Abstract
Phytic acid is a main reservoir of phosphorous (P) in plants and contributes to about 80% of the total P in cereal seeds. However, it is well known to possess anti-nutritional behavior. Because it has strong affinity to chelate divalent ions e.g. calcium, magnesium, and especially with iron and zinc. Therefore, it is extremely poor as a dietary source of P. To enhance bio-availability of micronutrients, an enzyme namely phytase is known to hydrolyze phytic acid. Unfortunately, phytase is not produced in the stomach of monogastric animals and humans. Thus, the presence of phytic acid in cereal foods has become major concern about the deficiency of essential micronutrients in developing countries. To address this problem, various types of phytase have been isolated, purified and characterized from different varieties of cereal till date. Therefore, the present article discusses about catalytic properties, gene regulation of such cereal phytases and their importance in ensuring food safety.
Keywords: Phytase, Phytic acid, Micronutrients, Phosphorous, Cereal
Introduction
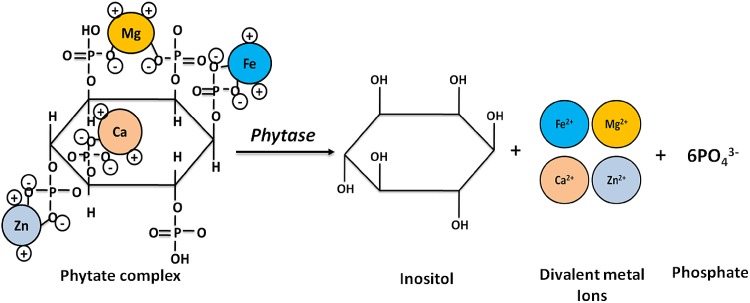
Phytase (main enzyme classes E.C.3.1.3.8, E.C. 3.1.2.26; myo-inositol hexakisphosphate phosphohydrolases) initiates the partial or complete hydrolytic removal of orthophosphates from the phytate (myo-inositol 1,2,3,4,5,6-hexakisphosphate). Phytate is usually exploited in commercial animal feeds and human food diet. Reddy et al. (1982) reported that phytate constitutes about 65–90% of the total phosphorous content in plants. Phytate is degraded by phytase enzyme into one molecule of inositol and six molecules of inorganic phosphate (Fig. 1). Monogastric animals have a negligible phytase activity in their gastro-intestinal tract. Therefore, phytate passes largely undigested through the digestive system. To overcome the loss of phytate P bio-available phosphate is added to feed. According to an environmental perspective, exploitation of cereal phytases is most common method where undigested phytate P leads to run-off of phosphorous and other divalent ions in aquatic ecosystem. It leads to the phenomenon of eutrophication which has severe environmental health risk. Furthermore, phytate is a strong chelator of divalent cations and minerals such as Ca2+, Fe2+ and Zn2+ (Lopez et al. 2002; Vats and Banerjee 2004), making them unavailable for the absorption in the digestive system. Moreover, phytate is known to form complexes with proteins under both acidic and alkaline pH conditions. These interactions were found to affect the proteins structure, thus reducing the enzymatic activity, protein solubility and proteolytic digestibility (Kies et al. 2006; Yao et al. 2011). Moreover, phytase enzyme reacts upon phytate complex; as a result inositol, phosphate and other micronutrients get released as depicted in Fig. 1.
Fig. 1.
Schematic representation of phytate hydrolysis in catalytic reaction
Supplemental microbial phytases in corn-soybean meal diets for monogastric animals may reduce these problems and can improve the animal’s utilization of the phytate phosphorous and reduce their faecal phosphorous excretion by up to 50% (Leytem et al. 2008; Kim et al. 2010). Although microbial phytases have been used in food processing, but cereal phytases are thought to be better alternatives due to higher acceptance among consumers and their assumed lower allergenic potential. Therefore, cereal phytases have been mainly used as a feed supplement in diets for swine, poultry and to some extent for fish. Svanberg et al. (1993) observed a significant degradation of phytate content in plant based food by the process of lactic acid fermentation with a concomitant improvement of micronutrient bio-availability. Furthermore, Oliver et al. (2014) reported the importance of fermentation in the whole grain bread to reduce the content of phytate. Both Lactobacillus amylovorus and Lactobacillus plantarum were observed to produce significant extracellular phytase (Sreeramulu et al. 1996). Gunashree and Govindarajulu (2015) explained the method of dephytinization in cereals and pulses using phytase producing lactic acid bacteria. The field trials and laboratory experiments have demonstrated that 500–1000 units of phytase can replace 1 g of inorganic phosphorous supplementation and reduce total P excretion by 30–50% (Yi et al. 1996; Kemme et al. 1997). Thus, phytase performs dual function; (a) conserve expensive and non-renewable inorganic phosphorous resources by reducing their use in animal feed; (b) prevention of water pollution due to excessive manure phosphorous run-off. For these reasons, phytases are used globally as a phosphate-mobilizing feed supplement in the diets of swine and poultry (Ketaren et al. 1993). Recently, the effect of cereal phytase enzyme was observed in the intestinal microflora and gut morphology of broilers by Kalantar et al. (2016). Phytases have potential to enhance iron absorption from 0.6–23% to 5.5–42% in cereal meals for daily physiological iron requirements (Nielsen et al. 2013). The problem summarized above have led to increased interest in improved phytate P utilization from cereal feed stuffs and on reducing anti-nutritional effect of undigested phytate in the digestive tract. Five strategies have been applied to resolve the discussed problems. (1) Cereal grains can be soaked in water, germinated or cooked whereby endogenous grain or exogenous phytase is activated (Egli et al. 2002; Marero et al. 1991; Svanberg et al. 1993). (2) Cereal crops can be genetically engineered to enhance phytase production in their grains (Brinch-Pedersen et al. 2002). (3) Impairing phytate biosynthesis can be useful in cereals such as wheat, barley, rice and maize (Raboy 2009). (4) Pigs have been genetically modified to produce heterologous phytases in their salivary glands (Golovan et al. 2001). (5) Addition of microbial phytases to feed and food (up to 2200 FTU/kg, where 1 FTU is the activity of enzyme which releases one micro molar orthophosphate from phytate per minute at pH 5.3) significantly enhances the release of phosphate and minerals from phytate (Kornegay 2001; Troesch et al. 2009). Currently, above discussed five approaches, four are commonly used and can be prove to the most adaptable solution to enhance the bio-availability of micronutrients in the intestine of monogastric animals. Use of cereal phytases has been proved to be very successful in improving the nutritional value of cereal based foods. Several review articles in recent years have been published on microbial phytases, still there are only few reports on cereal phytases. So to fulfill this gap, in this review we attempted to understand about cereal phytases and their importance.
Nutritional importance of phytate
Phytate, also known as phytin salt, is stored in the form of phosphate, inositol and bounded micronutrients in the intestine. The phytate is formed during maturation of cereal seed and plays an important role in the constituent of cereal derived foods (Table 1). Depending on the amount of different cereal foods in the diet and the grade of food processing, the daily intake of phytate can be high i.e. 4500 mg (Reddy 2002). He reported that daily intake of phytate was estimated to be 2000–2600 mg for vegetarian diet and other inhabitants of rural areas in developing countries and 150–1400 mg for mixed diets. The phytate works at a broad range of pH due to the presence of highly negatively charged ions on six phosphate groups and show very strong affinity for food components e.g. minerals, proteins and other trace elements (Cheryan 1980; Konietzny and Greiner 2003). This interaction does not have only nutritional consequences, but also affects yield and quality of food ingredients, such as starch and protein.
Table 1.
A different range of phytate content in cereal derived foods
(Source Greiner and Konietzny 2006)
| Food | Phytate (mg/g) |
|---|---|
| Cereal-based | |
| Whole wheat bread | 3.2–7.3 |
| Unleavened wheat bread | 10.6–3.2 |
| Whole rye bread | 1.9–4.3 |
| Mixed flour bread (70% wheat, 30% rye) | 0.4–1.1 |
| Mixed flour bread (70% rye, 30% wheat) | 0–0.4 |
| Sourdough rye bread | 0.1–0.3 |
| Maize | 9.8–21.3 |
| Maize bread | 4.3–8.2 |
| Unleavened maize bread | 12.2–19.2 |
| Rice (polished cooked) | 1.2–3.7 |
| Wild rice (cooked) | 12.7–21.6 |
| Cornflakes | 0.4–1.5 |
| Pasta | 0.7–9.1 |
| Oat bran | 2.1–7.3 |
| Oat flakes | 8.4–12.1 |
| Oat porridge | 6.9–10.2 |
| French bread | 0.2–0.4 |
| Wild rice | 12.7–21.6 |
The presence of phytate in human food mainly affects the uptake of mineral ions and also their absorption in the body. As a result undigested phytate complex formed at physiological pH and it is the major reason for poor minerals bio-availability. Besides, the small intestine of human is not able to hydrolyze phytate due to the lacking of phytase activity and the limited microbial population in digestive tract.
Enzymatic dephosphorylation of phytate during food processing
Several strategies have been adopted to reduce the phytate content of the processed material. The given four different methods are also effective to degrade the phytate complex. The ability to dephosphorylate phytate varies greatly among different cereal plants and microbial species. It is due to the differences in their intrinsic phytate-degrading activity and it also depends on the properties of the enzyme such as protein solubility, pH, temperature optima for phytate hydrolysis (Konietzny and Greiner 2002).
Soaking
It is generally used as a pre-treatment to facilitate processing of cereal grains. Soaking may be for less time i.e. 15–20 min. or for a long time, usually 15–20 h. Domestically, cereal grains are normally soaked in water at room temperature for overnight. Because phytate is water soluble and a major phytate reduction can be visualized by discarding the soaked water. Furthermore, endogenous phytases play an important role to reduce the content of phytate. An optimum temperature and pH value have also shown good impact on enzymatic phytate hydrolysis during soaking (Greiner and Konietzny 1998; Fredlund et al. 1997). When soaking step is carried out at temperature of 45–65 °C and pH 5.0–6.0, which are very close to the optimal conditions of phytate dephosphorylation. It has been found that a significant content of phytate (35–100%) was found to enzymatically hydrolyzed (Greiner and Konietzny 1999).
Germination
It is used in cereals to increase the nutritional value and particularly to degrade anti-nutrient factors, such as phytate and protease inhibitors. Egli et al. (2002) and Eeckhout and Paepe (1994) reported that some cereal crops have only little intrinsic phytate hydrolysis capability. But during germination of cereal grains, some authors revealed that the activity of hydrolysis of phytate increased significantly (Greiner et al. 1997, 2001; Greiner 2002; Vidal-Valverde et al. 1998). Phytate is hydrolyzed during germination in sequential order by the required activity of phytase and phosphatases. To increase the activity of phytase enzyme, cereal grains would have to germinate for longer time, i.e. 6–10 days of germination. Because long time period reduces a significant level of phytate and it is generally applied in households for domestic uses.
Cooking
Phytate is stable at high temperature, so required degradation of phytate does not take place during cooking. Therefore, significant phytate dephosphorylation during cooking only takes place via two routes. (1) Discard the cooking water; (2) An enzymatic hydrolysis of phytate due to the activity of cereal phytases during early phase of cooking (Greiner and Konietzny 1998). Cereal phytases are inactivated at high temperature during prolonged heating. Moreover, the use of cereal phytases with heat stability could be the good alternative to improve phytate degradation during cooking.
Addition of isolated cereal phytases
It is the best method to hydrolyze phytate content using preformed enzymes in the raw material used for food processing. Greiner and Konietzny (1999) and Konietzny et al. (1995) demonstrated that supplemental phytase enzyme during food processing were reducing phytate content from cereal food products. It has been seen that the content of phytate was reduced by the added amount of enzyme activity, but the added phytase has to be more active during food processing or preparation. Both temperature and pH value are the major factors to determine enzyme activity. Isolated phytases should be of high phytate degrading capability even at room temperature, acceptable heat resistance and high activity over a broad pH range. To increase the nutritional value of cereal grains, it is needed to enhance the level of phytase by degrading phytic acid found in grains. But, there is little information of cereal phytases on molecular level yet. Some authors reported cereal phytase gene and their regulation to control the activity of phytase.
Cereal phytase gene
The different forms of cereal phytase gene can be classified into three major groups; (1) PAPhy gene; (2) HAPhy gene; (3) MINPhy gene. However, all classes are not capable of utilizing phytate as a substrate. Each phytase gene has its unique structural properties due to their distinct catalytic properties which make them capable to utilize phytate as a substrate in different environments. Cereal phytase belongs to given three classes of gene and the present article discusses the main features of these genes. Several authors identified different isoforms of phytase gene which are located on specific chromosome locus. Therefore, we emphasized mainly to understand the function and regulation of these phytase genes.
Purple acid phosphatase gene
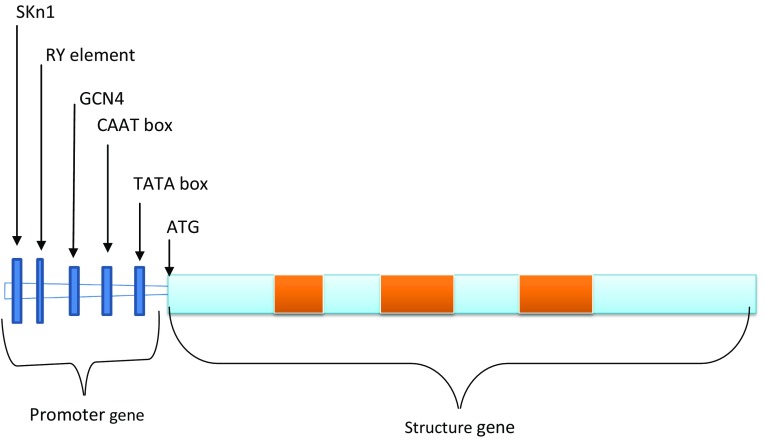
PAPhys genes were reported in the plant kingdom with 25 putative genes characterized in Arabidopsis thaliana (Li et al. 2002) and illustrated in Fig. 2. The data were retrieved from gene bank to know the presence of different cereal PAPhys gene. In wheat five coding DNA sequences of PAPhys were clustered to form a contig and finally cloned the gene to study the different isoforms of gene. PAPhys were first reported in soybean and, furthermore, about ten cereal PAPhys cDNA have been reported from wheat (5), maize (1), barley (3) and rice (1) (Dionisio et al. 2011). Moreover, the cDNA was synthesized after the isolation of mRNAs from germinating and developing grains. Wheat and barley PAPhys transcripts can be grouped as PAPhy_a and PAPhy_b on behalf of differences found in their C-terminal amino acid. Expression analysis showed that PAPhy_a genes were expressed during seed development and PAPhy_b were expressed during seed germination.
Fig. 2.
Schematic representations of cereal PAPhys gene. Annotations are exons (sky blue), intron (orange) and regulatory elements (blue)
Some authors also amplified the promoter sequence of phytase gene in few cereals which control the specific activity of phytase. Nakano et al. (1999) confirmed two phytases with N-terminal sequences which were found similar to PAPhy_a gene purified from wheat bran. Greiner et al. (2000) also purified two phytases from barley. One was from germinating seeds and other from both germinating and developing seeds. Both were considered as PAPhys on the basis of their catalytic properties. PAPhys cDNAs have been cloned from germinating maize, wheat, rice and their recombinant enzymes shown to be potent phytases (Dionisio et al. 2011). Two phytases were purified from rice bran and classified as PAPhys based on their molecular mass and color of the concentrated enzyme (Hayakawa et al. 1989).
Histidine acid phosphatase gene
HAPhys genes are widely distributed among bacteria, fungi and cereal crops. These genes are having high specific activity for phytate and their specific position to initiate the hydrolysis of phytate. HAPhys genes have been exploited for various industrial and biotechnological applications (Afinah et al. 2010; Lei and Porres 2003) and other biochemical properties (Oh et al. 2004). They also studied that all phytase genes have not similar and common active site, so the initial classification system is entirely based on their catalytic mechanism. The maize seedlings were used to isolate a HAPhys cDNA clone from expression library which was prepared from 3 to 4 days old maize seedling (Laboure et al. 1993). Two homologous genes PHYT1 and PHYTII were isolated using cDNA to screen a genomic library. Moreover, two linked phytase loci at maize chromosome 3 were mapped and suggested to correspond to the two clones (Maugenest et al. 1997, 1999). The authors confirmed by BLASTN against the maize genome, showing that PHYTI and PHYII reside 450 kb apart in the same orientation. Laboure et al. (1993) demonstrated that the reservoir of phytate present in maize is generally found in the germ.
Multiple inisitol phosphate phosphatase genes
MINPhys genes constitute a separate group within the HAPs (Chi et al. 1999). They seem to be localized in the endoplasmic reticulum (ER) and are assumed to have a central role, not only in providing bio-available phosphate to the growing cell, but also in yielding important downstream metabolites of the inositol phosphates. MINPhys gene was first discovered in animals and later Dionisio et al. (2007) reported that these genes expressed during grain development and germination. MINPhys also have been isolated from the genomic clones of barley MINPhys PHYIIa2 and rye. They reported 11 exons, which consisted of whole gene and their general structure was conserved. Expression studies of germination and developing grains of wheat and barley MINPhys genes were highly expressed and reported with the highest expression levels in the developing grains (Dionisio et al. 2007). Additionally, the expression of wheat MINPhys gene was higher than the barley MINPhys gene. It may be due to the presence of three homeoalleles in hexaploid wheat, in contrast to only one gene in diploid barley.
Applications of cereal phytases
Cereal phytases in food/feed play an important role mainly in enhancing mineral, phosphorous and energy uptake to fortify the fodder with these substances. The increased availability of cereal phytases at the same time reduces excretion and therefore, reducing the phosphate load in aquatic ecosystem. These cereal phytases used as commercially available phytases as fortifiers of pigs feed revealed that they satisfy the criteria for an ideal phytase for feed production, such as resistance from denaturation under extreme pH and temperature (Boyce and Walsh 2006). However, supplemental microbial phytase increased phosphorous availability by 12, 15 and 38% in pig diet containing wheat, triticale and maize, respectively (Dungelhoef et al. 1994). Mollgaard (1946) reported that the degradation of phytate during bread-making has been known to effect mineral bio-availability for many years. Therefore, several bread-making methodologies designed to reduce the phytate content have been reported. These include the isolation of commercial phosphoesterases (phytase or phosphatase) from wheat to whole wheat flour (Knorr et al. 1981) and the activation of naturally occurring phytase by soaking and malting the grain. Iron absorption from porridges based on flours from wheat, rice, maize, oat, sorghum and wheat-soy flour blend have been tested on humans. The results have shown that phytate degradation improves iron absorption from cereal porridges prepared with water and further addition of ascorbic acid is a better way to enhance iron absorption in child food than addition of phytase. Adding amylase to the porridge in combination with phytase also increasing the absorption of iron in digestive system (Hurrell et al. 2003).
Conclusions and future perspectives
It is very important to overcome the problem associated with poor micronutrient bio-availability in commercially processed cereal derived food products used in developing countries. Many of these cereal food products have high content of phytate and phytate-micronutrient molar ratios likely to reduce the absorption of Zinc, Iron and other essential mineral ions in gastro-intestinal tract. Dephytinization strategy could reduce the content of phytate in cereal based diet and improve the absorption of micronutrients. But, alone dephytinization is not enough to overcome the shortfalls in iron, calcium and zinc that have been consistently reported in cereal foods. Therefore, dephytinization should be combined with strategies such as addition of isolated cereal phytases and characterization of cereal phytase gene by molecular study. In low-income countries, molecular breeding may be a better strategy to improve the nutritional quality of staple cereals. Genetic manipulation and domestic four pre-treatment methods also improve nutritional quality. The main purpose of this article was to explore the potential of cereal phytases as better food processes enzymes due to their unique properties to hydrolyze phytate under optimum conditions. Cereal phytases are cost effective and highly accepted among the consumers. Still, more research is required to discover new more cereal phytases and engineer them to develop desired characteristics for better crop improvement to enhance the absorption of micronutrients.
Acknowledgements
The authors gratefully acknowledge all the staff of Quality and Basic Sciences Laboratory, ICAR-IIWBR, Karnal, India for the general support provided, when it was required.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
References
- Afinah S, Yazid AM, Anis MH, Shobirin M, Shuhaimi M. Phytase: application in food industry. Intern Food Res J. 2010;17:13–21. [Google Scholar]
- Boyce A, Walsh G. Comparison of selected physicochemical characteristics of commercial phytases relevant to their application in phosphate pollution abatement. J Env Sci Health Part A Toxic Hazard Subst Env Eng. 2006;41:789–798. doi: 10.1080/10934520600614397. [DOI] [PubMed] [Google Scholar]
- Brinch-Pedersen H, Sorensen LD, Holm PB. Engineering crop plants: getting a handle on phosphate. Trends Plant Sci. 2002;7:118–125. doi: 10.1016/S1360-1385(01)02222-1. [DOI] [PubMed] [Google Scholar]
- Cheryan M. Phytic acid interactions in food systems. Crit Rev Food Sci Nutr. 1980;13:297–355. doi: 10.1080/10408398009527293. [DOI] [PubMed] [Google Scholar]
- Chi HB, Tiller GE, Dasouki MJ, Romano PR, Wang J, O’Keefe RJ, Puzas JE, Rosier RN, Reynolds PR. Multiple inositol polyphosphate phosphatase: Evolution as a distinct group within the histidine phosphatase family and chromosomal localization of the human and mouse genes to chromosomes 10q23 and 19. Genomics. 1999;56:324–336. doi: 10.1006/geno.1998.5736. [DOI] [PubMed] [Google Scholar]
- Dionisio G, Holm PB, Brinch-Pedersen H. Wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) multiple inositol polyphosphate phosphatases (MINPPs) are phytases expressed during grain filling and germination. Plant Biotech J. 2007;5:325–338. doi: 10.1111/j.1467-7652.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- Dionisio G, Madsen CK, Holm PB, Welinder KG, Jorgensen M, Stoger E, Arcalis E, Brinch-Pedersen H. Cloning and characterization of purple acid phosphatase phytases from wheat, barley, maize, and rice. Plant Physiol. 2011;156:1087–1100. doi: 10.1104/pp.110.164756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungelhoef M, Rodehutscord M, Spiekers H, Pfeffer E. Effects of supplemental microbial phytase on availability of phosphorus contained in maize, wheat and triticale to pigs. Anim Feed Sci Tech. 1994;49:1–10. doi: 10.1016/0377-8401(94)90076-0. [DOI] [Google Scholar]
- Eeckhout W, Paepe MD. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim Feed Sci Tech. 1994;47:19–29. doi: 10.1016/0377-8401(94)90156-2. [DOI] [Google Scholar]
- Egli I, Davidsson L, Juillerat MA, Barclay D, Hurrell RF. The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. J Food Sci. 2002;67:3484–3488. doi: 10.1111/j.1365-2621.2002.tb09609.x. [DOI] [Google Scholar]
- Fredlund K, Asp NG, Larsson M, Marklinder I, Sandberg AS. Phytate reduction in whole grains of wheat, rye, barley and oats after hydrothermal treatment. J Cereal Sci. 1997;25:83–91. doi: 10.1006/jcrs.1996.0070. [DOI] [Google Scholar]
- Golovan SP, Meidinger RG, Ajakaiye A, Cottrill M, Wiederkehr MZ, Barney DJ, Plante C, Pollard JW, Fan MZ, Hayes MA, Laursen J, Hjorth JP, Hacker RR, Phillips JR, Forsberg CW. Pigs expressing salivary phytase produce low-phosphorus manure. Nat Biotechnol. 2001;19:741–745. doi: 10.1038/90788. [DOI] [PubMed] [Google Scholar]
- Greiner R. Purification and characterization of three phytate-degrading enzymes from germinated lupin seeds (Lupinus albus var. Amiga) J Agric Food Chem. 2002;50:6858–6864. doi: 10.1021/jf025619u. [DOI] [PubMed] [Google Scholar]
- Greiner R, Konietzny U. Endogenous phytate-degrading enzymes are responsible for phytate reduction while preparing beans (Phaseolus vulgaris) J Food Process Preserv. 1998;29:321–331. doi: 10.1111/j.1745-4549.1998.tb00353.x. [DOI] [Google Scholar]
- Greiner R, Konietzny U. Improving enzymatic reduction of myo-inositol phosphates with inhibitory effects on mineral absorption in black beans (Phaseolus vulgaris var. Preto) J Food Process Preserv. 1999;23:249–261. doi: 10.1111/j.1745-4549.1999.tb00383.x. [DOI] [Google Scholar]
- Greiner R, Konietzny U. Phytase for food application. Food Technol Biotech. 2006;44:125–140. [Google Scholar]
- Greiner R, Konietzny U, Jany KD (1997) Properties of phytate-degrading enzymes and their biotechnological application in phytate reduction. In: Cost 916: Bioactive inositol phosphates and phytosterols in foods, office for the official publications of the European communities. Luxembourg, pp 61–67
- Greiner R, Jany K, Larsson AM. Identification and properties of myo-inositol hexakisphosphate phosphohydrolases (phytases) from barley (Hordeum vulgare) J Cereal Sci. 2000;31:127–139. doi: 10.1006/jcrs.1999.0254. [DOI] [Google Scholar]
- Greiner R, Muzquiz M, Burbano C, Cuadrado C, Pedrosa MM, Goyoaga C. Purification and characterization of a phytate-degrading enzyme from germinated faba beans (Vicia faba var. Alameda) J Agr Food Chem. 2001;49:2234–2240. doi: 10.1021/jf0100806. [DOI] [PubMed] [Google Scholar]
- Gunashree BS, Govindarajulu V. Dephytinization of cereals and pulses by phytase producing lactic acid bacteria. Int J Curr Res Aca Rev. 2015;3:61–69. [Google Scholar]
- Hayakawa T, Toma Y, Igaue I. Purification and characterization of acid phosphatases with and without phytase activity from rice bran. Agric Biol Chem. 1989;53:1475–1483. [Google Scholar]
- Hurrell RF, Reddy MB, Juillerat MA, Cook JD. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am J Clin Nutr. 2003;77:1213–1219. doi: 10.1093/ajcn/77.5.1213. [DOI] [PubMed] [Google Scholar]
- Kalantar M, Khajal F, Yaghobfar A. Effect of cereal type and enzyme addition on performance, pancreatic enzyme activity, intestinal microflora and gut morphology of broilers. Poultry Sci J. 2016;4:63–71. [Google Scholar]
- Kemme PA, Jongbloed AW, Mroz Z, Beynen AC. The efficacy of Aspergillus niger phytase in rendering phytate phosphorus available for absorption in pigs is influenced by pig physiological status. Anim Sci. 1997;75:2129–2138. doi: 10.2527/1997.7582129x. [DOI] [PubMed] [Google Scholar]
- Ketaren PP, Batterham ES, Dettmann EB, Farrell DJ. Phosphorus studies in pigs. 3. Effect of phytase supplementation on the digestibility and availability of phosphorus in soya-bean meal for grower pigs. Brit J Nutr. 1993;70:289–311. doi: 10.1079/BJN19930123. [DOI] [PubMed] [Google Scholar]
- Kies A, De Jonge, Kemme P, Jongbloed A. Interaction between protein, phytate, and microbial phytase. In vitro studies. J Agric Food Chem. 2006;54:1753–1758. doi: 10.1021/jf0518554. [DOI] [PubMed] [Google Scholar]
- Kim OH, Kim YO, Shim JH, Jung YS, Jung WJ, Choi WC, Lee H, Lee SJ. Beta-propeller phytase hydrolyzes insoluble Ca2+-phytate salts and completely abrogates the ability of phytate to chelate metal ions. Biochem. 2010;49:10216–10227. doi: 10.1021/bi1010249. [DOI] [PubMed] [Google Scholar]
- Knorr D, Watkins TR, Carlson BL. Enzymatic reduction of phytate in whole wheat breads. J Food Sci. 1981;46:1866–1869. doi: 10.1111/j.1365-2621.1981.tb04506.x. [DOI] [Google Scholar]
- Konietzny U, Greiner R. Molecular and catalytic properties of phytate-degrading enzymes (phytases) Int J Food Sci Tech. 2002;37:791–812. doi: 10.1046/j.1365-2621.2002.00617.x. [DOI] [Google Scholar]
- Konietzny U, Greiner R. Phytic acid: Nutritional impact. In: Caballero B, Trugo L, Finglas P, editors. Encyclo of food Sci and Nutr. London: Elsevier; 2003. pp. 4555–4563. [Google Scholar]
- Konietzny U, Greiner R, Jany KD. Zur Biochemie und Anwendung von Phytasen aus Dinkel und Roggen (Biochem and Appl of Phytases from Spelt and Rye) Getreide Mehl und Brot. 1995;49:265–271. [Google Scholar]
- Kornegay ET (2001) Digestion of phosphorus and other nutrients: the role of phytases and factors influencing their activity. In: Bedford MR, Partridge GG (eds) Enzymes in farm animal nutrition. CABI Publishing, pp 237–272
- Laboure AM, Gagnon J, Lescure AM. Purification and characterization of a phytase (Myo-inositol-hexaphosphate phosphohydrolase) accumulated in maize (Zea-Mays) seedlings during germination. Biochem J. 1993;295:413–419. doi: 10.1042/bj2950413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei XG, Porres JM. Phytase enzymology, applications, and biotechnology. Biotechnol Lett. 2003;25:1787–1794. doi: 10.1023/A:1026224101580. [DOI] [PubMed] [Google Scholar]
- Leytem AB, Widyaratne GP, Thacker PA. Phosphorus utilization and characterization of ileal digesta and excreta from broiler chickens fed diets varying in cereal grain, phosphorus level, and phytase addition. Poultry Sci. 2008;87:2466–2476. doi: 10.3382/ps.2008-00043. [DOI] [PubMed] [Google Scholar]
- Li DP, Zhu HF, Liu KF, Liu X, Leggewie G, Udvardi M, Wang DW. Purple acid Phosphatases of Arabidopsis thaliana comparative analysis and differential regulation by phosphate deprivation. J Biol Chem. 2002;277:27772–27781. doi: 10.1074/jbc.M204183200. [DOI] [PubMed] [Google Scholar]
- Lopez HW, Leenhardt F, Coudray C, Remesy C. Mineral and phytic acid interactions: is it a real problem for human nutrition? Int J Food Sci Tech. 2002;37:727–739. doi: 10.1046/j.1365-2621.2002.00618.x. [DOI] [Google Scholar]
- Marero LM, Payumo EM, Aguinaldo AR, Matsumoto I, Homma S. Anti-nutritional factors in weaning foods prepared from germinated cereals and legumes. Food Sci Technol LEB. 1991;24:177–181. [Google Scholar]
- Maugenest S, Martinez I, Lescure AM. Cloning and characterization of a cDNA encoding a maize seedling phytase. Biochem J. 1997;322:511–517. doi: 10.1042/bj3220511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugenest S, Martinez I, Godin B, Perez P, Lescure AM. Structure of two maize phytase genes and their spatio-temporal expression during seedling development. Plant Mol Biol. 1999;39:503–514. doi: 10.1023/A:1006131506193. [DOI] [PubMed] [Google Scholar]
- Mollgaard H. On phytic acid, its importance in metabolism and its enzymic cleavage in bread supplemented with calcium. Biochem J. 1946;40:589–603. doi: 10.1042/bj0400589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Joh T, Tokumoto E, Hayakawa T. Purification and characterization of phytase from bran of Triticum aestivum L. cv. Nourin #61. Food Sci Techn Res. 1999;5:18–23. doi: 10.3136/fstr.5.18. [DOI] [Google Scholar]
- Nielsen AVF, Tetens I, Meyer AS. Potential of phytase-mediated iron release from cereal-based foods: a quantitative view. Nutrients. 2013;5:3074–3098. doi: 10.3390/nu5083074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh BC, Choi WC, Park S, Kim YO, Oh TK. Biochemical properties and substrate specificities of alkaline and histidine acid phytases. Appl Microbiol Biot. 2004;63:362–372. doi: 10.1007/s00253-003-1345-0. [DOI] [PubMed] [Google Scholar]
- Oliver B, Oliver AH, Jones Hugh J, Cornell Darryl M. The influence of fermentation processes and cereal grains in wholegrain bread on reducing phytate content. J Cereal Sci. 2014;59:3–8. doi: 10.1016/j.jcs.2013.11.006. [DOI] [Google Scholar]
- Raboy V. Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci. 2009;177:281–296. doi: 10.1016/j.plantsci.2009.06.012. [DOI] [Google Scholar]
- Reddy NR (2002) Occurrence, distribution, content, and dietary intake of phytate. In: Food phytates. CRC Press, Boca Raton, pp 25–51
- Reddy NR, Sathe SK, Salunkhe DK. Phytates in legumes and cereals. Adv Food Res. 1982;28:1–92. doi: 10.1016/S0065-2628(08)60110-X. [DOI] [PubMed] [Google Scholar]
- Sreeramulu G, Srinivasa DS, Nand K, Joseph R. Lactobacillus amylovorus as a phytase producer in submerged culture. Lett Appl Microbiol. 1996;23:385–388. doi: 10.1111/j.1472-765X.1996.tb01342.x. [DOI] [Google Scholar]
- Svanberg U, Lorri W, Sandberg AS. Lactic fermentation of non-tannin and high-tannin cereals effects on in vitro estimation of iron availability and phytase hydrolysis. J Food Sci. 1993;58:408–412. doi: 10.1111/j.1365-2621.1993.tb04286.x. [DOI] [Google Scholar]
- Troesch B, Egli I, Zeder C, Hurrell RF, De Pee S, Zimmermann MB. Optimization of a phytase-containing micronutrient powder with low amounts of highly bioavailable iron for in-home fortification of complementary foods. Am J Clin Nutr. 2009;89:539–544. doi: 10.3945/ajcn.2008.27026. [DOI] [PubMed] [Google Scholar]
- Vats P, Banerjee UC. Production studies and catalytic properties of phytasesn (myo-inositol hexakisphosphate phosphohydrolases): an overview. Enzyme Microb Tech. 2004;35:3–14. doi: 10.1016/j.enzmictec.2004.03.010. [DOI] [Google Scholar]
- Vidal-Valverde C, Frias J, Sotomayor C, Diaz-Pollan C, Fernandez M, Urbano G. Nutrients and antinutritional factors in faba beans as affected by processing. Z Lebensm Unters Forsch A. 1998;207:140–145. doi: 10.1007/s002170050308. [DOI] [Google Scholar]
- Yao MZ, Zhang YZ, Lu WL, Hu MQ, Wang W, Liang AH. Phytases: crystal structures, protein engineering and potential biotechnological applications. J Appl Micro. 2011;112:1–14. doi: 10.1111/j.1365-2672.2011.05181.x. [DOI] [PubMed] [Google Scholar]
- Yi Z, Kornegay ET, Ravindran V, Denbow DM. Improving phytate phosphorus availability in corn and soybean meal for broilers using microbial phytase and calculation of phosphorus equivalency values for phytase. Poultry Sci. 1996;75:240–249. doi: 10.3382/ps.0750240. [DOI] [PubMed] [Google Scholar]