Abstract
Pasireotide (SOM230) is currently under clinical evaluation as a successor compound to octreotide for the treatment of acromegaly, Cushing’s disease, and carcinoid tumors. Whereas octreotide acts primarily via the sst2A somatostatin receptor, pasireotide was designed to exhibit octreotide-like sst2A activity combined with enhanced binding to other somatostatin receptor subtypes. In the present study, we used phophosite-specific antibodies to examine agonist-induced phosphorylation of the rat sst2A receptor. We show that somatostatin and octreotide stimulate the complete phosphorylation of a cluster of four threonine residues within the cytoplasmic 353TTETQRT359 motif in a variety of cultured cell lines in vitro as well as in intact animals in vivo. This phosphorylation was mediated by G protein-coupled receptor kinases (GRK) 2 and 3 and followed by rapid cointernalization of the receptor and ß-arrestin into the same endocytic vesicles. In contrast, pasireotide failed to promote substantial phosphorylation and internalization of the rat sst2A receptor. In the presence of octreotide or SS-14, SOM230 showed partial agonist behavior, inhibiting phosphorylation, and internalization of sst2A. Upon overexpression of GRK2 or GRK3, pasireotide stimulated selective phosphorylation of Thr356 and Thr359 but not of Thr353 or Thr354 within the 353TTETQRT359 motif. Pasireotide-mediated phosphorylation led to the formation of relatively unstable ß-arrestin-sst2A complexes that dissociated at or near the plasma membrane. Thus, octreotide and pasireotide are equally active in inducing classical G protein-dependent signaling via the sst2A somatostatin receptor. Yet, we find that they promote strikingly different patterns of sst2A receptor phosphorylation and, hence, stimulate functionally distinct pools of ß-arrestin.
Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation, beta-arrestin trafficking and internalization.
Somatostatin regulates the release of many peptide hormones, including GH, thyrotropin, ACTH, glucagon, insulin, gastrin, and ghrelin (1). The biological actions of somatostatin are mediated by five G protein-coupled receptors designated sst1 through sst5. Two variants of the sst2 receptor are present: the unspliced sst2A form and the spliced sst2B form, which carries a different carboxyl terminus. Somatostatin is rapidly degraded in human plasma limiting its clinical utility. In the past, two metabolically stable somatostatin analogs, octreotide and lanreotide, were approved for clinical use. Octreotide and lanreotide bind with high subnanomolar affinity to sst2 only, have moderate affinity to sst3 and sst5, and show very low or absent binding to sst1 and sst4. Octreotide and lanreotide are first line agents in the medical management of neuroendocrine tumors such as GH-secreting adenomas and carcinoid tumors (2, 3). Octreotide provides initial control of symptoms caused by hormonal overproduction in about 90% of carcinoid patients. However, after 1 yr of treatment, some 50% of patients show an escape of response (3, 4). In acromegaly, octreotide normalizes GH levels in only 65% of patients (2). Octreotide response in these patients clearly depends on the presence of sst2A receptors (5). In one-third of acromegalic patients who do not respond to octreotide, there is diminished expression of sst2A but persistent sst5 expression (5). In Cushing’s disease, a condition with predominant sst5 expression, octreotide has no suppressive effect on ACTH (6, 7, 8).
Recently, the novel multireceptor somatostatin analog, pasireotide (SOM230), has been synthesized (9, 10). SOM230 is a cyclohexapeptide, which binds with high affinity to all somatostatin receptors except to sst4 (11). In contrast to octreotide, SOM230 exhibits improved metabolic stability and particularly high subnanomolar affinity to sst5 (12). SOM230 is currently under preclinical and clinical evaluation for use in the treatment of acromegaly, Cushing’s disease, and carcinoid tumors (13, 14, 15, 16, 17, 18, 19, 20, 21). However, little is known about the effects of SOM230 in the regulation of individual somatostatin receptor subtypes. In a recent in vitro study, we show that, when compared with octreotide, SOM230 is more potent in inducing internalization and signaling of human sst3 and sst5 receptors but less potent than octreotide in inducing internalization and signaling of the human sst2 receptor (22).
We recently showed that the rat sst2A receptor undergoes rapid agonist-induced phosphorylation (23). Analysis of serial truncation and site-directed mutants suggests that the sst2A receptor is predominantly phosphorylated at a cluster of four threonine residues, namely Thr353, Thr354, Thr356, and Thr359, within the cytoplasmic 353TTETQRT359 motif (24). Phosphorylation of this cluster of threonine residues was required for the formation of stable ß-arrestin complexes and subsequent cointernalization of the sst2A receptor and ß-arrestin into the same endocytic vesicles (24). In the present study, we generated and extensively characterized phophosite-specific antibodies directed against the 353TTETQRT359 motif. Generation of these phophosite-specific antibodies allowed us to examine the spatial and temporal dynamics of agonist-driven phosphorylation of individual phosphate acceptor sites within the rat sst2A receptor. Our findings reveal previously unappreciated differences in sst2A receptor phosphorylation and trafficking induced by SOM230 and octreotide.
Results
Agonist-selective phosphorylation and internalization of the sst2A receptor in vitro
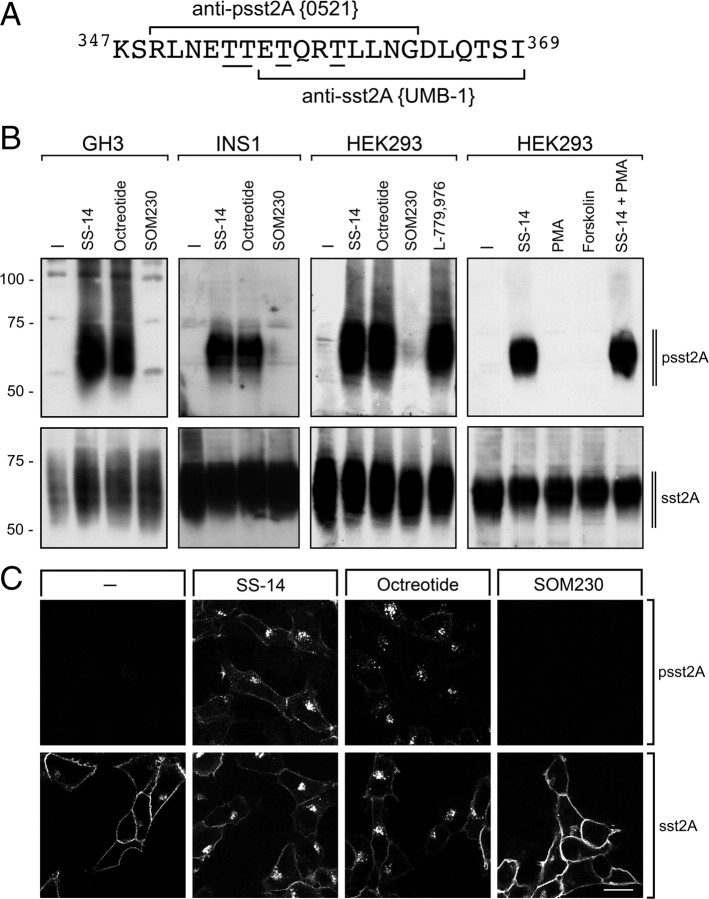
We recently demonstrated that the cytoplasmic 353TTETQRT359 motif serves as the primary site of agonist-dependent phosphorylation of the sst2A receptor (24). To examine the temporal and spatial dynamics of sst2A phosphorylation, we generated phosphosite-specific antibodies directed against phospho-threonine residues within the 353TTETQRT359 motif. Figure 1A shows the identity of the peptide used for immunizations. When rat pituitary adenoma GH3 cells were transiently transfected with the rat sst2A receptor and subjected to a 5-min treatment with different agonists, 1 μm SS-14 and 1 μm octreotide induced a robust phosphorylation of the sst2A receptor (Fig. 1B). In contrast, no phosphorylation was detectable in untreated cells. Unexpectedly, a saturating concentration of SOM230 (10 μm) did not cause any detectable phosphorylation within the 353TTETQRT359 motif. Similarly, in rat pancreatic insulinoma INS1 cells, which endogenously express the sst2A receptor, subjected to a 5-min treatment with different agonists, only SS-14 and octreotide but not SOM230 induced a robust phosphorylation of the sst2A receptor (Fig. 1B). We then examined sst2A phosphorylation in HEK293 cells stably expressing the rat sst2A receptor (Fig. 1B). Again, SS-14, octreotide, and the sst2-selective agonist L-779,976 induced strong sst2A phosphorylation. In contrast, no signal was detectable in untreated or SOM230-treated cells. Phosphorylation of the sst2A receptor was also not detectable after prolonged treatment with SOM230 (data not shown). Agonist-induced sst2A phosphorylation was dose-dependent. Whereas SS-14 in concentrations as low as 1 nm stimulated clearly detectable 353TTETQRT359 phosphorylation, 1 μm SS-14 stimulated a maximal phosphorylation of the sst2A receptor within 1 min of exposure (supplemental Figs. S1 and S2, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Phosphorylation of G protein-coupled receptors can occur via specific G protein-coupled receptor kinases (GRKs) or second messenger-activated kinases, e.g. protein kinase (PK) A or PKC. We therefore treated stable HEK293 cells with forskolin, phorbol 12-myristate 13-acetate (PMA), or the combination of PMA and SS-14 and examined sst2A phosphorylation. Neither forskolin nor PMA produced a detectable phosphorylation of the 353TTETQRT359 motif (Fig. 1B). Combined treatment with SS-14 and PMA also did not induce sst2A phosphorylation to an extent above that observed with SS-14 alone, indicating that the 353TTETQRT359 motif is not a substrate for phosphorylation by PKA or PKC. Nevertheless, previous evidence indicates that PMA-induced activation of PKC can stimulate substantial sst2A receptor phosphorylation and internalization (23, 25). In fact, a recent study suggests that PKC-mediated sst2A regulation is primarily mediated by phosphorylation of Ser343 (26). We next employed immunocytochemistry to examine the spatial distribution of phosphorylated sst2A receptors in HEK293 cells. As depicted in Fig. 1C, upper panel, 1 μm SS-14 and 1 μm octreotide stimulated a phosphorylation of the sst2A receptor. Phosphorylated sst2A receptors were initially seen at the plasma membrane. Within 5 min of treatment, phosphorylated sst2A receptors were predominantly confined to vesicle-like structures within the cytosol (Fig. 1C, upper panel). In contrast, phosphorylated sst2A receptors were not detectable in untreated cells or in cells exposed to 10 μm SOM230 (Fig. 1C, upper panel). Detection of total cellular receptors with a phosphorylation state-independent antibody revealed that SS-14 and octreotide induced rapid endocytosis of the sst2A receptor. In contrast, saturating concentrations of SOM230 were not able to stimulate the internalization of sst2A receptors under otherwise identical conditions (Fig. 1C, lower panel).
Fig. 1.
Agonist-selective phosphorylation and internalization of the sst2A receptor in vitro. A, Carboxyl-terminal amino acids of sst2A are depicted. Peptides used for immunization of rabbits are marked for anti-psst2A (0521) and anti-sst2A (UMB-1). The underlined phosphate acceptor sites were phosphorylated for generation of anti-psst2A. B, Rat pituitary GH3 cells were transiently transfected with the rat sst2A receptor. Cells were either not exposed or exposed to 1 μm SS-14, 1 μm octreotide, or 10 μm SOM230 for 5 min (left panel). B, Pancreatic insulinoma INS1 cells which endogenously express the rat sst2A receptor were either not exposed or exposed to 1 μm SS-14, 1 μm octreotide, or 10 μm SOM230 for 5 min (second panel). B, HEK293 cells stably expressing the rat sst2A receptor were either not exposed or exposed to 1 μm SS-14, 1 μm octreotide, 10 μm SOM230, 1 μm L-779,976, 100 nm PMA, 10 μm forskolin, or 1 μm SS-14 and 100 nm PMA for 5 min (third and fourth panels). The levels of phosphorylated sst2A receptors (upper panel) and total sst2A receptors (lower panel) were then determined by Western blot analysis. The Western blots shown are representative for two to three independent experiments each. The positions of the molecular mass markers are indicated on the left (in kilodaltons). C, HEK293 cells stably expressing the rat sst2A receptor were exposed to 1 μm SS-14, 1 μm octreotide, or 10 μm SOM230 for 5 min. Cells were then fixed and incubated with anti-psst2A (upper panel) or anti-sst2A (lower panel), processed for immunofluorescence, and examined by confocal microscopy. Shown are representative images from one of five independent experiments performed in duplicate. Scale bar, 20 μm.
Agonist-selective phosphorylation and internalization of the sst2A receptor in vivo
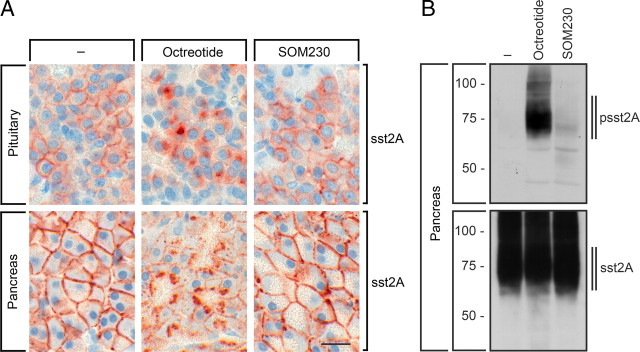
These striking differences of octreotide- and SOM230-induced sst2A phosphorylation and internalization observed in a variety of cultured cell lines were unexpected. We therefore examined in vivo internalization and phosphorylation of sst2A receptors after sc application of octreotide or SOM230 at doses known to inhibit pituitary hormone secretion (9). In untreated rats, sst2A receptors were localized at the plasma membrane both in the anterior pituitary and exocrine pancreas, as determined by immunohistochemistry using the phosphorylation state-independent sst2A antibody UMB-1 (Fig. 2A). A dramatic redistribution of sst2A receptors from the plasma membrane into the cytosol was observed 90 min after sc injection of octreotide (Fig. 2A). In contrast, no change in the subcellular distribution of sst2A receptors was observed 90 min after sc injection of SOM230 (Fig. 2A). We then examined in vivo phosphorylation of sst2A receptors in rat pancreas 30 min after sc administration of octreotide or SOM230. The results depicted in Fig. 2B reveal that octreotide stimulated a robust phosphorylation of the 353TTETQRT359 motif in intact animals. In contrast, sst2A receptor phosphorylation was not detectable in untreated or SOM230-treated rats (Fig. 2B).
Fig. 2.
Agonist-selective phosphorylation and internalization of the sst2A receptor in vivo. A, Rats were killed at 90 min after injection of octreotide (50 μg/kg) or SOM230 (50 μg/kg). Pituitary and pancreas of each animal were collected, fixed in formalin, and embedded in paraffin for immunohistochemical staining using the phosphorylation-state independent antibody UMB-1. Note that octreotide but not SOM230 stimulated a robust redistribution of sst2A receptors from the plasma membrane into the cytosol in both tissues. In contrast, SOM230 was not able to stimulate in vivo internalization of sst2A. Scale bar, 10 μm. B, Rats were killed at 30 min after injection of octreotide (50 μg/kg) or SOM230 (50 μg/kg). The pancreas of each animal was collected. The levels of phosphorylated sst2A receptors (upper panel) and total sst2A receptors (lower panel) were then determined by Western blot analysis. Note that octreotide stimulated a robust phosphorylation of sst2A receptors. In contrast, SOM230 was not able to stimulate in vivo phosphorylation of sst2A. The positions of the molecular mass markers are indicated on the left (in kilodaltons).
GRK2 and GRK3 are responsible for agonist-induced sst2A phosphorylation
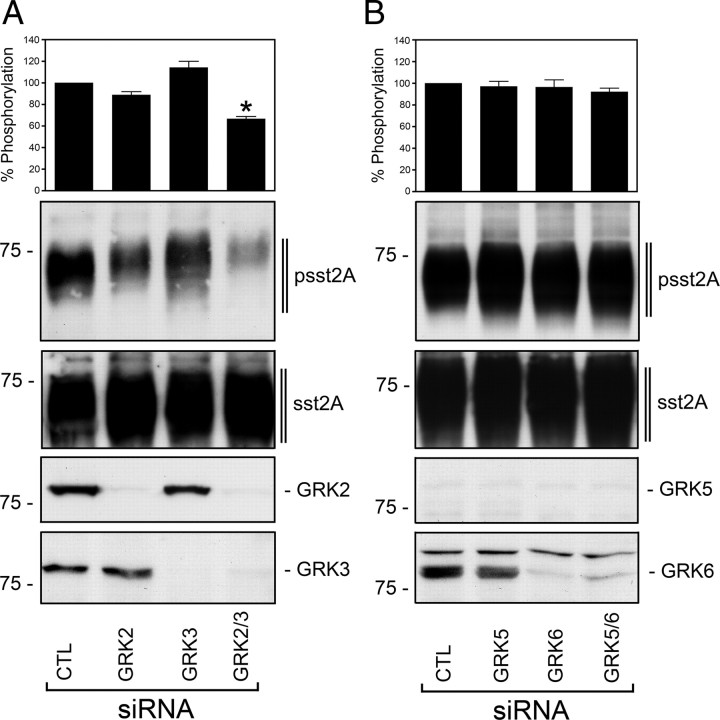
PKA and PKC did not appear to play a major role in agonist-induced phosphorylation of the carboxyl-terminal 353TTETQRT359 motif. We next used specific small interference RNA sequences to evaluate the contribution of individual GRKs to agonist-induced sst2A phosphorylation. Inhibition of GRK2 or GRK3 expression did not produce a significant reduction in SS-14-induced sst2A phosphorylation (Fig. 2A). Given the close relation of GRK2 and GRK3, it is conceivable that the loss of one GRK could be compensated by another GRK. We therefore examined the effect of inhibition of both GRK2 and GRK3 expression. The results show that the combined administration of GRK2 and GRK3 small interfering RNAs (siRNAs) led to a 40% reduction of sst2A phosphorylation, indicating that GRK2 and GRK3 function as a redundant phosphorylation system for sst2A (Fig. 2A). The same approach performed for GRK5 and GRK6 under otherwise identical conditions revealed no significant alteration of sst2A phosphorylation (Fig. 2B). It has recently been reported that low levels of Raf kinase inhibitory protein (RKIP) in GH-secreting pituitary adenomas correlate with poor response to octreotide treatment (27). Whereas nonphosphorylated RKIP binds to and inhibits Raf1 kinase, and thereby attenuates MAPK signaling, phosphorylated RKIP inhibits GRK2 (28). We have therefore examined the degree of regulation exerted by RKIP levels on 353TTETQRT359 motif phosphorylation. As depicted in supplemental Fig. S3, neither siRNA knockdown nor overexpression of RKIP modulated sst2A receptor phosphorylation in our cell system.
All four threonine residues within 353TTETQRT359 are phosphorylated
We exchanged different numbers of threonines to alanines by site-directed mutagenesis to examine the epitope of the anti-psst2A antibody in more detail (Fig. 3A). As expected, the exchange of all four threonine residues completely diminished sst2A phosphorylation. In contrast, the mutants in which the first two (353tteaqra359) or the last two (353AAETQRT359) threonines were retained still exhibited detectable phosphorylation, suggesting that all four threonine residues within 353TTETQRT359 are phosphorylated in an agonist-dependent manner (Fig. 3A). To facilitate the detection of either phospho-Thr353 and phospho-Thr354 or phospho-Thr356 and phospho-Thr359, we used the corresponding phospho-peptides for selective immunoaffinity purification. The identity of the peptides is given in Fig. 3B. Dot blot and Western blot analyses confirmed that this approach resulted in the purification of antibodies that exert selective specificity for either psst2A(Thr353/Thr354) or psst2A(Thr356/ Thr359) (Fig. 3B).
Fig. 3.
GRK2 and GRK3 are responsible for agonist-induced sst2A phosphorylation. HEK293 cells stably expressing sst2A were transfected with the indicated siRNAs for 72 h and then exposed to 1 μm of SS-14 for 5 min. A, Transfection with siRNA targeted to GRK2, GRK3, GRK2 and GRK3, or nonsilencing siRNA control (CTL). B, Transfection with siRNA targeted to GRK5, GRK6, GRK5 and GRK6, or nonsilencing siRNA control. Equal protein levels were used for visualization of total and phosphorylated receptor levels and GRKs by Western blot analyses as described in Materials and Methods. The sst2A phosphorylation was quantified and expressed as percentage of the maximal phosphorylation in stimulated CTL siRNA-transfected cells. Data correspond to the mean ± sem from at least four independent experiments performed in duplicate. The results were analyzed by two-way ANOVA followed by the Bonferroni posttest (*, P < 0.05). Note that coexpression of GRK2 and GRK3 siRNAs resulted in a significant decrease of receptor phosphorylation. The positions of molecular mass markers are indicated on the left (in kilodaltons).
Agonist-selective patterns of sst2A phosphorylation
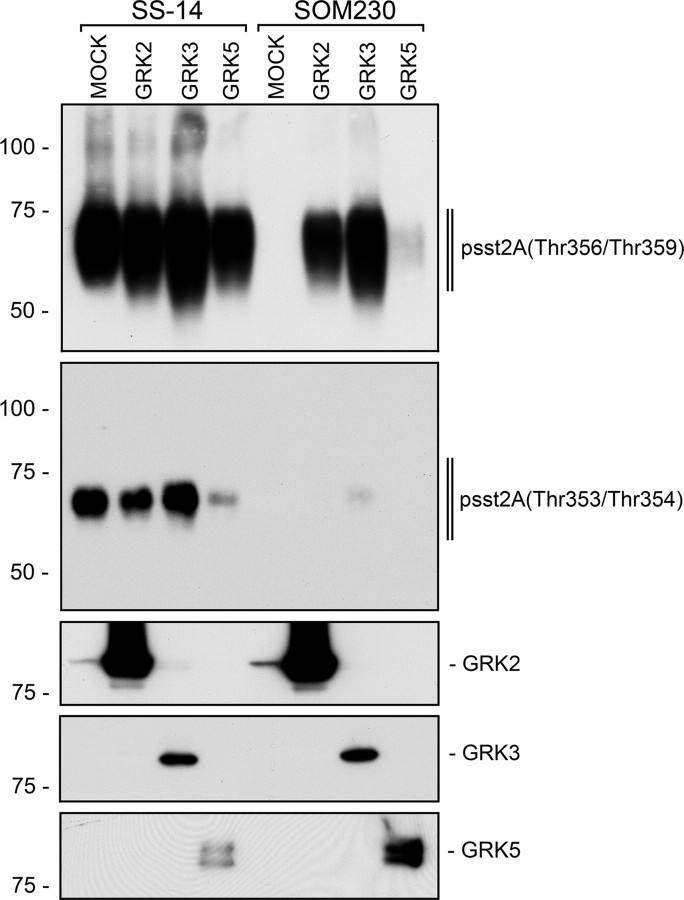
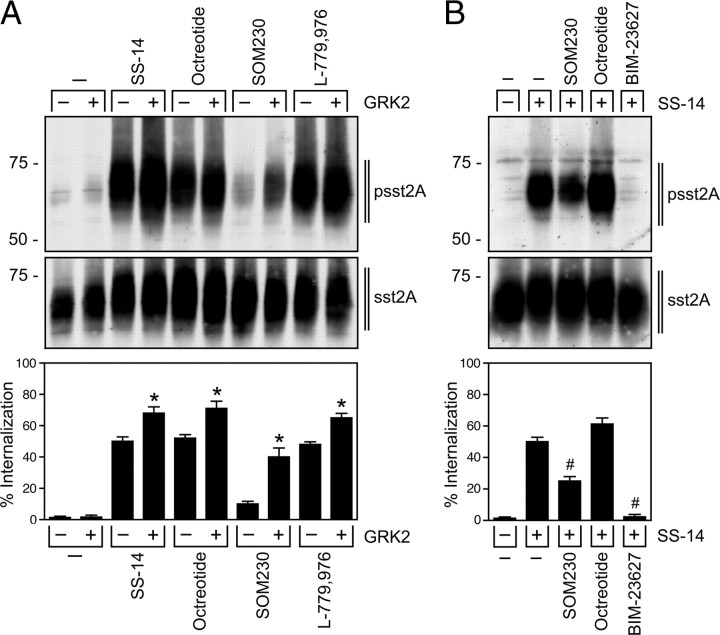
Next, we examined the effect of overexpression of GRK2, GRK3, or GRK5 on phosphorylation of the 353TTETQRT359 motif in SS-14- and SOM230-treated cells. Overexpression of GRK2 or GRK3 but not GRK5 resulted in increased SS-14-induced phosphorylation and internalization of sst2A (Figs. 4 and 5A). Interestingly, overexpression of GRK2 or GRK3 in SOM230-treated cells induced a robust phosphorylation of Thr356 and Thr359 but not of Thr353 or Thr354, indicating the existence of agonist-selective patterns of sst2A phosphorylation (Fig. 4). In the presence of endogenous GRK2, the SOM230-activated sst2A receptor showed only limited internalization. Overexpression of GRK2 not only facilitated the SOM230-stimulated phosphorylation of Thr356 and Thr359 but also the internalization of the sst2A receptor (Fig. 6A). In the presence of SS-14, SOM230 showed partial agonist behavior, inhibiting the SS-14-induced phosphorylation and internalization of sst2A (Fig. 6B). Interestingly, SOM230 also blocked octreotide-induced sst2A phosphorylation (supplemental Fig. S4). The antagonist BIM-23627 completely blocked SS-14-medited phosphorylation and internalization of sst2A (Fig. 6B).
Fig. 4.
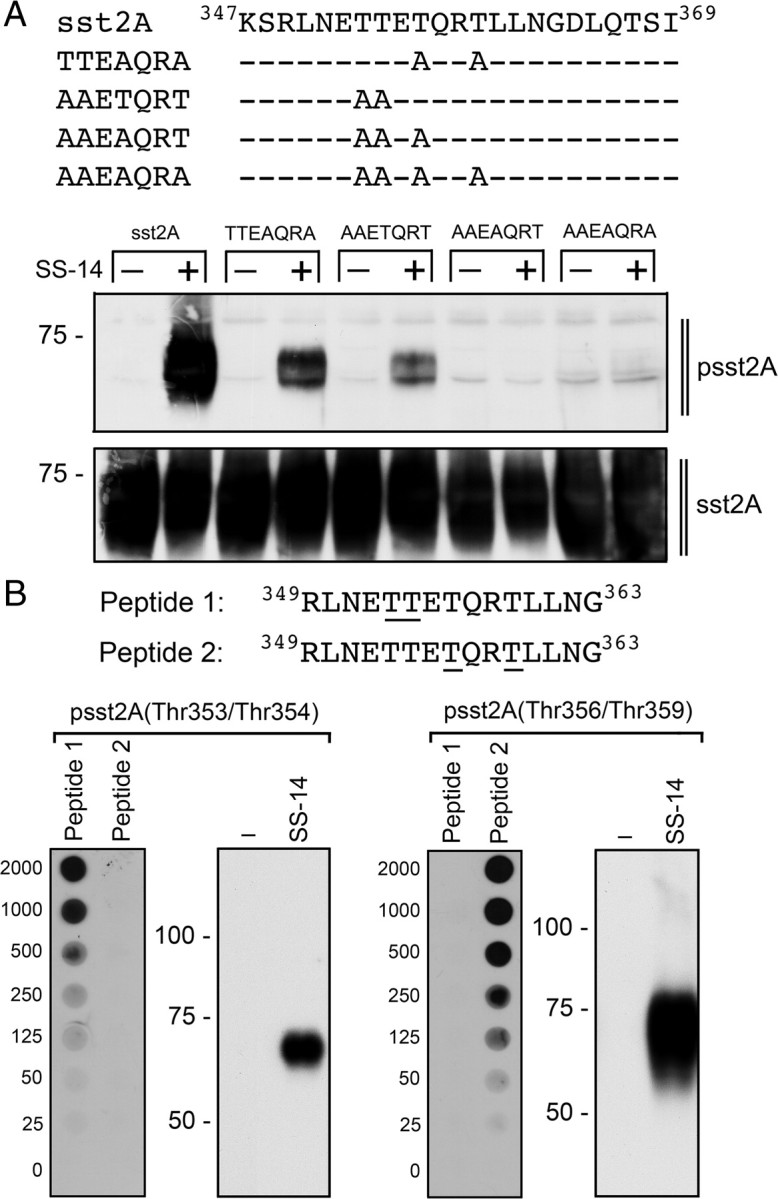
Mutational analysis of the carboxyl-terminal 353TTETQRT359 phosphorylation motif. A, Carboxyl-terminal amino acid sequences and site-directed mutants of sst2A (upper panel). HEK293 cells were stably transfected with wild-type sst2A, TTEAQRA, AAETQRT, AAEAQRT, or AAEAQRA and then treated with or without 1 μm SS-14 for 5 min (lower panel). The levels of phosphorylated sst2A receptors and total sst2A receptors were then determined by Western blot analysis. Note that the polyclonal anti-psst2A antiserum detects the phosphorylation of Thr353 and Thr354 in the TTEAQRA mutant as well as Thr356 and Thr359 in the AAETQRT mutant. B, Amino acid sequences of peptides used for immunoaffinity purification to produce anti-psst2A(Thr353/Thr354) or anti-psst2A(Thr356/Thr359) antibodies (upper panel). The underlined phosphate acceptor sites in peptide 1 and peptide 2 were phosphorylated. B, Characterization of anti-psst2A(Thr353/Thr354) and anti-psst2A(Thr356/Thr359) antibodies using Dot blot and Western blot analyses (lower panel). Dot blot: Serial dilutions (0–2000 ng) of peptide 1 and peptides 2 were blotted, and membranes were incubated with anti-psst2A(Thr353/Thr354) or anti-psst2A(Thr356/Thr359) antibodies. Western blot: HEK293 cells stably expressing sst2A were either not exposed or exposed to 1 μm SS-14 for 5 min. The levels of phosphorylated sst2A receptors were then determined using anti-psst2A(Thr353/Thr354) or anti-psst2A(Thr356/Thr359) antibodies. The positions of molecular mass markers are indicated on the left (in kilodaltons).
Fig. 5.
Agonist-selective patterns of sst2A phosphorylation. HEK293 cells stably expressing sst2A were transfected with empty vector (MOCK), GRK2, GRK3, or GRK5 for 2 d. Cells were then treated with either 1 μm SS-14 or 10 μm SOM230 for 5 min. The levels of phosphorylated sst2A receptors were determined using anti-psst2A(Thr356/Thr359) (upper panel) and anti-psst2A(Thr353/Thr354) (lower panel) antibodies. The positions of molecular mass markers are indicated on the left (in kilodaltons).
Fig. 6.
Partial agonistic properties of SOM230. A, HEK293 cells stably expressing sst2A were transfected with empty vector (MOCK) or GRK2 for 2 d. Cells were either not exposed or exposed to 1 μm SS-14, 1 μm octreotide, 10 μm SOM230, or 1 μm L-779,976 for 5 min. The levels of phosphorylated sst2A receptors and total sst2A receptors were determined by Western blot analysis (upper panel). A, HEK293 cells stably expressing sst2A were transfected with empty vector (MOCK) or GRK2 for 2 d. Cells were either not exposed or exposed to 1 μm SS-14, 1 μm octreotide, 10 μm SOM230, or 1 μm L-779,976 for 20 min. Receptor sequestration, quantified as the percent loss of cell-surface receptors in agonist-treated cells, was measured by ELISA (lower panel). B, HEK293 cells stably expressing sst2A were either not exposed or exposed to 1 μm octreotide, 10 μm SOM230, or 10 μm BIM-23627 for 5 min, and then treated with 1 μm SS-14 for an additional 5 min in the presence of octreotide, SOM230, or BIM-23627. The levels of phosphorylated sst2A receptors and total sst2A receptors were determined by Western blot analysis (upper panel). B, HEK293 cells stably expressing sst2A were either not exposed or exposed to 1 μm octreotide, 10 μm SOM230, or 10 μm BIM-23627 for 5 min and then treated with 1 μm SS-14 an additional 20 min in the presence of octreotide, SOM230, or BIM-23627. Receptor sequestration, quantified as the percent loss of cell-surface receptors in agonist-treated cells, was measured by ELISA (lower panel). The Western blots shown are representative for two independent experiments each. ELISA data are presented as the mean ± sem from four independent experiments performed in quadruplicate. The results were analyzed by two-way ANOVA followed by the Bonferroni posttest (*, P < 0.05, significant increase compared with untreated cells; #, P < 0.05, significant decrease compared with SS-14-stimulated cells). Note that overexpression of GRK2 facilitated phosphorylation and internalization of the SOM-activated sst2A receptor, and that SS-14-induced phosphorylation and internalization was effectively inhibited by SOM230.
SS-14 and SOM230 induce distinct patterns of ß-arrestin trafficking
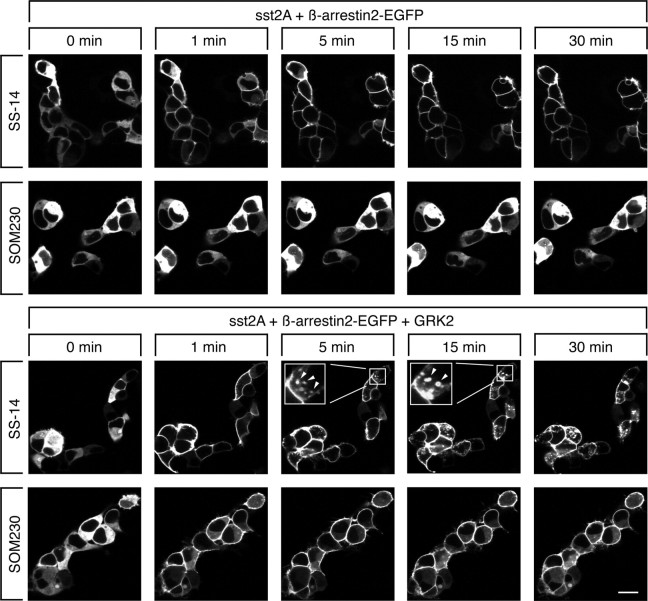
Given the capacities of SS-14 and SOM230 to stimulate different patterns of sst2A receptor phosphorylation and endocytosis, we employed functional ß-arrestin-2 conjugated to enhanced green fluorescent protein (EGFP) to visualize the patterns of agonist-induced ß-arrestin mobilization in live HEK293 cells. In the absence of an agonist, ß-arrestin-2-EGFP was uniformly distributed throughout the cytoplasm of the cells. In the presence of endogenous GRK2, the addition of saturating concentrations of SS-14 but not of SOM230 induced a rapid redistribution of ß-arrestin-2 from the cytoplasm to the plasma membrane resulting in fluorescence outlining the shape of the cells (Fig. 7, upper panel). In the presence of overexpressed GRK2, the addition of both SS-14 and SOM230 induced a rapid redistribution of ß-arrestin-2 from the cytoplasm to the plasma membrane (Fig. 7, lower panel). After extended agonist exposure, internalization of ß-arrestin-2-EGFP into endocytic vesicles was observed only in SS-14- but not in SOM230-treated cells (insets in Fig. 7, lower panel). Given the function of β-arrestin as scaffold to facilitate signaling pathways, we sought to compare octreotide and SOM230 for their ability to activate ERK. Surprisingly, we did not observe differential phospho-ERK levels between octreotide and SOM230 (supplemental Fig. S5). However, sst2A-mediated ERK activation was completely ablated by preincubation with pertussis toxin, indicating that it required G protein activation (supplemental Fig. S6).
Fig. 7.
SS-14 and SOM230 induce distinct patterns of ß-arrestin trafficking. HEK293 cells were transiently transfected with 2 μg of ß-arrestin-2-EGFP, 6 μg of sst2A, and 4 μg of GRK2 or 4 μg empty vector. The distribution of ß-arrestin-2 was visualized sequentially in the same live cells before (0 min) and after (1, 5, 15, and 30 min) the addition of either 1 μm SS-14 or 10 μm SOM230 to the culture medium. Shown are representative images from one of four independent experiments performed in duplicate. Scale bar, 20 μm.
Discussion
Agonist-dependent regulation of the sst2A somatostatin receptor involves rapid phosphorylation of a series of phosphate acceptor sites within the carboxyl-terminal tail of the receptor. We have previously employed of serial truncation and site-directed mutagenesis to determine that a cluster of four threonine residues within the cytoplasmic 353TTETQRT359 motif serves as the primary site of agonist-driven sst2A receptor phosphorylation (24). In the present study, we generated and extensively characterized phophosite-specific antibodies directed against the 353TTETQRT359 motif. Generation of these phophosite-specific antibodies enabled us to demonstrate that the presence of a full agonist is required for phosphorylation of the 353TTETQRT359 motif. Upon addition of SS-14, a rapid and dose-dependent sst2 receptor phosphorylation was detectable in pituitary GH3, in pancreatic insulinoma INS1, as well as in HEK293 cells. Phosphorylated sst2A receptors were initially seen at the plasma membrane; within 5 min, these receptors were translocated into vesicle-like structures within the cytosol. The sst2A receptor phosphorylation was agonist selective, i.e. SS-14, octreotide, and L-779,976 but not SOM230 were able to stimulate a substantial phosphorylation of the 353TTETQRT359 motif. There was excellent correlation between the extent of 353TTETQRT359 phosphorylation after exposure to various agonists and sst2A receptor internalization. Overexpression and siRNA knockdown experiments clearly demonstrated that 353TTETQRT359 phosphorylation is mediated by GRK2 and GRK3 but not by GRK5 or GRK6. These results not only indicate that antibody {0521} specifically detects the phosphorylated sst2A receptor but also unequivocally demonstrate that agonist-driven sst2A receptor phosphorylation primarily occurs at threonine residues within the cytoplasmic 353TTETQRT359 motif. The generation of antibodies that exert selective specificity for either psst2A(Thr353/Thr354) or psst2A(Thr356/Thr359) revealed that all four threonine residues are likely to serve as phosphate acceptor sites for GRK-mediated phosphorylation of the sst2A receptor. In contrast, the 353TTETQRT359 motif is not a substrate for second messenger-activated kinases.
Perhaps the most striking finding of the present study is that SOM230 modulates sst2A receptor phosphorylation and trafficking in a manner clearly distinct from SS-14 and octreotide. Octreotide suppresses hormonal hypersecretion in neuroendocrine tumors in a manner dependent upon the presence of functional sst2A receptors (4, 5). SOM230 is currently under clinical evaluation as a successor compound to octreotide for treatment of acromegaly, Cushing’s disease, and carcinoid tumors (2, 20, 29). SOM230 was designed to exhibit octreotide-like sst2A activity combined with enhanced binding to other somatostatin receptors (10, 11). We demonstrate that exposure to SS-14 or octreotide leads to rapid and robust phosphorylation of all four threonine residues within the 353TTETQRT359 motif; this is followed by ß-arrestin mobilization and subsequent cointernalization of the receptor and ß-arrestin into the same endocytic vesicles. In contrast, SOM230 failed to promote substantial phosphorylation and internalization of the rat sst2A receptor. In the presence of GRK2 or GRK3 overexpression, SOM230 stimulated a selective phosphorylation of Thr356 and Thr359 but not of Thr353 or Thr354 within the 353TTETQRT359 motif. SOM230-mediated phosphorylation led to the formation of relatively unstable ß-arrestin-sst2A complexes that dissociated at or near the plasma membrane. These findings indicate that SS-14 or octreotide-induced activation of the sst2A receptor produces a conformational change that facilitates phosphorylation of a number of carboxyl-terminal phosphate acceptor sites by GRK2 or GRK3. In contrast, the SOM230-activated sst2A receptor acquires a conformation in which only Thr356 and Thr359 are accessible for GRK-mediated phosphorylation. The fact that overexpression of GRK2 or GRK3 was required to drive SOM-induced sst2A phosphorylation and internalization indicates that sst2A receptor regulation crucially depends on the cellular content of these GRKs. Moreover, in the presence of full agonists, SOM230 behaved like a partial agonist and strongly inhibited SS-14- and octreotide-induced phosphorylation and internalization of sst2A.
These findings have important implications for the clinical utility of octreotide and SOM230. 1) Tumors which predominantly express sst2A receptors and exhibit long-lasting responses to octreotide, e.g. the majority of GH-secreting ademonas, should remain stable on octreotide. Given the partial agonistic properties of SOM230, it is conceivable that coadministration of SOM230 and octreotide may potentially limit the clinical benefit of octreotide. 2) Tumors which show an escape of response during octreotide treatment and exhibit high levels of sst5 receptors, e.g. octreotide-resistent GH ademonas and carcinoids, are likely to respond to SOM230. 3) Given the limited ability of SOM230 to internalize via the sst2A receptor, SOM230 may be less effective than octreotide for tumor imaging and sst2A receptor radiotherapy.
The present study reveals that SOM230 displays functional selectivity at the sst2A receptor. In this regard, SOM230 is similar to morphine, which activates the μ-opioid receptor without causing its rapid internalization. Interestingly, full phosphorylation of μ-opioid receptor in response to morphine required overexpression of GRK2 (30, 31). CCL21 is an endogenous CCR7 chemokine receptor ligand that failed to stimulate phosphorylation and desensitization of its cognate receptor (32, 33).
It is thought that the rate of receptor recycling and the mode of ß-arrestin-dependent signaling events are dictated by the pattern of ß-arrestin trafficking (34, 35, 36). The ß-arrestin-dependent trafficking of the SS-14-activated sst2A receptor resembles that of a class B receptor in that receptor activation resulted in the formation of stable complexes between ß-arrestin and the receptor, and these molecules internalized together into the same endocytic vesicles. In contrast, ß-arrestin mobilization of the SOM-activated sst2A receptor resembled that of a class A receptor in that upon receptor activation, ß-arrestin and the receptor formed relatively unstable complexes that dissociated at or near the plasma membrane. Consequently, in SOM-treated cells, ß-arrestin was excluded from sst2A-containing vesicles. This pattern was crucially dependent on GRK2- or GRK3-mediated phosphorylation of the 353TTETQRT359 motif. ß-Arrestins can serve as scaffolds that can bind and activate an array of signal transducers (37). Thus, the fact that SOM230 and octreotide stimulate functionally distinct pools of ß-arrestin could provide a potential explanation for the differential cellular responses observed after long-term administration.
In conclusion, we identify the cytoplasmic 353TTETQRT359 motif as the primary site of agonist-dependent phosphorylation of the sst2A receptor. We demonstrate that both SS-14 and octreotide stimulate the phosphorylation of all four threonine residues within 353TTETQRT359 motif in vitro and in vivo. We also identify GRK2 and GRK3 as kinases responsible for agonist-dependent sst2A receptor phosphorylation. SOM230 stimulated a selective phosphorylation of Thr356 and Thr359 only in the presence of GRK2 or GRK3 overexpression. Although octreotide and SOM230 have comparable effects on G protein activation, the distinct modes of sst2A receptor phosphorylation and ß-arrestin trafficking may explain some of the differences between octreotide and SOM230 observed in clinical settings.
Materials and Methods
Reagents and antibodies
SOM230 and octreotide were provided by Herbert Schmid (Novartis, Basel, Switzerland). L-779,976 was a gift from Susan Rohrer (Merck, Rahway, NJ). BIM-23627 was provided by Michael Culler (IPSEN, Milford, MA). Somatostatin (SS-14) was obtained from Bachem (Weil am Rhein, Germany). The phosphorylation-independent rabbit monoclonal anti-sst2A antibody (UMB-1) and the rabbit polyclonal anti-T7 antibody were generated and extensively characterized as previously described (24, 38, 39). Phosphosite-specific polyclonal anti-sst2A antisera were generated against the following sequence that contains four phosphorylated threonine residues: RLNE(pT)(pT)E(pT)QR(pT)LLNG. This sequence corresponds to amino acids 349–363 of the rat sst2A receptor. The peptide was purified and coupled to keyhole limpet hemocyanin. The conjugate was mixed 1:1 with Freund’s adjuvant and injected into four rabbits (0479, 0490, 0521, and 0522) for antisera production. Animals were injected at 4-wk intervals, and serum was obtained 2 wk after immunizations beginning with the second injection. The specificity of the antisera was initially tested using Dot blot analysis. For subsequent analysis, antibodies were affinity purified against their immunizing peptide as well as against the peptides, RLNE(pT)(pT)ETQRTLLNG (peptide 1) and RLNETTE(pT)QR(pT)LLNG (peptide 2), using the SulfoLink kit (Thermo Scientific, Rockford, IL). Antibodies (4465–4467) to RKIP were generated against the peptide CFQAEWDDSVPKLHDQLAGK, which corresponds to amino acids 168–187 of the rat RKIP.
Cell culture and transfection
Rat pancreatic insulinoma INS1 cells were kindly provided by Mathias Strowski (Charité, Berlin, Germany). Rat pituitary GH3 cells and human embryonic kidney HEK293 cells were obtained from the German Resource Centre for Biological Material (DSMZ, Braunschweig, Germany). INS1 cells were grown in RPMI 1640 medium supplemented 5% fetal calf serum, 1 mm sodium pyruvate, and 50 μm β-mercaptoethanol. GH3 cells were grown in Ham’s F10 medium supplemented with 15% horse serum. HEK293 cells were grown in DMEM supplemented with 10% fetal calf serum. Cells were transfected with a plasmid encoding for a T7-tagged rat sst2A receptor using LipofectAMINE 2000 according to the instructions of the manufacturer (Invitrogen, Carlsbad, CA). Stable transfectants were selected in the presence of 400 μg/ml G418. HEK293 cells stably expressing rat sst2A receptor were characterized using radioligand-binding assays, cAMP assays, Western blot analysis, and immunocytochemistry as described previously (23, 24, 39). The IC50 values of SS-14, octreotide, and SOM230 for ligand binding affinities and inhibition of forskolin-stimulated cAMP accumulation are given in Table 1. The level of sst2A receptor expression was 820 fmol/mg membrane protein. The level of sst2A receptor expression was between 1500 and 2000 fmol/mg membrane protein for all experiments using transiently transfected cells.
Table 1.
Functional properties of SS-14, octreotide, and SOM230 on the ratsst2A receptor
| SS-14 | Octreotide | SOM230 | |
|---|---|---|---|
| Binding affinity IC50 (nm) | 0.59 | 0.45 | 2.35 |
| Inhibition of cAMP accumulation IC50 (nm) | 0.48 | 0.39 | 1.91 |
Ligand binding and cAMP assays were carried out as described in Materials and Methods. The IC50 values were analyzed by nonlinear regression curve fitting using the computer program GraphPad Prism 4.0. Data are presented as the mean of three or four independent experiments. se values were smaller than 15%.
Immunocytochemistry
Cells were grown on poly-l-lysine-coated coverslips overnight. After the appropriate treatment with SS-14, SOM230, or octreotide, cells were fixed with 4% paraformaldehyde and 0.2% picric acid in phosphate buffer (pH 6.9) for 40 min at room temperature and washed several times. Specimens were permeabilized and then incubated with anti-psst2A (0521) or anti-sst2A (UMB-1) antibodies followed by cyanine 3.18-conjugated secondary antibodies (Amersham, Braunschweig, Germany). Specimens were mounted and examined using a Leica TCS SP5 laser scanning confocal microscope (22, 23, 24, 39).
Quantification of receptor internalization by ELISA
Cells expressing T7-tagged sst2A receptors were seeded onto poly-L-lysine-treated 24-well plates. The next day, the cells were preincubated with 1 μg/ml anti-T7 antibody for 2 h at 4 C. After the appropriate treatment with SS-14, SOM230, or octreotide, cells were fixed and incubated with peroxidase-conjugated antirabbit antibody for 2 h at room temperature. After washing, the plates were developed with 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid solution and analyzed at 405 nm using a microplate reader (22, 24).
In vivo internalization assay
Male Wistar rats weighing 150–200 g were used for in vivo internalization experiments. A first set of rats (three per group) was injected with octreotide (50 μg/kg body weight) or SOM230 (50 μg/kg body weight). Rats were killed at 90 min after injection. Pituitary and pancreas of each animal were collected, immersed in a 10% formalin solution for 72 h, and embedded in paraffin for immunohistochemical investigation as described (38). A second set of rats (two per group) was injected with octreotide or SOM230 as described above and killed at 30 min after injection. The pancreas of each animal was collected and immediately subjected to Western blot analysis. For all animal procedures, ethical approval was sought before the experiments according to national and institutional requirements.
ß-Arrestin-EGFP mobilization assay
HEK293 cells were seeded into 35-mm glass-bottom culture dishes (Mattek, Ashland, MA). The next day, cells were transiently cotransfected with 2 μg ß-arrestin-2-EGFP, 6 μg rat sst2A, and 4 μg GRK2 or 4 μg empty vector. After 48 h, cells were transferred onto a temperature-controlled microscope stage set at 37 C of a Leica TCS NT laser scanning confocal microscope. Images were collected sequentially using single line excitation at 488 nm with 515–540-nm band pass emission filters. Saturating concentrations of SS-14 (1 μm), SOM230 (10 μm), or octreotide (1 μm) were applied directly into the culture medium immediately after the initial image was taken (22, 24).
Western blot analysis
Cells were plated onto poly-l-lysine-coated 100-mm dishes and grown to 80% confluence. After the appropriate treatment with SS-14, SOM230, or octreotide, cells and tissues were lysed in detergent buffer [50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 5 mm EDTA, 10 mm NaF, 10 mm disodium pyrophosphate, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 0.2 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 1 μg/ml pepstatin A, 1 μg/ml aprotinin, and 10 μg/ml bacitracin]. Glycosylated proteins were partially enriched using wheat germ-lectin-agarose beads as described (5, 40, 41). Proteins were eluted from the beads using SDS-sample buffer for 20 min at 60 C and then resolved on 8% SDS-polyacrylamide gels. After electroblotting, membranes were incubated with 1 μg/ml anti-psst2A (0521) or anti-sst2A (UMB-1) antibodies followed by detection using an enhanced chemiluminescence detection system (Amersham).
siRNA silencing of gene expression
Chemically synthesized double-stranded siRNA duplexes (with 3′ dTdT overhangs) were purchased from QIAGEN (Hilden, Germany) for the following mRNA targets: GRK2 (5′-CCGGGAGAUCUUCGACUCAUA-3′), GRK3 (5′-AAGCAAGCUGUAGAACACGUA-3′), GRK5 (5′-AGCGUCAUAACUAGAACUGAA-3′), and GRK6 (5′-AACACCUUCAGGCAAUACCGA-3′), RKIP (5′-CAGGUCUACAGUGAUAGAGCA-3′) and a nonsilencing RNA duplex (5′-GCUUAGGAGCAUUAGUAAA-3′) (42). HEK293 cells were transfected with HiPerFect (QIAGEN), according to the instructions of the manufacturer for 3 d. Silencing was quantified by immunoblotting. All experiments showed protein levels reduced by ≥80%.
Measurements of cAMP accumulation
Transfected cells were seeded onto poly-l-lysine-treated 22-mm 12-well dishes. On the next day, cells were washed and then incubated with 0.5 ml of serum-free RPMI 1640 medium containing 25 μm forskolin or 25 μm forskolin plus SS-14, SOM230, or octreotide in concentrations ranging from 10−12 to 10−6 m for 15 min at 37 C. The reaction was terminated by removal of the culture medium and subsequent addition of 1 ml of ice-cold HCl/ethanol (1 volume of 1 N HCl, 100 volumes of ethanol). After centrifugation, the supernatant was evaporated, the residue was dissolved in TE buffer [50 mm Tris-EDTA (pH 7.5)], and the cAMP content was determined using a RIA as described (39).
Data analysis
ImageJ 1.39s software was used to densitize and quantify protein bands detected on Western blots. Data from cAMP and internalization assays were analyzed by nonlinear regression curve fitting using GraphPad Prism 4.0 software. Statistical analysis was carried out with two-way ANOVA followed by the Bonferroni posttest. P values below 0.05 were considered statistically significant.
Acknowledgments
We thank Abhi Rao and Dan Thorngren for assistance in the preparation of this manuscript; Chin Yo Lin for providing information about the locations of PR48, PR205, and PR221 before publication; and Karen Kieser for the pS2 vector.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft Grant SCHU924/10-1,2.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 5, 2010
Abbreviations: EGFP, Enhanced green fluorescent protein; GRK, G protein-coupled receptor kinase; PK, protein kinase; PMA, phorbol 12-myristate 13-acetate; RKIP, Raf kinase inhibitory protein; SDS, sodium dodecyl sulfate; siRNA, small interfering RNA.
References
- 1.Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C2003. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2:999–1017 [DOI] [PubMed] [Google Scholar]
- 2.Donangelo I, Melmed S2005. Treatment of acromegaly: future. Endocrine 28:123–128 [DOI] [PubMed] [Google Scholar]
- 3.Oberg K2005. Neuroendocrine tumors of the gastrointestinal tract: recent advances in molecular genetics, diagnosis, and treatment. Curr Opin Oncol 17:386–391 [DOI] [PubMed] [Google Scholar]
- 4.Asnacios A, Courbon F, Rochaix P, Bauvin E, Cances-Lauwers V, Susini C, Schulz S, Boneu A, Guimbaud R, Buscail L2008. Indium-111-pentetreotide scintigraphy and somatostatin receptor subtype 2 expression: new prognostic factors for malignant well-differentiated endocrine tumors. J Clin Oncol 26:963–970 [DOI] [PubMed] [Google Scholar]
- 5.Plöckinger U, Albrecht S, Mawrin C, Saeger W, Buchfelder M, Petersenn S, Schulz S2008. Selective loss of somatostatin receptor 2 in octreotide-resistant growth hormone-secreting adenomas. J Clin Endocrinol Metab 93:1203–1210 [DOI] [PubMed] [Google Scholar]
- 6.Labeur M, Theodoropoulou M, Sievers C, Paez-Pereda M, Castillo V, Arzt E, Stalla GK2006. New aspects in the diagnosis and treatment of Cushing disease. Front Horm Res 35:169–178 [DOI] [PubMed] [Google Scholar]
- 7.Silva AP, Schoeffter P, Weckbecker G, Bruns C, Schmid HA2005. Regulation of CRH-induced secretion of ACTH and corticosterone by SOM230 in rats. Eur J Endocrinol 153:R7–R10 [DOI] [PubMed]
- 8.van der Hoek J, Lamberts SW, Hofland LJ2004. The role of somatostatin analogs in Cushing’s disease. Pituitary 7:257–264 [DOI] [PubMed] [Google Scholar]
- 9.Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G2002. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 146:707–716 [DOI] [PubMed] [Google Scholar]
- 10.Weckbecker G, Briner U, Lewis I, Bruns C2002. SOM230: a new somatostatin peptidomimetic with potent inhibitory effects on the growth hormone/insulin-like growth factor-I axis in rats, primates, and dogs. Endocrinology 143:4123–4130 [DOI] [PubMed] [Google Scholar]
- 11.Lewis I, Bauer W, Albert R, Chandramouli N, Pless J, Weckbecker G, Bruns C2003. A novel somatostatin mimic with broad somatotropin release inhibitory factor receptor binding and superior therapeutic potential. J Med Chem 46:2334–2344 [DOI] [PubMed] [Google Scholar]
- 12.Ma P, Wang Y, van der Hoek J, Nedelman J, Schran H, Tran LL, Lamberts SW2005. Pharmacokinetic-pharmacodynamic comparison of a novel multiligand somatostatin analog, SOM230, with octreotide in patients with acromegaly. Clin Pharmacol Ther 78:69–80 [DOI] [PubMed] [Google Scholar]
- 13.Hofland LJ, van der Hoek J, Feelders R, van Aken MO, van Koetsveld PM, Waaijers M, Sprij-Mooij D, Bruns C, Weckbecker G, de Herder WW, Beckers A, Lamberts SW2005. The multi-ligand somatostatin analogue SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor type 5. Eur J Endocrinol 152:645–654 [DOI] [PubMed] [Google Scholar]
- 14.Hofland LJ, van der Hoek J, Feelders R, van der Lely AJ, de Herder W, Lamberts SW2005. Pre-clinical and clinical experiences with novel somatostatin ligands: advantages, disadvantages and new prospects. J Endocrinol Invest 28:36–42 [PubMed] [Google Scholar]
- 15.Hofland LJ, van der Hoek J, van Koetsveld PM, de Herder WW, Waaijers M, Sprij-Mooij D, Bruns C, Weckbecker G, Feelders R, van der Lely AJ, Beckers A, Lamberts SW2004. The novel somatostatin analog SOM230 is a potent inhibitor of hormone release by growth hormone- and prolactin-secreting pituitary adenomas in vitro J Clin Endocrinol Metab 89:1577–1585 [DOI] [PubMed] [Google Scholar]
- 16.Murray RD, Kim K, Ren SG, Lewis I, Weckbecker G, Bruns C, Melmed S2004. The novel somatostatin ligand (SOM230) regulates human and rat anterior pituitary hormone secretion. J Clin Endocrinol Metab 89:3027–3032 [DOI] [PubMed] [Google Scholar]
- 17.Schmid HA2008. Preclinical evidences suggest new treatment options for endocrine disorders: Pasireotide (SOM230) and Everolimus (RAD001). Ann Endocrinol 69:162–163 [DOI] [PubMed] [Google Scholar]
- 18.Schmid HA, Schoeffter P2004. Functional activity of the multiligand analog SOM230 at human recombinant somatostatin receptor subtypes supports its usefulness in neuroendocrine tumors. Neuroendocrinology 1(Suppl 80):47–50 [DOI] [PubMed] [Google Scholar]
- 19.Schmid HA, Silva AP2005. Short- and long-term effects of octreotide and SOM230 on GH, IGF-I, ACTH, corticosterone and ghrelin in rats. J Endocrinol Invest 28:28–35 [PubMed] [Google Scholar]
- 20.van der Hoek J, de Herder WW, Feelders RA, van der Lely AJ, Uitterlinden P, Boerlin V, Bruns C, Poon KW, Lewis I, Weckbecker G, Krahnke T, Hofland LJ, Lamberts SW2004. A single-dose comparison of the acute effects between the new somatostatin analog SOM230 and octreotide in acromegalic patients. J Clin Endocrinol Metab 89:638–645 [DOI] [PubMed] [Google Scholar]
- 21.van der Hoek J, Waaijers M, van Koetsveld PM, Sprij-Mooij D, Feelders RA, Schmid HA, Schoeffter P, Hoyer D, Cervia D, Taylor JE, Culler MD, Lamberts SW, Hofland LJ2005. Distinct functional properties of native somatostatin receptor subtype 5 compared with subtype 2 in the regulation of ACTH release by corticotroph tumor cells. Am J Physiol Endocrinol Metab 289:E278–E287 [DOI] [PubMed]
- 22.Lesche S, Lehmann D, Nagel F, Schmid HA, Schulz S2009. Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro J Clin Endocrinol Metab 94:654–661 [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer M, Koch T, Schröder H, Laugsch M, Höllt V, Schulz S2002. Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J Biol Chem 277:19762–19772 [DOI] [PubMed] [Google Scholar]
- 24.Tulipano G, Stumm R, Pfeiffer M, Kreienkamp HJ, Höllt V, Schulz S2004. Differential β-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem 279:21374–21382 [DOI] [PubMed] [Google Scholar]
- 25.Hipkin RW, Wang Y, Schonbrunn A2000. Protein kinase C activation stimulates the phosphorylation and internalization of the sst2A somatostatin receptor. J Biol Chem 275:5591–5599 [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Bee MS, Schonbrunn A2009. Site specificity of agonist and second messenger-activated kinases for sst2A somatostatin receptor phosphorylation. Mol Pharmacol 76:68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fougner SL, Bollerslev J, Latif F, Hald JK, Lund T, Ramm-Pettersen J, Berg JP2008. Low levels of raf kinase inhibitory protein in growth hormone-secreting pituitary adenomas correlate with poor response to octreotide treatment. J Clin Endocrinol Metab 93:1211–1216 [DOI] [PubMed] [Google Scholar]
- 28.Lorenz K, Lohse MJ, Quitterer U2003. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature 426:574–579 [DOI] [PubMed] [Google Scholar]
- 29.de Bruin C, Feelders RA, Lamberts SW, Hofland LJ2009. Somatostatin and dopamine receptors as targets for medical treatment of Cushing’s Syndrome. Rev Endocr Metab Disord 10:91–102 [DOI] [PubMed] [Google Scholar]
- 30.Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Höllt V2004. Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J 23:3282–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG1998. Role for G protein-coupled receptor kinase in agonist-specific regulation of μ-opioid receptor responsiveness. Proc Natl Acad Sci USA 95:7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS2004. Differential desensitization, receptor phosphorylation, β-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem 279:23214–23222 [DOI] [PubMed] [Google Scholar]
- 33.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ2009. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA 106:9649–9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG2001. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-β-arrestin complexes after receptor endocytosis*. J Biol Chem 276:19452–19460 [DOI] [PubMed] [Google Scholar]
- 35.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS2000. Differential affinities of visual arrestin, β arrestin1, and β arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275:17201–17210 [DOI] [PubMed] [Google Scholar]
- 36.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ2004. Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem 279:35518–35525 [DOI] [PubMed] [Google Scholar]
- 37.Lefkowitz RJ, Shenoy SK2005. Transduction of receptor signals by β-arrestins. Science 308:512–517 [DOI] [PubMed] [Google Scholar]
- 38.Fischer T, Doll C, Jacobs S, Kolodziej A, Stumm R, Schulz S2008. Reassessment of sst2 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-1. J Clin Endocrinol Metab 93:4519–4524 [DOI] [PubMed] [Google Scholar]
- 39.Pfeiffer M, Koch T, Schroder H, Klutzny M, Kirscht S, Kreienkamp HJ, Höllt V, Schulz S2001. Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of sst(3) receptor function by heterodimerization with sst(2A). J Biol Chem 276:14027–14036 [DOI] [PubMed] [Google Scholar]
- 40.Mundschenk J, Unger N, Schulz S, Höllt V, Schulz S, Steinke R, Lehnert H2003. Somatostatin receptor subtypes in human pheochromocytoma: subcellular expression pattern and functional relevance for octreotide scintigraphy. J Clin Endocrinol Metab 88:5150–5157 [DOI] [PubMed] [Google Scholar]
- 41.Schulz S, Pauli SU, Schulz S, Händel M, Dietzmann K, Firsching R, Höllt V2000. Immunohistochemical determination of five somatostatin receptors in meningioma reveals frequent overexpression of somatostatin receptor subtype sst2A Clin Cancer Res 6:1865–1874 [PubMed] [Google Scholar]
- 42.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ2005. Different G protein-coupled receptor kinases govern G protein and β-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA 102:1448–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]