Abstract
Pituitary function has been shown to be regulated by an increasing number of factors, including cytokines and hormones, such as TNFα and T3. Both the proinflammatory cytokine TNFα and T3 have been suggested to be involved in the maintenance of tissue homeostasis in the anterior pituitary gland. In this report we show that T3 negatively interferes with MAPK p38 and nuclear factor-κB (NF-κB) activation by TNFα in GH4C1 cells. Our data demonstrate that MAPK p38 is specifically activated upon exposure to TNFα and that T3 abolishes this activation in a time-dependent manner by a mechanism that involves the induction of the MAPK phosphatase, DUSP1. Our data show that the pool of up-regulated DUSP1 by T3 is mainly localized to the cytosol, and that TNFα does not affect this localization. On the other hand, we show that T3 impairs the activation of the NF-κB pathway induced by TNFα, producing a significant decrease in NF-κB-dependent transcription, phosphorylation of IκBα, translocation of p65/NF-κB to the nucleus, and p65/NF-κB transactivation potential. Interestingly, the overexpression of DUSP1 inhibits the NF-κB activation achieved by either TNFα or ectopic expression of the upstream inducer of MAPK p38. Conversely, DUSP1 depletion abrogates the inhibitory effect of T3 on the induction of NF-κB-dependent transcription by TNFα. Overall, our results indicate that T3 antagonizes TNFα signaling in rat pituitary tumor cells through the induction of DUSP1.
Thyroid hormone inhibits TNF-alpha-mediated activation of both MAPK p38 and NF-κB pathways by a mechanism requiring the induction of the DUSP1 phosphatase.
The thyroid hormone T3 influences a variety of physiological processes in mammals, including cell growth and metabolism (1). Most, if not all, of these actions are mediated by nuclear T3 receptors, which are widely expressed in mammalian tissues. Pituitary somatolactotrope cells are a well established target of T3, and GH4C1 cells, which are derived from a rat pituitary tumor, have been widely used as a model to understand the mechanisms of T3 action (2).
TNFα is a proinflammatory cytokine that regulates cell proliferation, differentiation, and apoptosis and induces production of other cytokines (3). TNFα is mainly produced by macrophages, but also by a broad variety of other tissues, including the pituitary, in particular by the intermediate lobe and by the somatotroph cells (4, 5). The presence of TNFα receptors has also been reported both in normal pituitary cells (6, 7) and in the pituitary GH3 cell line (8), suggesting that this cytokine might well play a role as an autocrine or paracrine regulator of the pituitary cell function. Indeed, TNFα has been suggested to be involved in the maintenance of tissue homeostasis in the anterior pituitary gland (8) and is likely to be a major paracrine modulator of lactotroph function (9).
In many cell types, TNFα affects different cellular functions by regulating the phosphorylation cascade of members of the MAPK family, in particular the stress kinases, c-Jun N-terminal kinase (JNK) and MAPK p38 (10). The activation of MAPK is tightly regulated by MAPK phosphatases, which dephosphorylate MAPK at Thr/Tyr residues critical for activation, thereby contributing to the down-regulation of MAPK activity. DUSP1, also known as MAPK phosphatase 1 (MKP1), the founder member of this family, is induced by a variety of stimuli including growth factors, nuclear receptors, and stress stimuli (11), and localizes to the nucleus through a LXXLL motif in its amino terminus (12). The balance between the activation and the inactivation of MAPK is a critical determinant of biological outcomes in many cell types. For example, MAPK p38 activation in the pituitary has been shown to regulate GH gene transcription and expression (13, 14) or the GnRH-signaling cascade (15).
TNFα can also exert its effects by triggering the activation of nuclear factor-κB (NF-κB)-signaling cascade (10). NF-κB transcription factors consist of dimers assembled from p50 and p65 subunits, which are normally found in the cytosol, retained by interaction with an inhibitory molecule called IκB. Activation of IκB kinases (IκK) leads to phosphorylation and degradation of IκB, allowing nuclear translocation of NF-κB dimers, where they regulate target gene transcription (16). It has been shown previously that TNFα activates NF-κB in pituitary GH3 cells (9, 17) and that this activation can be blocked by coexpressing dominant-negative isoforms of components of the NF-κB-signaling pathway (9).
Interestingly, TNFα can simultaneously activate both MAPK p38 and NF-κB pathways in different cellular contexts. For example, it has been previously described that MAPK p38 is required for NF-κB-dependent gene expression (18, 19) and contributes to TNFα-dependent NF-κB activation through the downstream effector, mitogen- and stress-associated protein kinase 1 (MSK1) (20). The activation of MAPK and/or NF-κB pathways by TNFα switches many key proapoptotic, antiapoptotic, proliferative, or inflammatory genes that result in the desired cellular responses. Many ligands of different nuclear receptors have been shown to inhibit TNFα signaling. Among them, dexamethasone is widely used as TNFα antagonist in diverse cellular contexts (21). To a lesser extent, other nuclear receptors such as the peroxisome proliferator-activated receptor α (22) or the androgen receptor (23) negatively interfere with inflammatory gene expression triggered by the activation of TNFα-signaling pathway. Interestingly, the progesterone receptor antagonizes the permissive action of estrogens on TNFα-induced apoptosis of anterior pituitary cells, whereas dexamethasone fails to do it (24). However, no evidence of regulation of TNFα effects by T3 receptors has been described so far.
We have recently reported that T3 induces the expression of DUSP1 in pituitary GH4C1 cells by a posttranscriptional mechanism (25). In this study, we have analyzed the possibility that T3 could also affect the response of these cells to TNFα, and we show for the first time that T3 blocks the effects of the cytokine on both phosphorylation of MAPK p38 and NF-κB activity. The inhibitory effect of T3 on the TNFα pathway involves the up-regulation of the cytosolic pool of DUSP1, which, in turn, impairs the phosphorylation of MAPK p38 achieved by TNFα. Moreover, DUSP1 also controls the induction of NF-κB pathway by either TNFα or ectopic expression of the upstream inducer of MAPK p38 in GH4C1 cells.
Given the crucial role of TNFα in several physiopathological processes, deregulation of TNFα function by T3 is likely to be biologically important in the pituitary.
Results
T3 selectively inhibits TNFα-mediated activation of MAPK p38 and induces the expression of DUSP1
TNFα exerts its effects through the regulation of MAPK-signaling pathway in different cellular contexts. Considering that this cytokine plays a pivotal role in the maintenance of the homeostasis in the pituitary, our first aim was to test whether TNFα was able to regulate MAPK activation in pituitary cells. To that purpose, we first analyzed the phosphorylation level of the classical MAPK (ERK, JNK, and MAPK p38), after treatment with TNFα by using phospho-specific antibodies. As shown in Fig. 1A, MAPK p38 was phosphorylated in response to TNFα in a time-dependent manner, showing a peak of activation at 15 min incubation. By contrast, TNFα did not induce the phosphorylation level of either ERK or JNK (data not shown). The lack of induction of JNK by TNFα was confirmed by kinase assays using c-Jun as substrate (data not shown). Different nuclear receptors have been shown to block the activation of MAPK-signaling pathways; therefore, we next investigated the effect of T3 on MAPK phosphorylation after TNFα treatment. For these experiments, MAPK phosphorylation levels were measured by Western blotting in cells pretreated with T3 for different times and with TNFα for the last 15 min. As shown in Fig. 1B, T3 blocked TNFα-induced MAPK p38 phosphorylation in a time-dependent manner. By contrast, T3 did not affect the low levels of the phosphorylated ERK or JNK under the same conditions (Fig. 1B).
Fig. 1.
T3 selectively inhibits TNFα-mediated activation of MAPK p38 and induces the expression of DUSP1. A, GH4C1 cells were incubated with TNFα (10 ng/ml) for different times, and total cell lysates were analyzed by Western blotting with antibodies against phosphorylated and total MAPK p38. B, Cells were treated with T3 (5 nm) for different times and then incubated with 10 ng/ml TNFα for the last 15 min. Total cell lysates were prepared and analyzed by Western blotting with antibodies against the phosphorylated and total forms of the three MAPKs. The blots shown are from a representative experiment performed twice with similar results. C, Cells were incubated with T3 (5 nm) for different times, and the total cell extracts were analyzed by Western blotting using antibodies against DUSP1. Equal protein loading was evaluated by assessing β-actin. D, Cells were maintained in the presence or absence of T3 (5 nm) for 24 h and then incubated with 10 ng/ml TNFα for 15 min. The cytosolic extracts (CE) and the nuclear extracts (NE) were analyzed by Western blotting using antibodies against DUSP1. The levels of tubulin and poly ADP ribose polymerase (PARP) were used as controls to validate the integrity of the cytosolic and nuclear fractions, respectively.
DUSP1 is a phosphatase that regulates MAPK activity by controlling its phosphorylation state in diverse cell lines. Furthermore, we have previously reported that T3 can induce DUSP1 levels in pituitary cells (25). Therefore, we next tested whether T3 was able to regulate TNFα-mediated activation of MAPK p38 phosphorylation through the induction of DUSP1 in pituitary cells. To test this hypothesis, we first measured the levels of DUSP1 after T3 treatment up to 24 h. The expression of DUSP1 protein was induced rapidly by incubation with T3 and was sustained for at least 24 h (Fig. 1C). Because it has been generally accepted that DUSP1 localizes to the nucleus (12), we next investigated the subcellular localization of DUSP1 after T3 treatment with or without TNFα. Surprisingly, control GH4C1 cells showed low expression of DUSP1 in cytosolic fractions, whereas the levels of DUSP1 in nuclear extracts were almost undetectable (Fig. 1D). Moreover, the pool of up-regulated DUSP1 by T3 was mainly localized to the cytosol, and the treatment of the cells in the presence of TNFα did not induce major changes in the subcellular distribution of DUSP1 both in control and T3-treated cells (Fig. 1D).
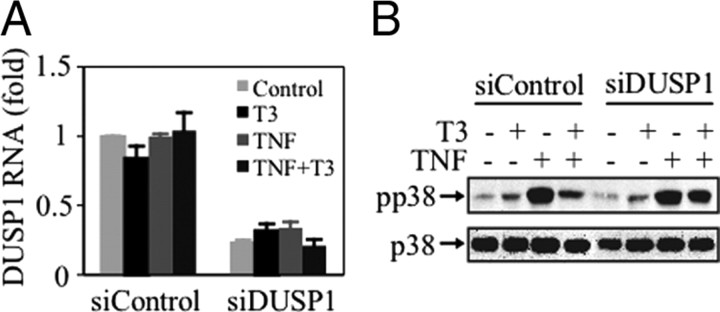
We next investigated whether DUSP1 was involved in the regulation of MAPK p38 activity by T3. To address this question, cells were transiently transfected with either siControl [a control small interfering RNA (siRNA)] or with two specific siRNAs to knock down DUSP1 expression (siDUSP1), as previously described (25). After incubation in the absence or presence of T3, and/or TNFα, the levels of DUSP1 mRNA were monitored by quantitative RT-PCR (QRT-PCR). The siDUSP1 reduced DUSP1 mRNA levels by about 80% of those found in cells transfected with the siControl, without any effect of the treatments (Fig. 2A). Figure 2B shows that although T3 markedly reduced the induction of MAPK p38 phosphorylation by TNFα in cells transfected with siControl, the effect of T3 was blunted in the cells transfected with the specific siDUSP1. These data demonstrate that DUSP1 mediates the inhibition of TNFα-induction of MAPK p38 activity caused by T3 in GH4C1 cells.
Fig. 2.
DUSP1 controls the T3-mediated inhibition of TNFα-induced MAPK p38. GH4C1 cells were transfected with the siControl or with the siDUSP1, incubated for 24 h with T3, and then stimulated with TNFα for 15 min. A, Total RNA was extracted, and the levels of DUSP1 mRNA were monitored by QRT-PCR. DUSP1 mRNA levels were normalized with glyceraldehyde-3-phosphate dehydrogenase mRNA levels, and the results are expressed as the fold change in mRNA expression. The values represent the mean ± sd. B, Total cell lysates were prepared and analyzed by Western blotting with antibodies against phospho-p38 and total p38. The blots shown are from a representative experiment performed twice with similar results.
T3 blocks the NF-κB pathway induced by TNFα
TNFα effects are mediated, not only by regulating MAPK pathways, but also by activation of the NF-κB pathway in diverse cell types. Because NF-κB is a known downstream target of MAPK p38 and we have recently shown that T3 blocks the activity of the NF-κB pathway in serum-deprived-GH4C1 cells (25), we tested whether T3 was also able to prevent the effects of TNFα on NF-κB activation. For this purpose, GH4C1 cells were transiently transfected with a thymidine kinase (TK)-Luc reporter containing three repeats of the NF-κB consensus-binding sequence (3×NF-κB-Luc). Treatment of the cells with TNFα for 6 h caused a significant increase in luciferase activity, and preincubation for 24 h with T3 reduced TNFα-mediated activation by about 50% (Fig. 3A). By contrast, the TK-Luc control reporter showed a low activity, which was not modified in the presence of either TNFα or T3 (Fig. 3A). NF-κB may be activated by stimulation of the transactivation domain in the p65/NF-κB subunit. Therefore, we studied whether the differences observed after T3 treatment were also dependent on the transcriptional activity of p65/NF-κB. To address this question, we used a plasmid encoding the Gal4-p65 fusion protein, in which the sequences encoding the DNA-binding domain of Gal4 have been fused with sequences encoding p65/NF-κB. Its cotransfection with a Gal4-Luc reporter plasmid allowed us to determine whether cellular signals triggered by TNFα and T3 regulate gene expression by specifically targeting the p65/NF-κB protein. Like NF-κB-dependent transcription, TNFα increased the basal activity of Gal4-p65 about 8-fold, and incubation of the cells with T3 decreased it by about 50% (Fig. 3B). By contrast, the Gal4 control reporter activity was not modified in the presence of either TNFα or T3 (Fig. 3B). These results indicate that TNFα activates the NF-κB transcriptional activity by increasing p65/NF-κB transactivation, and that T3 is able to decrease this effect. However, in agreement with our previous results (25), it seems that T3 is not only blocking TNFα-mediated induction of NF-κB, because it also significantly inhibits the basal activity of both NF-κB-dependent transcription (Fig. 3A) and p65 transactivation (Fig. 3B).
Fig. 3.

T3 antagonizes the activation of the NF-κB pathway by TNFα. A, GH4C1 cells were transiently transfected with a NF-κB-driven luciferase reporter plasmid (3× NF-κB-TK-Luc) or the parental TK-Luc plasmid (TK-Luc), and pRL-TK-Renilla, treated with T3 (5 nm) for 24 h and then incubated in the absence or presence of 10 ng/ml TNFα for 6 h. Cell extracts were prepared and assayed for luciferase and Renilla activities. The luciferase levels were normalized to those of Renilla and expressed as the induction over the controls. The data represent the mean ± sd of five independent experiments performed in duplicate. B, Cells were transiently transfected with the pGal4-Luc reporter and the pRL-TK-Renilla, together with Gal4–p65 or the parental Gal4-DBD plasmid (DBD-Gal). Cells were then treated as in panel A, and luciferase levels in the cell extracts were assayed, normalized with Renilla, and expressed as the induction over controls. The data shown represent the mean ± sd of five independent experiments performed in duplicate. C, Cells were incubated with T3 (5 nm) for 24 h and then treated at different times with 10 ng/ml TNFα. The cytosolic extracts (CE) and the nuclear extracts (NE) were analyzed by Western blotting using antibodies against IκBα, the phosphorylated form of IκBα (pIκBα), and p65. Equal protein loading was evaluated with β-actin. The blots shown are from one representative experiment of three. D, Cells were treated as in panel C, and nuclear extracts were prepared and tested for NF-κB binding activity by EMSA. The asterisk indicates a nonspecific band. A representative experiment of three replicates is shown. DBD, DNA-binding domain; Ren, Renilla.
As mentioned previously, NF-κB is regulated, in part, by a cellular process that involves phosphorylation and degradation of its inhibitory subunit IκBα, permitting active NF-κB complexes to translocate to the nucleus and activate transcription. Thus, we measured the kinetics of IκBα degradation in cytosolic cell extracts upon exposure to TNFα in the absence or presence of T3. As expected, incubation of the cells with TNFα produced a transient decrease of IκBα levels, which were not significantly modified by treatment with T3 (Fig. 3C). We next analyzed the effect of TNFα and T3 on IκBα phosphorylation and showed that phosphorylated IκBα levels transiently increased after TNFα treatment, whereas T3 abrogated this effect (Fig. 3C). We then examined the effect of TNFα and T3 on p65/NF-κB levels in nuclear extracts and found that p65/NF-κB levels increased after TNFα incubation in control cells. However, the translocation of p65/NF-κB to the nucleus induced by TNFα was strongly reduced after 24 h exposure to T3. In contrast, p65/NF-κB levels in cytosolic extracts remained quite high and unchanged in the presence of T3 (Fig. 3C).
Because we detected differences in the levels of nuclear p65/NF-κB after exposure to T3, we determined the NF-κB-binding activity in nuclear extracts from cells that were incubated with T3 for 24 h and then with TNFα for the indicated time periods (Fig. 3D). Specific NF-κB-DNA-binding complexes were observed when cells were incubated in the presence of TNFα for 15 min to 6 h, whereas complex formation was reduced when these cells were previously exposed to T3. However, although T3 treatment appears to reduce complex formation, significant translocation into the nuclear compartment is still observed in the presence of T3.
T3 blocks the NF-κB pathway induced by ectopic activation of MAPK p38
The above-mentioned results suggest that T3 could down-regulate NF-κB activity through the induction of DUSP1 and the inhibition of MAPK p38 activity. If MAPK p38 is causally involved in the regulation of the NF-κB response to TNFα by T3 in GH4C1 cells, then its activation should be sufficient for NF-κB stimulation and for T3-dependent repression. Accordingly, experiments were carried out to determine the effect of T3 on NF-κB activity in cells transfected with a constitutively active form of the activating kinase of MAPK p38, MAPK kinase 6 (MKK6)EE. As shown in Fig. 4, MKK6EE was able to induce both NF-κB-dependent transcription (Fig. 4A) and p65 transactivation potential (Fig. 4B) significantly, and incubation with T3 significantly reduced both responses, indicating that the hormone is able to inhibit the activation of NF-κB mediated by MAPK p38. As a control, the MKK6EE-induced activation of NF-κB was reversed by the specific inhibitor SB203580, confirming that NF-κB is a target for the MAPK p38 pathway in GH4C1 cells (Fig. 4, A and B). The kinase MSK1 is phosphorylated and activated by MAPK p38 and has been suggested to be the main downstream effector for TNFα-mediated p65 transactivation potential (20). To further investigate the mode of action of T3 on NF-κB pathway activity, p65 transactivation was first measured in cells that were transfected either with a vector encoding for wild-type MSK1, the MKK6EE mutant alone, or the MKK6EE mutant plus wild-type MSK1 or two different dominant-negative mutants of MSK1 (dn195 and dn565). Although their overexpression was quite high, neither basal nor MKK6EE-induced p65 transactivation potential was modified by either the wild-type MSK1 or the two dominant-negative mutants, and T3 was still able to repress the response to MKK6EE in all groups (Fig. 4D). The activity of the MSK1 proteins was tested by analyzing the transcriptional activity of a luciferase reporter governed by the cAMP response element binding protein (CREB-Gal), which is a known MSK1 downstream target (26). As expected, the activity of this reporter was increased by MKK6EE plus wild-type MSK1, whereas the expression of any of the MSK1 dominant-negative mutants abrogated the activity (Fig. 4E). Furthermore, in agreement with our previous findings showing that T3 can antagonize CREB-dependent transcription (27), we found that T3 also reduced CREB-Gal activity. The overexpression of all MSK1 proteins was verified in parallel Western blots (Fig. 4, D and E, insets). To further verify that MAPK p38 was indeed involved in the regulation of NF-κB by TNFα and T3, we examined the effects of the specific inhibitor, SB203580, on the p65 transactivation potential (Fig. 4C). As expected, the reduction of TNFα-induced p65 transactivation potential achieved by treatment with either SB203580 or T3 was very similar and close to 50% (Fig. 4C).
Fig. 4.
T3 blocks the NF-κB pathway induced by ectopic activation of MAPK p38. A and B, GH4C1 cells were transiently cotransfected with a vector encoding MKK6EE and pRL-TK-Renilla, together with either a NF-κB-driven luciferase reporter plasmid (3× NF-κB-TK-Luc) (A) or the pGal4-Luc reporter and Gal4-p65 (B). After the incubation with T3 (5 nm) or SB203580 (5 μm) for 24 h, cell extracts were prepared and assayed for luciferase and Renilla activities. The luciferase levels were normalized to those of Renilla and expressed as the induction over the controls. The data show the mean ± sd of three independent experiments performed in duplicate. C, Cells were transiently transfected with the pGal4-Luc reporter, Gal4-p65, and pRL-TK-Renilla. Cells were incubated with T3 (5 nm) or SB203580 (5 μm) for 24 h and then with 10 ng/ml TNFα for 6 h. Cell extracts were prepared and assayed for luciferase and Renilla activities as in panel A. D and E, Cells were transiently cotransfected with the pRL-TK-Renilla, the pGal4-Luc reporter, and Gal4-p65 (D) or Gal4-CREB (E), together either with a vector encoding wild type MSK1, MKK6EE alone, or MKK6EE plus a vector encoding wild type MSK1, or the mutants MSK1dn195 or MSK1dn565. Cell incubations, cell extracts, and measurement of luciferase and Renilla activities were performed as in panel A. The expression level of MSK1 proteins is shown in the insets. Ren, Renilla; SB, SB203580; wt, wild type.
DUSP1 mediates the inhibitory effect of T3 on stimulation of NF-κB activity by TNFα or by MKK6EE expression
Considering that our data demonstrate that MAPK p38 is involved in the regulation of NF-κB activity by TNFα (Fig. 4), and that T3 blocks TNFα-mediated activation of MAPK p38 through the induction of DUSP1 (Fig. 2), we next investigated whether DUSP1 affected the NF-κB activity in TNFα-stimulated cells or in MKK6EE-transfected cells. Our results show that overexpression of a plasmid encoding DUSP1 reduced the NF-κB-dependent transcription induced by constitutively active MKK6 (Fig. 5A) or by TNFα (Fig. 5C). Similarly, DUSP1 overexpression reduced the p65 transactivation potential induced by transfecting cells with MKK6EE (Fig. 5B) or by treatment with TNFα (Fig. 5D). In all cases, T3 was still able to reduce the NF-κB activity, albeit to a lesser extent than in control cells. To demonstrate the specificity of the effect of DUSP1 overexpression on the NF-κB-related-reporter genes, we performed similar experiments using either the TK-Luc control reporter or a luciferase reporter gene driven by the collagenase promoter (−73Col-Luc). As shown in Fig. 5E, the activity of TK-Luc was not modified by either cell treatments or DUSP1 overexpression. On the other hand, the activity of −73Col-Luc was not changed by either TNFα or T3 treatment, whereas DUSP1 overexpression, rather than inhibit the response to TNFα, significantly up-regulated it by about 4-fold (Fig. 5F). These results demonstrate that DUSP1 overexpression specifically down-regulates the activity of the NF-κB-related-reporter genes employed in this study.
Fig. 5.

DUSP1 overexpression reduces the stimulation of NF-κB activity. A and B, GH4C1 cells were transiently cotransfected with a vector encoding MKK6EE, a vector encoding DUSP1, and pRL-TK-Renilla, together with either a NF-κB-driven luciferase reporter plasmid (3× NF-κB-TK-Luc) (A) or the pGal4-Luc reporter and Gal4-p65 (B). After the incubation with T3 (5 nm) for 24 h, cell extracts were prepared and assayed for luciferase and Renilla activities as in Fig. 4. The data shown represent the mean ± sd of three independent experiments performed in duplicate. C and D, GH4C1 cells were transiently cotransfected with a vector encoding DUSP1, and pRL-TK-Renilla, together with either the NF-κB-driven luciferase reporter plasmid (3× NF-κB-TK-Luc) (C) or the pGal4-Luc reporter and Gal4-p65 (D). Cells were incubated with T3 (5 nm) for 24 h and then with 10 ng/ml TNFα for 6 h. Cell extracts and measurement of luciferase and Renilla activities were performed as in panel A. E and F, GH4C1 cells were transiently cotransfected with a vector encoding DUSP1, pRL-TK-Renilla, together with either the TK-Luc plasmid (E) or the collagenase-driven luciferase reporter plasmid (−73Col-Luc) (F). Cells were incubated with T3 (5 nm) for 24 h and then with 10 ng/ml TNFα for 6 h. Cell extracts and measurement of luciferase and Renilla activities were performed as in panel A. G, Cells were transfected with a vector encoding DUSP1 and then incubated in the absence or presence of 10 ng/ml TNFα for 1 h. The cytosolic extracts (CE) and the nuclear extracts (NE) were analyzed by Western blotting using antibodies against p65 or DUSP1. Equal protein loading was evaluated with β-actin. Ren, Renilla.
To analyze the mechanism by which DUSP1 overexpression reduced NF-κB activity, we examined the effect of DUSP1 on TNFα-induced nuclear translocation of p65/NF-κB. As shown in Fig. 5G, TNFα significantly increased p65/NF-κB levels in nuclear extracts from control cells, whereas the overexpression of DUSP1 partially suppressed the effect of TNFα. Accordingly, p65/NF-κB levels in cytosolic extracts were increased in DUSP1-expressing cells (Fig. 5G). In agreement with the results shown in Fig. 1D, the phosphatase was essentially absent from nuclei in GH4C1 cells (Fig. 5G). These data suggest that T3 inhibits, at least in part, the NF-κB activity through the induction of DUSP1 expression.
To assess the role of DUSP1 on inhibition of TNFα-mediated stimulation of NF-κB by T3, cells were cotransfected with the 3×NF-κB-Luc reporter and the siControl or siDUSP1. The levels of DUSP1 mRNA were monitored by QRT-PCR, demonstrating that siDUSP1 attenuated DUSP1 mRNA expression by 60% (Fig. 6A). In addition, to confirm the knockdown of the protein, DUSP1 protein levels were analyzed by Western blotting. As expected, the transfection with the siDUSP1 abrogated the T3-mediated-induction of DUSP1 both in control- and TNFα-treated cells (Fig. 6B). On the other hand, when the cells were incubated with T3, the TNFα-induced-NF-κB transcription was reduced to about 50% of control cells, whereas the inhibition by T3 was abolished in siDUSP1 transfected cells (Fig. 6C). These results demonstrate that T3 regulates the TNFα-activated NF-κB pathway through the induction of DUSP1.
Fig. 6.
DUSP1 mediates the inhibitory effect of T3 on TNFα-stimulated NF-κB activity. A, Cells were transfected with the siControl or the siDUSP1, total RNA was extracted, and the levels of DUSP1 mRNA were monitored by QRT-PCR. DUSP1 mRNA levels were normalized with glyceraldehyde-3-phosphate dehydrogenase mRNA levels, and the results are expressed as the fold change in mRNA expression. The values represent the mean ± sd. B, Cells were transfected as in panel A, incubated for 24 h with T3, and then treated with 10 ng/ml TNFα for 6 h. and DUSP1 and β-actin protein levels were analyzed in total cell lysates by Western blotting. C, Cells were cotransfected with the control siRNA or the DUSP1 siRNAs, together with the NF-κB-driven luciferase reporter plasmid (3× NF-κB-TK-Luc), and treated as in B. Cell extracts were prepared and assayed for luciferase and Renilla activities as in Fig. 5.
Discussion
In this report we show, for the first time, that T3 is able to repress the activation of MAPK p38 by TNFα in pituitary cells. Previous reports indicate that MAPK-dependent pathways are present and active in pituitary cells and are involved in gene transcription regulation stimulated by some hormones and factors, such as IGF-I (28), GnRH (29), or IL-1β (30). In many cells, MAPK p38 is described as being activated by proinflammatory cytokines, such as TNFα, controlling diverse cellular functions. For example, in the pituitary, it has been demonstrated that TNFα regulates apoptosis (8) or prolactin gene expression (9). Here we demonstrate that this cytokine activates MAPK p38 in pituitary cells without affecting ERK or JNK activity, and, more interestingly, we show that T3 blocks the stimulation of MAPK p38 activity by TNFα. To date, different nuclear receptors, such as the glucocorticoid receptor (31, 32, 33) or the peroxisome proliferator-activated receptor-γ (34), have been shown to be antagonists of the MAPK p38 pathway, but, to our knowledge, this is the first report showing that the T3 receptors also have this effect.
Many signaling pathways can interact with each other through biochemical cross talk. For example, MAPK p38 and NF-κB are known to be activated in response to similar stimuli, including inflammatory cytokines, phorbol esters, bacterial toxins, viruses, or UV light in different cell types. In fact, it is well known that NF-κB is a MAPK p38 target in response to TNFα in different cell types (35). Stimulation of NF-κB activates the IκK signalsome that phosphorylates IκB and NF-κB proteins, leading to ubiquitination and degradation of IκB, and to subsequent translocation of free NF-κB dimers to the nucleus. In agreement with this, we found rapid IκBα phosphorylation and transient nuclear translocation of p65/NF-κB, together with an increase in NF-κB-mediated transcription and p65/NF-κB transactivation potential in TNFα-stimulated GH4C1 cells. These results are in agreement with those obtained in other pituitary cell lines (9, 17) and reinforce the idea that TNFα might act as an autocrine or paracrine regulator of pituitary cell function (6, 7, 8).
However, the interactions between MAPK p38 and NF-κB are not well understood, and they have never been investigated in pituitary cells. Here we demonstrate that MAPK p38 plays an important role on NF-κB activation in GH4C1 cells. Previous reports have shown that overexpression or activation of MAPK p38 leads to the degradation of IκBα and its dissociation from NF-κB, thereby allowing p65/NF-κB nuclear translocation and DNA binding in NIH3T3 cells (36), in C2C12 cells (37), or in L929 cells (18). In agreement with these reports, our data show that ectopic activation of MAPK p38 induces the NF-κB pathway in GH4C1 cells. Interestingly, this activation does not appear to be controlled by the downstream effector MSK1, which has been described as the main mediator of NF-κB regulation by MAPK p38 in other cellular systems (20).
Functional cross talk between nuclear receptors and NF-κB has been reported for various classes of receptors and has been shown to be crucial for regulation of many cellular functions (36, 38, 39, 40). Nuclear receptors can inhibit the action of NF-κB in the presence of their cognate ligands by a variety of mechanisms, and we show here that T3 inhibits NF-κB-dependent transcription induced by TNFα in GH4C1 cells by affecting the phosphorylation of IκBα, the translocation of active NF-κB complexes from the cytosol to the nucleus, the binding of p65/NF-κB to κB sequences in DNA, and p65/NF-κB transactivation. Surprisingly, T3 impaired the phosphorylation of IκBα without affecting its degradation. Although we do not have a good explanation for this observation, we can speculate that this apparent discrepancy could be explained in light of the rapid turnover of this protein by resynthesis after TNFα treatment, which could hinder observation of the differences between control- and T3-treated cells. Alternatively, T3 could affect the activation of NF-κB by a mechanism that is independent of the degradation of IκBα, as previously described (41, 42, 43). Similar to what happened in TNFα-stimulated cells, we show that T3 is able to inhibit both the NF-κB-dependent transcription and the p65/NF-κB transactivation induced by expression of the constitutively active mutant of MKK6. These findings altogether indicate that the effect of T3 on the NF-κB pathway is mediated, at least in part, by regulation of MAPK p38 and seems to be proximal to MAPK p38 itself. The hormone must block MAPK p38 activation, even when the stimulus is provided by a constitutively active upstream kinase. In addition, these results strongly suggest that the inhibitory effect of T3 on TNFα-induced NF-κB activity could be also mediated by MAPK p38 regulation. In fact, this hypothesis is very plausible, taking into account that TNFα specifically activates MAPK p38 in GH4C1 cells and that T3 significantly reduces this induction in a time-dependent manner. Moreover, the fact that both T3 and the specific MAPK p38 inhibitor SB203580 repress TNFα-induced p65/NF-κB transactivation to a similar extent reinforces this hypothesis. Different nuclear receptors have been shown to regulate both the NF-κB pathway and the MAPK p38-signaling cascade. For example, dexamethasone synergistically enhances IL-1β-induced Toll-like receptor 2 expression via negative cross talk with the MAPK p38 pathway and NF-κB activation (44). On the other hand, PPARγ reduces proinflammatory cytokines expression by antagonizing the activity of MAPK p38 (34) and by interfering with NF-κB (45). Moreover, estradiol blocks the hypoxia-mediated activation of both MAPK p38 and NF-κB- dependent transcription (46). In agreement with all these reports, our data show that T3 can also block both the NF-κB pathway and MAPK p38 phosphorylation induced by TNFα in pituitary cells.
DUSP1 is a phosphatase that is gaining interest as an antiinflammatory molecule because it impairs the response achieved by diverse proinflammatory agents including TNFα (47, 48). Using the siRNA approach to selectively inhibit DUSP1 expression, we demonstrate in this report that the effect of T3 on TNFα-induced MAPK p38 and NF-κB activation is mediated by this phosphatase, because DUSP1 depletion abrogates the effect of the hormone. We also used a converse approach, namely DUSP1 overexpression, and showed that NF-κB activation by either TNFα or ectopic expression of the upstream inducer of MAPK p38 was almost abrogated, whereas T3 continued to inhibit the NF-κB activity. Taking the effects of DUSP1 knockdown and DUSP1 overexpression together, we conclude that DUSP1 is an important mediator of the T3-induced inhibition of both the MAPK p38- and the NF-κB-signaling pathways. Several reports have shown an inhibition of the NF-κB signaling pathway concomitant with an induction of the expression of DUSP1 in diverse cellular systems (49, 50, 51). However, the existence of both events at the same time was only circumstantial in all cases. By contrast, we had shown that DUSP1 governs T3-mediated inhibition of the NF-κB pathway in serum-deprived GH4C1 cells (25), and in this report we demonstrate that this is also true for TNFα-stimulation. Regulation of the NF-κB pathway by DUSP1 is not surprising considering that this protein has been identified as one of the components of the IKK signalsome. Indeed, DUSP1 coassociates with IKKα, and it was speculated that DUSP1 (or the DUSP1-reactive protein identified in the IKK signalsome) might be the phosphatase responsible for down-regulating IKKα/β activity (52, 53, 54). This hypothesis could explain our data showing that T3 inhibits NF-κB through a mechanism dependent on DUSP1 expression. To study this possibility, we have performed experiments to analyze the possible interaction between DUSP1 and IKKα/β in GH4C1 cells, and our preliminary data show that TNFα, alone or in combination with T3, is able to induce the association between DUSP1 and IKKα/β (data not shown). On the other hand, our results show that the pool of up-regulated DUSP1 by T3 is mainly localized to the cytosol, suggesting that T3-induced-DUSP1 specifically affects proteins that are located at this compartment. Subcellular localization is an important regulatory feature of DUSP1. Despite the fact that the nuclear distribution of DUSP1 is known in many systems, the mechanisms regulating its localization remain poorly defined. It has been suggested that the two basic clusters in the amino terminus of DUSP1, which are critical for binding to MAPK p38 (55), also serve to target DUSP1 to the nucleus (12). Thus, it is conceivable that, upon T3 treatment, the basic clusters of DUSP1 bind to the cytosolic pool of MAPK p38, hindering complete nuclear localization of DUSP1. Alternatively, DUSP1 may interact with other proteins through its LXXLL motif, which may retain DUSP1 in the cytosolic compartment. These findings altogether strongly suggest that DUSP1 could inhibit the NF-κB pathway by regulating IκK activity through its interaction with the IκK signalsome, although the underlying mechanism requires further investigation.
In conclusion, we have shown, for the first time, that T3 can antagonize the TNFα signaling pathway in pituitary cells by a mechanism that involves the induction of DUSP1. Both T3 and TNFα have been shown to be produced locally in the pituitary gland, suggesting that this effect could be important for pituitary physiology in vivo. Moreover, the fact that T3 regulates the effects of TNFα on both MAPK p38 and NF-κB activation in GH4C1 cells opens new perspectives in the study of the role of T3 as an antiinflammatory ligand controlling TNFα effects in the pituitary.
Materials and Methods
Materials and plasmids
Tissue culture media and sera were obtained from Life Technologies, Inc. (Gaithersburg, MD). The antibodies used were: anti-NF-κB/p65, anti-IκBα, anti-MKP1, anti-p38 MAPK, anti-ERK2, anti-JNK1, and anti-poly ADP ribose polymerase (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); antiphospho-IκBα, antiphospho-ERK and antiphospho-p38 MAPK (Cell Signaling Technology, Danvers, MA); antihemagglutinin (Covance Research Products, Princeton, NJ); antiphospho-JNK (Promega Corp., Madison, WI); anti-β-actin, anti-Flag, and antitubulin (Sigma Chemical Co., St. Louis, MO); and peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology). SB203580 was from Calbiochem (La Jolla, CA). T3 was from Sigma. Rat TNFα was from PreProTech (Rocky Hill, NJ). The 3×NF-κB-TK-Luc reporter plasmid, which contains a three tandem repeat of the NF-κB-binding motif of the H-2k gene upstream of the thymidine kinase minimal promoter (56), was kindly provided by Dr. M. Fresno (Centro de Biología Molecular Severo Ochoa, Consejo Superior de Investigaciones Cientificas-UAM, Madrid, Spain). The plasmid containing the Gal4-DNA-binding domain fused to the full-length human p65/NF-κB coding sequence (pGal4-p65) (57) was obtained from the Belgian Coordinated Collections of Microorganisms (BCCM/LMBP Plasmid Collection) (Ghent, Belgium). The plasmid pGal4-Luc and the probe for EMSAs (58) were kindly provided by Dr. R. Perona (Instituto de Investigaciones Biomédicas, CSIC-UAM, Madrid, Spain). The plasmids pCMV-Flag-MKP1 and pcDNA3-HA-MKK6EE (with Ser-to-Glu and Thr-to-Glu mutations at codons 207 and 211) were kindly provided by Dr. A. Clark (Imperial College, London, UK). The plasmids pCMV5-Flag-Msk1 wild type, pCMV5-Flag-D195/A-Msk1 (with an Asp-to-Ala mutation at codon 195), and pCMV5-Flag-D565/A-Msk1 (with an Asp-to-Ala mutation at codon 565) were kindly provided by Dr. D. Alessi (The University of Dundee, Scotland, UK).
Cell culture and transfection
GH4C1 cells were cultured in DMEM supplemented with 10% fetal bovine serum. In the experiments, the cell medium was replaced by fresh medium containing 0.1% AG1×8 resin and charcoal-stripped fetal bovine serum, and cells were incubated in the presence or absence of T3 (5 nm) and with or without TNFα (10 ng/ml) as indicated. Control cells were incubated with the same volume of the vehicle used to dissolve the different compounds.
GH4C1 cells were transfected by electroporation, as described previously (59) with minor modifications (25).
NF-κB reporter assays
The NF-κB reporter assay was performed as described previously (25) with minor modifications. Cells were transfected as described, and the reporter assays were performed 24 h later. Each experiment was repeated at least three times with similar differences in regulated expression, and all data are expressed as the mean ± sd.
Cell extracts and Western blot analysis
Nuclear and total cell extracts were prepared as described previously (25). For Western blot analysis, cells were incubated as described in the figure legends and then harvested in lysis buffer (25). The protein content of the cell or nuclear extracts was normalized, and the samples were separated by SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were probed with the primary antibodies indicated and were detected with a peroxidase-coupled secondary antibody. Antibody binding was visualized using the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ).
EMSA
After cell treatment, DNA binding was assessed by EMSA as previously described (25).
Real-time quantitative RT-PCR
They were performed as previously described (25).
Statistical analysis
All data are expressed as means ± sd. In statistical analysis, Student’s t test was performed using the SSC-Stat software (version 2.18; University of Reading, Reading, UK). The statistical significance of difference between groups was expressed by asterisks (*, 0.01 < P < 0.05; **, 0.001 < P < 0.01; ***, P < 0.001).
Acknowledgments
We thank Dr. Fresno and Dr. Perona for the probe for the EMSAs. We thank M. Sánchez-Prieto (Instituto de Investigaciones Biomédicas, CSIC-UAM, Madrid, Spain) for technical help.
NURSA Molecule Pages:
Ligands: Thyroid hormone.
Footnotes
This work was supported by grants from the Fundación Mutua Madrileña (2005X0615), from Fondo de Investigaciones Sanitarias (PI070832), from Ministerio de Educación y Ciencia (BFU2007-62402), from Fondo de Investigaciones Sanitarias (RD06/0020/0036) and the European grant CRESCENDO (FP-018652). M.L. is recipient of a grant from the Spanish Ministerio de Educación y Ciencia (“Ramón y Cajal” Program).
Present address for M.P.: Cancer Epigenetics and Biology Program (PEBC), Bellvitge Biomedical Research Institute (IDIBELL), Barcelona, Spain.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 23, 2009
Abbreviations: CREB, cAMP response element binding protein; DUSP1, MAPK phosphatase 1; IκK, IκB kinase; JNK, c-Jun N-terminal kinase; MKK6, MAPK kinase 6; MSK1, mitogen- and stress-associated protein kinase 1; NF-κB, nuclear factor-κB; QRT-PCR, quantitative RT-PCR; siRNA, small interfering RNA; TK, thymidine kinase.
References
- 1.Aranda A, Pascual A2001. Nuclear hormone receptors and gene expression. Physiol Rev 81:1269–1304 [DOI] [PubMed] [Google Scholar]
- 2.Tashjian Jr AH1979. Clonal strains of hormone-producing pituitary cells. Methods Enzymol 58:527–535 [DOI] [PubMed] [Google Scholar]
- 3.Li H, Lin X2008. Positive and negative signaling components involved in TNFα-induced NF-κB activation. Cytokine 41:1–8 [DOI] [PubMed] [Google Scholar]
- 4.Arras M, Höche A, Bohle R, Eckert P, Riedel W, Schaper J1996. Tumor necrosis factor-α in macrophages of heart, liver, kidney, and in the pituitary gland. Cell Tissue Res 285:39–49 [DOI] [PubMed] [Google Scholar]
- 5.Nadeau S, Rivest S1999. Regulation of the gene encoding tumor necrosis factor alpha (TNF-α) in the rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol 58:61–77 [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H, Fukata J, Murakami N, Usui T, Ebisui O, Muro S, Hanaoka I, Inoue K, Imura H, Nakao K1997. Tumor necrosis factor receptors in the pituitary cells. Brain Res 758:45–50 [DOI] [PubMed] [Google Scholar]
- 7.Wolvers DA, Marquette C, Berkenbosch F, Haour F1993. Tumor necrosis factor-α: specific binding sites in rodent brain and pituitary gland. Eur Cytokine Network 4:377–381 [PubMed] [Google Scholar]
- 8.Candolfi M, Jaita G, Pisera D, Ferrari L, Barcia C, Liu C, Yu J, Liu G, Castro MG, Seilicovich A2006. Adenoviral vectors encoding tumor necrosis factor-α and FasL induce apoptosis of normal and tumoral anterior pituitary cells. J Endocrinol 189:681–690 [DOI] [PubMed] [Google Scholar]
- 9.Friedrichsen S, Harper CV, Semprini S, Wilding M, Adamson AD, Spiller DG, Nelson G, Mullins JJ, White MR, Davis JR2006. Tumor necrosis factor-α activates the human prolactin gene promoter via nuclear factor-κB signaling. Endocrinology 147:773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karin M, Gallagher E2009. TNFR signaling: ubiquitin-conjugated TRAFfic signals control stop-and-go for MAPK signaling complexes. Immunol Rev 228:225–240 [DOI] [PubMed] [Google Scholar]
- 11.Keyse SM2000. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 12:186–192 [DOI] [PubMed] [Google Scholar]
- 12.Wu JJ, Zhang L, Bennett AM2005. The noncatalytic amino terminus of mitogen-activated protein kinase phosphatase 1 directs nuclear targeting and serum response element transcriptional regulation. Mol Cell Biol 25:4792–4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong FY, Shi YF, Deng JY2006. The regulatory mechanism by which interleukin-6 stimulates GH-gene expression in rat GH3 cells. J Endocrinol 190:397–406 [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Jiang Y, Ko WK, Li W, Wong AO2005. Paracrine regulation of growth hormone gene expression by gonadotrophin release in grass carp pituitary cells: functional implications, molecular mechanisms and signal transduction. J Mol Endocrinol 34:415–432 [DOI] [PubMed] [Google Scholar]
- 15.Roberson MS, Zhang T, Li HL, Mulvaney JM1999. Activation of the p38 mitogen-activated protein kinase pathway by gonadotropin-releasing hormone. Endocrinology 140:1310–1318 [DOI] [PubMed] [Google Scholar]
- 16.Israël A2000. The IKK complex: an integrator of all signals that activate NF-κB? Trends Cell Biol 10:129–133 [DOI] [PubMed] [Google Scholar]
- 17.Grandison L, Nolan GP, Pfaff DW1994. Activation of the transcription factor NF-κB in GH3 pituitary cells. Mol Cell Endocrinol 106:9–15 [DOI] [PubMed] [Google Scholar]
- 18.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G1998. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κB p65 transactivation mediated by tumor necrosis factor. J Biol Chem 273:3285–3290 [DOI] [PubMed] [Google Scholar]
- 19.Carter AB, Knudtson KL, Monick MM, Hunninghake GW1999. The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression. The role of TATA-binding protein (TBP). J Biol Chem 274:30858–30863 [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G2003. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J 22:1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark AR2007. Anti-inflammatory functions of glucocorticoid- induced genes. Mol Cell Endocrinol 275:79–97 [DOI] [PubMed] [Google Scholar]
- 22.Vanden Berghe W, Vermeulen L, Delerive P, De Bosscher K, Staels B, Haegeman G2003. A paradigm for gene regulation: inflammation, NF-κB and PPAR. Adv Exp Med Biol 544:181–196 [DOI] [PubMed] [Google Scholar]
- 23.Norata GD, Tibolla G, Seccomandi PM, Poletti A, Catapano AL2006. Dihydrotestosterone decreases tumor necrosis factor-α and lipopolysaccharide-induced inflammatory response in human endothelial cells. J Clin Endocrinol Metab 91:546–554 [DOI] [PubMed] [Google Scholar]
- 24.Candolfi M, Jaita G, Zaldivar V, Zárate S, Ferrari L, Pisera D, Castro MG, Seilicovich A2005. Progesterone antagonizes the permissive action of estradiol on tumor necrosis factor-α-induced apoptosis of anterior pituitary cells. Endocrinology 146:736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiloeches A, Sánchez-Pacheco A, Gil-Araujo B, Aranda A, Lasa M2008. Thyroid hormone-mediated activation of the ERK/dual specificity phosphatase 1 pathway augments the apoptosis of GH4C1 cells by down-regulating nuclear factor-κB activity. Mol Endocrinol 22:2466–2480 [DOI] [PubMed] [Google Scholar]
- 26.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183 [DOI] [PubMed] [Google Scholar]
- 27.Méndez-Pertuz M, Sánchez-Pacheco A, Aranda A2003. The thyroid hormone receptor antagonizes CREB-mediated transcription. EMBO J 22:3102–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castillo AI, Aranda A1997. Differential regulation of pituitary-specific gene expression by insulin-like growth factor 1 in rat pituitary GH4C1 and GH3 cells. Endocrinology 138:5442–5451 [DOI] [PubMed] [Google Scholar]
- 29.Kanasaki H, Yonehara T, Yamada Y, Takahashi K, Hata K, Fujiwaki R, Yamamoto H, Takeuchi Y, Fukunaga K, Miyamoto E, Miyazaki K2002. Regulation of gonadotropin α subunit gene expression by dopamine D(2) receptor agonist in clonal mouse gonadotroph αT3-1 cells. Biol Reprod 67:1218–1224 [DOI] [PubMed] [Google Scholar]
- 30.Gong FY, Deng JY, Shi YF2005. Stimulatory effect of interleukin-1β on growth hormone gene expression and growth hormone release from rat GH3 cells. Neuroendocrinology 81:217–228 [DOI] [PubMed] [Google Scholar]
- 31.Lasa M, Brook M, Saklatvala J, Clark AR2001. Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol Cell Biol 21:771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR2002. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol 22:7802–7811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR2006. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med 203:1883–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desreumaux P, Dubuquoy L, Nutten S, Peuchmaur M, Englaro W, Schoonjans K, Derijard B, Desvergne B, Wahli W, Chambon P, Leibowitz MD, Colombel JF, Auwerx J2001. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor γ (PPARγ) heterodimer. A basis for new therapeutic strategies. J Exp Med 193:827–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R2008. TLR-4, IL-1R and TNF-R signaling to NF-κB: variations on a common theme. Cell Mol Life Sci 65:2964–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calleros L, Lasa M, Toro MJ, Chiloeches A2006. Low cell cholesterol levels increase NFκB activity through a p38 MAPK-dependent mechanism. Cell Signal 18:2292–2301 [DOI] [PubMed] [Google Scholar]
- 37.Baeza-Raja B, Munoz-Cánoves P2004. p38 MAPK-induced nuclear factor-κB activity is required for skeletal muscle differentiation: role of interleukin-6. Mol Biol Cell 15:2013–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Bosscher K, Vanden Berghe W, Haegeman G2003. The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev 24:488–522 [DOI] [PubMed] [Google Scholar]
- 39.Kalkhoven E, Wissink S, van der Saag PT, van der Burg B1996. Negative interaction between the RelA(p65) subunit of NF-κB and the progesterone receptor. J Biol Chem 271:6217–6224 [DOI] [PubMed] [Google Scholar]
- 40.Palvimo JJ, Reinikainen P, Ikonen T, Kallio PJ, Moilanen A, Jänne OA1996. Mutual transcriptional interference between RelA and androgen receptor. J Biol Chem 271:24151–24156 [DOI] [PubMed] [Google Scholar]
- 41.Bui NT, Livolsi A, Peyron JF, Prehn JH2001. Activation of nuclear factor κB and Bcl-x survival gene expression by nerve growth factor requires tyrosine phosphorylation of IκBα. J Cell Biol 152:753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Béraud C, Henzel WJ, Baeuerle PA1999. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-κB activation. Proc Natl Acad Sci USA 96:429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF1996. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell 86:787–798 [DOI] [PubMed] [Google Scholar]
- 44.Sakai A, Han J, Cato AC, Akira S, Li JD2004. Glucocorticoids synergize with IL-1β to induce TLR2 expression via MAP kinase phosphatase-1-dependent dual inhibition of MAPK JNK and p38 in epithelial cells. BMC Mol Biol 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubuquoy L, Dharancy S, Nutten S, Pettersson S, Auwerx J, Desreumaux P2002. Role of peroxisome proliferator-activated receptor γ and retinoid X receptor heterodimer in hepatogastroenterological diseases. Lancet 360:1410–1418 [DOI] [PubMed] [Google Scholar]
- 46.Lee MY, Jung SC, Lee JH, Han HJ2008. Estradiol-17β protects against hypoxia-induced hepatocyte injury through ER-mediated upregulation of Bcl-2 as well as ER-independent antioxidant effects. Cell Res 18:491–499 [DOI] [PubMed] [Google Scholar]
- 47.Fürst R, Schroeder T, Eilken HM, Bubik MF, Kiemer AK, Zahler S, Vollmar AM2007. MAPK phosphatase-1 represents a novel anti-inflammatory target of glucocorticoids in the human endothelium. FASEB J 21:74–80 [DOI] [PubMed] [Google Scholar]
- 48.Kiemer AK, Weber NC, Fürst R, Bildner N, Kulhanek-Heinze S, Vollmar AM2002. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-α-induced actin polymerization and endothelial permeability. Circ Res 90:874–881 [DOI] [PubMed] [Google Scholar]
- 49.Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S2008. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-κB signaling pathways. J Biol Chem 283:11535–11540 [DOI] [PubMed] [Google Scholar]
- 50.Jang BC, Lim KJ, Suh MH, Park JG, Suh SI2007. Dexamethasone suppresses interleukin-1β-induced human β-defensin 2 mRNA expression: involvement of p38 MAPK, JNK, MKP-1, and NF-κB transcriptional factor in A549 cells. FEMS Immunol Med Microbiol 51:171–184 [DOI] [PubMed] [Google Scholar]
- 51.Issa R, Xie S, Khorasani N, Sukkar M, Adcock IM, Lee KY, Chung KF2007. Corticosteroid inhibition of growth-related oncogene protein-α via mitogen-activated kinase phosphatase-1 in airway smooth muscle cells. J Immunol 178:7366–7375 [DOI] [PubMed] [Google Scholar]
- 52.Kosaka Y, Calderhead DM, Manning EM, Hambor JE, Black A, Geleziunas R, Marcu KB, Noelle RJ1999. Activation and regulation of the IκB kinase in human B cells by CD40 signaling. Eur J Immunol 29:1353–1362 [DOI] [PubMed] [Google Scholar]
- 53.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV1997. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science 278:866–869 [DOI] [PubMed] [Google Scholar]
- 54.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860–866 [DOI] [PubMed] [Google Scholar]
- 55.Tanoue T, Yamamoto T, Nishida E2002. Modular structure of a docking surface on MAPK phosphatases. J Biol Chem 277:22942–22949 [DOI] [PubMed] [Google Scholar]
- 56.Yano O, Kanellopoulos J, Kieran M, Le Bail O, Israël A, Kourilsky P1987. Purification of KBF1, a common factor binding to both H-2 and β2-microglobulin enhancers. EMBO J 6:3317–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmitz ML, Baeuerle PA1991. The p65 subunit is responsible for the strong transcription activating potential of NF-κB. EMBO J 10:3805–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perona R, Montaner S, Saniger L, Sánchez-Pérez I, Bravo R, Lacal JC1997. Activation of the nuclear factor-κB by Rho, CDC42, and Rac-1 proteins. Genes Dev 11:463–475 [DOI] [PubMed] [Google Scholar]
- 59.Flug F, Copp RP, Casanova J, Horowitz ZD, Janocko L, Plotnick M, Samuels HH1987. cis-acting elements of the rat growth hormone gene which mediate basal and regulated expression by thyroid hormone. J Biol Chem 262:6373–6382 [PubMed] [Google Scholar]