Abstract
The phenolic content, antioxidant, antitumor, and enzyme inhibitory activities of commonly used medicinal herbs from a Unani system of medicine were investigated using four different extraction methods. Among the plants studied, the Hyssopus officinalis L, Origanum vulgare L, and Portulaca oleracea L. extracts showed the highest amount of total phenolics (64.40, 60.35, and 58.81 mg GAE/g) and revealed significant antioxidant activities. The plants also showed a maximum cytotoxic activity as indicated by H. officinalis (82%), O. vulgare (75%), and P. oleracea (72%) showed more than 70% cytotoxicity for breast cancer cells, 82% of the cells were dead at the concentration of 500 mg/mL. The plants H. officinalis, P. oleracea, O. vulgare, and Rubia cordifolia L. revealed more than 80% inhibition towards xanthine oxidase and comprising maximum 70% of inhibition for superoxide dismutase. From results we conclude that there is a strong correlation between phenolic content, antioxidant, and enzyme inhibitory activity among these plants, indicating phenolics are the major compounds for these biological activities. Furthermore, this study provides the basis for the therapeutic importance of studied plants as latent inhibitors of oxidative stress and antitumor cell proliferation which correlate with the ethnobotanical data contained in the Unani system of medicine.
Keywords: Unani medicine, Total phenolics, Antioxidant, Xanthine oxidase, Antitumor
Introduction
Plants have been found of great importance due to their medicinal and nutritional properties with a primary source of bioactive compounds. The plants and plant products are found throughout human history as herbal supplements as botanicals, nutraceuticals, and drugs (Ekor 2014). About 60–80% of the world’s population still relies on traditional medicine for the treatment of common illnesses (Ravishankar and Shukla 2007). India is one of the world’s largest producer of medicinal plants and herb products and with recognized systems of traditional medicines such as Naturopathy, Homeopathy, Ayurveda, Siddha, and Unani (Samal 2016). The Indian medicinal plants have also been utilized in the number of modern system of medicines for the development of polyherbal formulations and various drugs (Ravishankar and Shukla 2007). The ethnobotanical survey for India showed that about 20,000 medicinal plants were recorded and only 7500 plants were utilized by the traditional practitioners for drug development, and about 25,000 herb-based formulations were prepared and provided in traditional Indian medicines for curing various disorders (Pandey et al. 2013). The Unani System of Medicine was first introduced in India by Arab, Greek, and Persian settlers which were originated in Greece. Unani Medicine is a synthesis of traditional medicine in Egypt, Syria, Iraq, Iran, China, India, and other Far East countries (Ansari 1985). The Arabs first introduced the Unani system of medicine in India. Nowadays, the Unani system of medicine is practiced India, Pakistan, and Bangladesh and in the case of India, the maximum rural and some urban population are dependent for their health care on Unani system of medicine (Izhar 1989). In the Unani system of medicine, mostly the polyherbal plant formulations were preferred over single drugs or combinations of drugs which are considered to have maximum activity without any side effects for human (Ravishankar and Shukla 2007). Many aromatic and medicinal plants contain a broad range of bioactive compounds such as phenolic compounds (phenolic acids, flavonoids, coumarins, tannins, stilbenes, and lignans), nitrogen compounds (amines, alkaloids, and betalains), vitamins, carotenoids, and other endogenous metabolites having different biological activities (Cai et al. 2003, 2004). Concerning these properties of plants and herbs, their bioactive constituents have been widely used for the preparation of various formulations and drugs in the food and pharma industry for their flavoring and biological activities (Kosar et al. 2008). Plant extracts are complex mixtures which consist of numerous bioactive compounds which can be extracted using various solvents and several extraction techniques. Different in screening models such as such as plant bioassays, tissue or cell culture, receptor enzyme and bio-chromatography were used for instigation biological activities of secondary metabolites from plants ( Wang et al. 2011; Skotti et al. 2014). Aqueous extracts of herbs and medicinal plants have been attracting wide attention since they are consumed on a daily basis as decoctions or infusions, due to their remedial actions designated by bioactive polyphenolic components including phenolic acids and flavonoids (Pietta 2000; Surveswaran et al. 2007). However, it is crucial to check the toxicity of any crude extract before given orally, and care should be taken during supplementation in high doses as they have been not entirely authenticated (Tajkarimi et al. 2010). In this context, the present work formulated to analyze antioxidant, anticancer, and enzyme inhibitory activity together with the characterization of phenolics from plants used in the Unani system of medicine. Also, the current study reports a screening program of anticancer activity against breast cancer MCF-7 human carcinoma.
Materials and methods
Plant samples
The plant species were selected based on pharmacological and economical use with health benefit claims as per Unani system of medicine. The fully grown matured plant samples were collected in the month of September–October, 2015, from Marathwada region, Maharashtra, India, and identified by Dr. Datta Bandapalle (Manjara Ayurved Medical College, Maharashtra, India). A voucher specimen (Accession# Plant Name/UMP/COCSIT/101–110) was deposited in College herbarium. For every reference, full particulars of the experimental plants are presented in Table 1. All the plant materials were washed and rinsed with distilled water repeatedly to remove any soil or solid particulates and shade dried at 40 °C for 2 days. After drying, the moisture content (below 10%) was checked and ground to a fine powder using a mechanical blender and stored in desiccators at 30 °C, before extraction.
Table 1.
Commonly used herbs in Unani system of medicine selected for this study
| Botanical name | Family | Local name | Unani name | Part used | Voucher specimen |
|---|---|---|---|---|---|
| Rubia cordifolia Linn. | Rubiaceae | Manjith | Majeeth | Roots | RC/UMP/COCSIT/101 |
| Rauwolfia serpentina Benth. ex Kurz | Apocynaceae | Sarpagandha | Asrol | Roots | RS/UMP/COCSIT/102 |
| Origanum vulgare Linn. | Lamiaceae | Basla-ghas | Mirzanjosha | Aerial | OV/UMP/COCSIT/103 |
| Hyssopus officinalis Linn. | Lamiaceae | Jufa | Zufa | Aerial | HO/UMP/COCSIT/104 |
| Cichorium intybus Linn. | Asteraceae | Kasni | Hindaba | Whole | CI/UMP/COCSIT/105 |
| Malva sylvestris Linn. | Malvaceae | Gulkhair | Khubazi | Whole | MS/UMP/COCSIT/106 |
| Portulaca oleracea Linn. | Portulacaceae | Kulfa | Khurfah | Whole | PO/UMP/COCSIT/107 |
| Aristolochia indica Linn. | Aristolochiaceae | Isharmul | Zaravand | Roots | AI/UMP/COCSIT/108 |
| Achyranthes aspera Linn. | Amaranthaceae | Puthkanda | Chirchita | Roots | AS/UMP/COCSIT/109 |
| Symplocos racemosa Roxb. | Symplocaceae | Lodhra | Lodh Pathani | Stems | SR/UMP/COCSIT/110 |
Materials
Folin–Ciocalteu’s reagent, DPPH (2,2-diphenyl-1-picryhydrazyl), ferric chloride, β-Carotene, linoleic acid, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were purchased from Aldrich (Mumbai, India). Superoxide dismutase (SOD) assay kit (Cayman Chemicals, MI, USA). Potassium persulfate (K2S2O8), sodium carbonate (Na2CO3), absolute ethanol and petroleum ether were supplied by Merck Chemicals (Mumbai, India). All the other chemicals were of analytical grade.
Types of cancer cell lines
MCF-7 human carcinoma cell lines obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA).
Extraction of plant material
The dried plant samples were extracted using four different extraction methods: microwave assisted extraction (MAE), ultrasound-assisted extraction (UAE), Soxhlet extraction (SE), and Maceration (MC). The herbal infusions were then filtered through a Whatman filter No. 1, before analysis. The extracts obtained after evaporation of the organic solvent were weighed for calculation of extraction yield. Three individual preparations of each extract were prepared and used to determine the total phenolics and various biological activities.
Extraction yield
The yield for particular extraction method using different solvents is nothing but the measurement of the potency of solvent to extract components from the crude plant material, and it was defined as the amount of extract recovered in mass compared with the initial amount of plant material. Extraction yield presented with % value and was determined by each one of techniques tested here in this study (Zhang et al. 2007).
Microwave-assisted extraction (MAE)
About 5 g of each powdered plant material was extracted with 100 mL of absolute ethanol in a focused microwave (CEM Discover) for 10–40 min. On a mass yield basis, an extraction time of 20 min at 150 W microwave powers and 60 °C temperatures was taken as optimum. The extracts were filtered and concentrated to dryness under vacuum (temperature, 40–45 °C) and then subjected to lyophilization until a constant weight was obtained (Karabegovic et al. 2014).
Ultrasound-assisted extraction (UAE)
About 5 g each of powdered plant material was extracted with ethanol (500 mL). Sonication was performed for 20 min in an ultrasonic cleaning bath (Elma Ultrasonic, Germany). The temperature was controlled and maintained at 60 °C (±1 °C) by water circulating from a thermostated bath using a pump. An extraction time of 1 h was considered as optimum on a mass yield basis. The extracts were filtered and concentrated to dryness under vacuum (temperature, 45 °C) and then subjected to lyophilization until a constant weight was obtained (Karabegovic et al. 2014).
Soxhlet extraction
About 5 g each of powdered plant material was extracted with 200 mL of absolute ethanol for 5–10 h in a Soxhlet apparatus. An extraction time of 8 h was taken as optimum by mass yield. The extracts were filtered and concentrated to dryness under vacuum (temperature, 45 °C) and then subjected to lyophilization until a constant weight was obtained (Aspé and Fernández 2011).
Maceration
About 5 g each of powdered plant material was macerated overnight in 100 mL of absolute ethanol, ≥99.8% (GC), at 70 °C. The extract obtained was filtered and concentrated entirely under vacuum (temperature, 45 °C) and lyophilized until a constant weight was obtained (Aspé and Fernández 2011).
Determination of total phenolic content
Folin–Ciocalteu colorimetric method was used for total phenolic determination. The results were calculated as mg gallic acid equivalents (GAE) mg GAE/100 g extract (Nile and Park 2013).
Determination antioxidant activity
DPPH radical scavenging assay
The DPPH radical scavenging potential of the each plant extracts was conducted by mixing. The DPPH radical (DPPH•) solution (60 µM) was prepared in ethanol. The DPPH• solution had an absorbance of 0.680 ± 0.050 at 515 nm. For this assay, 0.5-mL of the DPPH• solution was mixed with 100 µL of the tested extracts. The obtained mixture was properly shaken using vortex mixture for 5 min, and absorbance was recorded (515 nm) against a blank after 45 min of incubation (UV–vis Shimadzu) when the reaction reached a steady state. The radical scavenging activity was calculated (% inhibition) as: % inhibition = [A S (o) − A E (t)/A S (o)] × 100, where A S (o) is the absorbance of standard at t = 0 min and A E(t) is the absorbance of extract at t = 1 h and the % of DPPH radical inhibition converted to µmol Trolox/g extract (Nile and Park 2014). All determinations were carried out three times.
Reducing power assay
The Fe3+-reducing power of each plant extracts was determined by the method of Nile and Park (2015), with a slight modification. The reaction flask consists of 100 µL extracts, 0.5% v/v dimethyl sulfoxide, and 5 mL of potassium ferricyanide (1 mM) solution which was incubated for 30 min in water bath at (50 °C). Finally, the reaction was terminated using 3 mL of TCA (10%). Further, the upper portion of the reaction mixture (5 mL) was mixed with 5 mL distilled water, and 1 mL FeCl3 solution (0.01%). The absorbance of the sample was measured at 593 nm spectrophotometrically after cooling for 10 min at room temperature, using an appropriate blank solution. The calibration curve was constructed using Trolox (100–2000 μM) and the results were expressed in µmol Trolox/g extract. All values were taken in triplicates, and mean ± SD values were calculated.
β-Carotene–linoleic acid assay
The antioxidant activity of the ethanol extracts, based on coupled oxidation of β-carotene and linoleic acid emulsion, was evaluated following a modified method by Husein et al. (2014). The calibration curve was constructed using Trolox (100–2000 μM), and the results were expressed in µmol Trolox/g extracts. All determinations were carried out three times.
Xanthine oxidase (XO) enzyme inhibition
Bovine milk XO activity was measured based on colored compounds formation called formazan in the reaction mixture as end product at 550 nm using a UV–visible spectrophotometer at 25 °C. The reaction mixture in the sample tube consisted of xanthine oxidase (obtained from bovine milk, grade 1, ammonium sulfate suspension, Sigma-Aldrich, 500 μM final concentration) in phosphate buffer (0.01 M, pH 8.75, 30 μL), NBT (50 μL, 100 μM, final concentration), PMS (50 μL, 100 μM final concentration), Triton X-100 (20 μL, 0.5%) and plant extracts 5 g each. After 2 h of incubation at 37 °C in a water bath, the absorbance at 550 nm was read. All values were expressed as the means of three experiments. Xanthine was omitted from blank samples. Allopurinol was used as a positive control. The percent inhibition was calculated using inhibitor concentration–activity curve (Nile and Khobragade 2011; Nile and Park 2013).
Superoxide dismutase (SOD) enzyme inhibition
For SOD assay 5 g of powdered plant materials extracted was taken in Erlenmeyer flask and placed in a thermostatic water bath for 1 h at 60 ± 1 °C. After incubation, the extracts were dried after filtration and concentrated to dryness under vacuum (temperature, 45 °C) and then subjected to lyophilization until a constant weight was obtained. Final extract was collected and used for SOD assay. WST (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulphophenyl)-2H-tetrazolium, monosodium salt) reduction method is used for superoxide anion scavenging activity of plant extracts which was determined by SOD assay kit-WST; vitamin C was used as a standard. In this, the WST-1 salt is reduced by O2•− with the formation of a yellow-colored complex called formazan and color absorbance was measured spectrophotometrically at 450 nm (Dudonne et al. 2009). The % inhibition of superoxide radicals was calculated using the formula: % inhibition = [(A B−A E)/A B] × 100, where A B = absorbance of the blank sample and A E = absorbance of the plant extract. All measurements were done in triplicate.
Cytotoxicity assay
The MCF-7 human carcinoma cells were cultured in RPMI 1640 medium and maintained as the per the method described by Husein et al. (2014) and the cell viability was determined using Trypan blue exclusion, and it exceeds 96% as counted in a hemocytometer. Stock cultures were duplicated weekly after inoculation. The cell line was cultured in 96-well tissue culture plates (9.8 cm2) and incubated at 35 °C in a humidified atmosphere containing 5% CO2. After 24 h, the cells were treated with 50 µL extracts (50 µg/mL). The MTT assay determined the anticancer activity of samples on MCF-7 human carcinoma cells. Cells (1 × 105/well) were added in plates using 0.5 mL of medium/well in 96-well plates. After addition and incubation of sample, the medium was removed, and the cells were washed with minimum essential medium (MEM; w/o) fetal calf serum (FCS) for 4–5 times, and 200 µL of MTT (5 mg/mL) was added. The plates were incubated for 6–7 h after addition of diluted extract (500 mg/mL) in 5% CO2 incubator for cytotoxicity. After incubation, 1 mL of DMSO (solubilizing reagent) was added to each well and mixed well by micropipette and left for 1 min. The presence of viable cells was visualized by the development of purple color due to the formation of formazan crystals. The absorbance was measured spectrophotometrically (595 nm) by using DMSO as a blank (Senthilraja and Kathiresan, 2015). Measurements were performed in triplicates, and the extent of cell death was expressed as the percentage of cell viability in comparison with control cells using the following formula: Cell viability (%) = Mean OD/control OD × 100%.
Statistical analysis
Data were expressed as mean ± SD. One-way analysis of variance (ANOVA) was applied followed by Tukey’s test, and the values were considered significant at p < 0.05. The correlation between the antioxidant activity, toxicity, and total phenolic content was determined using Pearson’s correlation method. All statistical analysis was calculated using SPSS version 14.0 statistical software.
Result and discussion
Extraction and extraction yield
Many methods were employed for the extraction and isolation of plant phytochemicals such as solvent extraction, homogenization, maceration, soxhlet extraction, grinding, and ultrasound treatment (Stalikas 2007). Biologically active compounds usually occur in very low concentration in plants and extraction yield and the quality of herbal extracts were mainly affected by extraction methods, nature of phytochemicals, particle size, composition, nature of the solvent, and the presence of interfering substances (Quispe-Candori et al. 2008). Therefore, it is necessary to select the suitable extraction method as well as solvent based on sample matrix properties, chemical properties of the analytes, matrix-analyte interaction, efficiency, and desired properties (Hayouni et al. 2007). Although extraction of bioactive compounds from the plants has been extensively investigated using conventional extraction methods, in the present study we selected some traditional and advanced methods such as microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), Soxhlet extraction (SE), and maceration (MC). In this study, the extraction yields ranged from 24.9 (P. oleracea) to 2.6% (R. serpentina) by all extractions methods. The order for extraction yield for all plants samples with respect to methods was decreased in the following manner: microwave-assisted extraction (MAE) > ultrasound-assisted extraction (UAE) > Soxhlet extraction (SE) > maceration (MC). Considering the individual plant species the extraction yield decreased in the following order: P. oleracea) > H. officinalis > O. vulgare > M. sylvestris > C. intybus > S. racemosa > A. indica > R. cordifolia > A. aspera > R. serpentine for all studied extractions methods (Table 2). It is evident from results that the conventional and advanced methods are more superior to classical methods of extractions for plant samples. Possibly, this could be due to the higher polarity of ethanol and also at elevated extraction temperature as in the case of MAE and UAE; ethanol has a similar dielectric constant as organic solvents like methanol and acetonitrile (Dhanani et al. 2017). That the heat kinetics and pressure effects on plant cell wall through MAE and UAE resulted in the faster diffusion or increased partition rate for the solute from the solid matrix into solvent may be the probable reason for the highest yield in MAE and UAE (Terigar et al. 2011). Also improved extract yield in the case of MAE and UAE may be explained regarding the hyperinflation effects caused due to the high intensity of ultrasound (Li et al. 2004). Among the different extraction methods, the successive MAE and UAE method were found to have greater recovery over other extraction methods.
Table 2.
Extraction yield and total phenolic content in selected medicinal plants with different extraction methods
| Samples | Extraction yield (g/100 g of DW) | Total phenolic content (mg GAE/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| MAE | UAE | SE | MC | MAE | UAE | SE | MC | |
| R. cordifolia | 9.6 ± 0.42c | 5.5 ± 0.52a | 4.9 ± 0.22c | 3.4 ± 0.41b | 47.65 ± 1.10c | 40.44 ± 1.08b | 35.99 ± 0.99c | 31.65 ± 1.41b |
| R. serpentina | 6.4 ± 0.24b | 4.6 ± 0.28d | 3.2 ± 0.16b | 2.6 ± 0.36d | 35.88 ± 1.64d | 27.12 ± 1.15a | 23.54 ± 1.26d | 18.88 ± 1.62c |
| O. vulgare | 21.5 ± 0.67a | 18.1 ± 0.45b | 12.8 ± 0.44d | 10.2 ± 0.46a | 65.40 ± 1.58b | 56.22 ± 2.11d | 50.88 ± 1.32b | 46.89 ± 1.49d |
| H. officinalis | 23.4 ± 0.36b | 20.6 ± 0.48a | 15.1 ± 0.25d | 12.7 ± 0.14c | 60.35 ± 1.12a | 53.75 ± 1.98b | 47.44 ± 2.01a | 42.80 ± 1.34a |
| C. intybus | 13.7 ± 0.24c | 10.8 ± 0.61c | 7.2 ± 0.33a | 5.0 ± 0.12b | 30.57 ± 1.77c | 26.01 ± 1.03c | 19.67 ± 1.48b | 15.55 ± 1.12a |
| M. sylvestris | 20.2 ± 0.42a | 16.6 ± 0.61b | 11.6 ± 0.31c | 8.5 ± 0.23b | 45.26 ± 0.42a | 39.98 ± 1.39b | 35.92 ± 1.01d | 30.12 ± 1.08b |
| P. oleracea | 24.9 ± 0.36c | 18.8 ± 0.38a | 12.5 ± 0.34b | 9.6 ± 0.50d | 58.81 ± 1.39d | 50.92 ± 2.10d | 45.90 ± 1.91a | 40.25 ± 1.65c |
| A. indica | 9.8 ± 0.22b | 7.8 ± 0.64d | 6.5 ± 0.71c | 4.9 ± 0.41b | 37.33 ± 1.53c | 30.56 ± 1.75a | 26.90 ± 1.11c | 23.33 ± 1.03 |
| A. aspera | 8.5 ± 0.44d | 6.9 ± 0.22b | 5.8 ± 0.26a | 4.3 ± 0.65a | 27.85 ± 1.42b | 20.12 ± 1.11b | 15.95 ± 1.05d | 12.66 ± 0.99d |
| S. racemosa | 10.5 ± 0.58c | 9.8 ± 0.43a | 8.2 ± 0.58c | 6.5 ± 0.61c | 39.65 ± 1.22a | 33.22 ± 1.09d | 28.15 ± 1.77a | 25.43 ± 1.54c |
Values are mean ± SD (n = 3). The letters a, b, c, d, e indicate if there is a significant difference between microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), Soxhlet extraction (SE), and maceration (MC) extracts
Total phenolics
Plant phenolics are nothing but the secondary metabolites from plants with various pharmacological and functional properties. These phenolic compounds neutralize reactive oxygen species or free radicals by donating a hydrogen atom or an electron chelating metal ion in aqueous solutions (Nile and Park 2015). In this study, the analysis of phenolic content in plants from the Unani system of medicine was measured using the Folin–Ciocalteu (FC) method (Table 2). The total phenolic content for these plants ranged from 65.40 to 15.55 mg GAE/g, with greater variation coefficient between the plants of up to 420.5-fold. The extraction methods also affected the concentrations of phenolics in plants showing highest percent of phenolics to lowest percent as per the following manner: microwave-assisted extraction (MAE) > ultrasound-assisted extraction (UAE) > Soxhlet extraction (SE) > maceration (MC). O. vulgare showed the highest phenolic content (64.40–46.89 mg GAE/g), followed by H. officinalis (60.35–42.80 mg GAE/g), P. oleracea (58.81–40.25 mg GAE/g), R. cordifolia (47.65–31.65 mg GAE/g), M. sylvestris (45.26–30.12 mg GAE/g), S. racemosa (39.65–25.43 mg GAE/g), A. indica (37.33–23.33 mg GAE/g), R. serpentina (35.88–18.88 mg GAE/g), and C. intybus (30.57–15.55 mg GAE/g), whereas A. aspera (27.85–12.66 mg GAE/g) showed the lowest phenolic content of these plants. The phenolic compounds extracted from plants possess multiple biological properties such as antimicrobial, antioxidant, anti-inflammatory, anticancer, antidiabetic, and anti-mutagenic properties, related to functional groups present on each phenolic compound (Shui and Leong 2002).
Antioxidant activity
The bioactive compounds extracted from plants such as phenolics and flavonoids possess multifunctional properties. Hence it is important to check its biological activities using different methods, and herein for this study, we assessed antioxidant activity of the plant extracts using DPPH, FRAP, and β-carotene/linoleic acid assay. Trolox calibration curves had first to be plotted for each of the methods tested. In all the methods the slopes, intercepts, and R 2 values are high for all the methods studied, showing a significant dose–response curve. Due to high stability and hydrogen atom/electron donation capacity, the DPPH radical was widely used to monitor the antioxidant abilities in the solution for various samples or extracts (Ferreira et al. 2006). DPPH-free radical has a red/dark violet color due to its impaired electron which has been scavenged with the generation of yellow-colored complex and detected spectrophotometrically by the loss of absorbance at 517 nm (Nile and Park 2015). The fundamental principle of FRAP assay is a reduction of ferric ions (Fe3+) to ferrous ions (Fe2+) at low pH with the formation of the blue color complex (Fe2+/TPTZ) which may serve as a measure of the antioxidant ability of the extracts (Samaradivakara et al. 2016). In β-carotene/linoleic acid assay, there is discoloration of β-carotene in reaction with linoleic acid-free radical which was generated upon the increased temperature due to the removal of hydrogen atoms coupled in two double bonds of linoleic acid (Amarowicz et al. 2004). This reaction leads the loss of conjugation which automatically affects the decrease in absorbance at 470 nm for β-carotene, and thus the antioxidants present in solution protect reduction of β-carotene through reacting with other free radicals or linoleate-free radical in reaction system (Mraihi et al. 2013). In this study, we found a considerable variation in the antioxidant capacity measured with DPPH, FRAP, and β-carotene/linoleic acid antioxidant assays using different extractions methods for medicinal plants selected from the Unani system of medicine (Table 3). The values ranged from 296.5 to 45.8, 285.6 to 41.9, 226.8 to 35.6, and 206.8 to 30.1 μmol Trolox (TE)/g extracts dry weight for microwave-assisted extraction (MAE) > ultrasound-assisted extraction (UAE) > Soxhlet extraction (SE) > maceration (MC) extracts as per the order showed by DPPH, FRAP, and β-carotene/linoleic acid antioxidant assays, respectively. Highest levels of antioxidant activity were revealed by of ethanol extracts of H. officinalis 296.5–206.8 μmol TE/g extract dry weights, P. oleracea 255.6–188.1 μmol TE/g extract dry weight, and O. vulgare 210.5–150.2 μmol TE/g extract dry weights. The lowest levels of antioxidant activity were obtained from the ethanol extracts R. serpentina 56.8–40.8 μmol TE/g extract dry weights; C. intybus 50.1–31.9 μmol TE/g extract dry weight, and A. aspera 45.8–30.1 μmol TE/g extract dry weight. Many plant extracts in this study with different antioxidant assays and extraction methods showed good levels of antioxidant activity when compared with Trolox, well-known standard antioxidants. In this study, we observed that the antioxidant activity of each plant extract was influenced by choice of extractions and antioxidant methods used for analysis.
Table 3.
Antioxidant activities estimated by DPPH•, reducing ability, and β-carotene–linoleic acid assays for selected plant extracts
| Samples | Antioxidant assays | Antioxidant activities (μmol Trolox/g) | |||
|---|---|---|---|---|---|
| MAE | UAE | SE | MC | ||
| R. cordifolia | DPPH | 198.2 ± 2.33a | 185.7 ± 1.22c | 162.5 ± 1.10a | 120.3 ± 2.10b |
| Reducing ability | 142.3 ± 1.45d | 138.6 ± 2.10b | 113.8 ± 1.65c | 98.9 ± 1.06a | |
| β-Carotene–linoleic acid | 70.4 ± 1.64c | 63.6 ± 1.98b | 56.9 ± 1.24d | 50.9 ± 1.36a | |
| R. serpentina | DPPH | 98.3 ± 1.02a | 91.8 ± 1.32a | 88.5 ± 1.88b | 81.7 ± 1.65c |
| Reducing ability | 84.6 ± 1.84b | 79.8 ± 1.63c | 71.6 ± 1.02d | 65.9 ± 0.98a | |
| β-Carotene–linoleic acid | 56.8 ± 1.31d | 50.7 ± 1.42b | 46.9 ± 0.99c | 40.8 ± 1.03a | |
| O. vulgare | DPPH | 210.8 ± 2.36b | 201.6 ± 2.01d | 182.4 ± 1.65a | 150.2 ± 1.39c |
| Reducing ability | 165.3 ± 1.91a | 152.6 ± 2.13b | 140.8 ± 1.08c | 126.3 ± 1.44b | |
| β-Carotene–linoleic acid | 92.2 ± 2.11b | 81.9 ± 3.01c | 75.4 ± 1.24a | 66.8 ± 0.94d | |
| H. officinalis | DPPH | 296.5 ± 3.12b | 285.6 ± 2.14a | 226.8 ± 2.56d | 206.8 ± 2.64c |
| Reducing ability | 190.5 ± 1.99d | 185.6 ± 2.01b | 170.8 ± 1.55c | 161.3 ± 1.39a | |
| β-Carotene–linoleic acid | 105.6 ± 2.10b | 90.6 ± 1.34d | 84.7 ± 1.12a | 76.2 ± 1.04d | |
| C. intybus | DPPH | 92.6 ± 1.31a | 85.1 ± 1.58 | 74.9 ± 1.76d | 62.8 ± 1.33b |
| Reducing ability | 75.4 ± 1.44 | 70.6 ± 1.79 | 65.9 ± 1.34c | 60.7 ± 1.69 | |
| β-Carotene–linoleic acid | 50.1 ± 1.77c | 42.5 ± 1.09d | 37.2 ± 0.88a | 31.9 ± 0.97b | |
| M. sylvestris | DPPH | 150.3 ± 1.67b | 145.7 ± 1.31b | 136.5 ± 1.01c | 125.1 ± 1.07d |
| Reducing ability | 110.6 ± 1.79d | 100.2 ± 1.51a | 95.8 ± 1.23b | 88.7 ± 1.33c | |
| β-Carotene–linoleic acid | 75.2 ± 2.01b | 70.6 ± 1.65c | 67.4 ± 1.02c | 60.3 ± 0.95d | |
| P. oleracea | DPPH | 255.3 ± 2.34a | 250.8 ± 2.21b | 215.3 ± 3.01a | 188.1 ± 1.81d |
| Reducing ability | 175.8 ± 1.14b | 168.2 ± 1.33a | 150.6 ± 1.41d | 138.5 ± 1.31c | |
| β-Carotene–linoleic acid | 100.1 ± 1.77a | 95.6 ± 1.61c | 84.2 ± 1.39d | 75.6 ± 1.50b | |
| A. indica | DPPH | 110.3 ± 1.91b | 100.5 ± 1.36d | 89.6 ± 0.86b | 80.7 ± 0.97a |
| Reducing ability | 87.2 ± 1.34d | 81.6 ± 1.16c | 74.8 ± 1.19c | 65.8 ± 1.30b | |
| β-Carotene–linoleic acid | 65.3 ± 1.70a | 56.8 ± 1.64d | 50.6 ± 0.92b | 44.1 ± 0.97c | |
| A. aspera | DPPH | 87.1 ± 1.31c | 84.5 ± 1.20b | 73.9 ± 1.60a | 65.8 ± 1.71d |
| Reducing ability | 65.5 ± 1.77b | 60.9 ± 1.09a | 55.7 ± 0.96c | 50.2 ± 0.91a | |
| β-Carotene–linoleic acid | 45.8 ± 0.89c | 40.9 ± 0.94b | 35.6 ± 0.87d | 30.1 ± 0.99a | |
| S. racemosa | DPPH | 122.5 ± 1.23b | 118.9 ± 1.50c | 102.7 ± 1.33a | 90.6 ± 1.71d |
| Reducing ability | 99.1 ± 1.11d | 90.5 ± 1.01a | 84.6 ± 1.84c | 71.2 ± 1.03b | |
| β-Carotene–linoleic acid | 60.3 ± 1.03a | 57.9 ± 0.91b | 48.4 ± 0.85d | 40.5 ± 0.99c | |
Values are mean ± SD (n = 3). Microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), soxhlet extraction (SE), and maceration (MC) extracts, Different letter indicates significant differences (p < 0.05) among treatments for the same herb sample
Correlation studies for total phenolic content (TPC), antioxidant activity and toxicity
Regardless of the presence of a wide range of phenolics and significant antioxidant capacities, we found a positive linear correlation between the Trolox equivalent antioxidant capacity (TEAC) values for the studied plants. High correlations were observed between the results obtained for plants using four different extraction methods such as MAE, UAE, SE, and MC and measurement of antioxidant capacity with three assays (DPPH, reducing power and β-carotene–linoleic acid assay) with total phenolic content (Table 4). From this study, it was shown that phenolic compounds largely contribute to the antioxidant activities and enzyme inhibitory activities from these plants. However, there were some plants, such as H. officinalis, P. oleracea, and O. vulgare, which exhibited relatively high antioxidant capacity with comparable phenolic content. This shows the presence of other antioxidant compounds in some of the medicinal plants, which meets the general agreement that the extracts of these plants often contain complex mixtures of different kinds of active compounds, and the contribution from other compounds besides phenolic should not be neglected.
Table 4.
Correlation between total phenolic content (TPC), antioxidant activity and toxicity
| Parameters | DPPH | Reducing ability | β-Carotene-linoleic acid | TPC | Toxicity |
|---|---|---|---|---|---|
| DPPH | – | 0.990 | 0.996 | 0.981 | 0.008 |
| Reducing ability | 0.990 | – | 0.990 | 0.992 | 0.010 |
| β-Carotene-linoleic acid | 0.996 | 0.990 | – | 0.996 | 0.012 |
| TPC | 0.981 | 0.992 | 0.996 | 0.998 | 0.014 |
| Toxicity | 0.008 | 0.010 | 0.012 | 0.014 | – |
Xanthine oxidase (XO) enzyme inhibition
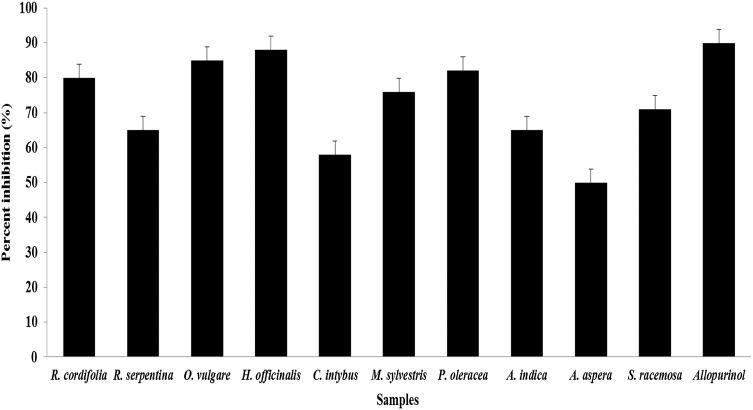
The MAE plant extracts were used for xanthine oxidase inhibition, as the MAE samples showed high phenolic composition and antioxidant activity of all plant samples and which forced to utilize this MAE extracts for the xanthine oxidase inhibition. In this study, all the plant extracts obtained through microwave-assisted extraction (MAE) methods revealed good to excellent inhibition of xanthine oxidase (Fig. 1). The values ranged from 88 to 50% as compared to (90%) allopurinol (commercial drug). The plants H. officinalis (88), P. oleracea (85), O. vulgare (82), and R. cordifolia (80) revealed more than 80% inhibition towards xanthine oxidase compared to other plants like C. intybus (58%) and A. aspera (50) revealed less than 60% inhibition towards xanthine oxidase. To our knowledge, so far there are no study or report on the activity of medicinal plants from Unani system of medicine against xanthine oxidase, but many scientists studied the xanthine oxidase inhibition using various aromatic and medicinal plant extracts (Nile and Park 2013). Recent findings revealed that the incidence of gout and hyperuricemia is increasing worldwide drastically and the possible reason may change in unusual habits of food, smoking, and drinking also, intake of foods which rich in nucleic acids, including meat, pork, and seafood (Nile and Park 2015). Hypouricemic agents like allopurinol, including xanthine oxidase inhibitors and uricosuric agents, are commonly used for the treatment of chronic gouty arthritis. In general, allopurinol is the drug of choice; however, it has serious side effects. Thus, novel alternatives with increased therapeutic activity and fewer side effects are desired (Nile and Park 2013; 2014). Thus, we attempted to identify these plants from Unani system of medicine against xanthine oxidase inhibition.
Fig. 1.
Xanthine oxidase inhibition by plant extracts. aThe data are expressed as the mean ± SD, n = 3
Superoxide dismutase (SOD) enzyme inhibition
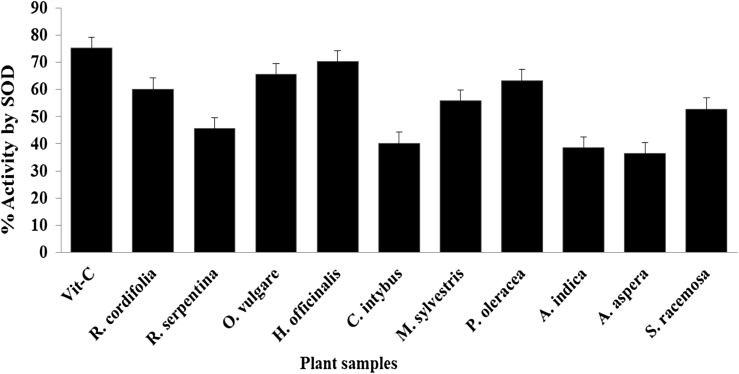
The enzyme superoxide dismutase (SOD) is a most thermo-stable and well-recognized indicator for oxidative stress defense mechanism in a human cell. This enzyme is having a most heat stable property followed by ascorbate peroxidase among the antioxidant enzymes working on reduction of reactive oxygen species and free radicals (Khanna-Chopra and Semwal, 2011). Most of the tested plant extracts induced SOD inhibition activity, ranging from 38.5 to 70.2% by all plant extracts which was extracted using microwave assisted extraction (MAE) > ultrasound-assisted extraction (UAE) > Soxhlet extraction (SE) > maceration (MC) methods as order provided herein and compared with Vitamin C (75.2), as standard. The plants H. officinalis (70.2), O. vulgare (65.5), P. oleracea (63.3), and R. cordifolia (60.1) revealed higher activity comprising more than 60% inhibition towards SOD compared to other studied plants (Fig. 2).
Fig. 2.
Superoxide dismutase (SOD) enzyme inhibition by plant extracts. aThe data are expressed as the mean ± SD, n = 3
Cytotoxicity study
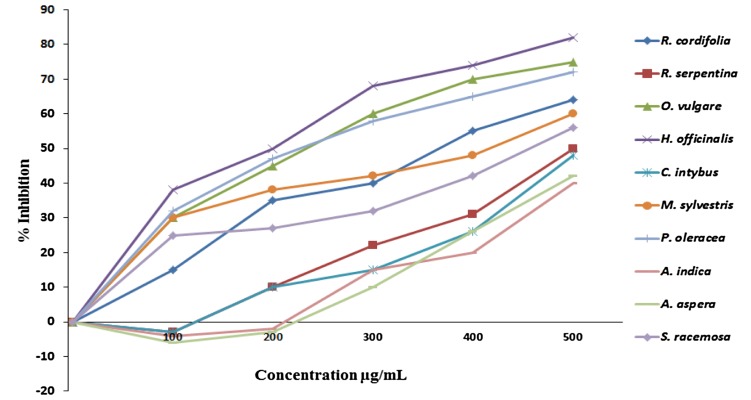
To evaluate anti-carcinogenic activity, the plant extract was completed with cell viability study using MTT assay. MTT reagent is a yellowish water-soluble tetrazolium salt which is reduced to a purple-colored complex called formazan through succinate dehydrogenase system of mitochondria in a human cell (Shoemaker et al. 2004). Epithelial cancer cells (MCF-7) were used for cytotoxicity study which is most accessible normal mammalian cell types and widely used as a model for cancer initiation and progression mechanism (Andrade et al. 2009). This study showed the percent inhibition of the ethanol extracts on breast cancer cells (MCF-7) by each plant at different concentrations (Fig. 3). The results obtained vary with the choice of plant extract and method used for extraction. H. officinalis (82), O. vulgare (75), and P. oleracea (72) showed more than 70% cytotoxicity for breast cancer cells; maximum 82% of the cells were dead at the concentration of 500 mg/mL. In contrast, C. intybus (48%), A. aspera (42), and A. indica (40) revealed less than 50% cytotoxicity towards breast cancer cells (MCF-7). These results may be attributed to the presence of bioactive compounds like phenolics and flavonoids since these phenolics and flavonoids are known to have various biological activities including inhibition of mutagenesis in human cells when ingested from different plants, vegetables, and fruits through diet (Husein et al. 2014). This study shows that the selected herbs from the Unani system of medicine probably could be used as the cytotoxic and anti-carcinogenic agent. Further research is necessary for identification and characterization of active compounds present in these plant extracts, which are responsible for the above-mentioned biological activities.
Fig. 3.
Cytotoxic effects of the extracts on breast cancer cell (MCF-7) lines by MTT assay
Conclusions
In conclusion, the results obtained suggested that the selected plants demonstrated a significant amount of phenolics with different biological activities including antioxidant, antitumor, and enzyme inhibitory activities which were strongly correlated with each studied parameter. However, further in vivo and clinical studies must be carried out for identification and characterization of active phytochemicals which are responsible for these biological properties. Based on this research study we must say that these plants from the Unani system of medicine might be used against various disorders and diseases associated with free radical or reactive oxygen species generated stress, inhibition of xanthine oxidase, superoxide dismutase, and cell cytotoxic studies. Furthermore, we conclude that these plants may be useful for the treatment of diseases like urolithiasis, hyperuricemia, gout, and cancer, which are correlated with the ethnobotanical data on the use of this plant in Indian folklore and Unani system of medicine.
Acknowledgements
This research was supported by KU-Research Professor Program-2017, Konkuk University, Seoul, Republic of Korea.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest.
References
- Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84(4):551–562. doi: 10.1016/S0308-8146(03)00278-4. [DOI] [Google Scholar]
- Andrade D, Gil C, Breitenfeld L, Domingues F, Duarte AP. Bioactive extracts from Cistus ladanifer and Arbutus unedo L. Ind Crops Prod. 2009;30:165–167. doi: 10.1016/j.indcrop.2009.01.009. [DOI] [Google Scholar]
- Ansari AA. Prospects of Unani System of Medicine in Primary Health Care in India. Indian J Hosp Adm New Delhi. 1985;XXII(1–2):223–225. [Google Scholar]
- Aspé E, Fernández K. The effect of different extraction techniques on extraction yield, total phenolic, and anti-radical capacity of extracts from Pinus radiata Bark. Ind Crops Prod. 2011;34:838–844. doi: 10.1016/j.indcrop.2011.02.002. [DOI] [Google Scholar]
- Cai YZ, Sun M, Corke H. Antioxidant activity of betalains from plants of the Amaranthaceae. J Agric Food Chem. 2003;51(8):2288–2294. doi: 10.1021/jf030045u. [DOI] [PubMed] [Google Scholar]
- Cai YZ, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanani T, Shah S, Gajbhiye N, Kumar S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab J Chem. 2017;10:S1193–S1199. doi: 10.1016/j.arabjc.2013.02.015. [DOI] [Google Scholar]
- Dudonne S, Vitrac X, Coutiere P, Woillez M, Merillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57(5):1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4(177):1–10. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Proença C, Serralheiro MLM, Araujo MEM. The in vitro screening for acethylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol. 2006;108:31–37. doi: 10.1016/j.jep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Hayouni EA, Abedrabba M, Bouix M, Hamdi M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenica L. fruit extracts. Food Chem. 2007;105:1126–1134. doi: 10.1016/j.foodchem.2007.02.010. [DOI] [Google Scholar]
- Husein AI, Ali-Shtayeh MS, Jondi WJ, Zatar NA, Abu-Reidah IM, Jamous RM. In vitro antioxidant and antitumor activities of six selected plants used in the Traditional Arabic Palestinian herbal medicine. Pharm Biol. 2014;52:1249–1255. doi: 10.3109/13880209.2014.886274. [DOI] [PubMed] [Google Scholar]
- Izhar N. The Unani traditional medical system in India: a case study in health behavior. Geographia Medica. 1989;19:163–185. [PubMed] [Google Scholar]
- Karabegovic IT, Stojičević SS, Veličković DT, Todorović ZB, Nikolić NČ, Lazić ML. The effect of different extraction techniques on the composition and antioxidant activity of cherry laurel (Prunus laurocerasus) leaf and fruit extracts. Ind Crops Prod. 2014;54(2):142–148. doi: 10.1016/j.indcrop.2013.12.047. [DOI] [Google Scholar]
- Khanna-Chopra R, Semwal VK. Superoxide dismutase and ascorbate peroxidase are constitutively more thermotolerant than other antioxidant enzymes in Chenopodium album. Physiol Mol Biol Plants. 2011;17(4):339–346. doi: 10.1007/s12298-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosar M, Demirci B, Demirci F, Baser KHC. Effect of maturation on the composition and biological activity of the essential oil of a commercially important Satureja species from Turkey: satureja cuneifolia Ten. J Agric Food Chem. 2008;56:2260–2265. doi: 10.1021/jf0732253. [DOI] [PubMed] [Google Scholar]
- Li H, Pordesimo L, Weiss J. High intensity ultrasound: assisted extraction of oil from soybeans. Food Res Int. 2004;37:731–738. doi: 10.1016/j.foodres.2004.02.016. [DOI] [Google Scholar]
- Mraihi M, Journi M, Chérif JK, Sokmen M, Sokmen A, Ayadi MT. Phenolic contents and antioxidant potential of Crataegus fruits grown in Tunisia as determined by DPPH, FRAP, and β-carotene/linoleic acid assay. J Chem Article ID. 2013;378264:6. [Google Scholar]
- Nile SH, Khobragade CN. In vitro anti-inflammatory and xanthine oxidase inhibitory activity of Tephrosia purpurea shoot extract. Nat Prod Commun. 2011;6:1437–1440. [PubMed] [Google Scholar]
- Nile SH, Park SW. Total phenolics, antioxidant and xanthine oxidase inhibitory activity of three colored onions (Allium cepa L.) Front Life Sci. 2013;7:224–228. doi: 10.1080/21553769.2014.901926. [DOI] [Google Scholar]
- Nile SH, Park SW. Antioxidant: α-glucosidase and xanthine oxidase inhibitory activity of bioactive compounds from maize (Zea mays L.) Chem Biol Drug Des. 2014;83:119–125. doi: 10.1111/cbdd.12205. [DOI] [PubMed] [Google Scholar]
- Nile SH, Park SW. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind Crop Prod. 2015;70:238–244. doi: 10.1016/j.indcrop.2015.03.033. [DOI] [Google Scholar]
- Pandey MM, Rastogi S, Rawat AKS. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid Based Complement Alternat Med. 2013;2013:376327. doi: 10.1155/2013/376327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Quispe-Candori S, Foglio MA, Rosa PTV, Meireles MAA. Obtaining β-caryophyllene from Cordia verbenacea de Candolle by super critical fluid extraction. J Supercrit Fluids. 2008;46:27–32. doi: 10.1016/j.supflu.2008.02.015. [DOI] [Google Scholar]
- Ravishankar B, Shukla VJ. Indian System of Medicine: a brief profile. Afr J Tradit Complement Altern Med. 2007;4(3):319–337. doi: 10.4314/ajtcam.v4i3.31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samal J. Medicinal plants and related developments in India: a peep into 5-year plans of India. Ind. J Health Sci. 2016;9:14–19. doi: 10.4103/2349-5006.183698. [DOI] [Google Scholar]
- Samaradivakara SP, Samarasekera R, Handunnetti SM, Weerasena OVDSJ. Cholinesterase, protease inhibitory and antioxidant capacities of Sri Lankan medicinal plants. Ind Crops Prod. 2016;83:227–234. doi: 10.1016/j.indcrop.2015.12.047. [DOI] [Google Scholar]
- Senthilraja P, Kathiresan K. In vitro cytotoxicity MTT assay in Vero, HepG2 and MCF -7 cell lines study of Marine Yeast. J App Pharm Sci. 2015;5(3):080–084. doi: 10.7324/JAPS.2015.50313. [DOI] [Google Scholar]
- Shoemaker M, Cohen I, Campbell M. Reduction of MTT by aqueous herbal extracts in the absence of cells. J Ethnopharmacol. 2004;93:381–384. doi: 10.1016/j.jep.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Shui G, Leong LP. Separation and determination of organic acids and phenolic compounds in fruit juices and drinks by high-performance liquid chromatography. J Chromatogr A. 2002;977:89–96. doi: 10.1016/S0021-9673(02)01345-6. [DOI] [PubMed] [Google Scholar]
- Skotti E, Anastasaki E, Kanellou G, Polissiou M, Tarantilis PA. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind Crops Prod. 2014;53:46–54. doi: 10.1016/j.indcrop.2013.12.013. [DOI] [Google Scholar]
- Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci. 2007;30:3268–3295. doi: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- Surveswaran S, Cai YZ, Corke H, Sun M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007;102:938–953. doi: 10.1016/j.foodchem.2006.06.033. [DOI] [Google Scholar]
- Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- Terigar BG, Balasubramanian S, Sabliov CM, Lima M, Boldor D. Soybean and rice bran oil extraction in a continuous microwave system: from laboratory to pilot scale. J Food Eng. 2011;104:208–217. doi: 10.1016/j.jfoodeng.2010.12.012. [DOI] [Google Scholar]
- Wang B, Deng J, GaoY ZhuL, He R, Xu Y. The screening toolbox of bioactive substances from natural products: a review. Fitoterapia. 2011;82:1141–1151. doi: 10.1016/j.fitote.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Zhang SQ, Bi HM, Liu CJ. Extraction of bio-active components from Rhodiola sachalinensis under ultrahigh hydrostatic pressure. Sep Purif Technol. 2007;57:277–282. doi: 10.1016/j.seppur.2007.04.022. [DOI] [Google Scholar]