Abstract
The tomato fruits during different stages of ripening have been extensively characterized for nutritionally important bioactives; however, changes in fatty acid composition are not available. Thus, in the present study, changes in fatty acid, along with carotenoid and α-tocopherol, were studied during the six stages of ripening. Fruits were harvested at the green, breaker, turning, pink, light red, and red stages, which occurred at means of 30, 35, 40, 46, 50, and 55 days after anthesis (DAE), respectively. During the ripening process, profiles of all the metabolites altered significantly (p < 0.05). All-E-lycopene content increased from the breaker (0.21 μg/g FW) to the red stage (30.6 μg/g FW), while all-E-lutein was slightly increased during initial stages of ripening and then decreased significantly, with the highest (4.15 μg/g FW) in the fruits of the pink stage. Furthermore, the contents of α-tocopherol increased during ripening, and its increase was highest between light red to the red stages. In all the ripening stages, linoleic acid (C18:2n6c) was found in the highest quantity (42.3–49.2%), followed by oleic (C18:1n9c; 20.1–26.6%) and palmitic acids (C16:0; 16.6–17.7%). With fruit ripening, the ratio of polyunsaturated fatty acids and saturated fatty acids (PUFAs:SFAs) was increased significantly from 1.89 (green) to 2.19 (red). Interestingly, the oleic acid proportions correlated inversely with linoleic (r = −0.450) and α-linolenic acid (r = −0.904), during all the stages of ripening. The highest and lowest contents of oleic acid and linoleic acid (26.7 and 42.3%, respectively) were recorded in the fruits of stage 3 (turning). In conclusion, ripening in tomatoes is accompanied by significant increases in carotenoids and α-tocopherol, as well as by concomitant increases in PUFAs.
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0666-0) contains supplementary material, which is available to authorized users.
Keywords: Lycopersicon esculentum, Lycopene, β-carotene, Linoleic acid, α-Linolenic acid
Introduction
Tomato (Solanum lycopersicum L., syn: Lycopersicon esculentum Mill) is one of the most widely consumed vegetables, being the second most important vegetable crop in the world, next to potato. In the United States, in the year 2015, tomatoes, sweet corn, and snap beans together accounted for 93% of the total production of processing crops (United States Department of Agriculture 2016). The consumption of tomatoes is strongly associated with a reduced risk of chronic degenerative diseases, owing to the high content of health beneficial phytochemicals, such as, the carotenoids (β-carotene and lycopene), the glycoalkaloids (dehydrotomatine and α-tomatine), ascorbic acid, tocopherols, and many phenolic and flavonoid compounds (Choi et al. 2014; Perveen et al. 2015; Saini et al. 2015). In the United States and many Western countries, tomato is the major dietary source of vitamin A and C because of high per capita consumption (Klee and Giovannoni 2011).
The tomato fruit has emerged as the preeminent model for the study of ethylene signaling in fruit ripening and quality preservation. Tomato is a climacteric fruit, with an essential requirement for the phytohormone ethylene to ripen (Alexander and Grierson 2002). The ripening of tomato fruit involves several biochemical, physiological, and structural changes, including the production of secondary metabolites which influence flavor, aroma, texture, and appearance (Konozy et al. 2012). Accumulation of large quantities of pigments, especially lycopene and β-carotene, inside the plastoglobules of chromoplast provides a visual indication that the fruit is mature and suitable for consumption (Klee and Giovannoni 2011). Lipid components of fruits, though occurring in minor quantities, are supposed to contribute to the development of typical aromas and flavors during ripening as fatty acids are the major precursors for aroma volatiles in most fruits, including tomato (Baldwin et al. 2000).
Tomato fruits are usually consumed at the full red ripe stage, however, before excessive softening. The color of tomato fruits is the most important external characteristic to assess ripeness and postharvest life. Based on the visible color, the USDA establishes six ripening stages: green, breaker, turning, pink, light red, and red (Camelo and Gómez 2004). Harvesting of tomato fruits depends upon the purpose for which they are used, e.g., Tomato fruits are harvested at mature green for long distance marketing, and full ripen stage for fresh consumption (Moneruzzaman et al. 2009). The nutritional and sensory quality of tomato-based product largely depends on variety, maturity stage at harvest, transport conditions, and supermarket storage (Verheul et al. 2015). The tomato fruits during storage and different developmental stages of ripening have been extensively characterized for the contents of carotenoids, especially β-carotene and lycopene (Moco et al. 2007; Park et al. 2016). Unlike fruits of other species, changes in fatty acid composition during the vine-ripening of tomato fruits are still not reported. Thus, in the present study, we investigated the fatty acid composition, along with carotenoid and α-tocopherols during the ripening of tomato fruits, commercially cultivated in the Republic of Korea. This information adds to our understanding of temporal differentiation of nutritionally significant phytochemical during ripening of tomato fruits.
Materials and methods
Plant material, reagents, and standards
Authentic standard of all-E-lutein was purchased from Cayman Chemical Company, Michigan, USA. All-E-β-carotene, all-E-lycopene, fatty acid standard mix (CRM47885- Supelco 37 Component FAMES Mix), and certified reference material (BCR-485) were purchased from Sigma-Aldrich, St. Louis, MO, USA. All organic solvents used for extraction of fatty acids were of HPLC grade (Daejung, Korea).
The tomato vines of commercial F1-hybrid cultivar “Sunglove” were cultivated in an open commercial farm located in Paju, South Korea from May to August 2016. The wines were cultivated following the natural farming practices, without the use of any chemical herbicide, pesticides, and fertilizers. The mature green (Stage 1) fruits appeared after 30 days after anthesis (DAE), which turn to the breaker, turning, pink, light red, and red stages, at means of 35, 40, 46, 50, and 55 DAE, respectively. For each stage, five fruits were harvested from five different vines (biological replicates). Also, the fruits of all the ripening stages were harvested simultaneously in a single occasion. The fruits were brought to the laboratory, washed with ordinary tap water, cleaned with tissue paper, and stored at −80 °C in the ultra-low temperature deep freezer (Ilshin Biobase Co. Ltd., Korea) until analysis. The fruits of six different ripening stages studied in the present investigation are shown in Fig. 1.
Fig. 1.
Six different ripening stages of tomato fruits and the pattern of accumulation of red color
Extraction and quantification of carotenoids and tocopherols
The carotenoids and tocopherols were extracted in triplicates and quantified according to previously established protocol with minor modifications (Rodriguez-Amaya 2001; Saini et al. 2012). All the preparations were performed in low-light conditions to avoid the light-mediated degradation. Three independent biological samples were extracted separately. Briefly, one whole tomato fruit was finely chopped and homogenized in a food processor, and five gram of homogenized fruit (exact to 0.001 g) from each ripening stage was separately transferred into test tubes containing 20 ml of acetone and 0.1% butylated hydroxytoluene (BHT: w/v). The samples were again homogenized with a mechanical homogenizer and centrifuged at 5000×g (5 min at 4 °C). The supernatant was recovered, and pelleted samples were repeatedly extracted until the pellets became colorless. Supernatants from all extractions were pooled and vacuum-dried in a rotary evaporator (temperature <35 °C) (Büchi RE 111, Switzerland). The extract was recovered with 10 ml of methylene chloride (CH2Cl2) containing 0.1% BHT and transferred to an amber color HPLC vial for HPLC analysis.
The chromatographic separation was achieved using an Agilent Model 1100 HPLC instrument (Agilent Technologies Canada Inc., Mississauga, ON, Canada) equipped with a degasser, autosampler, dual pump, and diode-array detector (DAD). Samples were scanned (200–800 nm) with 0.05 min (1 s) response time, 8.0-mm slit width, and a detection wavelength of 295 (for tocopherols), 450 (for lutein and β-carotene), and 470 nm (for lycopene). The bandwidth was ±16 nm for all detection wavelengths. Similarly, 600 nm was used as reference wavelength with ±50 nm bandwidth in all detections. The column used was a YMC, C30 carotenoid column, 250 × 4.6 mm, 5 μm (YMC, Wilmington, NC), and the chromatographic data were recorded with ChemStation LC 3D software. The column thermostat was maintained at 20 °C. 20 μl of standards and samples were injected with autosampler. The solvent system consisted of methanol: water (95:5) (mobile phase A) and methyl tertiary butyl ether (MTBE): Methanol (91:9) (Mobile phase B). The gradient elution was 0–100% B in 50-min and 5-min post run at a flow rate of 1 ml/min.
Lipid extraction and fatty acid methyl esters (FAMEs) preparation
Lipid extraction was performed (in triplicate) using chloroform–methanol extraction, based on methodology reported by Bligh and Dyer (1959), and Sivanesan et al. (2016) with minor modifications. Briefly, one whole tomato fruit was finely chopped and homogenized in a food processor, and five grams of homogenized fruit (exact to 0.001 g) from each ripening stage were separately transferred into a test tube containing 20 ml mixture of chloroform and methanol (2:1 v/v). The samples were again homogenized, centrifuged at 5000×g (5 min at 4 °C), and the supernatant was recovered. The pelleted sample was extracted again with 10 ml mixture of chloroform and methanol (2:1 ratio). Supernatants from both the extractions were pooled and dried in a rotary vacuum evaporator, and total lipid contents were then determined gravimetrically. Subsequently, fatty acid methyl esters (FAMEs) were prepared from extracted lipids by the conventional anhydrous methanolic hydrochloric acid (HCl) method, using 5% acetyl chloride in methanol (v/v) (Saini et al. 2014).
GC-FID analysis of FAMEs
Fatty acid methyl esters (FAMEs) were analyzed by Gas Chromatograph (GC) (Agilent 7890B, Agilent Technologies Canada Inc.,) equipped with a flame ionization detector (FID), autoinjector, and a fused silica Rtx-2330 column (Restek make, 30 m length, 0.32 mm ID, and 0.20-μm film thickness). Injector port and the detector temperatures were set up at 250 and 260 °C, respectively, Nitrogen (N2) was used as carrier gas. Initially, column temperature was maintained at 50 °C for 2 min, followed by increasing to 250 °C in 50 min by linear temperature program of 4 °C/min. The FAMEs were identified by comparing their retention time with authentic standards of 37 Component FAMEs Mix (CRM47885-Supelco).
Statistical analysis, method validation, and quality control
For GC and HPLC analysis, all the samples were extracted in triplicates and analyzed separately in duplicates. The values from all determinations (n = 6) of each sample were averaged and represented as means with standard deviations. The data were analyzed statistically by the SPSS 17.0 software using one-way ANOVA.
The accuracy of the carotenoid extractions was evaluated using certified reference material (CRM) (BCR-485, Sigma-Aldrich, St. Louis, MO, USA). Since the contents of α-tocopherol are not mentioned in this CRM, additional recovery experiment was conducted for α-tocopherol (Cruz and Casal 2013). The HPLC analytical method used for quantification of carotenoids and tocopherols was also validated in terms of accuracy, linearity, precision, and stability as previously described. The instrumental precision (Intra-day and Inter-day) for retention time and area count was also determined using multiple injections (n = 6) of same concentration and expressed as the percentage of coefficient of variation (% CV) (US Food and Drug Administration 2001; Cruz and Casal 2013). The identities of carotenoids were confirmed by retention times, peak symmetry, and average peak spectrum compared with authentic standards.
Results and discussions
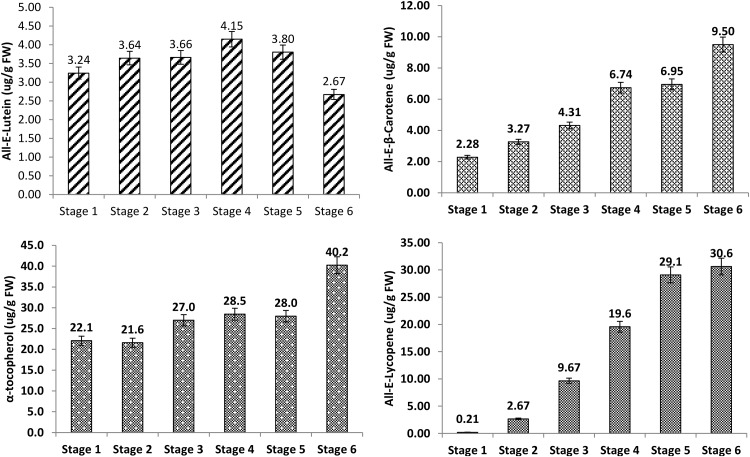
The gradient elution system with C30 stationary phase applied in this study for the quantification of carotenoids and tocopherols provided good resolution, precision, and repeatability (Supplementary material Figure S1). Carotenoids were quantified without saponification, as most of the monohydroxy carotenoids in studied samples were found non-esterified in preliminary studies (data not shown). In the quality control study, the mean contents of major carotenoids, all-E-lutein, all-E-β-carotene, and all-E-lycopene (11.5, 21.3 and 12.2 μg/g, respectively) in certified reference material (CRM) (BCR-485), were found in accordance with certified values (12.5, 23.7 and 13.8 μg/g, respectively). The mean recovery percentage for α-tocopherol was 94.2%. Thus, these carotenoids and α-tocopherol data validated the accuracy of applied extraction methods from the tomato fruits. In HPLC method validation of carotenoids and tocopherols, the low coefficients of variation (% CV; always below 3.80 and 0.80 for peak areas and retention times, respectively) were recorded for intra-day (n = 6) and inter-day (n = 6 × 2) values assessed for peak area and retention time. The linear calibration curves also showed a high coefficient of correlation between area counts and standard concentrations (R 2; >0.992–0.994). With regard to these parameters, the currently used HPLC method shows good accuracy, linearity, precision, and stability. Using this approach, all-E- lutein, all-E- β-carotene, and all-E- lycopene were identified as the major carotenoids in tomato fruits of different ripening stages, on the basis of retention time with authentic standards and also by comparing the peak spectra recorded with a diode-array detector (DAD) during the analysis. The chromatograms (470 nm) and the peak spectra of major identified peaks were shown in Supplementary material Figure S1. The other minor carotenoids were not quantified due to the unavailability of standard compounds. During the ripening process, profiles of all the studied metabolites altered significantly (p < 0.05). All-E-lycopene content increased from the breaker (0.21 μg/g FW) to the red stage (30.6 μg/g FW), while all-E-lutein was slightly increased during initial ripening stages and then decreased significantly, with highest values during the pink stage (Fig. 2).
Fig. 2.
The content of all-E-lutein, all-E-β-carotene, all-E-lycopene, and α-tocopherol in tomato fruits of different ripening stages. Values above bars displaying the data labels. Values are mean ± standard deviation from six replicates (triplicate extractions with duplicate analysis). Different superscript letters indicate statistically significant differences among the various ripening stages (p < 0.05)
Regulation of carotenoid biosynthesis and high-accumulation lycopene during tomato fruit development is widely studied (Ronen et al. 1999; Moco et al. 2007; Su et al. 2015). Moco et al. (2007) observed an increase in β-carotene, reduction in neoxanthin contents, while lutein was virtually constant during tomato fruit development. In the present study, we have also recorded an increase in all-E-β-carotene and all-E-lycopene contents during ripening. However, all-E-lutein showed a profile that was slightly different from these carotenoids. This carotenoid first increased up to the pink stage (stage 4) and then decreased to the red stage. This may be due to the difference in cultivars, as, during tomato fruit ripening, the cultivar-specific pattern of accumulation of β-carotene is reported (Bhandari and Lee 2016). Authors recorded the higher β-carotene content in pink and light red stages of cherry tomatoes. However, cultivar Dafnis showed a higher level of β-carotene content in red stage than in other stages. During tomato fruit development, the mRNA levels for the lycopene-producing enzymes phytoene synthase (PSY) and phytoene desaturase (PDS) increase significantly, while the mRNA levels of the genes for the lycopene β- and ε-cyclases, which convert lycopene to either β- or δ-carotene, respectively, decline and completely disappear (Ronen et al. 1999). Thus, lycopene is produced as major carotenoid in cell-localized phytochromes and confers the red color to ripe tomato fruits.
In nature, vitamin E (tocochromanols or tocols) consists of four tocopherols (α-, β-, γ-, and δ-tocopherol) and four tocotrienols (α-, β-, γ-, and δ-tocotrienols), well-known for its potent antioxidant and anticancer activities (Saini and Keum 2016). Among various tocols, α-tocopherol was recorded as the major tocopherol in tomato fruits of various developmental stages. The level of α-tocopherol increased during ripening in all tissues, though its increase was largest between light red to red stages (Fig. 2). Similar trends of α-tocopherol accumulation were previously recorded in tomato fruits during development (Moco et al. 2007). α-Tocopherol plays a significant role in cell signaling toward environmental and developmental signals (Traber and Atkinson 2007). In non-climacteric fruit (such as grape, Vitis vinifera), tocopherol contents decline gradually along with its development (Horvath et al. 2006), whereas in climacteric fruits (such as mango and tomato), exhibits an inverse pattern (Singh et al. 2011). The intense respiration and ethylene production in climacteric fruits are associated with an oxidative phenomenon with increased hydrogen peroxide content, lipid peroxidation, and protein oxidation (Jimenez et al. 2002). As a result, within this oxidative environment, the maintenance or increase in tocopherol levels by activation of the tocopherol biosynthetic pathway is clearly advantageous to balance their levels (Quadrana et al. 2013). Additionally, the chlorophyll degradation during ripening also favors the tocols biosynthesis by utilizing chlorophyll degradation-derived phytyl-diphosphate in tocopherol biosynthesis (Almeida et al. 2015). Tomato fruit ripening is also reported to increase the total phenolics, flavonoids, vitamin C, and antioxidant potential (Periago et al. 2009).
A large number of studies have been conducted to analyze the temporal changes in the contents of carotenoid and tocopherol during the ripening of tomato fruits. This study extends their results by analyzing the fatty acid composition during the various developmental stages of tomato fruits. The GC–FID analysis revealed the presence of 10 major fatty acids (C13–C24) in the unripe and ripened fruits (Supplementary material Figure S2). Among all the ripening stages, linoleic acid (C18:2n6c) was found in the highest quantity (42.3–49.2%), followed by oleic (C18:1n9c; 20.1–26.6%) and palmitic acids (C16:0; 16.6-17.7%) (Table 1). Similar compositions of fatty acids were reported in red ripened tomato fruits cultivated in greenhouses (Guil-Guerrero and Rebolloso-Fuentes 2009). The improvement in contents of linoleic and α-linolenic acids were previously reported in mature green tomato fruits stored at non-chilling and chilling temperatures (2 and 15 °C, respectively) for 12 days (Whitaker 1991). To our knowledge, no reports are available on the changes of fatty acid compositions during ripening of tomato fruits on the vine; however, this phenomenon has been widely studied in other climacteric fruits, including mango (Lalel et al. 2003) and avocado (Villa-Rodríguez et al. 2011). Lalel et al. (2003) studied the production of aroma volatiles during fruit ripening of ‘Kensington Pride’ mango and found that most of the fatty acids increased during fruit ripening. The linoleic and linolenic acids were also found to increase during ripening. However, their proportion (% fatty acid to total fatty acids) was first increased during initial ripening stages (0–6 days) and then decreased in further ripening stages (7–10 days). Mango fruits were ripe on the seventh day of the ripening period, which corresponded to the fruit being eaten soft and a skin color that was 75% yellow. The production of major aroma volatiles, monoterpene, and sesquiterpene hydrocarbons was also found concordant with fatty acids. Thus, it is considered that fatty acids are the primary precursors of volatile compounds responsible for plant aromas, modulated by ethylene and storage conditions during fruit ripening (García-Rojas et al. 2016). Villa-Rodríguez et al. (2011) studied the variations in fatty acid content during ripening of ‘Hass’ avocado and observed significant increase in total content of monounsaturated (MUFAs) and saturated fatty acids (SFAs) and a decrease of polyunsaturated fatty acids (PUFAs) during the ripening period, resulting in decrease of PUFAs: SFAs ratio. Contrasting to this, in the present study, PUFAs: SFAs ratio was increased significantly during ripening, from 1.89 in green fruits (stage 1) to 2.19 in red fruits (stage 6). Therefore, according to these results, it appears that the nutrition value of tomato fruit increased during ripening. α-Linolenic acid and linoleic acid (ω-3 and ω-6 FAs, respectively) are the essential fatty acids (EFAs) for the human diet. Though in tomato fruits, fatty acids are the minor component, the high proportion of PUFAs in fully ripened fruits can add their health benefits. Interestingly, the oleic acid proportions correlated inversely with linoleic (r = −0.945) and α-linolenic acid (r = −0.904) during all the stages of ripening. The contents of linoleic acid were lowest (42.3%) in fruits of stage 3 (turning) and increased in red ripened fruits (49.3%). This is probably due to the enhancement of enzymatic activities of desaturases, especially Δ12-desaturase responsible of linoleic acid biosynthesis, using 18 carbon fatty acids as substrate stimulation. Aidi Wannes et al. (2009) studied the variations in essential oil and fatty acid composition during Myrtus communis fruit maturation and found that linoleic acid proportions correlated inversely with palmitic and oleic acids during all the stages of ripening. In another study, SFAs and PUFAs decreased significantly, and MUFAs increased during maturation of coriander fruit (Msaada et al. 2009). Results of this study and also previous studies indicate that the variation in the fatty acid composition of fruit during ripening/maturation might be useful in understanding the source of nutritionally and industrially important phytochemicals.
Table 1.
Composition of fatty acids in tomato fruits of six ripening stages
| S. No. | Fatty acids | RT | Green | Breaker | Turning | Pink | Light red | Red |
|---|---|---|---|---|---|---|---|---|
| 1 | C13:0 (Tridecanoic) | 21.7 | 2.03a | 1.56b | 1.62b | 0.90c | 0.51d | 0.51d |
| 2 | C14:0 (Myristic) | 23.0 | 0.29b | 0.24c | 0.27b | 0.25c | 0.37d | 0.36d |
| 3 | C16:0 (Palmitic) | 26.8 | 16.8ab | 16.9ab | 16.8ab | 16.6b | 17.5ab | 17.7a |
| 4 | C18:0 (Stearic) | 30.3 | 5.97a | 6.24a | 5.87a | 5.99a | 4.87b | 5.03b |
| 5 | C18:1n9c (Oleic) | 31.0 | 23.6b | 25.7ab | 26.6a | 24.6ab | 24.8ab | 20.1c |
| 6 | C18:2n6c (Linoleic) | 32.3 | 44.7b | 43.0b | 42.3b | 45.5ab | 45.6ab | 49.3a |
| 7 | C20:0 (Arachidic) | 33.5 | 0.52bc | 0.56b | 0.63a | 0.53bc | 0.48c | 0.50c |
| 8 | C18:3n3 (α-Linolenic) | 33.7 | 5.21ab | 4.94b | 4.97b | 4.86b | 4.98b | 5.61c |
| 9 | C22:0 (Behenic) | 36.4 | 0.30ab | 0.24c | 0.27b | 0.30ab | 0.28ab | 0.30a |
| 10 | C24:0 (Lignoceric) | 39.2 | 0.60ab | 0.61a | 0.65a | 0.55ab | 0.60ab | 0.64a |
| Total saturated fatty acids (SFAs) | 26.5a | 26.3a | 26.1a | 25.1a | 24.6ab | 25.0a | ||
| Total monounsaturated fatty acids (MUFAs) | 23.6ab | 25.7ab | 26.6a | 24.6ab | 24.8ab | 20.1c | ||
| Total polyunsaturated fatty acids (PUFAs) | 50.0b | 48.0b | 47.3b | 50.3ab | 50.5ab | 54.9a | ||
| PUFAs:SFAs | 1.89bcd | 1.82cd | 1.81d | 2.00bc | 2.05ab | 2.19a | ||
| PUFAs:MUFAs | 2.12b | 1.87cd | 1.78d | 2.05bc | 2.03bc | 2.73a | ||
| Total lipids | 0.52b | 0.54b | 0.51b | 0.80a | 0.82a | 0.85a |
Green (Stage 1), breaker (Stage 2), turning (Stage 3), pink (Stage 4), light red (Stage 5), and red (Stage 6). Values are percentages of the fatty acids in the total lipids (fresh weight basis), from an average of six replicates (triplicate extractions with duplicate analysis). Superscript letters indicate statistically significant subsets among different fruits of the various ripening stages (p < 0.05)
RT retention time
Conclusions
These results of the present investigation indicate that each metabolite has a unique pattern of accumulation during the ripening of tomato fruit. In general, ripening in tomatoes is accompanied by significant increases in carotenoids and α-tocopherol, as well as PUFAs. Thus, the ripening of tomato fruits is a favorable process to improve the nutritional value.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This paper was supported by KU Research Professor Program of Konkuk University.
Compliance with ethical standards
Conflict of interest
The authors have declared that there is no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0666-0) contains supplementary material, which is available to authorized users.
Contributor Information
Ramesh Kumar Saini, Phone: +82-2450-3739, Email: saini_1997@yahoo.com.
Young-Soo Keum, Email: rational@konkuk.ac.kr.
References
- Aidi Wannes W, Mhamdi B, Marzouk B. Variations in essential oil and fatty acid composition during Myrtus communis var. italica fruit maturation. Food Chem. 2009;112:621–626. doi: 10.1016/j.foodchem.2008.06.018. [DOI] [Google Scholar]
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- Almeida J, Asís R, Molineri VN, Sestari I, Lira BS, Carrari F, Peres LEP, Rossi M. Fruits from ripening impaired, chlorophyll degraded and jasmonate insensitive tomato mutants have altered tocopherol content and composition. Phytochemistry. 2015;111:72–83. doi: 10.1016/j.phytochem.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Baldwin EA, Scott JW, Shewmaker CK, Schuch W. Flavor trivia and tomato aroma: biochemistry and possible mechanisms for control of important aroma components. HortScience. 2000;35:1013–1021. [Google Scholar]
- Bhandari SR, Lee JG. Ripening-dependent changes in antioxidants, color attributes, and antioxidant activity of seven tomato (Solanum lycopersicum L.) cultivars. J Anal Methods. 2016 doi: 10.1155/2016/5498618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Camelo LFA, Gómez PA. Comparison of color indexes for tomato ripening. Hortic Bras. 2004;22:534–537. doi: 10.1590/S0102-05362004000300006. [DOI] [Google Scholar]
- Choi SH, Kim D-S, Kozukue N, Kim HJ, Nishitani Y, Mizuno M, Levin CE, Friedman M. Protein, free amino acid, phenolic, β-carotene, and lycopene content, and antioxidative and cancer cell inhibitory effects of 12 greenhouse-grown commercial cherry tomato varieties. J Food Compos Anal. 2014;34:115–127. doi: 10.1016/j.jfca.2014.03.005. [DOI] [Google Scholar]
- Cruz R, Casal S. Validation of a fast and accurate chromatographic method for detailed quantification of vitamin E in green leafy vegetables. Food Chem. 2013;141:1175–1180. doi: 10.1016/j.foodchem.2013.03.099. [DOI] [PubMed] [Google Scholar]
- García-Rojas M, Morgan A, Gudenschwager O, Zamudio S, Campos-Vargas R, González-Agüero M, Defilippi BG. Biosynthesis of fatty acids-derived volatiles in “Hass” avocado is modulated by ethylene and storage conditions during ripening. Sci Hortic. 2016;202:91–98. doi: 10.1016/j.scienta.2016.02.024. [DOI] [Google Scholar]
- Guil-Guerrero JL, Rebolloso-Fuentes MM. Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties. J Food Compos Anal. 2009;22:123–129. doi: 10.1016/j.jfca.2008.10.012. [DOI] [Google Scholar]
- Horvath G, Wessjohann L, Bigirimana J, Monica H, Jansen M, Guisez Y, Caubergs R, Horemans N. Accumulation of tocopherols and tocotrienols during seed development of grape (Vitis vinifera L. cv. Albert Lavallée) Plant Physiol Biochem. 2006;44:724–731. doi: 10.1016/j.plaphy.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Jimenez A, Creissen G, Kular B, Firmin J, Robinson S, Verhoeyen M, Mullineaux P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta. 2002;214:751–758. doi: 10.1007/s004250100667. [DOI] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ. Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet. 2011;45:41–59. doi: 10.1146/annurev-genet-110410-132507. [DOI] [PubMed] [Google Scholar]
- Konozy EHE, Causse M, Faurobert M. Cell wall glycosidase activities and protein content variations during fruit development and ripening in three texture contrasted tomato cultivars. Saudi J Biol Sci. 2012;19:277–283. doi: 10.1016/j.sjbs.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalel HJD, Singh Z, Tan SC. Aroma volatiles production during fruit ripening of “Kensington Pride” mango. Postharvest Biol Technol. 2003;27:323–336. doi: 10.1016/S0925-5214(02)00117-5. [DOI] [Google Scholar]
- Moco S, Capanoglu E, Tikunov Y, Bino RJ, Boyacioglu D, Hall RD, Vervoort J, De Vos RC. Tissue specialization at the metabolite level is perceived during the development of tomato fruit. J Exp Bot. 2007;58:4131–4146. doi: 10.1093/jxb/erm271. [DOI] [PubMed] [Google Scholar]
- Moneruzzaman KM, Hossain A, Sani W, Saifuddin M, Alenazi M. Effect of harvesting and storage conditions on the post harvest quality of tomato (Lycopersicon esculentum Mill) cv. Roma VF. Aust J Crop Sci. 2009;3:113. [Google Scholar]
- Msaada K, Hosni K, Ben Taarit M, Chahed T, Hammami M, Marzouk B. Changes in fatty acid composition of coriander (Coriandrum sativum L.) fruit during maturation. Ind Crops Prod. 2009;29:269–274. doi: 10.1016/j.indcrop.2008.05.011. [DOI] [Google Scholar]
- Park M-H, Sangwanangkul P, Baek D-R. Changes in carotenoid and chlorophyll content of black tomatoes (Lycopersicone sculentum L.) during storage at various temperatures. Saudi J Biol. 2016 doi: 10.1016/j.sjbs.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periago MJ, García-Alonso J, Jacob K, Belén Olivares A, José Bernal M, Dolores Iniesta M, Martínez C, Ros G. Bioactive compounds, folates and antioxidant properties of tomatoes (Lycopersicum esculentum) during vine ripening. Int J Food Sci Nutr. 2009;60:694–708. doi: 10.3109/09637480701833457. [DOI] [PubMed] [Google Scholar]
- Perveen R, Suleria HAR, Anjum FM, Butt MS, Pasha I, Ahmad S. Tomato (Solanum lycopersicum) carotenoids and lycopenes chemistry; metabolism, absorption, nutrition, and allied health claims—a comprehensive review. Crit Rev Food Sci Nutr. 2015;55:919–929. doi: 10.1080/10408398.2012.657809. [DOI] [PubMed] [Google Scholar]
- Quadrana L, Almeida J, Otaiza SN, Duffy T, Da Silva JV, de Godoy F, Asís R, Bermúdez L, Fernie AR, Carrari F, Rossi M. Transcriptional regulation of tocopherol biosynthesis in tomato. Plant Mol Biol. 2013;81:309–325. doi: 10.1007/s11103-012-0001-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Amaya DB. A guide to carotenoid analysis in foods. Washington: ILSI press; 2001. [Google Scholar]
- Ronen G, Cohen M, Zamir D, Hirschberg J. Regulation of carotenoid biosynthesis during tomato fruit development: expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J Cell Mol Biol. 1999;17:341–351. doi: 10.1046/j.1365-313X.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- Saini RK, Keum Y-S. Tocopherols and tocotrienols in plants and their products: a review on methods of extraction, chromatographic separation, and detection. Food Res Int. 2016;82:59–70. doi: 10.1016/j.foodres.2016.01.025. [DOI] [Google Scholar]
- Saini RK, Shetty NP, Giridhar P, Ravishankar GA. Rapid in vitro regeneration method for Moringa oleifera and performance evaluation of field grown nutritionally enriched tissue cultured plants. 3. Biotech. 2012;2:187–192. [Google Scholar]
- Saini RK, Shetty NP, Giridhar P. GC-FID/MS analysis of fatty acids in Indian cultivars of Moringa oleifera: potential sources of PUFA. J Am Oil Chem Soc. 2014;91:1029–1034. doi: 10.1007/s11746-014-2439-9. [DOI] [Google Scholar]
- Saini RK, Nile SH, Park SW. Carotenoids from fruits and vegetables: chemistry, analysis, occurrence, bioavailability and biological activities. Food Res Int . 2015;76(3):735–750. doi: 10.1016/j.foodres.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Singh RK, Ali SA, Nath P, Sane VA. Activation of ethylene-responsive p-hydroxyphenylpyruvate dioxygenase leads to increased tocopherol levels during ripening in mango. J Exp Bot. 2011;62:3375–3385. doi: 10.1093/jxb/err006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanesan I, Saini RK, Noorzai R, Zamany AJ, Kim DH. In vitro propagation, carotenoid, fatty acid and tocopherol content of Ajuga multiflora Bunge. 3. Biotech. 2016;6:91. doi: 10.1007/s13205-016-0376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Diretto G, Purgatto E, Danoun S, Zouine M, Li Z, Roustan JP, Bouzayen M, Giuliano G, Chervin C. Carotenoid accumulation during tomato fruit ripening is modulated by the auxin–ethylene balance. BMC Plant Biol. 2015;15:114. doi: 10.1186/s12870-015-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture . Vegetables 2015 summary. Washington: National Agricultural Statistics Service; 2016. [Google Scholar]
- US Food and Drug Administration . FDA guidance for industry: bioanalytical method validation. Rockville: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2001. [Google Scholar]
- Verheul MJ, Slimestad R, Tjøstheim IH. From producer to consumer: greenhouse tomato quality as affected by variety, maturity stage at harvest, transport conditions, and supermarket storage. J Agric Food Chem. 2015;63:5026–5034. doi: 10.1021/jf505450j. [DOI] [PubMed] [Google Scholar]
- Villa-Rodríguez JA, Molina-Corral FJ, Ayala-Zavala JF, Olivas GI, González-Aguilar GA. Effect of maturity stage on the content of fatty acids and antioxidant activity of “Hass” avocado. Food Res Int. 2011;44:1231–1237. doi: 10.1016/j.foodres.2010.11.012. [DOI] [Google Scholar]
- Whitaker BD. Changes in lipids of tomato fruit stored at chilling and non-chilling temperatures. Phytochemistry. 1991;30:757–761. doi: 10.1016/0031-9422(91)85247-W. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.