Abstract
Unrestricted and reckless use of antibiotics has resulted in their accumulation in environment. This, in turn, has led to the emergence of multiple drug-resistant microbes. The present study focuses on degradation of ciprofloxacin (CIP) by an edible white rot fungus Pleurotus ostreatus. Effect of CIP was determined on radial growth and biomass of P. ostreatus. Titrimetric and spectrophotometric assays were carried out to assess the degrading potential of P. ostreatus towards CIP. It was found that CIP has a stimulatory effect on growth and enzyme activity of P. ostreatus. Maximum enzyme (glucanase, ligninases, laccase) production was observed at the highest concentration of CIP (500 ppm). Antibiotic degradation of about 68.8, 94.25 and 91.34% was estimated after 14 days of incubation at 500 ppm CIP using Titrimetric, Indigo carmine and Methyl orange assay, respectively. Degradation of CIP was further validated by high performance liquid chromatography (HPLC) and microbiological analysis. HPLC analysis revealed 95.07% degradation while microbiological test also exhibited a decreased antimicrobial activity of degraded products against Escherichia coli, Staphylococcus aureus and Streptococcus pyogenes. To the best of our knowledge, this is the first study wherein P. ostreatus was used for the degradation of ciprofloxacin.
Keywords: Antibiotic resistance, Bioremediation, CIP, Pleurotus ostreatus, HPLC
Introduction
Antibiotics are among the most frequently prescribed medicaments in modern day medicine (Hernandez et al. 2012). Between 2005 and 2009, the units of antibiotics sold in India specifically increased by about 40% . Increased sales of cephalosporins were particularly striking, with sales increasing by 60% though some increase was seen in most antibiotic classes, making India world’s largest consumer of antibiotics (Shah et al. 2015). Being non-degradable in nature, most of these antibiotics persist in the environment for long periods. Antibiotics like ciprofloxacin (CIP) degrade very slowly and may persist in soil in its original form for up to 1–4 months, thus creating a microenvironment for the development of antibiotic resistant strains (Laxminarayan et al. 2013). CIP is one of the most widely used second-generation broad spectrum quinolone which has been detected in domestic wastewaters in concentrations of up to 1000–6000 ng/l, that causes the possible occurrence of selective pressures and the consequent selection of resistant bacteria (Batt et al. 2007). This problem of extensive and unrestricted use of antibiotics has aggravated to such magnanimous proportions that it is becoming increasingly difficult to treat diseases caused by such resistant strains. It has now, therefore, become necessary to search for new methods for effective degradation of antibiotics persisting in the environment.
A number of methods including physical and chemical have been adopted for treatment of water contaminated with antibiotics (Hernandez et al. 2012; Hubicka et al. 2013). However, these methods are largely ineffective and end up adding more pollutants into the environment. An alternative lies in the use of living organisms for remediation of these antibiotics. Bioremediation involves the use of microbes to remove or breakdown complex hazardous substances into simpler, less toxic or nontoxic substances. The process is generally 60–70% less costly than other technologies (Laxminarayan et al. 2013). Fungi such as Gloeophyllum striatum (Wetzstein et al. 1999), Phanerochaete chrysosporium (Guo et al. 2014; Martens et al. 1996) and Trametes versicolor (Rodríguez-Rodríguez et al. 2012) have been reported for their use in bioremediation of antibiotics. The aim of this study was to evaluate the potential use of mycelium of a basidiomycetous fungus Pleurotus ostreatus for degradation of CIP.
Pleurotus ostreatus (P. ostreatus), is a temperate edible mushroom which forms oyster shaped fruiting bodies that can be grown on different agricultural wastes in a temperature range of 25–28 °C (Ahmed et al. 2009). The ability of P. ostreatus as bioremediation agent has been attributed to the production of various enzymes such as laccase, manganese peroxide, lignin peroxidases, xylanases, etc. which are important for various metabolic reactions such as substrate utilization as well as degradation of pollutants (Martens et al. 1996; Espindola et al. 2007; Rana and Rana 2011; Jegatheesan et al. 2012; Singh et al. 2012).
Materials and methods
Growth studies
Mycelium of P. ostreatus was maintained on Potato dextrose agar (PDA) medium at 25 ± 2 °C. The effect of different concentrations of CIP (100, 200, 300, 400 and 500 ppm) on growth of P. ostreatus mycelium and biomass production was studied by inoculating mycelium bits (5 mm) in PDA and Potato dextrose broth (PDB), respectively. One-way ANOVA was used to determine whether there was any significant effect of concentration of CIP used on the radial growth of P. ostreatus with increase in incubation time by calculating Critical Difference (CD) at 5%.
Enzymatic studies
Effect of different concentrations (100–500 ppm) of CIP on Endo-β-d-1,4-glucanase (Miller 1959), Laccase (Jegatheesan et al. 2012), Hemicellulase (Bucht and Erikson 1968), Lignin peroxidase (Tien and Kirk 1984) and Manganese peroxidase (Kuwahara et al. 1983) produced by P. ostreatus was determined after 7 and 14 days of incubation. Culture broth devoid of CIP was used as control.
Degradation of CIP
Two different approaches, viz., titrimetric and spectrophotometric were used to estimate the amount of CIP degraded by P. ostreatus.
Titrimetric analysis
The amount of CIP degraded by P. ostreatus was determined according to Basavaiah et al. (2006). A 10 ml aliquot of standard solution containing 100–500 ppm of CIP was placed in a 100 ml volumetric flask. The solution was acidified by adding 5 ml of 5 M sulfuric acid. 10 ml of 0.025 M cerium sulfate was added to content in the flask, mixed well and kept aside for 15 min. Finally, the unreacted oxidant was back titrated with 0.025 M Ferrous Ammonium Sulfate (FAS) solution using one drop of ferroin indicator. Simultaneously, a blank titration was performed and the amount of drug left in the aliquot was calculated from the amount of cerium sulfate reacted. Standard curve of FAS was used to calculate the amount of CIP degraded.
Spectrophotometric analysis
Spectrophotometric determination of degraded CIP was carried out by methyl orange method and Indigo carmine method (Basavaiah et al. 2006; Nijhu et al. 2011).
Indigo carmine method
20 µg of spent broth was added to 4 ml distilled water. 1 ml of 5 M sulfuric acid was added to each tube followed by addition of 500 µg/ml of cerium sulfate solution (0.025 M). The contents were mixed well and kept aside for 10 min with occasional swirling at room temperature. 1 ml of indigo carmine was added to each tube and absorbance was measured at 610 nm after 5 min. Standard curve was prepared for determination of degraded amount of CIP.
Methyl orange method
10 µg of spent broth was added to 4 ml of distilled water. 1 ml of 5 M sulfuric acid and 250 µg/ml of cerium sulfate (0.025 M) were added to above mixture and kept at room temperature for 10 min with occasional swirling. Finally, 1 ml of methyl orange was added and absorbance was measured at 520 nm. Standard curve was prepared for determination of degraded amount of CIP.
Validation of CIP bioremediation
High performance liquid chromatography (HPLC)
HPLC was carried out to validate the degradation results obtained in the above mentioned assays. AC18 column with acetonitrile as mobile phase was used for chromatography (Singh et al. 2013). A standard run of pure CIP was performed to comparatively assess the samples taken, i.e., 100 and 500 ppm concentrations of CIP.
Antimicrobial activity of degraded products
Antibacterial activity of degraded products formed post incubation with P. ostreatus was evaluated against Escherichia coli, Staphylococcus aureus and Streptococcus pyogenes on Mueller–Hinton agar plates by disc diffusion method and compared with antibacterial activity of different CIP concentrations (Thillaimaharani et al. 2013; Hernandez et al. 2012).
Results and discussion
Radial growth studies
Radial growth studies of P. ostreatus on PDA at different concentrations of CIP revealed that there was no inhibitory effect of CIP on the growth of the fungus. On the contrary, as the concentration of the antibiotic was increased, a subsequent increase was observed in the radial growth. It was also seen that with the increase in incubation period, there was an increase in the radial growth of the fungus, with maximum growth being observed on the 6th day at all concentrations. However, 4th day onwards, there was no substantial change in the growth rate even when the concentration of CIP was increased (Table 1). Maximum growth was observed at an antibiotic concentration of 500 ppm, exhibiting a 27% increase as compared to the control. CD at 5% showed a significant effect of concentrations of CIP used on the radial growth of P. ostreatus. Such significant increase in the growth of the fungi in presence of antibiotics has not been reported before. Use of antibiotics as growth promoters in animal feeds has been well documented (Dibner and Richards 2005; Butaye et al. 2003; Miles et al. 2006; Wegener et al.1999) but the stimulatory effect of antibiotics specifically ciprofloxacin on the growth of P. ostreatus has not been reported before. The stimulatory effect of CIP on the fungus can be supported by the fact that increased fungal microbiota growth is a common side effect of antibiotic therapy (Noverr et al. 2004). This can be probably due to decrease in competition with bacterial species for food and space and reduction in microbial metabolites that depress growth (Dibner and Richards, 2005), there by stimulating fungal growth. Further, work needs to be carried out to understand the mechanism behind the growth stimulatory effect of ciprofloxacin on P. ostreatus.
Table 1.
Effect of different concentrations of CIP on radial growth of P. ostreatus
| Concentration of CIP used (ppm) | Radial Growth of P. ostreatus (mm)a | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
| 0 (control) | 7.5 ± 0.228 | 9.0 ± 0.000 | 19.0 ± 0.577 | 34.0 ± 0.000 | 46.0 ± 1.154 | 55.0 ± 1.154 |
| 100 | 8.0 ± 1.154 | 11.0 ± 0.000 | 22.5 ± 1.443 | 34.0 ± 3.464 | 50.0 ± 4.612 | 74.0 ± 0.000 |
| 200 | 8.5 ± 0.866 | 11.5 ± 0.866 | 23.0 ± 1.410 | 36.0 ± 1.732 | 50.0 ± 1.154 | 74.0 ± 0.577 |
| 300 | 9.5 ± 0.288 | 12.0 ± 1.154 | 26.0 ± 1.154 | 39.0 ± 0.577 | 53.0 ± 0.577 | 75.0 ± 0.000 |
| 400 | 11.0 ± 0.000 | 13.0 ± 0.000 | 26.5 ± 1.443 | 40.0 ± 0.000 | 55.0 ± 5.196 | 75.5 ± 0.288 |
| 500 | 11.5 ± 0.288 | 14.0 ± 0.000 | 26.5 ± 1.154 | 40.5 ± 4.33 | 55.5 ± 0.288 | 75.5 ± 0.288 |
| CD 5% | 1.967 | 1.816 | 3.559 | NS | NS | 1.703 |
NS not significant
aAverage of three replicates with standard error; Medium used-PDA; Temperature: 25 ± 2 °C
Biomass studies
Biomass studies showed that after 7 days of incubation, maximum growth (3.703 gm wet weight/1.436 gm dry weight) was observed in medium having concentration of 500 ppm of CIP while lowest biomass production (2.448 g wet weight/1.194 g dry weight) was seen at 100 ppm. The trend continued up to 14 days of incubation where maximum growth was observed at 500 ppm (3.965 g wet weight/3.965 g dry weight) and lowest at 100 ppm (3.128 g wet weight/1.398 g dry weight). CD (5%) revealed a significant increase in mycelial growth at increasing concentrations of CIP (Table 2). Biomass studies also exhibited a pattern similar to the one observed in radial mycelium growth studies.
Table 2.
Effect of different concentrations of CIP on wet and dry weight of P. ostreatus after 7 and 14 days of incubation
| Concentration of CIP used (ppm) | Wet weight(gm)a | Dry weight (gm)a | ||
|---|---|---|---|---|
| 7 Days | 14 Days | 7 Days | 14 Days | |
| 0 (control) | 2.448 ± 0.002 | 2.686 ± 0.000 | 1.194 ± 0.000 | 1.389 ± 0.002 |
| 100 | 2.503 ± 0.000 | 3.128 ± 0.000 | 1.254 ± 0.000 | 1.596 ± 0.000 |
| 200 | 2.521 ± 0.000 | 3.143 ± 0.000 | 1.291 ± 0.000 | 1.604 ± 0.000 |
| 300 | 2.901 ± 0.000 | 3.457 ± 0.000 | 1.400 ± 0.001 | 1.609 ± 0.000 |
| 400 | 3.094 ± 0.000 | 3.816 ± 0.000 | 1.415 ± 0.001 | 1.614 ± 0.000 |
| 500 | 3.703 ± 0.000 | 3.965 ± 0.000 | 1.436 ± 0.001 | 1.647 ± 0.001 |
| CD 5% | 0.008 | 0.003 | 0.003 | 0.002 |
Medium used-PDB; Incubation temperature-25 ± 2 °C
aAverage of three replicates with standard error
Endo-β-d-1,4-glucanase, hemicellulase, lignin peroxidase, manganese peroxidase and laccase activity of Pleurotus ostreatus
P. ostreatus produces different extracellular enzymes to utilize the substrate. The effect of various concentrations of CIP on production of endo-β-d-1,4-glucanase, hemicellulase, laccase, lignin peroxidase and manganese peroxidase was determined. It was observed that with the increase in antibiotic concentration, the enzymatic activity of the fungus also increases. In case of endo-β-d-1,4-glucanase, hemicellulase, laccase, lignin peroxidase and manganese peroxidase, maximum activity was observed at 500 ppm CIP after 14 days of incubation. P. ostreatus exhibited a maximum endo-β-d-1,4-glucanase specific activity 392.94 μmol/min/mg as compared to 58 μmol/min/mg of specific activity observed in the absence of antibiotic in the control. Similarly, maximum specific activity of 331.40, 0.0256 and 23.864 μmol/min/mg, was observed for hemicellulase, lignin peroxidase and manganese peroxidase, respectively, after 14 days of incubation at 500 ppm antibiotic concentration (Table 3). Enzymatic activities were determined after 7 and 14 days only so as to evaluate the profile of enzymes being produced by the fungus. The work was not designed to optimize the conditions for enzyme production. A similar pattern was observed in the enzyme activities of laccase with maximum enzyme activity at 500 ppm CIP after 14 days of incubation (Data Not Provided). CD at 5% revealed significant increase in the activities of all the enzymes at different concentrations of CIP.
Table 3.
Effect of different concentrations of CIP on Enzyme activity of P. ostreatus after 7 and 14 days of incubation
| Concentration of CIP used (ppm) | Enzyme activity (in µmol/min/mg)a | |||||||
|---|---|---|---|---|---|---|---|---|
| Endo-β-d-1,4-glucanase | Hemicellulase | Lignin peroxidase | Manganese peroxidase | |||||
| 7 Days | 14 Days | 7 Days | 14 Days | 7 Days | 14 Days | 7 Days | 14 Days | |
| 0 (control) | 35.53 | 58.00 | 43.40 | 62.84 | 0.0117 | 0.0129 | 4.295 | 13.779 |
| 100 | 51.93 | 99.73 | 58.68 | 105.99 | 0.0134 | 0.0159 | 4.704 | 13.863 |
| 200 | 53.81 | 164.86 | 65.02 | 144.10 | 0.0161 | 0.0184 | 4.805 | 15.454 |
| 300 | 94.20 | 185.52 | 71.78 | 164.82 | 0.0184 | 0.0212 | 5.426 | 15.909 |
| 400 | 94.30 | 269.95 | 82.73 | 235.97 | 0.0208 | 0.0222 | 6.231 | 21.786 |
| 500 | 112.87 | 392.94 | 93.69 | 331.40 | 0.0244 | 0.0256 | 7.441 | 23.864 |
| CD 5% | 0.101 | 0.162 | 0.121 | 0.181 | 0.001 | 0.001 | 0.029 | NS |
Medium used-PDB; Incubation temperature-25 ± 2 °C
NS non significant
aAverage of three replicates with standard error
Sandhu and Arora (1985) reported the induction of laccase production in Polyporus sanguineus in the presence of different phenolic compounds and protein synthesis inhibitors. They further proposed that laccase thus represents a mechanism to eliminate these toxic compounds by enzymatic transformation. Hence the possibility that the white rot fungi might sense the antibiotic as phenolic substrates and detoxify them, cannot be ruled out. Similarly, Phlebia radiata has been shown to produce lignin-modifying enzymes for detoxification purposes when toxic compounds are present in its environment (Rogalski et al. 1991) Froehner and Eriksson (1974) have reported that inhibition of extracellular protein synthesis in Neurospora crassa, stimulated laccase production in the presence of cycloheximide, owing to normal protein turnover. Dhawan et al. (2005) also reported the stimulatory effect of Apramycin sulfate (200 mg/l) on laccase production (18.2 U/ml) in Pycnoporus cinnabarinus. Similalry, Praveen et al. (2012) reported an increase in Lac, LiP and MnP production by Stereum ostrea and Phanerochaete chrysosporium in media supplemented with Tetracycline at a concentration of 200 ppm. The stimulation of ligninolytic enzyme production by certain antibiotics could be attributed to the fact that the fungi might be treating antibiotics as phenolic compounds and adopting the mechanism used for their detoxification for degradation antibiotics.
Degradation of CIP
Titrimetric analysis
Titrimetric analysis was used as one of the methods for the determination of degradation of CIP by P. ostreatus. On determining the concentration of FAS remaining after oxidation by cerium sulfate and calculating the % degradation thereof, it was found that the maximum degradation (68.8%) was obtained after 14 days of incubation at 500 ppm CIP (Table 4). This can be attributed to the fact that with the increase in CIP concentration, an increase in radial growth and enzyme production was observed, which in turn may have led to the increased biodegradation of the antibiotic. These results also suggest that the concentration of CIP in environment may significantly affect the biodegradation of antibiotic by the fungus.
Table 4.
Percent degradation of CIP by P. ostreatus after 7 and 14 days of incubation by Titrimetric and Spectrophotometric Analysis
| Concentration of CIP used (ppm) | Percent degradation of CIP | |||||
|---|---|---|---|---|---|---|
| Titrimetric analysis | Spectrophotometric analysis | |||||
| Indigo carmine method | Methyl orange method | |||||
| 7 Days | 14 Days | 7 Days | 14 Days | 7 Days | 14 Days | |
| 0 (control) | ND | ND | ND | ND | ND | ND |
| 100 | 12.0 | 46.0 | 81.60 | 86.50 | 79.20 | 85.80 |
| 200 | 41.0 | 52.0 | 83.40 | 90.90 | 81.05 | 88.00 |
| 300 | 57.3 | 61.3 | 87.13 | 91.00 | 85.37 | 90.26 |
| 400 | 60.5 | 63.0 | 90.87 | 93.00 | 88.97 | 90.30 |
| 500 | 65.4 | 68.8 | 93.50 | 94.25 | 88.92 | 91.34 |
| CD 5% | 4.847 | 4.691 | 0.55 | 0.75 | 0.168 | 0.161 |
ND not detected
Spectrophotometric analysis
Indigo carmine assay
Spectrophotometric determination of CIP using Indigo carmine method also exhibited a pattern similar to that obtained in titrimetric analysis. Percent degradation increased with the increasing concentration of CIP. 81.50% degradation of CIP was carried out by P. ostreatus after 7 days of incubation when 100 ppm CIP was initially taken. This increased to 86.50% after 14 days of incubation. A maximum degradation of 94.25% was achieved at CIP concentration of 500 ppm, after 14 days of incubation (Table 4).
Methyl orange assay
Showed that after 14 days of incubation, about 86.50% of CIP was degraded when 100 ppm of CIP was originally supplemented in the broth. As observed above, degradation increased with the increase in antibiotic concentration with highest degradation (91.34%) obtained at 500 ppm of CIP, after 14 days of incubation (Table 4).
These results suggest that spectrophotometric methods are more sensitive in determination of CIP as compared to titrimetric method and Indigo carmine assay was a more sensitive method than methyl orange assay.
Validation of CIP bioremediation
High performance liquid chromatography (HPLC)
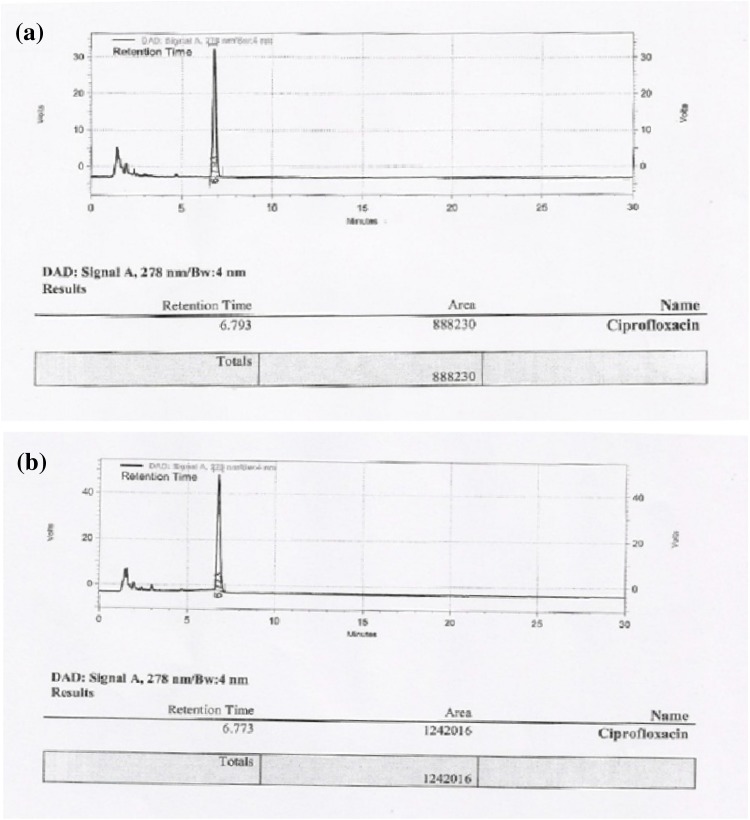
Validation of the results obtained by titrimetric and spectrophotometric methods was carried out by HPLC. On comparison with CIP standard, a degradation of 82.3% of 100 ppm CIP and 95.07% for 500 ppm CIP was observed after 14 days of incubation with P. ostreatus (Fig. 1). These results are in agreement with the results obtained by titrimetric and spectrophotometric analysis.
Fig. 1.
Chromatogram for CIP a 100 ppm b 500 ppm after treatment with P. ostreatus for 14 days
Antimicrobial activity of degraded products
Antimicrobial activity of degraded CIP was determined against Escherichia coli, Staphylococcus aureus and Streptococcus pyogenes. It was observed that the inhibitory action of CIP degradation products produced after incubation with P. ostreatus for 14 days was less as compared to standard CIP (100 and 500 ppm CIP solution). When 100 ppm CIP was used, a zone of inhibition of 2.5, 2.0 and 2.8 cm for E. coli, S. aureus and S. pyogenes, respectively, was observed whereas when degradation product obtained after incubation of 100 ppm CIP with P. ostreatus for 14 days was used, an inhibition zone of about 1.9, 1.7 and 2.3 cm for E. coli, S. aureus and S. pyogenes was observed. This corresponds to a 24, 15 and 17% decrease in the antimicrobial activity against E. coli, S. aureus and S. pyogenes, respectively. Similarly, for 500 ppm CIP used, a zone of 4.0, 3.6 and 3.4 cm for E. coli, S. aureus and S. pyogenes was obtained while with degradation product obtained after incubation of 500 ppm CIP with P. ostreatus for 14 days, a zone of inhibition of 2.9, 2.6 and 2.6 cm for E. coli, S. aureus and S. pyogenes was observed, exhibiting an average decrease of 26.3% in the antimicrobial activity of CIP (Table 5).
Table 5.
Antimicrobial activity of degradation products of ciprofloxacin against Escherichia coli, Staphylococcus aureus and Streptococcus pyogenes
| Concentration of CIP used ( in ppm) | Zone of inhibition (in cm)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | S. pyogenes | |||||||
| CIP degradation products | CIP | CD 5% | CIP degradation products | CIP | CD 5% | CIP degradation products | CIP | CD 5% | |
| D/w | ND | ND | – | ND | ND | – | ND | ND | – |
| 0(Control) | 1.5 | 3.0 | 0.226 | 1.6 | 3.8 | 0.358 | 2.0 | 3.6 | 0.358 |
| 100 | 1.9 | 2.5 | 0.226 | 1.7 | 2.0 | 0.226 | 2.3 | 2.8 | 0.240 |
| 200 | 1.9 | 3.0 | 0.226 | 2.5 | 2.8 | 0.226 | 2.5 | 3.0 | 0.226 |
| 300 | 2.3 | 3.4 | 0.358 | 2.5 | 3.0 | 0.321 | 2.5 | 3.2 | 0.330 |
| 400 | 2.6 | 3.6 | 0.321 | 2.6 | 3.4 | 0.330 | 2.1 | 3.2 | 0.160 |
| 500 | 2.9 | 4.0 | 0.179 | 2.6 | 3.6 | 0.330 | 2.6 | 3.4 | 0.320 |
Medium used—Mueller Hinton Agar; Temperature conditions 37 ± 2 °C
D/w distilled water, CIP ciprofloxacin, ND not detected
aAverage of triplicates
When these results were compared by calculating CD5% using one-way ANOVA, they suggested a significant decrease in the antimicrobial activity of degraded products produced when CIP was incubated with P. ostreatus. These results are supported by Wetzstein et al. (1999) who found that another fungus Gloeophyllum striatum reduced the activity of CIP in liquid culture to 0–33% after 13 weeks.
These results are also in agreement with the growth and enzymatic studies of P. ostreatus in presence of CIP. The stimulatory effect of CIP concentration on the growth of P. ostreatus can be attributed to an increase in amount of enzyme production in the presence of CIP. Extracellular enzymes of P. ostreatus have been found to have a degradative effect on various environmental pollutants in past studies (Tellez et al. 2013; Collins et al. 1997) and can possibly be involved in the degradation of CIP using similar metabolic pathways. These results are of much significance since they indicate that treatment of CIP containing effluent with P. ostreatus might effectively decrease antimicrobial activity of the antibiotic, thereby leading to a negative effect on the development of antibiotic resistant bacteria in nature.
Conclusion
Pleurotus ostreatus, a basidiomycetous fungus has shown a considerable biodegradation potential towards antibiotic ciprofloxacin. Not only was P. ostreatus found to have a high tolerance to CIP, but it was also observed that CIP has a stimulatory effect on the fungus as observed by increased mycelial growth and enzyme activity.
Spectrophotometric methods were better at detection of CIP than titrimetric methods with Indigo carmine assay being the most sensitive assay in detection of CIP.
The products produced after degradation of CIP were found to have a reduced antimicrobial activity against test microorganisms as detected from their zones of inhibition.
To the best of our knowledge, the effect of ciprofloxacin on growth of P. ostreatus and its subsequent degradation by the fungus has not been reported before.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Ahmed SA, Kadam JA, Mane VP, Patil SS, Baig MMV. Biological efficiency and nutritional contents of Pleurotus florida (Mont.) Singer cultivated on different agro-wastes. Nat Sci. 2009;7(1):44–48. [Google Scholar]
- Basavaiah K, Nagegowda P, Somashekar BC, Ramakrishna V. Spectrophotometric and titrimetric determination of CIP based on reaction with cerium (IV) Sulfate. Sci Asia. 2006;32:403–409. doi: 10.2306/scienceasia1513-1874.2006.32.403. [DOI] [Google Scholar]
- Batt AL, Kim S, Aga DS. Comparison of the occurrence of antibiotics in four fullscale wastewater treatment plants with varying designs and operations. Chemosphere. 2007;68(3):428–435. doi: 10.1016/j.chemosphere.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Bucht B, Eriksson KE. Extracellular enzyme system utilized by the rot fungus Stereum sanguinolentum for the breakdown of cellulose. I. Studies on the enzyme production. Arch Biochem Biophys. 1968;124(1):135–141. doi: 10.1016/0003-9861(68)90312-3. [DOI] [PubMed] [Google Scholar]
- Butaye P, Devriese LA, Haesebrouck F. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin Microbiol. 2003;16(2):175–188. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PJ, Field JA, Teunissen P, Dobson ADW. Stabilization of lignin peroxidases in white rot fungi by tryptophan. Appl Environ Microbiol. 1997;63(7):2543–2548. doi: 10.1128/aem.63.7.2543-2548.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan S, Lal R, Hanspal M, Kuhad RC. Effect of antibiotics on growth and laccase production from Cyathus bulleri and Pycnoporus cinnabarinus. Biores Technol. 2005;96:1415–1418. doi: 10.1016/j.biortech.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84(4):634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Espindola LHS, Espindola FS, Freitasi GRD, Brandeburgo MAM. Biodegradation of red 40 dye by the mushroom Pleurotus florida. Biosci J. 2007;23(3):90–93. [Google Scholar]
- Froehner SC, Eriksson KEL. Induction of Neurospora crassa laccase with protein synthesis inhibitors. J Bacteriol. 1974;120(1):450–457. doi: 10.1128/jb.120.1.450-457.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhu Z, Li H. Biodegradation of sulfamethoxazole by Phanerochaete chrysosporium. J Mol Liq. 2014;198:169–172. doi: 10.1016/j.molliq.2014.06.017. [DOI] [Google Scholar]
- Hernandez F, Rivera A, Ojeda A, Zayas T, Cedillo L. Photochemical degradation of the CIP antibiotic and its microbiological validation. J Environ Sci Eng. 2012;A1:448–453. [Google Scholar]
- Hubicka U, Zmudzki P, Talik P, Witek BZ, Krzek J. Photodegradation assessment of CIP, moxifloxacin, norfloxacin and ofloxacin in the presence of excipients from tablets by UPLC-MS/MS and DSC. Chem Cent J. 2013;7:133. doi: 10.1186/1752-153X-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegatheesan M, Kumaran MS, Eyini M. Enhanced production of laccase enzyme by the white-rot mushroom fungus Pleurotus florida using response surface methodology. Int J Curr Res. 2012;4(10):025–031. [Google Scholar]
- Kuwahara M, Glenn JK, Morgan MA, Gold MH. Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. KEBS Lett. 1984;69(2):247–250. [Google Scholar]
- Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- Martens R, Wetzstein HG, Zadrazil F, Capelari M, Hoffmann P, Schmeer N. Degradation of the fluoroquinolone enrofloxacin by wood-rotting fungi. Appl Environ Microbiol. 1996;62(11):4206–4209. doi: 10.1128/aem.62.11.4206-4209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles RD, Butcher GD, Henry PR, Littell RC. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult Sci. 2006;85(3):476–485. doi: 10.1093/ps/85.3.476. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Nijhu RS, Jhankar YM, Sutradhar KB. Development of an assay method for simultaneous determination of CIP and naproxen by UV spectrophotometric method. Stamford J Pharma Sci. 2011;4(1):84–90. [Google Scholar]
- Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72(9):4996–5003. doi: 10.1128/IAI.72.9.4996-5003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praveen K, Usha KY, Rajasekhar Reddy B. Effect of antibiotics on ligninolytic enzymes production from Stereum ostrea and Phanerochaete chrysosporium under submerged fermentation. Int J Pharm Pharm Sci. 2012;4(3):135–138. [Google Scholar]
- Rana IS, Rana AS. Lignocellulolytic enzyme profile of Agaricus and Pleurotus species cultured on used tea leaves substrate. Adv Bio Tech. 2011;11(6):10–14. [Google Scholar]
- Rodríguez-Rodríguez CE, García-Galán MA, Blánquez P, Díaz-Cruz MS, Barceló D, Caminal G, Vicent T. Continuous degradation of a mixture of sulfonamides by Trametes versicolor and identification of metabolites from sulfapyridine and sulfathiazole. J Hazard Mater. 2012;213–214:347–354. doi: 10.1016/j.jhazmat.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Rogalski J, Lundell TK, Leonowicz A, Hatakka AI. Influence of aromatic compounds and lignin on production of ligninolytic enzymes by Phlebia radiata. Phytochem. 1991;30(9):2869–2872. doi: 10.1016/S0031-9422(00)98215-3. [DOI] [Google Scholar]
- Sandhu DK, Arora DS. Laccase production by Polyporus sanguineus under different nutritional and environmental conditions. Experimentia. 1985;41:355–356. doi: 10.1007/BF02004501. [DOI] [Google Scholar]
- Shah Z, Dighe A, Londhe V. Pharmacoeconomic study of various brands of antibiotic medications in India. World J Parma Res. 2015;4(3):1600–1606. [Google Scholar]
- Singh MP, Pandey AK, Vishwakarma SK, Srivastava AK, Pandey VK. Extracellular xylanase production by Pleurotus species on lignocellulosic wastes under in vivo condition using novel pretreatment. Cell Mol Biol (Noisy-le-grand) 2012;58(1):170–173. [PubMed] [Google Scholar]
- Singh RN, Sahoo S, Mishra U, Garnaik B, Sahoo SK, Hati D. Stability indicating RP-HPLC method development and validation of norfloxacin. Am J Adv Drug Deliv. 2013;1(5):743–758. [Google Scholar]
- Tellez MT, Diaz R, Sanchez C, Godinez GD. Hydrolytic enzymes produced by Pleurotus species. Afr J Microbiol Res. 2013;7(4):276–281. doi: 10.5897/AJMR12x.016. [DOI] [Google Scholar]
- Thillaimaharani KA, Sharmila K, Thangaraju P, Karthick M, Kalaiselvam M. Studies on antimicrobial and antioxidant properties of oyster mushroom Pleurotus florida. Int J Pharm Sci Res. 2013;4(4):1540–1545. [Google Scholar]
- Tien M, Kirk TK. Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci. 1984;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener HC, Aarestrup FM, Jensen LB, Hammerum AM, Bager F. Use of antimicrobial growth promoters in food animals and Enterococcus faecium resistance to therapeutic antimicrobial drugs in Europe. Emerg Infect Dis. 1999;5(3):329–335. doi: 10.3201/eid0503.990303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzstein HG, Stadler M, Tichy HV, Dalhoff A, Karl W. Degradation of CIP by basidiomycetes and identification of metabolites generated by the brown rot fungus Gloeophyllum striatum. Appl Environ Microbiol. 1999;65(5):1556–1563. doi: 10.1128/aem.65.4.1556-1563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]