Abstract
Food security and safety are the major concern in ever expanding human population on the planet earth. Each and every year insect pests cause a serious damage in agricultural field that cost billions of dollars annually to farmers. The loss in term of productivity and high cost of chemical pesticides enhance the production cost. Irrespective use of chemical pesticides (such as Benzene hexachloride, Endosulfan, Aldicarb, and Fenobucarb) in agricultural field raised several types of environmental issues. Furthermore, continuous use of chemical pesticides creates a selective pressure which helps in emerging of resistance pest. These excess chemical pesticide residues also contaminate the environment including the soil and water. Therefore, the biological control of insect pest in the agricultural field gains more importance due to food safety and environment friendly nature. In this regard, bacterial insecticides offer better alternative to chemical pesticides. It not only helps to establish food security through fighting against insect pests but also ensure the food safety. In this review, we have categorized insect pests and the corresponding bacterial insecticides, and critically analyzed the importance and mode of action of bacterial pesticides. We also have summarized the use of biopesticides in integrated pest management system. We have tried to focus the future research area in this field for the upcoming scientists.
Keywords: Bacterial insecticides, Insect pest, Integrated pest management, Mode of action, Bioremediation
Introduction
The total annual economic losses caused by insect pests reach US$ 17.7 billion (Oliveira et al. 2014). Every year worldwide, more than $40 billion dollars are expended for chemical pesticides to control lepidopteron pests (Pan-UK 2003; Khan and Law 2005). The usage of pesticide in India is about 0.5 kg/ha of which major contribution is from organochlorine pesticides (Bhat and Padmaja 2014). Irrespective of that, India loses about 25% rice and 50% cotton production every year due to insect pests (Singh 2007). In addition to that, during the last couple of decades, the use of such synthetic chemicals has raised a number of environmental issues causing health hazards (Aktar et al. 2009). Few chemical pesticides were already banned by EU and USA due to environment and human health related issue (Pesticides Safety Directorate 2008). Remaining chemical pesticides were appeared to be weak in continuous battle with the insect pests due to arrival of resistance (Siegwart et al. 2015). Low biodegradability of chemical pesticides has classified these as persistent toxic substances (Tayade et al. 2013). Detrimental effects of chemical pesticides on soil microbiota may be directly related to loss of biodiversity and functions such as the recycling of nutrients (Su et al. 2014). Besides contaminating the environment, including the soil and water, pesticide residues also affect useful organisms like earth worms, bees, spiders, plants (Singh et al. 2014). Therefore, bioremediation of the agricultural field from the harmful residue of chemical pesticides has put forward another challenge to the combat against insect pest.
Cultivation methods, like crop rotation for avoidance of the target host and by stimulating the growth of adventitious root system, can partially address the pest problem (Smith et al. 2000; Bailey et al. 2009). Biological control offers better alternative to synthetic chemical pesticides, because biopesticides (both live organisms and their components) are target specific, easy biodegradability, having less self life and user friendly for sustainable agriculture (Sayyed and Patel 2011; Kumar and Singh 2015). About 100 bacteria were identified as exo- and endo-pathogens of arthropods (Thacker and Jonathan 2002). But only few of the bacterial entomopathogens are available commercially (Johnson et al. 2001; Roh et al. 2007; Jeong et al. 2010).
Multiple activities of biopesticides are now considered under ICM (Vimaia Devi et al. 2012). For example, the strain S. entomophila AB2 reported from epizootic Heliothis sp. (Lepidoptera) exhibited both fungicidal and nutrient solubilizing ability (Chattopadhyay and Sen 2013; Chattopadhyay et al. 2014a, b). Bioremediation is a waste management technique that involves the use of organisms to remove or neutralize pollutants from a contaminated site. Microbial bioremediation is the use of prokaryotes (or microbial metabolism) to remove pollutants. Bioremediation has been used to remove agricultural chemicals (pesticides, fertilizers) that leach from soil into groundwater and the subsurface (Radhika and Kannahi 2014). With advances in biotechnology, bioremediation has become one of the most rapidly developing fields of environmental restoration, utilizing microorganisms to reduce the concentration and toxicity of various chemical pollutants, such as petroleum hydrocarbons, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, phthalate esters, nitroaromatic compounds, industrial solvents, pesticides and metals (Dua et al. 2002). Bacterial insecticides can also reduce the risk generated by chemical pesticides in agricultural field as they are (1) less toxic; (2) affects only the target pest; (3) effective in very small quantities; (4) decompose quickly; and (5) decrease the chemical pesticide residues in soil.
In this review, we have categorized insect pests, their corresponding bacterial insecticides, their mode of action and application. We also analyzed the total industrial pipeline of bacterial insecticides starting from isolation of novel strain to strain improvement/development (including genetic and protein engineering) and marketing. The environmental aspects of bacterial insecticides were also discussed.
Insect pests and insecticides
The insect pest can be defined as the insect that has potentiality to damage human purposes or natural habitats and ecosystems (Table 1). Pest insects can damage or kill agricultural crops, ornamental plants or native plants in situ; consume and/or damage harvested food; causing illness or non-productivity to agricultural animals; and vector for human diseases (Verkerk and Wright 1996). Some insects are considered to be a friend at one stage of life, but behave like a pest at another stage. For example many lepidopteron may be serious pests at their larval stage, while they may be pollinators in adulthood. Some insects that are considered pests (particularly in suburbia) are actually more beneficial than pestiferous, include wasps (predate or parasitize many pest insects) or bees (the main pollinators of human food supplies). Insect pests may be broadly classified into four major categories: (1) agricultural and horticultural, (2) medical, (3) veterinary and (4) domestic household (Chattopadhyay and Sen 2012). Among nearly one million known species of insects, about 15,000 species are considered as pests and about 300 gain special attentions.
Table 1.
A list of major categories of insect pests
| Types | Subcategory | Examples |
|---|---|---|
| Agricultural and horticultural pest insects | Root-eating insects | Root maggots, mole crickets, white grubs |
| Chewing insects | Caterpillar, chafer beetle, semi looper | |
| Piercing and sucking insects | Aphids, scales, mealy bugs, thrips, red mites | |
| Seed-, pod-, and fruit-eating insects | Corn earworm, pickleworm, pod borers | |
| Field crop pest insects | Cotton insect pests, sugarcane pest insects | |
| Flower pest insects | European corn borer, Spanish moth | |
| Forest tree pest insects | Bark beetles, gall makers | |
| Fruit and nut pest insects | Mango pest insects, fruit flies, stink bugs | |
| Greenhouse pest insects | Whiteflies, thrips, aphids, mealy bugs | |
| Nursery pest insects | Asian ambrosia beetle, tarnished plant bug | |
| Vegetable pest insects | Diamondback moth, potato pest insects | |
| Medical pest insects (vectors and stinging insects) | Biting flies | Sand flies, black flies, stable fly |
| Fire ants | Red imported fire ants, southern fire ants | |
| Mosquitoes | Aedes, Anopheles, Culex | |
| Wasps and bees | Honey bees, bumble bees, cicada killer wasps | |
| Veterinary pest insects | Cattle pest insects | Cattle grubs, cattle tail lice, horn flies |
| Horse pest insects | Stable flies, black flies, biting gnats, face flies | |
| Poultry pest insects | Litter beetles, poultry lice, bed bugs | |
| Sheep and goat pest insects | Sheep keds, sheep nose bots, African blue louse | |
| Swine pest insects | Hog lice, follicle mite, hog mange mites, flies | |
| Zoo pest insects | Black flies, biting gnats, horn flies, lice | |
| Domestic household pest insects | Turf-sod pests | Mites |
| Interiorscape pest insects | Ants, termites, bed bugs, moths, cockroaches | |
| Landscape plant pest insects, mites | Caterpillars, aphids, grasshoppers, mole crickets | |
| Urban tree pest insects | Gall wasps, oak treehopper, thorn bug | |
| Wood-destroying insects | Bamboo borer, wood-boring weevil |
Once it is established that an insect is causing economic loss, it becomes necessary to control it. Entomopathogens have been suggested as controlling agents of insect pests for over a century, and belong to species of fungi, viruses, bacteria, and protozoa. Insect pest control refers to the regulation or management of a species defined as a pest. Pesticides are chemicals and other agents (e.g., beneficial microorganisms) that are used to control or protect other organisms from pests. Moreover, a larvicide is an insecticide that can be targeted against the larval life stage of an insect. Larvicides may be contact poisons, stomach poisons, growth regulators, or (increasingly) biological control agents. Due to several health-related problems, EU has banded the use of different insecticides (particularly organophosphates) in the agricultural field (Pesticides Safety Directorate 2008). Furthermore, insecticides create a selective pressure for emergence of resistant pest. Resistance has been reported for all chemical insecticide classes of one or more key pest species, including stored product insects (Whalon et al. 2008; Sparks and Nauen (2015). Spider mite (Tetranychus urticae) was the first glasshouse pest to develop resistance in 1949. More recently, leafminers, aphids, whiteflies and thrips have been reported to develop resistance to a wide range of chemicals. The new strategy should be developed, as the range of chemical solutions becomes more limited. Pest control techniques have evolved over the past 50 years. In recent years, the chemical pesticides were replaced by biopesticides as a significant part of IPM. Biopesticides are living agents (virus, bacteria, fungi, protozoa, nematode and plant cell) or their extracts or toxins or enzymes or their combinations or even genes that are used to control or protect other organisms from pests. Among biopesticides only insecticide of bacterial origin has been summarized in this review.
Bacterial candidates used as insecticides
While about 100 bacteria were identified as exo- and endo-pathogens of arthropods (Thacker and Jonathan 2002), only a few are used commercially in pest management system (Table 2). Among commercially exploited bacteria, Bacillus popilliae, B. sphaericus, B. thuringiensis, Clostridium bifermentans, Pseudomonas alcaligenes, Pseudomonas aureofaciens, Saccharopolyspora spinosa, Serratia entomophila and Streptomyces avermitilis were considered to be the major stack holders. However, two bacterial candidates; spore-forming soil bacterium B. thuringiensis (Bt) (Bizzarri et al. 2008; Porcar et al. 2008) and non-spore-forming S. entomophila (Inglis and Lawrence 2001; O’Callaghan and Gerard 2005) gain more popularity as a pest control agents.
Table 2.
Commercially available bacterial species with insecticidal activity
| Bacterial sp. | Target pests | Effectors molecules | References |
|---|---|---|---|
| Subdivision: Firmicutes, Order: Bacillales | |||
| B. popilliae | Japanese beetle grubs | Cry and Cyt toxins | Kaya et al. 2008 |
| B. sphaericus | Mosquitoes | Cry and Cyt toxins | El-Bendary 2006 |
| B.t. subsp. aizawai | Lepidopteran larvae | Cry and Cyt toxins | Soberón et al. 2012 |
| B.t. subsp. israelensis | Mosquito | Cry and Cyt toxins | Bravo et al. 2007 |
| B.t. subsp. kurstaki | Lepidopteran larvae | Cry and Cyt toxins | Bravo et al. 2007 |
| B.t. subsp. tenebrionis | Coleoptera pests | Cry and Cyt toxins | Bravo et al. 2007 |
| Subdivision: Firmicutes, Order: Clostridiales | |||
| C. bifermentans | Mosquito | Qureshi et al. 2014 | |
| Subdivision: Firmicutes, Order: Actinomycetales | |||
| Saccharopolyspora spinosa | Two-spotted spider mites | Spinosyns | Sparks et al. 2012 |
| Streptomyces avermitilis | Colorado potato beetle | Doramectin congeners | Wang et al. 2011a, 2011b |
| Subdivision: Gracilicutes, Order: Pseudomonadales | |||
| P. alcaligenes | Locusts, grasshoppers | Insecticidal protein (Ppip) | Ruffner et al. 2015 |
| P. aureofaciens | Scrub, mildew | Insecticidal toxin (Fit) | Ruffner et al. 2015 |
| Subdivision: Gracilicutes, Order: Enterobacteriales | |||
| Serratia entomophila | New Zealand grass grub | sepA, sepB, sepC and Afp | Hurst et al. 2007 |
B. thuringiensis is often used for controlling Lepidoptera (Helicoverpa armigera, Spodoptera exempta, Cydia pomonella, etc.), Diptera (Aedes aegypti, Anopheles albimanus, Culex obscures, etc.), Coleoptera (Leptinotarsa decemlineatam, Popillia japonica, Tribolium confusum, etc.), and Hymenoptera (Megastigmus spermotrophus, Megachile frontalis, Xylocopa aruana, etc.) (Bravo et al. 2007). Specific endotoxin producing strains of B. thuringiensis var. israelensis and B. sphaericus have been used throughout the world to suppress or eliminate the larval stages of mosquitoes, particularly in malaria and filariasis endemic zones. Furthermore, B. thuringiensis var. israelensis is also effective against the larval stages of Simulium black fly, which serve as a vector of river blindness in man (onchocerciasis) in tropical Africa. There are over 40 Bt products (LARVECT 50®, Mosquito Dunks®, Montery B.t. ®) available in the market for controlling caterpillars, beetles and blood-feeding flies such as mosquitoes. Taking all together, it accounts for 1% of the total insecticide market (Bizzarri and Prabhakar 2008).
Whereas, strains of S. entomophila are effective natural bio-controlling agent for the grass grub, Costelytra zealandica (Coleoptera: Scarabaeidae), a major pasture pest of New Zealand (Jackson et al. 1992; Gerard and Popay 2016). Grimont et al. (1977) have first reported the S. entomophila as a natural biocontrol agent against C. zealandica. Later on a Mexican strain (S. entomophila Mor4.1) was also found to be active against another white grub, Phyllophaga blanchardi (Nunez-Valdez et al. 2008). S. entomophila AB2 was also reported to be a bio- control agent against Lepidopteran pest (Chattopadhyay and Sen 2013; Chattopadhyay et al. 2014b). The bio-controlling activity of S. entomophila against different insect genera (Anomala, Costelytra, and Phyllophaga) also has been reported by several researchers (Nunez-Valdez et al. 2008). Studies conducted to date have shown no significant effect of these bacteria and their toxins on the vertebrates, however, few toxic effects on some non-target arthropods and crustaceans have been recorded.
Genes from bacterial insecticides can be used in plant in making transgenic crops that will be protective against insect pests (National Academy of Science 2000). Genetic combination might be an effective tool for enhancing the efficacy of bacterial insecticides. For example, plant-colonizing pseudomonad can be used for the delivery of Bt genes to improve efficacy. Manker et al. (2002) have reported the combined effect of a Bt enhancer and Bt protein against the toughest Lepidopteran caterpillar. Future research will likely enhance these facts and find even more effective approaches for the stabilization of the Bts. Crops with seed engineered to contain a gene for insect control have been great commercial successes (National Academy of Science 2000). The advancement in biotechnological field will definitely find out more effective way to control the agricultural pests.
Why bacterial insecticides are environment friendly
Biopesticide is a potential tool to be utilized for environmental safety (Vimaia Devi et al. 2012). An uneasy truce exists between the insect pests and man, and this is termed as ‘balance in nature’. This balance is the result of two opposing phenomena; the ‘biotic potential’, i.e., the tremendous capacity of insects to reproduce and multiply and the environmental resistance that keeps their numbers under control. The environment resistance results in the death of adults and the mortality of eggs, larvae or pupae of the insects because of desiccation, starvation, parasites, predators, diseases and other adverse environmental conditions. The change in ‘environment resistance’ may take place owing to a number of causes, either natural or operated by different agencies. More rational approaches would be required to popularize biopesticide as one of the important inputs for safe and sustainable agriculture (Kamble et al. 2016). Training on production and quality control to manufacturers, organizational training to extension workers and farmers to popularize biopesticides would be essential for better adoption. As environmental safety is a global issue, we need to create awareness among the common men to switch over to biopesticides for their pest management requirements. Biopesticides are expected to provide predictable performance, and they must do so in an economically viable manner for their better acceptability and adaptability (Birthal and Sharma 2004). To be readily acceptable by the end users, biopesticides must be efficient enough in controlling the targeted pests. Biopesticides have tremendous potential to bring sustainability to agriculture and environmental safety. However, three distinct environmental benefits of bacterial insecticides are recognized. They are: (a) have a narrow target range and a very specific mode of action; (b) are slow acting; (c) have relatively critical application times; (d) suppress, rather than eliminate a pest population; (e) have limited field persistence and a short shelf life; (f) are safer to humans and the environment than conventional pesticide; (g) present no residue problems.
Isolation and commercialization of bacterial insecticides
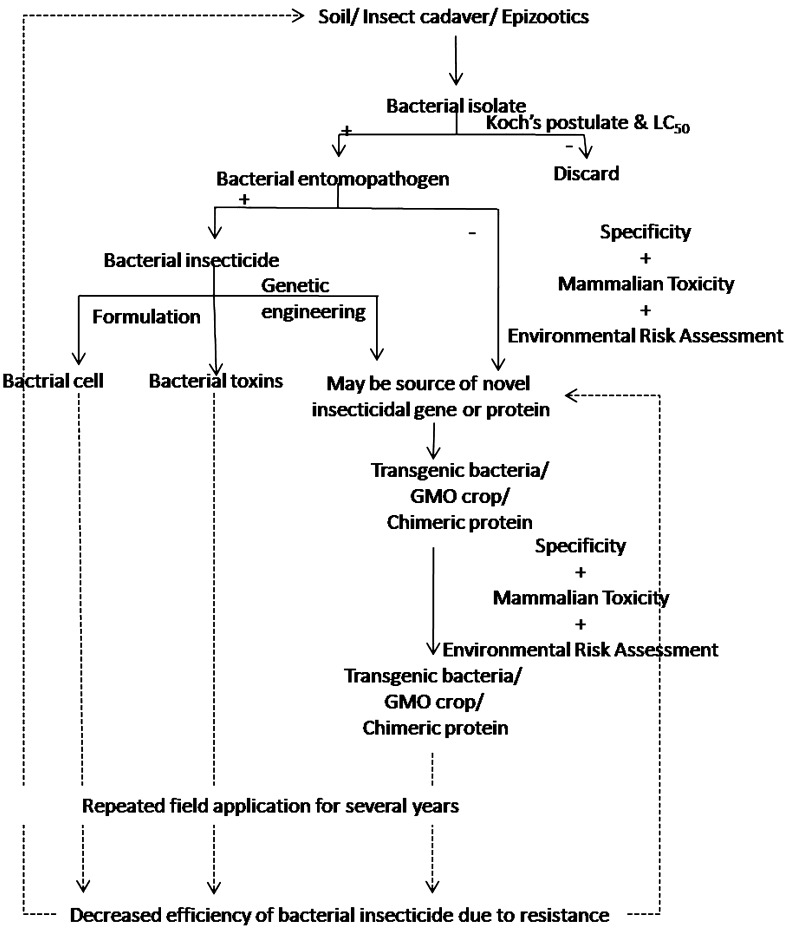
Efficiency of an insecticide depends upon its source, which means, whether it is derived from nature or prepared synthetically using certain formula. Natural biological control occurs where native or co-evolved natural enemies reduce native arthropod populations, whereas applied biological control involves human intervention to enhance natural enemy activities (Lacey and Shapiro-Ilan 2008). Several bacterial species cause deadly diseases in insect and lead to precipitous population declines when they become epizootic (analogous to an epidemic in a human population). The scope of bacterial pesticide is not limited to direct use only. Genetic and protein engineering have already opened a scope of tissue-specific chimeric protein expression either by the bacteria or by the vector or by the plant itself. Therefore, searching for new strain is important to enhance the existed gene pool to combat against developing resistance of insect pests (Fig. 1). As for example, Gray et al. (2009) reported Bt toxins produced by plant growth-promoting rhizobacteria, which also produce bacteriocin compounds of insecticidal attributes.
Fig. 1.
Industrial pipeline of bacterial insecticides starting from isolation of novel strain to strain improvement/development, quality control, environmental assessment, toxicity testing and marketing
Understanding and characterizing the bioactive compounds and optimizing their production in fermentation can increase the efficacy and consistency of a biopesticide in a way that is more comparable to that of synthetic pesticides, but their use will probably raise some questions around residues on food and potential of resistance development. The risk of resistance development might be considered as low if the mode of action is based on a combination of several bioactive compounds and sometimes also the living microorganisms, along with their physiological interaction with the target pest (Glare et al. 2012). In recent year, several modern techniques such as genes technology, high-throughput screening and bioinformatics have been introduced to screen potential insecticidal bacterial species (Ragunath et al. 2014). Soil and water are the richest sources of bacteria, but less than 1% of candidate isolates eventually make successful insecticidal products (Glare et al. 2012). However, the selection of potential candidate is an important process. First, a set of technical specifications (blueprint) for the desired bacterial insecticides have developed based on the knowledge of the life cycle of the target pest. The blueprint is then compared with the information available in database and the best 20–30 matches identified with the help of bioinformatic tools (Ragunath et al. 2014). These isolates are then subjected to a series of standardized bioassays to identify the biocontrol ability and field performance capability. This targeted screening strategy reduces the average length of time; thereby reduce the cost of developing biopesticides.
Different modes of action of bacterial insecticides
There are more than 100 bacterial species having entomopathogenic activity (Starnes et al. 1993). Among them, Bacillus thuringiensis, B. popilliae, B. lontimorbus, B. sphaericus, Saccharopolyspora spinosa and Serratia entomophila are the most studied species (Table 2). In this present review, we have categorically discussed the mode of action of commercially exploited bacterial insecticides.
Mode of action of insecticidal Bacillus
Different members of the bacilli family are in use as bacterial insecticide including: B. popilliae, B. sphaericus, B. thuringiensis, and B. popilliae (Bp). B. popilliae is an obligate pathogen on scarab larvae (cause ‘milky disease’) and is highly specific in nature. Causes of insect death due to Bp may be infection and physiological starvation. On the other hand, B. sphaericus activity is reported to be specific for few Diptera in aquatic environment. Its mode of action is quite similar to B. thuringiensis (Bt). Among them, B. thuringiensis (Bt) is the most used and described bacterial insecticide in the world (Peralta and Palma 2017). Therefore, the mode of action of Bt has been elucidated here with little detail. Bt is an aerobic, Gram-positive, rod-shaped bacterium belongs to the bacilli family and is widely distributed in soil, water, air and plants in its vegetative form (Raymond et al. 2010). Under deficient trophic conditions, Bt starts endospore formation. During this period, Bt produces proteins that are toxic for insects. Among these, the Cry and Cyt are most important insecticidal proteins that form parasporal crystals (Bravo et al. 2011). The most commonly used bacterial serovars are kurstaki against defoliating Lepidoptera larvae, aizawai against Lepidoptera larvae feeding on seed and sandiego and tenebrionis (=morrisoni) against Coleoptera larvae. An additional commonly used serovar, Bt var. israelensis, is used against mosquito vectors borne human diseases (Seleena et al. 1997). The anaerobic bacterium Clostridium bifermentans has also been reported to produce a number of proteins having mosquitocidal activity (Seleena et al. 1997; Lennox et al. 2016). Hence, toxins that target Anopheles are different from those expressed by the Cry operon (Qureshi et al. 2014).
There is a great variety of Cry toxins that belong to the class of pore-forming toxins (PFTs) (Pigott and Ellar 2007). The mode of action of Cry toxins in Hymenoptera (Cry5A, Cry5B) and Diptera (Cry11A, Cry4B) consists of similar steps as those described for Lepidopteran (Cry1A) specific Cry toxins (Garcia-Robles et al. 2001; Gomez et al. 2007). According to the commonly accepted understanding of its mode of action, there are five stages leading towards pest mortality, viz. (1) solubilization, (2) proteolysis, (3) specific binding, (4) pore formation and (5) larval death. The pores formed are permeable to K+ ions, other cations, and solutes such as sucrose, which induces a change in the membrane permeability. Due to disturbance in the osmotic balance, gut cell lyzed that allows gut contents to leak into the haemocoel, resulted into physiological starvation and lethal septicaemia (Heckel 2012). Recent molecular studies on nematode (Caenorhabditis elegans) and lepidoptera species have revealed alternative pathway of Cry toxin activity (Crickmore 2005). The activation of intracellular apoptotic pathways after Cry1A toxin binding to cadherin has been hypothesized to be involved in Cry1 toxicity (Raymond et al. 2010). Moreover, Cyt toxins (e.g., Cyt1A) have no protein binding receptor on the insect gut wall. However, it has detergent-like activity that allows to directly interact with membrane lipids and form pores in the membrane (Soberón et al. 2012). Specific perforation occurs at low toxin concentration or short exposure, whereas membrane disruption occurs at high levels or long times period. Cyt toxins may form pore by itself and even form pore synergistically with Cry proteins. As for example, Cyt1Aa, which functions as a membrane-bound receptor, inserts its β-sheet into the membrane and expose a binding domain for Cry11Aa.
Mode of action of insecticidal actinomycetes
Streptomyces avermitilis and Saccharopolyspora spinosa are Gram-positive, spore-forming bacteria belong to the group of actinomycete. Doramectin congeners isolated from S. avermitilis (Wang et al. 2011a, b) and spinosyns extracted from S. spinosa (Sparks et al. 2012) exhibited noticeable insecticidal activity. Doramectin congeners were reported to be active against adult two-spotted spider mites (Wang et al. 2011a, b). Moroever, spinosyns are mainly commercialized as two active substances: the spinosad and the spinetoram. Spinosad is a mixture of spinosyns A and D, the two most active metabolites that are produced by the species. This product was first approved in the United States in 1997 to control Lepidoptera larvae which were resistant to pyrethroids. Today, this insecticide is used in 40 countries on various crops, such as cotton, crucifer, apple, grapevine and peach, to control more than 50 pests (Sparks et al. 2012). Once ingested by the insect, spinosyns quickly reach the central nervous system, where they induce the depolarization of the neuron membranes that are connected to the muscles. This hyper excitation causes insect paralysis. Spinosyns fix at a specific site on the acetylcholine receptor (nAchR) that are different from the neonicotinoid sites. These compounds also act on the GABA (g-aminobutyric) receptors, but their functions are not yet clearly defined (Sparks et al. 2012).
Mode of action of insecticidal Gram-negative bacteria
Among different Gram-negative bacteria Clostridium bifermentans, Pseudomonas alcaligenes, Pseudomonas aureofaciens, and Serratia entomophila were commercially exploited. Certain strains of plant root-colonizing Pseudomonas bacteria display insect pathogenicity, and thus could be formulated to extend the present range of bioinsecticides for the protection of crop plants against root-feeding insects (Kupferschmied et al. 2013). Similarly, S. entomophila has been used in New Zealand to control grass grub (Costelytra zealandica) for 15 years with no indications of safety problems or unexpected environmental effects from widespread application. Despite widespread testing, no other insect species have been shown to be susceptible to the plasmid bearing strains of S. entomophila. A 155-kb plasmid, pADAP, carries the genes sepA, sepB, and sepC, which are essential for production of amber disease symptoms. Sequence data shows that the anti-feeding component is part of a large gene cluster that may form a defective prophage, and that six potential members of this prophage are present in Photorhabdus luminescens subsp. laumondii TTO1 (Hurst et al. 2004). The S. entomophila anti-feeding prophage (Afp) is thought to form a virus like structure that has activity towards C. zealandica (Hurst et al. 2007).
Bacterial insecticide formulation
It is important to note that only the bacterial agent is used neither as pesticide nor as the sole active agent. Pesticide formulation is the process of transforming a pesticide chemical into a product which can be applied by practical methods to permit its effective, safe and economic use. Formulation may contain live bacteria, bacterial spore, bacterial toxin, enzymes and even engineered protein along with the filler, matrix, binding material, etc. Development of microbial insecticide formulation closely paralleled that of chemical insecticides. However, the important differences do exist because microbial insecticides do not directly depend on the effect of a poisonous chemical but exploit the activity of living (or self-replicating) entities. An exception is the enterotoxicosis caused by Bacillus thuringiensis, where a pre-formed toxic glycoprotein is essential for infection. Commercialized Bt products are made of a mixture of spores and protein crystals (Raymond et al. 2010) and represented 2% of the total insecticides available in the market in 2011 (Bravo et al. 2011). They are applied on leaves or other environments where the insect larvae feed. Toxin genes from Bt have been genetically engineered into several crops. An attempt to utilize a particular species of microorganism in an insecticide formulation should be based on an intimate knowledge of the host–pathogen relationships (Chattopadhyay et al. 2014a), particularly, the multi-replication of a microorganism in the host tissues that leads to disease and death. As for example, a granular formulation of S. entomophila (BioShield™) was developed by Townsend et al. (2004).
Field application of bacterial insecticide
Effective biological control often requires a good understanding of the biology of the pest and its natural enemies, as well as the ability to identify various life stages of relevant insects in the field. Field scouting usually is necessary to monitor natural enemy activity, evaluate impact on pest populations, and anticipate the need for additional control measures. Generally, the application of biopesticides is not complicated; however, it requires proper training and knowledge about the pests/pathogens (Chattopadhyay et al. 2014a). In case of biopesticide, the appropriate time of application is essential to ensure the efficacy. The challenging task is to develop a balance between the broadly defined costs and the benefits of biopesticides, compared with the synthetic pesticides. Bt-based microbial insecticides have been used successfully in many cropping and forestry systems for years, but the effective life of Bt in the field is only a few hours owing to various derivative influences. The newer biopesticides may bring with them new regulatory and economic challenges that must be addressed jointly by the social and natural scientists, policy makers and the industry.
Bacterial insecticide in integrated pest and crop management (IPM and ICM) practice
Pest management includes a wide range of programs addressing human health, environmental and economic issues related to the management of pest populations through a variety of science based technologies. Normally, people want safe, pest- and disease-free apartments, and a wholesome pesticide-free environment. According to British Agrochemical Association, integrated crop management (ICM) is a method of farming that balances the requirements of running a profitable business with responsibility and sensitivity to the environment. It includes practices that avoid waste, enhance energy efficiency and minimize pollution (http://www.ecifm.rdg.ac.uk/integrated_crop_management.htm). ICM combines the best of modern technology with some basic principles of good farming practice and is a long term strategy. According to the Food and Agriculture Organization (FAO) of the United Nations, IPM is a part of ICM, while ICM is the solution for sustainable agriculture (http://www.ecpa.eu/information-page/ipm/what-ipm).
India has adopted the environment friendly integrated pest management (IPM) approach for combating with pests and diseases, as a cardinal principle of its plant protection strategy (Birthal and Sharma 2004). The emphasis is given in controlling and not in eradication. IPM holds that wiping out an entire pest population is often impossible, and the attempt is also expensive and environmentally unsafe. IPM programs work to establish the acceptable pest levels, called action thresholds, and to apply controls if the threshold values are crossed (Sayyed and Patel 2011). These values are pest- and site-specific. It means that it may be acceptable to have a weed such as white clover at one site, but at another site it may not be acceptable. The presence of susceptible pests dilutes resistant genes that appear with time. This stops the pest gaining resistance to chemicals produced by the plant or applied to the crops. IPM is applicable to all types of agriculture and sites such as residential and commercial structures, or is used in habitation purpose.
Conclusion
Bacterial insecticides have been used in agriculture for many years. It is established that biopesticides are eco-friendly, target‐specific, easily biodegradable and safer alternatives. In this review, we have critically analyzed several advantages of bacterial insecticides over synthetic chemicals, including biosafety (safe for non-target organisms including human), eco-friendly (tendency to biodegrade), economic (low cost to develop) and good compatibility with IPM programs. Still, bacterial insecticides are not without critics. Drawbacks that might suggest further areas of research include limited product shelf life, unpredictable efficacy and short effective life in the field. Use of genetically modified organisms (GMO) is still controversial. When one makes crops resistant to an insect pest, this can potentially result in over-use of that pesticide. Incorporation of natural insect toxicants into the plant material might cause health-related problems. There is also concern about distributing genetic material to places where it does not occur naturally. Therefore, there is an ample scope of research for bacterial insecticide industry to face these challenges. The registrant community and the EPA, U.S. Department of Agriculture, and U.S. Food and Drug Administration have developed high standards to ensure safety to man, animals, and the environment. In India, 478 products of the 14 microbial pesticides are registered under Sect. 9(3) of the Insecticide Act, 1968 as on 17/06/2011. However, bio‐pesticides may represent about 4.2% of the overall pesticides market in India. Presently, only 12 types of biopesticides formulations are registered under the Insecticide Act, 1968 in India.
Future perspective
In addition to the continuous search for new biomolecules and improving the efficiency of the known biopesticides, recombinant DNA technology is also being used for enhancing the efficacy of biopesticides. Better understanding of genes from microorganisms and crop plants has enabled the isolation of genes effective against particular pest. Fusion proteins are also being designed to develop next-generation biopesticides. This technology allows selected toxins to be combined with a carrier protein which makes them toxic to insect pests when consumed orally. The fusion protein may be produced as a recombinant protein in substitutes. The human and environmental safety of the biopesticides and compatibility with integrated pest management systems will drive continued expansion of this industry. The industry has recognized the need to work together and has formed the Biopesticide Industry Alliance (BPIA), with a mission to improve the global market perception of biopesticides as effective products. BPIA plans to develop industry standards for product quality and efficacy.
Acknowledgements
We are very much thankful to Gauhati University, University of Delhi, and Visva Bharati University for providing necessary supports.
Compliance with ethical standards
Conflict of interest
We declared that none of the authors have any types of conflict of interest.
References
- Aktar MW, Sengupta D, Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol. 2009;2:1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KL, Boyetchko SM, Längle T. Social and economic drivers shaping the future of biological control: a Canadian perspective on the factors affecting the development and use of microbial biopesticides. Biol Contl. 2009;52:221–229. doi: 10.1016/j.biocontrol.2009.05.003. [DOI] [Google Scholar]
- Bhat D, Padmaja P. Assessment of organic pesticides in ground and surface water in Bhopal India. IOSR J Environ Sci Toxicol Food Technol. 2014;8:51–52. doi: 10.9790/2402-08535152. [DOI] [Google Scholar]
- Birthal PS, Sharma OP. Integrated pest management in Indian agriculture. New Delhi: National Centre For Agricultural Economics and Policy Research (NCAP); 2004. [Google Scholar]
- Bizzarri MF, Prabhakar A, Bishop AH. Multiple-locus sequence typing analysis of Bacillus thuringiensis recovered from the phylloplane of clover (Trifolium hybridum) in vegetative form. Microbiol Ecol. 2008;55:619–625. doi: 10.1007/s00248-007-9305-3. [DOI] [PubMed] [Google Scholar]
- Bravo A, Gill S, Soberón M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicol. 2007;49:423–435. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A, Likitvivatanavong S, Gill S, Soberón M. Bacillus thuringiensis: a story of a successful bio-insecticide. Insect Biochem Mol Biol. 2011;41:423–431. doi: 10.1016/j.ibmb.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay P, Sen SK (2012) Development of bacterial biopesticide: isolation to product formulation. LAP Lambert Academic Publishing, ISBN-10: 3848410419
- Chattopadhyay P, Sen SK. Systemic infestation of Serratia entomophila AB2 through plant tissue inferred protection against insect pest and fungal pathogens. Afr J Microbiol Res. 2013;7:2651–2655. doi: 10.5897/AJMR2013.5743. [DOI] [Google Scholar]
- Chattopadhyay P, Karmakar N, Chatterjee S, Sen SK. Field efficacy of inorganic carrier based formulations of Serratia entomophila AB2 in Sesamum indicum var. Kanak. Afr J Biotechnol. 2014;13:3481–3488. doi: 10.5897/AJB2014.13648. [DOI] [Google Scholar]
- Chattopadhyay P, Karmakar N, Sen SK. Exploration of Serratia entomophila AB2 for lepidopteran pest control and productivity of groundnut. Afr J Microbiol Res. 2014;8:3250–3254. doi: 10.5897/AJMR2014.6648. [DOI] [Google Scholar]
- Crickmore N. Using worms to better understand how Bacillus thuringiensis kills insects. Trends Microbiol. 2005;13:347–350. doi: 10.1016/j.tim.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Dua M, Singh A, Sethunathan N, Johri AK. Biotechnology and bioremediation: successes and limitations. Appl Microbiol Biotechnol. 2002;59:143–152. doi: 10.1007/s00253-002-1024-6. [DOI] [PubMed] [Google Scholar]
- El-Bendary MA. Bacillus thuringiensis and Bacillus sphaericus biopesticides production. J Basic Microbiol. 2006;46:158–170. doi: 10.1002/jobm.200510585. [DOI] [PubMed] [Google Scholar]
- Garcia-Robles I, Sanchez J, Gruppe A, Martínez-Ramírez AC, Rausell C, Real MD, Bravo A. Mode of action of Bacillus thuringiensis PS86Q3 strain in hymenopteran forest pests. Insect Biochem Mol Biol. 2001;31:849–856. doi: 10.1016/S0965-1748(01)00030-3. [DOI] [PubMed] [Google Scholar]
- Gerard PJ, Popay AJ (2016) Climate change effects on biological control in grasslands. In: Johnson SN, Jones TH (eds) Global climate change and terrestrial invertebrates, Wiley-Blackwell, New York, p 92
- Glare T, Caradus J, Gelernter W, Jackson T, Keyhani N, Köhl J, Marrone P, Morin L, Stewart A. Have biopesticides come of age? Trends Biotechnol. 2012;30:5250–5258. doi: 10.1016/j.tibtech.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Gomez I, Pardo-Lopez L, Munoz-Garay C, Fernandez LE, Pérez C, Sánchez J, Soberón M, Bravo A. Role of receptor interaction in the mode of action of insecticidal Cry and Cyt toxins produced by Bacillus thuringiensis. Peptides. 2007;28:169–173. doi: 10.1016/j.peptides.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Gray ME, Sappington TW, Miller NJ, Moeser J, Bohn MO. Adaptation and invasiveness of western corn rootworm: intensifying research on a worsening pest. Annu Rev Entomol. 2009;54:303–321. doi: 10.1146/annurev.ento.54.110807.090434. [DOI] [PubMed] [Google Scholar]
- Grimont PAD, Grimont F, De Rosnay HL. Taxonomy of the genus Serratia. J Gen Microbiol. 1977;98:39–66. doi: 10.1099/00221287-98-1-39. [DOI] [PubMed] [Google Scholar]
- Heckel DG. Insecticide resistance after Silent Spring. Science. 2012;337:1612–1614. doi: 10.1126/science.1226994. [DOI] [PubMed] [Google Scholar]
- Hurst MRH, Glare TR, Jackson TA. Cloning Serratia entomophila anti-feeding genes—a putative defective prophage active against the grass grub Costelytra zealandica. J Bacteriol. 2004;186:5116–5128. doi: 10.1128/JB.186.15.5116-5128.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst MR, Beard SS, Jackson TA, Jones SM. Isolation and characterization of the Serratia entomophila antifeeding prophage. FEMS Microbiol Lett. 2007;270:42–48. doi: 10.1111/j.1574-6968.2007.00645.x. [DOI] [PubMed] [Google Scholar]
- Inglis GD, Lawrence AM. Effects of Serratia marcescens on the F1 generation of laboratory-reared Heliothis virescens (Lepidoptera: Noctuidae) J Econ Entomol. 2001;94:362–366. doi: 10.1603/0022-0493-94.2.362. [DOI] [PubMed] [Google Scholar]
- Jackson TA, Pearson JF, O’Callaghan M, Mahanty HK, Willock MJ. Pathogen to product development of Serratia entomophila Enterobacteriaceae as a commercial biological control agent for the New Zealand grass grub Costelytra zealandica. In: Jackson TA, Glare TR, editors. Use of pathogens in scarab pest management, intercept Ltd. UK: Andover; 1992. pp. 191–198. [Google Scholar]
- Jeong HU, Mun HY, Oh HK, Kim SB, Yang KY, Kim I, Lee HB. Evaluation of insecticidal activity of a bacterial strain, Serratia sp. EML-SE1 against diamondback moth. J Microbiol. 2010;48:541–545. doi: 10.1007/s12275-010-0221-9. [DOI] [PubMed] [Google Scholar]
- Johnson VW, Pearson JF, Jackson TA. Formulation of Serratia entomophila for biological control of grass grub. N Z Plant Prot. 2001;54:125–127. [Google Scholar]
- Kamble KJ, Thakor NJ, Sonawane SP, Sawant AA (2016) Review on need of utilization of biopesticides in agriculture for safe environment. In: 3rd International Conference on “Latest Concepts in Science, Technology and Management”. Maharashtra, India, pp 16–23
- Kaya HK, Klein MG, Burlando TM. Impact of Bacillus popilliae, Rickettsiella popilliae and entomopathogenic nematodes on a population of the scarabaeid, Cyclocephala hirta. Biocontrol Sci Technol. 2008;3:443–453. doi: 10.1080/09583159309355299. [DOI] [Google Scholar]
- Khan MZ, Law FCP. Adverse effects of pesticides and related chemicals on enzyme and hormone systems of fish, amphibians and reptiles: a review. Proc Pak Acad Sci. 2005;42:315–323. [Google Scholar]
- Kumar S, Singh A. Biopesticides: present status and the future prospects. J Biofertil Biopestici. 2015;6:e129. doi: 10.4172/2471-2728.1000e129. [DOI] [Google Scholar]
- Kupferschmied P, Maurhofer M, Keel C. Promise for plant pest control: root-associated pseudomonads with insecticidal activities. Front Plant Sci. 2013;4:287. doi: 10.3389/fpls.2013.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey LA, Shapiro-Ilan DI. Microbial control of insect pests in temperate orchard systems: potential for incorporation into IPM. Ann Rev Entomol. 2008;53:121–144. doi: 10.1146/annurev.ento.53.103106.093419. [DOI] [PubMed] [Google Scholar]
- Lennox JA, Kalu O, Egbe JG. Screening of bacteria isolated from the environment for the capability to control mosquito larva. Afr J Bacteriol Res. 2016;8:29–34. [Google Scholar]
- Manker DC, Lidster WD, MacIntosh SC, Starnes RL. Potentiator of Bacillus pesticidal activity. US Patent. 2002;6(406):691. [Google Scholar]
- National Academy of Science . The future role of pesticides in US agriculture. Washington, DC: National Academy Press; 2000. pp. 168–169. [Google Scholar]
- Nunez-Valdez ME, Calderon MA, Aranda E, Hernández L, Ramírez-Gama RM, Lina L, Rodríguez-Segura Z, Gutiérrez MDC, Villalobos FJ. Identification of a putative Mexican strain of Serratia entomophila pathogenic against root-damaging larvae of Scarabaeidae (Coleoptera) Appl Environ Microbiol. 2008;74:802–810. doi: 10.1128/AEM.01074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan M, Gerard EM. Establishment of Serratia entomophila in soil from a granular formulation. N Z Plant Prot. 2005;58:122–125. [Google Scholar]
- Oliveira CM, Auad AM, Mendes SM, Frizzas MR. Crop losses and the economic impact of insect pests on Brazilian agriculture. Crop Prot. 2014;56:50–54. doi: 10.1016/j.cropro.2013.10.022. [DOI] [Google Scholar]
- Pan-UK (2003) Current pesticide spectrum, global use and major concerns. http://www.pan-uk.org/ briefing/SIDA_Fil/Chap1.htm
- Peralta C, Palma L. Is the insect world overcoming the efficacy of Bacillus thuringiensis? Toxins. 2017;9:39. doi: 10.3390/toxins9010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesticides Safety Directorate (2008) Assessment of the impact on crop protection in the UK of the ‘cut-off criteria’ and substitution provisions in the proposed Regulation of the European Parliament and of the Council concerning the placing of plant protection products in the market. Pesticides Safety Directorate, UK, p 46
- Pigott CR, Ellar DJ. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol Rev. 2007;71:255–281. doi: 10.1128/MMBR.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcar M, Gómez F, Gruppe A, Gómez-Pajuelo A, Segura I, Schröder R. Hymenopteran specificity of Bacillus thuringiensis strain PS86Q. Biol Control. 2008;45:427–432. doi: 10.1016/j.biocontrol.2008.02.002. [DOI] [Google Scholar]
- Qureshi N, Chawla S, Likitvivatanavong S, Lee HL, Gill SS. The Cry toxin operon of Clostridium bifermentanssubsp. malaysia Is highly toxic to Aedes larval mosquitoes. Appl Environ Microbiol. 2014;80:5689–5697. doi: 10.1128/AEM.01139-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhika M, Kannahi M. Bioremediation of pesticide (Cypermethrin) using bacterial species in contaminated soil. Int J Curr Microbiol Appl Sci. 2014;3:427–435. [Google Scholar]
- Ragunath PK, Abhinand PA, Archanna K. Relevance of bioinformatics in biopesticide management: a comparative comprehensive review. Basic Appl Asp Biopestic. 2014 [Google Scholar]
- Raymond B, Johnston P, Nielsen-Leroux C, Lereclus D, Crickmore N. Bacillus thuringiensis: an impotent pathogen? Trends Microbiol. 2010;18:189–194. doi: 10.1016/j.tim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Roh JY, Choi JY, Li MS. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J Microbiol Biotechnol. 2007;17:547–559. [PubMed] [Google Scholar]
- Ruffner B, Péchy-Tarr M, Höfte M, Bloemberg G, Grunder J, Keel C, Maurhofer M. Evolutionary patchwork of an insecticidal toxin shared between plant-associated pseudomonads and the insect pathogens Photorhabdus and Xenorhabdus. BMC Genimics. 2015;16:609. doi: 10.1186/s12864-015-1763-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyed RZ, Patel PR. Biocontrol potential of siderophore producing heavy metal resistant Alcaligenes sp. and Pseudomonas aeruginosa RZS3 vis-à-vis organophosphorus fungicide. Ind J Microbiol. 2011;51:266–272. doi: 10.1007/s12088-011-0170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleena P, Lee HL, Lecadet MM. A novel insecticidal serotype of Clostridium bifermentans. J Am Mosq Control Assoc. 1997;13:395–397. [PubMed] [Google Scholar]
- Siegwart M, Graillot B, Blachere Lopez C, Besse S, Bardin M, Nicot PC, Lopez-Ferber M. Resistance to bio-insecticides or how to enhance their sustainability: a review. Front Plant Sci. 2015;6:381. doi: 10.3389/fpls.2015.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK (2007) Insecticidal methods of pest control. In: Singh DK, Trivedi TP (eds) Applied entomology. http://nsdl.niscair.res.in/jspui/handle/123456789/416
- Singh R, Singh P, Sharma R. Microorganism as a tool of bioremediation technology for cleaning environment: a review. Proc Int Acad Ecol Environ Sci. 2014;4:1–6. [Google Scholar]
- Smith R, Lanini WT, Gaskell M (2000) Weed management for organic crops. Vegetable Research and Information Center—University of California—Division of Agriculture and Natural Resources, Davis CA Publication, California, USA
- Soberón M, López-Díaz JA, Bravo A. Cyt toxins produced by Bacillus thuringiensis: a protein fold conserved in several pathogenic microorganisms. Peptides. 2012;41:87–93. doi: 10.1016/j.peptides.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Sparks TC, Nauen R. IRAC: Mode of action classification and insecticide resistance management. Pest Biochem Physiol. 2015;121:122–128. doi: 10.1016/j.pestbp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Sparks T, Dripps J, Watson G, Paroonagian D. Resistance and cross-resistance to the spinosyns—a review and analysis. Pestic Biochem Physiol. 2012;102:1–10. doi: 10.1016/j.pestbp.2011.11.004. [DOI] [Google Scholar]
- Starnes RL, Lui CL, Marone PG. History, use and future of microbial insecticides. Am Entomol. 1993;39:83–91. doi: 10.1093/ae/39.2.83. [DOI] [Google Scholar]
- Su C, Jiang L, Zhang W. A review on heavy metal contamination in the soil world wide: situation, impact and remediation techniques. Environ Skeptics Critics. 2014;3:24–38. [Google Scholar]
- Tayade S, Patel ZP, Mutkule DS, Kakde AM. Pesticide contamination in food: a review. IOSR J Agri Vet Sci. 2013;6:7–11. doi: 10.9790/2380-0610711. [DOI] [Google Scholar]
- Thacker, Jonathan RM (2002) An introduction to arthropod pest control. Cambridge University Press, Cambridge
- Townsend RJ, Ferguson CM, Proffitt JR, Slay MWA, Swaminathan J, Day S, Gerard E, O’Callaghan M, Johnson VW, Jackson TA. Establishment of Serratia entomophila after application of a new formulation for grass grub control. N Z Plant Prot. 2004;57:310–313. [Google Scholar]
- Verkerk RHJ, Wright DJ. Common cabbage resistance mechanisms against the diamondback moth: still an open book? Ann Appl Biol. 1996;128:571–573. doi: 10.1111/j.1744-7348.1996.tb07116.x. [DOI] [Google Scholar]
- Vimaia Devi PS, Ranga Rao GV, Gopalakrishnan S, Sivakumar G. Environmental impact of microbial pesticides. In: Dhillon MK, Sehrawat KL, editors. Sharma HC. Houston: Environmental Safety of Biotech and Conventional IPM Technologies, Studium Press LLC; 2012. pp. 261–272. [Google Scholar]
- Wang XJ, Wang JJ, Wang JD, Zhang J, Xu MD, Xiang WS. Two new doramectin analogs from Streptomyces avermitilis NEAU1069: fermentation, isolation and structure elucidation. J Antibiot. 2011;64:591–594. doi: 10.1038/ja.2011.48. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Zhang J, Wang JD, Huang SX, Chen YH, Liu CX, Xiang WS. Four new doramectin congeners with acaricidal and insecticidal activity from Streptomyces avermitilis NEAU1069. Chem Biodivers. 2011;8:2117–2125. doi: 10.1002/cbdv.201000295. [DOI] [PubMed] [Google Scholar]
- Whalon ME, Mota-Sanchez D, Hollingworth RM, et al. (2008) The MSU arthropod pesticide resistance database. http://www.pesticideresistance.org