Abstract
Cuticle collagens form a major part of the nematode cuticle and are responsible for maintaining the overall shape of the animal and its protection from the external environment. Although substantial research on cuticle collagen genes has been carried out in Caenorhabditis elegans, their isolation and characterization in plant parasitic nematodes have been limited to a few genes only. In this study, a cuticle collagen gene, Mi-col-5, was isolated from root-knot nematode, Meloidogyne incognita. A partial segment of 402 bp was first cloned and analyzed on Gbrowse followed by subsequent cloning of the 1047 bp long full cDNA specifying the open reading frame. The deduced amino acid sequence showed 92% sequence identity with that of Mj-col-5. However, a transmembrane helix was predicted in Mi-col-5 which was not present in Mj-col-5. The conserved pattern of cysteine residues in Mi-col-5 suggested that it belonged to group 2 of nematode cuticle collagens but with a longer carboxy terminal region as was the case with Mj-col-5. Domain prediction revealed the presence of a nematode cuticle collagen N terminal domain and a pfam collagen domain along with collagen triple helix repeats. A phylogenetic tree based on the amino acid sequences showed evolutionary relationship of Mi-col-5 with cuticle collagens genes of other nematodes. 3D models for Mi-col-5 were predicted with the best confidence score of −2.78. Expression of Mi-col-5 transcript was found to be maximum in egg masses followed by adult females and J2s suggesting its role in the early stages of the development of the nematode during its life cycle.
Keywords: Cuticle collagens, Mi-col-5, Meloidogyne incognita, Egg masses
Introduction
Structural proteins, collagens are involved in the formation of cuticle in nematodes which maintains the shape of the animal and protects it from the external environment. The collagens constitute around 80% of total protein content of the nematode cuticle (Kingston 1991). Cuticle collagens possess characteristic Gly-X-Y repeats with frequent appearance of proline and hydroxyproline in place of X and Y, respectively (Page and Johnstone 2007). They also share conserved patterns of cysteine residues which forms the basis of their classification into different groups (Johnstone 2000). The collagen genes encode procollagens with molecular masses of approximately 30 kDa, which undergo post-translational modifications and trimerisation in the endoplasmic reticulum. The triple helicle structure is brought about by tyrosine–tyrosine bonds while disulphide bonds are involved in cross linking between the triple helices (Koltai et al. 1997; Kramer 1994). In the free living nematode, C. elegans, more than 150 collagen genes are found to be involved in the formation of the cuticle. Individual collagen genes are expressed at different stages of the life cycle of the nematode and exoskeletal defects may result from mutation in these individual collagen genes leading to defects in the shape of the animal which corresponds to lethality (Page et al. 2014; Page and Winter 2003). In plant parasitic nematodes (PPNs) like root-knot nematodes (RKNs) and cyst nematodes (CNs), the cuticle is also involved in their interaction with soil environment as well as with the host (Davies and Curtis 2011). However, not much is known about the genomic organization of the cuticle collagens of PPNs.
Root-knot nematode, Meloidogyne incognita, is amongst the most devastating and economically important PPNs. These PPNs have a wide host range and are biotrophic in nature completing most of their life cycle inside the host. The nematode molts multiple times inside the host bringing about spectacular changes in its morphology during development of J2s to adult female. Hence, the cuticle collagen genes and their developmentally regulated expression may play an important role in the establishment of the nematode inside the host. The expression of cuticle collagen genes and their role in synthesis and maintenance of the cuticle has been well studied in C. elegans; but in PPNs, it is still an understudied domain. However, the availability of the M. incognita whole genome sequence has facilitated ways for identification and cloning of crucial genes like cuticle collagens (Abad et al. 2008). In this study, we have isolated, cloned and characterized a cuticle collagen gene, Mi-col-5 from M. incognita.
Materials and methods
Nematode culture
For the maintenance of pure culture of M. incognita chitwood race 1, young tomato plants (Solanum lycopersicum L. cv. Pusa Ruby) were inoculated with fresh second-stage juveniles (J2s). The roots of the infected tomato plants were uprooted 30 days post inoculation and washed with double distilled water, and the egg masses were handpicked and kept in a cavity block. These egg masses were treated with 0.1% HgCl2 for 1 min for surface sterilization and then washed thrice with double distilled water to remove the surface sterilizing agent. The egg masses were then allowed to hatch at 26–28 °C through a wire gauze covered with double-layered tissue paper into a petri plate filled with double distilled water (Hooper 1986). Freshly hatched J2s were used for further experiments. Adult females were also isolated from the roots of the infected tomato plants 30 days post inoculation under the microscope using a needle.
Isolation of total RNA from different stages of M. incognita
Total RNA was isolated from egg masses, adult females, and freshly hatched J2s of M. incognita using TRIzol (Thermofisher). 1 mL of TRIzol was added to egg masses, J2s and adult females per 100 mg of tissue sample and frozen in liquid nitrogen. The samples were crushed in 1.5-mL centrifuge tubes using a tissue crusher. Finely crushed samples in TRIzol were incubated at room temperature for 5 min followed by addition of 0.2 mL of chloroform/mL of TRIzol reagent. The tubes were vigorously shaken by hand for 15 s and incubated for 5 min at room temperature. The samples were then centrifuged for 15 min at 12000×g at 4 °C for phase separation. The aqueous phases of the samples were taken in new 1.5-mL microcentrifuge tubes and 0.5 mL of 100% isopropanol was added per mL of the TRIzol used. The samples were then incubated at –20 °C for 2 h for precipitation of RNA followed by their centrifugation at 12000×g for 10 min at 4 °C, and the supernatants were removed. The pellets were washed with 1 mL of 75% ethanol/mL of TRIzol used by centrifugation at 7500×g for 5 min at 4 °C. The RNA pellets were air dried for 20 min and then dissolved in 50 µL of nuclease free water per sample. DNAse treatment was given to the RNAs and quantification was done using a nanodrop spectrophotometer (Thermo Scientific).
cDNA synthesis, amplification, and cloning of partial and full Mi-col-5 gene from M. incognita
First strand cDNA was synthesized from 300 ng of total RNA of egg masses, J2s, and adult females using Verso first strand cDNA synthesis kit (Thermo Scientific). A 402 bp segment of Mi-col-5 gene was amplified from the first strand cDNA using primers Col-5-F and Col-5-R (Table 1), designed from already available sequence of Mj-col-5 from M. javanica (Accession No. AF289026.1). The volume of each PCR amplification reaction was 25 µL containing 100 ng of first strand cDNA, 1 × Taq buffer, 10 mmol/L dNTP, 20 µmol/L of each primer, 3.5 mmol/L MgCl2, and 1.5 U Taq DNA polymerase (Fermentas). The PCR product was purified using geneJET gel extraction kit (Thermo scientific) and cloned into pGEMT easy vector (Promega) using manufacturer’s protocol. The recombinant plasmids were transformed into freshly prepared competent cells of E. coli DH5α. The positive clones were selected by blue-white screening using ampicillin (50 mg/L), IPTG (0.5 mM), and X-gal (80 µg/mL) and sequenced using ABI solid sequencing platform. The partial sequence of Mi-col-5 thus obtained was submitted to Genbank (Accession No. KF411439.1).
Table 1.
List of primers used and their sequences
| S. n. | Primer name | Primer sequence |
|---|---|---|
| 1 | Col-5-F | CAAATCGGAAAAGGACACGTAG |
| 2 | Col-5-R | TGGTCCTTTAGCACCAGCAG |
| 3 | Col-5-full-F | ATGGAACCTAAAGAGCAGTT |
| 4 | Col-5-full-R | TTAATGATATCCACCACTTT |
| 5 | Col-5-qrt-F | ACCGAGTTAAACGTGGTTGG |
| 6 | Col-5-qrt-R | GGGCCTTGAGATATTGCTGA |
| 7 | MI-18 s rRNA-F | TCAACGTGCTTGTCCTACCCTGAA |
| 8 | MI-18 s r RNA-R | TGTGTACAAAGGGCAGGGACGTAA |
Full Mi-col-5 sequence was retrieved from G-browse available at Meloidogyne incognita resources (http://www6.inra.fr/meloidogyne_incognita). Primers Col-5-full-F and Col-5-full-R (Table 1) were designed manually, and full gene was amplified from first strand cDNA of the adult females. Advantage 2 PCR kit was used for amplification of the full Mi-col-5 gene using manufacturer’s protocol. A 1047 bp long PCR product was purified and cloned into pGEMT easy vector and sequenced as described above. The full gene sequence of Mi-col-5 was submitted to NCBI database (Accession No. KX372291).
In silico analysis of Mi-col-5
The amino acid sequence of Mi-col-5 was deduced from the nucleotide sequence using expasy translate tool (http://web.expasy.org/translate/). The amino acid and nucleotide sequences of Mi-col-5 were analyzed using NCBI blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Physical and chemical parameters of the predicted amino acid sequence were computed using ProtParam (Gasteiger et al. 2005). Clustal Omega was used for alignment between amino acid sequences of Mi-col-5 and Mj-col-5 (Sievers et al. 2011). Domain architecture analysis was done using SMART and MOTIF search (Letunic et al. 2015). SOPMA was used for the analysis of secondary structure of the predicted amino acid sequence of Mi-col-5 (Combet et al. 2000). Multiple sequence alignment of the deduced amino acid sequence with cuticle collagen proteins identified in M. incognita and other species was done using Clustal Omega (Roy et al. 2010). A phylogenetic tree was constructed by maximum likelihood method using MEGA6 (Tamura et al. 2013). Intrinsic folding and unfoldability of Mi-col-5 was predicted using Fold Index (Prilusky et al. 2005). Prediction of transmembrane domain for Mi-col-5 and other cuticle collagen proteins was performed using TMHMM server V. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/), and signal peptide prediction was performed through SignalP 4.1 server (Petersen et al. 2011). Subcellular localisation predictor CELLO v2.5 server (Yu et al. 2006) was used for prediction of subcellular localization of Mi-col-5. Three-dimensional structure of the protein Mi-col-5 was predicted by using I-TASSER server (Roy et al. 2010; Yang et al. 2015). The predicted model was evaluated using PROCHECK server (Laskowski et al. 1996).
Differential expression analysis of Mi-col-5 through q-PCR
Expression of Mi-col-5 at different stages of the life cycle of M. incognita was quantified and analyzed through real-time q-PCR. Total RNA was isolated from egg masses, J2s, and adult females and first strand cDNA was synthesized as described above. qPCR was performed with gene-specific primers for Mi-col-5 (Table 1) in a CFX96 real-time system (Biorad) using 2X brilliant III SYBR Green q-PCR master mix (Agilent). 18 s rRNA was used as a reference gene for normalization of gene expression levels (Table 1). The amplification reactions were run using the following program: Initial denaturation of 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s and 57 °C for 1 min. After 35 cycles, a melt curve analysis or dissociation program (95 °C for 15 s, 57 °C for 15 s followed by a slow ramp from 57 to 95 °C) was acquired to ensure the specificity of amplification. Two biological and three technical replicates were used for each of the samples. After obtaining the Ct values, 2−ΔΔCT method was used to quantify the relative fold change in gene expression and Student’s t test (p < 0.05) was performed (Livak and Schmittgen 2001).
Results and discussion
Isolation and cloning of Mi-col-5 from M. incognita
Among root knot nematodes, only three cuticle collagen genes viz. Lemmi-5, mi-col-1, and mi-col-2 have been identified in M. incognita, while Mjcol-3 and Mj-col-5 have been identified in M. javanica (Koltai et al. 1997; Liu et al. 2001; Ray and Hussey 1995; Van Der Eycken et al. 1994; Wang et al. 1998). In potato cyst nematode Globodera pallida, cuticle collagen genes gp-col-1, gp-col-2 and gp-col-8 have been isolated and characterized (Gray et al. 2001; Jones et al. 1996). In this study, we have isolated and characterized a putative cuticle collagen gene, Mi-col-5, in M. incognita. Initially a partial segment of 402 bp of the gene Mi-col-5 was amplified from adult female cDNA of M. incognita using the primers designed based on the already available sequence of Mj-col-5 (Liu et al. 2001). After cloning and sequencing of this partial segment (Fig. 1), its sequence was submitted to NCBI Genbank which showed 99% sequence identity with Mj-col-5. The partial sequence was further analyzed on G-browse platform of ‘Resources for Meloidogyne incognita” to predict the full sequence of the gene based on whole genome sequence data of M. incognita. A 1593 bp long DNA segment was predicted to code for Mi-col-5 out of which the exonic sequence consisted of 1047 bp specifying the open reading frame. The full cDNA sequence was amplified (Fig. 2), cloned in pGEMT easy vector, sequenced and submitted to NCBI. At the nucleotide level, this sequence showed 98% sequence identity with Meloidogyne enterolobii collagen mRNA (accession no. KU350654.1) and 96% sequence identity with M. javanica Mj-col-5 gene.
Fig. 1.

Restriction digestion of pGEMT clones with EcoRI releasing a 402 bp partial fragment of Mi-col-5. Lane M 1 Kb ladder. lanes 1–4 positive pGEMT clones. lanes 5–7 negative plasmids
Fig. 2.

Amplification of full Mi-col-5 gene from adult female cDNA. Lane M, 1 Kb plus ladder. lane 1 adult female cDNA
In silico analysis of Mi-col-5
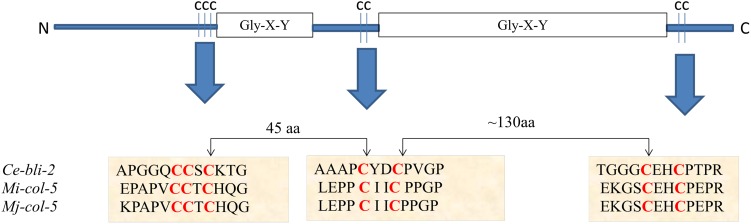
A predicted amino acid sequence of 348 residues was deduced using the cDNA sequence of Mi-col-5 with a calculated molecular mass of 35.12 kD and a theoretical pI of 5.79. Primary structural properties of Mi-col-5 are listed in Table 2. The predicted protein sequence showed 92% identity with Mj-col-5 from M. javanica (accession no. AAK83075.1), 67% identity with CRE-ROL-8 protein from Caenorhabditis remanei (accession no. XP_003113796.1) and 66% identity with ROL-8 protein from C. elegans (accession no. NP_495582.1). Predicted amino acid sequence of Mi-col-5 suggests that it belongs to group 2 of the cuticle collagen genes based on the pattern of conserved cysteine residues according to the classification proposed by Johnstone 2000 (Fig. 3). However, Like Mj-col-5, Mi-col-5 also has a 12 amino acid longer carboxy terminal than other members of the group 2 with an additional tyrosine residue. Since the tyrosine residues in the carboxy terminus of the nematode cuticle genes are supposedly involved in the collagen cross linking, the presence of an extra tyrosine residue in Mi-col-5 suggests a different pattern of its cross linking compared to other members of the group 2 (Cox 1992).
Table 2.
Primary structural properties of Mi-col-5
| S. n. | Parameter | Theoretical prediction |
|---|---|---|
| 1 | Molecular weight (kDa) | 35.12 |
| 2 | Isoelectric point | 5.79 |
| 3 | Total no. of negatively charged residues (Asp + Glu) | 33 |
| 4 | Total no. of positively charged residues (Arg + Lys) | 27 |
| 5 | Extinction coefficient (M−1 cm−1, at 280 nm) | 27,430 |
| 6 | Instability index | 49.76 |
| 7 | Aliphatic index | 49.08 |
| 8 | Grand average of hydropathicity (GRAVY) | −0.625 |
Fig. 3.
Conserved pattern of cysteine residues classifies Mi-col-5 in group 2 of cuticular collagen genes of nematodes. The positions of Gly-X-Y domains along three cysteine containing domains are represented here. This system of classification of cuticular collagen genes was proposed by Johnstone (2000) on the basis of conserved patterns of cysteine residues. In C. elegans, group 2 has 38 members
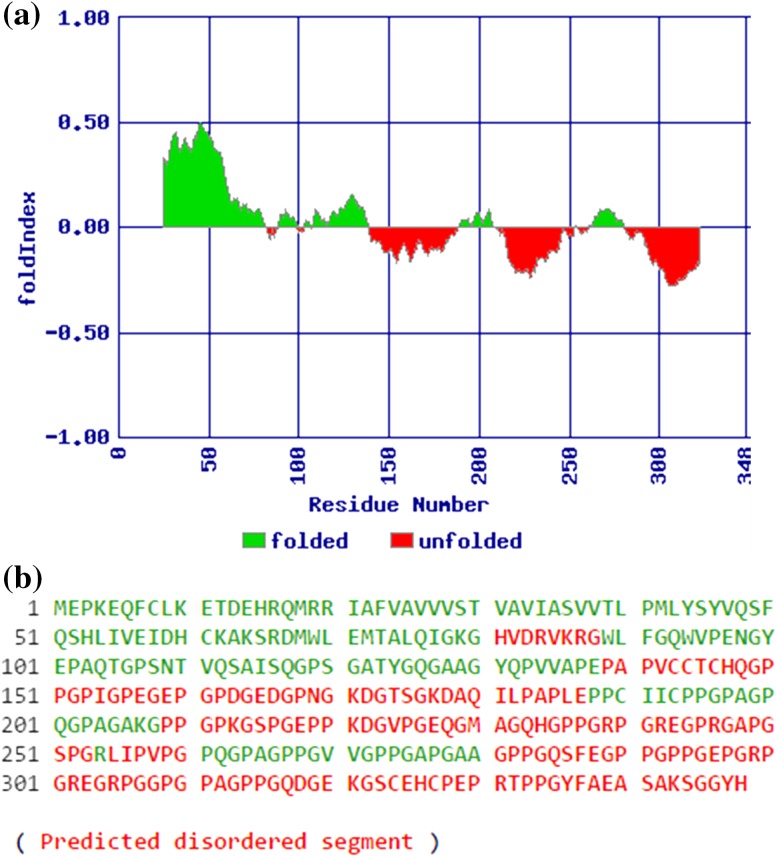
Extinction coefficient (at 280 nm) was calculated to be 27430 (M−1 cm−1) assuming all pairs of cysteine residues form cystines and 27930 (M−1 cm−1) assuming all cysteine residues were reduced. The aliphatic index and Grand Average of Hydropathicity (GRAVY) were found to be 49.08 and −0.625, respectively. 63.5% of the amino acids in the predicted protein are hydrophobic (Ala, Cys, Gly, Ile, Leu, Met, Phe, Pro, Val) while 36.5% of the amino acids were found to be polar (Arg, Asn, Asp, Gln, Glu, His, Lys, Ser, Thr, Trp, Tyr). Secondary structure prediction revealed that 50 amino acid residues (14.37%) were involved in alpha helices, 44 residues (12.64%) in extended strand, 16 residues (4.60%) in β-turn, and 238 residues (68.39%) in random coil. On comparison of the predicted secondary structures of Mi-col-5 and Mj-col-5, most of the difference was observed within the first 50 amino acids with deviation in the distribution of α helices in this region (Fig. 4). TMHMM results indicate the presence of a transmembrane helix in Mi-col-5 between amino acids positions 21–43 involving approximately 22.5 amino acid residues (Fig. 5). Interestingly, most of the difference between Mi-col-5 and Mj-col-5 lies in the amino acid composition of this region with the latter lacking a transmembrane helix (Fig. 6). However, transmembrane helices are also predicted in the amino acid sequences of the other three cuticle collagen genes, col-1, col-2, and Lemmi-5 identified in M. incognita. The subcellular localization of the putative protein Mi-col-5 was predicted to be extracellular by CELLO predictive system. Further, five disordered regions were predicted in the putative protein sequence of Mi-col-5 with 68 residues in the longest disordered region and a total of 176 residues in the entire disordered region (Fig. 7). Nematode cuticle collagen N-terminal domain spanning from amino acid residue 20-72 was predicted by SMART analysis of the deduced amino acid. Another pfam collagen domain was identified consisting of 62 amino at 194–255 amino acid position. Collagen triple helix repeats were identified by Motif Search between amino acid positions 149–176, 196–253, and 260–320. Phylogenetic tree involving the cuticle collagens isolated from PPNs and few free living and animal parasitic nematodes showed two clusters (Fig. 8). Mi-col-5 and Mj-col-5 were closely related and placed in cluster I. Cluster II included cuticle collagens from Globodera pallida grouped with Lemmi-5, col-1, and col-2 from Meloidogyne incognita. Mj-col-3, however, placed in cluster I is distantly related to Mi-col-5 and Mj-col-5.
Fig. 4.
Secondary structure prediction of deduced amino acid sequence of Mi-col-5. Comparison of Mi-col-5 and Mj-col-5 secondary structure predictions. Helices, extended strands, turns, and coils are represented by longest (blue), second longest (red), second shortest (green), and shortest (violet) vertical lines, respectively (a), Deduced amino acid sequence of Mi-col-5 is represented by upper cases and lower cases represent the corresponding secondary structural characteristics such as random coils (c), extended strand (e), α helices (h), and β turns (t) (b)
Fig. 5.
TMHMM predictions for Mi-col-5 (a), Mj-col-5 (b). Blue, red, and pink colors represent the portions of the amino acids inside, in the transmembrane helix and outside the cell, respectively
Fig. 6.
Clustal Omega analysis of the Mi-col-5 and Mj-col-5 amino acid sequences. Area under the black box shows the transmembrane helix region of Mi-col-5 and its difference with Mj-col-5
Fig. 7.
Folding predictions for Mi-col-5. Predicted folded and unfolded regions of Mi-col-5 (a), amino acid sequence for Mi-col-5 showing ordered segments in green and disordered segments in red (b)
Fig. 8.
Phylogenetic tree constructed by maximum likelihood method using MEGA 6 showing evolutionary relationship of Mi-col-5 with cuticle collagens of other plant parasitic, animal parasitic, and free living nematodes
Molecular modeling of Mi-col-5
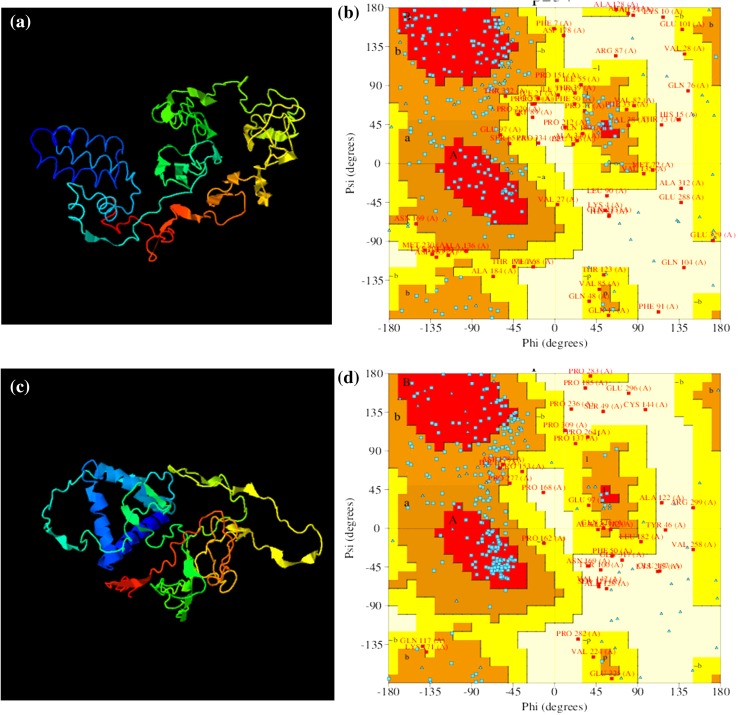
The 3D models for Mi-col-5 were generated by using I-TASSER (Iterative Threading ASSEmbly Refinement) (Fig. 9). This server generated models using threading approach. The I-TASSER server generated five models for the given amino acid sequence of Mi-col-5, out of which model 1 had the best C-score (confidence score) of −2.78. C-score reflects the quality estimation of the models generated by I-TASSER. It is calculated based on the significance of threading template alignments and the convergence parameters of the structure assembly simulations. Typically, it lies in the range of −5 to 2. The higher the C-score, higher is the quality and confidence of the corresponding predicted model. The PROCHECK analysis of model 1 showed that only 32.4% of the amino acid residues were in the most favored region, while 43.5% of the residues were in the additionally allowed region, 17.1% residues in the generously allowed region, and 6.9% residues in the disallowed region. However, PROCHECK analysis of Model 3 with a C-score of −4.47 showed 52.8% residues in the most favored region, 34.7% residues in the additionally allowed region, 6% residues in the generously allowed region, and 6.5% residues in the disallowed region. An important aspect of the Ramachandran plots for the predicted models is G factors, which is a combination of different parameters like psi–psi distribution, chi1–chi2 distribution, etc. The overall average G factors value for model 1 is −1.45 which falls into highly unusual category, while that for model 3 is −0.99 falling into unusual category.
Fig. 9.
Molecular modeling of Mi-col-5 using I-TASSER server. Predicted 3D models of Mi-col-5 representing model 1 and model 3, respectively, (a) and (c), Ramachandran plots for predicted model 1 and model 3, respectively, (b) and (d)
Expression analysis of Mi-col-5 by qRT PCR
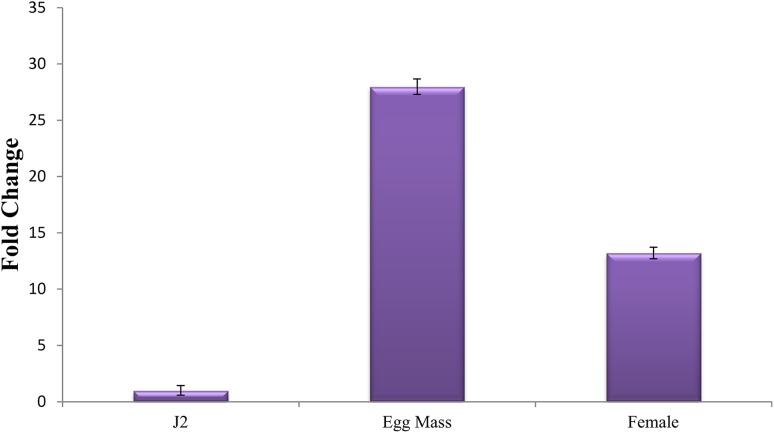
Stage-specific expression analysis of Mi-col-5 transcript was investigated in egg masses, J2s, and adult females. Taking expression levels of Mi-col-5 at J2 stage as reference, 27-fold upregulation was observed in the expression in egg masses, whereas a 12-fold upregulation was observed in the adult females (Fig. 10). In C. elegans, increased expression of cuticle collagen genes has been observed during cuticle synthesis just before molting (Johnstone and Barry 1996). Similarly, a higher expression in the egg masses suggests the role of Mi-col-5 in the earlier stages of nematode cuticle development, while the expression in the adult females may be attributed to the role of Mi-col-5 in maintenance and thickening of adult female cuticle. These results are in agreement with the expression pattern of Mj-col-5 (Liu et al. 2001). Wang et al. (1998) reported expression patterns of Mi-col-1, Mi-col-2, and Lemmi-5 showing highest level of expression in adult females followed by parasitic J3s or J4s and parasitic J2 s for all the three genes. Expression of none of these genes could be detected in pre-parasitic J2 s. Similarly, a higher expression of gp-col-1 and gp-col-2 was detected in virgin and adult females of G. pallida (Gray et al. 2001). In M. javanica, the expression pattern of Mj-col-3 showed a higher expression in developing eggs followed by J4 while the transcription level was very low in adult females (Koltai et al. 1997). Interestingly, cuticle collagen genes gp-col-1, gp-col-2, Lemmi-5, Mi-col-1, and Mi-col-2 having similar expression patterns were placed in the same cluster (cluster II) in the phylogenetic tree while Mi-col-5, Mi-col-3, and Mj-col-5 representing cluster I exhibited similar expression patterns. This suggests a strong correlation between the phylogenetic relationship and expression patterns of cuticle collagen genes in plant parasitic nematode. The differential expression of different cuticle collagen genes at different stages in root-knot nematodes suggests the involvement of these genes at different developmental stages and molting of the nematodes.
Fig. 10.
Expression of Mi-col-5 in different developmental stages of M. incognita
Conclusion
A full length cuticle collagen gene from M. incognita was isolated, characterized with the help of bioinformatic tools and its differential expression was studied at various developmental stages in the life cycle of the nematode. A comparison of this gene with other characterized cuticle collagen genes from PPNs was also made leading to understanding of its evolutionary relationships and classification. Further studies focused on RNAi-based silencing of Mi-col-5 can provide a more detailed insight into the role of this gene in the development and maintenance of the cuticle. Development of transgenic plants expressing dsRNAs of this cuticle collagen gene can be another future approach for engineering resistance against M. incognita by hindering the structural organization of the nematode cuticle.
Acknowledgements
The authors are thankful to Indian Council of Agricultural Research for providing financial support through NFBSFARA project.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, Caillaud MC, Coutinho PM, Dasilva C, De Luca F, Deau F, Esquibet M, Flutre T, Goldstone JV, Hamamouch N, Hewezi T, Jaillon O, Jubin C, Leonetti P, Magliano M, Maier TR, Markov GV, McVeigh P, Pesole G, Poulai J, Robinson-Rechavi M, Sallet E, Segurens B, Steinbach D, Tytgat T, Ugarte E, van Ghelder C, Veronico P, Baum TJ, Blaxter M, Bleve-Zacheo T, Davis EL, Ewbank JJ, Favery B, Grenier E, Henrissat B, Jones JT, Laudet V, Maule AG, Quesneville H, Rosso MN, Schiex T, Smant G, Weissenbach J, Wincker P. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol. 2008;26:909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- Combet C, Blanchet C, Geourjon C, Deleage G. NPS@: network protein sequence analysis. Trends Biochem Sci. 2000;25:147–150. doi: 10.1016/S0968-0004(99)01540-6. [DOI] [PubMed] [Google Scholar]
- Cox GN. Molecular and biochemical aspects of nematode collagens. J Parasitol. 1992;78:1–15. doi: 10.2307/3283678. [DOI] [PubMed] [Google Scholar]
- Davies KG, Curtis RSG. Cuticle surface coat of plant-parasitic nematodes. Ann Rev Phytopathol. 2011;49:135–156. doi: 10.1146/annurev-phyto-121310-111406. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools on the expasy server. In: Walker JM, editor. The proteomics protocols handbook. Totowa: Humana; 2005. pp. 571–607. [Google Scholar]
- Gray LJ, Curtis RH, Jones JT. Characterisation of a collagen gene subfamily from the potato cyst nematode Globodera pallida. Gene. 2001;263:67–75. doi: 10.1016/S0378-1119(00)00558-8. [DOI] [PubMed] [Google Scholar]
- Hooper DJ. Extraction of free-living stages from soil. In: Southey JF, editor. Laboratory methods for work with plant and soil nematodes. London: Ministry of agriculture, fisheries and food; 1986. pp. 5–30. [Google Scholar]
- Johnstone IL. Cuticle collagen genes expression in Caenorhabditis elegans. Trends Genet. 2000;16:21–27. doi: 10.1016/S0168-9525(99)01857-0. [DOI] [PubMed] [Google Scholar]
- Johnstone IL, Barry JD. Temporal reiteration of a precise gene expression pattern during nematode development. EMBO J. 1996;15:3633–3639. [PMC free article] [PubMed] [Google Scholar]
- Jones JT, Curtis RH, Wightman PJ, Burrows PR. Isolation and characterization of a putative collagen gene from the potato cyst nematode Globodera pallida. Parasitology. 1996;113:581–588. doi: 10.1017/S0031182000067639. [DOI] [PubMed] [Google Scholar]
- Kingston IB. Nematode collagen genes. Parasitol Today. 1991;7:11–15. doi: 10.1016/0169-4758(91)90077-2. [DOI] [PubMed] [Google Scholar]
- Koltai H, Chejanovsky N, Raccah B, Spiegel Y. The first isolated collagen gene from Meloidogyne javanica is developmentally regulated. Gene. 1997;196:191–199. doi: 10.1016/S0378-1119(97)00227-8. [DOI] [PubMed] [Google Scholar]
- Kramer JM. Structures and functions of collagens in Caenorhabditis elegans. FASEB J. 1994;8:329–336. doi: 10.1096/fasebj.8.3.8143939. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- Letunic I, Doerks T, Bork P. SMART: recent updates, new developments and status in. Nucleic Acids Res. 2015;43:D257–D260. doi: 10.1093/nar/gku949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Koltai H, Chejanovsky N, Spiegel Y. Isolation of a novel collagen gene (MJ-COL-5) in Meloidogyne javanica and analysis of its expression pattern. J Parasitol. 2001;87(4):801–807. doi: 10.1645/0022-3395(2001)087[0801:IOANCG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Page AP, Johnstone IL (2007) The cuticle. In: The C. elegans research community (eds.) WormBook. doi:10.1895/wormbook.1.138.1
- Page AP, Winter AD. Enzymes involved in the biogenesis of the nematode cuticle. Adv Parasit. 2003;53:85–148. doi: 10.1016/S0065-308X(03)53003-2. [DOI] [PubMed] [Google Scholar]
- Page AP, Stepek G, Winter AD, Pertab D. Enzymology of the nematode cuticle: a potential drug target? Int J Parasitol Drugs Drug Resist. 2014;4:133–141. doi: 10.1016/j.ijpddr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(785–786):1–15. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Prilusky J, Felder CE, Zeev-Ben-Mordehai T, Rydberg EH, Man O, Beckmann JS, Silman I, Sussman JL. FoldIndex: a simple tool to predict whether a given protein sequence is intrinsically unfolded. Bioinformatics. 2005;21(16):3435–3438. doi: 10.1093/bioinformatics/bti537. [DOI] [PubMed] [Google Scholar]
- Ray C, Hussey RS. Evidence for proteolytic processing of a cuticle collagen in a plant-parasitic nematode Mol. Biochem Parasit. 1995;72:243–246. doi: 10.1016/0166-6851(95)00082-C. [DOI] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Eycken W, Dealmeidaengler J, Vanmontagu M, Gheysen G. Identification and analysis of a cuticular collagen coding gene from the plant-parasitic nematode Meloidogyne incognita. Gene. 1994;151:237–242. doi: 10.1016/0378-1119(94)90663-7. [DOI] [PubMed] [Google Scholar]
- Wang T, Deom CM, Hussey RS. Identification of a Meloidogyne incognita cuticle collagen gene and characterization of the developmental expression of three collagen genes in parasitic stages. Mol Biochem Parasit. 1998;93:131–134. doi: 10.1016/S0166-6851(98)00018-8. [DOI] [PubMed] [Google Scholar]
- Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CS, Chen YC, Lu CH, Hwang JK. Prediction of protein subcellular localization. Proteins. 2006;64:643–651. doi: 10.1002/prot.21018. [DOI] [PubMed] [Google Scholar]