Abstract
White rot fungus Marasmius sp. BBKAV79 (GenBank accession number-KP455496, KP455497) exhibited decolourization and degradation of Navy blue HER dye (concentration 50 mg l−l) within 24 h under shaking condition. In the present study, various investigated parameters like initial dye concentration, pH, temperature, carbon and nitrogen sources were optimized to develop a faster decolourization process by Marasmius sp. BBKAV79. High-performance liquid chromatography, Fourier transform infrared spectroscopy and gas chromatography and mass spectroscopy analysis of the extracted product confirmed the biodegradation of Navy blue HER. The microbial toxicity and phytotoxicity assay revealed that the degradation of Navy blue HER produced nontoxic metabolites.
Keywords: Decolourization, FTIR, GCMS: HPLC, Marasmius sp. BBKAV79
Introduction
Increasing industrial development and urbanisation have resulted in the generation of large quantities of toxic and persistent pollutants in water courses that cause deleterious ecological effects and pose a serious threat to animals and humankind (Shannon et al. 2008). Considering both the volume and the composition of the effluents, the textile industry is rated as the most polluting among all industrial sectors. Textile effluents are one of the most difficult-to-treat wastewaters on account of the considerable amount of suspended solids and of the massive presence of dyes, salts, additives, detergents and surfactants (Prigione et al. 2008). The physical–chemical technologies for wastewater treatment, including the advanced oxidation processes (i.e. application of ozone, hydrogen peroxide and ultraviolet), are expensive, not always effective and often do not reduce the toxicity of the effluents (Robinson et al. 2001).
In recent years, many studies on innovative biological approaches have investigated the possibility to use selected microorganisms to degrade dyes in wastewaters. Fungi, particularly white rot fungi, have long been recognised for their ability to degrade a wide range of recalcitrant compounds, such as synthetic dyes, through the use of relatively non-specific, extracellular oxidative enzymes (Kaushik and Malik 2009; Diwaniyan et al. 2010). This extracellular enzymatic system, which in nature is involved in lignin degradation, consists mainly of oxidative enzymes like laccases (Lac), lignin peroxidases (LiP) and manganese peroxidases (MnP) that have been demonstrated effective against a wide range of industrial dyes (Husain 2006).
However, fungal treatments have not yet found a real application, mainly due to the difficulty in selecting organisms able to grow and remain active in the very variable and harsh conditions of wastewaters (Anastasi et al. 2010). In fact, fungi should be able to live with the scarce nutrient resources present in the effluents, to win the competition with the autochthonous microflora and to survive in the presence of high concentrations of salts, dyes, detergents and heavy metals. Moreover, the requirement of low pH values (4–5), optimum enzyme activity and long hydraulic retention times for complete decolourization are other concerns in fungal treatments application (Singh and Arora 2011).
Hence, the present study was to develop a potential fungal culture capable to decolourize and degradation of Navy blue HER. Moreover, the characterization of its metabolic products and toxicity assessment by seed germination method were also studied.
Materials and methods
Microorganism
The pure culture of Marasmius sp. BBKAV79 (GenBank accession number KP455496, KP455497) was isolated and identified at Agharkar Research Institute, Pune (Adiveppa and Basappa 2015). The strain was maintained on potato dextrose agar plate at 30 °C and stored at 4 °C (Vantamuri and Kaliwal 2016).
Decolourization of Navy blue HER
Decolourization experiment was conducted in 250 ml Erlenmeyer flask containing 100 ml of production medium. The Marasmius sp. BBKAV79 was tested for its ability to decolourize selected Navy blue HER dye over a period of 24 h. The final concentrations of the dye in the medium without inoculation with Marasmius sp. BBKAV79 was considered as a control. The extent of decolourization was recorded as residual colour. The dyes Navy blue HER (50 mg l−1) was monitored at their absorbance maxima at 620 nm (Saratale et al. 2009).
The effect of dye decolourization was determined by the decrease in absorbance under the maximum wavelength of the dye. The efficiency of decolourization was expressed in terms of percentage (Saratale et al. 2009).
Effect of various concentrations on dye decolourization by Marasmius sp. BBKAV79
To study the effect of different initial dye concentrations on decolourizing process, measured quantities of dyes as 50, 100, 150 and 200 mg l−1 were added to the culture flask. These flasks were than incubated at 40 °C to observe the time taken for decolourization at various dye concentration.
Effect of physicochemical parameters on dye decolourization by Marasmius sp. BBKAV79
To study the effect of pH on decolourization process, production medium having different pH 4, 5, 6, 8, 9 and 10 were inoculated with Marasmius sp. BBKAV79, 50 mg l−l dye was added to observe the pattern of decolourization. The effect of temperature on decolourization process was studied by keeping inoculated flasks, at different temperature 30, 40 and 50 °C.
Effect of carbon and nitrogen sources on dye decolourization by Marasmius sp. BBKAV79
The effect of different carbon sources namely maltose, sucrose, glucose, lactose and nitrogen sources namely peptone, yeast extract, urea and ammonium chloride were added to dye (50 mg l−1) containing medium flask was studied by inoculating the Marasmius sp. BBKAV79 and the flasks were incubated at 40 °C under shaking condition and used for decolourization studies.
Bio-decolourization and biodegradation analysis
Decolourization was monitored by high performance liquid chromatography (HPLC), Fourier transform infrared spectroscopy (FTIR) and Gas chromatography and mass spectroscopy (GCMS). Decolourized sample (100 ml, at 24 h) was centrifuged at 10,000×g for 20 min. The extraction of metabolites was carried from the supernatant using equal volume of ethyl acetate. The extracts were dried over anhydrous Na2SO4 and evaporated to dryness in rotary evaporator. The crystals obtained were dissolved in small volume of HPLC grade methanol and used for HPLC, FTIR and GCMS.
HPLC analysis was carried out (Waters model no. 2690) on C18 column (symmetry, 4.6 × 0250 mm) with 1 ml min−1 flow rate and ultraviolet (UV) detector at 248 nm. The biodegraded Navy blue HER was characterized by FTIR (PerkinElmer, Spectrum one). The analysis results were compared with that of the control dye. The FTIR analysis was done in the mid- IR region of 400–4000 cm−1 with 16 scan speed. The samples were mixed with spectroscopically pure KBr in the ratio of 5:95, pellets were fixed in sample holder, and the analyses were carried out. A QP2010 gas chromatography coupled with mass spectrometer (Shimadzu) was used for GC–MS analysis. The analysis was performed in the temperature-programming mode at an ionization voltage 70 eV. Temperature of the Restek column (0.25 mm, 60 m; XTI-5) was kept 80 °C for initial 2 min, then raised up to 280 °C with rate of 10 °C min−1, and held for 7 min. The temperature of injection port and the GC/MS interface was maintained at 280 and 290 °C, respectively. The flow rate for helium as a carrier gas was 1.0 ml min−1. NIST spectral library stored in the computer software (version 1.10 beta, Shimadzu) of the GC–MS was used for comparison of retention times and mass spectra of degradation metabolites based on their fragmentation pattern.
Phytotoxicity studies
Phytotoxicity tests were performed in order to assess the toxicity of the untreated and treated dye. The ethyl acetate extracted products of Navy blue HER degradation were dried and dissolved in 10 ml sterile distilled water to make a final concentration of 1500 ppm for phytotoxicity studies. The phytotoxicity study was carried out (at room temperature) on two kinds of seeds commonly used in Indian agriculture (Pourbabaee et al. 2006) Phaseolus mungo and Sorghum vulgare (10 seeds) by watering separately 10 ml sample of control Navy blue HER and its degradation products (1500 ppm) per day. Control set was carried out using distilled water at the same time. Germination (%) as well as the length of plumule and radical was recorded after 7 days.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) with Tukey–Kramer multiple comparisons test. Readings were considered significant when P was ≤0.05.
Results and discussion
Influence of different concentrations of Navy blue HER
The rate and extent of decolourization was affected by the addition of dye ranging from 50 to 200 mg l−l. Navy blue HER concentration 50 mg l−l gets decolourized up to 91.25% followed by 100 mg l−l (56.66%), 150 mg l−l (37.50%) and 200 mg l−l (12.90%) (Fig. 1). Dhanve et al. (2008) reported the effective decolorization of Navy blue HE2R dye by utilizing Exiguobacterium sp.
Fig. 1.
Effect of concentration of dye on decolourization of Navy blue HER
Influence of pH and temperature on dye decolourization from Marasmius sp. BBKAV79
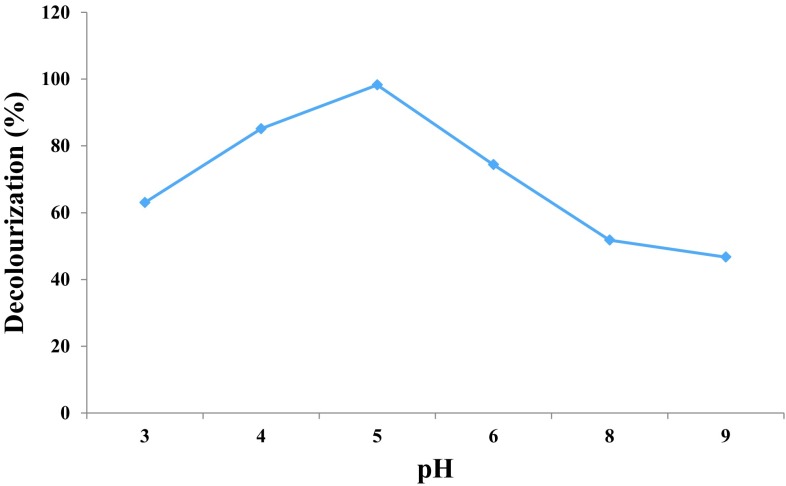
The maximum decolourization was observed at pH 5 (97.27%) for Navy blue HER followed by pH 4 (81.30%), pH 6 (71.12%), pH 3 (53.87%), pH 8 (50.14%) and pH 9 (32.64%). by Marasmius sp. BBKAV79 (Fig. 2). Saratale et al. (2009) have reported that the optimum pH at 7 was observed in the decolourization of Navy blue HER by Trichosporon beigelii NCIM-3326.
Fig. 2.
Effect of pH on decolourization of Navy blue HER
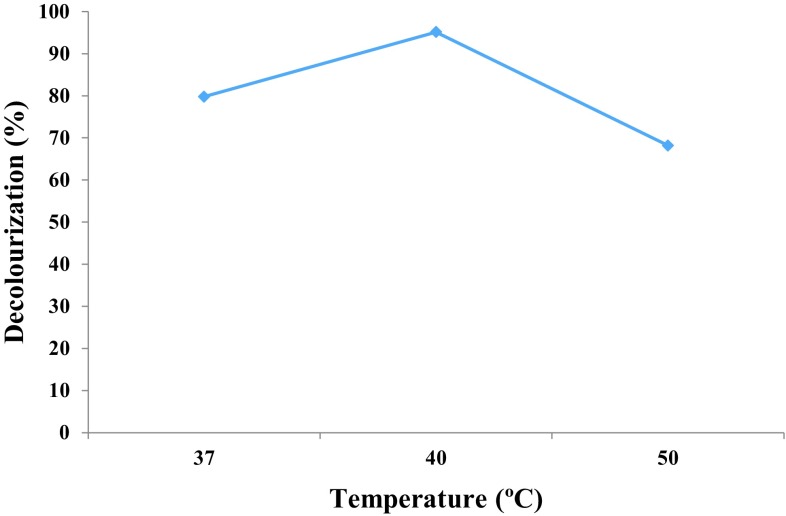
The maximum decolourization was observed at 40 °C (95.12%) for Navy blue HER followed by 37 °C (78.96%) and 50 °C (72.55%) by Marasmius sp. BBKAV79 (Fig. 3). Maximum decolourization of malachite green was observed at 30 °C by Penicillium ochrochloron (Utkarsha and Jyoti 2011).
Fig. 3.
Effect of temperature on decolourization of Navy blue HER
Influence of carbon and nitrogen sources on decolourization of Navy blue HER by Marasmius sp. BBKAV79
While trying to enhance decolourization performance of Navy blue HER, extra carbon sources were supplied in medium. Percent of decolourization was observed maximum with sucrose (100%), glucose and (75.68%) while less decolourization with other supplements of carbon sources maltose (35.14%) and lactose (25.86%) within 24 h (Fig. 4). Wang et al. (2009) have reported a Citrobacter sp. decolorized by 96.2% of reactive red 180 dyes with glucose as carbon source.
Fig. 4.
Effect of carbon sources on decolourization of Navy blue HER
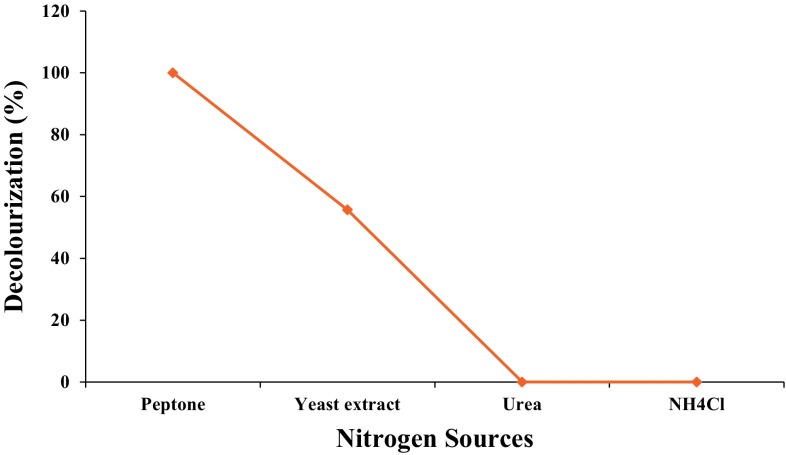
Percent of decolourization was observed maximum with peptone (100%) and yeast extract (55.68%), while there is no decolourization in the presence of urea and ammonium chloride within 24 h (Fig. 5). Maryam et al. (2014) have reported that the addition of using ammonium chloride supported decolourization.
Fig. 5.
Effect of nitrogen sources on decolourization of Navy blue HER
Analysis of metabolites resulting from decolourization and biodegradation of Navy blue HER by Marasmius sp. BBKAV79
HPLC analysis of control displayed a peak at a retention time of 3.12 min (Fig. 6), whereas that of the extracted metabolites after degradation there is the appearance of new peaks at retention times of 4.01 and 4.10 min. HPLC analysis of the mixture of dyes showed the peaks at retention times of 1.01, 2.22, 2.49, 2.90, 3.12 4.50 and 5.69 min (Fig. 6). Thus, HPLC analysis showed prominent peak at retention time at 3.12 min when products were separated from the sample obtained after decolourization, compared to control peaks. There are appearances of new peaks at different retention times. As the degradation process started from 0 h until completion, retention time was noted for each peak.
Fig. 6.
a HPLC chromatogram of Navy blue HER. b HPLC elution profile of Navy blue HER and metabolites formed from on its degradation by Marasmius sp. BBKAV79
The HPLC analysis of 0 h extracted dye sample appeared as a peak at 2.24 min (Fig. 6). Following the decolourization, the parent compound yielded in to seven different peaks in 24 h extracted sample (Fig. 6; Rt: 2.48, 2.73, 3.42, 3.91, 4.12, 5.39 and 5.71 min). The metabolites formed at 24 h were further degraded and only three detectable peaks were observed in the 72 h extracted sample, with retention time of 2.58, 2.80 and 5.37 min.
Overall HPLC study concluded that compare to control the degradation product showed major peaks at 1.01, 2.22, 2.49, 2.90, 3.12 4.50 and 5.69 which are not seen in case of controls that confirms the degradation of dye.
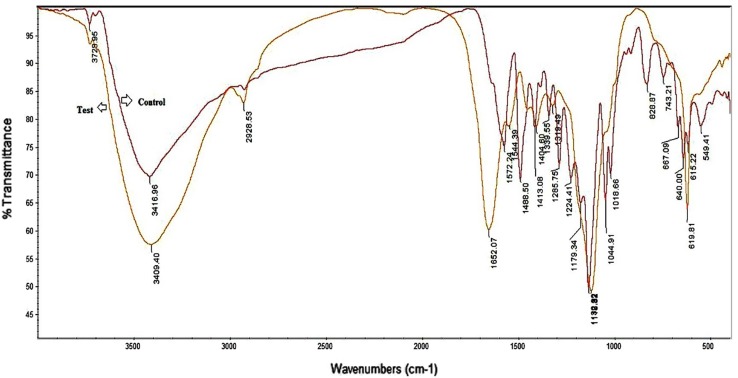
According to the FTIR spectral data, Navy blue HER reveals that control dye with treated enzyme, in both tested and control dye, the sharp absorption peak at 1132 cm−1 were observed due to aliphatic amines with C–N stretching. There will be degradation observed from control (1572 cm−1) to tested compound (1652 cm−1), it may due to the enzyme catalytic activity, also there will be a sharp band at 1488 cm−1 indicates the asymmetric stretching of nitro groups. After the treatment of enzyme the absence of Diazo dye peak was observed, viz., 1404, 1319, 1118 and 619 cm−1 peaks represent the presence of aromatic, aliphatic amines, alkenes and alkyl halides group, respectively (Fig. 7).
Fig. 7.
FTIR spectrum of Navy blue HER and its degradation product
According to the GCMS experiment was carried out in order to verify degradation product formed during the dye decolourization by Marasmius sp. BBKAV79, According to the TIC. The molecular weight compounds like Nitrous acid. Butyl ester n-butyl nitrite (molecular weight 103 m/z with retention time 8.70) observed as a final product of Navy blue HER (data not shown), Here we have investigated that primary reductive cleavage in diazo bonds of Navy blue HER followed by the sequential denitration reaction giving rise to the final identified dye. Which present in tiny amount (0.01) in the decolourization solution (data not given).
Phytotoxicity studies
Disposal of untreated dyeing effluents in water bodies might cause serious environmental and health hazards and this water is being used for an agriculture purpose shows toxic effect on the germination rates and biomass of several plant species, which play an important role in ecological function such as providing the habitat for wildlife, protecting soil from erosion and providing bulk of organic matter that is so significant to soil fertility (Kalyani et al. 2009; Tan et al. 2005; Dhanve et al. 2008). Thus, it was of concern to assess the phytotoxicity of the dye before and after degradation. The relative sensitivity towards the dye Navy blue HER and its degradation products in relation to P. mungo and S. vulgare were studied. The mean of plumule length and radical length of P. mungo were 11.26 ± 0.24 and 6.14 ± 0.16 cm, and 12.18 ± 0.40 and 15.07 ± 0.45 cm in case of S. vulgare, respectively, of ten seeds in distilled water as a control with 100% germination. The germination of both plant seeds inhibited 90% when seeds treated with 1500 ppm concentration of Navy blue HER, whereas the plumule length and radical length was found in P. mungo (7.22 ± 0.30 and 4.48 ± 0.178 cm) and in S. vulgare (10.31 ± 0.26 and 11.38 ± 0.24 cm), respectively, with 100% germination when treated with 1500 ppm degradation products (Table 1). Parshetti et al. (2006) have also found that germination of P. mungo was less with Malachite green treatment as compared to its degradation products and distilled water. Thus, phytotoxicity studies showed good germination rate as well as significant growth in the plumule and radical for both the plants, in the metabolites extracted after decolourization, as compared to dye sample. This study indicates the detoxification of Navy blue HER by Marasmius sp. BBKAV79.
Table 1.
Phytotoxicity studies of Navy blue HER and metabolites produced after complete decolourization (24 hours) by Marasmius sp. BBKAV79
| Parameters | Phaseolus mungo | Sorghum vulgare | ||||
|---|---|---|---|---|---|---|
| Distilled water | Navy blue HER (1500 ppm) | Extracted metabolites (1500 ppm) | Distilled water | Navy blue HER (1500 ppm) | Extracted metabolites (1500 ppm) | |
| Germination (%) | 100 | 10 | 100 | 100 | 10 | 100 |
| Plumule (cm) | 11.26 ± 0.24 | 1.19 ± 0.08 | 7.22 ± 0.30 | 12.18 ± 0.40 | 1.90 ± 0.10 | 10.31 ± 0.26 |
| Radical (cm) | 6.14 ± 0.16 | 0.70 ± 0.18 | 4.48 ± 0.17 | 15.07 ± 0.45 | 1.30 ± 0.08 | 11.38 ± 0.24 |
Values are mean of germinated seeds of two experiments, SEM (±) significantly different from the control (seeds germinated in distilled water) at P ≤ 0.05 by one-way ANOVA with Tukey–Kramer multiple comparisons test
Conclusion
The investigation demonstrates that Marasmius sp. BBKAV79 was capable of decolourizing and degrading the diazo dye Navy blue HER (Reactive Blue 171) effectively under shaking condition. Addition of carbon and nitrogen sources appeared to enhance decolourization activity of Marasmius sp. BBKAV79. FTIR and HPLC analysis of extracted products confirmed the biodegradation of Navy blue HER. The results suggest the potential of Marasmius sp. BBKAV79 for future application towards treatment of real dye bearing wastewaters by using appropriate bioreactor.
Acknowledgements
The authors are thankful to the Department of Biotechnology, New Delhi, for providing Bioinformatics laboratory facility, DBTKUD- IPLS (BT/PR14555/INF/22/126/2010), Purse program from Department of Science and Technology, New Delhi. The authors also acknowledge, Post Graduate Department of Studies in Biotechnology and Microbiology, Karnatak University Dharwad for providing all the necessary facilities and financial assistance through UPE (University with Potential for Excellence) fellowship.
Compliance with ethical standards
Conflict of interest
The authors hereby declare no conflict of interest.
References
- Adiveppa BV, Basappa BK. Isolation, screening and identification of laccase producing fungi. Int J Pharm Bio Sci. 2015;6:242–250. [Google Scholar]
- Anastasi A, Spina F, Prigione V, Tigini V, Giansanti P, Varese GC. Scale-up of a bioprocess for textile wastewater treatment using Bjerkandera adusta. Biores Technol. 2010;101:3067–3075. doi: 10.1016/j.biortech.2009.12.067. [DOI] [PubMed] [Google Scholar]
- Dhanve RS, Shedbalkar UU, Jadhav JP. Biodegradation of diazo reactive dye Navy Blue HE2R (Reactive Blue 172) by an isolated Exiguobacterium sp. RD3. Biotechnol Bioprocess Eng. 2008;13:53–60. doi: 10.1007/s12257-007-0165-y. [DOI] [Google Scholar]
- Diwaniyan S, Kharb D, Raghukumar C. Decolorization of synthetic dyes and textile effluents by basidiomycetous fungi. Water Air Soil Pollut. 2010;210:409–419. doi: 10.1007/s11270-009-0263-x. [DOI] [Google Scholar]
- Husain Q. Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol. 2006;26:201–221. doi: 10.1080/07388550600969936. [DOI] [PubMed] [Google Scholar]
- Kalyani DC, Telke AA, Dhanve R, Jadhav JP. Ecofriendly biodegradation and detoxification of Reactive Red 2 textile dye by newly isolated Pseudomonas sp. SUK1. J Hazard Mater. 2009;163:735–742. doi: 10.1016/j.jhazmat.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Kaushik P, Malik A. Fungal dye decolourisation, recent advances and future potential. Environ Int. 2009;35:127–141. doi: 10.1016/j.envint.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Maryam MT, Mahnaz MA, Hamid R. Biodecolorization of textile effluents by autochthonous fungi. J Appl Biotechnol Rep. 2014;1:161–165. [Google Scholar]
- Parshetti GK, Kalme SD, Saratale GD, Govindwar SP. Biodegradation of Malachite green by Kocuria rosea MTCC 1532. Acta Chim Slov. 2006;53:492–498. [Google Scholar]
- Pourbabaee AA, Malekzadeh F, Sarbolouki MN, Najafi F. Aerobic decolorization and detoxification of a disperse dye in textile effluent by a new isolate of Bacillus sp. J Biotechnol Bioeng. 2006;93:631–635. doi: 10.1002/bit.20732. [DOI] [PubMed] [Google Scholar]
- Prigione V, Pezzella C, Anastasi A. Decolourization and detoxification of textile effluents by fungal biosorption. Water Res. 2008;42:2911–2920. doi: 10.1016/j.watres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Robinson T, Chandran B, Nigam P. Studies on the production of enzymes by white-rot fungi for the decolorisation of textile dyes. Enzyme Microb Technol. 2001;29:575–579. doi: 10.1016/S0141-0229(01)00430-6. [DOI] [Google Scholar]
- Saratale RG, Saratale GD, Chang JS, Govindwar SP. Decolorization and biodegradation of textile dye Navy blue HER by Trichosporon beigelii NCIM-3326. J Hazard Mater. 2009;166:1421–1428. doi: 10.1016/j.jhazmat.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Mariñas BJ, Mayes AM. Science and technology for water purification in the coming decades. Nature. 2008;452:301–310. doi: 10.1038/nature06599. [DOI] [PubMed] [Google Scholar]
- Singh K, Arora S. Removal of synthetic textile dyes from wastewaters: a critical review on present treatment technologies. Crit Rev Environ Sci Technol. 2011;41:807–878. doi: 10.1080/10643380903218376. [DOI] [Google Scholar]
- Tan NCG, van Leeuwen A, van Voorthuizen EM, Slenders P, Prenafeta- Boldu FX, Temmink H, Lettinga G, Field JA. Fate and biodegradability of sulfonated aromatic amines. Biodegradation. 2005;16:527–537. doi: 10.1007/s10532-004-6593-x. [DOI] [PubMed] [Google Scholar]
- Utkarsha S, Jyoti PJ. Detoxification of Malachite Green and Textile Industrial Effluent by Penicillium ochrochloron. Biotechnol Bioprocess Eng. 2011;16:196–204. doi: 10.1007/s12257-010-0069-0. [DOI] [Google Scholar]
- Vantamuri AB, Kaliwal BB. Purification and characterization of laccase from Marasmius species BBKAV79 and effective decolorization of selected textile dyes 3. Biotechnology. 2016;6:189. doi: 10.1007/s13205-016-0504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Su JQ, Zheng XW, Tian Y, Xiong XJ, Zheng TL. Bacterial decolourization and degradation of the reactive dye Reactive Red 180 by Citrobacter sp. CK3q. Int Biodeterior Biodegrad. 2009;63:395–399. doi: 10.1016/j.ibiod.2008.11.006. [DOI] [Google Scholar]