google Scholar]