Abstract
Thermophilic enzymes have many potential benefits in industrial production with increased flexibility related to process configurations. A thermostable β-glucosidase from Thermotoga naphthophila RUK-10 was found to possess catalytic activity for cellobiose hydrolysis with a high potential for application in biomass conversion. The aggregation of cellobiose often has an inhibitory effect on cellobiohydrolases and endoglucanases during cellulose hydrolysis. The presence of β-glucosidases has a significant effect on reducing inhibition from hydrolytic products by hydrolysing the intermedia cellobiose. In this study, β-glucosidase TN0602 exhibited a high tolerance to glucose and high thermostability even after a long incubation (>72 h). Additionally, supplementing β-glucosidase TN0602 with microcrystalline cellulose, untreated corn straw and steam-exploded corn straw hydrolysis reactions containing a commercial cellulase led to an increased conversion rate in released glucose compared to hydrolysis without the addition of β-glucosidase (15.82, 30.62 and 35.21%, respectively); the increase of conversion rates were 61.86, 93.50 and 94.55%. It was thus shown that an obvious synergistic effect exists between TN0602 and cellulases for cellulose hydrolysis, suggesting its potential as a component of enzymatic cocktails for the conversion of lignocellulosic biomass to other chemicals.
Keywords: Glucose tolerance, Cellulase, β-Glucosidase, Intermedia inhibition
Introduction
Lignocellulose is considered to be a renewable resource for biofuel production; it contributes to easing oil shortages (Wyman et al. 2005; Takashima et al. 1999; Li et al. 2014a). The current technology gap is the most crucial limiting factor in cellulose hydrolysis before fermentation (Tilbeurgh et al. 1986). However, hundreds of microorganisms exist that degrade plant cellulose in nature, thus laying the basis for cellulose hydrolysis. Among these microbes, Trichoderma reesei is the most commonly used strain and is the first choice for cellulase production (Saha and Bothast 1996). It has a high capacity for producing cellulases and degrades cellulose in the presence of insoluble cellulose, semicellulose and some plant biomasses (Janbon et al. 1995; Coughlan 1985).
Reese (1977) proposed the C1–Cx hypothesis to illustrate the mechanism of cellulose enzymatic hydrolysis. Cellulase is a multienzyme system, not a monomeric enzyme system (Sun and Cheng 2002). Cellulose hydrolysis involves the synergistic actions of three enzymes in the multienzyme system, acting in different ways, but coordinating to catalyse the same reactions (Li et al. 2014b). (1) Endoglucanases: endoglucanases act on amorphous regions in cellulose molecules and hydrolyse β-1,4-glucosidic bonds to produce short-chain cellulose, releasing abundant small molecules of cellulose. (2) Exoglucanases: exoglucanase cleaves long cellulose chains at the extremities and hydrolyses β-1,4-glucosidic bonds to release soluble cellobiose. (3) β-Glucosidases: β-glucosidases hydrolyse cellobiose and soluble cellulose oligosaccharides to produce glucose (Singh et al. 2016; Keerti et al. 2014).
β-Glucosidases belong to the hydrolases family that includes β-d-glucoside hydrolases. It is one of the most important components in the cellulose hydrolases family. β-Glucosidases hydrolyse nonreductive β-d-glucosidic bonds in cellobiose and liberate glucose and its relevant ligands (Meinke et al. 1995). This is also the rate-limiting step of the entire hydrolysis process. Therefore, β-glucosidases is a key factor that determines the efficiency of cellulose hydrolysis (Yang et al. 2015a).
The low activity and low content of β-glucosidases reduce the cellulose hydrolysis efficiency to a great extent (Jabbour et al. 2012). Preliminary studies have reported that cellobioses strongly inhibit β-glucosidase activity (Bothast and Saha 1997; Turan and Zheng 2005; Woodward and Wiseman 1982), which explains why the hydrolysis of celluloses is always inefficient. Because of the inhibition patterns of these saccharogenic products, the hydrolytic yields from lignocellulosic biomass to a monosaccharide are often improved by supplying enzymatic cocktail components to cellulases using high enzyme loading that is accurate per gram of cellulose as well as an excessive amount of β-glucosidase (Fang et al. 2010; Singh et al. 2016; Meleiroa et al. 2015; Sun and Cheng 2002).
Thermostable β-glucosidase offers potential advantages, especially in industrial processes, such as biorefining, where heat treatment of substrates is desirable, and has an excellent specific activity for reducing the supplementation of enzymes, higher temperature stability and improved pH flexibility related to process configurations (Bhat and Bhat 1997; Harnpicharnchai et al. 2009). Therefore, constructing engineered bacteria that can express β-glucosidases with high activity and glucose tolerance is of great significance in cellulose hydrolysis (Teeri et al. 1983; Sun and Cheng 2002). In our previous study, we cloned β-glucosidase TN0602 from Thermotoga naphthophila RKU-10 and overexpressed it in Escherichia coli BL21 (DE3), which has a high tolerance to glucose and high thermostability, even after a long incubation (Kong et al. 2015). The main purpose of this work is to investigate the synergistic effect between β-glucosidase TN0602 and commercial cellulase, which may provide a potential approach for improving the economy of the process and the cellulose hydrolysis efficiency with good prospects.
Materials and methods
Materials
Cellulase (Trichoderma reesei ATCC26921 Solid, purchased from Sigma-Aldrich, USA); thermostable β-glucosidase TN0602 (expressed and purified by Key Laboratory for Molecular Enzymology and Engineering in Jilin university); cellobiose (Shanghai Moclin Biotech Co., LTD.); microcrystalline cellulose (Shanghai Ruiyong Biotech Co., LTD.); BCA Assay Kit (Beijing Aidlab Biotech Co., LTD.); Glucose Assay Kit (Shanghai Rongsheng Biomed Co., LTD.).
Protein concentration and enzyme activity assay
The protein concentration was assayed according to the BCA method, using 5 mg/ml BSA as the standard. The absorbance was measured using a spectrophotometer at 540 nm.
One unit of enzyme activity was defined as the amount of enzyme required to release 1 μmol of glucose per minute at 50 °C and pH 4.5 under the assay conditions.
β-Glucosidase assay: for the determination of β-glucosidase TN0602 activity, crude enzyme was diluted with 100 mM Na2HPO4–citric acid buffer (pH 4.5). 50 μl of the dilution was added to 950 μl of 15 mmol/l cellobiose substrate and was incubated at 50 °C for 30 min. Then, the mixture was heated in a boiling water bath for 5 min and cooled quickly after heating. The amount of released glucose was determined by a standard glucose assay kit.
Effects of temperature and pH on cellulase and β-glucosidase TN0602
The effects of temperature on cellulase and β-glucosidase TN0602 were investigated using the same method described above. The hydrolysis efficiency was investigated in the range of 30–90 °C for different times until reaching 48 h. The release of reducing sugars was determined by a standard glucose assay kit at room temperature. The effects of pH on cellulase were investigated at different pH values ranging from 3.0 to 10.0. Half a gram of microcrystalline cellulose was dissolved in buffers with different pH levels. A mixture of 50 μl of the enzyme preparation and 950 μl of the prepared solution was incubated at 50 °C for 48 h. The amount of released reducing sugars was examined by a standard glucose assay kit. To determine the influence of pH on β-glucosidase TN0602, the same method as described above was used and the substrate was changed to cellobiose.
Glucose tolerance of β-glucosidase TN0602
Glucose tolerance of β-glucosidase TN0602 was measured at 50 °C in a 0.1 M Na2HPO4–citric acid buffer solution (pH 6.5). First, the diluted enzyme solution was incubated with a gluconate-δ-lactone solution at different concentrations for 5 min, followed by incubation with pNP-β-Glc solutions in the range of 0.125–1 mmol/l. The kinetic parameters were then measured under the absorbance at 420 nm.
Effect of the cellulase dosage and substrate concentrations on the hydrolysis of microcrystalline cellulose
A mixture of 40 ml of 5% microcrystalline cellulose and different additions of a cellulase solution from 0.125 to 0.875 U/ml was shaken in an incubator at 140 rpm, 50 °C, and pH 5.0. The amount of reducing sugars was assayed at seven time points.
To investigate the influence of the substrate concentrations on hydrolysis productivity, we designed a single factor test. The cellulase solution was added to substrate solutions at different concentrations ranging from 1 to 11% at pH 5.0. The glucose liberated during the reaction was quantified per 48 h under standard assay conditions.
Synergistic effect of β-glucosidase TN0602 and cellulase in the hydrolysis of microcrystalline cellulose and corn straw
To investigate the synergistic effect of β-glucosidase TN0602 and cellulase, the reaction was performed at 50 °C for 48 h using 5% microcrystalline cellulose, 0.1 M citric acid buffer at pH 5.0, and 0.75 U/ml of cellulase with different amounts of β-glucosidase (2.09 mg/ml) supplementation levels from 0 to 0.75 U/ml.
One gram of untreated corn straw and steam-exploded corn straw was hydrolysed in a 40-ml reaction system containing 0.1 M citrate buffer (pH 5.0) and 0.75 U/ml of cellulase (based on activity toward cellulose) with or without 0.5 U/ml of β-glucosidase TN0602 and was incubated at 50 °C for 48 h.
Results and discussion
Protein concentration and enzyme activity assay
The protein concentrations of cellulase and β-glucosidase TN0602 were 0.83 and 0.02 mg/ml, respectively. As shown in Table 1, the specific activity of β-glucosidase TN0602 was 42.64 U/mg, using cellobiose as a substrate. It is well accepted that cellulase always exhibits different activity towards different substrates. Our results are in accordance with this theory. The specific activity of cellulase was 0.15 U/mg for cellobiose and 0.08 U/mg for natural cellulose.
Table 1.
Protein concentration and enzyme activity assay of cellulase and β-glucosidase TN0602
| Entry | Enzyme | Total protein (mg) | Substrates | Total activity (IU) | Specific activity (IU/mg) |
|---|---|---|---|---|---|
| 1 | Cellulase | 0.83 | Cellobiose | 0.13 | 0.15 |
| Cellulose | 0.07 | 0.08 | |||
| 2 | β-Glucosidase TN0602 | 0.02 | Cellobiose | 2132.00 | 42.64 |
Effects of temperature and pH on cellulase and β-glucosidase TN0602
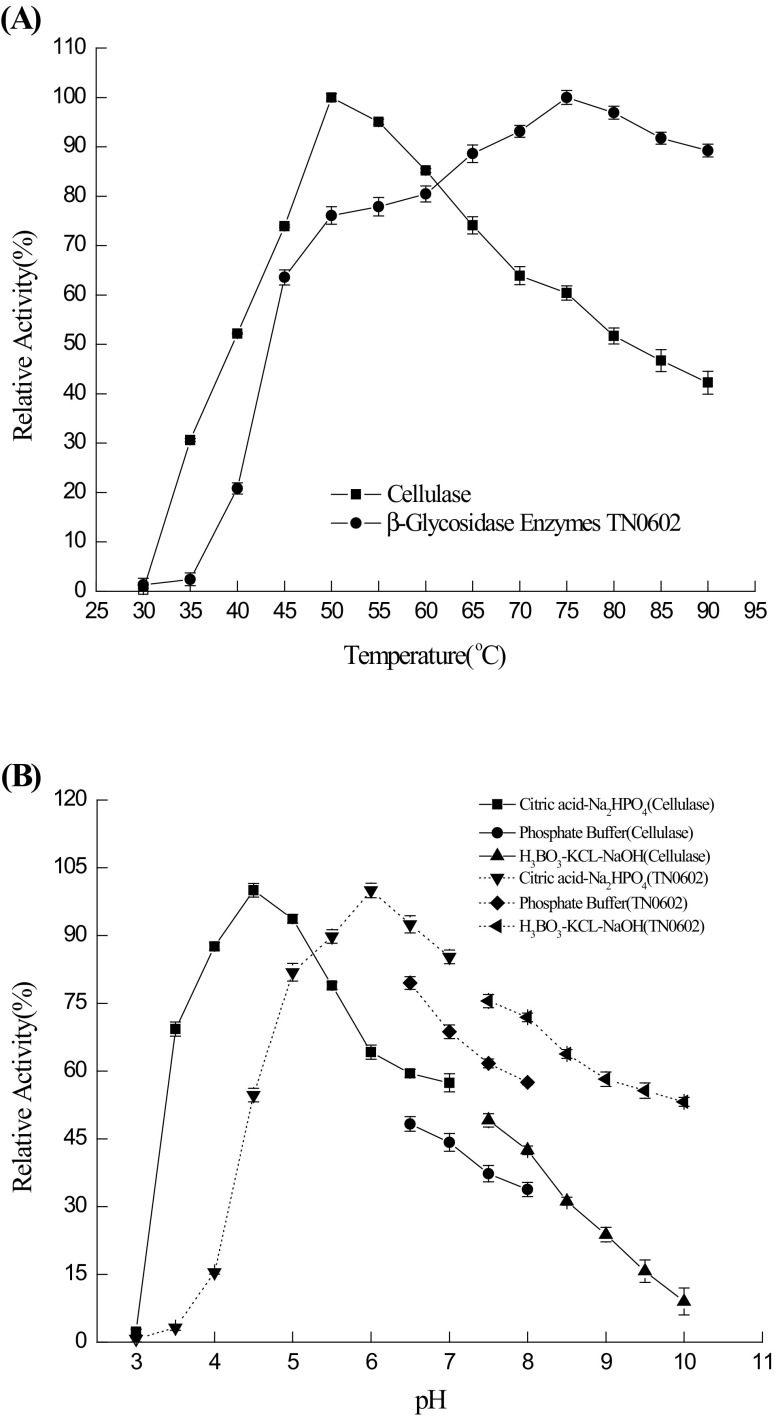
Kong et al. (2015) previously reported that each enzyme has different optimum temperatures for various substrates. Temperature changes have a strong influence on the affinity between enzymes and substrates or even the K m values of reactions (Zhang et al. 2010). Cellulase is highly sensitive to the change in temperature, and the maximum activity was achieved at 50 °C (Fig. 1a). Cellulase maintains half of its initial activity over the range of 40–80 °C. Furthermore, β-glucosidase TN0602 exhibited its maximum activity at 75 °C, and the maximum activity was achieved at K m values of reactions, in accordance with this theory.
Fig. 1.
Optimal temperature (a) and pH (b) of cellulase and β-glucosidase TN0602
It is well accepted that cellulase can only catalyse reactions in a certain pH range. To ensure that both cellulases and β-glucosidase maintain their high activity in the same solution, we investigated the optimum pH for these two enzymes (Fig. 1b). The optimum pH for cellulase was 4.5, while it was 6.0 for β-glucosidase TN0602. Both retained most of their activity at pH 5.0. Therefore, we regarded pH 5.0 to be the optimum pH for these two enzymes. Additionally, we found that the activity of β-glucosidase remained 55% at pH 4.5 and decreased slowly at pH levels ranging from 6.5 to 10.0, suggesting that TN0602 was relatively stable in neutral or slight alkali environments.
Glucono-δ-lactone and glucose tolerance of β-glucosidase TN0602
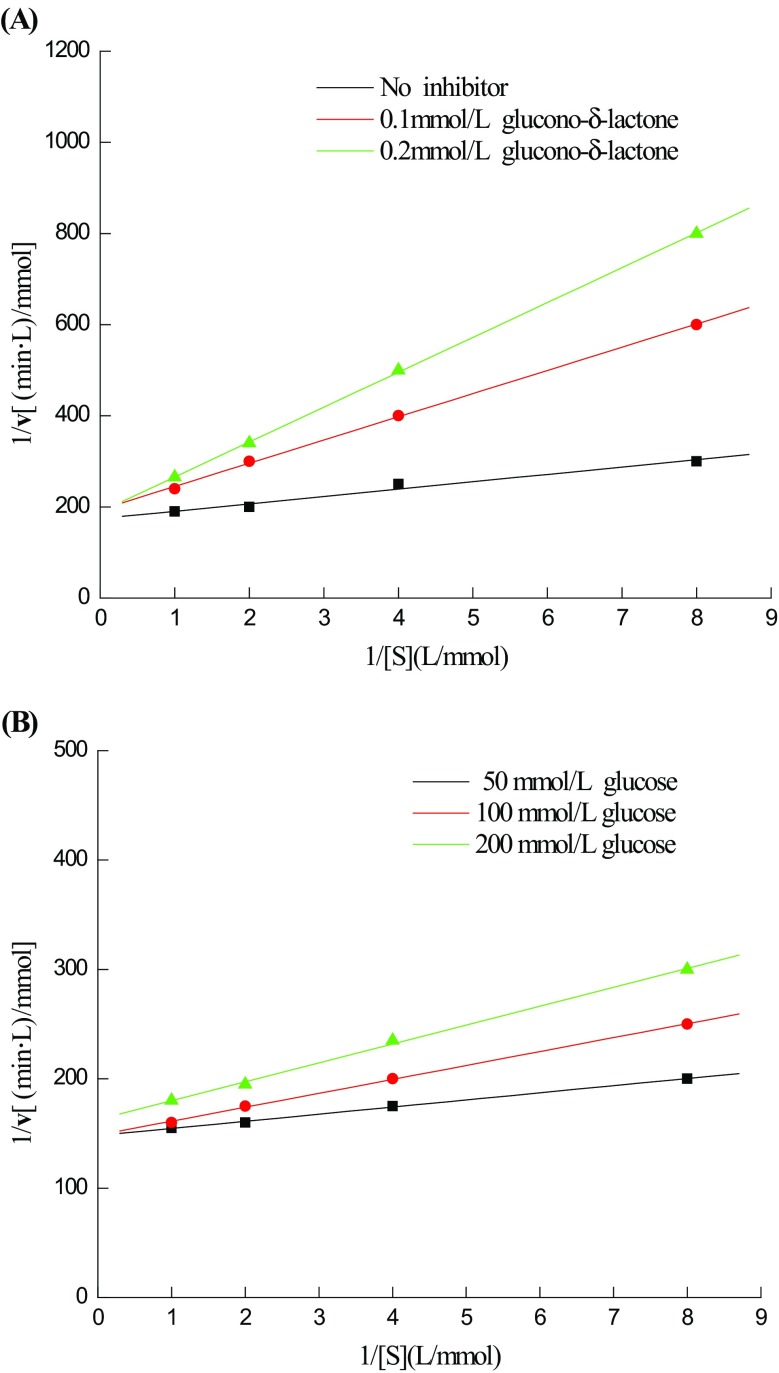
At present, the inhibition effect of the final products is the main obstacle for cellulases in industrial applications. When hydrolysing cellulose, the accumulation of cellobiose always inhibits the activity of endoglucanases and exoglucanases. Though β-glucosidases can hydrolyse cellobiose and overcome this limitation, the subsequently produced inhibition of glucose can make the entire system stop working. A previous study found that the low yield of β-glucosidases in T. reesei was the main obstacle to effectively hydrolysing celluloses (Guo et al. 2010). Thus, we mainly concentrated on the glucose tolerance of β-glucosidase TN0602. According to the Michaelis–Menten equation, the apparent K i value of glucono-δ-lactone was 0.03 mmol/l (Fig. 2a), indicating its strong inhibition of the activity of β-glucosidase TN0602 (Pei et al. 2015). As shown in Fig. 2b, the K i value of glucose was 75 mmol/L, suggesting that the released glucose had a minimal influence on β-glucosidase TN0602 activity. Moreover, Fang et al. (2010) expressed β-glucosidase bgl1A in marine microorganisms; in this study, when the concentration of glucose reached 400 mM, the activity of bgl1A began to be inhibited. In comparison with Fang’s research, we could easily conclude that β-glucosidase TN0602 showed excellent glucose tolerance.
Fig. 2.
Lineweaver–Burk plot of β-glucosidase TN0602 activities in the presence of glucono-δ-lactone (a) and glucose (b)
Effect of the cellulase dosage and substrate concentrations on hydrolysis
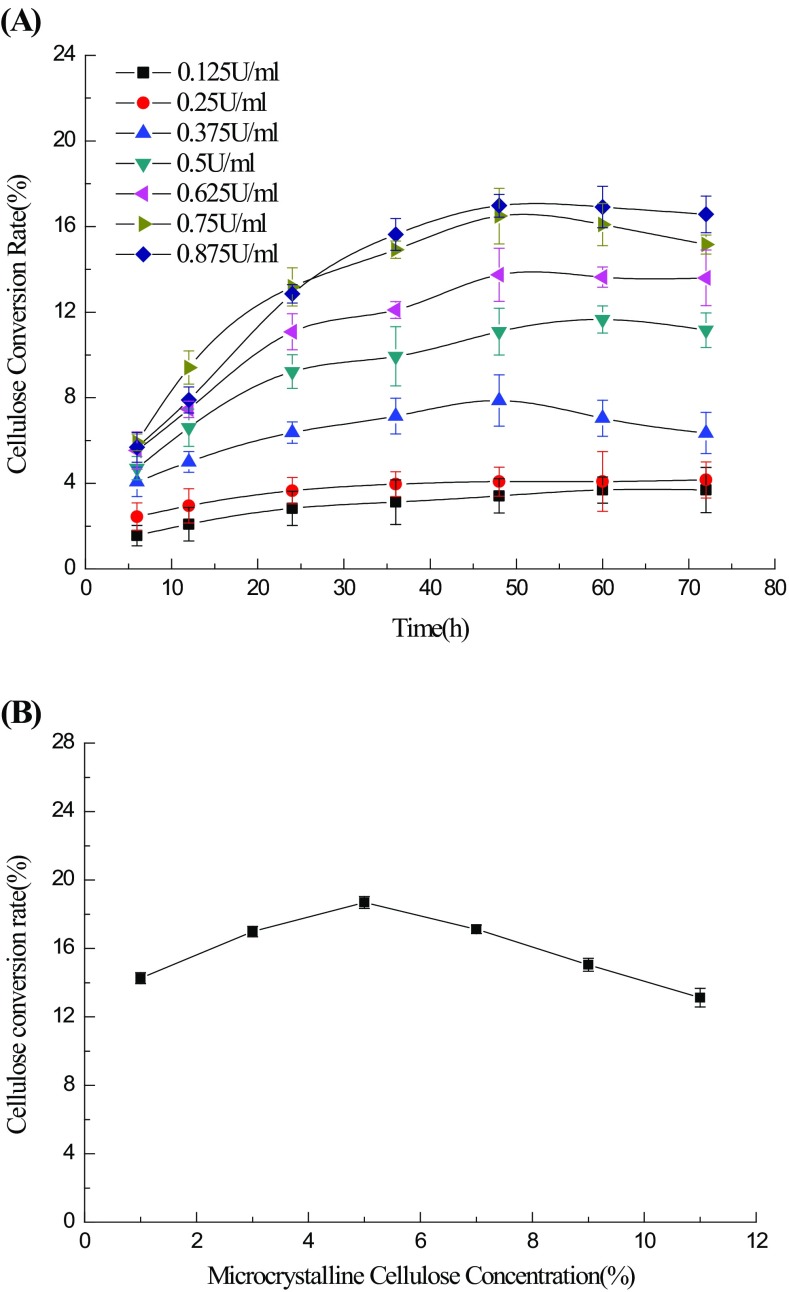
As mentioned above, the high cost of cellulase for cellulose degradation is considered to be a crucial limiting factor in industrial applications (Wyman 1994). There are two approaches for minimizing the cost: one is to reduce the productivity cost and increase the enzymatic activity, the other is to reduce the addition of cellulases by supplying accessory enzymes (Mullings 1985). Thus, determining the optimal additions of enzymes is the priority when hydrolysing cellulose. The results in Fig. 3a show that the amount of liberated glucose reached a peak with a 16.51% conversion rate after 48 h of incubation when the addition of cellulase was 0.75 U/ml. However, as the concentration of cellulase increased, the released glucose did not show significant improvement.
Fig. 3.
Effect of the enzyme dosage (a) and substrate concentrations (b) on the hydrolysis of microcrystalline cellulose
The substrate concentration is the main factor contributing to the efficiency of hydrolysis reactions. Over a certain range, a higher solid content could reduce the hydrolysis time and enhance the productivity of enzymatic saccharification (Yang et al. 2010; Cheung and Anderson 1997). Nevertheless, when the concentration reaches a certain point, the final glucose product inhibits hydrolytic processes (Ramos et al. 1993). In this study, we found, as shown in Fig. 3b, that cellulose had an obvious effect on enzymatic reactions over a range of concentrations; the highest conversion rate was obtained at a 5% substrate concentration. However, a slight decrease in productivity was also observed when the solid content was >5%; this result could be explained by product inhibition (Bothast and Saha 1997; Turan and Zheng 2005; Woodward and Wiseman 1982). This might result from the special structure of cellulose and efficient enzyme additions. When the initial substrate concentration was 5%, the cellulose conversion rate reached 18.68% after incubation for 48 h.
Synergistic effect of β-glucosidase TN0602 and cellulase
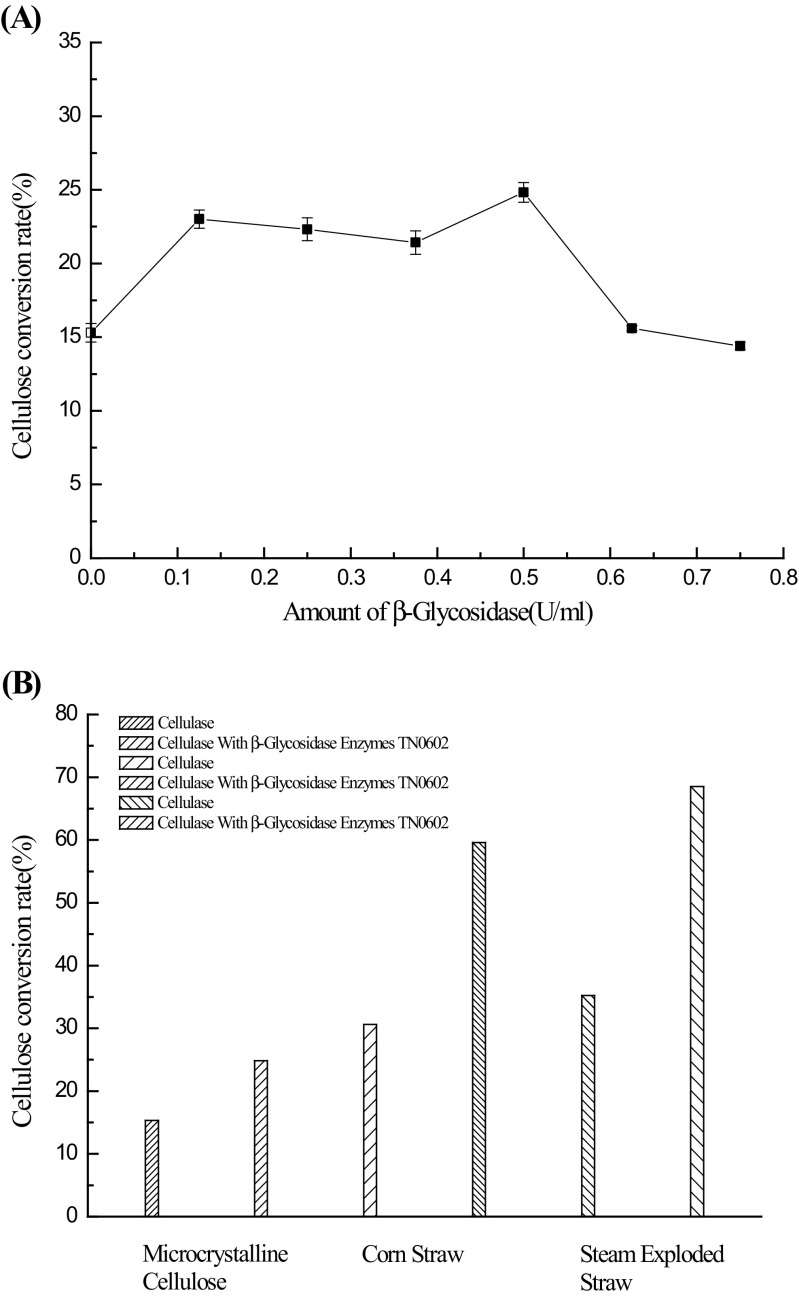
To identify whether β-glucosidase TN0602 affects cellulose saccharification and glucose production, the enzyme was supplemented into the hydrolysis reaction. As shown in Fig. 4a, supplementing TN0602 increased the yield of reducing sugar over an enzyme dosage of 0–0.75 U/ml; the increasing rate of cellulose conversion reached 24.83% when the addition of TN0602 was 0.5 U/ml. Then, an obvious drop in the conversion rate was observed when the enzyme dosage was >0.5 U/ml, which might be explained by a complex inhibition pattern (Gusakov and Sinitsyn 1992; Gan et al. 2003). A multienzyme cellulase system (e.g., cellulase and β-glucosidase) was used in a product inhibition study; however, nonlinear and different inhibition patterns (noncompetitive, competitive, or mixed type) might be observed. These inhibition patterns depended on the cellulase binding constant, enzyme concentration, maximum adsorption of the enzyme (cellulose surface area accessible to the enzyme), range in which substrate concentration is varied, and β-glucosidase activity.
Fig. 4.
Synergistic effect of β-glucosidase TN0602 and cellulase. a Effect of the enzyme dosage on the enzymatic hydrolysis of β-glucosidase TN0602. b Hydrolysis of corn straw
Corn straw is an abundant resource in nature; hydrolysis of these lignocellulosic biomasses is not only an important process in the conversion of agricultural residues to value-added products, but also takes advantage of the important components of sustainable development. It is commonly found that a low activity and minimal content of β-glucosidases reduce cellulose hydrolysis efficiency to a great extent. To solve this problem, β-glucosidase TN0602 with a high glucose tolerance was added to the hydrolysis reaction of untreated corn straw and steam-exploded corn straw to investigate the conversion rate of cellulose (Fig. 4b). When combined with a commercial cellulase, the hydrolysis of each substrate in the presence of TN0602 showed a greater efficiency for cellulose conversion compared with reactions without TN0602. The highest conversion rate (68.51%) was found when the steam-exploded straw was used as a substrate, up to a 94% increase in the conversion rate. It was superior in sugar conversion to previously reported conversion rates when supplementing β-glucosidases with cellulase to improve the biomass hydrolysis efficiency (Table 2), suggesting that β-glucosidase TN0602 could be an efficient component of enzymatic cocktails with cellulases in a complex lignocellulosic biomass.
Table 2.
Heterogeneously expressed β-glucosidases isolated from other organisms compared with TN0602 with an increasing conversion rate
| Source | T (°C) | pH | Increase of conversion rate (%) | References |
|---|---|---|---|---|
| Thermotoga naphthophila RUK-10 | 75 | 6 | 97 | This study |
| Thermoanaerobacterium aotearoense P8G3#4 | 60 | 6 | 20 | Yang et al. (2015b) |
| Aspergillus unguis NII-08123 | 60 | 6 | 20 | Rajasree et al. (2013) |
| Nasutitermes takasagoensis | 65 | 5.5 | 27 | Uchima et al. (2012) |
| Thermococcus sp. | 78 | 5–6.8 | 80 | Sinha and Datta (2016) |
| Periconia sp. | 70 | 5–6 | 70 | Harnpicharnchai et al. (2009) |
| Penicillium decumbens | 65–70 | 4.5–5.0 | 80 | Ma et al. (2011) and Chen et al. (2010) |
| Metagenomic library | 60 | 6 | 67 | Matsuzawa and Yaoi (2016) |
Conclusion
In conclusion, this is the first evidence that a β-glucosidase from thermophilic bacteria Thermotoga naphthophila RUK-10 not only has a great tolerance to glucose, but also contributes greatly to eliminating intermediate inhibition. The hydrolysis results indicated that the highly efficient synergistic effect can be achieved by combining cellulase and β-glucosidase TN0602. The conversion rate of glucose was 68.51% after 48 h of incubation, up to a 94% improvement compared with the reaction catalysed by cellulase only. Further studies on its potential for application as an accessory enzyme in industrial production for the hydrolysis of lignocellulosic biomasses are still in progress.
Acknowledgements
First author thanks Jilin Agriculture University, College of Life Science, Changchun and Jilin University, Key Laboratory for Molecular Enzymology and Engineering Ministry of Education for providing research facility. The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (nos. 20772046 and 21072075) and High-end technology innovation platform of straw comprehensive utilization of colleges and universities in Jilin province ((2014) C–1).
Compliances with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Contributor Information
Xiaoxiao Yu, Phone: +86 43185155212, Email: yx8751@sina.com.
Guang Chen, Phone: +86 43184532942, Email: chg61@163.com.
References
- Bhat MK, Bhat S. Cellulose degrading enzymes and their potential industrial applications. Biotechnol Adv. 1997;15(3–4):583–620. doi: 10.1016/S0734-9750(97)00006-2. [DOI] [PubMed] [Google Scholar]
- Bothast RJ, Saha BC. Ethanol production from agricultural biomass substrates. Adv Appl Microbiol. 1997;44(8):261–286. doi: 10.1016/S0065-2164(08)70464-7. [DOI] [Google Scholar]
- Chen M, Qin YQ, Liu ZY, Liu K, Wang FS, Qu YB. Isolation and characterization of a β-glucosidase from Penicillium decumbens and improving hydrolysis of corncob residue by using it as cellulase supplementation. Enzyme Microbiol Technol. 2010;46:444–449. doi: 10.1016/j.enzmictec.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Anderson BC. Laboratory investigation of ethanol production from municipal primary wastewater solids. Bioresour Technol. 1997;59(1):81–96. doi: 10.1016/S0960-8524(96)00109-5. [DOI] [Google Scholar]
- Coughlan MP. The properties of fungal and bacterial cellulases with comment on their production and application. Biotechnol Genet Eng Rev. 1985;3(1):39–110. doi: 10.1080/02648725.1985.10647809. [DOI] [Google Scholar]
- Fang ZM, Fang W, Liu JJ, Hong YZ, Peng H, Zhang XC, Sun BL, Xiao YZ. Cloning and characterization of a beta-glucosidase from marine microbial metagenome with excellent glucose tolerance. J Microbiol Biotechnol. 2010;20(9):1351–1358. doi: 10.4014/jmb.1003.03011. [DOI] [PubMed] [Google Scholar]
- Gan Q, Allen SJ, Taylorn G. Kinetic dynamics in heterogeneous enzymatic hydrolysis of cellulose: an overview, an experimental study and mathematical modelling. Process Biochem. 2003;38(7):1003–1018. doi: 10.1016/S0032-9592(02)00220-0. [DOI] [Google Scholar]
- Guo H, Bian YN, Liu R. Enzymatic characterization of a novel glucose-tolerant β-glucosidase from Agrobacterium tumefaciens. Microbiology China. 2010;37(9):1356–1361. [Google Scholar]
- Gusakov AV, Sinitsyn AP. A theoretical analysis of cellulase product inhibition: effect of cellulase binding constant, enzyme/substrate ratio, and β-glucosidase activity on the inhibition pattern. Biotechnol Bioeng. 1992;40(6):663–671. doi: 10.1002/bit.260400604. [DOI] [PubMed] [Google Scholar]
- Harnpicharnchai P, Champreda V, Sornlake W, Eurwilaichitr L. A thermotolerant beta-glucosidase isolated from an endophytic fungi, Periconia sp., with a possible use for biomass conversion to sugars. Protein Expr Purif. 2009;67(2):61–69. doi: 10.1016/j.pep.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Jabbour D, Klippel B, Antranikian G. A novel thermostable and glucose-tolerant β-glucosidase from Fervidobacterium islandicum. Appl Microbiol Biotechnol. 2012;93(5):1947–1956. doi: 10.1007/s00253-011-3406-0. [DOI] [PubMed] [Google Scholar]
- Janbon G, Derancourt J, Chemardin P, Arnaud A, Galzy P. A very stable β-glucosidase from a Candida molischiana mutant strain: enzymatic properties, sequencing, and homology with other yeast β-glucosidases. Biosci Biotechnol Biochem. 1995;59(7):1320–1322. doi: 10.1271/bbb.59.1320. [DOI] [PubMed] [Google Scholar]
- Keerti, Gupta A, Kumar V, Dubey A, Verma AK (2014) Kinetic characterization and effect of immobilized thermostable β-glucosidase in alginate gel beads on sugarcane juice. ISRN Biochem 178498. doi:10.1155/2014/178498 [DOI] [PMC free article] [PubMed]
- Kong FS, Yang JW, Zhen Z, Liang TT, Zhu DL, Gao RJ, Xie GQ. Gene cloning and molecular characterization of a β-glucosidase from Thermotoga naphthophila RUK-10: an effective tool for synthesis of galacto-oligosaccharide and alkyl galactopyranosides. Chem Res Chin Univ. 2015;31(5):774–780. doi: 10.1007/s40242-015-5179-y. [DOI] [Google Scholar]
- Li JB, Zhou PF, Liu HM, Xiong CJ, Lin JH, Xiao WJ, Gong YX, Liu ZH. Synergism of cellulase, xylanase, and pectinase on hydrolyzing sugarcane bagasse resulting from different pretreatment technologies. Bioresour Technol. 2014;155:258–265. doi: 10.1016/j.biortech.2013.12.113. [DOI] [PubMed] [Google Scholar]
- Li JB, Zhou PF, Liu HM, Lin JH, Gong YX, Xiao WJ, Liu ZH. Monosaccharides and ethanol production from superfine ground sugarcane bagasse using enzyme cocktail. BioResources. 2014;9(2):2529–2540. [Google Scholar]
- Ma L, Zhang J, Zou G, Wang CS, Zhou ZH. Improvement of cellulase activity in Trichoderma reesei by heterologous expression of a beta-glucosidase gene from Penicillium decumbens. Enzyme Microb Technol. 2011;49:366–371. doi: 10.1016/j.enzmictec.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Matsuzawa T, Yaoi K. Screening, identification, and characterization of a novel saccharide-stimulated β-glycosidase from a soil metagenomic library. Appl Microbiol Biotechnol. 2016;122(4):1–14. doi: 10.1007/s00253-016-7803-2. [DOI] [PubMed] [Google Scholar]
- Meinke A, Damude HG, Tomme P, Kwan E, Kilburn DG, Miller RC, Jr, Warren RA, Gilkes NR. Enhancement of theendo-beta-1,4-glucanase activity of an exocellobiohydrolase by deletion of a surface loop. J Biol Chem. 1995;270(9):4383–4386. doi: 10.1074/jbc.270.9.4383. [DOI] [PubMed] [Google Scholar]
- Meleiroa LP, Carlos J, Salgado JCS, Maldonadoc RF, Alpontia JS, Zimbardi ALRL, Jorge JA, Ward RJ, Furriel RPM. A Neurospora crassa ß-glucosidase with potential for lignocellulose hydrolysis shows strong glucose tolerance and stimulation by glucose and xylose. J Mol Catal B Enzym. 2015;122:131–140. doi: 10.1016/j.molcatb.2015.09.003. [DOI] [Google Scholar]
- Mullings R. Measurement of saccharification by cellulases. Enzyme Microb Technol. 1985;7(12):586–591. doi: 10.1016/0141-0229(85)90025-0. [DOI] [Google Scholar]
- Pei X, Zhao JQ, Cai PL, Sun WL, Ren J, Wu QQ, Zhang SH, Tian CG. Heterologous expression of a GH3 β-glucosidase from Neurospora crassa in Pichia pastoris with high purity and its application in the hydrolysis of soybean isoflavone glycosides. Protein Expr Purif. 2015;119:75–84. doi: 10.1016/j.pep.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Rajasree KP, Mathew GM, Pandey A, Sukumaran RK. Highly glucose tolerant β-glucosidase from Aspergillus unguis: NII 08123 for enhanced hydrolysis of biomass. J Ind Microbiol Biotechnol. 2013;40:967–975. doi: 10.1007/s10295-013-1291-5. [DOI] [PubMed] [Google Scholar]
- Ramos LP, Breuil C, Saddler JN. The use of enzyme recycling and the influence of sugar accumulation on cellulose hydrolysis by Trichoderma cellulases. Enzyme Microb Technol. 1993;15(1):19–25. doi: 10.1016/0141-0229(93)90111-E. [DOI] [Google Scholar]
- Reese E. Enzymatic hydrolysis of the walls of yeast cells and germinated fungal spores. Biochim Biophys Acta. 1977;499(1):10–23. doi: 10.1016/0304-4165(77)90223-9. [DOI] [PubMed] [Google Scholar]
- Saha BC, Bothast RJ. Purification and characterization of a highly glucose-tolerant novel β-glucosidase from Candida peltata. Appl Environ Microbiol. 1996;62(9):3165–3170. doi: 10.1128/aem.62.9.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Verma AK, Kumar V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech. 2016;6(1):1–14. doi: 10.1007/s13205-015-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha SK, Datta S. β-Glucosidase from the hyperthermophilic archaeon Thermococcus sp. is a salt-tolerant enzyme that is stabilized by its reaction product glucose. Appl Microbiol Biotechnol. 2016;100:8399–8409. doi: 10.1007/s00253-016-7601-x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Cheng JY. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83(1):1–11. doi: 10.1016/S0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Takashima S, Nakamura A, Hidaka M, Masaki H, Uozumi T. Molecular cloning and expression of the novel fungal β-glucosidase genes from Humicolagrisea and Thichodermareesei. J Biochem. 1999;125(4):728–736. doi: 10.1093/oxfordjournals.jbchem.a022343. [DOI] [PubMed] [Google Scholar]
- Teeri T, Salovuori I, Knowles J. The molecular cloning of the major cellulase gene from Trichoderma reesei. Nat Biotechnol. 1983;1(8):696–699. doi: 10.1038/nbt1083-696. [DOI] [Google Scholar]
- Tilbeurgh HV, Tomme P, Claeyssens M, Bhikhabhai R, Pettersson G. Limited proteolysis of the cellobiohydrolase I from Trichoderma reesei: separation of functional domains. FEBS Lett. 1986;204(2):223–227. doi: 10.1016/0014-5793(86)80816-X. [DOI] [Google Scholar]
- Turan Y, Zheng M. Purification and characterization of an intracellular β-glucosidase from the methylotrophic yeast Pichia pastoris. Biochem (Moscow) 2005;70(12):1363–1368. doi: 10.1007/s10541-005-0270-5. [DOI] [PubMed] [Google Scholar]
- Uchima CA, Tokuda G, Watanabe H, Kitamoto K, Arioka M. Heterologous expression in Pichia pastoris and characterization of an endogenous thermostable and high-glucose-tolerant_β-glucosidase from the termite Nasutitermes takasagoensis. Appl Environ Microbiol. 2012;78(12):4288–4293. doi: 10.1128/AEM.07718-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J, Wiseman A. Fungal and other β-d-glucosidases-their properties and applications. Enzyme Microb Technol. 1982;4(2):73–79. doi: 10.1016/0141-0229(82)90084-9. [DOI] [Google Scholar]
- Wyman CE. Ethanol from lingocellulosic biomass: technology, economics, and opportunities. Bioresour Technol. 1994;50(1):3–15. doi: 10.1016/0960-8524(94)90214-3. [DOI] [Google Scholar]
- Wyman CE, Dale BE, Elander RT, Holtzapple M, Ladisch MR, Lee YY. Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour Technol. 2005;96(18):2026–2032. doi: 10.1016/j.biortech.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang X, Yong Q, Yu S. Three-stage hydrolysis to enhance enzymatic saccharification of steam-exploded corn stover. Bioresour Technol. 2010;101(13):4930–4935. doi: 10.1016/j.biortech.2009.09.079. [DOI] [PubMed] [Google Scholar]
- Yang JW, Di XJ, Wang M. Gene clone and characterization of a novel thermostable β-galactosidase with transglycosylation activity from Thermotoga naphthophila RUK-10. Chem Res Chin Univ. 2015;31(4):564–568. doi: 10.1007/s40242-015-5032-3. [DOI] [Google Scholar]
- Yang F, Yang XF, Li Z, Du CY, Wang JF, Li S. Overexpression and characterization of a glucose-tolerant β-glucosidase from T. aotearoense with high specific activity for cellobiose. Appl Microbiol Biotechnol. 2015;99:8903–8915. doi: 10.1007/s00253-015-6619-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu JL, Yuan ZH, Xu HJ, Yu Q. Artificial neural network-genetic algorithm based optimization for the immobilization of cellulase on the smart polymer Eudragit L-100. Bioresour Technol. 2010;101(9):3153–3158. doi: 10.1016/j.biortech.2009.12.080. [DOI] [PubMed] [Google Scholar]