Abstract
Background:
Age-disaggregated analyses of prevention of mother-to-child transmission (PMTCT) program data to assess the uptake of HIV services by pregnant adolescent women are limited but are critical to understanding the unique needs of this vulnerable high-risk population.
Methods:
We conducted a retrospective analysis of patient-level PMTCT data collected from 2011 to 2013 in 36 health facilities in 5 districts of Zimbabwe using an electronic database. We compared uptake proportions for PMTCT services between adolescent (≤19 years) and adult (>19 years) women. Multivariable binomial regression analysis was used to estimate the association of the women's age group with each PMTCT service indicator.
Results:
The study analyzed data from 22,215 women aged 12–50 years (22.5% adolescents). Adolescents were more likely to present to antenatal care (ANC) before 14 weeks of gestational age compared with older women [adjusted relative risk (aRR) = 1.34; 95% confidence interval: 1.22 to 1.47] with equally low rates of completion of 4 ANC visits. Adolescents were less likely to present with known HIV status (aRR = 0.34; 95% confidence interval: 0.29 to 0.41) but equally likely to be HIV tested in ANC. HIV prevalence was 5.5% in adolescents vs 20.1% in adults. While >84% of both HIV-positive groups received antiretroviral drugs for PMTCT, 44% of eligible adolescents were initiated on antiretroviral therapy vs 51.3% of eligible adults, though not statistically significant.
Conclusions:
Pregnant adolescents must be a priority for primary HIV prevention services and expanded HIV treatment services among pregnant women to achieve an AIDS-free generation in Zimbabwe and similar high HIV burden countries.
Key Words: adolescents, antenatal care, antiretroviral therapy, elimination, pregnant women, prevention of mother-to-child transmission of HIV
INTRODUCTION
HIV infections remain high among women of childbearing age.1,2 In Sub-Saharan Africa, young women, in particular, are at the epicenter of the HIV epidemic as they contribute approximately 30% of all new HIV infections in the region.3–5 Young women face multiple legal, economic, and social vulnerabilities that interact to affect their sexual behaviors, decisions, and circumstances, making them more susceptible to acquiring new HIV infections.3,5 A number of studies have found that young people are less likely to take an HIV test and that those who are HIV infected have poorer HIV treatment uptake, retention, and outcomes than adults.6–14 Less is known about adolescent uptake of prevention of mother-to-child transmission (PMTCT) of HIV services, where new HIV infections, low HIV testing uptake, and poor initiation of and retention on treatment are significant barriers to elimination of HIV infection in children.2,9–14
Zimbabwe is one of the 21 sub-Saharan Africa countries that continue to experience a high HIV burden in the adult population and high HIV transmission risk in adolescent women. HIV prevalence among the adult population aged 15–49 years was estimated through the Spectrum model to be 14.7% in 2015 and prevalence among women attending antenatal care (ANC) to be 16.1% while the mother-to-child transmission rate was estimated at 6.7% in 2014.15–17 The Zimbabwe Demographic and Health Survey of 2010–2011 showed that adolescent women faced high HIV acquisition risks as HIV prevalence among women 15–19 years was 4.2% but increased to 10.6% in the 20–24 years age group and to 20.0% in the 25–30 years age group.18
Higher HIV risk factors among adolescent women have important implications for the goal of eliminating new pediatric HIV infections in any country. Given the challenges that adolescent women face in controlling sexual decisions, accessing reproductive health services and protecting themselves against infection with HIV, understanding differences in service uptake among adolescent and adult women is critical for strengthening PMTCT programs. This study compared PMTCT service uptake among adolescent and adult women in selected ANC clinics in Zimbabwe to identify possible gaps in service uptake by adolescent women. The findings will inform the scale-up of the current PMTCT guidelines, which aim to provide antiretroviral therapy (ART) to all HIV-infected pregnant and breastfeeding women to minimize the risk of HIV transmission to children and to improve the health of the mothers.
METHODS
We conducted a retrospective analysis on patient-level data from pregnant women accessing ANC services collected using an electronic database in 36 of 145 primary health centers and hospitals from 5 districts of Zimbabwe between September 2011 and December 2013. The 5 districts where the database was implemented were purposively selected to represent the major ethnic and geographical characteristics of the female population of Zimbabwe from the Manicaland, Mashonaland, and Matabeleland provinces, including urban, rural, and highly mobile border populations. Health facilities serving at least 40 HIV-positive pregnant women per year were selected and the selected health facilities served approximately 3 in every 4 of the estimated HIV-positive women in the districts. Figure 1 displays the location of the districts and study health facilities. All the health facilities were operated by government or faith-based organizations.
FIGURE 1.

Map of Zimbabwe showing the districts and health facilities where the electronic database was implemented and the study data were collected.
Zimbabwe implemented the World Health Organization (WHO) 2010 “Option A” PMTCT guidelines during this period, with all pregnant women being encouraged to attend first ANC visit by 14 weeks of gestational age so that HIV-infected women would be identified and would start taking antiretroviral drugs (ARVs) for PMTCT early.19 HIV-infected women underwent WHO clinical staging and/or CD4 cell count assessment to determine eligibility for lifelong ART (WHO stage 3 or 4 or CD4 count ≤350 cells/μL). Women who were not clinically eligible for ART (WHO stage 1 or 2) took Zidovudine (AZT) prophylaxis while awaiting results of their CD4 cell count. Women who were eligible for ART by CD4 cell count switched to lifelong ART while women who were not eligible (CD4 cell count >350 cells/μL) continued to take AZT prophylaxis throughout pregnancy and a single dose of Nevirapine at the onset of labor. Their infants received daily Nevirapine throughout breastfeeding.
Study Population
The study population consisted of pregnant women who attended ANC at the selected health facilities during the study period. Women who attended their first ANC visit at a nonstudy facility for the current pregnancy were excluded because of missing first ANC visit information.
Data Collection
The Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) supported the Zimbabwe Ministry of Health and Child Care (MoHCC) to implement an electronic patient-level database (EDB) in the selected districts and health facilities using the International Quality Care electronic medical record system developed by the Futures Group.20 Clinic nurses routinely completed patient registers as they provided PMTCT services to the women. The registers recorded patient demographic data such as age, parity, gravida, gestational age, and expected date of delivery and PMTCT service data including HIV testing, WHO staging, CD4 count, and ARV uptake. Data entry clerks entered all patient data from the registers into the EDB. The data entry clerks verified all data at entry and followed up missing and inaccurate information with the nurses, assisted by inbuilt EDB data validation functionalities. Additional data included in the analysis were type of health facility (hospital or health center) and location (rural, urban).
Data Analysis
We categorized the study population into adolescents—defined as women ≤19 years of age and adults—women >19 years of age. We summarized the demographic characteristics of the study population using proportions and means or medians for each group—adolescent and adult women. We calculated uptake proportions for various PMTCT service outcomes (HIV status before ANC, testing in ANC, ART eligibility, and ARV uptake) and compared them between the adolescent and adult women. We used multivariable binomial regression analysis to estimate relative risk (RR) for each PMTCT service outcome, comparing adolescent women with adult women. We performed exploratory analyses, comparing younger adolescents (≤16 years) and older adolescents (17–19 years), to assess if younger adolescents were driving the observed differences between adolescents and adults. Risks ratios for ANC attendance and ANC visit outcomes were adjusted for facility type, gravidity, gestational age, HIV status before ANC, and being on ART before ANC while risk ratios for knowledge of and HIV status, ART eligibility, and ARV uptake were adjusted for facility type and gravidity. All analyses were performed using Stata version 12.0.21
The Medical Research Council of Zimbabwe gave ethical approval for analysis of the data and dissemination of results from the EDB.
RESULTS
Study Facilities
The study facilities included 5 district hospitals, 7 rural hospitals, 6 urban health centers, and 18 rural health centers.
Characteristics of the Study Population
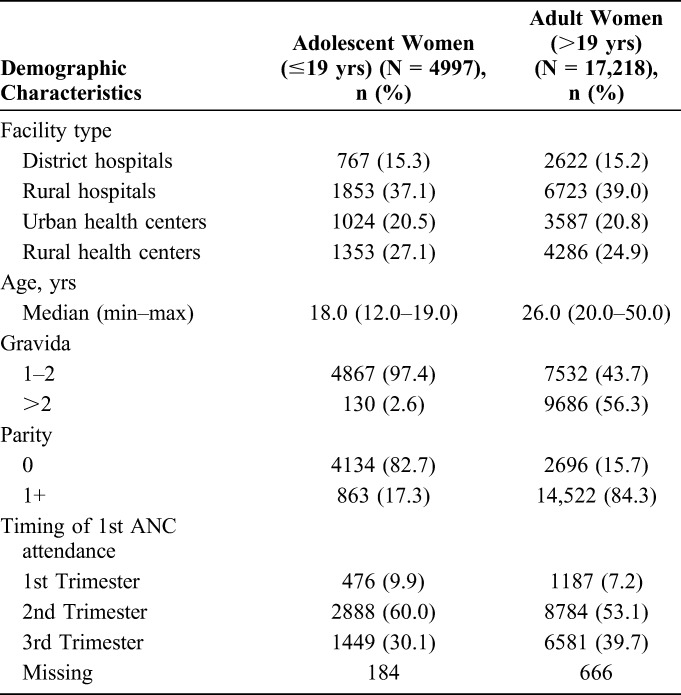
We extracted data from the records of 22,215 women attending ANC at the study sites, 22.5% (n = 4997) of whom were adolescents and 77.5% (n = 17218) were adults (Fig. 2). The age range for adolescent women was 12–19 years (median = 18 years), and the age range for adult women was 20–50 years (median = 26 years) (Table 1). Of the 22,215 women, 64% presented at rural health facilities, 20.8% at urban health facilities, and 15.3% at the district hospital with no differences between adolescents and adults. Among adolescent women, 82.7% had not given birth before compared with 15.7% of adult women.
FIGURE 2.

Flow diagram for pregnant women accessing PMTCT services from September 2011 to December 2013 in 36 ANC clinics in Zimbabwe and those included in the study analysis. EDB, electronic patient-level database.
TABLE 1.
Characteristics of Study Women Attending ANC in 36 Health Facilities Implementing the PMTCT EDB in Zimbabwe, September 2011–December 2013
ANC Attendance
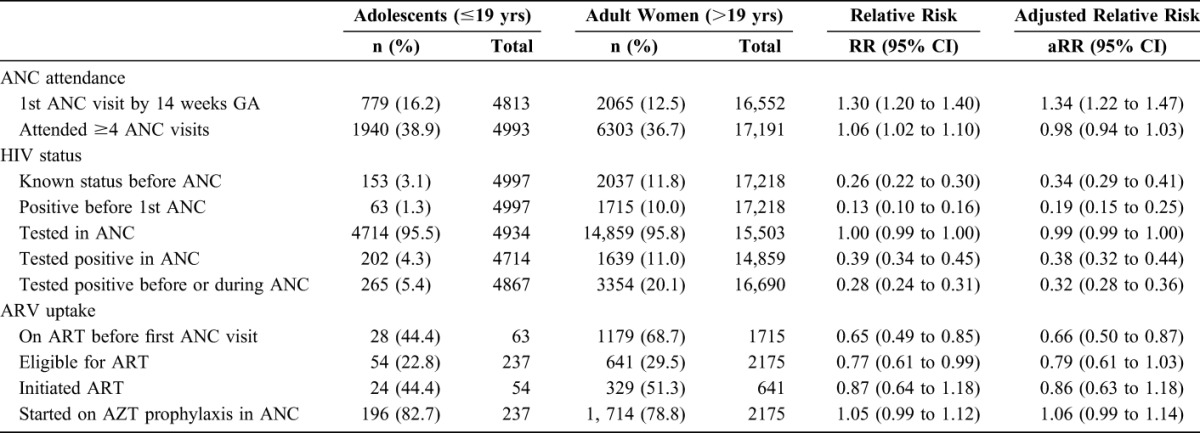
Among adolescent women, 9.9% had their first ANC visit in the first trimester, 60.0% in the second trimester, and 30.1% in the third trimester (Table 1). Among adult women, 7.2% had their first ANC visit in the first trimester, 53.1% in the second trimester, and 39.7% in the third trimester. Sixteen percent (16.2%) of adolescent women compared with 12.5% of adult women attended their first ANC visit by 14 weeks of gestational age (Table 2). Adjusting for gravidity, health facility type, and knowledge of HIV status before first ANC visit, adolescent women were 34% more likely to attend first ANC by 14 weeks gestational age compared with adult women [adjusted relative risk (aRR) = 1.34; 95% confidence interval (CI): 1.22 to 1.47]. However, adolescent (38.9%) and adult (36.7%) women were equally likely to attend at least 4 ANC visits (aRR = 0.98; 95% CI: 0.94 to 1.03).
TABLE 2.
ANC Attendance and HIV Service Utilization Among Adolescent and Adult Pregnant Women Booking for ANC in the 36 Health Facilities Implementing the PMTCT EDB in Zimbabwe, September 2011–December 2013
HIV Testing
Only 3.1% of adolescent women knew their HIV status before their first ANC visit in contrast to 11.8% of adult women. Adjusting for parity, gravida, and facility type, adolescents were 66% less likely to know their HIV status before ANC (aRR = 0.34; 95% CI: 0.29 to 0.41). Acceptance of HIV testing in ANC was equally high in both adolescent (95.5%) and adult (95.8%) women. Overall HIV prevalence among all the women attending ANC was 16.3%. HIV prevalence was significantly lower in adolescent women (5.4%) compared with adult women (20.1%). Adjusting for gravidity, adolescents were 68% less likely to be HIV infected than adult women (aRR = 0.32; 95% CI: 0.28 to 0.36).
ARV Uptake
Approximately 44.4% of HIV-infected adolescent women who knew their status before first ANC visit were already on ART compared with 68.7% of HIV-infected adult women who knew their status (Fig. 2 and Table 2). Among the HIV-infected women who were not already on ART at first ANC visit, 82.7% of the adolescents and 78.8% of the adult women were initially started on AZT prophylaxis. Among HIV-positive women not on ART and assessed for ART eligibility, 22.8% of adolescent women compared with 29.5% of adult women were eligible for ART. After adjusting for gravidity, gestational age at first ANC, and health facility type, adolescent women were less likely to be eligible for ART compared with adult women, although this did not reach statistical significance (aRR = 0.79; 95% CI: 0.61 to 1.03). Among those eligible for ART initiation in ANC, 44.4% of the adolescent women and 51.3% of the adult women were initiated on ART, similarly this was not significantly different.
Comparison of Younger and Older Adolescents
There were no statistically significant differences between younger and older adolescents in all PMTCT service outcomes except in HIV prevalence (Table 3). Older adolescents were 64% more likely to be HIV positive than younger adolescents (aRR = 1.64; 95% CI: 1.14 to 2.36).
TABLE 3.
ANC Attendance and HIV Service Utilization Among Younger (<17 Years) and Older (17–19 Years) Adolescent Pregnant Women Booking for ANC in the 36 Health Facilities Implementing the PMTCT EDB in Zimbabwe, September 2011–December 2013

DISCUSSION
In this study with more than 20,000 antenatal attendees in Zimbabwe, nearly one-quarter (4997) were adolescents, highlighting the importance of understanding ANC and HIV health-seeking behaviors in this group, as preventing new HIV infections in children depends on early and optimal uptake of PMTCT services. This study found that adolescents were more likely than adult women to attend ANC early but both groups had very low rates of completion of the WHO recommended 4 antenatal visits. By contrast, compared with adult women, adolescents were less likely to know their HIV status or to be on ART at the time of their first ANC visit. Although not significant, there were trends toward fewer adolescents being eligible for ART in ANC and lower ART initiation during ANC among eligible adolescent women compared with adult women.
The finding of early ANC attendance by adolescents is consistent with studies in Ethiopia where pregnant women aged ≤25 years were nearly twice as likely to commence ANC at the recommended time as women older than 25.22,23 By contrast, a cross-sectional survey of postnatal MCH attendees in Kenya found no difference in timing of first ANC between adolescents and adult women but higher rates of attending 4 ANC visits among adult women.24 This may be due to the smaller sample size and the difference in study population between all ANC attendees in this study and those attending postnatal services in the Kenya study. The absence of complications in previous pregnancies has been associated with older women's confidence in delaying ANC attendance.25–28 On the contrary, adolescents may have a higher pregnancy risk perception and start seeking ANC services early. The overall proportion of women attending at least 4 ANC visits was low in this study in part because of the initiation of ANC late in the third trimester by about one-third of attendees. The low rates of early ANC attendance overall indicate the need to focus efforts on improving early ANC attendance among pregnant women of all ages.
Several studies in South Africa also found that adolescent women were less likely to know their HIV status before their first ANC visit compared with adult women.11,29,30 In a sentinel surveillance cohort study of women attending ANC in the Eastern Cape, Fatti et al found that 75.3% of adolescent women were unaware of their HIV status compared with 44.7% of older women.30 Similarly, Woldesenbet and others found that adolescent women were twice as likely as adult women to be unaware of their HIV status.11 Older women had more of a chance to be tested for HIV during previous pregnancies compared with adolescents who were more likely to be presenting with their first pregnancy.22 In addition, adolescent women may have less access to voluntary HIV counseling and testing services because of barriers such as need for parental consent, unfriendly HIV testing environments in health facilities, lack of knowledge of where and how to get an HIV test, fear of the test itself, and fear of discovering their HIV status.2–4,26,28,31,32 Failure to know their HIV status before pregnancy is a missed opportunity to adolescents for pregnancy planning, initiation of ART before pregnancy, and active choices about how to reduce the risk of HIV transmission to the unborn baby. This further supports efforts to improve access to HIV testing for adolescents.
The acceptance of HIV testing for both adolescent and adult women was equally high in this (>95%) and the South Africa and Kenya studies, surpassing the UNAIDS target of 90% of people living with HIV knowing their status.24,29,30,33,34 This confirms that PMTCT programs are now achieving high HIV testing rates in ANC, enabling the identification of nearly all HIV-infected pregnant women who attend ANC and providing them the opportunity to receive ARVs for their own health and prevention of HIV transmission to their unborn babies. If more pregnant women were to attend ANC early and regularly, the UNAIDS goal of putting more than 90% of HIV-infected people on ART would be achieved for pregnant women.33,34 This would make possible the achievement of eliminating new HIV infections in children.
Although adolescents are less likely to be tested in the general population than adults, this and the similar studies identified demonstrate that adolescents are as likely to accept HIV testing as adults when the testing is offered to them in appropriate, supportive environments.24,29,30,35 ANC remains a significant entry point for HIV testing for adolescent women and provides an opportunity for expanded adolescent-focused HIV prevention and treatment services. The significant differences in the HIV sero-prevalence among women ≤19 years compared with women >19 years (5.4% vs 20.1%) in this study demonstrates the critical need for interventions (such as PreP) to decrease the incidence of new HIV infections in the vulnerable and high-risk group of pregnant adolescents while they are in contact with the health system.
The proportion of known HIV-positive adolescents who came for first ANC visit already on ART was significantly lower than that of adult women in this study (44.4% vs 68.7%). A greater differential rate between adolescents and adults was seen in the Ronen study in Kenya (4.8% vs 43.1%) but with a much lower proportion of those on ART in both groups in Kenya compared with Zimbabwe.24 Our results should be interpreted with caution as we did not know the proportion of adolescents and adults who were eligible for ART at the time of HIV diagnosis. Hence, the difference could be due to fewer adolescents being eligible for ART during the time of the study when eligibility was determined by clinical or immunologic disease status through testing of CD4 cell count or WHO staging. In contrast to other studies, we did not find a significant difference in the proportions of HIV-infected adolescent and adult women who were eligible for ART in ANC possibly because of lack of power because of the small number of HIV-infected adolescents.24,29 We would expect fewer eligible adolescents as the duration of HIV infection in these young women was likely to be significantly shorter than older adult women. This difference will not be relevant with the change of guidelines to include initiation of ART in all HIV-positive individuals (Option B+ for pregnant women, test and start for general population).36,37
Overall, there was very high uptake of ARVs for PMTCT among both adolescents and adult women in this study (>84%) compared with the 33% of adolescents in the Kenya study who did not receive any ARV for PMTCT.24 However, some of the difference is related to the postnatal study population in Kenya in which some of the adolescents may not have been diagnosed until after delivery. We found that adolescent women were as equally likely to receive AZT prophylaxis for PMTCT as adult women. However, there was a trend toward lower ART initiation among eligible adolescent women in ANC compared with adult women. While not statistically significant in our study, similar results have been reported in other studies from the Southern Africa region. In both the Horwood and the Fatti studies, lower rates of ART initiation were found in adolescent women in ANC compared with adult women.29,30 Fitzgerald and colleagues found that the women who had not received ARVs for PMTCT were significantly younger than those who were started on ART.12 It is possible that AZT prophylaxis is more acceptable to adolescent women who are naive to lifelong ART. Adolescent women are likely to face more challenges with adherence to lifelong ART including difficulties with the disclosure of their HIV status to their partners and families that could result in adolescent women being less likely to be initiated on ART yet equally likely to accept AZT prophylaxis as adult women. At the time that our data were collected, all HIV-infected women were initiated on AZT prophylaxis on diagnosis while they were being assessed for ART eligibility and initiated on ART if eligible. Women who were not offered or did not accept ART continued on AZT, thereby offering some protection to their unborn babies.
We suspected that the observed differences in PMTCT service uptake between adolescent and adult women could be driven by the more vulnerable younger adolescents (<16). However, our exploratory analyses did not find any significant differences between younger and older adolescents in all PMTCT outcomes analyzed except HIV prevalence where older adolescents were 64% more likely to be HIV positive than younger adolescents.
The findings of this study are strengthened by the large number of adolescents in the study population included from a diverse set of health facilities fairly distributed across the country. However, there are limitations to the interpretation of the study results. The electronic patient database was not able to track women across health facilities and record PMTCT services that women might have received at other health facilities which could lead to underreporting of services received. The absence of data on adolescent and adult women who did not seek ANC services and the use of program data that included few demographic characteristics limit the ability to determine true associations with health-seeking behaviors for pregnant women.
This study highlights the importance of identifying and addressing the unique HIV testing, prevention, and treatment needs of adolescent women in all 4 prongs of the WHO PMTCT strategy to achieve elimination of pediatric AIDS.2 This includes improved access to HIV testing and family planning services for adolescent women before pregnancy, targeted community awareness-raising on adolescent vulnerabilities and unique health needs particularly for those who are HIV positive, and critical primary HIV prevention services for pregnant/postpartum adolescents.
ACKNOWLEDGMENTS
The authors acknowledge the efforts of the health workers who provided PMTCT services to the women and recorded the data in the registers; the data entry clerks who entered the data into the electronic database; the Zimbabwe Ministry of Health and Child Care for collaborating with EGPAF in the implementation of the EDB, the Children's Investment Fund Foundation who sponsored the implementation of the electronic database, Futures Group for providing the IQ Care database, EGPAF colleagues who reviewed this manuscript.
Footnotes
Supported by the Children's Investment Fund Foundation.
Oral presentation at the 18th International Conference on AIDS and STIs in Africa; November 19, 2015; Harare, Zimbabwe.
The authors have no funding or conflicts of interest to disclose.
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the Children's Investment Fund Foundation who funded the database through which these data were collected or the Ministry of Health and Child Care in Zimbabwe.
REFERENCES
- 1.Joint United Nations Program on HIV/AIDS. The Gap Report. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2013. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf. Accessed November 14, 2014. [Google Scholar]
- 2.Joint United Nations Program on HIV/AIDS. Countdown to Zero: Global Plan towards the Elimination of New HIV Infections among Children by 2015 and Keeping Their Mothers Alive, 2011-2015. Geneva, Switzerland: 2011. Available at: http://www.unaids.org/sites/default/files/media_asset/20110609_JC2137_Global-Plan-Elimination-HIV-Children_en_1.pdf. Accessed November 14, 2014. [Google Scholar]
- 3.Dellar RC, Dlamini S, Karim QA. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc. 2015;18(2 suppl 1):19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. HIV and Adolescents: Guidance for HIV Testing and Counseling and Care for Adolescents Living With HIV. Recommendations for a Public Health Approach and Considerations for Policy-Makers and Managers. Geneva, Switzerland: 2013. Available at: http://apps.who.int/iris/bitstream/10665/94334/1/9789241506168_eng.pdf?ua=1. Accessed November 14, 2014. [PubMed] [Google Scholar]
- 5.Fleishman J, Peck K. Addressing HIV Risk in Adolescents and Young Women: A Report of the ICIS Global Health Policy Center. Center for Strategic and International Studies; 2015. Available at: https://www.csis.org/analysis/addressing-hiv-risk-adolescent-girls-and-young-women. Accessed January 27, 2016. [Google Scholar]
- 6.Auld AF, Agolory SG, Shiraishi RW, et al. Antiretroviral therapy enrolment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults–seven African countries, 2004–2013. Morbid Mortal Weekly Rep. 2014;63:1097–1103. [PMC free article] [PubMed] [Google Scholar]
- 7.Koech E, Teasdeale CA, Wang C, et al. Characteristics and outcomes of HIV-infected youth and young adolescents enrolled in HIV care in Kenya. AIDS. 2014;28:2729–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans D, Menezes C, Mahomed K, et al. Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retrovir. 2013;29:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tweya H, Gugsa S, Hosseinipour M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health. 2014;19:1360–1366. [DOI] [PubMed] [Google Scholar]
- 10.Mitiku I, Arefayne M, Mesfin Y, et al. Factors associated with loss to follow-up among women in Option B+ PMTCT programme in northeast Ethiopia: a retrospective cohort study. J Int AIDS Soc. 2016;19:206–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woldesenbet S, Jackson D, Lombard C, et al. Missed opportunities along the prevention of mother-to-child transmission services cascade in South Africa: uptake, determinants, and attributable risk (the SAPMTCTE). PLoS One. 2015;10:e0132425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald FC, Bekker LG, Kaplan R, et al. Mother-to-child transmission of HIV in a community-based antiretroviral clinic in South Africa. S Afr Med J. 2010;100:827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidze LK, Faye A, Tetang SN, et al. Different factors associated with loss to follow-up of infants born to HIV-infected or uninfected mothers: observations from the ANRS 12140-PEDIACAM study in Cameroon. BMC Public Health. 2015;15:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mnyani CN, Simango A, Murphy J, et al. Patient factors to target for elimination of mother-to-child transmission of HIV. Global Health. 2014;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health and Child Care, Zimbabwe. Zimbabwe National HIV and AIDS Estimates. Harare, Zimbabwe: MOHCC; 2015. [Google Scholar]
- 16.Ministry of Health and Child Care. Survey of HIV and Syphilis Among Women Attending Antenatal Care Clinics in Zimbabwe. Harare, Zimbabwe: MOHCC; 2012. [Google Scholar]
- 17.Buzdugan R, Kang Dufour MS, McCoy SI, et al. Option A improved HIV-free infant survival and mother to child HIV transmission at 9–18 months in Zimbabwe. AIDS. 2016;30:1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ZimStat and ICF International, Inc. Zimbabwe Demographic and Health Survey 2010–2011. Harare, Zimbabwe: ZimStat; 2012. [Google Scholar]
- 19.WHO. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Recommendations for a Public Health Approach. 2010 version. Geneva, Switzerland: 2010. Available at: http://apps.who.int/iris/bitstream/10665/75236/1/9789241599818_eng.pdf. Accessed May 21, 2015. [PubMed] [Google Scholar]
- 20.Futures Group International. IQ Care/IQ Solutions. Available at: http://www.iqstrategy.net/products/iqcare/. Accessed April 28, 2016. [Google Scholar]
- 21.Stata Corporation, LTD. STATA software. 1985. Available at: http://www.stata.com. License 40120509681. [Google Scholar]
- 22.Ronen K, McGrath CJ, Langat AC, et al. Gaps in adolescent engagement in antenatal care and prevention of mother-to-child HIV transmission services in Kenya. J Acquir Immune Defic Syndr. 2017;74:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gudayu TW, Woldeyohannes SM, Abdo AA. Timing and factors associated with first antenatal care booking among pregnant mothers in Gondar Town, North West Ethiopia. BMC Pregnancy Childbirth. 2014;14:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regassa N. Antenatal and postnatal care service utilization in southern Ethiopia: a population-based study. Afr Health Sci. 2011;11:390–397. [PMC free article] [PubMed] [Google Scholar]
- 25.Ochako R, Fotso JC, Ikamari Khasakhala LA. Utilization of maternal health services among young women in Kenya: insights from the Kenya Demographic and Health Survey, 2003. BMC Pregnancy Childbirth. 2011;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tariku A, Melkamu Y, Kebede Z. Previous utilization of service does not improve timely booking in antenatal care: cross sectional study on timing of antenatal care booking at public health facilities in Addis Ababa. Ethiop J Health Dev. 2010;24:226–233. [Google Scholar]
- 27.Belayneh T, Adefris M, Andargie G. Previous early antenatal service utilization improves timely booking: cross-sectional study at University of Gondar hospital, Northwest Ethiopia. J Pregnancy. 2014. Available at: http://www.hindawi.com/journals/jp/2014/132494/. Accessed March 30, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nwagha UI, Ugwu OV, Nwagha TU, et al. The influence of parity on the gestational age at booking among pregnant women in Enugu, South East Nigeria. Niger J Physio Sci. 2008;23:67–70. [DOI] [PubMed] [Google Scholar]
- 29.Horwood C, Butler LM, Haskins L, et al. HIV-infected adolescent mothers and their infants: low coverage of HIV services and high risk of HIV transmission in KwaZulu-Natal, South Africa. PLoS One. 2013;8:e74568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fatti G, Shaikh N, Eley B, et al. Adolescent and young pregnant women at increased risk of mother-to-child transmission of HIV and poor maternal and infant health outcomes: a cohort study at public facilities in the Nelson Mandela Bay Metropolitan District, Eastern Cape, South Africa. SAMJ. 2014;104:12. [DOI] [PubMed] [Google Scholar]
- 31.Wachira T, Ndege S, Koech J, et al. HIV testing uptake and prevalence among adolescents and adults in a large home-based HIV testing program in Western Kenya. J Acquir Immune Defic Syndr. 2014;65:e58–e66. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization (2013). The Voices, Values and Preference of Adolescents on HIV Testing and Counseling: Consultation for the Development of the World Health Organization HIV Testing and Counseling Guidelines for Adolescents. Geneva, Switzerland: World Health Organization; 2013. WHO/HIV/2013.135. [Google Scholar]
- 33.UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. 2014. Available at: http://www.unaids.org/en/resources/documents/2014/90-90-90UNICEF’s. Accessed March 30, 2016.
- 34.Zimbabwe Factsheet. Countdown to zero: elimination of new HIV infections among children by 2015 and keeping their mothers alive. Available at: http://www.emtct-iatt.org/wp-content/uploads/2012/10/PMTCT-Factsheet-Zimbabwe.pdf. Accessed January 27, 2016.
- 35.Mlilo-Chaiba CN. Factors Influencing Adolescents' Utilization of Antenatal Care Services in Bulawayo, Zimbabwe [PhD Thesis]. Pretoria, South Africa: University of South Africa; 2007. [Google Scholar]
- 36.World Health Organization (WHO). Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach. Geneva, Switzerland: WHO; 2013. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. Accessed March 30, 2016. [Google Scholar]
- 37.World Health Organization (WHO). Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. Geneva, Switzerland: WHO; 2015. Available at: http://who.int/hiv/pub/guidelines/earlyrelease-arv/en/. Accessed March 30, 2016. [PubMed] [Google Scholar]


