Abstract
Mannanases, one of the important enzyme group for industry, are produced by numerous filamentous fungi, especially Aspergillus species with different fermentation methods. The aim of this study was to show the best fermentation method of β-mannanase production for fungal growth in fermenter. Therefore, different fermentation strategies in fed-batch fermentation (suspended, immobilized cell, biofilm and microparticle-enhanced bioreactor) were applied for β-mannanase production from glucose medium (GM) and carob extract medium (CEM) by using recombinant Aspergillus sojae. The highest β-mannanase activities were obtained from microparticle-enhanced bioreactor strategy. It was found to be 347.47 U/mL by adding 10 g/L of Al2O3 to GM and 439.13 U/mL by adding 1 g/L of talcum into CEM. The maximum β-mannanase activities for suspended, immobilization, and biofilm reactor remained at 72.55 U/mL in GM, 148.81 U/mL in CEM, and 194.09 U/mL in GM, respectively. The reason for that is the excessive, and irregular shaped growth and bulk formation, inadequate oxygen transfer or substrate diffusion in bioreactor. Consequently, the enzyme activity was significantly enhanced by addition of microparticles compared to other fed-batch fermentation strategies. Also, repeatable β-mannanase activities were obtained by controlling of the cell morphology by adding microparticle inside the fermenter.
Keywords: Suspended cells, Immobilized cells, Biofilm reactor, Microparticles, β-Mannanase, Fed-batch fermentation
Introduction
Microbial enzymes are commonly utilized in many industries such as textile, detergent, leather, beverage, cosmetics, medicine, food, etc., due to their catalytic activity, stability, and easier production and optimization of production conditions according to animal and plant enzymes. Endo-1,4-β-mannanases (1,4-β-d-mannan mannohydrolase; 3.2.1.78) are hydrolyzed β-1,4-d-mannosidic linkages in mannans and heteromannans such as galactomannans, glucomannans, and galactoglucomannans. Also, the microbial enzyme applications are increasing day by day due to decreased processing time, low energy input, lower cost, eco-friendly properties, and non-toxicity (Singh et al. 2016).
β-mannanases are widely distributed in the nature and produced by a vast variety of bacteria, actinomycetes, yeasts, and fungi (Chauhan et al. 2012). Various Aspergillus species such as Aspergillus fumigatus (Puchart et al. 2004), Aspergillus sojae (Duruksu et al. 2009), Aspergillus terrus (Soni et al. 2016) are well known as the best mannanase producers. The genus Aspergillus is a considerable industrial fungus for large-scale production of numerous enzymes (Ozturk et al. 2010) and also used in food fermentation industry for production of fermented foods (de Vries 2003), especially with A. sojae that is considered as safe (Duruksu et al. 2009; Ozturk et al. 2010). In previous studies, A. fumigatus IMI 385708 has been reported to be a β-mannanase producer. However, due to its pathogenic nature, β-mannanase produced by A. fumigatus is not used in food industry (Puchart et al. 2004). Therefore, Duruksu et al. (2009) isolated the region encoding the protein of the β-mannanase gene from A. fumigatus and expressed in A. sojae (ATCC 11906). Additionally, the optimization of production conditions of β-mannanase by using recombinant A. sojae (ATCC 11906) in shake flask fermentation with molasses was also performed by Ozturk et al. (2010).
In the literature, the production of β-mannanase has been performed using cheaper alternative substrate sources such as sugar beet molasses (Ozturk et al. 2010), palm seed, orange seed, apple peel, corn cob, wheat straw, potato peel, mango peel, and rice husk (Mabrouk and El Ahwany 2008) instead of pure carbon sources for cost-effective production. Besides, the carob pod used in this study was also utilized a low-cost alternative carbon source for mannanase production (Yatmaz et al. 2016) due to its high carbohydrate content, which consisted of 50–65% sugars of 62–67% of total soluble solids (Oziyci et al. 2014).
In order to increase enzyme activity in fermenter, the batch, fed-batch and continuous fermentations can be utilized. In general, fed-batch fermentation system is applied to overcome substrate inhibition or catabolite repression by intermittent feeding of substrate. Besides, fed-batch fermentation ensures higher cell concentration and product formation in bioreactor in comparison to batch fermentation. (Shulter and Kargi 2000). The cultivation of filamentous microorganisms in bioreactor led to some problems such as clumpy, excessive, and irregular shaped growth (although they are natural forms of filamentous fungi) and inadequate oxygen transfer or substrate diffusion. Moreover, the batch processes have some disadvantages such as limitation of operating time, re-inoculation requirement for each fermentation, time loss due to cleaning and sterilization for each fermentation, substrate or product inhibition as well as low resistance against share force. All those things cause low fermentation efficiency, weakness of cell viability or stability, high recovery cost, low production yield and productivity (Bai et al. 2008; Coban et al. 2015a; Germec et al. 2015). To overcome such problems, different fermentation methods such as immobilization (El-Naggar et al. 2006), biofilm formation using support materials (Ercan and Demirci 2015a; Germec et al. 2015), and the usage of microparticles such as aluminum oxide (Al2O3), magnesium silicate (Talcum, 3MgO·4SiO2·H2O), and titanium silicate oxide (Titanate, TiSiO4) have been studied (Coban et al. 2015a; Yatmaz et al. 2016). Immobilization processes such as biofilm, are natural forms of cell immobilization or passive immobilization, can be applied in reactors. At the same time, they are called as microbial cell layers, which are attached on the support materials. Due to high biomass concentration, stability, and potential of long-term fermentation of cells attached on the surface materials, biofilm reactors can be used for production of value-added products such as human lysozyme (Ercan and Demirci 2015b), ethanol (Germec et al. 2015, 2016), lactic acid (Ho et al. 1997), kojic acid (Liu et al. 2016b), isoflavone aglycones (Liu et al. 2016a), etc. Nonetheless, microparticles used as a novel approach to improve morphology engineering of filamentous microorganisms and increase their productivity are also applied. In addition, the reduction of pellet size up to single hyphal growth during fermentation is a significant issue for the more product formation. Also, it ensures more easily diffusion of substrate within the fungal aggregates, as well as an effective agitation in the bioreactors. Therefore, microparticles can ensure more precise control of fungal morphology in bioreactor by eliminating bulk fungal growth (Walisko et al. 2012).
The main objectives of this study were not only to use different fermentation strategies in bioreactor in order to obtain higher enzyme activity and reproducible process, but also to realize cost-effective β-mannanase production from carob pod extract by using recombinant A. sojae.
Materials and methods
Microorganism and inoculum preparation
The recombinant A. sojae ATCC 11906 (A. sojae transformant 1: AsT1) was obtained from Prof. Dr. Z.B. OGEL laboratory (Middle East Technical University, Ankara, Turkey). The stock culture was grown on potato dextrose agar (PDA) at 30 °C for 4–5 days, maintained at 4 °C and renewed monthly. Besides, spore suspensions were prepared by pouring the 15 mL of sterile solution of 0.85% NaCI and 0.05% Tween-80 on top of stock cultures on the PDA agar plates and the mixture containing spores of AsT1 in 15-mL sterile falcon tubes. The number of spores in the mixture was counted in a microscope using Thoma slide (Ozturk et al. 2010). The resulting suspension solution has about 1.0 × 107 spores per mL.
Plastic composite support (PCS)
PCS materials containing 50% (w/w) agricultural materials and 50% (w/w) polypropylene were used to be solid supports in biofilm reactors. The agricultural products (Table 1) were also ensured essential nutrients to continue cell growth at the surface of PCS (Ho et al. 1997). PCS materials were produced at Iowa State University (Ames, IA) by high-temperature extrusion in a Brabender PL2000 co-rotating twin-screw extruder (Model CTSE-V, CW Brabender Instruments, Inc., South Hackensack, NJ, USA) as described by Ho et al. (1997).
Table 1.
Composition of plastic composite supports used in this study
| PCS | Minor agricultural material(s) (w/w) |
|---|---|
| SH–SF–YE–S | 5% soybean flour–5% yeast extract |
| SH–SF–YE–BA–S | 5% soybean flour–5% yeast extract–5% bovine albumin |
| SH–SF–S | 10% soybean flour |
| SH–SF–YE–RBC–S | 5% soybean flour–5% yeast extract–5% red blood cells |
SH ground 20 mesh and vacuum-dried soybean hulls (Cargill Soy Processing Plant, Iowa Falls, IA, USA), SF vacuum-dried soybean flour (Archer Daniel Midland Co, Decatur, IL, USA), YE yeast extract (Ardamine-Z, Champlain Industries, Chifton, NJ, USA), BA dried bovine albumin (American Protein Corporation, Ames, IA, USA), RBC red blood cells (American Protein Corp., Ames, IA, USA), S salts
Carob pod extraction
Broken and seedless carob pods were obtained from local sources in Antalya, Turkey. They were stored at 4 °C until extraction. In order to extract the sugars, carob pod extractions were performed at 80 °C for 2 h using 25% (w/v) solid loading with water in a water bath (Turhan et al. 2010).
Immobilization
Immobilization of AsT1 (~1×107 spores) was carried out as follows: suspended AsT1 spores were transferred into 1 L of 0.85% sterile NaCI solution and centrifuged at 3800 rpm and 4 °C for 25 min. After the liquid phase was removed, AsT1 spores were aseptically mixed with 20 mL of 2.5% alginate solution and well mixed. Then, the mixture was placed in a sterile 50 mL syringe (with 16G 1½ needle) and slowly dropped into a sterile 0.05 M CaCI2 solution, which was continuously agitated. AsT1 spores were allowed to harden for 12 h after the formation of beads. The CaCI2 solution was then aseptically removed from alginate beads, which were washed with 500 mL of sterile 0.85% NaCI solution to remove non-adherent cells and residual CaCI2. Afterwards, the irrigating solution was removed and beads were inoculated in a stirred tank bioreactor (Lee et al. 2011).
Biofilm formation
PCS selection
Before the production of β-mannanase in fed-batch biofilm reactor with GM and CEM, four different PCS discs (Table 1) were used to determine the best PCS material in terms of the production of β-mannanase. For this purpose, they were used for biofilm formation in test tube fermentation. For test tube fermentation, 2.9 g of the PCS discs were placed in 25 × 150 mm screw-cap culture tubes and autoclaved at 121 °C for 30 min in deionized water for surface sterilization of PCS discs. After cooling, 10 mL of sterile medium was added. The tubes were incubated at 30 °C for 24 h, and then decanted to remove the sloughing-off excess particles from the surface of PCS during incubation. After adding 10 mL of fresh medium, the tubes were inoculated with 0.1 mL (1%, v/v) of spore suspensions of AsT1 and incubated at 30 °C. Every other day, the culture medium was decanted aseptically and 10 mL of sterile medium was added for seven transfers. At the end of the seventh transfer, the spent culture broth of each tubes were analyzed for β-mannanase activity (Demirci et al. 1997).
Constructed biofilm reactor with selected PCS
For the biofilm reactor, 12 PCS materials (65 mm long and 10.5 mm width) were bound to the agitator shaft in a grid-like fashion, with six rows of two parallel tubes. The PCS bioreactor was autoclaved at 121 °C for 1 h in 3-L deionized water. After autoclaving and cooling down to the room temperature, water was pumped out from the reactor with a peristaltic pump, then 3-L sterile medium was added aseptically into the sterile biofilm reactor vessel. After the bioreactor was incubated at 30 °C for overnight to check sterility, the initial leachate solution was replaced with fresh sterile fermentation medium. Prepared inoculum (~1.0 × 107 spores) was used to inoculate the reactor at 1% (v/v) ratio (Demirci et al. 1997). Fermentation conditions for biofilm formation were adjusted to 30 °C, pH 5.0, 200 rpm (Ozturk et al. 2010), and 1 vvm aeration. The pH was controlled by automatic addition of 4N NaOH. Seven repeated-batch fermentations were performed to establish biofilm on selected PCS materials (Demirci et al. 1997). After biofilm formation at the end of the seventh repeated-batch fermentation, β-mannanase production was carried out in fed-batch biofilm reactor with GM and CEM.
Microparticles and preparation of microparticles
In order to test the effect of different microparticles on enzyme production by using fungal microorganisms, aluminum oxide (≥64% Al2O3 powder, Sigma-Aldrich, Seelze, Germany), magnesium silicate (talcum with ~10 µm particle size, 3MgO·4SiO2·H2O, Sigma-Aldrich, Seelze, Germany), and titanium silicate oxide (titanate, TiSiO4 powder, Sigma-Aldrich, Seelze, Germany) were added into the fermentation media prior to inoculation. Before adding into the medium, the microparticles were prepared in 100 mM citrate buffer (pH 6.5), 50 mM Na-acetate buffer (pH 6.5), and 50 mM Na-acetate buffer (pH 6.5), respectively. In our preliminary experiments, the effect of different microparticle concentrations (0–25 g/L) on β-mannanase production was evaluated and determined that the best results were with 1 g/L of talcum, 10 g/L of aluminum oxide, and 25 g/L of titanate (data not shown). Therefore, microparticle concentrations applied in this study were 1 g/L for talc powder, 10 g/L for Al2O3, and 25 g/L for titanate. After autoclaving at 121 °C for 20 min, they were aseptically added into the medium prior to inoculation (Kaup et al. 2008).
Fed-batch fermentations
Fed-batch fermentations for β-mannanase production were performed in a Sartorius Biostat B-Plus bioreactor with twin configuration (Sartorius Stedim, Biostat B-Plus, Göttingen, Germany) equipped with a 2-L vessel with 1.5-L working volume, but 5-L vessel with 3-L working volume for biofilm reactor (Single Sartorius Stedim, Biostat B-Plus Bioreactor, Göttingen, Germany). The base-line fermentation medium contained 4% glucose, 0.4% yeast extract, 0.05% MgSO4·7H2O, and 0.1% K2HPO4 per liter of deionized water (Ozturk et al. 2010; Yatmaz et al. 2016). Nevertheless, to produce β-mannanase from CEM, 4ºBx CEM, which is equal to basal medium sugar content, was used in placed of glucose. Then the reactors were autoclaved at 121 °C for 30 min (for biofilm reactor, sterilization time was 1 h) and were inoculated with 1% (v/v) (~1.0 × 107 spores) of prepared inoculum after cooling. The reactors were run at 30 °C, 200 rpm, pH 5.0 (Ozturk et al. 2010), and 1.0 vvm aeration. Fed-batch fermentations were carried out to study the effect of GM and CEM on β-mannanase production. Based on the reduction of sugar concentration (to 1.0 g/L sugar level) and the level of dissolved oxygen (to 30% level), glucose and carob extract solutions were aseptically and gradually added into the fermentation media at specified times with residual sugar analysis. The pH was automatically controlled with 4 N NaOH. Nonetheless, as long as micelles excessive grow and dissolved oxygen concentration decreases (to 30% level), the agitation speed was increased up to 600 rpm by automatic control system of bioreactor. Samples were collected from the reactors every 24 h for 5–12 days and immediately feeding and analyzed for β-mannanase activity and residual sugar concentration.
Experimental design
Four different production profiles of β-mannanase in fed-batch fermentation were, respectively, evaluated as follows:
β-mannanase production in suspended cell fed-batch bioreactor with GM and CEM.
Effect of immobilization on β-mannanase production in fed-batch bioreactor with GM and CEM.
β-mannanase production in fed-batch PCS biofilm reactor with GM and CEM.
β-mannanase production in microparticle-enhanced fed-batch bioreactor with GM and CEM.
Analysis
The collected samples were analyzed for β-mannanase activity and residual sugar concentration in the fermentation broth.
Enzyme activity
To determined β-mannanase activity, 0.2 mL of cell-free fermentation broth and 1.8 mL of 0.05% (w/v) locust bean gum solution (prepared in 0.1 M Na-citrate buffer, pH 6.0) as substrate were mixed and incubated at 50 °C for 5 min. After incubation, 3 mL of DNS (3,5-dinitrosalicylic acid) was added the test tubes immediately, and the mixture was heated at 90 °C for 15 min to stop activity and to accelerate the reaction for the color change and was swiftly cooled. Deionized water instead of enzyme solution used in analysis was used as a blank (Bailey et al. 1992). Absorbance values measured at 540 nm by using a spectrophotometer (ThermoScientific Evolution, Shanghai, China) were converted to β-mannanase activity using the generated standard curve equations (Eqs. 1 and 2).
| 1 |
| 2 |
where Y is the mannose concentration (µmol/mL), , DF is dilution factor, and t is the time (min). One unit of enzyme was defined as the amount of enzyme releasing reducing sugars equivalent to 1 µmol d-mannose at 50 °C per min (Ozturk et al. 2010).
Residual sugar
In order to determine the residual sugar concentration in fermentation broth, 3,5-dinitrosalicylic acid (DNSA) method was used (Miller 1959). Two calibration curves for the spectrophotometric measurements at 575 nm were obtained using a sucrose and glucose solution. Deionized water was used as a negative control. Then, absorbance values measured at 575 nm were converted to residual sugar concentration by using the sucrose standard curve for carob extract (Eq. 3) and glucose standard curve (Eq. 4).
| 3 |
| 4 |
Kinetic parameters
After measuring the residual sugar concentration and β-mannanase activity in fermentation broth of each sample, the kinetic parameters such as β-mannanase activity (U/mL), maximum sugar consumption rate (Q S, g/L/h) and maximum β-mannanase production rate (Q P, U/mL/h) were calculated.
Statistical analysis
The data were evaluated by using SAS statistical software (SAS Institute INC., Carry, NC, USA). Duncan’s multiple comparison test was used at a significance level, p < 0.05.
Results and discussion
β-mannanase productions in fed-batch bioreactors with GM and CEM were performed. For this purpose, suspended-cell, immobilized-cell, PCS biofilm reactor, and microparticle-enhanced fed-batch fermentations were implemented.
Mannanase production in suspended cell fed-batch bioreactor
β-mannanase productions were performed in suspended cell fed-batch bioreactor with GM and CEM. The kinetic parameters for suspended cell fed-batch fermentations are given in Table 2. During the fed-batch fermentation, glucose was added into the medium at 134, 148 and 175 h of fermentation when the glucose concentration decreased to 1.0 g/L. Although, sugar consumption was quite well by AsT1 (data not shown), β-mannanase activity was very low, which resulted in 72.55 U/mL (Table 2). On the other hand, Q P and Q S were calculated to be 2.33 U/mL/h and 0.61 g/L/h, respectively.
Table 2.
The kinetic parameters of different fermentation strategies from glucose and carob extract mediums
| Different fermentation strategies | Carbon sources | Kinetic parameters | ||
|---|---|---|---|---|
| P (U/mL) | Q S (g/L/h) | Q P (U/mL/h) | ||
| Suspended cells | Glucose | 72.55f ± 1.77 | 0.61bc ± 0.04 | 2.33cd ± 0.06 |
| Carob extract | 50.89f ± 3.82 | 1.76a ± 0.20 | 1.06ef ± 0.03 | |
| Immobilized cells | Glucose | 57.51f ± 3.85 | 0.17d ± 0.01 | 0.83f ± 0.03 |
| Carob extract | 148.81d ± 11.27 | 0.12d ± 0.01 | 2.43cd ± 0.10 | |
| Biofilm reactor | Glucose | 194.09c ± 11.43 | 0.41c ± 0.05 | 1.42def ± 0.08 |
| Carob extract | 139.41d ± 11.20 | 0.16d ± 0.02 | 1.03ef ± 0.04 | |
| Microparticle addition | ||||
| 1 g/L talcum | Glucose | 107.68e ± 10.52 | 0.65b ± 0.05 | 2.02de ± 0.10 |
| Carob extract | 439.13a ± 11.48 | 0.16d ± 0.04 | 8.69a ± 1.00 | |
| 10 g/L Al2O3 | Glucose | 347.47b ± 11.43 | 0.73b ± 0.04 | 6.06b ± 0.25 |
| Carob extract | 215.24c ± 10.84 | 0.14d ± 0.04 | 3.39c ± 0.39 | |
| 25 g/L titanate | Glucose | 68.01f ± 2.13 | 0.52bc ± 0.02 | 1.47def ± 0.13 |
| Carob extract | 331.13b ± 9.73 | 0.12d ± 0.02 | 3.22c ± 0.22 | |
Values are given as the mean ± standard deviation of two replicates. Different letters in the same column indicate statistically significance between mean values (p < 0.05)
4ºBx CEM was used for β-mannanase production in suspended cell fed-batch bioreactor. During the fed-batch fermentation, carob extract was added into the medium at 85 and 96 h of fermentation when the sugar concentration in carob extract reduced to 1.0 g/L. According to results, β-mannanase activity, Q S, and Q P were obtained to be 50.89 U/mL, 1.76 g/L/h, and 1.06 U/mL/h, respectively (Table 2). While the sugars in carob extract was quite well consumed by AsT1 (data not shown), β-mannanase production was fairly low due to insufficient substrate diffusion and reduced surface area of molds based on the excessive growth of molds in fed-batch bioreactor with GM and CEM (Fig. 1). On the other hand, the β-mannanase activity produced in fed-batch bioreactor with CEM was slightly lower than GM while the Q P in CEM was approximately 2.2-fold lower than GM. On the contrary, the Q S in CEM was approximately 2.89-fold higher than GM. Accordingly, β-mannanase activities in GM and CEM were not statistically different (p > 0.05). However, the Q P in CEM was statistically significant while the Q S in GM was statistically important (p < 0.05) (Table 2). In conclusion, since high initial sugar concentrations caused excessive growth of molds, fermentation conditions were not suitable for β-mannanase production (Fig. 2). Therefore, fed-batch fermentations were performed with suitable initial sugar content by performing preliminary experiments prior to each fermentation (data not shown). Accordingly, several fermentations were carried out by using 1 or 4ºBx sugar content for future fermentations.
Fig. 1.

The excessive growth of AsT1 in the fermentation medium
Fig. 2.
a Diagram of the biofilm reactor. b Biofilm formation at the end of the 7th repeated-batch fermentations. c The image of biofilms attached on the surface of selected PCS material at the end of the fed-batch fermentation in biofilm reactor
Up to now, β-mannanase productions in submerged fermentations such as shake flask, batch, and fed-batch were carried out. For instance, Ozturk et al. (2010) optimized the production conditions of β-mannanase in shake flasks with sugar beet molasses by using AsT1. The maximum β-mannanase activity was 482 U/mL which is the higher than this study presented in bioreactor. The main reason for that scale-up processes need to be more optimized. Furthermore, Blibech et al. (2011) studied β-mannanase production in shake flask, batch, and fed-batch bioreactors with acacia seeds by using Penicillium occitanis. They reported that the maximum β-mannanase activity was 7.9, 41.1, and 76.1 U/mL, respectively. Wang et al. (2010) produced the β-mannanase in shake flasks with konjac powder by using Pantoena agglomerans A021 and reported that the maximum β-mannanase activity of purified Man26P was 514 U/mg. It has also been reported that the β-mannanase activity was found to be 2195.5 U/mL in fed-batch bioreactor with corn steep liquor dextrose medium by recombinant Pichia pastoris (Zheng et al. 2012). Großwindhager et al. (1999) studied the production of endo-β-1,4-d-mannanase by Sclerotium rolfsii under de-repressed conditions and reported that the activity reached 462 U/mL in fed-batch fermentation compared to batch fermentation (240 U/mL). Additionally, Roth et al. (2009) optimized the endo-β-1,4-mannanase production by recombinant Yarrowia lipolytica in fed-batch fermentation and reported that fed-batch fermentation resulted in 3.9-fold increase in endo-β-1,4-mannanase activity in comparison to batch fermentation, and maximum activity was 1.57 U/mL.
Mannanase production in immobilized-cell fed-batch bioreactor
In order to determine the effect of cell immobilization on the production of β-mannanase, AsT1 was immobilized in Ca-alginate and the β-mannanase was produced in immobilized cell fed-batch bioreactor with GM and CEM. The results are indicated in Table 2. The growth of AsT1 within the fermentation media was inhibited as a result of immobilized mold spores in Ca-alginate. Therefore, the sugar in the media was inadequately consumed by immobilized AsT1. Accordingly, the highest β-mannanase activity was measured as 57.51 U/mL (Table 2). Although the substrate was added into the medium, the growth of molds was very low and consequently, the low β-mannanase activity was produced. In conclusion, it was determined that the immobilization of molds in Ca-alginate was not efficient for β-mannanase production.
β-mannanase was produced in fed-batch bioreactor with CEM by using immobilized AsT1 spores in Ca-alginate. During the fermentation, sugars in carob extract were swiftly utilized by the immobilized AsT1 spores, but β-mannanase was not produced for the first 72 h (data not shown). However, when CEM was added into the fermentation media at 96 and 120 h of fermentation, immobilized AsT1 spores began β-mannanase production. The maximum β-mannanase activity (148.81 U/mL) was produced at the end of the fermentation (168 h). In conclusion, although the most of sugar was consumed by the immobilized AsT1, the growth of molds was very low and hence the β-mannanase activity was not further increased. According to results, β-mannanase production in fed-batch bioreactor with CEM was better (p < 0.05). The β-mannanase activity in CEM was ~2.59-fold higher than GM. On the other hand, although the Q S in CEM was relatively lower than the Q S in GM (p > 0.05), the Q P in CEM was considerably higher than GM, ~2.93-fold (p < 0.05) (Table 2).
El-Naggar et al. (2006) investigated the β-mannanase production from coconut fibers by the immobilized A. niger in alginate. β-mannanase activity was ~3.7-fold lower with immobilization process compared to suspended cell culture. In another study, β-mannanase activity was increased fourfold by using alkaliphilic Bacillus halodurans immobilized in Na-alginate in the presence of 1% defatted copra meal and 0.5% peptone or feather hydrolysate at pH 11 and 40 °C (Vijayalaxmi et al. 2013). In conclusion, CEM can be utilized as an alternative carbon source for β-mannanase production using immobilized AsT1 spores. β-mannanase activity was 2.92-fold further than suspended cells. However, β-mannanase activity in GM with immobilized AsT1 was ~1.26-fold lower than suspended cells. On the other hand, β-mannanase activity in immobilized cell fed-batch bioreactor with CEM was ~2.59-fold higher than suspended cell fed-batch bioreactor with GM. Accordingly, β-mannanase production in immobilized cell fed-batch bioreactor with CEM was more advantageous with regard to GM in terms of both high β-mannanase activity and low cost.
Mannanase production in fed-batch biofilm reactor
Firstly, in order to produce the β-mannanase in fed-batch PCS biofilm reactor with GM and CEM, the best PCS material was selected among four different PCS materials. When the PCSs were used in the culture tube repeated-batch fermentation media (Table 1), the β-mannanase activities were determined as 260.29 U/mL with the use of SH-SF-YE-RBC-S, 215.16 U/mL with the use of SH-SF-YE-BA-S, 161.01 U/mL with the use of SH-SF-S, and 84.33 U/mL with the use of SH-SF-YE-S. Therefore, the highest activity was obtained with the use of SH-SF-YE-RBC-S and was statistically significant (p < 0.05). This result was consistent with those obtained by Cheng et al. (2009). Nonetheless, SH-SF-YE-BA-S was selected as the best biofilm support material for ethanol production from carob extract (Germec et al. 2015) and lysozyme production from lactose (Ercan and Demirci 2013) in biofilm reactor, respectively, which consists of soybean hull, soybean flour, yeast extract, bovine albumin, and salt in trace amount. After selecting the best biofilm support material, a biofilm reactor was set up for β-mannanase production (Fig. 2a).
β-mannanase production in fed-batch PCS biofilm reactor with GM was carried out after forming the biofilms on the selected support material (Fig. 2b). During the fermentation, GM was added into vessel at 96 and 168 h of fermentation. The highest β-mannanase activity was 194.09 U/mL at the end of the fermentation (216 h). On the other hand, the Q S and Q P kinetic parameters were also calculated to be 0.41 g/L/h and 1.42 U/mL/h, respectively (Table 2).
Also, β-mannanase in the same fed-batch PCS biofilm reactor with CEM was produced. During the fermentation, CEM was added into the fermentation media at 120 and 192 h of fermentation. The maximum enzyme activity was 139.41 U/mL at 144 h of fermentation. Also, the Q S and Q P values were computed to be 0.16 g/L/h and 1.03 U/mL/h, respectively (Table 2).
Further comparison was also performed in terms of statistical significant level, p < 0.05 (Table 2). Results shown that β-mannanase production and Q S in GM were statistically significant (p < 0.05), but the Q P did not (p > 0.05). The kinetic parameters belong to β-mannanase production in fed-batch PCS biofilm reactor with GM were relatively higher than CEM (Table 2). However, since the molds attached on the surface of biofilm support material, β-mannanase activities in GM and CEM were low at the end of the fed-batch fermentations in biofilm reactor. However, it was a positive result, such that the β-mannanase activities in GM and CEM were higher than the results of suspended cell fed-batch fermentations. On the other hand, due to decreasing of the surface area contacting with substrate of the molds in fed-batch PCS biofilm reactor (Fig. 2c), the diffusion of substrate to microorganism was considerably restricted. Accordingly, this drawback led to low β-mannanase activity. Consequently, it was found that β-mannanase production in fed-batch PCS biofilm reactor can be further investigated.
In the literature, β-mannanase production in fed-batch PCS biofilm reactor by using AsT1 was not performed yet. However, some similar studies related to enzyme production in biofilm reactor have been performed. For instance, Ercan and Demirci (2014) aimed to enhance the human lysozyme production by Kluyveromyces lactis K7 in PCS biofilm reactor with lactose medium. It was reported that the human lysozyme production by K. lactis K7 was increased to 173 U/mL in PCS biofilm reactor, which is about 57% improvement compared to the suspended cell fermentation. In another study of same researchers, human lysozyme was produced in fed-batch PCS biofilm reactor with GM and reported that its production was increased from 173 U/mL (batch fermentation) to 187 U/mL (Ercan and Demirci 2015a). Additionally, Aspergillus oryzae kojic acid production was performed in PCS biofilm reactor via repeated-batch fermentation (Liu et al. 2016b). The efficiency of immobilized culture and the effect of A. oryzae morphology on kojic acid production was also evaluated in PCS biofilm reactor. In repeated-batch PCS bioreactor, 83.47 g/L of kojic acid was produced in nitrogen-deficient media, which is approximate twofold higher than the suspended cell batch fermentation. A. oryzae morphology was converted in the form of feather-like mycelium with increasing kojic acid production. In our study, β-mannanase production in fed-batch PCS biofilm reactor with GM and CEM was increased from 72.55 and 50.89 U/mL to 194.09 and 139.41 U/mL compared to suspended cell fed-batch fermentation, respectively (Table 2).
Mannanase production in microparticle-enhanced fed-batch bioreactor
Different microparticles (talcum, aluminum oxide, and titanate) were used to improve β-mannanase production in fed-batch bioreactor with GM and CEM.
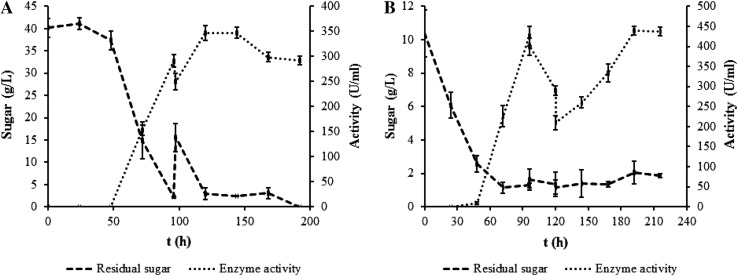
1 g/L of talcum was added to increase the β-mannanase production in microparticle-enhanced fed-batch bioreactor with GM and CEM. It provided a smaller pellet structure, more homogenous medium (Fig. 3), and better substrate diffusion into the molds. During the fermentation, the addition of glucose was carried out at 96, 120, 144, and 168 h of fermentation while the addition of CEM was performed at 96 and 120 h of fermentation. The results indicated that the highest β-mannanase activities were measured as 107.68 U/mL at the end of the fermentation with GM (216 h) and 439.13 U/mL at 192 h of fermentation with CEM, respectively (Table 2). On the other hand, β-mannanase production and Q P in talcum-enhanced fed-batch fermentation with CEM were ~4.08- and 4.30-fold higher than GM, respectively (p < 0.05). However, the Q S in GM was 4.06-fold further compared with that obtained from CEM (p < 0.05).
Fig. 3.
Single hyphae formation in the form of beads by addition of microparticles in the fermentation media
With the addition of 10 g/L of aluminum oxide into the GM and CEM, the excessive growth of molds was hindered (Fig. 3) and hence β-mannanase activity was significantly increased compared to suspended cell fed-batch fermentation, immobilized cell fed-batch fermentation, and fed-batch PCS biofilm reactor. During the fermentation, GM was added at 96, 120, and 168 h of fermentation into the medium. It was realized at 96 and 120 h of fermentation for CEM. Maximum β-mannanase activities were found to be 347.47 U/mL at 216 h of fermentation using GM; 215.24 U/mL at 144 h of fermentation using CEM. On the other hand, the Q S values were calculated to be 0.73 and 0.14 g/L/h in aluminum oxide-enhanced fed-batch bioreactor with GM and CEM, respectively. Similarly, the Q P values were also computed as 6.06 and 3.39 U/mL/h, respectively (Table 2). β-mannanase activity, Q S, and Q P in GM were ~1.61-, 5.21-, and 1.79-fold higher than CEM, respectively (p < 0.05).
To improve the β-mannanase production and surface area of the molds and to facilitate the nutrient transfer in the molds (Fig. 3), 25 g/L of titanate was added in fed-batch bioreactor system with GM and CEM. The addition of glucose into the fermentation media was performed at 96, 120 and 144 h of fermentation and the highest β-mannanase activity was 68.02 U/mL at 144 h of fermentation. β-mannanase production was very low due to low glucose consumption by molds. In conclusion, the glucose addition did not increase the β-mannanase production in titanate-enhanced fed-batch fermentation. For the fermentation with CEM, the addition of carob extract was carried out at 96 and 129 h of fermentation and maximum β-mannanase activity was 331.13 U/mL at the end of the fermentation (216 h). Therefore, the amount of enzyme-produced in CEM was ~4.87-fold higher compared to GM (p < 0.05). Besides, the other kinetic parameters were also estimated and compared (Table 2). The Q P in CEM was ~2.19-fold further than GM (p < 0.05) while the Q S in CEM was ~4.33-fold lower (p < 0.05). In conclusion, β-mannanase activity was continuously increased with the addition of CEM into the fermentation media, but fermentation time was quite long due to slow consumption by molds of sugar when titanate was used into the media (data not shown).
In the literature, there is a study with related to β-mannanase production in microparticle-enhanced shake flask fermentation. Yatmaz et al. (2016) performed the β-mannanase production in shake flask fermentation with GM and CEM by adding talcum and aluminum oxide as microparticles into the media. It was reported that the highest β-mannanase activity was found to be 514 U/mL by adding 1 g/L of aluminum oxide into the GM while the maximum β-mannanase titer was measured to be 568.7 U/mL with the addition of 5 g/L of talcum into the CEM. For comparison, they also produced β-mannanase in the absence of microparticle in the mediums, which were yielded as 199.3 U/mL in GM and 310.5 U/mL in CEM, respectively. However, β-mannanase production in microparticle-enhanced fed-batch fermentation with GM and CEM in bioreactor has not been performed yet. On the other hand, several studies related to production of various metabolites (especially enzymes) in microparticle-enhanced fermentation system were carried out. For instance, fructofuranosidase and glucoamylase activities were increased to 150 U/mL (3.7-fold) and 190 U/mL (9.5-fold) with the addition of 25 g/L of titanate compared to control, respectively (Driouch et al. 2012). Moreover, Coban et al. (2015a) performed the production of fungal phytase in microparticle-enhanced fed-batch bioreactor. The highest phytase activities were found as 9.6 U/mL with the addition of 15 g/L of talcum while it was 4.9 U/mL without microparticle. Similarly, it indicated that the lactic acid concentration increased from 6.02 to 13.88 and 24.01 when 15 g/L of aluminum oxide and 10 g/L of talcum was utilized in the shake flask fermentation, respectively. Also, the lactic acid concentration further enhanced to 75.1 g/L with the addition of 10 g/L of talcum in batch fermentation (Coban and Demirci 2016). Additionally, Yang et al. (2016) controlled the fungal morphology and improved the curvulamine production with the addition of talc powder under submerged fermentation and reported that by supplementation of 5 g/L of talcum, the curvulamine production was increase about 1.9-fold in shake flask fermentation and increased about 3.3-fold in 5-L bioreactor. In addition, the effect of different concentrations of talcum and Al2O3 on A. ficuum phytase production in submerged fermentation was studied. The phytase activity was increased from 1.02 U/mL (control fermentation) to 2.93 and 2.01 U/mL with the addition of 15 g/L of talcum and Al2O3 microparticles into the media, respectively. Then, the activity was increased from 3.45 U/mL within 120 h of fermentation (control) to 6.49 U/mL within 96 h of fermentation by addition of 15 g/L of talcum in bioreactor (Coban et al. 2015b). In conclusion, β-mannanase activities in GM and CEM were significantly increased with microparticle-enhanced fed-batch fermentation strategy (Table 2; Fig. 4a, b) since the bulk fungal growth was prevented (Fig. 3). Also, since the enzyme release and nutrient transfer are increased by addition of different microparticles, β-mannanase activity in microparticle-enhanced fed-batch bioreactor was significantly increased in comparison with suspended cell fed-batch, immobilized cell fed-batch, and fed-batch PCS biofilm reactor systems (Table 2).
Fig. 4.
Plots belong to highest mannanase productions from carob extract and glucose mediums via fed-batch fermentations. a The highest mannanase production plot produced from carob extract medium with the addition of 1 g/L talcum. b The highest mannanase production plot produced from glucose medium with the addition of 10 g/L Al2O3
Comparison of different fed-batch fermentation strategies
The comparison of different fed-batch fermentation strategies in terms of β-mannanase production was discussed. β-mannanase activities in immobilized cell fed-batch bioreactor with GM and CEM were 57.51 U/mL (~1.26-fold lower) and 148.81 U/mL (~2.92-fold higher) when compared to suspended cell fed-batch fermentation system, respectively. Similarly, in fed-batch PCS biofilm reactor with GM and CEM, β-mannanase activities were higher than suspended cell fed-batch fermentation system, which were 194.09 and 139.41 U/mL (~2.68- and 2.74-fold higher, respectively). In addition, the maximum enzyme activities in microparticle-enhanced fed-batch fermentation (439.13 and 347.47 U/mL) were obtained when 1 g/L of talcum and 10 g/L of Al2O3 were added into the CEM and GM, respectively (Fig. 4a, b; Table 2). Besides, with the addition of 1 g/L of talcum, 10 g/L of Al2O3, and 25 g/L of titanate into the CEM, the enzyme activities were 439.13, 215.24, and 331.13 U/mL, which were ~8.63-, 4.23-, and 6.51-fold higher than suspended cell fed-batch fermentation, respectively. Also, β-mannanase activities with the addition of 1 g/L of talcum and 10 g/L of Al2O3 into the GM were 107.68 and 347.47 U/mL, which resulted in ~1.48- and 4.79-fold higher enzyme activities compared to suspended cell fed-batch fermentation, respectively. But, with the addition of 25 g/L of titanate, β-mannanase activity in GM was slightly lower than control (Table 2). In conclusion, it indicated that the microparticle-enhanced fed-batch fermentation was determined as the best fermentation technique among the other fed-batch fermentation strategies. Besides, it also revealed that the microbial growth homogenously dispersed in the microparticle-enhanced fermentation medium (Fig. 3), whereas big pellets occurred in the media without microparticles (Fig. 1). However, as enhancing the formation of single hypha or as long as increasing the surface area of microorganism with the addition of microparticles into the media (Fig. 3), the diffusion of substrate and release of enzyme-produced were significantly increased according to suspended cell, immobilized cell, and biofilm reactor systems, and hence enzyme activity was significantly increased (p < 0.05) (Table 2).
Conclusions
In this study, the effect of different fed-batch fermentation strategies on β-mannanase production was investigated. It was understood that suspended cell and immobilized cell fed-batch bioreactors with GM and CEM were not suitable for β-mannanase production by AsT1 due to excessive mold growth, slow substrate diffusion and product releasing, and decreasing surface area of molds. Also, β-mannanase production in fed-batch PCS biofilm reactor can be further investigated. However, β-mannanase activities in immobilization and biofilm reactor systems resulted in higher than suspended cell fed-batch system. Besides, when the microparticles were added into the media, bulk fungal growth was prevented and the homogeneity in media was ensured compared to the other fed-batch fermentations, which led to higher β-mannanase activities (347.47 and 439.13 U/mL). Overall, this study indicated that microparticle addition into fermenter can be successfully applied to increase β-mannanase production and to control fungal morphology in fed-batch bioreactor. Also, the more repeatable results by addition of microparticles into the medium were obtained due to a more homogenous fermentation media, a better agitation and aeration, no excessive cell growth, further nutrient transfer since increasing surface area of microorganism. In addition, for cost-effective β-mannanase production CEM was an alternative substrate source to GM.
Acknowledgements
This study was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) foundation (Grant No. #112O167).
Compliance with ethical standards
Conflict of interest
All the authors in this study mutually agree for submitting our manuscript to 3 Biotech and declare that they have no conflict of interest in the publication.
References
- Bai F, Anderson W, Moo-Young M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol Adv. 2008;26(1):89–105. doi: 10.1016/j.biotechadv.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23(3):257–270. doi: 10.1016/0168-1656(92)90074-J. [DOI] [Google Scholar]
- Blibech M, Ellouz Ghorbel R, Chaari F, Dammak I, Bhiri F, Neifar M, Ellouz Chaabouni S. Improved mannanase production from Penicillium occitanis by fed-batch fermentation using acacia seeds. ISRN Microbiol. 2011 doi: 10.5402/2011/938347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan PS, Puri N, Sharma P, Gupta N. Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl Microbiol Biotechnol. 2012;93(5):1817–1830. doi: 10.1007/s00253-012-3887-5. [DOI] [PubMed] [Google Scholar]
- Cheng K-C, Catchmark JM, Demirci A. Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J Biol Eng. 2009;3(1):12. doi: 10.1186/1754-1611-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban HB, Demirci A. Enhancement and modeling of microparticle-added Rhizopus oryzae lactic acid production. Bioprocess Biosyst Eng. 2016;39(2):323–330. doi: 10.1007/s00449-015-1518-0. [DOI] [PubMed] [Google Scholar]
- Coban HB, Demirci A, Turhan I. Enhanced Aspergillus ficuum phytase production in fed-batch and continuous fermentations in the presence of talcum microparticles. Bioprocess Biosyst Eng. 2015;38(8):1431–1436. doi: 10.1007/s00449-015-1384-9. [DOI] [PubMed] [Google Scholar]
- Coban HB, Demirci A, Turhan I. Microparticle-enhanced Aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess Biosyst Eng. 2015;38(6):1075–1080. doi: 10.1007/s00449-014-1349-4. [DOI] [PubMed] [Google Scholar]
- de Vries R. Regulation of Aspergillus genes encoding plant cell wall polysaccharide-degrading enzymes; relevance for industrial production. Appl Microbiol Biotechnol. 2003;61(1):10–20. doi: 10.1007/s00253-002-1171-9. [DOI] [PubMed] [Google Scholar]
- Demirci A, Pometto A, III, Ho KG. Ethanol production by Saccharomyces cerevisiae in biofilm reactors. J Ind Microbiol Biotechnol. 1997;19(4):299–304. doi: 10.1038/sj.jim.2900464. [DOI] [PubMed] [Google Scholar]
- Driouch H, Hänsch R, Wucherpfennig T, Krull R, Wittmann C. Improved enzyme production by bio-pellets of Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng. 2012;109(2):462–471. doi: 10.1002/bit.23313. [DOI] [PubMed] [Google Scholar]
- Duruksu G, Ozturk B, Biely P, Bakir U, Ogel ZB. Cloning, expression and characterization of endo-β-1,4-mannanase from Aspergillus fumigatus in Aspergillus sojae and Pichia pastoris. Biotechnol Prog. 2009;25(1):271–276. doi: 10.1002/btpr.104. [DOI] [PubMed] [Google Scholar]
- El-Naggar MY, El-Aassar S, Youssef AS, El-Sersy NA, Beltagy E. Extracellular β-mannanase production by the immobilization of the locally isolated Aspergillus niger. Int J Agric Biol. 2006;8:57–62. [Google Scholar]
- Ercan D, Demirci A. Production of human lysozyme in biofilm reactor and optimization of growth parameters of Kluyveromyces lactis K7. Appl Microbiol Biotechnol. 2013;97(14):6211–6221. doi: 10.1007/s00253-013-4944-4. [DOI] [PubMed] [Google Scholar]
- Ercan D, Demirci A. Enhanced human lysozyme production in biofilm reactor by Kluyveromyces lactis K7. Biochem Eng J. 2014;92:2–8. doi: 10.1016/j.bej.2014.04.013. [DOI] [Google Scholar]
- Ercan D, Demirci A. Effects of fed-batch and continuous fermentations on human lysozyme production by Kluyveromyces lactis K7 in biofilm reactors. Bioprocess Biosyst Eng. 2015;38(12):2461–2468. doi: 10.1007/s00449-015-1483-7. [DOI] [PubMed] [Google Scholar]
- Ercan D, Demirci A. Enhanced human lysozyme production by Kluyveromyces lactis K7 in biofilm reactor coupled with online recovery system. Biochem Eng J. 2015;98:68–74. doi: 10.1016/j.bej.2015.02.032. [DOI] [Google Scholar]
- Germec M, Turhan I, Karhan M, Demirci A. Ethanol production via repeated-batch fermentation from carob pod extract by using Saccharomyces cerevisiae in biofilm reactor. Fuel. 2015;161:304–311. doi: 10.1016/j.fuel.2015.08.060. [DOI] [Google Scholar]
- Germec M, Turhan I, Demirci A, Karhan M. Effect of media sterilization and enrichment on ethanol production from carob extract in a biofilm reactor. Energy Sources Part A Recovery Util Environ Effects. 2016;38(21):3268–3272. doi: 10.1080/15567036.2015.1138004. [DOI] [Google Scholar]
- Großwindhager C, Sachslehner A, Nidetzky B, Haltrich D. Endo-β-1, 4-d-mannanase is efficiently produced by Sclerotium (Athelia) rolfsii under derepressed conditions. J Biotechnol. 1999;67(2):189–203. doi: 10.1016/S0168-1656(98)00176-X. [DOI] [Google Scholar]
- Ho K, Pometto AL, Hinz PN, Dickson JS, Demirci A. Ingredient selection for plastic composite supports for l-(+)-lactic acid biofilm fermentation by Lactobacillus casei subsp. rhamnosus. Appl Environ Microbiol. 1997;63(7):2516–2523. doi: 10.1128/aem.63.7.2516-2523.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup BA, Ehrich K, Pescheck M, Schrader J. Microparticle-enhanced cultivation of filamentous microorganisms: increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol Bioeng. 2008;99(3):491–498. doi: 10.1002/bit.21713. [DOI] [PubMed] [Google Scholar]
- Lee KH, Choi IS, Kim Y-G, Yang D-J, Bae H-J. Enhanced production of bioethanol and ultrastructural characteristics of reused Saccharomyces cerevisiae immobilized calcium alginate beads. Bioresour Technol. 2011;102(17):8191–8198. doi: 10.1016/j.biortech.2011.06.063. [DOI] [PubMed] [Google Scholar]
- Liu CT, Erh MH, Lin SP, Lo KY, Chen KI, Cheng KC. Enrichment of two isoflavone aglycones in black soymilk by Rhizopus oligosporus NTU 5 in a plastic composite support bioreactor. J Sci Food Agric. 2016;96(11):3779–3786. doi: 10.1002/jsfa.7569. [DOI] [PubMed] [Google Scholar]
- Liu JM, Yu TC, Lin SP, Hsu RJ, Hsu KD, Cheng KC. Evaluation of kojic acid production in a repeated-batch PCS biofilm reactor. J Biotechnol. 2016;218:41–48. doi: 10.1016/j.jbiotec.2015.11.023. [DOI] [PubMed] [Google Scholar]
- Mabrouk ME, El Ahwany AM. Production of 946-mannanase by Bacillus amyloliquifaciens 10A1 cultured on potato peels. Afr J Biotechnol. 2008 [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Oziyci HR, Tetik N, Turhan I, Yatmaz E, Ucgun K, Akgul H, Gubbuk H, Karhan M. Mineral composition of pods and seeds of wild and grafted carob (Ceratonia siliqua L.) fruits. Sci Hortic. 2014;167:149–152. doi: 10.1016/j.scienta.2014.01.005. [DOI] [Google Scholar]
- Ozturk B, Cekmecelioglu D, Ogel ZB. Optimal conditions for enhanced β-mannanase production by recombinant Aspergillus sojae. J Mol Catal B Enzym. 2010;64(3):135–139. doi: 10.1016/j.molcatb.2010.02.009. [DOI] [Google Scholar]
- Puchart V, Vršanská M, Svoboda P, Pohl J, Ögel ZB, Biely P. Purification and characterization of two forms of endo-β-1, 4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708 (formerly Thermomyces lanuginosus IMI 158749) Biochim Biophys Acta (BBA) Gen Subj. 2004;1674(3):239–250. doi: 10.1016/j.bbagen.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Roth R, Moodley V, van Zyl P. Heterologous expression and optimized production of an Aspergillus aculeatus endo-1, 4-β-mannanase in Yarrowia lipolytica. Mol Biotechnol. 2009;43(2):112–120. doi: 10.1007/s12033-009-9187-3. [DOI] [PubMed] [Google Scholar]
- Shulter M, Kargi F. Bioprocess engineering basic concept. New Delhi: Parentice-Hall of India Pvt Ltd; 2000. [Google Scholar]
- Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3 Biotech. 2016;6(2):174. doi: 10.1007/s13205-016-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni H, Rawat HK, Pletschke BI, Kango N. Purification and characterization of β-mannanase from Aspergillus terreus and its applicability in depolymerization of mannans and saccharification of lignocellulosic biomass. 3 Biotech. 2016;6(2):1–11. doi: 10.1007/s13205-016-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turhan I, Bialka KL, Demirci A, Karhan M. Ethanol production from carob extract by using Saccharomyces cerevisiae. Bioresour Technol. 2010;101(14):5290–5296. doi: 10.1016/j.biortech.2010.01.146. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi S, Prakash P, Jayalakshmi S, Mulimani V, Sreeramulu K. Production of extremely alkaliphilic, halotolerent, detergent, and thermostable mannanase by the free and immobilized cells of Bacillus halodurans PPKS-2. Purification and characterization. Appl Biochem Biotechnol. 2013;171(2):382–395. doi: 10.1007/s12010-013-0333-9. [DOI] [PubMed] [Google Scholar]
- Walisko R, Krull R, Schrader J, Wittmann C. Microparticle based morphology engineering of filamentous microorganisms for industrial bio-production. Biotechnol Lett. 2012;34(11):1975–1982. doi: 10.1007/s10529-012-0997-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Shao Z, Hong Y, Li C, Fu X, Liu Z. A novel β-mannanase from Pantoea agglomerans A021: gene cloning, expression, purification and characterization. World J Microbiol Biotechnol. 2010;26(10):1777–1784. doi: 10.1007/s11274-010-0358-y. [DOI] [Google Scholar]
- Yang J, Jiao R-H, Yao L-Y, Han W-B, Lu Y-H, Tan R-X. Control of fungal morphology for improved production of a novel antimicrobial alkaloid by marine-derived fungus Curvularia sp. IFB-Z10 under submerged fermentation. Process Biochem. 2016;51(2):185–194. doi: 10.1016/j.procbio.2015.11.025. [DOI] [Google Scholar]
- Yatmaz E, Karahalil E, Germec M, Ilgin M, Turhan I. Controlling filamentous fungi morphology with microparticles to enhanced β-mannanase production. Bioprocess Biosyst Eng. 2016 doi: 10.1007/s00449-016-1615-8. [DOI] [PubMed] [Google Scholar]
- Zheng J, Zhao W, Guo N, Lin F, Tian J, Wu L, Zhou H. Development of an industrial medium and a novel fed-batch strategy for high-level expression of recombinant β-mananase by Pichia pastoris. Bioresour Technol. 2012;118:257–264. doi: 10.1016/j.biortech.2012.05.065. [DOI] [PubMed] [Google Scholar]