Abstract
In the current study, the PL7 strain was isolated from soil and identified as Raoultella planticola based on its physiological characteristics and 16S rDNA sequence. By the 10th day, the PL7 strain degraded 52.0% of the pyrene (PYR) content and 50.8% of the benzo[a]pyrene (BaP) content in 20 mg L−1 PYR and 10 mg L−1 BaP in the liquid matrix. The half-life of PYR and BaP by PL7 degradation was 8.59 and 9.46 days, respectively. At pH 8.0, the degradation rates of PYR and BaP by PL7 were significantly higher at 30 °C than at 20 and 40 °C. The degradation ability of PL7 differed in red soil, paddy soil and fluvo-aquic soil; red soil produced the fastest degradation rates. The half-life of PYR and BaP by PL7 degradation in red soil was 21.7 and 11.9 days, respectively; however, without PL7 the half-life of PYR in red soil was 91.2 days. This study demonstrated the significant potential of the PL7 strain for bioremediation applications in the liquid matrix and soil contaminated by PAHs.
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0704-y) contains supplementary material, which is available to authorized users.
Keywords: Pyrene, Benzo[a]pyrene, Raoultella planticola, Identification, Biodegradation
Introduction
Polycyclic aromatic hydrocarbons (PAHs) consist of fused aromatic rings and are biodegradation resistant. PAHs are produced by natural sources including forest fires and anthropogenic processes, such as urban and industrial activities and are, therefore, prevalent in the environment (Fetzer 2007; Marusenko et al. 2011). PAHs are amplified in soil and water and are accumulated by plant and animal uptake. High concentrations of PAHs in the environment affect the quality of agricultural products and pose risks to human health (Nacher-Mestre et al. 2014). Due to their carcinogenicity, mutagenicity and toxicity, seven PAHs have been placed on the black list of China’s priority pollutants. Sixteen PAH compounds have been recommended as priority pollutants by the United States Environmental Protection Agency (USEPA) and have been designated as persistent organic pollutants (POP) by the United Nations Environment Programme (UNEP). Over the past 150 years, the environmental concentrations of PAHs gradually increased in industrially developed countries. PAH pollution is a common problem in all countries (Elgh-Dalgren et al. 2011).
The presence of PAHs in the environment significantly affects environmental and ecological functions, agricultural product safety and human health. Therefore, it is necessary to pay attention to PAH pollution in the environment. Recently, there has been increased interest in developing methods to remove or degrade PAHs in the environment (Chen et al. 2015). The cost and efficiency are the first considerations in the implementation of remediation technologies. Bioremediation involves the utilization of microorganisms to breakdown or mineralize pollutants into less harmful or non-toxic compounds (Kumar and Maitra 2016). Its efficiency and cost-effectiveness make it a promising technology for the removal of pollutants. Although PAHs are stable and resistant to biodegradation, a variety of PAH-degrading bacteria isolated from PAH-contaminated soil or sediment have been discovered (Kanaly and Harayama 2000). The biodegradation of PAHs by microorganisms has received widespread attention (Shi et al. 2010). Screening PAH-degrading strains is the key to the successful implementation of bioremediation. It is not difficult to degrade PAHs with lower molecular weights (Ghoshal et al. 1996). Many bacterial strains that possess the ability to biodegrade PAHs consisting of two or three rings have been isolated and characterized (Song et al. 2011). PAHs containing four or more rings are relatively stable and are, therefore, difficult to degrade. To efficiently degrade or eliminate PAHs containing four or more rings, it is necessary to screen and identify microorganisms with high degradation abilities. Some studies have identified bacterial strains capable of degrading PAHs with four or more rings; however, the degradation efficiencies of these strains are relatively low (Wang et al. 2012; Wongwongsee et al. 2013).
Pyrene (PYR) and benzo[a]pyrene (BaP, the most common cause of cancer) are representative PAHs with four and five rings, respectively. They account for a large proportion of the sixteen PAH priority compounds in the environment (Machín-Ramírez et al. 2010) and are model compounds of high-molecular mass PAHs. Previous studies have demonstrated microbial degradation of PYR and BaP (Arulazhagan and Vasudevan 2011); however, resources of degrading strains remain scarce. The aim of this study was to isolate strains that are capable of efficiently degrading PYR and BaP. One strain, R. planticola PL7, was identified through screening based on its physiological characteristics and 16S rDNA sequence. The application of R. planticola PL7 in the degradation of PYR and BaP in liquid matrix and soil was also studied in detail. The biodegradation of PYR and BaP by R. planticola PL7 was higher in the soil.
Materials and methods
Chemicals
Pyrene (PYR, 92.2% of purity) and benzo[a]pyrene (BaP, 99.0% of purity) were purchased from the Supelco Corporation (Bellefonte, PA, USA). Stock solutions with a final concentration of 1000 mg L−1 (PYR) and 500 mg L−1 (BaP) were prepared in acetone and kept in a brown bottle at 4 °C to avoid any light exposure prior to use. All other chemicals were of analytical grade and commercially available. The Gel extraction and Plasmid extraction kits were provided by Axygen Biotech Ltd. (Hangzhou, China). Primers for 16S rDNA fragment amplification were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The TaKaRa Mini Best bacterial genomic DNA extraction kit and PCR reagent were obtained from TaKaRa Biotechnology Co., Ltd. (Dalian, China).
Soil samples
Soil samples were collected from the surface layer (0–20 cm) for bacterial isolation near a car repair station in Hangzhou City, Zhejiang Province of China. For the degradation study, the soil samples were collected from the 0–20 cm layer of agricultural soils in Zhejiang Province of China. After the debris was removed, the air-dried soil was passed through a 1 mm sieve and kept at room temperature. The physical and chemical properties of the soils were as follows: (1) Fluvo-aquic soil (taxonomy of China, Gleyic Cambisols for taxonomy of World Reference Base for Soil Resources) from Hangzhou City: organic matter content (dry basis) 1.20%, pH 6.24, CEC/(me/100 g) 3.83, sandy soil texture. (2) Red soil (taxonomy of China, Orthic Acrisol for taxonomy of World Reference Base for Soil Resources) from Quzhou City: organic matter content (dry basis) 0.79%, pH 5.28, CEC/(me/100 g) 17, heavy loam soil texture. (3) Paddy soil (taxonomy of China, Gleysol for taxonomy of World Reference Base for Soil Resources) from Jinhua City: Organic matter content (dry basis) 2.02%, pH 5.64, CEC/(me/100 g) 10.1, medium loam soil texture.
Culture medium
The minimal medium (MM) contains (per L): 8.0 g of sucrose, 1.0 g of (NH4)2SO4, 2.0 g of K2HPO4, 0.5 g of MgSO4·7H2O, 0.1 g of NaCl, 0.5 g of yeast, 0.5 g of CaCO3 and 1000 mL of distilled water. The solid MM plate (per L) was composed of 20.0 g agar. The MM without sucrose was amended by PYR and BaP mixture solution in acetone as the degradation medium. The solid MM plate without sucrose was amended by PYR and BaP mixture solution in acetone as the solid isolation medium. The solid lysogeny broth (LB) plate was composed of (per L): 10.0 g NaCl, 10.0 g peptone, 5.0 g yeast extract and 15.0 g agar.
Strain isolation and identification
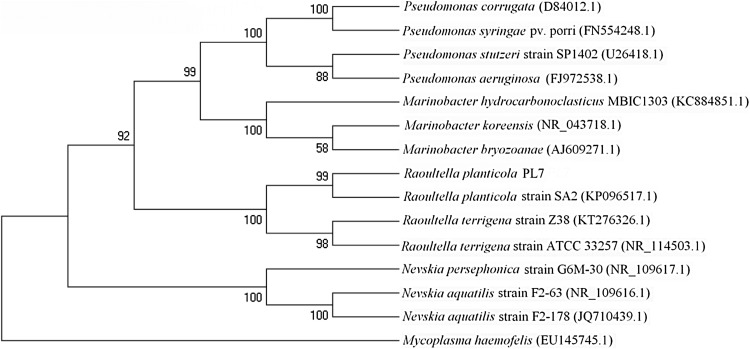
The strain isolation and inoculation methods were carried out according to the procedures described by Ping et al. (2011, 2014). The detailed physiological characteristics of the PL7 strain were studied. The cell morphology of the strain was observed using a light microscope (Olympus CH20, Japan) and a field-emission scanning electron microscope (FEI/Philips XL30, Holand, USA). The carbon source utilization was examined using a standardized method with the Biolog microstation (GP2; Biolog Hayward, CA, USA). For genotypic identification, the genomic DNA of the PL7 strain was isolated according to the methods described previously (Ping et al. 2014). The 16S rDNA nucleotide sequence was enzymatically amplified using the polymerase chain reaction (PCR) method with genomic DNA isolated from Raoultella sp. PL7 as the template. The PCR products were extracted and purified from the agarose gel using the AxyPrep DNA Gel Extraction Kit (Axygen Biotech Ltd.) according to the manufacturer’s instructions. The resulting PCR fragment was ligated with pGEM-T (Promega, Madison, WI, USA) using the T/A cloning procedure (Liu et al. 2011). The pGEM-T-PL7 recombinant plasmid was transferred into competent cell Escherichia coli JM109, and then spread onto the LB plate containing 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal), isopropyl-1-thio-α-d-galactoside (IPTG) and ampicillin (50 g mL−1). The positive clone, E. coli JM109/pGEM-T-PL7, was obtained. After isolation using the AxyPrep Plasmid Miniprep Kit (Axygen Biotech Ltd.), both strands of the pGEM-T-PL7 plasmid were sequenced using ABI 3730XL DNA Analyzer (Sangon Biotech Co. Ltd., Shanghai, China). The sequence obtained in this study was compiled and compared to sequences deposited in the GenBank databases using the BLAST program (Altschul et al. 1997). The sequences were aligned using multiple sequence alignment software, CLUSTAL W ver. 1.81 (Thompson et al. 1994). A phylogenetic tree was constructed using MegAlign software (DNASTAR Inc., Madison, WI, USA). Construction of the phylogenetic tree was based on the partial 16S rDNA sequences using similarity as the index.
PYR and BaP degradation by PL7
Two sets of experiments including the control were performed to inoculate the boiled PL7 cells and the liquid matrix containing 20 mg L−1 PYR and 10 mg L−1 BaP with the PL7 strain for the biodegradation of PYR and BaP in liquid matrix. 2 mL of PL7 pre-culture (approximately 2.0 × 108 cells mL−1) was transferred into 8 mL of the degradation culture. The flasks were incubated at 30 °C with shaking at 180 rpm in a culture incubator in the dark. Samples from each treatment were collected 9 times during the time interval from 10th to 240th hour to determine the number of bacteria and the concentrations of PYR and BaP.
Degradation solutions with different pHs ranging from 5 to 10 adjusted by 0.1 M NaOH or HCl were incubated at 20, 30 and 40 °C in the dark to investigate the effects of pH and temperature on the biodegradation of PYR and BaP. Twenty grams of soil were placed in Erlenmeyer flasks and autoclaved at 121 °C for 20 min. The unsterilized soil was artificially contaminated with PYR and BaP. Two treatments including the control (no inoculants) and inoculants (10%) were used to investigate the biodegradation efficiency. PYR and BaP at concentrations of 10 and 5 mg kg−1 (dry matter basis), respectively, were mixed with the tested soil (20.0 g) for each treatment. The mixtures were stored at room temperature overnight to evaporate the acetone. After the acetone evaporated, the inoculants were then added to the Erlenmeyer flasks. The Erlenmeyer flasks were placed at 30 °C in a culture incubator and incubated in the dark. Sterile water was added to maintain the soil moisture content at 65%. Soil samples from each treatment were collected after 0, 10, 20, 32, 44, 56, 68, 92, 140, 188, and 240 h to determine the PYR and BaP concentrations.
In this study, all treatments were carried out in triplicate if not specially noted.
PYR and BaP analysis
Extraction of PAHs from the liquid culture medium was carried out according to the following steps: 10 mL of degradation solution was transferred into a 250 mL separatory funnel and extracted with four 30 mL additions of dichloromethane. For each extraction, the separatory funnel was shaken for 30 s. The combined dichloromethane portions were reduced to dryness under a stream of nitrogen in a 40 °C water bath. The dried samples were redissolved in 10 mL of acetonitrile. The extraction of PYR and BaP from the soil was performed according to the methods described previously (Ping et al. 2014). The concentrations of PYR and BaP were analyzed using HPLC (Waters Corporation, Milford MA, USA) with a Waters PAH C18 column (4.6 mm × 250 mm, 5 μm particle size; Waters Corporation) under the condition of 1.0 mL min−1 acetonitrile–water mobile phase in gradient elution mode. The gradient elution mode was set to 60% acetonitrile at 0 min, 100% acetonitrile at 12.0 min and 60% acetonitrile at 20.0 min. The excitation and emission wavelengths were 250 and 270 nm at 0 min, 250 and 385 nm from 7.6 to 9.0 min, and 296 and 404 nm from 12.2 to 20.0 min, respectively. The retention times for PYR and BaP were 10.3 and 17.4 min, respectively.
The total PYR and BaP recoveries were within the acceptable ranges: the mean PYR recovery ranged from 95 to 105% in 2.0 and 20.0 mg L−1, and the mean BaP recovery was 91 and 98% in 1.0 and 10.0 mg kg−1, respectively, in the degradation solution. The mean PYR recovery values obtained from the soil were 89 and 101% in 1.0 and 10 mg kg−1, respectively. The mean BaP recovery values obtained from the soil were 85 and 97% in 0.5 and 5.0 mg kg−1, respectively. The results of the blanks (degradation culture with strains and soils but without spiking PAHs) extracted under the same conditions were below the detection limits. The sample results without the blank correction are presented.
Results
Isolation and identification of microorganisms
The competitive strains capable of degrading PYR and BaP were continuously enriched and formed a relatively stable microbial community after repeated enrichment. The bacterial strain was isolated from the soil using plate-screening of microorganisms and pure bacterial culture isolation techniques. The isolated strain was identified as PL7. PL7 was capable of using PYR or BaP as the sole carbon source when incubated in liquid culture. The PL7 strain was deposited in the China Center for Type Culture Collection (CCTCC), Wuhan, China, and assigned the accession number M 2016061. This strain was selected for further study based on its ability to utilize PYR and BaP as a carbon source. The strain was Gram-positive, aerobic, possessed a capsule, non-motile, typically round, pink, 0.5–0.8 × 1.0–1.5 mm in size, convex with a smooth margin and non-flagellated (Fig. S1 in the Supporting Information). The diameter of the colony incubated at 30 °C for 24 h was 2–3 mm. The colony was sticky when picked up from the plate. The carbon source utilization and sensitivity to chemicals were determined using a standardized method employing the Biolog microstation (Table S1 and S2 in the Supporting Information). According to the biochemical and morphological characterization, and the Biolog GP2 tests, the similarity of the PL7 strain to R. planticola was 99.9%. The partial 16S rDNA sequence of PL7 was determined and the sequence was deposited in the GenBank database with the accession number KU668567. Analysis of its 16S rDNA sequence revealed that PL7 had the highest sequence identity (100%) with R. planticola (AJ251467). The phylogenetic analysis indicated that these two strains shared the closest relationship (Fig. 1). Thus, based on the identification results, this newly isolated strain is considered to be a strain of R. planticola and is named R. planticola PL7.
Fig. 1.
The phylogenetic dendrogram of Raoultella planticola PL7 and related strains based on the 16S rDNA sequence. The accession numbers of published sequences are given in the parentheses. The bootstrap values were based on 1000 iterations. The Mycoplasma haemofelis was used as the outgroup
Effects of temperature and pH on PYR and BaP degradation by PL7
To investigate the effects of temperature on biodegradation by PL7, the degradation tests were carried out at various temperatures ranging from 20 to 40 °C and the pH was maintained at 7.0. As shown in Fig. 2, the degradation rates of PYR and BaP by PL7 at 30 °C were significantly higher than those at 20 °C. On the 10th day, the degradation rate of PYR and BaP at 30 °C increased by 17.89 and 27.13%, respectively, when compared to those at 40 °C. On the 10th day, the degradation rates of PYR and BaP at 20 °C decreased by 36.77 and 24.30%, respectively, when compared to those at 30 °C.
Fig. 2.

PYR and BaP degradation by PL7 under different temperatures. a PYR degradation by PL7 under different temperatures. b BaP degradation by PL7 under different temperatures. c PYR degradation rate by PL7 under different temperatures. d BaP degradation rate by PL7 under different temperatures
According to the kinetic model (El-Mansi et al. 2007), we obtained kinetic equation as follows: −dc/dt = Kc, where c is the concentration of PYR, t is the reaction time, and K is the kinetic coefficient. The degradation of CK did not obey first-order reaction kinetics (R 2 < 6). The first-order reaction kinetics of PYR degradation were c = 19.416e−0.0126t, R 2 = 0.8406 (20 °C), c = 18.032e−0.0807t, R 2 = 0.8202 (30 °C) and c = 17.851e−0.068t, R 2 = 0.9462 (40 °C). The half-life of PYR in liquid matrix containing PL7 was 26.26 (20 °C), 8.59 (30 °C) and 10.19 days (40 °C). The first-order reaction kinetics of the PL7 strain for BaP were c = 9.7744e−0.0386t, R 2 = 0.945 (20 °C); c = 8.3645e−0.0733t, R 2 = 0.8488 (30 °C) and c = 9.3213e−0.0346t, R 2 = 0.8097 (40 °C). The half-life of BaP in liquid matrix containing PL7 at varying temperatures was 17.96 (20 °C), 9.46 (30 °C) and 20.03 days (40 °C). On the 10th day, the degradation rates of PYR and BaP by PL7 at 30 °C were higher than those at 20 and 40 °C.
Degradation tests were carried out at various pHs. As shown in Fig. 3, the optimal pH for degradation was 8.0. At a pH 8.0, 64.6 and 58.9% of PYR and BaP, respectively, were removed after 10 days. This was significantly higher than under other pH conditions. The first-order reaction kinetics of PL7 degradation at various pHs are shown in Table 1. The half-lives of PYR and BaP by PL7 degradation at a pH of 8.0 were 5.91 and 7.30 days, respectively, whereas the half-lives of PYR and BaP at a pH of 10.0 were 9.99 and 10.24 days, respectively.
Fig. 3.
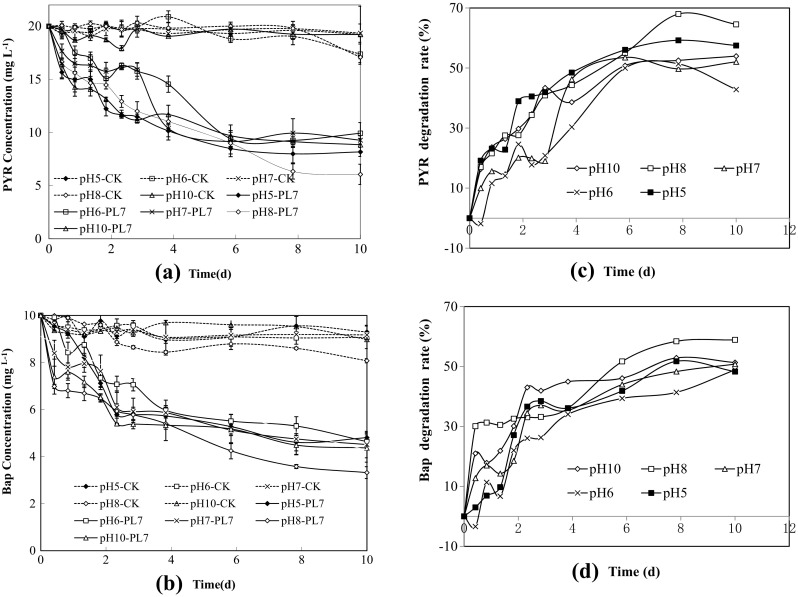
PYR and BaP degradation by PL7 under different pHs. a PYR degradation by PL7 under different pHs. b BaP degradation by PL7 under different pHs. c PYR degradation rate by PL7 under different pHs. d BaP degradation rate by PL7 under different pHs
Table 1.
The first-order reaction regression equation in different pH conditions
| Strains | PYR | BaP | ||||
|---|---|---|---|---|---|---|
| Condition | Regression equation | T 1/2 (days) | R 2 | Regression equation | T 1/2 (days) | R 2 |
| pH 5 | c = 15.735e−0.0841t | 7.37 | 0.8281 | c = 8.6314e−0.0767t | 9.04 | 0.7605 |
| pH 6 | c = 19.064e−0.0823t | 7.94 | 0.8699 | c = 9.1e−0.0754t | 9.19 | 0.8944 |
| pH 7 | c = 18.032e−0.0807t | 8.59 | 0.8202 | c = 8.3645e−0.0733t | 9.46 | 0.8488 |
| pH 8 | c = 17.606e−0.1173t | 5.91 | 0.9702 | c = 7.7992e−0.0949t | 7.30 | 0.9147 |
| pH 10 | c = 15.634e−0.0694t | 9.99 | 0.7990 | c = 7.6281e−0.0677t | 10.24 | 0.7470 |
Degrading characteristics of the PL7 strain in liquid matrix
PL7 effectively degraded PYR and BaP in the solution under the conditions of 30 °C and a pH of 7.0. The results are detailed in Figs. 4 and 5. The degradation rate (DR) was calculated according to the following equation: DR (%) = (C c−C s)/C c × 100%, where C c is the concentration of PYR or BaP with the boiled cells of the strain (CK) and C s is the concentration of PYR or BaP with PL7 (PL7). At the initial concentrations of 20 and 10 mg L−1 of PYR and BaP, respectively, PL7 degraded 52.0% of PYR and 50.8% of BaP after 10 days, demonstrating that PL7 degraded PYR and BaP equally as well.
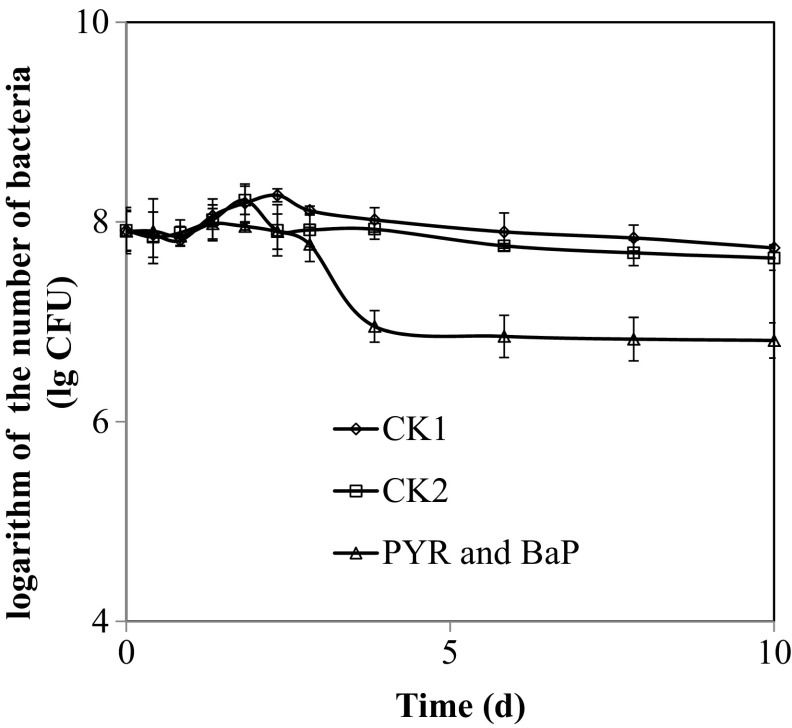
Fig. 4.
The number of bacteria in the degradation process. CK1: No addition of PAHs and acetone; CK2: Addition of acetone only; PYR and BaP: Degradation solution with PYR and BaP
Fig. 5.
PYR and BaP degradation by PL7 in the liquid matrix
According to the kinetic model (El-Mansi et al. 2007), the degradation reaction of PL7 fit to the first-order reaction kinetics according to the following equation: c = 18.0321e−0.0807t, R 2 = 0.8202 (PYR) and c = 8.3645e−0.0733t, R 2 = 0.8488 (BaP), where c is the concentration of PYR and BaP, t is the reaction time of degradation and k is the kinetic coefficient. The degradation reaction of the control was not described by the first-order reaction kinetics (R 2 < 5). The half-life of PYR and BaP in liquid matrix containing PL7 was 8.59 and 9.46 days, respectively. Figure 5 shows the number of bacteria in the liquid culture during the 240 h biodegradation process. These data indicated that the number of bacteria gradually decreased as the culture time continued. The mixture of PYR and BaP had the greatest impact on the number of bacteria. At 240th hour, the number of bacteria in the mixture of PYR and BaP solution was 0.65 × 107 CFU. However, in the CK1 (no addition of PAH and acetone added) and CK2 (only acetone added) solutions, the number of bacteria was 5.5 × 107 and 4.35 × 107 CFU, respectively.
Degrading characteristics of the PL7 strain in soil
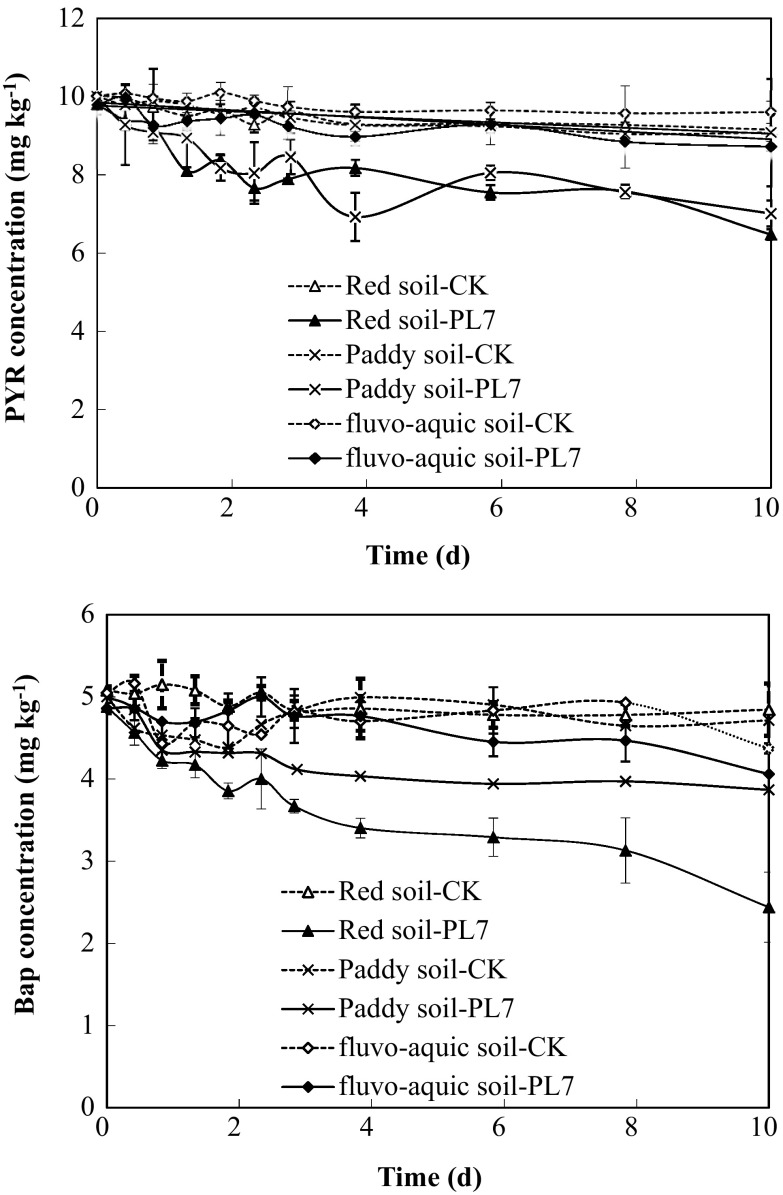
The degradation of PYR and BaP was significantly higher (p < 0.05) in soils inoculated with live PL7 cell than that in the non-inoculated soils (Fig. 6). The results demonstrated that BaP and PYR were degraded by PL7 in soils with a pH of 7.0 and a temperature of 30 °C during incubation period. The degradation rates of BaP and PYR by the PL7 strain were 49.7 and 29.3% in red soil, 18.0 and 32.0% in paddy soil, and 7.1 and 9.1% in fluvo-aquic soil, respectively, on the 10th day. This study demonstrated that the degradation ability of PL7 was different in different soils and the rate of degradation was highest in the red soil. Just as the pH of the liquid affected the degrading characteristics of the strain (the order of degradation pH 8.0 > pH 5.0 > pH 6.0 > pH 7.0 > pH 10.0), the pH of the soil may also exert similar effects. The soil pH was 5.28, 5.64 and 6.24 for red soil, paddy soil and fluvo-aquic soil, respectively. The effects of soil pH on the degradation abilities were more obvious than in the liquid matrix.
Fig. 6.
Pyrene and BaP degradation by PL7 in soils
The PYR degradation by PL7 fit the first-order reaction kinetics model (El-Mansi et al. 2007). The equations were as follows: c = 9.0755e−0.0319t, R 2 = 0.7647 (red soil); c = 9.1027e−0.0286t, R 2 = 0.6400 (paddy soil); and c = 9.6510e−0.0106t, R 2 = 0.7104 (fluvo-aquic soil). The PYR degradation reaction in soil without PL7 (CK) was described by the following equation: c = 9.7691e−0.0076t, R 2 = 0.6564 (red soil CK); c = 9.8465e−0.0100t, R 2 = 0.8601 (paddy soil CK); and c = 9.9915e−0053t, R 2 = 0.7064 (fluvo-aquic soil CK). The half-life of PYR by PL7 degradation was 21.7, 24.3, and 65.4 days in red soil, paddy soil and fluvo-aquic soil, respectively. However, the half-life of PYR without PL7 was 91.2, 69.3, and 130.8 days in red soil, paddy soil and fluvo-aquic soil, respectively. PL7 degradation shortened the half-life of PYR to 69.5, 45.0, and 65.4 days in red soil, paddy soil and fluvo-aquic soil, respectively. The degradation reaction of BaP by PL7 fits the first-order reaction kinetics according to the following equations: c = 4.5328−0.0584t, R 2 = 0.9336 (red soil); c = 4.5807e−0.0210t, R 2 = 0.6802 (paddy soil); and c = 4.9256e−0.0157t, R 2 = 0.7683 (fluvo-aquic soil). The half-life of BaP by PL7 degradation was 11.9, 33.0, and 44.1 days in red soil, paddy soil and fluvo-aquic soil, respectively.
Discussion
PAHs are the primary pollutants of the environment; therefore, the removal and degradation of these hydrocarbons are of the utmost importance. Previous studies have reported that the removal of PAHs from the environment mainly relies on the biodegradation processes of microorganisms (Song et al. 2011; Thavamani et al. 2012). The identification of strains that degrade PAHs is critical for successful implementation of environmental bioremediation. Thavamani et al. (2012) reported that degradation by the consortium-5 consisted of four different bacterial species. Sixty-seven percent of BaP was degraded after 60 days of incubation, while only 50% was degraded in the presence of Cd (Thavamani et al. 2012). After cultivation with 50 mg L−1 of PYR and BaP, the P14 strain removed 34% of PYR and 30% of BaP in 30 days (Song et al. 2011). The percentage of BaP degradation in 5 days ranged from 1 to 35% in bacterial cultures with 25–75 mg L−1 of BaP (Machín-Ramírez et al. 2010). Wongwongsee et al. (2013) demonstrated that the Novosphingobium and Microbacterium sp. strains degraded 98 and 71% of the PYR content, respectively, after 2 weeks. Sphingobium sp. FB3 degraded 72 ± 8% of the PYR content (100 mg L−1) and 6 ± 2% of the BaP content (10 mg L−1) in 10 days (Fu et al. 2014). After 40-day cultivation with Pseudomonas sp. JP1, the anaerobic biodegradation rate of BaP, fluoranthene and phenanthrene was 30, 47, and 5%, respectively (Liang et al. 2014). However, Pseudomonas sp. JP1 hardly degraded PYR under aerobic or anaerobic conditions. Sodium sulfite, iron oxide, manganese dioxide, potassium chlorate, maltose, glycine and a salinity of 20% significantly stimulated anaerobic degradation of BaP. Amycolatopsis sp. Poz14 exhibited degradation percentages of 100% for naphthalene, 37.87% for anthracene, 25.10% for PYR and 18.18% for fluoranthene within 45 days (Ortega-González et al. 2015). Luo et al. (2009) reported that Ochrobactrum sp. BL01 and Pseudomonas fluorescens BL03 degraded 20.98 and 44.07% of the BaP content, respectively, after 14 days of incubation. These two strains also effectively degraded PYR; however, neither exhibited BaP degradation activity (Wongwongsee et al. 2013). Ping et al. (2014) showed that after 10 days, the PL1 strain degraded 63.4% of the PYR content and 55.8% of the BaP content in the presence of 20 mg L−1 PYR and 10 mg L−1 BaP (Ping et al. 2014). The R. planticola PL7 strain obtained in the current study efficiently degraded PYR and BaP. The degradation rates of PYR and BaP by R. planticola PL7 were similar to those reported in previous studies (Ping et al. 2014). The PL7 strain is a new candidate for the biodegradation of PYR and BaP.
PYR and BaP persist in the environment due to their resistance to biodegradation and slow degradation rates during bioremediation. In this study, the PL7 strain was able to efficiently degrade high-molecular-weight PAHs (PYR and BaP). This is the first study to report that R. planticola degrades PYR and BaP, though previous reports have found that R. planticola was able to degrade atrazine, organic compounds of molasses melanoidin, lipids and 2,4,5-trichlorophenoxyacetic acid (Swissaa et al. 2014; Sugimori et al. 2013). This study indicated that the R. planticola PL7 strain is a promising PYR and BaP degradation strain due to its high tolerance of PYR and BaP and demonstrated its potential in the remediation of mixed PAH contamination. This study also found that the rate of PYR and BaP degradation in liquid culture was rapid during the first 4 days and then slowed. Slow-growing bacteria due to nutritional deficiencies, intermediate metabolites produced during PYR and BaP degradation and changes in the environmental conditions may have contributed to this phenomenon (Zhong et al. 2010).
Environmental conditions have an important influence on the growth of microorganisms and their biodegradation functions. Commonly, the reproduction speed of bacteria was faster under higher temperatures because the growth and enzymatic activity of microorganisms in vivo are relatively high at higher temperatures. Biological activity (such as degradation) increased with higher temperatures. The optimum temperature for enzymatic activity typically ranges from 35 to 40 °C. On the 10th day, the degradation rate of BaP by PL7 was higher at 30 °C than at 20 and 40 °C. The degradation rate of PYR by PL7 was higher at 30 and 40 °C than at 20 °C. After 188 h, the degradation rates of PYR by PL7 at 30 and 40 °C were similar. PYR and BaP were not degraded equally possibly due to differences in the underlying mechanisms and different degradation enzymes.
The characteristics of PAH-degrading strains are influenced by the environmental pH and temperature. In contrast to previous studies, the highest amounts of PYR and BaP degradation were achieved when the pH was 8.0. The degradation of phenanthrene and anthracene by Sphingomonas paucimobilis was more sensitive to the pH of the growth media (Kastner et al. 1998). Cell numbers may increase rapidly under weakly acidic or neutral conditions; however, in an alkaline environment the cell growth is restrained further affecting degradation (Tao et al. 2007). The regulation of the pH and temperature is very important for PAH degradation by PL7 in the actual contaminated environment. The degradation ability of the PL7 strain varied in different soils. The rate of degradation in red soil was higher than that in paddy soil and fluvo-aquic soil, which may have been due to their different pHs. The degradation capacity of PL7 was higher under weak alkali and acidic conditions, and the degradation rate was highest in red soil with a pH of 5.28. Therefore, the degradation capacity of PL7 can be improved by regulating the pH of the polluted environment. Contrary to a previous report, BaP degradation was higher than PYR degradation in red soil (Zeng et al. 2010). The nutrient and environmental conditions are better in red soil than in paddy soil and fluvo-aquic soil. For these reasons, degradation by the PL7 strain in red soil was the fastest, further confirming that the environmental conditions greatly influence microbial metabolic activity. Similar to the PL1 strain, the half-life of BaP degradation was shorter than that of PYR degradation in red soil and fluvo-aquic soil with PL7 (Ping et al. 2014). Typically, the biodegradation of PAHs with low-molecular weights is more extensive and occurs much more rapidly than the high-molecular weight PAHs (Lors et al. 2012). However, high degradation rates have been observed with high-molecular weight PAHs in some cases. In the bioremediation of aged PAH-contaminated soil with a microbial consortium, the biodegradation of PAHs with two to four rings was significantly lower than that of PAHs with five to six rings (Mao et al. 2012). In this study, the degradation of BaP by PL7 was more efficient than that of PYR in red soil and fluvo-aquic soil.
Thavamani et al. (2012) reported that approximately 77% of bacterial consortium degradation was observed in PYR compared to 48% in BaP during the 60-day incubation period. Wu et al. (2013) reported that the maximum degradation rate was 38.2 and 26.4% for PYR and BaP, respectively, using six different PAH-degrading consortia over a 30-day period. Studies also showed that Ochrobactrum sp. from marine sediment degraded and utilized BaP (over 20% during 2 weeks of incubation) as a sole carbon and energy source (Wu et al. 2009). During a 70-day experiment, Contreras-Ramos et al. (2008) demonstrated that E. foetida removed 16% of BaP and 99% of phenanthrene from soil compared to 3 and 95%, respectively, from unamended soil. Chen and Ding (2012) reported that after 90 days of incubation the biodegradation percentage of PYR was 52% in unsterilized soil without microorganisms. The percentages increased to 95 and 91% when amended with live P. chrysosporium. In soils contaminated with 1000 mg kg−1 of a three-PAH mixture, Trichoderma asperellum H15 was shown to degrade 74% of phenanthrene, 63% PYR and 81% BaP after 14 days (Zafra et al. 2015). Compared to reports using other microorganisms, the rate of degradation of PYR and BaP in red soil using PL7 was relatively faster.
Conclusions
This study demonstrated that a newly isolated and identified strain (R. planticola PL7) could degrade 52.0% of PYR and 50.8% of BaP in 10 days. The degradation rate of PYR and BaP by PL7 at 30 °C was significantly higher than that at 20 and 40 °C. From the 32nd hour to the 10th day, the degradation rate of PYR at 40 °C was significantly higher than that at 20 °C. The degree of degradation by PL7 was influenced by the pH in the following order: pH 8.0 > pH 5.0 > pH 6.0 > pH 7.0 > pH 10.0. After 10 days, the percentage of PYR and BaP removal (64.56 and 58.89%, respectively) was significantly higher at a pH of 8.0. The study showed that the degradation ability of PL7 varied in different soils such that the rate of degradation in red soil was better than that in paddy soil and fluvo-aquic soil. The half-life of PYR degradation by PL7 was 21.7, 24.3, 65.4 days in red soil, paddy soil and fluvo-aquic soil, respectively. The half-life of BaP degradation by PL7 was 11.9, 33.0, and 44.1 days in red soil, paddy soil and fluvo-aquic soil, respectively, suggesting that the half-life of BaP degradation was shorter than that of PYR degradation when using PL7. This study indicated that that the capacity of PL7 to degrade PYR and BaP in red soil was relatively high. These findings demonstrate that R. planticola PL7 is a new PYR- and BaP-degrading strain and has significant potential for the bioremediation of PAHs in soil.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 21007061, No. 21307115), the opening foundation of the Key Laboratory of Recycling and Eco-treatment of Waste Biomass of the Zhejiang Province (No. 2016REWB08), the China Spark Program (2015GA710001), the Scientific Research Foundation of Zhejiang University of Science and Technology (No. F701104F07), the Program for International S&T Cooperation Projects of China (2014DFE90040) and the Key Project in the Youth Elite Support Plan of the Zhejiang Academy of Agricultural Sciences.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0704-y) contains supplementary material, which is available to authorized users.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulazhagan P, Vasudevan N. Biodegradation of polycyclic aromatic hydrocarbons by a halotolerant bacterial strain Ochrobactrum sp. VA1. Mar Pollut Bull. 2011;62:388–394. doi: 10.1016/j.marpolbul.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Chen B, Ding J. Biosorption and biodegradation of phenanthrene and pyrene in sterilized and unsterilized soil slurry systems stimulated by Phanerochaete chrysosporium. J Hazard Mater. 2012;229:159–169. doi: 10.1016/j.jhazmat.2012.05.090. [DOI] [PubMed] [Google Scholar]
- Chen M, Xu P, Zeng GM, Yang CP, Huang DL, Zhang JC. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications, microbes and future research needs. Biotechnol Adv. 2015;33:745–755. doi: 10.1016/j.biotechadv.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Contreras-Ramos SM, Álvarez-Bernal D, Dendooven L. Removal of polycyclic aromatic hydrocarbons from soil amended with biosolid or vermicompost in the presence of earthworms (Eisenia foetida) Soil Biol Biochem. 2008;40:954–1959. doi: 10.1016/j.soilbio.2008.04.009. [DOI] [Google Scholar]
- Elgh-Dalgren K, Arwidsson Z, Ribé V, Waara S, Kronhelm TV, Patrick AW. Bioremediation of a soil industrially contaminated by wood preservatives-degradation of polycyclic aromatic hydrocarbons and monitoring of coupled arsenic translocation. Water Air Soil Pollut. 2011;214(1):275–285. doi: 10.1007/s11270-010-0422-0. [DOI] [Google Scholar]
- El-Mansi EMT, Bryce CFA, Demain AL, Allman AR. Fermentation microbiology and biotechnology. 2. London, New York: CRC Press; 2007. [Google Scholar]
- Fetzer JC. The chemistry and analysis of the large polycyclic aromatic hydrocarbons. Polycycl Aromat Compd. 2007;27:143–162. doi: 10.1080/10406630701268255. [DOI] [Google Scholar]
- Fu B, Li QX, Xu T, Cui ZL, Sun Y, Li J. Sphingobium sp. FB3 degrades a mixture of polycyclic aromatic hydrocarbons. Int Biodeterior Biodegrad. 2014;87:44–51. doi: 10.1016/j.ibiod.2013.10.024. [DOI] [Google Scholar]
- Ghoshal S, Ramaswami A, Luthy RG. Biodegradation of naphthalene from coal tar and heptamethylnonane in mixed batch system. Environ Sci Technol. 1996;30:1282–1291. doi: 10.1021/es950494d. [DOI] [Google Scholar]
- Kanaly RA, Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol. 2000;182:2059–2067. doi: 10.1128/JB.182.8.2059-2067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner M, Breuer-Jammali M, Mahro B. Impact of inoculation protocols, salinity, and pH on the degradation of polycyclic aromatic hydrocarbons (PAHs) and survival of PAH-degrading bacteria introduced into soil. Appl Environ Microbiol. 1998;64:359–362. doi: 10.1128/aem.64.1.359-362.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Maitra SS. Biodegradation of endocrine disruptor dibutyl phthalate (DBP) by a newly isolated Methylobacillus sp. V29b and the DBP degradation pathway. 3 Biotech. 2016;6:200. doi: 10.1007/s13205-016-0524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Song XH, Kong J, Shen CH, Huang TW, Hu Z. Anaerobic biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by a facultative anaerobe Pseudomonas sp. JP1. Biodegradation. 2014;25:825–833. doi: 10.1007/s10532-014-9702-5. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Dong LZ, Cheng F, Xue YP, Wang YS, Ding JN, Zheng YG, Shen YC. Gene cloning, expression, and characterization of a nitrilase from Alcaligenes faecalis ZJUTB10. J Agric Food Chem. 2011;62:11560–11570. doi: 10.1021/jf202746a. [DOI] [PubMed] [Google Scholar]
- Lors C, Damidot D, Ponge JF, Périé F. Comparison of a bioremediation process of PAHs in a PAH-contaminated soil at field and laboratory scales. Environ Pollut. 2012;165:11–17. doi: 10.1016/j.envpol.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Luo YR, Tian Y, Huang X, Yan CL, Hong HS, Lin GH, Zheng T. Analysis of community structure of a microbial consortium capable of degrading benzo(a)pyrene by DGGE. Mar Pollut Bull. 2009;58:1159–1163. doi: 10.1016/j.marpolbul.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Machín-Ramírez C, Morales D, Martínez-Morales F, Okoh AI, Trejo-Hernández MR. Benzo[a]pyrene removal by axenic- and co-cultures of some bacterial and fungal strains. Int Biodeterior Biodegrad. 2010;64:538–544. doi: 10.1016/j.ibiod.2010.05.006. [DOI] [Google Scholar]
- Mao J, Luo YM, Teng Y, Li ZG. Bioremediation of polycyclic aromatic hydrocarbon-contaminated soil by a bacterial consortium and associated microbial community changes. Int Biodeterior Biodegrad. 2012;70:141–147. doi: 10.1016/j.ibiod.2012.03.002. [DOI] [Google Scholar]
- Marusenko Y, Herckes P, Hall SJ. Distribution of polycyclic aromatic hydrocarbons in soils of an arid urban ecosystem. Water Air Soil Pollut. 2011;219:473–487. doi: 10.1007/s11270-010-0721-5. [DOI] [Google Scholar]
- Nacher-Mestre J, Serrano R, Portoles T, Berntssen MHG, Pérez Sánchez J, Hernández F. Screening of pesticides and polycyclic aromatic hydrocarbons in feeds and fish tissues by gas chromatography coupled to high-resolution mass spectrometry using atmospheric pressure chemical ionization. J Agric Food Chem. 2014;62:2165–2174. doi: 10.1021/jf405366n. [DOI] [PubMed] [Google Scholar]
- Ortega-González DK, Martínez-González G, Flores CM, Zaragoza D, Cancino-Diaz JC, Cruz-Maya JA, Roblero JJ. Amycolatopsis sp. Poz14 isolated from oil-contaminated soil degrades polycyclic aromatic hydrocarbons. Int Biodeterior Biodegrad. 2015;99:165–173. doi: 10.1016/j.ibiod.2015.01.008. [DOI] [Google Scholar]
- Ping LF, Zhang CR, Zhu YH, Wu M, Hu XQ, Li Z, Zhao H. Biodegrading of pyrene by a newly isolated Pseudomonas putida PL2. Biotechnol Bioprocess Eng. 2011;16:1000–1008. doi: 10.1007/s12257-010-0435-y. [DOI] [Google Scholar]
- Ping LF, Zhang CR, ZhangCP ZhuYH, He HM, Wu M, Tang T, Li Z, Zhao H. Isolation and characterization of pyrene and benzo[a] pyrene-degrading Klebsiella pneumonia PL1 and its potential use in bioremediation. Appl Microbiol Biotechnol. 2014;98:3819–3828. doi: 10.1007/s00253-013-5469-6. [DOI] [PubMed] [Google Scholar]
- Shi ZP, Tian L, Zhang YG. Molecular biology approaches for understanding microbial polycyclic aromatic hydrocarbons (PAHs) degradation. Acta Ecol Sin. 2010;30:292–295. doi: 10.1016/j.chnaes.2010.08.002. [DOI] [Google Scholar]
- Song XH, Xu Y, Li GM, Zhang Y, Huang TW, Hu Z. Isolation, characterization of Rhodococcus sp. P14 capable of degrading high-molecular-weight polycyclic aromatic hydrocarbons and aliphatic hydrocarbons. Mar Pollut Bull. 2011;62:2122–2128. doi: 10.1016/j.marpolbul.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Sugimori D, Watanabe M, Utsu T. Isolation and lipid degradation profile of Raoultella planticola strain 232-2 capable of efficiently catabolizing edible oils under acidic conditions. Appl Microbiol Biotechnol. 2013;97:871–880. doi: 10.1007/s00253-012-3982-7. [DOI] [PubMed] [Google Scholar]
- Swissaa N, Nitzan Y, Langzam Y, Cahan R. Atrazine biodegradation by a monoculture of Raoultella planticola isolated from a herbicides wastewater treatment facility. Int Biodeterior Biodegrad. 2014;92:6–11. doi: 10.1016/j.ibiod.2014.04.003. [DOI] [Google Scholar]
- Tao XQ, Lu GN, Dang Z, Chen Y, Xiao YY. Aphenanthrene-degrading strain Sphingomonas sp. GY2B isolated from contaminated soils. Process Biochem. 2007;42:401–408. doi: 10.1016/j.procbio.2006.09.018. [DOI] [Google Scholar]
- Thavamani P, Megharaj M, Naidu R. Bioremediation of high molecular weight polyaromatic hydrocarbons co-contaminated with metals in liquid and soil slurries by metal tolerant PAHs degrading bacterial consortium. Biodegradation. 2012;23:823–835. doi: 10.1007/s10532-012-9572-7. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. Clustal-W-improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SX, Li XJ, Liu W, Li PJ, Kong LX, Ren WJ, Wu HY, Tu Y. Degradation of pyrene by immobilized microorganisms in saline-alkaline soil. J Environ Sci. 2012;24:1662–1669. doi: 10.1016/S1001-0742(11)60963-7. [DOI] [PubMed] [Google Scholar]
- Wongwongsee W, Chareanpat P, Pinyakong O. Abilities and genes for PAH biodegradation of bacteria isolated from mangrove sediments from the central of Thailand. Mar Pollut Bull. 2013;74:95–104. doi: 10.1016/j.marpolbul.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Wu Y, He T, Zhong M, Zhang Y, Li E, Huang T, Hu Z. Isolation of marine benzo[a]pyrene-degrading Ochrobactrum sp. BAP5 and proteins characterization. J Environ Sci. 2009;21:1446–1451. doi: 10.1016/S1001-0742(08)62438-9. [DOI] [PubMed] [Google Scholar]
- Wu ML, Chen LM, Tian YQ, Ding Y, Dick WA. Degradation of polycyclic aromatic hydrocarbons by microbial consortia enriched from three soils using two different culture media. Environ Pollut. 2013;178:152–158. doi: 10.1016/j.envpol.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Zafra G, Montaño AM, Absalón ÁE, Cortés-Espinosa DV. Degradation of polycyclic aromatic hydrocarbons in soil by a tolerant strain of Trichoderma asperellum. Environ Sci Pollut Res. 2015;22:1034–1042. doi: 10.1007/s11356-014-3357-y. [DOI] [PubMed] [Google Scholar]
- Zeng J, Lin XG, Zhang J, Li XG. Isolation of polycyclic aromatic hydrocarbons (PAHs)-degrading Mycobacterium sp. and the degradation in soil. J Hazard Mater. 2010;183:718–723. doi: 10.1016/j.jhazmat.2010.07.085. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Zou SH, Lin L, Luan TG, Qiu RL, Tam NFY. Effects of pyrene and fluoranthene on the degradation characteristics of phenanthrene in the cometabolism process by Sphingomonas sp. strain PheB4 isolated from mangrove sediments. Mar Pollut Bull. 2010;60:2043–2049. doi: 10.1016/j.marpolbul.2010.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.