Abstract
The accumulation of selenium (Se) by Lactobacillus delbrueckii ssp. bulgaricus (Lb) and Streptococcus thermophilus (St) at the different cultivation conditions, including initial pH, inoculum dose (%), and temperature (°C), was investigated in this work. Se enrichment efficiency was optimized using the Design-Expert software for response surface methodology on a basis of single-factor experiment. The antioxidant activities of Se-enriched Lactic acid bacteria (LAB) were also investigated. The qualitative analysis of Se-enriched LAB was performed by FT-IR spectra. The cell morphology and chemical element components were measured by a scanning electron microscope (SEM) equipped with energy-dispersive X-ray spectroscopy. The results indicated that the optimum initial pH, inoculum doses, and temperatures of Lb and St were 5.96, 6.73%, 33.24 °C, and 6.37, 6%, 40 °C, respectively. Under the optimal conditions, the ratios of Se enrichment reached 94.34% for Lb and 97.05% for St. Furthermore, Se-enriched LAB enhanced scavenging rates on DPPH, ABTS free radical, and also heightened reducing activity. The FT-IR results showed that the two Se-enriched strains had similar characteristic absorption peaks, which were further demonstrated that both Se biomasses had the same carbonyl, carboxyl, and hydroxyl groups. Elemental selenium nanoparticles were verified around cell surfaces of Se-enriched LAB, which implied that both strains had detoxification ability when grown in liquid media containing selenite.
Keywords: Selenium, Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Optimize, Antioxidant activity, Scanning electron microscope
Introduction
Selenium (Se) is one of the most important essential trace elements which has a recommended dietary allowance (RDA) for adults of 55 µg/day (tolerable upper level is 400 µg/day) (Monsen 2000), and it plays crucial roles in human health by its metabolic regulation, immunoregulation, fertility promotion, and anticarcinoma activity and also exerts remarkable antioxidant, antiaging, and detoxification activity (Kieliszek and Blazejak 2016; Mater et al. 2005; Navarro-Alarcon and Lopez-Martinez 2000). Se deficiency is associated with inflammation and immune responses. A recent study from Gao et al. (2016) indicated that Se deficiency resulted in increased inflammatory lesions in Staphylococcus aureus mastitis at the level of histopathological changes. Previous studies have shown that the physiological and biochemical functions of Se are closely related to its valence. Inorganic Se such as selenite (IV) or selenate (VI) is prohibited to be applied in food because of its toxicity and poor bioavailability (Kieliszek and Blazejak 2013). Reports showed that 85–95% of Se in food was absorbed in the intestine. The organic forms could be assimilated up to 90–95%, while inorganic compounds were less accessible by 10% on average (Kieliszek and Blazejak 2016). Although organic Se could be easily absorbed and utilized in most areas (Nuttall 2006; Zhang et al. 2005; Zhu et al. 2000), Se deficiency still exists in some areas, especially in developing countries such as China. In fact, artificially synthetic Se could be an alternative way to fulfill the needs of human body, but the production procedure is extremely complicated and costly, which could not meet the demand of Se utilization and resolve the problem of Se deficiency in some areas. Therefore, it has drawn increasing public attention in recent years how to get high nutrition quality organic Se through cheap and simple production procedure.
Some studies have shown that inorganic Se could be transformed into a wide variety of organic forms by microorganism, so these lower cost and high-security organic Se could be applied widely in different areas (Garbisu et al. 1996; Kessi and Hanselmann 2004; Nelson et al. 1996). Bioaccumulation is the main way to save organic Se for most organisms by incorporating the inorganic ions into cellular biomass from their environment (Kieliszek and Blazejak 2013). By means of biotransformation, organic Se, such as selenoprotein and selenocystine, are generated, and become more absorbable for the human body (Benko et al. 2012; Mogna et al. 2012). As probiotic, yeast has been widely investigated in the Se-enriched field over the past decades (Dumont et al. 2006; Kieliszek et al. 2015, 2016a, b). Lactic acid bacteria (LAB), generally recognized as safe probiotics, have also been confirmed they could absorb and transform Se into different valences (Andreoni et al. 2000; Galano et al. 2013). In fact, Se coupled with probiotics has already been applied in the production of fermented foods to meet the nutritional element needs (Kim and Milner 2001; Li et al. 2002; Penas et al. 2012; Reid and Burton 2002).
The accumulation of Se by microorganism, during production procedure, was substantially affected by culture conditions (Xia et al. 2007). Suzuki et al. found that Se bioaccumulation by S. cerevisiae yeast was greatly influenced by active acidity and level of oxygen dissolved in the medium (Suzuki et al. 2006). However, to date, only a few reports about the effects of culture conditions as temperature, pH, and inoculum dose on the enrichment of Se by LAB, so Se-enriched foods could not be popularized in daily life due to the lack of the corresponding data. It is necessary to grasp more details about the relationship between culture factors and Se enrichment of LAB. Therefore, the aim of this study was to explore how the three factors affect the biological enrichment of Se in Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus and achieve the optimized conditions, as well as to investigate the antioxidant property of Se-enriched LAB and reveal their cell surface characteristics through FT-IR spectra and a scanning electron microscope (SEM) equipped with energy-dispersive X-ray spectroscopy.
Materials and methods
Strains and reagents
Lactobacillus delbrueckii ssp. bulgaricus (Lb) and Streptococcus thermophilus (St) were gained from the College of Food Science and Engineering in Northwest Agriculture & Forestry University.
Sodium selenite (Na2SeO3) was purchased from the Tianjin Zhiyuan Chemical Engineering Factory (Tianjin, China), and 3,3′-diaminobenzidine (DAB), 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) were purchased from the Tokyo Chemical Industry (Tokyo, Japan). Other chemicals were purchased from local commercial sources and were of analytical grade.
Culture medium for lactic acid bacteria
The Mann, Rogosa, Sharp (MRS) medium contained the following ingredients: glucose, 20.0 g/L; beef extract, 10.0 g/L; K2HPO4, 2.0 g/L; MnSO4·H2O, 0.05 g/L; peptone, 10.0 g/L; CH3COONa, 5.0 g/L; MgSO4·7H2O, 0.2 g/L; Tween 80, 1.0 g/L; triammonium citrate, 2.0 g/L; yeast extract, 4.0 g/L.
Determination of the suitable selenite concentration and detection of selenium enrichment efficiency for LAB
The suitable selenite concentration was determined by measuring the viabilities of Lb and St in MRS containing different sodium selenite concentrations. Lb and St were cultivated in MRS broth at 35 °C for 24 h. Then, 2 mL of the fermented broth was, respectively, inoculated into 100 mL Erlenmeyer flasks containing 30 mL fresh MRS broth with selenite concentrations at 0, 20, 40, 60, 80, 100, 120, 140, and 160 µg/mL. When their growth reached the post-log phase, 1 mL fermented broth from each Erlenmeyer flask was applied for progressive dilutions, which were subsequently plated on MRS agar and incubated at 35 °C until the colonies were clearly observed. The bacterial viability was determined by counting colony forming units (cfu) in 1 mL sample.
The ratios of Se enrichment of Lb and St were achieved through total Se before inoculation subtracting the Se content in the supernatant of LAB fermented broth, which was detected by mixed acid digestion method followed by surveying the optical density of the reaction product of Se (IV) and 3,3′-diaminobenzidine hydrochloride (DAB) with Ultraviolet Spectrophotometer (UV-1240, Shimadzu, Japan). The supernatant of both strains was collected by centrifugation at 6000 r/min for 10 min, and then, DAB was added. The Se (IV) concentration in the supernatant was determined by reading the absorbance of the stable yellow-colored piazselenol complex formed by the reaction of Se (IV) and DAB in methylbenzene at 420 nm (Deng et al. 2015; Suhajda et al. 2000; Wang 2007). Se (IV) concentration of supernatant was worked out, so the rates of Se bioconversion could be calculated by the formula (1) as follows:
| 1 |
Effect of initial pH, inoculum dose, and temperature on the ratio of Se enrichment by LAB and design of response surface optimization
Single-factor experiments were carried out to determine the appropriate levels of the parameters, such as initial pH (A), inoculum dose (B), and cultivation temperature (C). Lb and St, with 2% (v/v) cultures from post-log phase growing in MRS, were inoculated in MRS broth containing sodium selenite at different initial pH of 3, 4, 5, 6, 7, and 8, respectively, followed by culturing at 35 °C for 48 h. For inoculum dose assay, 2, 4, 6, 8, 10, and 12% cultures (v/v) of Lb and St from post-log phase were, respectively, inoculated in the same volume of MRS broth with sodium selenite and cultivated at 35 °C for 48 h. At the same time, the two strains were inoculated in MRS broth containing sodium selenite at the dose of 2% and, respectively, cultivated at 20, 25, 30, 35, 40, and 45 °C for 48 h to investigate the effect of temperature. The suitable selenite concentration added in MRS broth was selected from previous result. The ratios of Se enrichment in all treatment were measured as described previously.
The experiment was designed with 15 trials according to the Box-Behnken Design (BBD) Approach (Tahmouzi 2014). Each trial was performed in duplicates. Response surface methodology (RSM) was used to determine the effects of several variables and to optimize the processes. Initial pH, inoculum dose (%), and temperature (°C) were three independent variables, and the ratio of Se enrichment (%) was regarded as the response value. Under optimal values of process parameters, verification test was carried out.
Determination of antioxidant activity with DPPH, ABTS, radical scavenging assay
Se-enriched Lb and St were obtained from optimum conditions and inoculated in no-Se fresh MRS broth, cultivated to their post-log phase at 35 °C. After 6000 r/min for 10 min, the supernatant and cell pellets were collected, respectively. The pellets were washed six times by sterile saline to ensure that there was no sodium selenite residue. Meanwhile, all the cell pellets were further diluted to 109 cfu/mL suspensions with sterile saline. The strains (Lb and St) grown in selenite-free MRS were enrolled as the control.
The scavenging of DPPH by Se-enriched strains was analyzed by a modified method reported by Lin and Chang (2000). 2 mL of cell suspension (or supernatant) and 2 mL of freshly prepared DPPH solution (0.2 mmol/L in ethyl alcohol) were mixed and reacted for 30 min in the dark. Blank samples contained sterile distilled water. The scavenged DPPH rate was monitored by measuring the decrease in absorbance at 517 nm. The DPPH free radical scavenging rate was defined as follows:
| 2 |
With a modification of the method reported by Wootton-Beard et al. (2011), the scavenging of ABTS by Se-enriched strains was analyzed. Stable, dark blue-green ABTS radical solution was generated after ABTS (7 in 20 mmol/L sodium acetate buffer, pH 4.5) reacted with an oxidant (2.45 mmol/L potassium persulfate) in the dark for 12–16 h at 4 °C, which was then diluted to the practical reagent with an absorbance of 0.7 ± 0.02 at 734 nm. In our assay on scavenging of ABTS, 2 mL of sample and 2 mL of reagent were mixed for 10 min at room temperature. The sterile distilled water took place of bacteria solution as blank samples. The strains grown in selenite-free MRS were used as the control. The ABTS scavenging was monitored by measuring the decrease of absorbance at 734 nm. The ABTS free radical scavenging rate was defined as follows:
| 3 |
Reducing activity
The reducing activity of Se-enriched LAB was determined essentially according to the method reported by Lin and Yen (1999). 0.5 mL of potassium ferricyanide (1%) was mixed with the same volume of phosphate buffer (0.02 mol/L) and cell suspension or supernatant and incubated for 20 min at 50 °C. With the mixture being cooled rapidly, 0.5 mL of TCA (10%) was added in 1.5 mL of upper phase was obtained by centrifugation at 3000 r/min for 5 min, and 0.2 mL of FeCl3 (0.1%) was added in. Absorbance of reaction solution was read at 700 nm. Sterile distilled water was used as blank sample. The strains grown in selenite-free MRS were used as the control.
Analysis of Se-enriched Lb and St with an FT-IR and a scanning electron microscope equipped with energy dispersion X-ray spectrometry
Under the optimal culture conditions, Se-enriched Lb and St were obtained. The cells of both strains were collected by centrifugation at 6000 r/min for 20 min, and then dehydrated by freeze drying. Half of these samples were used for FT-IR. The IR spectra of Se-enriched Lb and St were obtained by IR spectroscopy and recorded at 4 °C in the wave number range of 4000–400 cm−1 with an FT-IR spectrophotometer (Bruker, Germany). The other half were used for SEM analysis. The cell precipitates were mixed with 2.5% (w/v) glutaraldehyde for 3 h. Following being washed twice for 20 min with phosphate buffer (pH 7.2), these samples were then dehydrated by gradient concentration alcohol solution from 50 to 100% and subsequently subjected to ion sputtering coating (IB-3, Eiko, Tokyo, Japan). Finally, the surface morphology of cells was examined using an SEM (S-4800, Hitachi Ltd., Tokyo, Japan), and the chemical composition of the selected area was determined using X-ray spectrometry (EX-250, Horiba Ltd., Korea).
Statistical analysis
Data were analyzed with Design-Expert 8.0.5 program. Analysis of variance (ANOVA) was used to obtain the interaction between the variables and the response. The probability values (P value) were utilized to check the significant and effective levels of the developed model. Three-dimensional curves of the response surfaces were developed according to the same program. Each value was expressed as mean ± standard deviation.
Results and discussion
Determination of the suitable selenite concentration on the growths of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus
Effects of sodium selenite concentration on cell growths of Lb and St were determined by counting colony (cfu/mL). Both of them were inoculated into MRS broth containing different sodium selenite concentrations and cultivated to their post-log phase at 37 °C. The cultures were then smeared on MRS plates after diluted and cultivated at 37 °C until colonies were clearly visible. The results showed that the growths of both LABs had a steady increase with the increase of selenite concentration up to 80 µg/mL, but their growths reduced gradually with the further heightening of selenite concentrations from 80 to 160 µg/mL (Table 1). Kieliszek et al. reported that Candida utilis yeast reached the highest biomass yield after 24 h cultivation in the presence of 10 mg/L Se4+, but was strongly inhibited by higher concentrations (60 mg/L Se4+) (Kieliszek et al. 2016b). Nevertheless, a little different observed in this experiment was that low selenite concentrations (under 80 µg/mL) promoted the growths of both strains. It could most probably result from different biological structures between LAB and yeast, because the former is prokaryote and the latter is eucaryon. Another study has also revealed that high concentration of selenite is pernicious, but lower concentrations of selenite can accelerate the growth of LAB (Penas et al. 2012). Interestingly, there also existed some exceptions that Enterococcus durans accumulated sodium selenite from the medium at considerably high levels (Pieniz et al. 2014).
Table 1.
Effect of different concentration sodium selenite on LAB growth
| Sodium selenite (µg/mL) | Lb (cfu/mL) | St (cfu/mL) |
|---|---|---|
| 0 | (6.27 ± 0.14) × 108 | (6.87 ± 0.27) × 108 |
| 20 | (6.20 ± 0.18) × 108 | (6.93 ± 0.36) × 108 |
| 40 | (6.20 ± 0.31) × 108 | (6.80 ± 0.50) × 108 |
| 60 | (6.33 ± 0.51) × 108 | (6.93 ± 0.46) × 108 |
| 80 | (6.63 ± 0.60) × 108 | (7.37 ± 0.46) × 108 |
| 100 | (5.63 ± 0.36) × 108 | (6.47 ± 0.49) × 108 |
| 120 | (5.33 ± 0.19) × 108 | (6.40 ± 0.94) × 108 |
| 140 | (5.20 ± 0.18) × 108 | (5.87 ± 0.51) × 108 |
| 160 | (3.63 ± 0.63) × 107 | (4.83 ± 0.36) × 107 |
Values are mean ± SD or means, n = 3
The living cells of Lb and St in this study reached the maximum of (6.63 ± 0.60) × 108/mL and (7.37 ± 0.46) × 108/mL at the 80 µg/mL concentration of selenite, respectively. Therefore, 80 µg/mL of sodium selenite was regarded as the most suitable selenite concentration for Lb and St to accumulate selenium in following experiments. In addition, the phenomenon of deepening reddish color was observed in culture when the selenite concentration in MRS was increased from 40 to 160 µg/mL, which suggested that both strains produced more elemental Se at the higher selenite concentrations. A similar phenomena was reported in other studies that an excess amount of Se was confirmed to be transformed into Se(0) imparting a distinct red color (Xia et al. 2007). This feature indicated higher efficiency of the detoxification processes inside the cells when cultivated in culture media containing selenite (Kieliszek et al. 2016b).
Effect of initial pH, inoculum dose, and culture temperature on the ratios of Se enrichment by LAB
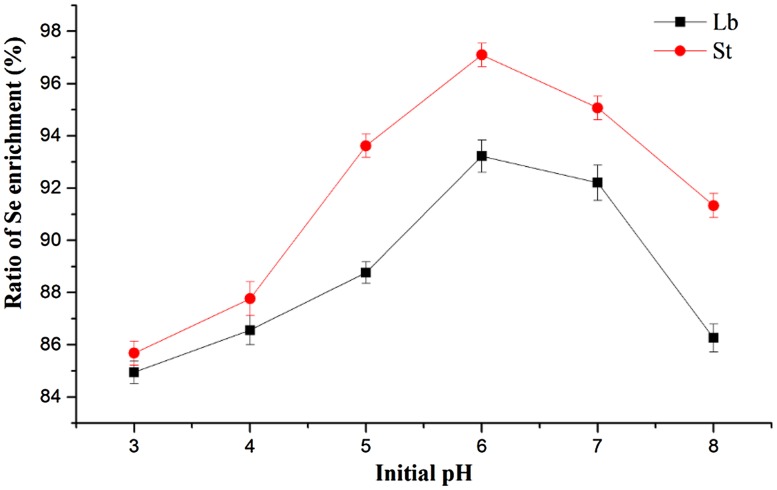
Data from initial pH assay showed that the Se enrichment ratio of Lb had a steady increase from pH 3 to pH 6, reached its peak at pH 6, but it decreased with the continued increase of pH value (Fig. 1). The trend of Se enrichment ratio of St was similar to that of Lb, with the highest ratio at pH 6.
Fig. 1.
Effect of initial pH value on the ratio of Se enrichment by LAB
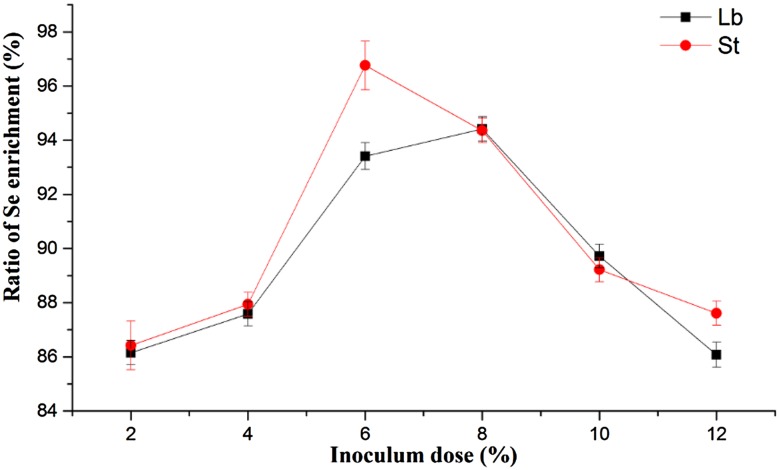
The effects of different inoculum doses were showed in Fig. 2. The Se enrichment ratio of Lb increased and reached its peak when the inoculum dose was 8%, then declined after this peak. The peak of Se enrichment ratio of St was at the dose of 6% and decreased rapidly between 6 and 12%.
Fig. 2.
Effect of inoculum dose on the ratio of Se enrichment by LAB
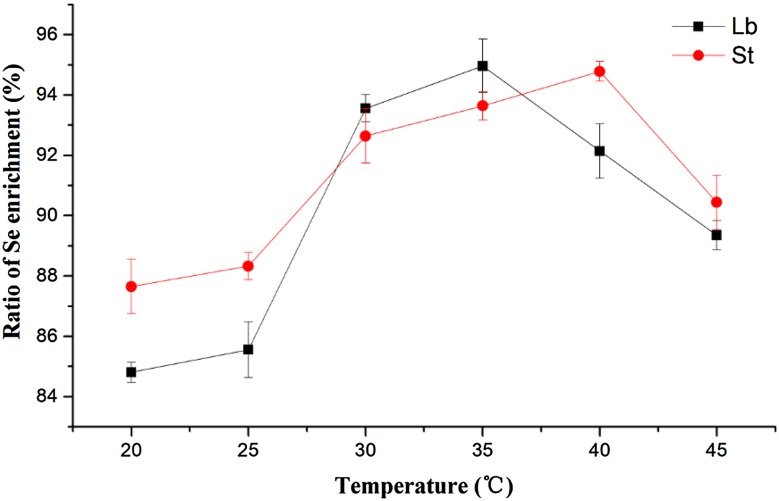
At the different culture temperatures, Se enrichment ratios of Lb and St were showed in Fig. 3. Their Se enrichment ratios increased at first and then decreased with the rising temperature, reaching its peak at 35 °C (for Lb) and 40 °C (for St). When Sofu et al. assessed the removal capacity of iron and zinc from aqueous solution with dairy wastewater in three different biomasses such as Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophillus, and a combination of both bacteria cultures, they found biomass concentration, pH, and temperature made important effects in the removal of Fe(II) and Zn(II); furthermore, under optimal values, 90–100% Fe(II) and 70–90% Zn(II) removals were obtained with all tested biomasses (Sofu et al. 2015). Zhang et al. (2016) found that intracellular organic selenium content in Se-enriched Candida utilis was improved markedly by acid stress (pH 3.5). The three factors, initial pH, inoculum dose, and temperature, showed a significant influence on the ratio of Se enrichment for both strains, in this single-factor experiment.
Fig. 3.
Effect of temperature on the ratio of Se enrichment by LAB
Analysis of response surface optimization and regression equation
Factors and levels investigated in the Se enrichment test were chosen in consideration of the results of a single-factor experimental test (Table 2). Tested variables (initial pH, inoculum dose, and culture temperature) were denoted as A, B, and C, respectively. The contour plots of the model-predicted responses were utilized to assess the interactive relationships between the significant variables. Results with less than 95% confidence interval (p > 0.05) were considered not statistically significant for the model (Sayilgan and Cakmakci 2013). The experimental conditions and Se enrichment results were shown in Table 3, which indicated that St exerted better Se enrichment performance than Lb in most cases. It probably depended on the physiological state of cultures from LAB, and the same inference was also observed in biosorption of Cu by wine-relevant lactobacilli (Schut et al. 2011).
Table 2.
Factors and levels investigated in Se enrichment tests of RSM (Lb, St)
| Code | Factor (variable) | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A | Initial pH | 5 | 6 | 7 |
| B | Inoculum dose (%) | 6 | 8 | 10 |
| C | Temperature (°C) | 30 | 35 | 40 |
Table 3.
Program and experimental result of RSM
| Test no. | A (Initial pH) | B (Inoculum dose, %) | C (Temperature, °C) | Ratio of St (%) | Ratio of Lb (%) |
|---|---|---|---|---|---|
| 1 | 0 | −1 | −1 | 96.79 | 94.21 |
| 2 | 1 | 0 | 1 | 95.78 | 93.11 |
| 3 | 0 | 0 | 0 | 95.22 | 94.76 |
| 4 | −1 | −1 | 0 | 94.57 | 93.98 |
| 5 | 0 | 0 | 0 | 95.52 | 94.02 |
| 6 | 0 | 0 | 0 | 94.91 | 94.26 |
| 7 | 0 | 1 | 1 | 95.72 | 91.22 |
| 8 | 1 | 0 | −1 | 94.59 | 93.28 |
| 9 | −1 | 0 | 1 | 94.55 | 91.42 |
| 10 | 0 | −1 | 1 | 96.88 | 93.06 |
| 11 | 1 | −1 | 0 | 95.81 | 93.99 |
| 12 | −1 | 0 | −1 | 94.02 | 93.55 |
| 13 | 0 | 1 | −1 | 94.22 | 93.01 |
| 14 | 1 | 1 | 0 | 93.17 | 92.95 |
| 15 | −1 | 1 | 0 | 92.98 | 91.05 |
The ratio of Se enrichment of Lb varied between 91 and 95%, and for St, between 92 and 97%. The experimental design used for the modeling of Se enrichment from MRS broth by Lb and St was carried out on basis of three factors, namely: initial pH (A), inoculum dose (B, %), and temperature (C, °C). A three-factor three-level response surface design was used to develop correlation between the selected factors and the ratio of Se enrichment. In this study, the A, B, and C variables in the regression equations were coded (non-dimensional) factors representing the three tested factors. The correlation between the coded factors and real values was chosen as follows:
| 4 |
| 5 |
| 6 |
A initial pH, B inoculum dose (%), and C temperature(°C)
Main effects and interaction effects of the variables were estimated using the following equation:
| 7 |
Y ratio of Se enrichment (%), A 1, B 1, and C 1 coded non-dimensional factors of initial pH, inoculum dose and temperature, respectively. a 0 the constant term, a 1, a 2, and a 3 the main effect terms. a 12, a 13, a 23, a 11, a 22, and a 33 the interaction effect terms.
The regression equations were submitted to the F test to determine the coefficient R 2 (Anupam et al. 2011). The predictive model was insured a representatively of the experimental data of about 95%, which means that the regression was <0.05. The regression coefficients for the ratio of Se enrichment by Lb and St were not given in tables. The ANOVA results have been used to derive simple empirical equations using the design expert program to predict the ratio of Se enrichment. Eliminating the insignificant interaction terms from the regression tables and refining the above empirical model equations could be simplified in terms of actual factors to
| 8 |
| 9 |
F value of Se(Lb) and Se(St) was 21.05 and 42.65, respectively (Tables 4, 5), indicates that both models were significant. The lack of fit about Se(Lb) and Se(St) was 0.7479 (>0.05) and 0.9014 (>0.05), which suggests that the two regression equations were in a good coincidence with the test, and could describe the relationship between these factors.
Table 4.
ANOVA result of enrichment ratio of Se by Lb
| Source | Sum of squares | df | Mean square | F value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 17.97 | 9 | 2.00 | 21.05 | 0.0019 | Significant |
| A (pH) | 1.39 | 1 | 1.39 | 14.61 | 0.0123 | |
| B (Inoculum dose) | 6.14 | 1 | 6.14 | 64.74 | 0.0005 | |
| C (Temperature) | 3.43 | 1 | 3.43 | 36.17 | 0.0018 | |
| AB | 0.89 | 1 | 0.89 | 9.41 | 0.0278 | |
| AC | 0.96 | 1 | 0.96 | 10.12 | 0.0245 | |
| BC | 0.10 | 1 | 0.10 | 1.08 | 0.3465 | |
| A 2 | 1.78 | 1 | 1.78 | 18.77 | 0.0075 | |
| B 2 | 1.61 | 1 | 1.61 | 16.93 | 0.0092 | |
| C 2 | 2.44 | 1 | 2.44 | 25.66 | 0.0039 | |
| Residual | 0.47 | 5 | 0.095 | |||
| Lack of fit | 0.19 | 3 | 0.063 | 0.44 | 0.7479 | Insignificant |
| Pure error | 0.29 | 2 | 0.14 | |||
| Cor total | 18.45 | 14 |
Table 5.
ANOVA result of enrichment ratio of Se by St
| Source | Sum of squares | df | Mean square | F value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 18.16 | 9 | 2.02 | 42.65 | 0.0003 | Significant |
| A (pH) | 1.30 | 1 | 1.30 | 27.57 | 0.0033 | |
| B (Inoculum dose) | 7.92 | 1 | 7.92 | 167.42 | <0.0001 | |
| C (Temperature) | 1.37 | 1 | 1.37 | 28.95 | 0.0030 | |
| AB | 0.28 | 1 | 0.28 | 5.83 | 0.0606 | |
| AC | 0.11 | 1 | 0.11 | 2.30 | 0.1897 | |
| BC | 0.50 | 1 | 0.50 | 10.51 | 0.0229 | |
| A 2 | 4.68 | 1 | 4.68 | 98.93 | 0.0002 | |
| B 2 | 6.410E−003 | 1 | 6.410E−003 | 0.14 | 0.7279 | |
| C 2 | 1.53 | 1 | 1.53 | 32.39 | 0.0023 | |
| Residual | 0.24 | 5 | 0.047 | |||
| Lack of fit | 0.050 | 3 | 0.017 | 0.18 | 0.9014 | Insignificant |
| Pure error | 0.19 | 2 | 0.093 | |||
| Cor total | 18.40 | 14 |
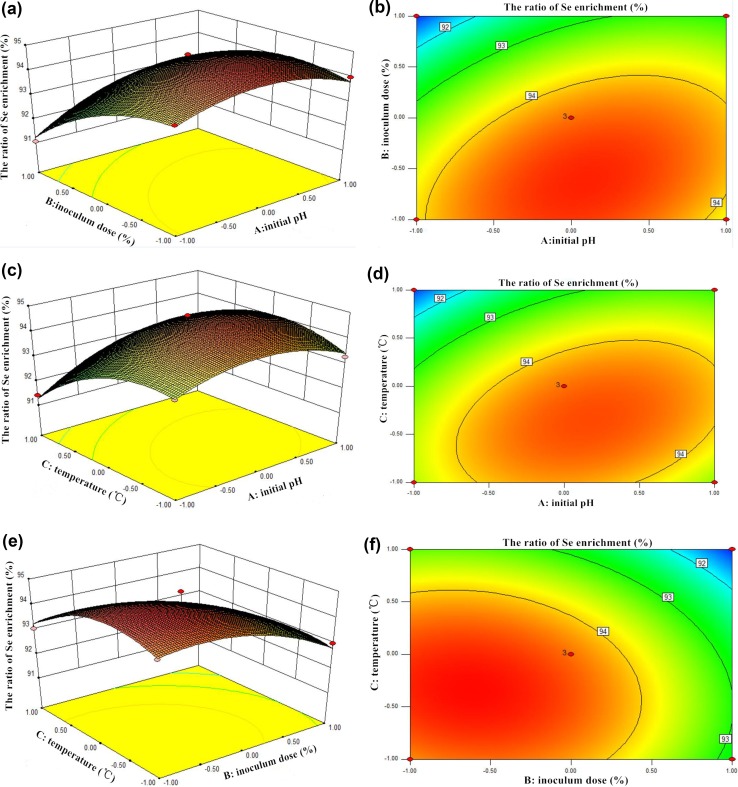
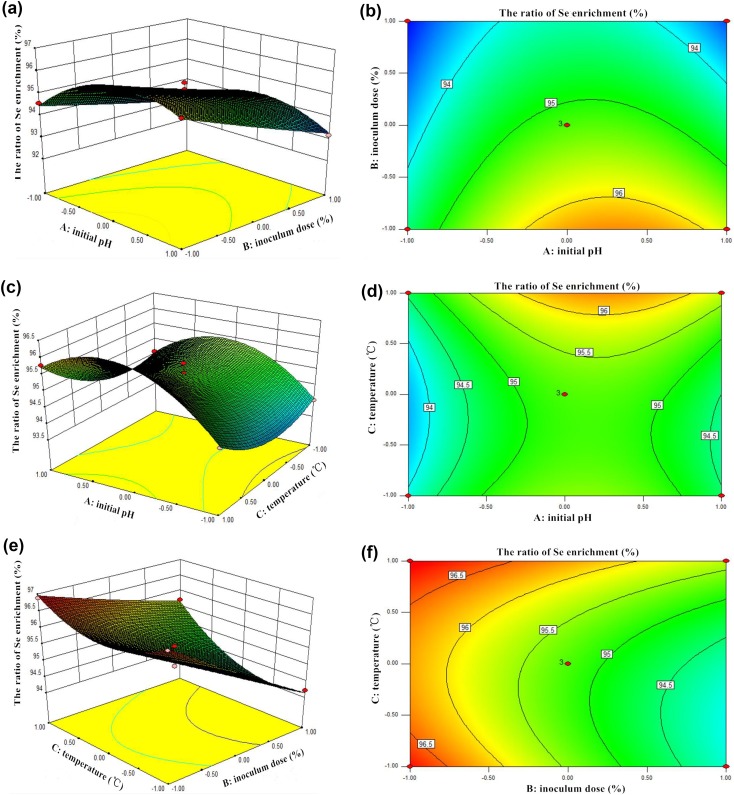
The graphical interpretation of main effects and interactions such as three-dimensional surfaces provided convenient information about the ratio of Se enrichment within the experimental design, and facilitated to show the effects of the experimental factors on the responses and contour plots between the factors (Ahmad and Hameed 2010; Anupam et al. 2011). The response surface contour plots of the ratio of Se enrichment over independent variables such as initial pH (A) and inoculum dose (B) with temperature were shown in Figs. 4a, b, and 5a, b, which indicated the relative effects of two variables (A and B), while the temperature of solution (C) was kept constant at its center point level (35 °C). The 3D-response surfaces and contour plot in Figs. 4 and 5 showed the main effect of inoculum dose (B) significantly enhanced the ratios of Se enrichment with both Lb and St. Furthermore, the effect of temperature (C) and initial pH came second for these two strains. The optimum initial pH, inoculum dose, and temperature for Lb and St were found to be 5.96, 6.73%, 33.24 °C and 6.37, 6%, 40 °C, respectively. The expected ratio values were 94.73% (Lb) and 97.11% (St). Under these conditions, the ratios of Se enrichment of Lb and St could be considered as the most efficient results among the tested groups in this study.
Fig. 4.
3D-response surfaces and contour plot of the ratio enrichment of Se by Lb
Fig. 5.
3D-response surfaces and contour plot of the ratio enrichment of Se by St
Even if with the lowest inoculum dose, initial pH or temperature, the ratios of Se enrichment for Lb and St were closed to 90%. This result might be due to the fact that both Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophillus in this study possessed original excellent properties. In fact, previous researches have demonstrated that bioaccumulation capacity of LAB for beneficial element or harmful heavy metal ions was inherently, which closely related to the biological structures of bacterial cells (Mrvcic et al. 2012; Wang and Chen 2009; Zhai et al. 2015). Therefore, as a representative of probiotics, LAB was widely used in almost every areas, including food processing and sewage disposal (Sofu et al. 2015).
In our optimization experiment, under optimal values of process parameters, verification test was finished, and the Se absorptivity was 94.34% (expected value was 94.73%) for Lb and 97.05% (expected value was 97.11%) for St. These results which indicate the model were accurate.
Determination of DPPH, ABTS radical, and reducing activity
The results of scavenging DPPH and ABTS and reducing activity by cell suspension and supernatant of Se-enriched Lb and St were shown in Table 6. The scavenged DPPH was monitored by measuring absorbance at 517 nm. It was found that Se-enriched LAB had higher levels of scavenging ability than the control (without Se) in both cell suspension and supernatant, respectively. Similar phenomenon was observed in ABTS scavenged and reducing activity tests. Analysis of both the control and Se-enriched groups showed a significant difference (p < 0.05) between these strains. As a whole, Se-enriched LAB had higher antioxidant activities in comparison with the control. The outstanding antioxidant activities of Se-enriched LAB may be due to two main reasons (the antioxidant enzymes of LAB and Se nanoparticles). The report from Drutel et al. (2013) told that selenium is an integral part of several antioxidant enzymes such as glutathione peroxidase (GPx), thioredoxin reductase (TRxR), and iodothyronine deiodinase (DIO), which contributed organisms to defend the harmful effects of free radicals that were generated during the oxidation process. Therefore, it could be speculated that the activities of these antioxidant enzymes in cells might have greatly improved by the stimulation of selenite ions in the culture media. Specifically, when the strains were cultivated in the MRS broth containing sodium selenite, these inorganic selenium ions in the solution were transferred into the cells through the biotransformation, and a part of these ions accompanied with the change of the valence state were integrated into related antioxidant enzymes in cells, which resulted in the antioxidant activities enhancement of these enzymes. This kind of relationship between selenium and antioxidant enzymes in cells has been found in some yeast cells such as Saccharomyces cerevisiae, Candida utilis, and Yarrowia lipolytica (Kieliszek et al. 2015). Another product, elemental Se nanoparticles, which were generated during the process of organism biological accumulation, could not be neglected. It was reported that lambs were fed with elemental Se nanoparticles derived from probiotic bacteria to improve Se nutrition status of lamb meat, and the total antioxidant capacity of blood had a great improvement (Ungvari et al. 2014). In the antioxidant assay, it was found that the antioxidant activities of supernatant were better than that of cell suspension, probably because most antioxidant substances were secreted into supernatant (Table 6). Some data indicated that extracellular secretion from LAB like exopolysaccharides has outstanding antioxidant activities in scavenging DPPH free radical rates (Wang et al. 2015). Therefore, the stronger antioxidant activity of Se-enriched supernatant may not only result from antioxidant extracellular secretion and antioxidant enzymes of strains, but also from Se nanoparticles, which were detached from the surface of cells in the process of centrifugation.
Table 6.
Antioxidant activities of Se-enriched LAB
| Strain | DPPH scavenged (%) | ABTS scavenged (%) | Reducing activity (OD700) | |||
|---|---|---|---|---|---|---|
| Lba | Control | Se-enriched | Control | Se-enriched | Control | Se-enriched |
| Cell suspension | 34.33 ± 0.30 | 39.11 ± 0.23* | 34.12 ± 0.47 | 37.95 ± 0.58* | 0.2707 ± 0.0100 | 0.3016 ± 0.0087 |
| Supernatant | 50.80 ± 0.61 | 57.42 ± 0.85* | 49.64 ± 0.93 | 57.35 ± 0.61* | 0.3408 ± 0.0084 | 0.5110 ± 0.0117* |
| Stb | ||||||
| Cell suspension | 46.02 ± 0.47 | 52.15 ± 1.18* | 36.90 ± 0.73 | 45.37 ± 0.18* | 0.2111 ± 0.0089 | 0.3155 ± 0.0085 |
| Supernatant | 53.60 ± 0.88 | 70.65 ± 0.41* | 56.30 ± 0.56 | 68.09 ± 0.49* | 0.4016 ± 0.0028 | 0.6055 ± 0.0133* |
Values in the same line with ‘*’ are significantly different (p < 0.05). Values are mean ± SD or means, n = 3
a Lactobacillus delbrueckii ssp. bulgaricus
b Streptococcus thermophilus
FT-IR analysis
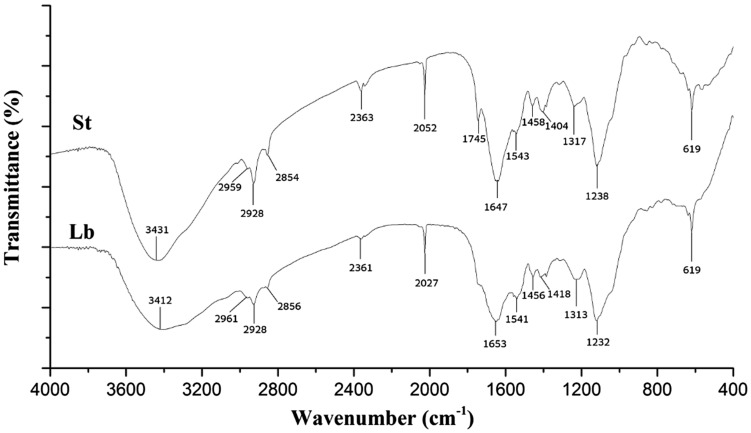
The FT-IR spectra of biomasses from Se-enriched Lb and St were shown in Fig. 6. Both Se-enriched LAB had a broad strong band at 3412–3431 cm−1, corresponding to stretching vibration of the O–H and N–H bonds. These kinds of peaks were observed also in other studies (Jimenez-Cedillo et al. 2013; Tan et al. 2010); strong bands at 2854–2961 and 2027–2052 cm−1 indicate the presence of CH (–CH2, –CH3) and C≡N stretching vibrations, respectively. The absorption peak around 1232–1745 cm−1 is indicative of C–H bending vibrations and C=O stretching vibrations. Furthermore, the FT-IR spectra were indicated in a number of absorption peaks at 619 cm−1 (indicative of C–H groups) (Selatnia et al. 2004). It was reported that the IR spectrum of selenium (Na2SeO3) rich exopolysaccharide (Se-EPS) by Pseudomonas was similar to that of EPS (control) except a little shift. Specifically, the characteristic absorption of Se-EPS at 614 cm−1 demonstrated the existence of selenium ester bond (Se–O–C), which was an important piece of evidence showing the presence of Se embedded in the EPS molecule (Ye et al. 2016). In our study, FT-IR results showed that the investigated Se biomasses contain carbonyl, carboxyl, and hydroxyl groups, and these groups may be involved in coordination with elemental Se (Veneu et al. 2013). In fact, Lin et al. (2005) found that the carbonyl groups form amino acid residues and peptides of proteins have the strong ability to bind metal a few decades ago; the amide linkages between the amino acid residues in polypeptides and proteins could give rise to the well-known signatures in the infrared region of the electromagnetic spectrum. An earlier report from Gole et al. (2001) showed that proteins could bind to gold nanoparticles through either free amine groups or cysteine residues in the proteins.
Fig. 6.
FTIR spectra of Se-enriched LAB
SEM analysis
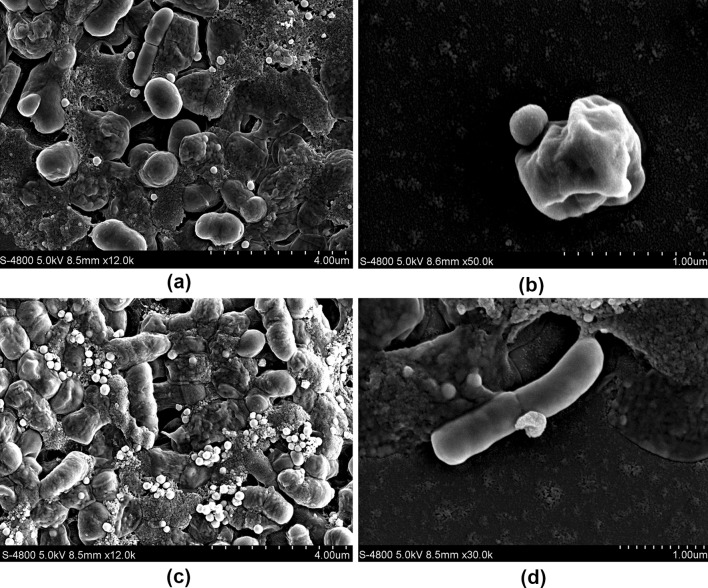
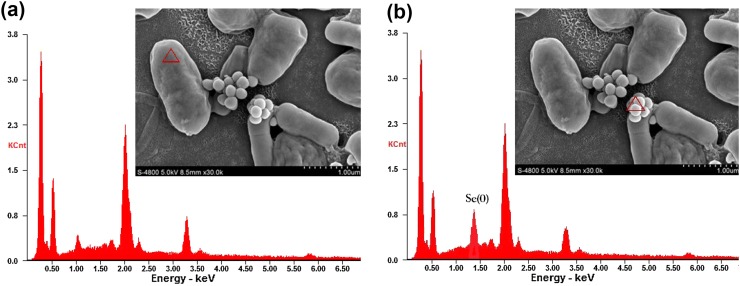
Lb and St could produce reddish cell suspensions in selenite-enriched MRS broth under the optimum cultivation conditions. These phenomena might associate with a detoxification mechanism against excess of selenite by reducing it to insoluble red elemental Se (Kieliszek et al. 2015, 2016b). To investigate this red phenotype, SEM was used to observe the cells grown in MRS broth with selenite, which showed obvious particles deposits around the cells (Fig. 7). To further determine the chemical characteristics of these deposits, the elemental analysis of selenite-enriched Lb was performed through an SEM equipped with an EDX spectroscope and the characteristic energy peaks for Se near 1.42 keV were found near the surface of the cells, as shown in Fig. 8. When other parts away from these nanoparticles of the cell surface were analyzed, no characteristic energy peaks for Se appeared. Similar SEM images and characteristic energy peaks were also obtained from those cells of Se-enriched St (data not shown). These phenomena suggested that Lb and St reduced toxic selenite to elemental Se, and similar properties and phenomena were found in others studies (Bajaj et al. 2012; Hnain et al. 2013). A summary from Kielisezek et al. showed that there are two mechanisms during the process of selenium bioaccumulation and selenium metabolism in yeast cells: one is extracellular binding by ligands of membrane assembly, and another is intracellular accumulation accompanied with the ions transportation across the cytoplasmic membrane into the cell interior. The metabolism of selenium bioaccumulation of yeast cells has been characterized as a four main step pathway. In step 1, selenate (VI) is converted into selenite (IV) by ATP sulfurylase, PAPSe reductase, and NADPH. In step 2, selenite (IV) reacts with the reduced form of glutathione (GSH), generates selenodiglutathione (GS–Se–SG), and the oxidized form of glutathione (GSSG) which is subsequently transformed to a reduced form (GSH) by glutathione reductase. In step 3, GS–Se–SG in cells is changed to glutathionyselenol (GS–Se–H), which is further translated into hydrogen selenide (H2Se/HSe−) accompanied by the formation of GSSG. In step 4, under the catalysis of superoxide dismutase, GS-Se-H is transformed into elemental selenium (0) and glutathione (Kieliszek et al. 2015). In our study, elemental selenium nanoparticles were observed obviously, which represent the direct visual evidence for the last step (Se(0)) of the selenium metabolism (Figs. 7, 8). Therefore, on the basis of data in this study and previous reports, it could be speculated that LAB had the similar or same selenium metabolic pathways.
Fig. 7.
Morphology of Se-enriched Streptococcus thermophiles (a, b) and Lactobacillus delbrueckii ssp. bulgaricus (c, d) by scanning electron microscope analysis
Fig. 8.
Nano-particles around the cells in 80 µg mL−1 selenite analyzed using a scanning electron microscope equipped with energy dispersion X-ray spectroscopy (EDX). a One part of the cells (non-nanoparticle area); EDX spectrum analysis indicates no Se (0) characteristic peak. b Selected nanoparticle area around the cells; EDX spectrum analysis indicates Se (0) characteristic peak (near 1.42 keV)
Conclusions
This study indicated that the optimum initial pH, inoculum doses, and cultivation temperatures for Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus to enrich selenium were 5.96, 6.73%, 33.24 °C and 6.37, 6%, 40 °C, respectively. Under optimal value of process parameters, the ratios of Se enrichment were 94.34% for Lb and 97.05% for St. It was showed that the antioxidant activities of Se-enriched LAB were improved in free radical scavenging rates of DPPH, ABTS, and reducing capacity. The FT-IR spectroscopy supported that the two strains possessed outstanding Se enriching capacities, which may be related to their carbonyl, carboxyl and hydroxyl groups. In addition, selenite could be reduced to elemental selenium nanoparticles through the biotransformation of strains, which proved that LAB have selenite detoxification function. The EDX spectrum analysis indicated that these elemental selenium (0) nanoparticles had a characteristic peak near 1.42 keV. In conclusion, the Se enrichment process for Lb and St was found to be simple and efficient. Both strains may have a potential commercial advantage for the production of Se-enriched probiotics and fermented dairy products.
Acknowledgements
This work was supported by the Grant No. 2016NY-148 from the Department of Science & Technology of Shaanxi Province.
Compliance with ethical standards
Conflict of interest
No conflict of interest was declared.
References
- Ahmad AA, Hameed BH. Effect of preparation conditions of activated carbon from bamboo waste for real textile wastewater. J Hazard Mater. 2010;173:487–493. doi: 10.1016/j.jhazmat.2009.08.111. [DOI] [PubMed] [Google Scholar]
- Andreoni V, Luischi MM, Cavalca L, Erba D, Ciappellano S. Selenite tolerance and accumulation in the Lactobacillus species. Ann Microbiol. 2000;50:77–88. [Google Scholar]
- Anupam K, Dutta S, Bhattacharjee C, Datta S. Adsorptive removal of chromium (VI) from aqueous solution over powdered activated carbon: optimisation through response surface methodology. Chem Eng J. 2011;173:135–143. doi: 10.1016/j.cej.2011.07.049. [DOI] [Google Scholar]
- Bajaj M, Schmidt S, Winter J. Formation of Se (0) Nanoparticles by Duganella sp and Agrobacterium sp. isolated from Se-laden soil of North-East Punjab, India. Microb Cell Fact. 2012;11:64. doi: 10.1186/1475-2859-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko I, et al. Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ Toxicol Chem. 2012;31:2812–2820. doi: 10.1002/etc.1995. [DOI] [PubMed] [Google Scholar]
- Deng Y, et al. Preparation of elemental selenium-enriched fermented milk by newly isolated Lactobacillus brevis from kefir grains. Int Dairy J. 2015;44:31–36. doi: 10.1016/j.idairyj.2014.12.008. [DOI] [Google Scholar]
- Drutel A, Archambeaud F, Caron P. Selenium and the thyroid gland: more good news for clinicians. Clin Endocrinol. 2013;78:155–164. doi: 10.1111/cen.12066. [DOI] [PubMed] [Google Scholar]
- Dumont E, Vanhaecke F, Cornelis R. Selenium speciation from food source to metabolites: a critical review. Anal Bioanal Chem. 2006;385:1304–1323. doi: 10.1007/s00216-006-0529-8. [DOI] [PubMed] [Google Scholar]
- Galano E, et al. Privileged incorporation of selenium as selenocysteine in Lactobacillus reuteri proteins demonstrated by selenium-specific imaging and proteomics. Mol Cell Proteom. 2013;12:2196–2204. doi: 10.1074/mcp.M113.027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XJ, Zhang ZC, Li Y, Shen P, Hu XY, Cao YG, Zhang NS. Selenium deficiency facilitates inflammation following S. aureus infection by regulating TLR2-related pathways in the mouse mammary gland. Biol Trace Elem Res. 2016;172:449–457. doi: 10.1007/s12011-015-0614-y. [DOI] [PubMed] [Google Scholar]
- Garbisu C, Ishii T, Leighton T, Buchanan BB. Bacterial reduction of selenite to elemental selenium. Chem Geol. 1996;132:199–204. doi: 10.1016/S0009-2541(96)00056-3. [DOI] [Google Scholar]
- Gole A, Dash C, Ramakrishnan V, Sainkar SR, Mandale AB, Rao M, Sastry M. Pepsin-gold colloid conjugates: preparation, characterization, and enzymatic activity. Langmuir. 2001;17:1674–1679. doi: 10.1021/la001164w. [DOI] [Google Scholar]
- Hnain A, Brooks J, Lefebvre DD. The synthesis of elemental selenium particles by Synechococcus leopoliensis. Appl Microbiol Biotechnol. 2013;97:10511–10519. doi: 10.1007/s00253-013-5304-0. [DOI] [PubMed] [Google Scholar]
- Jimenez-Cedillo MJ, Olguin MT, Fall C, Colin-Cruz A. As(III) and As(V) sorption on iron-modified non-pyrolyzed and pyrolyzed biomass from Petroselinum crispum (parsley) J Environ Manag. 2013;117:242–252. doi: 10.1016/j.jenvman.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Kessi J, Hanselmann KW. Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirillum rubrum and Escherichia coli. J Biol Chem. 2004;279:50662–50669. doi: 10.1074/jbc.M405887200. [DOI] [PubMed] [Google Scholar]
- Kieliszek M, Blazejak S. Selenium: significance, and outlook for supplementation. Nutrition. 2013;29:713–718. doi: 10.1016/j.nut.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Kieliszek M, Blazejak S. Current knowledge on the importance of selenium in food for living organisms: a review. Molecules. 2016;21:609. doi: 10.3390/molecules21050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M, Blazejak S, Gientka I, Bzducha-Wrobel A. Accumulation and metabolism of selenium by yeast cells. Appl Microbiol Biotechnol. 2015;99:5373–5382. doi: 10.1007/s00253-015-6650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M, Blazejak S, Bzducha-Wrobel A, Kurcz A. Effects of selenium on morphological changes in Candida utilis ATCC 9950 yeast cells. Biol Trace Elem Res. 2016;169:387–393. doi: 10.1007/s12011-015-0415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M, Blazejak S, Placzek M. Spectrophotometric evaluation of selenium binding by Saccharomyces cerevisiae ATCC MYA-2200 and Candida utilis ATCC 9950 yeast. J Trace Elem Med Biol. 2016;35:90–96. doi: 10.1016/j.jtemb.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Kim YS, Milner J. Molecular targets for selenium in cancer prevention. Nutr Cancer Int J. 2001;40:50–54. doi: 10.1207/S15327914NC401_10. [DOI] [PubMed] [Google Scholar]
- Li X, Liu Y, Wu J, Liang HG, Qu SS. Microcalorimetric study of Staphylococcus aureus growth affected by selenium compounds. Thermochim Acta. 2002;387:57–61. doi: 10.1016/S0040-6031(01)00825-5. [DOI] [Google Scholar]
- Lin MY, Chang FJ. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci. 2000;45:1617–1622. doi: 10.1023/A:1005577330695. [DOI] [PubMed] [Google Scholar]
- Lin MY, Yen CL. Antioxidative ability of lactic acid bacteria. J Agric Food Chem. 1999;47:1460–1466. doi: 10.1021/jf981149l. [DOI] [PubMed] [Google Scholar]
- Lin ZY, Wu JM, Xue R, Yang Y. Spectroscopic characterization of Au3+ biosorption by waste biomass of Saccharomyces cerevisiae. Spectrochim Acta A. 2005;61:761–765. doi: 10.1016/j.saa.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Mater DDG, Bretigny L, Firmesse O, Flores MJ, Mogenet A, Bresson JL, Corthier G. Streptococcus thermophilus and Lactobacillus delbrueckii subsp bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. Fems Microbiol Lett. 2005;250:185–187. doi: 10.1016/j.femsle.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Mogna L, Nicola S, Pane M, Lorenzini P, Strozzi G, Mogna G. Selenium and zinc internalized by Lactobacillus buchneri Lb26 (DSM 16341) and Bifidobacterium lactis Bb1 (DSM 17850): improved bioavailability using a new biological approach. J Clin Gastroenterol. 2012;46:S41–S45. doi: 10.1097/MCG.0b013e318268861d. [DOI] [PubMed] [Google Scholar]
- Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100:637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- Mrvcic J, Stanzer D, Solic E, Stehlik-Tomas V. Interaction of lactic acid bacteria with metal ions: opportunities for improving food safety and quality. World J Microbiol Biotechnol. 2012;28:2771–2782. doi: 10.1007/s11274-012-1094-2. [DOI] [PubMed] [Google Scholar]
- Navarro-Alarcon M, Lopez-Martinez MC. Essentiality of selenium in the human body: relationship with different diseases. Sci Total Environ. 2000;249:347–371. doi: 10.1016/S0048-9697(99)00526-4. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Casey WH, Sison JD, Mack EE, Ahmad A, Pollack JS. Selenium uptake by sulfur-accumulating bacteria. Geochimica et Cosmochimica Acta. 1996;60:3531–3539. doi: 10.1016/0016-7037(96)00221-9. [DOI] [Google Scholar]
- Nuttall KL. Evaluating selenium poisoning. Ann Clin Lab Sci. 2006;36:409–420. [PubMed] [Google Scholar]
- Penas E, et al. Se improves indole glucosinolate hydrolysis products content Se-methylselenocysteine content, antioxidant capacity and potential anti-inflammatory properties of sauerkraut. Food Chem. 2012;132:907–914. doi: 10.1016/j.foodchem.2011.11.064. [DOI] [Google Scholar]
- Pieniz S, Andreazza R, Anghinoni T, Camargo F, Brandelli A. Probiotic potential, antimicrobial and antioxidant activities of Enterococcus durans strain LAB18s. Food Control. 2014;37:251–256. doi: 10.1016/j.foodcont.2013.09.055. [DOI] [Google Scholar]
- Reid G, Burton J. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect. 2002;4:319–324. doi: 10.1016/S1286-4579(02)01544-7. [DOI] [PubMed] [Google Scholar]
- Sayilgan E, Cakmakci O. Treatment of textile dyeing wastewater by biomass of Lactobacillus: lactobacillus 12 and Lactobacillus rhamnosus. Environ Sci Pollut Res. 2013;20:1556–1564. doi: 10.1007/s11356-012-1009-7. [DOI] [PubMed] [Google Scholar]
- Schut S, Zauner S, Hampel G, Koenig H, Claus H. Biosorption of copper by wine-relevant lactobacilli. Int J Food Microbiol. 2011;145:126–131. doi: 10.1016/j.ijfoodmicro.2010.11.039. [DOI] [PubMed] [Google Scholar]
- Selatnia A, Boukazoula A, Kechid N, Bakhti MZ, Chergui A. Biosorption of Fe3+ from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Process Biochem. 2004;39:1643–1651. doi: 10.1016/S0032-9592(03)00305-4. [DOI] [Google Scholar]
- Sofu A, Sayilgan E, Guney G. Experimental design for removal of Fe(II) and Zn(II) ions by different lactic acid bacteria biomasses. Int J Environ Res. 2015;9:93–100. [Google Scholar]
- Suhajda A, Hegoczki J, Janzso B, Pais I, Vereczkey G. Preparation of selenium yeasts I. Preparation of selenium-enriched Saccharomyces cerevisiae. J Trace Elem Med Biol. 2000;14:43–47. doi: 10.1016/S0946-672X(00)80022-X. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Kurasaki K, Ogawa S, Suzuki N. Metabolic transformation of methylseleninic acid through key selenium intermediate selenide. Toxicol Appl Pharmacol. 2006;215:189–197. doi: 10.1016/j.taap.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Tahmouzi S. Optimization of polysaccharides from Zagros oak leaf using RSM: antioxidant and antimicrobial activities. Carbohydr Polym. 2014;106:238–246. doi: 10.1016/j.carbpol.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Tan G, Yuan H, Liu Y, Xiao D. Removal of lead from aqueous solution with native and chemically modified corncobs. J Hazard Mater. 2010;174:740–745. doi: 10.1016/j.jhazmat.2009.09.114. [DOI] [PubMed] [Google Scholar]
- Ungvari E, et al. Protective effects of meat from lambs on selenium nanoparticle supplemented diet in a mouse model of polycyclic aromatic hydrocarbon-induced immunotoxicity. Food Chem Toxicol. 2014;64:298–306. doi: 10.1016/j.fct.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Veneu DM, Torem ML, Pino GAH. Fundamental aspects of copper and zinc removal from aqueous solutions using a Streptomyces lunalinharesii strain. Miner Eng. 2013;48:44–50. doi: 10.1016/j.mineng.2012.11.015. [DOI] [Google Scholar]
- Wang LF. Determination of trace selenium in organic samples. Food Machin. 2007;23(1):115. [Google Scholar]
- Wang J, Chen C. Biosorbents for heavy metals removal and their future. Biotechnol Adv. 2009;27:195–226. doi: 10.1016/j.biotechadv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao X, Yang Y, Zhao A, Yang Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int J Biol Macromol. 2015;74:119–126. doi: 10.1016/j.ijbiomac.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Wootton-Beard PC, Moran A, Ryan L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res Int. 2011;44:217–224. doi: 10.1016/j.foodres.2010.10.033. [DOI] [Google Scholar]
- Xia SK, Chen L, Liang JQ. Enriched selenium and its effects on growth and biochemical composition in Lactobacillus bulgaricus. J Agric Food Chem. 2007;55:2413–2417. doi: 10.1021/jf062946j. [DOI] [PubMed] [Google Scholar]
- Ye S, Zhang J, Liu Z, Zhang Y, Li J, Li YO. Biosynthesis of selenium rich exopolysaccharide (Se-EPS) by Pseudomonas PT-8 and characterization of its antioxidant activities. Carbohydr Polym. 2016;142:230–239. doi: 10.1016/j.carbpol.2016.01.058. [DOI] [PubMed] [Google Scholar]
- Zhai Q, et al. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control. 2015;54:23–30. doi: 10.1016/j.foodcont.2015.01.037. [DOI] [Google Scholar]
- Zhang ES, Wang HL, Yan XX, Zhang LD. Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci. 2005;76:1099–1109. doi: 10.1016/j.lfs.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Zhang GC, Wang DH, Wang DH, Wei GY. The mechanism of improved intracellular organic selenium and glutathione contents in selenium-enriched candida utilis, by acid stress. Appl Microbiol Biotechnol. 2016;101(5):2131. doi: 10.1007/s00253-016-8016-4. [DOI] [PubMed] [Google Scholar]
- Zhu ZJ, Jiang WQ, Ganther HE, Ip C, Thompson HJ. Activity of se-allylselenocysteine in the presence of methionine gamma-lyase on cell growth. DNA integrity, apoptosis, and cell-cycle regulatory molecules. Mol Carcinog. 2000;29:191–197. doi: 10.1002/1098-2744(200012)29:4<191::AID-MC1000>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]