Abstract
Objective
In 2001, the province of Ontario expanded cancer genetic testing eligibility to include all women with high-grade serous ovarian carcinoma (HGSC) of the ovary, fallopian tube, and peritoneum. The aim of this study was to determine the proportion of women who attended genetics counseling for consideration of BRCA1/2 gene analysis. We also sought to examine if regional differences in consultation rate exist across administrative health regions in the province of Ontario.
Methods
We identified all women with a pathological diagnosis of HGSC in the province of Ontario between 1997 until 2011. Our primary outcome was the 2-year rate of genetics consultation following a diagnosis of HGSC. We compared consultation rates over time and geographical regions and applied multiple logistic regression to identify predictors of genetics consultation.
Results
Of the 5412 women with a diagnosis of HGSC over the study period, 6.6% were seen for genetics consultation within 2 years of diagnosis. Factors predictive of genetics consultation included history of breast cancer (odds ratio [OR], 3.56; 95% confidence interval [CI], 1.87–6.78), era of diagnosis (2009–2011 vs 1997–2000; OR, 10.59; 95% CI, 5.02–22.33), and younger age at diagnosis (OR, 0.95; 95% CI, 0.94–0.97 for each additional year). No regional differences in consultation rate were seen.
Conclusions
Despite an increasing rate across eras, a small proportion of women with HGSC undergo genetics consultation. Efforts are required to increase cancer genetics consultation in patients with HGSC in the province of Ontario.
Key Words: BRCA mutation, Genetics consultation, Ovarian carcinoma, Risk reducing
Epithelial ovarian carcinoma is among the top 15 most diagnosed cancers in the Canadian province of Ontario.1 Compared with other gynecologic malignancies, it has the highest mortality rate and is the fifth leading cause of cancer-related mortality in Canada.2 The dominant histologic subtype of ovarian carcinoma is high-grade serous ovarian carcinoma (HGSC), which includes serous ovarian, peritoneal, and fallopian tube carcinomas. The majority of HGSCs present at an advanced stage, with overall 5-year survival rates ranging between 35% and 40%.3 To date, no screening modality has been shown to be effective at identifying HGSC at a stage where intervention decreases mortality.4,5
Women at highest risk of developing HGSC are those who harbor a BRCA gene mutation. Traditionally, ethnicity, family history of HGSC, or family history of breast cancer has been used to screen women for referral onto genetics counseling for consideration of BRCA1/2 mutation testing. While family history may be used to guide referral for BRCA1/2 testing, 19% to 44% of women with HGSC and a documented BRCA1 or BRCA2 gene mutation report having no family history of ovarian cancer.6–11
There are multiple benefits to cancer genetics consultation. The identification of a BRCA1/2 mutation in an affected individual allows for consideration of treatment with a PARP (poly-ADP-ribose polymerase) inhibitor, a class of medications found to have activity in women with HGSC.12–15 Furthermore, it allows for testing of family members where the finding of a BRCA1 or BRCA2 gene mutation may be followed by a prophylactic bilateral salpingo-oophorectomy, an action associated with a 77% reduction in all-cause mortality.16
Recognizing the gap in BRCA mutation testing, in 2001 Ontario expanded public health coverage eligibility for BRCA1/2 testing to include all women with a diagnosis of HGSC of the ovary. Since that time, 3 studies have reported on the experience with cancer genetics consultation rates among individuals with HGSC in Ontario.17–19 In the 2 single institution reports, 23% and 32% of patients with a diagnosis of HGSC completed a genetics consultation.18,19
Whether these findings are generalizable to the larger province of Ontario or whether practices have changed over time remains uncertain. The aim of this population-based study was to determine the 2-year rate of genetics consultation following a diagnosis of HGSC in the province of Ontario over a 15-year period. We also sought to characterize possible regional differences in this practice across administrative health regions.
METHODS
Study Design and Setting
We conducted a population-based, secular trend study of women with newly diagnosed HGSC from January 1, 1997, through December 31, 2011, using linked health care databases in Ontario, Canada. Analysis was limited up to 2011, because this represented a time point prior to the widespread launch of PARP inhibitor trials in our region. In the province of Ontario, Local Health Integration Networks are responsible for regional health care administration and funding; the province is divided into 14 such geographically defined administrative health regions. Based on the 2011 census, Ontario has approximately 6.58 million female residents older than 18 years. All Ontario residents are eligible to receive universal access to hospital care and physician services. We conducted this study in accordance with a prespecified protocol that was approved by the institutional review board at Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada. This study used datasets that were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences (ICES). Our report conforms to guidelines for observational studies.20
Data Sources
We used records from 6 linked databases to identify patient characteristics, covariate information, and outcome data: (1) Registered Persons Database, which contains demographic and vital statistics information on all beneficiaries of the single-payer, publicly funded health insurer in Ontario; (2) Ontario Health Insurance Plan (OHIP), which tracks all fee-for-service health claims for inpatient and outpatient physician services; (3) Ontario Cancer Registry (OCR), which maintains records on diagnoses of invasive cancer; (4) Canadian Institute for Health Information (CIHI) discharge abstract database and same-day surgery database, which contain information about all discharges and procedures conducted during admission from all acute care facilities and hospital-based same-day surgery units; (5) ICES Physician Database, which allows for identification of physician specialty; (6) the yearly Ontario intercensal and postcensal population estimates (IntelliHEALTH Ontario), which was used to identify population estimates of adult women over the study period.21 In addition to the ICES Physician Database, we utilized the methodology of Elit et al22 to identify gynecologic oncologists in the province of Ontario. Ontario Health Insurance Plan billing codes were used to identify genetics consultations. To determine patient comorbidity, we utilized the John Hopkins adjusted disease groupings (ADGs).23
Patients
To derive the cohort, all women 18 years or older with a diagnosis of HGSC were identified using the OCR. To separate the carcinomas from borderline and benign serous tumors, we restricted to the behavior code within the OCR signifying malignancy. Patients were excluded if they were coded as male, had a missing sex value, were older than 105 years old at time of diagnosis, had a recorded death preceding diagnosis, or were not a resident of Ontario at time of diagnosis. In the event where more than 1 record was identified, we restricted to the first date of diagnosis. Population incidence rates were calculated per study year (number of incident HGSC patients divided by the year-specific estimated adult female population in Ontario).
Outcomes
We used a retrospective period of 5 years to identify genetics consultations occurring prior to diagnosis and an additional 2-year period after diagnosis. Ascertainment of a genetics consultation per patient was therefore a composite of a 5-year look-back and 2-year follow-up, until the study endpoint of December 31, 2013. A genetics consultation was identified through a billing claim by a medical geneticist. In the province of Ontario, genetics consultations can occur with a medical geneticist, genetics counselor, or a combination of both providers, depending on the location of the genetics clinic. When a patient has a consultation with a genetics counselor only, no billing claims are made, and the cost of this service is paid for through the global budget of the hospital where the genetics counselor is employed. For patients attending genetics clinics operating in this way, therefore, the referral rates might not be captured using our defined methodology. To address this issue, we surveyed all of the cancer genetics clinics within the province to gauge our ability to universally capture the utilization of billing claims for the provision of genetics consultations, including when the patient was seen by a genetics counselor (Panabaker K, personal communication, February 3, 2016). A multivariable analysis was performed using this restricted dataset.
Statistical Analysis
We performed a secular trend (time trend) analysis using the Cochran-Armitage test for trend over the 15-year period. A multivariable analysis was undertaken using logistic regression to identify predictors of genetics consultation using a restricted dataset of patients treated in centers where genetics consultation billings were universally captured in OHIP. Predictors included patient age, ADG score, surgeon specialty (gynecologic oncology, obstetrics/gynecology, or other), Local Health Integration Network of residence, rural/urban status, history of breast cancer, neighborhood income quintile, and era of diagnosis. Predictors were assessed for colinearity, and where substantial collinearity is observed, the investigators made the decision to exclude 1 factor or combine the dependent factors. Model results are expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs). We performed all hypothesis tests using a 2-sided test and interpreted a P < 0.05 as statistically significant. All statistical analyses were conducted with SAS for UNIX version 9.2 (SAS Institute, Cary, NC).
RESULTS
A total of 5419 patients with a diagnosis for HGSC between 1997 and 2011 were identified from the OCR. Following exclusions, 5412 remained for analysis. Baseline characteristics are outlined in Table 1, with the study period divided into 5 eras (1997–1999, 2000–2002, 2003–2005, 2006–2008, 2009–2011). The mean age at diagnosis across all years was 62 years, with 47.4% of patients having received a diagnosis between ages 45 and 64 years. Comorbidities as assessed using the ADG were consistent across groupings over the entire time period. Diagnosis did not discriminate across income quintiles; however, there was a trend toward HGSC diagnosis (22.4%) in the highest-income quintile (P = 0.057). Reflecting population distribution in Ontario, the majority of patients hailed from urban residences (86.6%).
TABLE 1.
Characteristics of patients with high-grade serous ovarian carcinoma in Ontario, 1997–2011
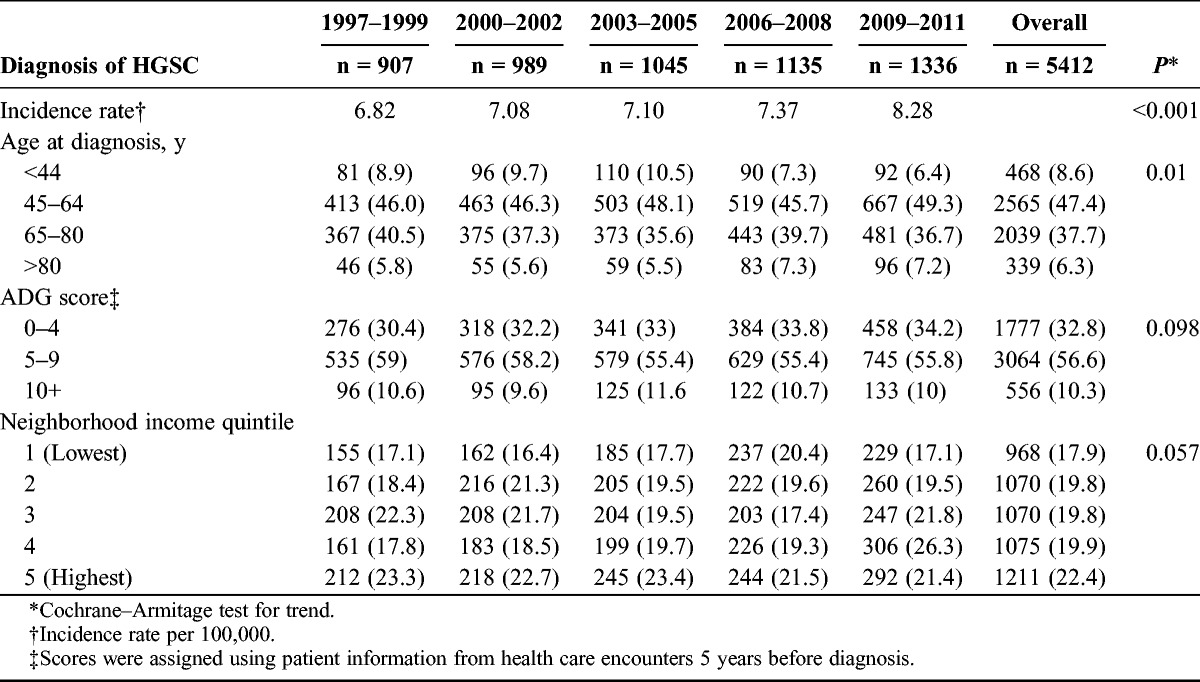
The population incidence of HGSC increased across the study period, from 6.82 per 100,000 in 1997–1999 to 8.28 per 100,000 adult women in 2009–2011 (Table 2). A diagnosis of breast cancer preceded the HGSC diagnosis in 5.7% of patients. For patients with a diagnosis of HGSC, the mean number of hospitalizations decreased from 6.68 in 1997–1999 to 3.84 in 2009–2011 (P < 0.001). An average of 30.9% of patients died within 2 years of HGSC diagnosis, and this did not change over the study time period (P = 0.068). Over the study time period, genetics consultations to a medical geneticist increased from 3.2% to 13.3% between 1997 and 2011 (P < 0.001), with 7.72% of all patients seeing a geneticist (Fig. 1). The mean time from diagnosis to genetics consultation was 11 months.
TABLE 2.
Consultation characteristics of patients with a diagnosis of HGSC in Ontario, 1997–2011
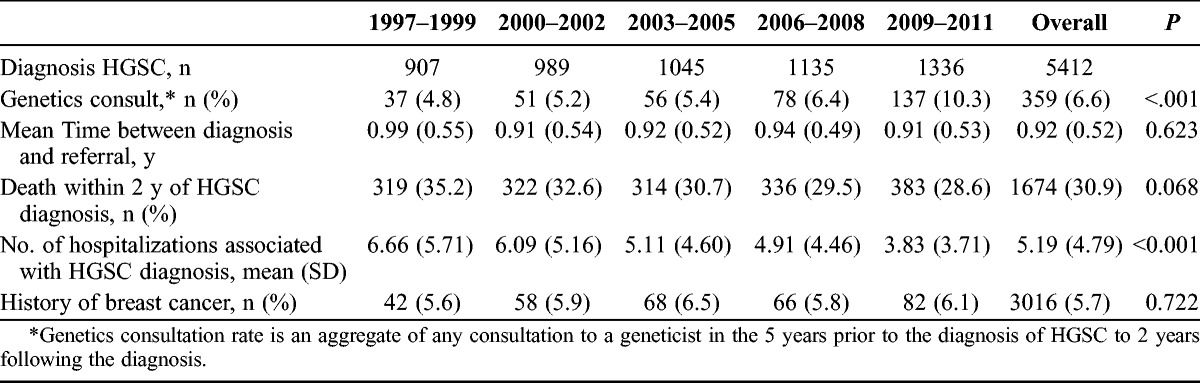
FIGURE 1.
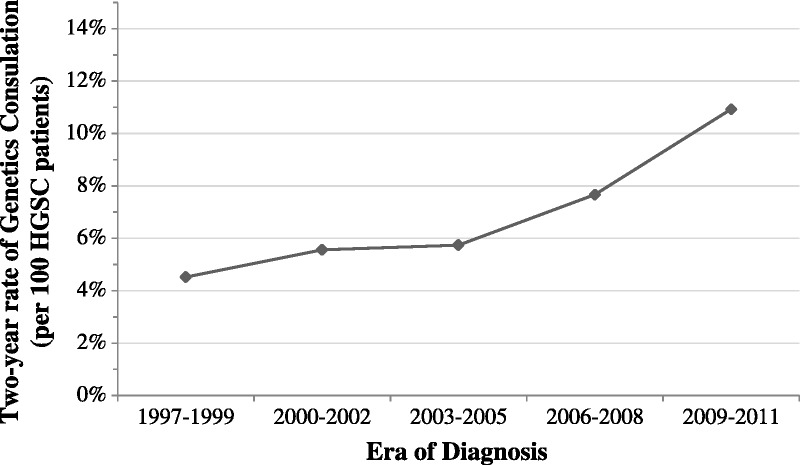
Rate of genetics consultation.
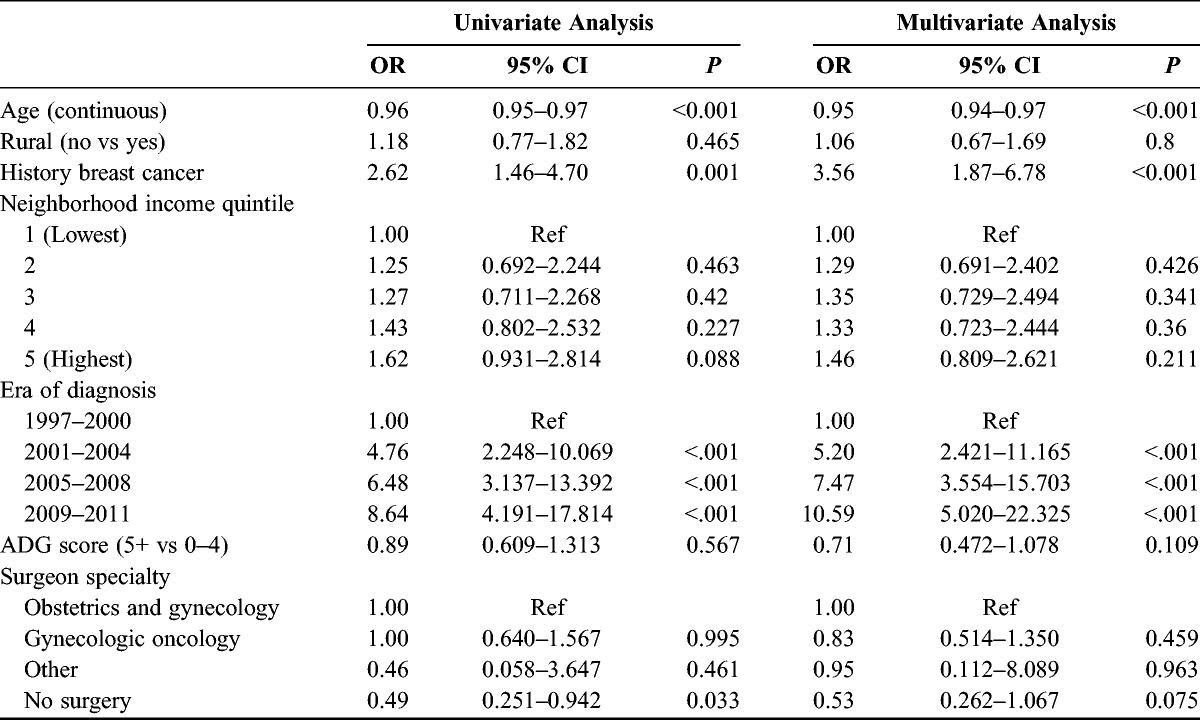
In a subgroup of patients where universal OHIP billing for genetics consultation was confirmed, 156 of the 1187 patients with HGSC (13%) underwent genetics consultation during the study time period. The univariate and multivariate analyses are presented in Table 3. Factors shown to be predictive of consultation included history of breast cancer (OR, 3.56; 95% CI, 1.87–6.78) and era of diagnosis (2009–2011 vs 1997–1999; OR, 10.59; 95% CI, 5.02–22.32). Advancing age was predictive of a lower likelihood of consultation (OR, 0.95; 95% CI, 0.94–0.97 for each year). Rural habitation, income quintile, patient comorbidity, and surgeon specialty did not influence referral rates for genetics consultation.
TABLE 3.
Predictors of genetics consultation in restricted dataset
DISCUSSION
Our analysis shows a positive increase in the provincial rate of genetics consultation since the late 1990s, peaking at 13.3% in 2011. Unfortunately, the rate of consultation was lower than the previously reported 32% and 23% at 2 individual tertiary care centers in the province.18,19 In a restricted dataset where genetics counseling and genetic testing services could more reliably be ascertained using OHIP billings, only 13% of patients with a diagnosis of HGSC completed a genetics consultation within 2 years of diagnosis. Predictors of consultation in this subset of women included later era of diagnosis, a personal history of breast cancer, and younger age at diagnosis. We saw no regional variations in referral rates across administrative health regions of treatment.
Our study has some limitations. With our methodology, it was not possible to capture those women who were referred to cancer genetics but never attended a cancer genetics appointment (i.e., patient was offered yet declined or passed away before the scheduled consultation). Furthermore, we were unable to capture the percentage of women seen by cancer genetics who pursued BRCA1/2 testing, nor could we ascertain the final test result in those patients who consented to have testing. We do know, however, from other published reports that women with HGSC who are referred for genetics counseling and genetic testing will accept BRCA1/2 gene analysis 86% to 100% of the time.18,19,24,25 Furthermore, for women with HGSC who have BRCA1/2 gene analysis in the province of Ontario, 32% were found to have a BRCA1 or BRCA2 gene mutation.19 Finally, because of the administrative nature of our data, predictors of referral such as positive family history or documented genetics discussion were not available for comparison to previous studies.
Despite an increase in frequency toward genetics consultation over the study time period, our analysis shows that the majority of women with HGSC in Ontario are not seen for cancer genetics consultation. Predictors of consultation in our study included later era of diagnosis, a prior personal history of breast cancer, and younger age. The latter 2 factors show tendencies among physicians to refer patients with traditional risk factors for a familial cancer syndrome. The increasing consultation rate across eras suggests increasing awareness by physicians of the association between HGSC and BRCA1/2 mutations. We saw a trend toward no genetics consultation in patients with HGSC who never underwent surgery.
Many barriers to cancer genetics consultation exist for the HGSC patient. Patients with HGSC undergo aggressive surgical debulking procedures and intensive chemotherapy regimens. Despite these efforts, prognosis overall for the disease is poor, as shown by a 30.9% mortality within the first 2 years of diagnosis in our cohort. Absence of referral, as seen in the study of Bell et al, suggests that with increasing acuity or complexity of the patient’s condition a genetics consultation may lose priority.18
Another potential barrier to consultation is the shared care for patients with HGSC that can exist in the province of Ontario, whereby patients return closer to home to receive chemotherapy in a center separate from where they received their surgical care. With no clear direction as to which care provider is responsible for making the referral to genetics, patients risk missing out on this important opportunity for themselves and their families. In Ontario, surgical care for patients with HGSC is highly concentrated in academic centers, in the hands of gynecologic oncologists.26 This centralization of care may provide an opportunity for assignment of responsibility for cancer genetics referrals to the gynecologic oncology team. Furthermore, as recognition of the link between primary peritoneal, fallopian tube, and serous ovarian carcinoma has broadened over time, potentially some of the cases diagnosed as primary peritoneal or fallopian tube serous carcinomas may not have been referred because of a lack of recognition of this association.
While care of HGSC patients is concentrated in academic centers, the model for referring patients and the process whereby patients are seen in genetics clinics are unique to each individual cancer center. Proposed solutions to the low attendance of HGSC patients to cancer genetics clinics have included adding a provision to the synoptic pathology report that outlines the Cancer Care Ontario recommendation for referral of all HGSC patients, as well as embedding an individual from cancer genetics into the multidisciplinary tumor board process.19 In addition, a role for testing of HGSC tumors for BRCA mutations has been suggested.27–30 Tumor testing will not, however, differentiate germline from somatic mutations. Prior to the widespread application of tumor testing, germline mutation testing must be maximized.
To address the low genetics consultation rate within the London Regional Cancer Program, the genetics referral process for patients with HGSC was altered in 2015 from an “opt-in” to an “opt-out” process. Each month, the pathology department generates a list of all new HGSC patients from the synoptic pathology report and forwards this list directly to the cancer genetics clinic. At a point 2 months from the surgery date, the patient is sent a letter acknowledging their cancer genetics referral by their surgeon, including an appointment date for a genetics consultation to discuss genetic testing. This 2-month lag allows ample time for the physician to see the patient postoperatively, discuss the diagnosis and future plans for treatment, and, if not already done, introduce the idea of genetic testing. At this first postoperative appointment, patients with a diagnosis of HGSC are provided with an information letter outlining the association between BRCA1/2 gene mutations and HGSC. In the first year of implementation of this opt-out strategy, 77% of patients with HGSC at the London Regional Cancer Program completed genetics consultation.31 As of 2012, 97.5% of hospitals in Ontario utilized synoptic pathology reporting, making this a feasible referral process for all tertiary care centers in the province.32
This study represents the real-world experience of women in the province of Ontario with a diagnosis of HGSC. The low consultation rates, as ascertained in our restricted dataset, suggest that a large gap exists between Cancer Care Ontario’s intention with the expansion of genetic testing in 2001 and the practice patterns in the province of Ontario. Improvements in pathways to cancer genetics consultation are required to maximize the benefits of BRCA1/2 gene analysis. We propose a novel opt-out strategy to enhance referral to cancer genetics.
Footnotes
This study was supported by ICES Western. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-term Care. Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario, the Schulich School of Medicine and Dentistry, Western University, and the Lawson Health Research Institute.
The authors declare no conflicts of interest.
The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES, Academic Medical Organization of Southwestern Ontario, Schulich School of Medicine and Dentistry, Lawson Health Research Institute, or the Ontario Ministry of Health and Long-term Care is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institutes for Health Information and Cancer Care Ontario. All analyses, conclusions, opinions, and statements expressed herein are those of the author and do not necessarily those of the Canadian Institutes for Health Information or the Cancer Care Ontario.
REFERENCES
- 1.Incidence & Mortality, Ontario—CCO [Internet] [cited December 23, 2015. Available at: https://www.cancercare.on.ca/ocs/csurv/stats/ontario/. Accessed December 21, 2016.
- 2. Canadian-Cancer-Statistics-2015-EN.pdf [Internet] [cited January 2, 2016]. Available at: https://www.cancer.ca/~/media/cancer.ca/CW/cancerinformation/cancer101/Canadiancancerstatistics/Canadian-Cancer-Statistics-2015-EN.pdf. Accessed December 21, 2016.
- 3.Berns EM, Bowtell DD. The changing view of high-grade serous ovarian cancer. Cancer Res. 2012;72:2701–2704. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387:945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305:2295–2303. [DOI] [PubMed] [Google Scholar]
- 6.Hirsh-Yechezkel G, Chetrit A, Lubin F, et al. Population attributes affecting the prevalence of BRCA mutation carriers in epithelial ovarian cancer cases in Israel. Gynecol Oncol. 2003;89(3):494–498. [DOI] [PubMed] [Google Scholar]
- 7.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst [Internet]. 2006;98:1694–1706. [DOI] [PubMed] [Google Scholar]
- 8.Schrader K, Hurlburt J, Kalloger SE, et al. Germline BRCA1 and BRCA2 mutations in ovarian cancer: utility of a histology-based referral strategy. Obstet Gynecol. 2012;120:235–240. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez AO, Llacuachaqui M, Pardo GG, et al. BRCA1 and BRCA2 mutations among ovarian cancer patients from Colombia. Gynecol Oncol [Internet]. 2012;124:236–243. [DOI] [PubMed] [Google Scholar]
- 10.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation–positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Royer R, Li S, et al. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol [Internet]. Elsevier Inc; 2011;121:353–357. [DOI] [PubMed] [Google Scholar]
- 12.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–2519. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–1392. [DOI] [PubMed] [Google Scholar]
- 15.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol [Internet]. Elsevier Ltd; 2011;12:852–861. [DOI] [PubMed] [Google Scholar]
- 16.Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547–1553.24567435 [Google Scholar]
- 17.Metcalfe KA, Fan I, McLaughlin J, et al. Uptake of clinical genetic testing for ovarian cancer in Ontario: a population-based study. Gynecol Oncol. 2009;112:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell K, Scott M, Pond G, et al. Genetic counselling referral rates and uptake of BRCA1 and BRCA2 testing among women diagnosed with serous ovarian cancer in a tertiary care cancer centre. J Genet Syndr Gene Ther [Internet]. OMICS Group; 2013;04(06) [cited November 23, 2015]. Available at: http://www.omicsonline.org/genetic-syndromes-gene-therapy-abstract.php?abstract_id=15568. Accessed December 21, 2016. [Google Scholar]
- 19.Demsky R, McCuaig J, Maganti M. Keeping it simple: genetics referrals for all invasive serous ovarian cancers. Gynecol Oncol [Internet] 2013;130:329–333. [DOI] [PubMed] [Google Scholar]
- 20.Vandenbroucke JP, von Elm E, Altman DG, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting. Ann Intern Med. 2007;147:573–578. [DOI] [PubMed] [Google Scholar]
- 21.Care: OM of H and L-T, IntelliHEALTH ONTARIO. No Title.
- 22.Elit LM, O'Leary EM, Pond GR. Impact of wait times on survival for women with uterine cancer. J Clin Oncol. 2014;32:27–33. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC, van Walraven C, Wodchis WP. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacour RA, Daniels MS, Westin SN, et al. What women with ovarian cancer think and know about genetic testing. Gynecol Oncol. 2008;111:132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meiser B, Gleeson M, Kasparian N, et al. There is no decision to make: experiences and attitudes toward treatment-focused genetic testing among women diagnosed with ovarian cancer. Gynecol Oncol. 2012;124:153–157. [DOI] [PubMed] [Google Scholar]
- 26.Fung-Kee_Fung M, Kennedy EB, Biagi J, et al. Organizational guideline for gynecologic oncology services in Ontario [Internet] [cited April 4, 2015]. Available at: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=282212. Accessed December 21, 2016. [DOI] [PubMed] [Google Scholar]
- 27.Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28:3570–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNeish IA, Oza AM, Coleman RL, et al. Results of ARIEL2: a phase 2 trial to prospectively identify ovarian cancer patients likely to respond to rucaparib using tumor genetic analysis. ASCO Meet Abstr [Internet] 2015;33(suppl 15):5508. [Google Scholar]
- 29.Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell D, Berchuck A, Birrer M, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Authors V. Proffered papers and posters presented at the Sixth International Symposium on Hereditary Breast and Ovarian Cancer—BRCA: challenges and opportunities. Curr Oncol. 2016;23(3). DO-103747/co233327 [Internet] [cited June 13, 2016. Available at: http://www.current-oncology.com/index.php/oncology/article/view/3327/2183. Accessed December 21, 2016. [Google Scholar]
- 32. Synoptic Pathology Reporting—CCO [Internet] [cited December 23, 2015]. Available at: https://www.cancercare.on.ca/ocs/clinicalprogs/pathnlabmed/pathproj_prof/. Accessed December 21, 2016.


