Abstract
Objective
The aim of this study was to determine the value of human papillomavirus (HPV) testing for primary cervical cancer screening in Japan.
Methods
In total, 5065 women who underwent primary screening with cytology and HPV between January 2005 and December 2006 were enrolled. In the baseline phase, these women were stratified by age, and the rates of HPV-positive and abnormal cytology were compared between women younger than and older than 30 years. In the follow-up phase, women aged 20 to 69 years and cytology negative for intraepithelial lesions or malignancy at baseline were followed up until December 2011 (n = 2383). Progression to grade 2/3 cervical intraepithelial neoplasia or worse (CIN2+/CIN3+) was compared between the HPV-positive and HPV-negative groups.
Results
In the baseline phase, HPV-positive rates were significantly higher in women younger than 30 years at 20.7% (95% confidence interval [CI], 18.4–22.9; 255/1234) compared with women 30 years or older at 7.2% (95% CI, 6.4%–8.0%; 275/3831; P < 0.001). However, there was no statistical difference for high-grade squamous intraepithelial lesion or worse rates between them, at 2.7% (95% CI, 1.8%–3.6%; 33/1234) and 2.4% (95% CI, 1.9%–2.9%; 91/3831), respectively, P = 0.55. In the follow-up phase, the rate of progression to CIN2+/CIN3+ was significantly higher in the HPV-positive group than in the HPV-negative group (P < 0.001). Moreover, relative risk of progression to CIN2+ was 15.9 times higher in the HPV-positive group, and that of progression to CIN3+ was 16.1 times higher in the HPV-positive group.
Conclusions
Human papillomavirus testing is a useful test for predicting progression to CIN and is recommended as a primary screening tool. However, screening with cytology alone is still appropriate for younger women, younger than 30 years, because HPV testing yields more false-positive results in younger women.
Key Words: Cervical cancer screening, Cytology, Disease progression, Human papillomavirus
High-risk human papillomavirus (HPV) infection is strongly correlated with cervical cancer, and persistent high-risk HPV infection is a major risk factor for the development of cervical intraepithelial neoplasia (CIN) that progresses to invasive cervical carcinoma. A previous study reported that 99.7% of patients with cervical cancer were high-risk HPV positive.1
Human papillomavirus–based screening yields a 60% to 70% higher detection rate of invasive cervical carcinoma compared with cytology screening.2 Worldwide large-scale randomized trials, such as Swedescreen,3 POBASCAM,4 ARTISTIC,5 NTCC,6 and ATHENA7 recommend that HPV-based screening should be initiated at the age of 30 years (only ATHENA at the age of 25 years).
Hybrid Capture 2 (HC2) assay-based HPV testing has demonstrated a high sensitivity (94.8%–96.4%) but a rather low specificity (86.0%–94.3%) compared with cytology (sensitivity: 56.9%–72.7%, specificity: 91.9%–94.3%) for the detection of CIN2.8–10 Several studies recommend that HPV testing should be the primary modality of cervical cancer screening worldwide.6,11–14
In 2011, the Japan Association of Obstetricians and Gynecologists proposed using cytology and HPV testing.15 In 2012, the guidelines established by the US Preventive Services Task Force, the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology also recommended using cytology and HPV cotesting for cervical cancer screening.13,16,17
With the introduction of conventional cytology in the 1950s, Japan has demonstrated a long history of population-wide cervical cancer screening. In light of new evidence regarding the effectiveness of HPV testing for cervical cancer screening, it is necessary to confirm the diagnostic value of cytology and HPV testing for primary cervical cancer screening and to determine the appropriate age and interval between screenings for a Japanese population. Therefore, we assessed the value of cytology and HPV testing to predict progression to CIN2+ or CIN3+ or invasive carcinoma. This is the first Japanese study to report long-term follow-up data of HPV testing and cytology as a primary screening method. We hope that the results obtained from this study might serve as important evidence to support using HPV testing for primary cervical cancer screening.
MATERIALS AND METHODS
Study Population
In total, 5065 women undergoing routine cervical cancer screening via an organized population-based program, conducted at Shimane Prefectural Central Hospital, were enrolled in this prospective observational study between January 2005 and December 2011.
Inclusion criteria were as follows: not being pregnant, having an intact uterus, no prior treatment for CIN, and no prior participation in a clinical trial for HPV treatment or HPV vaccination. All participants provided written informed consent before entering the study. In the baseline phase, all of the women (range, 14–95 years) participated in this study (n = 5065). Most studies to evaluate the effect of HPV testing have not included women 70 years or older. Most guidelines have not also recommended cervical cancer screening for women 70 years or older and women 19 years or younger. Although there is no upper age limit for cervical cancer screening in Japan, only women aged 20 to 69 years were included in the follow-up phase and the subanalysis (n = 4749). This study protocol was approved by Shimane Prefectural Central Hospital Ethical Committee.
Study Design and Interventions
Baseline Phase
The aim of the baseline phase was to assess the effectiveness of HPV testing as a primary screening modality for young women, younger than 30 years. All women were screened using cytology and HPV testing. After obtaining a brief medical history, cervical samples were collected from the women by obstetricians or gynecologists using a Cervical Sampler brush between January 2005 and December 2006. Conventional Papanicolaou staining results were reported using the 2001 Bethesda System. Abnormal cytology was defined as atypical squamous cells of undetermined significance or worse (≥ASC-US). We used the term high-grade squamous intraepithelial lesion (HSIL) CIN3+ (cytology estimated for CIN3+) as the value of evaluation for abnormal cytology in this study. The HC2 assay (Qiagen, Germantown, MD) was used to test for 13 high-risk HPV types according to the manufacturer’s instructions.
Follow-up Phase
The aim of the follow-up phase was to assess the factors associated with progression to CIN2+ or CIN3+ in women cytology negative at baseline.
Participants with negative cytology at baseline were then followed up until December 2011. Women with low-grade squamous intraepithelial lesion (LSIL) or worse cytology were referred to colposcopy, regardless of HPV test results. In the case of ASC-US cytology, HPV-positive women were also referred to colposcopy, whereas HPV-negative women underwent retesting 1 year later. Women cytology negative and HPV negative were rescreened in 3 years. A panel of 3 pathologists who were blinded to the screening results and participants’ information reviewed the histological diagnosis. When the pathologists disagreed about the diagnosis, the final diagnosis was decided on a majority vote. Standard CIN terminology was used for reporting the histology results. Women with a diagnosis of CIN2+ were excluded from the study and given the appropriate treatment or follow-up care. Women with CIN3+ underwent conization.
Statistical Methods
In the baseline phase, all women were divided into 7 age groups by decade: younger than 20 years, 20 to 29 years, 30 to 39 years, 40 to 49 years, 50 to 59 years, 60 to 69 years, and 70 years or older. The χ2 test was used to investigate differences in high-risk HPV infection rates and HSIL-CIN3+ for each age group.
During the follow-up phase, incidence rates and 95% confidence intervals (CIs) were calculated for CIN (per 1000 person-months) in the HPV-positive and HPV-negative groups, as well as the relative risk of progression to CIN2+ in both groups (95% CI estimation: formula 1). Sample size was determined by the need for a sufficient number in the follow-up phase to adequately evaluate the performance of cytology and HPV testing. This estimate was used, along with published 5-year cumulative incidence rates of CIN3+ (cytology−/HPV+: 5%, cytology−/HPV−: <0.1%), to arrive at a sample size of approximately 2000 women at a conventional significance level (α = 0.05) and power (1 − β = 0.80). Kaplan-Meier curves were used to investigate progression to CIN2+/CIN3+, and the log-rank test was used to examine difference in rates of progression between the HPV-negative and HPV-positive groups. Finally, to examine risk factors for progression to CIN2+/CIN3+, a Cox proportional hazards analysis was performed.

Where LN is the natural logarithm


Thus, P < 0.05 was considered to be statistically significant for all tests. Statistical analyses were performed with Microsoft Excel 2010 and SPSS (IBM SPSS Statistics 20J).
RESULTS
Baseline Phase
Participants’ ages ranged from 14 to 95 years, with a median age of 38 years (n = 5065). The overall HPV-positive rate was 10.5% (95% CI, 9.6%–11.3%; 530/5065), whereas the HSIL-CIN3+ rate was 2.4% (95% CI, 2.0%–2.9%; 124/5065). Table 1 shows the HPV status and HSIL-CIN3+ rates by age group at baseline. The invasive cancer rate was 0.5% (95% CI, 0.3%–0.7%; 24/5065).
TABLE 1.
Comparison of high-risk HPV infection and HSIL-CIN3+ rates in women younger or older than 30 years

Human papillomavirus–positive rates were significantly higher in women younger than 30 years at 20.7% (95% CI, 18.4%–22.9%; 255/1234) compared with women 30 years or older at 7.2% (95% CI, 6.4%–8.0%; 275/3831; P < 0.001) (Table 1). However, there was no statistical difference for HSIL-CIN3+ rates in the same ages groups, at 2.7% (95% CI, 1.8%–3.6%; 33/1234) and 2.4% (95% CI, 1.9%–2.9%; 91/3831), respectively, P = 0.555.
Follow-up Phase
A total of 4437 women aged 20 to 69 years with normal cytology, negative for intraepithelial lesions or malignancy, and either HPV positive or HPV negative at baseline were followed up. After excluding those lost to follow-up (n = 2054), a total of 2383 women were included in the final analysis. Of these, 15 and 10 women demonstrated progression to CIN2+ and CIN3+, respectively (Fig. 1). The age range of the 15 women who developed CIN2+ was 23 to 48 years, with a median age of 33 years. The age range of the 10 women who developed CIN3+ was 26 to 48 years, with a median age of 39 years. A total of 205 women (8.8%; 210/2383) were HPV positive, whereas 2178 women (91.4%; 2.178/2383) were HPV negative. Of the HPV-positive women, 9 women (4.4%; 9/205) and 6 women (2.9%; 6/205) developed CIN2+ and CIN3+, respectively. Of the HPV-negative women (n = 2178), 6 women (0.3%; 6/2178) and 4 women (0.2%; 4/2178) developed CIN2+ and CIN3+, respectively (Fig. 1). The differences in both CIN2+ and CIN3+ were statistically significant (P < 0.001).
FIGURE 1.
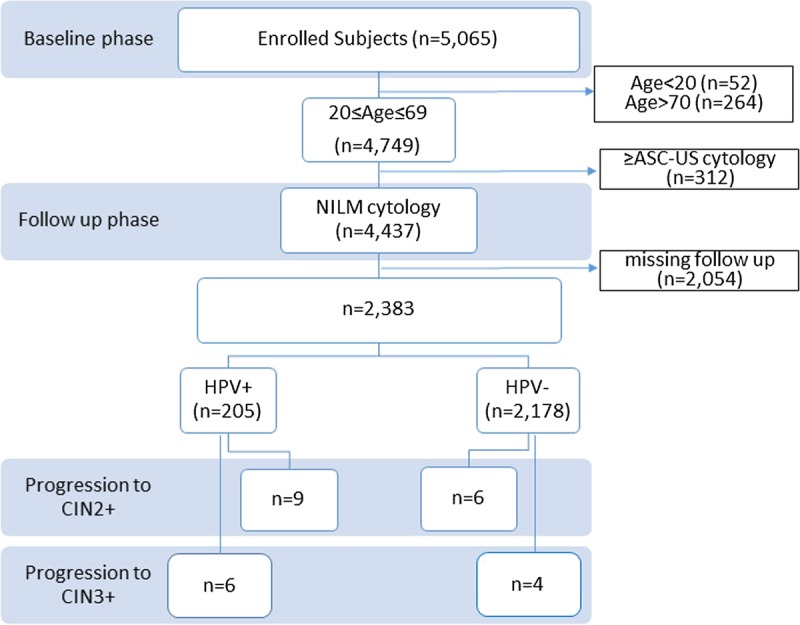
Consort diagram of flow of patients through the study. ≥ASC-US, atypical squamous cells of undetermined significance or worse; HPV+, HPV positive; HPV-, HPV negative; CIN2+, grade 2 CIN or worse; CIN3+, grade 3 CIN or worse; NILM, negative for intraepithelial lesion.
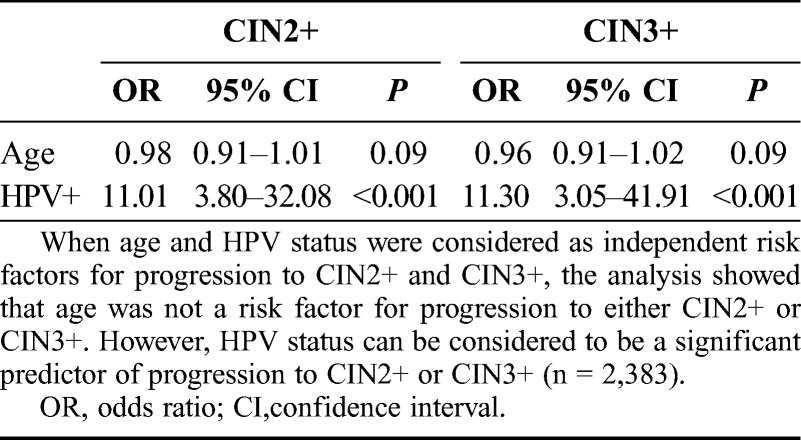
When age and HPV status were considered as independent risk factors for progression to CIN2+ and CIN3+, the analysis showed that age was not a risk factor for progression to either CIN2+ (P = 0.09) or CIN3+ (P = 0.21). However, compared with women who were HPV negative at baseline, women who were HPV positive had a more than 10-fold risk of developing CIN2+ or CIN3+ lesions (ORs, 11.01 [95% CI, 3.80–32.08] and 11.30 [95% CI, 3.05–41.91], respectively). Thus, HPV status can be considered to be a significant predictor of progression to CIN2+ or CIN3+ (Table 2).
TABLE 2.
Assessment of independent risk factors for progression to CIN2+/3+(Cox regression analysis)
Risk of Progression to CIN3+/CIN2+
When CIN2+ was used as the end point, the mean observation period for the HPV-negative and HPV-positive groups was 82.4 months (95% CI, 82.2–82.6 months) and 81.5 months (95% CI, 80.2–82.8 months), respectively. When CIN3+ was used as the end point, the mean observation period for the HPV-negative and HPV-positive groups was 82.4 months (95% CI, 82.3–82.5 months) and 80.4 months (95% CI, 78.8–82.1 months), respectively.
The incidence of CIN2+ and CIN3+ in the HPV-positive and HPV-negative groups is represented using Kaplan-Meier survival curves (Figs. 2 and 3). A significant difference was observed between the HPV-positive and HPV-negative groups in both CIN2+ and CIN3+ (log-rank test), with the HPV-positive group demonstrating a significantly higher incidence of CIN2+ and CIN3+ (P < 0.001).
FIGURE 2.

Survival analysis for incidence of CIN2+.
FIGURE 3.
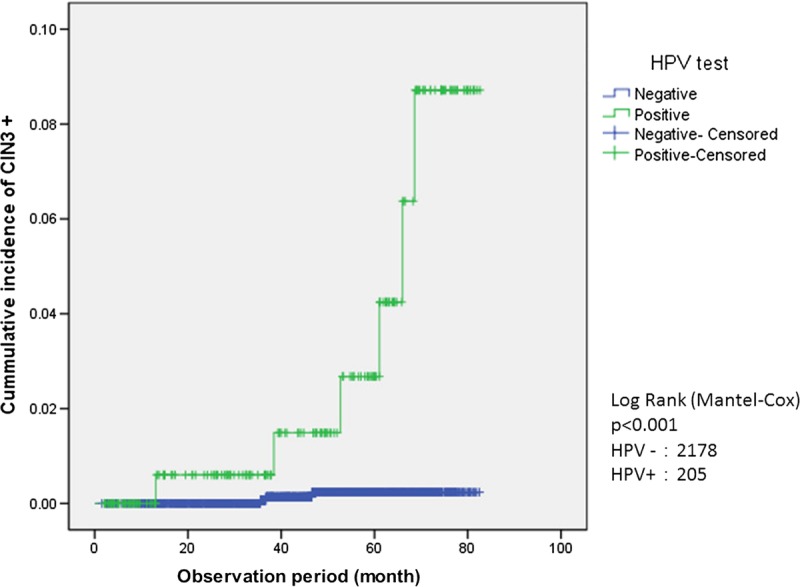
Survival analysis for incidence of CIN3+.
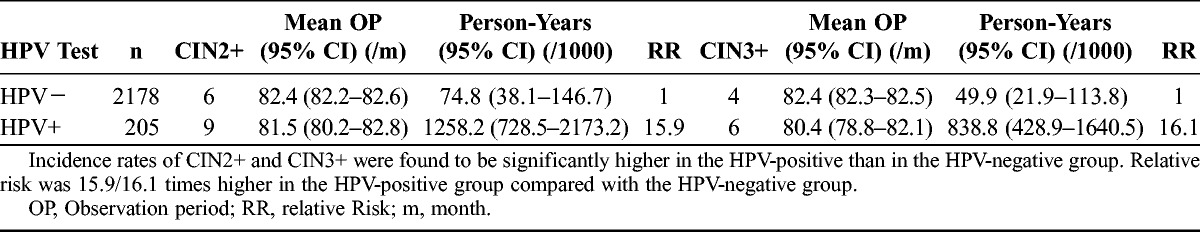
The incidence of CIN2+ was 74.8 (95% CI, 38.1–146.7) per 1000 person-years in the HPV negative group compared with 1258.2 (95% CI, 728.5–2173.2) per 1000 person-years in the HPV-positive group, indicating a 15.9-fold risk of progression to CIN2+ in the latter. Similarly, the incidence of CIN3+ was 49.9 (95% CI, 21.9–113.8) per 1000 person-years in the HPV-negative group compared with 838.8 (95% CI, 428.9–1640.5) per 1000 person-years in the HPV-positive group, once again indicating a 16.1-fold increased risk of progression to CIN3+ in HPV-positive women (Table 3).
TABLE 3.
Comparison of CIN2+/CIN3+ incidence per 1000 person-years at risk
DISCUSSION
Cervical cancer screening target age groups, testing intervals, and testing methods (the main theme of the present study) should take into account various factors such as differences in HPV type prevalence among countries, quality control, and screening compliance rates. Thus, screening protocols differ among different countries.
The findings of our study are as follows: HPV testing is a useful test for predicting progression to CIN and should be recommended as a primary screening tool. However, screening by cytology alone is appropriate for younger women, those younger than 30 years. There was a high lost-to-follow-up rate (46.3%); therefore, we could not estimate an appropriate screening interval. It may reflect the general low participation rate to cervical cancer screening in Japan. We checked whether participants in the follow-up phase (n = 2383) were representative of the population in the baseline phase (n = 5065) using the χ2 test. We found that there were no significant difference between participants in the follow-up phase and the lost-to-follow-up group in age; however, the sample followed up was not representative of the population at baseline because of the significant difference in HPV positive rates. This is the biggest limitation of this study.
Moreover, this was a prospective observational study and did not compare differences in CIN2+, CIN3+ detection rates using primary screening with conventional cytology alone versus HPV testing alone or conventional cytology versus cotesting; thus, the differences in sensitivity could not be analyzed. This study also did not compare differences between cotesting and HPV alone as a primary screening tool.
The results from the present study indicate that younger women have a higher HPV-positive rate, which is consistent with results from studies in other countries.13,18–20 This phenomenon originates from the fact that HPV, in its natural history, is frequently a transient infection in younger women,21 which explains the discrepancy in results between cytology and HPV testing. Thus, in younger women, HPV testing yields more false-positive results and is anticipated to decrease the specificity of testing, therefore making HPV unsuitable. Overdiagnosis not only causes mental anxiety on an individual level, but also increases medical costs. Screening with cytology alone is appropriate for younger women.
Although the present study stopped comparing cumulative incidences of CIN2+/3+ after a 5-year period, we believe that more evidence is necessary to determine the extent to which testing intervals can be prolonged for women who are both cytology negative and HPV negative. However, an important issue in Japan is the stagnation of screening uptake rates. While western nations boast uptake rates of 70% to 80%, the uptake rate in Japan is 42.1%.22 In order to achieve the goal of cervical cancer prevention, cost-effectiveness must be examined, as test precision cannot be further improved at present. Restricting target ages for testing and prolonging testing intervals naturally entails the risk of overlooking cases. However, it is important to minimize the harm caused by overtesting (burden placed on subjects, costs, and squandering of medical care resources) and to maximize the effect of testing within limited costs and medical care resources. In the future, the Japanese cervical cancer screening algorithm will require modeling and reanalyzing based on health economic theories if the HPV vaccination coverage increases.
This study has another minor limitation that has to be discussed. Data were collected from a single center. Therefore, the possibility of bias in terms of regional and population characteristics cannot be ruled out. Shimane Prefecture is located in the Chugoku Region of Japan and has a population of 695,489 (as of February 1, 2015). While Shimane Prefectural Central Hospital is a prefectural flagship hospital with more than 600 beds and provides highly specialized medical care, the hospital also provides routine cervical cancer screening for the region’s population base and careful management for cases referred to a colposcopy clinic. Another limitation is that we could not reach a conclusion on the most appropriate age at which HPV testing should be initiated (whether in women ≥30 or ≥25 years); this warrants further study.
There are some previous studies in other countries; however, this is the first Japanese study to report long-term follow-up data of cytology and HPV testing as a primary screening method.23
In conclusion, relative risk of progression to CIN2+/CIN3+ was 15.9/16.1 times higher in the HPV-positive group compared with the HPV-negative group in this study. Therefore, whereas women who are HPV positive require more careful management, screening intervals in cytology-negative, HPV-negative women can potentially be extended. Then HPV testing is a useful test for predicting progression to CIN, and it should be recommended as a primary screening tool. However, younger women have a high HPV-positive rate and discrepancy in results from cytology. This discrepancy suggests that HPV-based screening is not appropriate for younger women. It means that screening by cytology alone is appropriate for younger women, younger than 30 years.
ACKNOWLEDGMENTS
The authors thank the obstetricians and gynecologists at Shimane Prefectural Central Hospital for collecting the samples. The authors also thank the Department of Diagnostic Pathology at Shimane Prefectural Central Hospital for evaluating the large number of samples in the study and for providing the slides for sampling. They also thank Dr Sharon J. B. Hanley of Hokkaido University Graduate School of Medicine for editing the English.
Footnotes
The human papillomavirus test, Hybrid Capture 2 (Qiagen, Germantown, MD), used to test for high-risk human papillomavirus types in this study, was donated by Qiagen. Qiagen played no role in the research design, analysis, or interpretation of the data.
R.K. has received lecture fees from Qiagen, BD, Roche Diagnostics, GSK, MSD and Chugai Pharmaceutical, Co, Ltd. His institute received research funding from Chugai Pharmaceutical, Co, Ltd and Roche Diagnostics outside the submitted work. Those lecture fees and funding have no influence on the manuscript. All other authors declare no conflicts of interest.
REFERENCES
- 1.Franco EL, Duarte-Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. CMAJ. 2001;164:1017–1025. [PMC free article] [PubMed] [Google Scholar]
- 2.Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–532. [DOI] [PubMed] [Google Scholar]
- 3.Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597. [DOI] [PubMed] [Google Scholar]
- 4.Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78–88. [DOI] [PubMed] [Google Scholar]
- 5.Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672–682. [DOI] [PubMed] [Google Scholar]
- 6.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–257. [DOI] [PubMed] [Google Scholar]
- 7.Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189–197. [DOI] [PubMed] [Google Scholar]
- 8.Koliopoulos G, Arbyn M, Martin-Hirsch P, et al. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and meta-analysis of non-randomized studies. Gynecol Oncol. 2007;104:232–246. [DOI] [PubMed] [Google Scholar]
- 9.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Screening for cervical cancer: a systematic evidence review for the US Preventive Services Task Force. Evidence Report No. 86 Rockville, MD: Agency for Healthcare Research and Quality; 2011. [PubMed] [Google Scholar]
- 11.von Karsa L, Arbyn M, de Vuyst H, et al. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res. 2015;1:22–31. [Google Scholar]
- 12.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178–182. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Screening for cervical cancer: a decision analysis for the U.S. Preventive Services Task Force. Evidence Report No. 86. Rockville, MD: Agency for Healthcare Research and Quality; 2011. [PubMed] [Google Scholar]
- 14.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. [DOI] [PubMed] [Google Scholar]
- 15.Minakami H, Maeda T, Fujii T, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res. 2014;40:1469–1499. [DOI] [PubMed] [Google Scholar]
- 16.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. Am J Clin Pathol. 2012;137:516–542. [DOI] [PubMed] [Google Scholar]
- 17.ACOG Announcements. New Cervical Cancer Screening Recommendations from the U.S. Preventive Services Task Force and the American Cancer Society/American Society for Colposcopy and Cervical Pathology/American Society for Clinical Pathology. Available at: https://provider.carefirst.com/carefirst-resources/provider/pdf/acog-cervical-cancer-screening.pdf. Accessed December 8, 2016.
- 18.Garland SM, Cuzick J, Domingo EJ, et al. Recommendations for cervical cancer prevention in Asia Pacific. Vaccine. 2008;26(suppl 12):M89–M98. [DOI] [PubMed] [Google Scholar]
- 19.Peto J, Gilham C, Deacon J, et al. Cervical HPV infection and neoplasia in a large population-based prospective study: the Manchester cohort. Br J Cancer. 2004;91:942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;377:a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. [DOI] [PubMed] [Google Scholar]
- 22.OECD Health at a Glance 2015. Cervical cancer screening in women aged 20–69, 2003 to 2013 (or nearest years). Paris, France: OECD Publishing; 2015. [Google Scholar]
- 23.Sauvaget C, Nishino Y, Konno R, et al. Challenges in breast and cervical cancer control in Japan. Lancet Oncol. 2016;17:e305–e312. [DOI] [PubMed] [Google Scholar]


