Abstract
Mammalian reproduction depends on the release of a mature oocyte from the ovarian follicle. Maturation of the oocyte and rupture of the follicle wall constitute part of the responses to the preovulatory surge of LH, which also include cumulus expansion and granulosa cell luteinization. It was previously shown that the epidermal growth factor receptor (EGFR) mediates the ovulatory response to LH in the ovarian follicle. We hypothesized that it is a sustained activity of the EGFR that generates oocyte maturation and cumulus expansion. We demonstrated that, whereas a transient exposure of rat isolated, intact, preovulatory follicles to either LH or forskolin was sufficient to induce oocyte maturation and cumulus expansion, these LH-induced responses were only generated upon a prolonged activity of the EGFR. In addition, the continuous activity of the EGFR is essential for the chronic phosphorylation of the ERK1/2 downstream signaling molecules, which were shown to be essential for oocyte maturation and cumulus expansion. Interestingly, EGFR-sustained activity was also necessary to maintain the up-regulation of Ptgs2, a gene essential for cumulus expansion. The unusual prolonged duration of ERK1/2 activity may possibly be attributed to the late induction of the ERK-specific phosphatase 3, demonstrated herein. These new data shed light on the unique characteristics of EGFR-ERK1/2 activity in the ovarian follicle and emphasize the fact that the ovulatory process involves a nonclassical activation of this pathway.
A sustained activity of the EGFR is necessary to mediate LH-induced responses in the ovarian follicle such as ERK1/2 phosphorylation, oocyte maturation and cumulus expansion.
Ovulation is a complex process that culminates by the expulsion of a mature oocyte from the preovulatory follicle to the site of fertilization. In addition to the oocyte, the ovarian follicle consists of the somatic mural granulasa and theca cells. A subpopulation of the granulosa cells, the cumulus, encapsulates the oocyte.
The preovulatory surge of LH triggers the following major processes that are essential for successful ovulation: 1) resumption of meiosis (also known as oocyte maturation); 2) expansion and mucification of cumulus cells; 3) differentiation of the granulosa cells from estrogen to progesterone-producing cells, a process known as luteinization; and 4) rupture of the follicle wall. Upon binding to its Gs-coupled receptor, LH stimulates the adenylyl cyclase to produce cAMP, which, in turn, activates protein kinase A and the downstream ERK1 and ERK2 (also known as p44 and p42) signaling cascade (1, 2, 3). Subsequently, ERK1/2 induces the down-regulation of genes related to follicular development (4) concomitantly with up-regulation of the ovulation-related genes (5, 6, 7). Specifically, the preovulatory surge of LH up-regulates genes that are required for cumulus expansion, among which hyaluronan synthase 2 and prostaglandin-endoperoxide synthase 2 (Ptgs2, also known as Cox2), the rate-limiting enzyme in the synthesis of prostaglandin E2 (PGE2), are included (6, 7, 8, 9). Hyaluronic acid synthesis and cumulus expansion are required for the release of the ovum during ovulation (10). The precise role of Ptgs2 was demonstrated in Ptgs2-depleted mice, which fail to ovulate (11) and to undergo cumulus expansion in response to LH (12).
The role of ERK1/2 in gonadotropin-induced oocyte maturation and cumulus expansion was first demonstrated in mouse cumulus oocyte complexes (COCs) (13). It was later shown in rat ovarian follicles that ERK1/2 mediates the immediate effect of LH on gap junctional closure in granulosa cells (14). This stops the somatic cAMP influx to the oocyte, leading to a subsequent drop of the intraoocyte cAMP level, to allow the resumption of meiosis (15, 16). Additionally, it was recently shown that a genetically manipulated mouse, in which the granulosa ERK1 and ERK2 were depleted, did not ovulate (4). Hormonal administration in such mice failed to induce resumption of meiosis, cumulus expansion, and luteinization.
Two decades ago, we found that, similar to LH, the epidermal growth factor (EGF) stimulates rat large antral follicles and thereby promotes maturation of the oocyte (17), a fact that was further confirmed in mouse oocytes (18). We later demonstrated that the EGF-induced maturation produced fertilizable eggs (19). More recently, the epidermal growth factor (EGF) receptor (EGFR) was shown to mediate the effect induced by LH on oocyte maturation, cumulus expansion, and luteinization in mouse ovarian follicles (20, 21). These reports showed that LH increases the transcription of the epiregulin, amphiregulin, and betacellulin EGF-like molecules. These data were extended to the rat, further showing that in explanted follicles, metalloproteinases mediate the activation of the EGFR by LH (22). Furthermore, these authors have demonstrated that EGFR and metalloproteinases are involved in ovulation in vivo. The essential role of EGFR in mediating LH action was further confirmed in transgenic mice expressing a mutated EGFR, in which preovulatory follicles failed to respond to LH (20). Taken together, these data establish the indispensability of the EGFR pathway in the LH-induced ovulatory responses.
It was previously proposed that due to the lower density of LH receptors on the cumulus cells as compared with mural granulosa cells (23), the EGF-related growth factors produced at the periphery may serve as paracrine mediators that propagate the LH signal toward the center of the follicle (21). However, the fact that the EGF-like molecules are up-regulated similarly in granulosa and cumulus cells questions this possibility (24).
It is commonly established that the EGFR pathway is rapidly shut down (25, 26). However, in the ovary, EGFR and ERK1/2 are phosphorylated for few hours after LH stimulation (27, 28). In this study, we hypothesized that the sustained activity of the EGFR is required to mediate LH action in the ovary. We confirm herein that a short-term exposure of ovarian follicles to LH is sufficient to trigger the response to this gonadotropin (29). We further show that a forskolin-induced transient activation of the adenylyl cyclase is sufficient to generate the same responses. By contrast, we indeed demonstrate that termination of the activity of the EGFR at time points that are earlier than 3 h of exposure to LH severely impairs oocyte maturation and cumulus expansion. In addition, we report that the continuous activity of the EGFR is essential for the phosphorylation of its downstream effectors ERK1/2 and for maintaining these kinases in a phosphorylated state. A sustained activity of the EGFR is also required for the increase of the transcription of Ptgs2, an essential gene for cumulus expansion. Finally, in search for a mechanism that may be responsible for the prolonged ERK1/2 activity, we screened for the mRNA expression of MAPK phosphatases (MKPs) in the ovarian follicle upon exposure to LH, pointing toward MKP-3 as the potential enzyme responsible for ERK1/2 shutdown in this system.
Results
A transient exposure to LH is sufficient to induce oocyte maturation and cumulus expansion
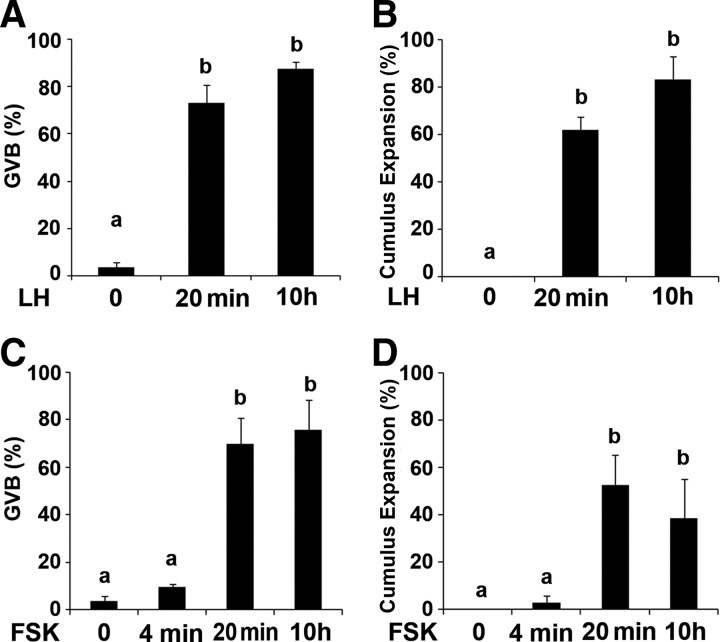
Our first experiment aimed at determining the time window during which the presence of LH is required for the induction of oocyte maturation and cumulus expansion. For this purpose, isolated intact ovarian follicles from 25-d-old pregnant mare’s serum gonadotropin (PMSG)-primed rats were exposed to LH for 20 min, after which time they were washed and placed in a hormone-free medium for a total incubation period of 10 h. Both germinal vesicle breakdown (GVB) and cumulus expansion (30) should have occurred at this time point. At the end of incubation, the follicles were incised and COCs were monitored for the meiotic status of the oocyte, as indicated by presence or the absence of a GV, as well as for the extent of the cumulus expansion. As positive and negative controls, ovarian follicles were exposed for 10 h to LH or vehicle, respectively. No significant difference was detected between the two groups incubated with LH, demonstrating that a 20-min pulse of LH is sufficient to induce resumption of meiosis and cumulus expansion (Fig. 1, A and B). Because these responses might be attributed to the classical, well-known, irreversible nature of ligand-receptor interaction, the effect of a transient activation of the adenylyl cyclase, the direct downstream effector of LH, was examined. Preovulatory follicles were incubated for 4 min, 20 min, and 10 h with forskolin, a reversible adenylyl cyclase activator. We found that exposure of follicles to forskolin for 20 min was sufficient to induce oocyte maturation and cumulus expansion in a maximal fraction of the incubated follicles (Fig. 1, C and D). Incubation with forskolin for only 4 min did not generate the same output, demonstrating in addition that the wash was indeed effective. Taken together, these data reveal that a short exposure to either LH or cAMP is sufficient to generate an irreversible commitment of this system for ovulatory changes.
Fig. 1.
Transient exposure to LH as well as a brief activation of adenylyl cyclase by forskolin induce oocyte maturation and cumulus expansion. A and B, Isolated intact ovarian follicles were exposed to LH for 20 min, after which time they were washed and placed in a hormone-free medium for a total incubation period of 10 h. As positive and negative controls, ovarian follicles were exposed to LH or vehicle for 10 h. C and D, A similar experiment was performed with forskolin (FSK), a reversible adenylyl cyclase activator. Ovarian follicles were exposed to FSK for 4 and 20 min, after which time they were washed and placed in FSK-free medium for 10 h. Other groups of follicles were exposed to either FSK or vehicle for 10 h. At the end of the incubation period, the follicles were incised. The COCs were monitored for oocyte maturation, as indicated by GVB (A and C) and for the extent of the cumulus expansion. The fraction of expanded, out of the total cumuli examined, is presented in panels B and D. The histograms show means ± se of three independent experiments. Columns with different superscripts differ significantly (P < 0.005 in A–C; P < 0.05 in D).
A sustained activity of the EGFR is necessary to mediate the LH-induced oocyte maturation and cumulus expansion
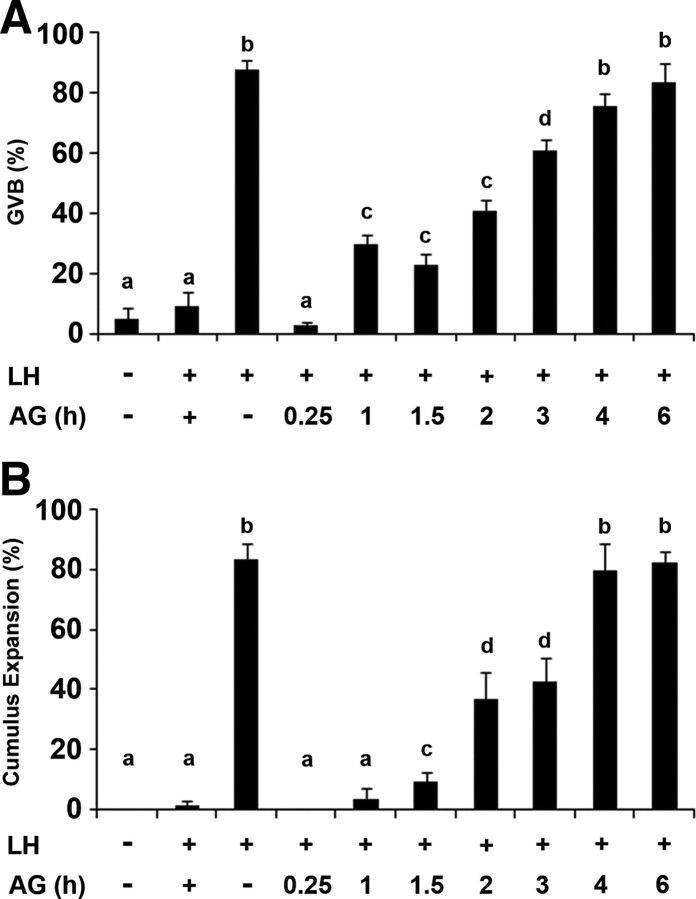
It was previously reported for mouse isolated intact follicles (27, 28), and herein confirmed for the rat (supplemental Fig. 1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), that in the ovary, EGFR stays phosphorylated for few hours after LH stimulation. To characterize the duration of the EGFR activity that is required to generate the response to LH, ovarian follicles were incubated with LH for 10 h, and the EGFR antagonist, AG1478, was added at different time points. At the end of incubation, the follicles were incised and the COCs were monitored for the meiotic status of the oocyte and for the extent of cumulus expansion. We found that blocking the EGFR, at any time point during the first 3 h after LH stimulation, partially inhibits oocyte maturation and cumulus expansion (Fig. 2, A and B). It is only after 4 h of continuous EGFR activity that the fractions of GVB oocytes and cumulus expansion were similar to that of ovarian follicles incubated without the EGFR inhibitor.
Fig. 2.
A sustained activity of the EGFR is necessary to mediate the LH-induced oocyte maturation and cumulus expansion. Isolated intact preovulatory follicles were incubated with LH for 10 h, and the EGFR antagonist AG1478 (AG) was added at different time points. As a positive control, AG1478 was not added to the incubation medium and as negative controls, ovarian follicles were incubated without LH or preincubated with AG1478 for 1 h before the addition of LH (lane 2). At the end of the incubation period, the follicles were incised, and the COCs were monitored for oocyte maturation, as indicated by GVB (A) and for the extent of cumulus expansion (B). The histograms show means ± se of at least three independent experiments. Columns with different superscripts differ significantly (P < 0.05).
Sustained activity of the EGFR is required for the LH-induced up-regulation of Ptgs2 expression
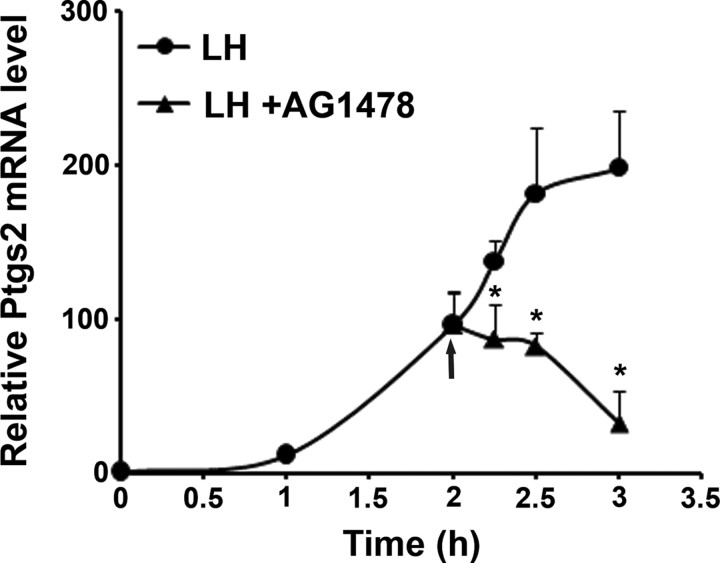
To decipher the mechanism involved in the requirement for the prolonged activity of EGFR, we examined the expression of Ptgs2, an essential gene for cumulus expansion (10, 11), which is up-regulated in response to LH. Consistent with previous publications (8), the expression of Ptgs2 was up-regulated at 1 h after LH stimulation and stayed elevated for at least 3 h (Fig. 3). The EGFR inhibitor was added 2 h after LH stimulation, a time that represents the midpoint of the ascending part of Ptgs2 expression curve. Ovarian follicles were therefore exposed to LH for 2 h, at which time either AG1478 or vehicle was added for different time intervals. At the end of incubation, RNA was extracted from these follicles; cDNA was synthesized and subjected to quantitative real-time PCR (Q-PCR). We found that the addition of AG1478 to the culture medium 2 h after LH stimulation for either 15 or 30 min (referred to in Fig. 3 as 2.25 and 2.5 h, respectively) blocked further up-regulation of Ptgs2; incubation with AG1478 for 1 h (referred to in Fig. 3 as 3 h) significantly reduced Ptgs2 mRNA level (Fig. 3). These observations reveal that termination of EGFR activity after 2 h immediately blocks further transcription of Ptgs2. Therefore, a continuous activity of the EGFR is required to achieve a full Ptgs2 up-regulation, pointing at the fact that EGFR activity is tightly coupled to cumulus expansion via the transcription Ptgs2.
Fig. 3.
A Sustained activity of the EGFR is required for the LH-induced elevation of Ptgs2 mRNA expression. Isolated intact preovulatory follicles were exposed to LH for 2 h, at which time either AG1478 or vehicle was added for 15, 30, and 60 min. At the end of incubation, the follicles were transferred into liquid nitrogen, their RNA was extracted, and cDNA was then synthesized and subjected to Q-PCR. The graph shows means ± se of three independent experiments, *, P < 0.05 vs. corresponding LH treatment.
A sustained activity of the EGFR induces prolonged ERK1/2 phosphorylation
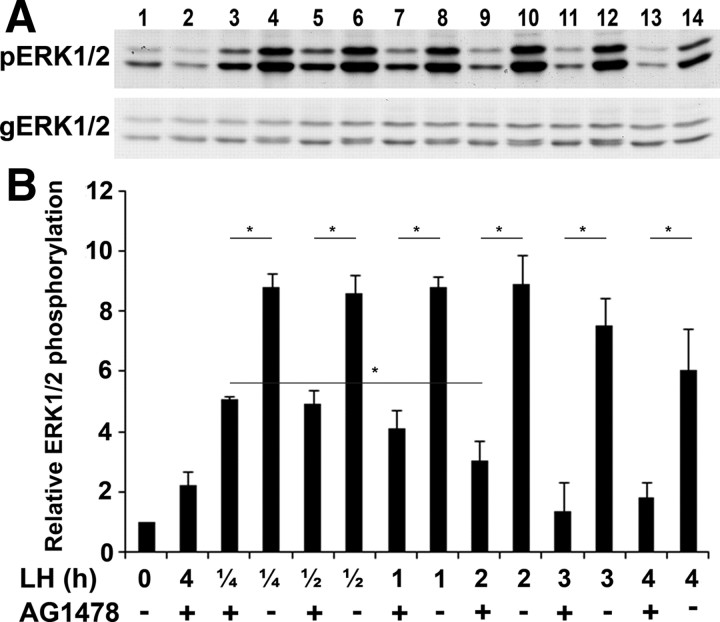
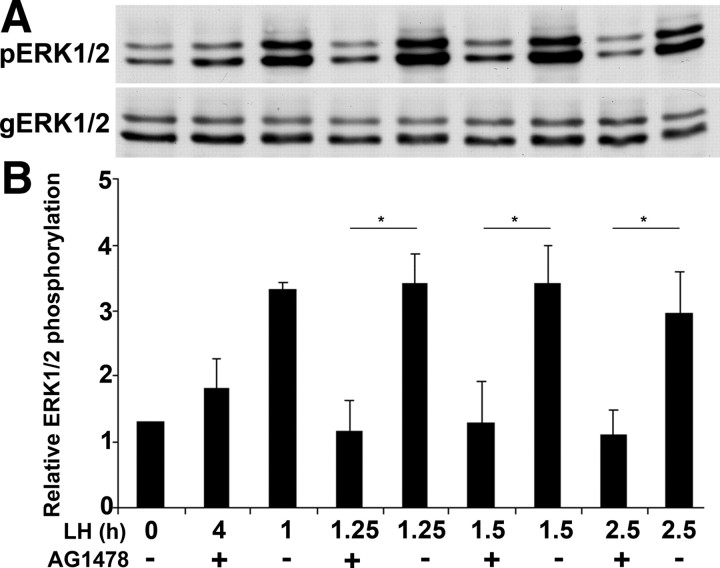
We have previously shown that after LH stimulation, ERK1/2 is activated within minutes and remains active for at least 4 h (27). It was further demonstrated that the EGFR is essential for the LH-induced ERK1/2 phosphorylation (28). The experiment described herein determines whether the long duration of ERK1/2 phosphorylation requires the continuous activity of the EGFR. For this purpose, preovulatory follicles were incubated with LH for different time intervals, and AG1478 was added at the last 15min of incubation. The follicles were then lysed, and the extracted proteins were subjected to SDS-PAGE, followed by Western blot analysis using antibodies recognizing the phosphorylated ERK1/2 (pERK1/2) as well as those raised against general ERK1/2 (gERK1/2). We found that blocking the activity of the EGFR for 15 min, at each time point examined, reduced the phosphorylation levels of ERK1/2, suggesting that EGFR continuous activity is essential to maintain the ERK1/2 in its phos-phorylated state for at least 4 h after LH stimulation (Fig. 4). These data establish a strong correlation between the continuous activity of the EGFR and the prolonged LH-induced ERK1/2 phosphorylation. It is interesting to note that whereas the EGFR antagonist totally blocked ERK1/2 phosphorylation from 2 h of exposure to LH, it only partially reduced ERK1/2 phosphorylation after 15 and 30 min of LH stimulation (as demonstrated in Fig. 4 by the significant difference between lanes 3 and 9, P = 0.03).
Fig. 4.
A sustained activity of the EGFR induces prolonged ERK1/2 phosphorylation. A, Preovulatory follicles were incubated with LH for different time intervals, and AG1478 or vehicle was added 15 min before the end of the incubation period. As a positive control, a group of follicles was treated only with LH (lane 3) and as negative controls, ovarian follicles were incubated without LH (lane 1) or preincubated with AG1478 for 1 h before the addition of LH (lane 2). The follicles were then lysed, and the extracted proteins were subjected to SDS-PAGE, followed by Western blot analysis using antibodies recognizing the phosphorylated, active ERK1/2 (pERK1/2) and those raised against general ERK1/2 (gERK1/2). B, Densitometric quantification of the results normalized against gERK1/2 protein levels. For quantification, intensity values of the bands were measured from three different repeats using image J program. The histograms show means ± se of three independent experiments (*, P < 0.05 vs. corresponding LH treatment).
The chronic ERK1/2 phosphorylation is exclusively maintained by EGFR
We showed that the prolonged duration of the ERK1/2 phosphorylation is dependent on the continuous activity of the EGFR. The aim of the present experiment was to examine whether, over time, an alternative pathway may compensate for EGFR inactivation to induce ERK1/2 phosphorylation. For this purpose, ovarian follicles were subjected to LH for 1 h, at which time AG1478 or vehicle was added for 15, 30, and 90 min. ERK1/2 phosphorylation was inhibited when EGFR was blocked for either 15, 30, or 90 min (Fig. 5). These results rule out the presence of a redundant pathway that bypasses the EGFR, to mediate the phosphorylation of ERK1/2 by LH.
Fig. 5.
The chronic EGFR activity maintains ERK1/2 in its phosphorylated state. A, Preovulatory follicles were incubated with LH for 1 h, at which time AG1478 or vehicle was added for 15, 30, and 90 min. At the end of the incubation period, the follicles were frozen. As a positive control, AG1478 was not added to the incubation medium and as negative controls, ovarian follicles were incubated without LH or preincubated with AG1478 for 1 h before the addition of LH (lane 2). B, Densitometric quantification of the results normalized against gERK1/2 protein levels. The histograms show means ± se of three independent experiments (*, P < 0.05 vs. corresponding LH treatment).
A sustained activity of ERK1/2 is necessary for the LH-induced oocyte maturation and cumulus expansion
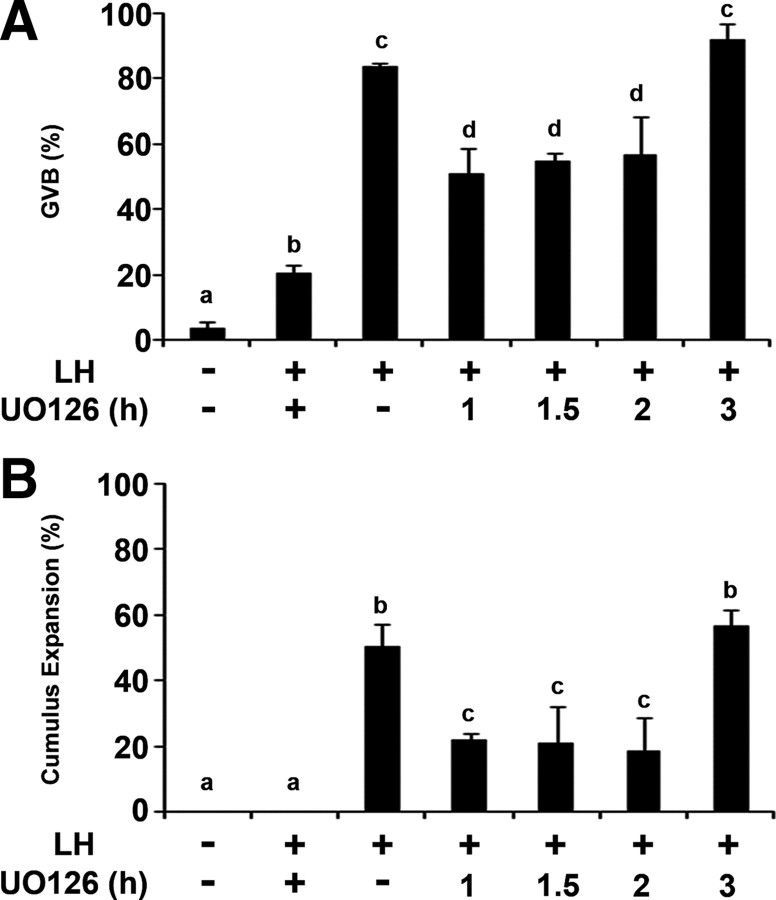
We have demonstrated that after LH stimulation, an active EGFR is essential to maintain ERK1/2 in its phosphorylated state. To further examine whether this prolonged ERK1/2 phosphorylation is essential for oocyte maturation and cumulus expansion, ovarian follicles incubated for different time periods with LH were subjected to UO126, a specific inhibitor of MAPK kinase (MEK), the direct upstream activator of ERK1/2. The follicles were incised after a total of 10 h incubation, and the recovered COCs were examined for meiosis resumption and cumulus expansion. We found that blocking ERK1/2 during the first 2 h after LH stimulation partially inhibits oocyte maturation and cumulus expansion (Fig. 6, A and B). It is only after 3 h of a sustained ERK1/2 activity that the fraction of GVB oocytes and that of expanded cumuli were similar to that of ovarian follicles incubated without the ERK1/2 inhibitor throughout the 10-h incubation.
Fig. 6.
A sustained activity of ERK1/2 is essential for the LH-induced oocyte maturation and cumulus expansion. Ovarian follicles were incubated with LH and UO126, the inhibitor of MEK, was added at different time points. As a positive control, UO126 was not added to the incubation medium, and as negative controls, ovarian follicles were incubated without LH or preincubated with UO126 for 1 h before the addition of LH (lane 2). After 10 h of incubation, the follicles were incised and the COCs were monitored for oocyte maturation, as indicated by GVB (A) and for the extent of cumulus expansion (B). The histograms show means ± se of at least three independent experiments. Columns with different superscripts differ significantly (P < 0.05).
Expression of different members of the MKP family upon LH stimulation
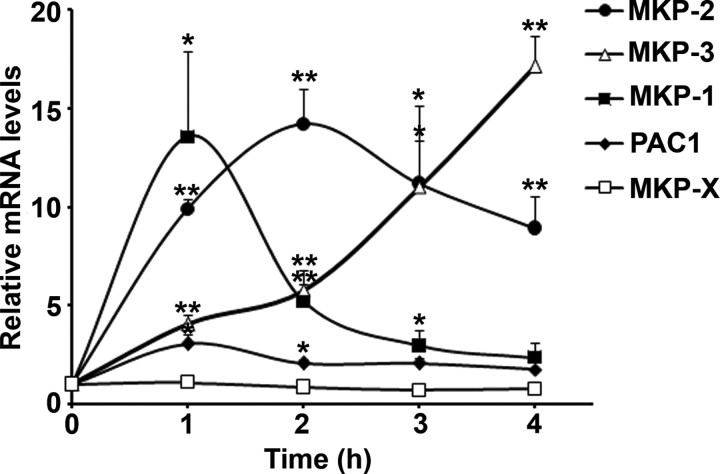
The continuous mode of ERK1/2 activity showed herein in the ovarian follicle is unusual, because in many other systems, the EGFR-induced ERK1/2 phosphorylation is rapidly reversed. It is well established that ERK1/2 signaling is mainly attenuated by the inducible family of MKPs, an atypical class of dual-specificity phosphatases (DUSPs), which specifically dephosphorylate the active ERK1/2. It was previously shown in many systems that MKPs are regulated at the transcriptional level (31). Taking this information into consideration, we screened the known MKPs that could possibly be up-regulated in temporal association with ERK1/2 dephosphorylation (4 h after LH exposure). For this purpose, ovarian follicles were exposed to LH for different periods, at the end of which RNA was extracted and the synthesized cDNA was subjected to Q-PCR for MKP-1 (DUSP1), MKP-2 (DUSP4), MKP-3 (DUSP6), and MKP-X (DUSP7) transcripts, as well as PACAP-preferring type 1 (PAC1; DUSP2), which inactivates ERK1/2 (32). This experiment revealed for the first time that upon LH stimulation, MKP-1,2,3 and PAC1 mRNA levels were significantly elevated whereas the expression MKP-X remained unchanged (Fig. 7). It is noteworthy that these phosphatases are differently expressed as follows: MKP-1 mRNA level is rapidly elevated, peaks at 1 h of exposure to LH, and is subsequently quickly reduced. Elevation of the MKP-2 mRNA is less abrupt, reaching a 14-fold induction within 2 h of exposure to LH with a reduced but still high level for at least 4 h. In a different manner, MKP-3 is up-regulated progressively to attain a 17-fold increase at 4 h after exposure to LH. These observations reveal that most MKPs are rapidly up-regulated in the ovarian follicle after LH stimulation, when ERK1/2 is strongly phosphorylated, whereas MKP-3 up-regulation after 4 h is temporally correlated with ERK1/2 dephosphorylation.
Fig. 7.
Expression of different members of the MKP family upon LH stimulation. Ovarian follicles were exposed to LH for 0, 1, 2, 3, and 4 h. At the end of incubation, RNA was extracted, and cDNA was synthesized and subjected to Q-PCR for MKP-1 (DUSP1), MKP-2 (DUSP4), MKP-3 (DUSP6), MKP-X (DUSP7), and PAC1 (DUSP2) transcripts. The graph shows means ± se of three independent experiments (*, P < 0.03; **, P < 0.005).
Discussion
We found that whereas the transient exposure to LH and the forskolin-induced short-term activation of the adenylyl cylase are sufficient to induce oocyte maturation and cumulus expansion in rat preovulatory follicles, a prolonged activity of the EGFR is absolutely required to generate the same responses. We further show that the sustained nature of EGFR activity is also essential for downstream responses such as up-regulation of the Ptsg2 expression, as well as for the ERK1/2 phosphorylation. Interestingly, it is also a chronic phosphorylation of ERK1/2 that is essential for oocyte maturation and cumulus expansion. In addition, our data demonstrate that ERK1/2 dephosphorylation is temporally correlated with MKP-3 up-regulation, suggesting that this phosphatase may be responsible for shutting down the ERK1/2 cascade in the ovarian follicle. Taken together, this study reveals that the ovulatory response is contingent on a nonclassical, sustained activity of the EGFR-ERK pathway.
A nonclassical mode of activity of the EGFR-ERK1/2 pathway
It is well established that the EGFR undergoes internalization within minutes after ligand binding, which is followed by its degradation (25, 26). This negative regulatory mechanism, which shuts down the EGFR-signaling pathway, apparently acts to protect the cells from excessive, harmful effect of the members of the EGF-like family. Indeed, various cancers are associated with mutations of the EGFR, which bring about its constitutive activity (33). The common transient nature of EGFR activity is associated with a phosphorylation of its downstream ERK1/2 that is immediately followed by their dephosphorylation (34). Surprisingly, in the ovary, the continuous activity of EGFR that lasts for at least 3 h is necessary to generate physiological responses such as oocyte maturation and cumulus expansion. This ovarian prolonged activity of the EGFR keeps ERK1/2 phosphorylated for an extended period of time. To our knowledge, it is the first time that the EGFR is shown to continuously activate ERK1/2.
Prolonged activity of ERK1/2, which has been demonstrated in PC12 cells, was triggered by nerve growth factor and led to differentiation. However, the effect of EGF in this system brought about a transient ERK1/2 activity leading to proliferation (35). Transient vs. sustained ERK1/2 activity that generates different outputs was also shown in other cell types (Refs. 36 and 37 ; reviewed by Ref. 38).
The prolonged EGFR activity in the ovary is also associated with a unique, sustained up-regulation of Ptgs2 that differs from the transient duration of the EGF-induced, Ptgs2-elevated expression in WISH cell line (39). A short kinetics of EGFR-induced up-regulation was also described for the early growth response factor 1 (EGR1), a zinc finger transcription factor. This gene was shown to be up-regulated within 20 min and further down-regulated 40 min after EGF stimulation in cell cultures (34), whereas an elevated expression that lasts for several hours was described previously in the ovary (40). Taken together, these findings point at the unique mode of regulation of the EGFR-signaling cascade in the ovarian physiology leading to ovulation.
The sustained activity of the EGFR maintains the transient LH stimulation
Ovulation is a tightly orchestrated process, in which a multitude of cells respond simultaneously to the surge of LH to release, 12 h later, mature oocytes surrounded by expanded cumuli. The fact that a short-term exposure to this gonadotropin generates this remote response agrees with the classical concept of ligand-receptor interaction. However, the fact that a transient exposure to forskolin is sufficient to stimulate oocyte maturation and cumulus expansion confirms that the response to LH is induced by a brief activation of adenylyl cyclase. It is the first time that a short pulse of forskolin is shown to bring about oocyte maturation and cumulus expansion to an extent that is similar to that obtained by a long-term exposure to this adenylyl cyclase activator. However, unlike the transient nature of LH action, a prolonged activity of the EGFR was found to be essential to induce oocyte maturation and cumulus expansion. We propose that, upon LH stimulation, the prolonged activity of EGFR in the granulosa cells maintains and synchronizes the many complex events that finally converge to ovulation. This mechanism allows translation of the short systemic surge of LH into a deferred response that involves the spatio-temporal coordination of the many processes, collectively defined as ovulation. According to this idea, the surge of LH could be compared with the starter of a car, the EGFR with the fuel, and the ERK1/2 with the engine; their combined activity will move ovulation forward.
It is interesting to note that the prolonged activity of EGFR-ERK1/2 appears to be specific to the female gonad because LH was shown to transiently activate the EGFR in a steroidogenic Leydig cell line (41).
EGFR activity and ERK1/2 phosphorylation are tightly coupled
We have shown that inhibition of the LH-induced EGFR activity for 15 min during the first hour of LH stimulation induced partial ERK1/2 dephosphorylation. However, a total ERK1/2 inactivation was obtained at later intervals of incubation. These findings may suggest that during the first hour of incubation with LH, the ERK1/2 phosphatases (MKPs) have not yet reached their maximal level of expression and are not fully effective. Alternatively, after the first hour, ERK1/2 could be exclusively phosphorylated by the EGFR, whereas during the first hour, another pathway may partially stimulate ERK1/2 phosphorylation. PKA might be responsible for the partial ERK1/2 phosphorylation during the first hour of LH stimulation, a possibility that remains to be validated. It is interesting to note that the immediate reduction of ERK1/2 phosphorylation upon EGFR inhibition suggests the presence of active phosphatases that rapidly dephosphorylate ERK1/2 in the absence of EGFR activation.
We noted that ERK1/2 reaches its maximal phosphorylation level within minutes of LH stimulation and that this phosphorylation depends on EGFR activity. Such a rapid response is unlikely to occur as a consequence of transcription of the EGF-like agonists. It seems that during the first minutes of exposure to LH, EGFR is activated as a consequence of metalloproteinases activity, which sheds the already existing membrane-bound EGF-like factors, allowing activation of the receptor (42). This initial effect could possibly be prolonged by further synthesis of EGF-like factors. The identity of the metalloproteinases and their mechanism of activation remain to be explored.
Presence of MKPs in the ovarian follicle
MKPs, which are induced by ERK activation, consist of a subclass of the DUSPs that selectively dephosphorylate ERKs. Some MKPs, including MKP-2 (DUSP4), MKP-3 (DUSP6), and -X (DUSP7) as well as PAC-1 (DUSP2) inactivate specifically ERK1/2 (reviewed by Refs. 43 and 44). In EGF-stimulated HeLa cells, MKPs are induced rapidly (MKP-2 peaks at 30 min whereas MKP-3 peaks at 2 h) (34), whereas in the mammalian preovulatory follicle, the expressions of MKP-2 and 3 are delayed. These data may explain the prolonged phosphorylation of ERK1/2 in the ovary.
Interestingly, 4 h after LH exposure, MKP-3 mRNA levels are substantially elevated, which temporally coincides with the time point of ERK1/2 dephosphorylation described herein. It suggests that, in the ovarian follicle, MKP-3 may be the phosphatase that plays a major role in shutting down the ERK1/2 cascade. It is noteworthy that MKP-3 is the most specific cytoplasm ERK1/2 phosphatase. Because its up-regulation is temporally correlated with ERK1/2 dephosphorylation, we propose that this phosphatase is the dominant regulator of ERK1/2 in the ovarian follicle.
MKP-1, which is considered as an immediate early gene (45), is also rapidly up-regulated in the ovarian follicle. The early up-regulation of MKP-1 and -2 after LH stimulation, which does not coincide with the ERK1/2 dephosphorylation, is surprising. Nevertheless, because MKP-1 and -2 are nuclear, cytoplasmatic ERK1/2 might be unaffected by these phosphatases. In addition, it is important to note that MKP-1 may be more specific to c-Jun N-terminal kinase and p38 than ERK1/2. The role of these phosphatases in the ovary remains to be clarified.
In conclusion, our results emphasize the different mode of action of the hormones controlling the ovulatory responses. Whereas a transient exposure to LH is sufficient to induce oocyte maturation and cumulus expansion in rat preovulatory follicles, a chronic activity of EGFR is necessary to generate the same responses. These findings raise the interesting novel notion that the physiological surge of LH requires a local sustained activity of the EGFR to not only mediate but also maintain its switch-like stimulation. Specifically, the EGFR continuously controls transcription, as reflected by the immediate decrease in Ptsg2 up-regulation upon inhibition of the EGFR. Equally interesting, we demonstrate that upon exposure to LH, EGFR continuous activity is essential for the ERK1/2-sustained phosphorylation. We further reveal that, 4 h after LH stimulation, ERK1/2 dephosphorylation temporally coincides with MKP-3 up-regulation. Collectively, we point at the fact that oocyte maturation and cumulus expansion involve a nonclassical, prolonged activity of the EGFR-ERK pathway.
Materials and Methods
Reagents
Leibovitz’s L-15 tissue culture medium and fetal bovine serum were purchased from Biological Industries (Kibbutz Beit Hemeek, Israel). Antibiotics were purchased from Bio-Lab Ltd. (Jerusalem, Israel), PMSG was supplied by Chronogest Intervet (Boxmeer, The Netherlands), and ovine LH (o-LH-26) was purchased from the National Hormone and Pituitary Program (Harbor-University of California Los Angeles Medical Center, Torrance, CA). The MEK inhibitor UO126 was supplied by Axxora (San Diego, CA), the EGFR blocker AG1478 was purchased from Calbiochem (San Diego, CA), and the adenylyl cyclase activator forskolin was supplied by Sigma-Aldrich Corp. (St. Louis, MO). Protease inhibitor cocktail, phenylmethylsulfonylfluoride, leupeptin, and pepstatin were from Sigma-Aldrich. Anti-phospho-ERK1/2, antigeneral ERK1/2, and anti-EGFR (sc-03) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-phospho-EGFR antibody (tyr1086) was purchased from Cell Signaling Technology (Beverly, MA). The secondary antibodies were purchased from The Jackson Laboratory (Bar Harbor, ME).
Animals
Sexually immature 25-d-old Wistar female rats were purchased from Harlan Laboratories (Rehovot, Israel) and handled according to the guidelines of the National Institute of Health and of the Weizmann Institute for management of laboratory animals. The rats were housed in a light- and temperature-controlled room, with food and water provided ad libitum.
Culture of follicles
The aforementioned rats were injected sc with 10 IU PMSG for induction of follicular development and killed 48 h later. The ovaries were removed and placed in L-15 medium, supplemented with 5% fetal bovine serum, penicillin (100 IU/ml), and streptomycin (100 mg/ml). The experiments assessing EGFR phosphorylation were conducted in serum-free medium. Isolated intact rat ovarian preovulatory follicles were separated and grown in suspension in L-15 tissue culture medium containing 5% fetal bovine serum in 25-ml flasks gassed with 50% O2-50% N2 as described previously (46). Each group comprised at least 35 preovulatory follicles. Incubations were carried out at 37 C in an oscillating water bath in the presence or absence of either 1 μg/ml LH, with or without 10 μm AG1478 or 10 μm UO126, doses that were previously shown to give an optimal effect in this system (14, 17, 22). At the end of the incubation period, the follicles were incised, and the oocyte COCs were recovered and microscopically examined for cumulus expansion using differential interference contrast optics as described by us previously (47). The oocytes were monitored for reinitiation of meiosis, as indicated by the disappearance of the visible large nucleus called germinal vesicle (GV).
RNA extraction and cDNA preparation
Liquid nitrogen frozen follicles were homogenized in 500 μl Tri-Reagent (Sigma Aldrich). Glycogen (10 μl) (Roche Applied Science, Mannheim, Germany) was added to allow better precipitation of RNA. After 100 μl of chloroform was added to allow phase separation by centrifugation (at 4 C), 250 μl of ispropanol was added to the aqueous phase. After several hours in −20 C, the RNA was precipitated by centrifugation. The pellet was washed in cold 70% ethanol and then dried. The RNA pellet was resuspended in deoxyribonuclease-ribonuclease-free water, and deoxyribonuclease treatment was performed according to manufacturer’s instructions (Ambion, Austin, TX). Quality and quantity of total RNA extracted were assessed using Nanodrop spectrophotometer. RNA samples (1.5 μg) were reverse transcribed using high-capacity reverse transcription kit (Applied Biosystems, Foster City, CA) as indicated in the manufacturer’s protocol. The cDNA was then diluted in a 1:100 ratio.
Quantitative real-time PCR (Q-PCR)
The reversed transcribed diluted cDNA was amplified by Q-PCR. The primers were chosen using primer express software (Applied Biosystems). They were checked by the NCBI-BLAST program for their specificity. Each of these primer pairs yielded only one sharp band of amplified product with the molecular weight of the desired amplicon. The nucleotide sequences for the different PCR primer pairs are as follows. MKP-1 (NM_053769): forward (F), GGACAACCACAAGGCAGACA; reverse (R), CAGTGCACAAACACCCTTCCT; MKP-2 (NM_022199): F, AGACTGCCCCAATCACTTTGA; R, CGATGGCTTCCATGAACCA; MKP-3 (NM_053883): F,CTGCCGGGCGTTCTACCT; R, CTGCACGAGCCGTCTAGATTG; PAC1 (NM_001012089): F, GCACTGCCAAGCTGGTATCTC; R, CAAAGTCAAAGGCCTCATCCA; MKP-X (X94186): F, CCTACAAGCAAATCCCCATCTC; R, GAGCGGGCTTCATCAATGA. B2m (NM_012512): F, ACATCCTGGCTCACACTGAA; R, ATGTCTCGGTCCCAGGTG. Ptgs2 (NM_017232): F, CCCTGAAACCTTACACATCGTTT; R, TGGCATCGATGTCATGGTAGA. Relative quantification of the mRNA was performed using the Stepone system v2.1 (Applied Biosystems). Q-PCRs (10 μl) were carried out using 5 μl of mix (Fast SYBR Green Master Mix; Applied Biosystems), 2 μl cDNA, and 2.5 pmol of each primer. β2-microglobulin (B2m) was used as internal control for normalization. The amplification process was monitored through the fluorescence of SYBR Green.
Protein extraction and Western blot analysis
Total protein was extracted from cultured follicles (15–25 per sample) by homogenization in radioimmune precipitation assay buffer supplemented with 1 mm phenylmethylsulfonylfluoride, 10 μg/ml leupeptin, 2 μg/ml pepstatin, protease inhibitor (according to manufacturer’s instructions), and 400 μm NaVO3. The lysates were then centrifuged for 20 min, after which the supernatants were collected. The samples were dissolved in protein sample buffer [2% β-mercaptoethanol; 2% sodium dodecyl sulfate; 50 mm Tris-HCl (pH 6.8); 10% glycerol; and 0.01% bromophenol blue] and boiled.
For ERK1/2, 15 μg of protein was loaded onto 12% SDS-PAGE. After electrophoretic separation, the proteins were transferred to polyvinylidene fluoride membranes (Millipore Corp, Bedford, MA), which were blocked for 1 h with a blocking solution [5% nonfat dry milk, 0.05% Tween in Tris-buffered saline (TBST)], and then incubated with primary antibodies (over night, 4 C). Two anti-ERK1/2 antibodies were used for the Western blot analysis: one antibody immunoreacted with the phosphorylated (active) ERK1/2 (pERK1/2), whereas the second immunoreacted with both the active and inactive ERK1/2 (general gERK1/2). The relative amount of the pERK1/2 in each sample represents the extent of ERK1/2 activation. The membranes were then incubated with antirabbit horseradish peroxidase (HRP)-conjugated antibodies (1:4000, 1 h, room temperature).
For EGFR, 30 μg of protein was loaded onto 7% SDS-PAGE. After electrophoretic separation, the proteins were transferred to polyvinylidene fluoride membranes (Millipore), which were blocked for 1 h in TBST plus 5% nonfat dry milk, washed with TBST, probed with an anti-phospho-EGFR antibody (specific for autophosphorylation site Tyr1068) diluted 1:1000 in TBST plus 5% BSA overnight at 4 C, and then incubated for 1 h at room temperature with an antirabbit IgG HRP-conjugated antibody diluted in TBST plus 0.5% nonfat dry milk. Afterward, the same membranes were incubated in a solution containing 62.5 mm Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, and 100 mm β-mercaptoethanol for 30 min at 50 C, washed in TBST, and reprobed for total EGFR. Membranes were blocked for 1 h at room temperature in TBST plus 5% nonfat dry milk, incubated with a polyclonal anti-EGFR antibody diluted 1:200 in TBST plus 0.2% nonfat dry milk overnight at 4 C, washed in TBST, and then incubated for 1 h at room temperature with an antirabbit IgG HRP-linked antibody diluted 1:5000 in TBST plus 0.2% nonfat dry milk.
Chemiluminescent signals were generated by incubation with the enhanced chemiluminescence reagent (Amersham, Buckinghamshire, UK). For quantification, intensity values of bands were measured from three different repeats for each experiment using Image J (National Institutes of Health, Bethesda, MD).
Statistical analysis
All experiments were repeated at least three times. All data were analyzed using Student’s unpaired two-tailed t test and presented as mean ± se. P < 0.05 was considered significant.
Acknowledgments
We thank Professor Alex Tsafriri, Professor Rony Seger, and Dr. Yaara Zwang for helpful discussions and deep insight in this project.
Footnotes
This work was supported by The Dwek Fund for Biomedical Research. N.D. is the incumbent of the Philip M. Klutnick Professorial Chair in Developmental Biology.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 15, 2009
Y.R. and J.E. contributed equally to this study.
Abbreviations: COCs, Cumulus oocyte complexes; DUSPs, dual-specificity phosphatases; EGF, epidermal growth factor; EGFR, EGF receptor; F, forward; GVB, germinal vesicle breakdown; HRP, horseradish peroxidase; MEK, MAPK kinase; MKP, MAPK phosphatase; PAC1, PACAP-preferring type 1; PMSG, pregnant mare’s serum gonadotropin; Ptgs2, prostaglandin-endoperoxide synthase 2; Q-PCR, quantitative real-time PCR; R, reverse; TBST, 5% nonfat dry milk, 0.05% Tween in Tris-buffered saline.
References
- 1.Cameron MR, Foster JS, Bukovsky A, Wimalasena J1996. Activation of mitogen-activated protein kinases by gonadotropins and cyclic adenosine 5′-monophosphates in porcine granulosa cells. Biol Reprod 55:111–119 [DOI] [PubMed] [Google Scholar]
- 2.Das S, Maizels ET, DeManno D, St Clair E, Adam SA, Hunzicker-Dunn M1996. A stimulatory role of cyclic adenosine 3′,5′-monophosphate in follicle-stimulating hormone-activated mitogen-activated protein kinase signaling pathway in rat ovarian granulosa cells. Endocrinology 137:967–974 [DOI] [PubMed] [Google Scholar]
- 3.Richards JS, Rolfes AI1980. Hormonal regulation of cyclic AMP binding to specific receptor proteins in rat ovarian follicles. Characterization by photoaffinity labeling. J Biol Chem 255:5481–5489 [PubMed] [Google Scholar]
- 4.Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS2009. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards JS2001. Perspective: the ovarian follicle—a perspective in 2001. Endocrinology 142:2184–2193 [DOI] [PubMed] [Google Scholar]
- 6.Richards JS2005. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 234:75–79 [DOI] [PubMed] [Google Scholar]
- 7.Robker RL, Russell DL, Yoshioka S, Sharma SC, Lydon JP, O'Malley BW, Espey LL, Richards JS2000. Ovulation: a multi-gene, multi-step process. Steroids 65:559–570 [DOI] [PubMed] [Google Scholar]
- 8.Espey LL, Richards JS2002. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod 67:1662–1670 [DOI] [PubMed] [Google Scholar]
- 9.Sirois J, Richards JS1992. Purification and characterization of a novel, distinct isoform of prostaglandin endoperoxide synthase induced by human chorionic gonadotropin in granulosa cells of rat preovulatory follicles. J Biol Chem 267:6382–6388 [PubMed] [Google Scholar]
- 10.Chen L, Russell PT, Larsen WJ1993. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev 34:87–93 [DOI] [PubMed] [Google Scholar]
- 11.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM1995. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378:406–409 [DOI] [PubMed] [Google Scholar]
- 12.Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R1999. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology 140:2685–2695 [DOI] [PubMed] [Google Scholar]
- 13.Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ2002. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 143:2221–2232 [DOI] [PubMed] [Google Scholar]
- 14.Sela-Abramovich S, Chorev E, Galiani D, Dekel N2005. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146:1236–1244 [DOI] [PubMed] [Google Scholar]
- 15.Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA2008. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135:3229–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N2006. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 147:2280–2286 [DOI] [PubMed] [Google Scholar]
- 17.Dekel N, Sherizly I1985. Epidermal growth factor induces maturation of rat follicle-enclosed oocytes. Endocrinology 116:406–409 [DOI] [PubMed] [Google Scholar]
- 18.Downs SM, Daniel SA, Eppig JJ1988. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool 245:86–96 [DOI] [PubMed] [Google Scholar]
- 19.Ben-Yosef D, Galiani D, Dekel N, Shalgi R1992. Rat oocytes induced to mature by epidermal growth factor are successfully fertilized. Mol Cell Endocrinol 88:135–141 [DOI] [PubMed] [Google Scholar]
- 20.Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M2007. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M2004. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- 22.Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A2005. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology 146:77–84 [DOI] [PubMed] [Google Scholar]
- 23.Lawrence TS, Dekel N, Beers WH1980. Binding of human chorionic gonadotropin by rat cumuli oophori and granulosa cells: a comparative study. Endocrinology 106:1114–1118 [DOI] [PubMed] [Google Scholar]
- 24.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS2006. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365 [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Villeneuve G, Wang Z2005. Control of epidermal growth factor receptor endocytosis by receptor dimerization, rather than receptor kinase activation. EMBO Rep 6:942–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zwang Y, Yarden Y2009. Systems biology of growth factor-induced receptor endocytosis. Traffic 10:349–363 [DOI] [PubMed] [Google Scholar]
- 27.Kalma Y, Granot I, Galiani D, Barash A, Dekel N2004. Luteinizing hormone-induced connexin 43 down-regulation: inhibition of translation. Endocrinology 145:1617–1624 [DOI] [PubMed] [Google Scholar]
- 28.Panigone S, Hsieh M, Fu M, Persani L, Conti M2008. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindner HR, Tsafriri A, Lieberman ME, Zor U, Koch Y, Bauminger S, Barnea A1974. Gonadotropin action on cultured Graafian follicles: induction of maturation division of the mammalian oocyte and differentiation of the luteal cell. Recent Prog Horm Res 30:79–138 [DOI] [PubMed] [Google Scholar]
- 30.Dekel N, Sherizly I, Tsafriri A, Naor Z1983. A comparative study of the mechanism of action of luteinizing hormone and a gonadotropin releasing hormone analog on the ovary. Biol Reprod 28:161–166 [DOI] [PubMed] [Google Scholar]
- 31.Keyse SM2008. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 27:253–261 [DOI] [PubMed] [Google Scholar]
- 32.Chu Y, Solski PA, Khosravi-Far R, Der CJ, Kelly K1996. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem 271:6497–6501 [DOI] [PubMed] [Google Scholar]
- 33.Hynes NE, Lane HA2005. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5:341–354 [DOI] [PubMed] [Google Scholar]
- 34.Amit I, Citri A, Shay T, Lu Y, Katz M, Zhang F, Tarcic G, Siwak D, Lahad J, Jacob-Hirsch J, Amariglio N, Vaisman N, Segal E, Rechavi G, Alon U, Mills GB, Domany E, Yarden Y2007. A module of negative feedback regulators defines growth factor signaling. Nat Genet 39:503–512 [DOI] [PubMed] [Google Scholar]
- 35.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ1998. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392:622–626 [DOI] [PubMed] [Google Scholar]
- 36.Bhalla US, Iyengar R1999. Emergent properties of networks of biological signaling pathways. Science 283:381–387 [DOI] [PubMed] [Google Scholar]
- 37.Shaul YD, Seger R2007. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta 1773:1213–1226 [DOI] [PubMed] [Google Scholar]
- 38.Ebisuya M, Kondoh K, Nishida E2005. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci 118:2997–3002 [DOI] [PubMed] [Google Scholar]
- 39.Perkins DJ, Kniss DA1997. Rapid and transient induction of cyclo-oxygenase 2 by epidermal growth factor in human amnion-derived WISH cells. Biochem J 321:677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Espey LL, Ujioka T, Russell DL, Skelsey M, Vladu B, Robker RL, Okamura H, Richards JS2000. Induction of early growth response protein-1 gene expression in the rat ovary in response to an ovulatory dose of human chorionic gonadotropin. Endocrinology 141:2385–2391 [DOI] [PubMed] [Google Scholar]
- 41.Evaul K, Hammes SR2008. Cross-talk between G protein-coupled and epidermal growth factor receptors regulates gonadotropin-mediated steroidogenesis in Leydig cells. J Biol Chem 283:27525–27533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A2001. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 20:1594–1600 [DOI] [PubMed] [Google Scholar]
- 43.Kondoh K, Nishida E2007. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta 1773:1227–1237 [DOI] [PubMed] [Google Scholar]
- 44.Patterson KI, Brummer T, O'Brien PM, Daly RJ2009. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J 418:475–489 [DOI] [PubMed] [Google Scholar]
- 45.Sun H, Charles CH, Lau LF, Tonks NK1993. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75:487–493 [DOI] [PubMed] [Google Scholar]
- 46.Dekel N, Galiani D, Beers WH1988. Induction of maturation in follicle-enclosed oocytes: the response to gonadotropins at different stages of follicular development. Biol Reprod 38:517–521 [DOI] [PubMed] [Google Scholar]
- 47.Dekel N, Hillensjo T, Kraicer PF1979. Maturational effects of gonadotropins on the cumulus-oocyte complex of the rat. Biol Reprod 20:191–197 [DOI] [PubMed] [Google Scholar]