Abstract
GH is generally believed to signal exclusively through Janus tyrosine kinases (JAK), particularly JAK2, leading to activation of signal transducers and activators of transcription (STAT), ERK and phosphatidylinositol 3-kinase pathways, resulting in transcriptional regulation of target genes. Here we report the creation of targeted knock-in mice wherein the Box1 motif required for JAK2 activation by the GH receptor (GHR) has been disabled by four Pro/Ala mutations. These mice are unable to activate hepatic JAK2, STAT3, STAT5, or Akt in response to GH injection but can activate Src and ERK1/2. Their phenotype is identical to that of the GHR−/− mouse, emphasizing the key role of JAK2 in postnatal growth and the minimization of obesity in older males. In particular, they show dysregulation of the IGF-I/IGF-binding protein axis at transcript and protein levels and decreased bone length. Because no gross phenotypic differences were evident between GHR−/− and Box1 mutants, we undertook transcript profiling in liver from 4-month-old males. We compared their transcript profiles with our 391-GHR truncated mice, which activate JAK2, ERK1/2, and STAT3 in response to GH but not STAT5a/b. This has allowed us for the first time to identify in vivo Src/ERK-regulated transcripts, JAK2-regulated transcripts, and those regulated by the distal part of the GHR, particularly by STAT5.
In vivo disabling of the Box1 motif demonstrated growth hormone receptor signaling through JAK activated STAT3, STAT5 and Akt, but not Src or ERK1/2.
For over a decade, the view has prevailed that the Janus tyrosine kinase JAK2 mediates signaling by the GH receptor (GHR) (1). JAK2 was shown to bind constitutively to the membrane proximal Box1 sequence of GHR and a distal acidic/hydrophobic sequence referred to as Box 2 (2). GH binding is thought to result in the repositioning of paired JAK2 in the receptor dimer to facilitate transphosphorylation and kinase activation (3). Activated JAK2 then phosphorylates key tyrosine residues on the cytoplasmic domain of the GHR, permitting SH2 domain interactions by signal adaptor proteins such as Sos, resulting in Ras/MAPK kinase/ERK activation, and docking of signal transducer and activator of transcription 5 (STAT5) for activation by JAK2. Activated JAK2 is also able to directly tyrosine phosphorylate STAT3 as well insulin receptor substrate-1/2, facilitating activation of the Akt/phosphatidylinositol 3-kinase pathway (4).
This monodimensional view of GH signaling contrasts with a report of patients with GH resistance where the ability of JAK2 to activate STAT5 is normal but ERK activation is impaired (5) and the in vitro demonstration by Zhu et al. (6) that Src kinase can be activated by GHR independent of JAK2. We have confirmed this finding in a myeloid cell line stably expressing GHR and shown activation of Src and ERK in fibrosarcoma cells lacking JAK2 (7). In this same study, we reported that the Src kinase Lyn binds directly to the membrane proximal part of the GHR even in the absence of JAK2, independent of hormone binding. Furthermore, we have identified a rearrangement of a loop in the lower cytokine receptor module of the GHR that occurs when GH agonist, but not antagonist, is bound, and this rearrangement is required for activation of Src and ERK by the GHR in myeloid cells (7). The use of both JAK and Src family kinases (SFK) by other homomeric class 1 cytokine receptors has been previously documented in vitro for prolactin (8) and erythropoietin (9) receptors, but no in vivo data supporting such a circumstance have been reported before our studies. Here we report the characterization of mice with targeted knock-in mutation of the Box1 sequence of the GHR that abrogates JAK2-STAT5 signaling in vivo but maintains other signaling pathways. We combine phenotypic and transcript profiling to document the existence of dual tyrosine kinase signaling by the GH receptor in vivo and the physiological role of SFK in gene regulation by the GHR. Finally, this model, together with our previous targeted knock-in mutations that debilitated STAT5 activation by the GHR, but not JAK2 activation (10, 11), allows us to conclude that JAK2 and STAT5 signaling is essential for normal postnatal growth and control of adiposity in the adult.
Results
Targeted mutation of the GHR
To elucidate the role of the Box1 domain of the GHR, transgenic mice bearing mutations in the Box1 region were created through targeted homologous recombination in embryonic stem (ES) cells as described in Materials and Methods (see also Fig. 1A). The four proline residues that bind the nonreceptor tyrosine kinase JAK2 were mutated to alanine by the mutation of five nucleotides along the Box1 sequence. Genomic restriction analysis was used to confirm homologous recombination of the cassette into ES cells (Fig. 1B), and expression of the mutant GHRs were demonstrated by Northern (Fig. 1C) and Western blotting (Fig. 1D) of hepatic tissue. Sequencing of the hepatic mRNA transcript was used to confirm the presence of the correct mutation in the Box1 sequence of homozygotes (Fig. 1E).
Fig. 1.
Panel A, Targeting vector used for creation of GHRBox1−/− mice showing location of mutations in exon 9; Panel B, genomic southern blot of ES cells after EcoR1 digestion and hybridization with an exon 10 probe showing predicted size difference (11 ); Panel C, Northern blot of mGHR from liver RNA of mice. GHRBox1−/− mice express normal-length receptor mRNA; Panel D, Western blot of GHR. GHRBox1−/− show normal-size GHR protein (110–120,000). Control shown is GHR−/− with no receptor expression. Panel E, Nucleotide sequences of WT and GHRΔBox1 constructs showing changes needed to convert the four prolines of Box1 to Ala and confirmation of mutant knock-in by determination of transcript sequence in this location (one of three homozygous mice shown for mutant). E, EcoR1; H, Hind III; Mut, mutant; Pro, proline; Val, valine.
Postnatal growth rate and organ weights
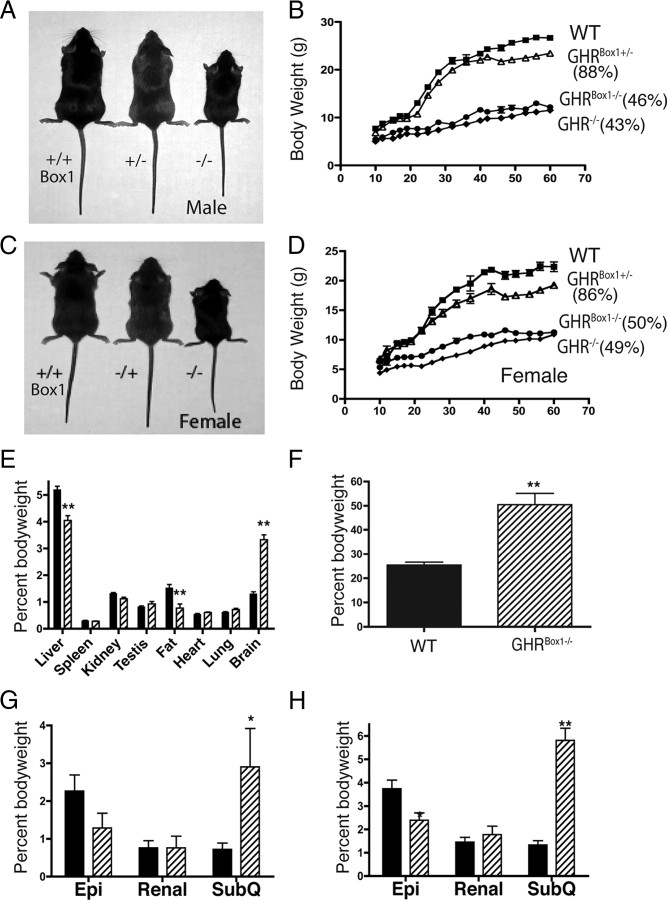
Homozygous GHRBox1−/− mice were found to be phenotypically normal at birth and not significantly different in birth weight, although they tended to be smaller. At 3 wk of age, deviation of the mutant’s growth from wild type (WT) became apparent, and by 2 months of age, the difference was striking (Fig. 2, A–D). Homozygous males were significantly smaller than WT males (46%, P < 0.001) but not different from GHR−/− (43%) at 2 months (Fig. 2B). Homozygous females showed the same trend and at 2 months of age were 50% of WT (P < 0.001) and similar to the GHR−/− at 49% (Fig. 2D). As reported previously in Rowland et al. (10, 11) for other GHR mutations, GHRBox1+/− mice exhibit a reduced weight from around 6 wk of age. At 2 months of age, the GHRBox1+/− heterozygous males and females were on average 87 and 86%, respectively, the weight of WT littermates.
Fig. 2.
Gross phenotypic analysis for GHRBox1−/−. For all histograms, GHRBox1−/− mice are shaded, and wild type are black. A and C, Male and female mice at 60 d of age. Shown in order from left to right is WT, GHRBox1+/−, and GHRBox1−/−. B, Growth curves for male mice (n = 8–12 per strain). Percentages shown are expressed as a percentage of WT weight at 2 months age. D, Growth curves for female mice (n = 6–10 per strain). E, Organ weights at 2 months age (n = 6–8 per strain). Liver and epididymal fat pads (fat) were significantly reduced in mutants when expressed as a percentage of body weight. F, Percent fat by PIXImus scan at 13 months of age (n = 4 per group). Various fat deposits were measured at 4 months (G) and 13 months (H) (n = 4–6 per strain). Epididymal fat depots (Epi) were smaller, and retroperitoneal fat deposits were similar, whereas sc fat pads (SubQ) were greatly increased at both ages. All values are mean ± sem. *, P < 0.05; **, P < 0.01, by ANOVA.
Organ weights were taken at 60 d and normalized against body weight to allow allometric comparison between the WT and GHRBox1−/− males (Fig. 2E). Liver (P < 0.01) and epididymal fat pads (P < 0.001) were found to be significantly decreased when compared with WT percentages and not different from the other GHR mutants reported previously (10). Brain size was found to be disproportionate in growth (P < 0.001) in accordance with other finding in other GHR mutants (10, 12).
Dual-energy x-ray absorptiometry (DEXA) analysis for the percent body fat revealed that GHRBox1−/− males at 13 months of age are strikingly obese compared with WT mice (Fig. 2F). We also quantified the epididymal fat pads, renal fat depots, and the inguinal sc fat pad in the GHRBox1−/− male mutants at two ages, 4 (Fig. 2G) and 13 (Fig. 2H) months, expressed as a percentage of total body weight. Epididymal fat pads tended to be smaller at 4 months, although this was not significant, whereas at 13 months, this fat depot was significantly smaller in the mutant males. Renal fat pads were not different at either age between WT and homozygous mutants. However, the sc fat depot was found to be significantly increased at both 4 months (P < 0.05) and 13 months (P < 0.001) of age.
IGF-I axis
IGF-I is the major mediator of the growth actions of GH, and striking decreases in the IGF-I axis were observed for GHRBox1−/− mutant similar to GHR−/− and mutant GHR 391-GHR mice unable to activate STAT5 in response to GH (11). Thus, hepatic IGF-I mRNA was reduced in the GHRBox1−/− mutant mice comparably to the levels observed in homozygote 391-GHR and GHR−/− (Fig. 3A), and serum levels of IGF-I were likewise reduced severely in the GHRBox1−/− when compared with WT (Fig. 3B). As expected from the low levels of circulating IGF-I, murine GH (mGH) serum levels were greatly increased due to the absence of the negative feedback loop of IGF-I on GH secretion (Fig. 3C). Serum IGF-binding protein-3 (IGFBP3) was also found to be strikingly reduced, which would contribute to the greatly reduced circulating levels of IGF-I by increasing clearance (Fig. 3D). Hepatic transcript profiling indicates that the low level of circulating IGFBP3 is likely a consequence of decreased ALS expression rather than a decrease in IGFBP3 transcript expression (IGFBP3, 1.4 ± 0.3-fold WT littermates; ALS, 0.36 ± 0.08-fold WT; P < 0.05; n = 3). Increased IGFBP1/2/5 in the ligand blot of Fig. 3D is consistent with an elevated hepatic IGFBP1 (5.7 ± 3.1-fold WT; n = 3; P < 0.05) and IGFBP2 transcript level (1.9 ± 0.3-fold WT; n = 3; P < 0.05).
Fig. 3.
GH/IGF-I axis parameters. A, Hepatic IGF-I transcript by quantitative PCR is strikingly decreased in GHRBox1−/− mice (n = 4 per group); B, circulating levels of IGF-I are reduced to the level of the GHR−/− mouse in the GHRBox1−/− mutant (n = 5 per group); C, plasma GH levels in the GHRBox1−/− are elevated to a similar extent as the GHR−/− mice at 60 d of age (n = 5 per group); D, Western ligand blot for IGFBP. IGFBP-3 is almost absent in GHRBox1−/− as also seen in the GHR−/−. E, Homozygous GHRBox1−/− show a significant (P < 0.01) decrease in long bone length at 42 d of age, whereas GHRBox1+/− heterozygotes do not (n = 6 each group); F, MUP expression in male mice is virtually absent in GHRBox1−/− and GHR−/− mice. All values shown are mean ± sem. BP, Binding protein. *, P < 0.05; ***, P < 0.001.
Consistent with the decreased IGF-I and loss of JAK2 signaling in the GHRBox1−/− mice, long bone growth was significantly decreased in homozygous but not heterozygous mice (Fig. 3E).
Sexually dimorphic gene expression
One of the major targets for STAT5 signaling are the sexually dimorphic major urinary proteins (MUP), which are urinary pheromones of hepatic origin. Other GHR mutants have shown a reduced amount of MUP relative to the reduction in STAT5 activity (11). As is evident in Fig. 3F, urinary MUP are greatly reduced in the GHRBox1−/− male mutants at the protein level, and within the liver, MUP transcripts are decreased in the GHRBox1−/− mice (Mup2, 15.7-fold decreased; Mup4, 7.2-fold decrease).
Signaling by GHRBox1−/−
One of the main aims of this study was to determine the effect of removing JAK2 activation of GHR signaling in vivo. Therefore, the relative phosphorylation of main effectors of GH stimulation, such as JAK2, STAT3/5, and ERK1/2 was compared after an ip injection of nonprimate GH [bovine GH (bGH)] or saline. Nineteen-day-old mice with low endogenous GH secretion (13) were used to avoid endogenous activation/phosphorylation of these effectors. As briefly reported earlier (7), whereas WT mice displayed a normal JAK2 and STAT5 tyrosine phosphorylation response to bGH stimulation, there was no activation of either protein in GHRBox1−/− mutants (Fig. 4, A and B). This supports the in vitro data that the Box1 sequence is required for activation of the JAK-STAT5 pathway (2). Moreover, STAT3 activation is abolished in the absence of JAK2 activation (Fig. 4C), indicating that in the liver, GH-dependent SFK do not activate this transformation-inducing factor, in contrast to its role in oncogenesis (14).
Fig. 4.
Immunoblots of signaling in WT and GHRBox1−/− mice. GHR signaling intermediates were quantified in 19-d-old homozygous mice in liver taken 5–15 min after injection of bGH (8 μg/g). Phosphospecific and load antibodies are given in Materials and Methods. Each lane represents an individual mouse. Densitometric quantification is expressed relative to untreated WT; n = 6 per group for JAK2, Src, STAT5, and ERK1/2, and n = 8 for STAT3. Black bars represent WT mice; hatched bars represent GHRBox1−/− mice. Mean ± sem is shown. *, P < 0.05; **, P < 0.01 by ANOVA. pY, Phosphotyrosine.
Akt can be activated by phosphorylation on either Thr308 or Ser473 by various receptors, and we have found that 15 min after bGH injection, Akt is phosphorylated on Ser 473 but not Thr 308. Similarly to STAT3, GH- induced activation of Akt is lost in GHRBox1−/− mice (Fig. 4D).
Because we have shown activation of ERK by SFK in vitro (7), we also examined ERK1/2 activation in response to bGH 15 min after injection. As Fig. 4E shows, the GHRBox1−/− mice do have normal ERK1/2 activation. The basal level of activated ERK1/2 in the GHRBox1−/− mutants appears to be elevated over the baseline WT levels, although this failed to reach statistical significance. ERK activation in the homozygous GHRBox1−/− mutants led us to examine potential upstream kinases that may be responsible, and we found that Src kinase was activated in the GHRBox1−/− mutants as well as WT with GH stimulation, again as observed in vitro (Fig. 4F).
Transcript profiling of the GHR transgenic mice
We have previously reported microarray results for the mutant 569-GHR, mutant 391-GHR receptor truncations, and GHR−/− mice at 6 wk of age (11) and found sets of hepatic genes that were altered and can be attributed to differences in signaling in these animals. Here we performed Illumina array analysis on livers of older, 4-month-old WT mice and compared the transcript profiles to age-matched GHRBox1−/− mutants and 391-GHR mutants lacking STAT5 activation but with JAK2 and Src activation by GH (Fig. 5). We also included matched GHR−/− mice, allowing us to fractionate genes whose expression is STAT5 regulated, JAK2 regulated, and Src/ERK regulated (see Table 1). These are presented as a Venn diagram (Fig. 5), which shows that of the 90 GH-regulated hepatic genes identified at high stringency, the majority (52%, 47 genes) are regulated specifically by the distal part of the receptor where STAT5 signaling originates, 14% (13 genes) are regulated by JAK2 directly, and 27% (23 genes) by Src/ERK signaling by the GHR. The STAT5/distal receptor-regulated genes include many previously identified genes (11, 15, 16, 17, 18) such as CYP, complement factors, and Spi genes. Based on DAVID gene ontology classification of gene function, 24% of Src/ERK-specific genes with identified function are small GTPase, and another 12.5% are involved in enzyme regulation (see Table 2). These genes include A1cf, which is part of the apolipoprotein B mRNA editing complex, Cdkn1a, which mediates p53-dependent cell cycle arrest in response to stress, and the GHR itself (4.8-fold decrease). The latter is known to be regulated by GH status, and particularly by hepatic nuclear fator-4 isoforms (19, 20), which are themselves regulated by MAPK phosphorylation (21). Direct JAK2-specific genes comprise those involved in signal transduction, such as the sphingosine kinase 2, which is involved in calcium signaling and apoptosis, and Accn5, which has a role in intestinal sodium ion transport. Direct growth-promoting actions of JAK2 could stem from its regulation of Mif4gd (SLIP1), which is needed for normal translation of histone mRNA and cell viability (22) and Pttg1 (securin), which is necessary for normal β-cell mass and insulin tolerance as well as pituitary hyperplasia (23). There are a substantial number of genes overlapping between JAK2 and Src/ERK categories in Fig. 5 but not with other categories, suggesting a specific phenomenon such as both JAK2 and ERK regulatory elements in use for these GH-regulated genes.
Fig. 5.
A, Venn diagram of transcripts altered in mutant mice assigning transcript number to particular signaling pathways by subtraction of transcript profiles. Most transcripts were regulated by the distal part of the receptor (STAT5 and other pathways) in these mature fasted mice. Whole mouse genome Illumina arrays were used for the analysis. B, Diagram of the mutations created in the GHR cytoplasmic domain in the mice used for the analysis (GHR−/− not shown). Y’s show tyrosine residues on the receptor.
Table 1.
Hepatic transcripts with altered expression in liver from 4-month-old male mice
| Common name | GenBank | Gene ID | Fold Change | P value |
|---|---|---|---|---|
| GHRBox1−/− vs. GHR−/− (Src) | ||||
| 2210010C17Rik | NM_027308.2 | 5720735 | 2.16 | 0.014 |
| 5033411D12Rik | NM_138654.2 | 2320471 | 0.11 | 0.002 |
| A1cf | NM_001081074.1 | 4150576 | 0.16 | 0.004 |
| A330081F11Rik | AK039667 | 104010364 | 0.42 | 0.004 |
| Abhd1 | NR_003522.1 | 6220685 | 5.19 | 0.019 |
| BC002216 | AK089229 | 106400035 | 3.12 | 0.006 |
| Blm | NM_007550.3 | 520619 | 2.92 | 0.019 |
| Cdkn1a | NM_007669.3 | 6400706 | 0.29 | 0.013 |
| Dhtkd1 | NM_001081131.1 | 104480451 | 2.18 | 0.013 |
| Epb4.1l2 | NM_013511 | 104570176 | 0.42 | 0.006 |
| Fgfr1op2 | NM_026218.2 | 60538 | 0.50 | 0.013 |
| Gdi2 | NM_008112.4 | 4780161 | 0.11 | 0.001 |
| Gfra1 | NM_010279.2 | 3610152 | 0.46 | 0.009 |
| Ghr | NM_010284.2 | 2260156 | 4.81 | 0.004 |
| H2-Bl | NM_008199.1 | 1450373 | 4.75 | 0.004 |
| LOC100046025 | XM_001475823.1 | 105860440 | 2.61 | 0.002 |
| Mif4gd | NM_027162.3 | 6130685 | 0.39 | 0.009 |
| Nedd4l | NM_031881.1 | 6380368 | 0.50 | 0.004 |
| Ngef | NM_019867 | 3190538 | 2.16 | 0.017 |
| Serpina11 | NM_199314.1 | 1980300 | 2.25 | 0.004 |
| Stip1 | NM_016737.1 | 6450390 | 0.50 | 0.019 |
| Ugt1a10 | NM_201641.2 | 100780102 | 4.49 | 0.001 |
| Vnn3 | NM_011979.1 | 730053 | 0.47 | 0.006 |
| WT vs. 391 (STAT5) | ||||
| 1100001G20Rik | NM_183249.2 | 3610368 | 40.61 | 0.004 |
| 1700007B13Rik | XM_913577.2 | 2850053 | 3.63 | 0.011 |
| 5730410E15Rik | NM_001032727.1 | 3840446 | 0.44 | 0.005 |
| Adh4 | NM_011996.2 | 6840301 | 2.24 | 0.019 |
| Agt | NM_007428.3 | 7000575 | 0.46 | 0.004 |
| AU018778 | NM_144930.1 | 1450707 | 2.57 | 0.017 |
| C6 | NM_016704.1 | 2900129 | 6.97 | 0.004 |
| C730007P19Rik | NM_009286.1 | 2650397 | 0.01 | 0.005 |
| C8a | NM_146148.1 | 2190601 | 13.05 | 0.009 |
| C8b | NM_133882.2 | 6770687 | 5.36 | 0.014 |
| Ccbl1 | NM_172404.2 | 2350082 | 3.87 | 0.011 |
| Csad | NM_144942.3 | 770215 | 4.32 | 0.002 |
| Cyp17a1 | NM_007809.2 | 1340408 | 0.09 | 0.014 |
| Cyp2a4 | NM_009997.2 | 106370722 | 0.16 | 0.019 |
| Cyp2a5 | NM_007812.4 | 1230253 | 0.24 | 0.019 |
| Cyp2b13 | NM_007813.1 | 5420672 | 0.01 | 0.014 |
| Cyp2b9 | NM_010000.2 | 360152 | 0.04 | 0.007 |
| Cyp2c54 | NM_206537.1 | 6130193 | 4.85 | 0.011 |
| Cyp4a12a | NM_177406.3 | 4210288 | 66.59 | 0.004 |
| Cyp4a12b | NM_172306.2 | 6660170 | 47.95 | 0.010 |
| E430021N18Rik | NM_144796 | 101230504 | 7.30 | 0.013 |
| EG13909 | NM_144511.1 | 6370064 | 41.56 | 0.004 |
| EG381806 | NR_003624.1 | 102230739 | 5.20 | 0.004 |
| EG434674 | NM_001013820.2 | 630368 | 3.26 | 0.010 |
| Elovl3 | NM_007703.1 | 6860050 | 8.53 | 0.001 |
| Es31 | XM_915050.2 | 3290458 | 36.06 | 0.010 |
| Igf1 | NM_184052.2 | 1990193 | 39.56 | 0.002 |
| Igfals | NM_008340.3 | 6350195 | 18.78 | 0.001 |
| LOC100039008 | XM_001472055.1 | 770088 | 31.37 | 0.010 |
| LOC100045280 | XM_001473988.1 | 105700164 | 0.45 | 0.017 |
| Lpl | NM_008509.2 | 2970706 | 0.12 | 0.020 |
| Mt1 | NM_013602.2 | 4850164 | 0.09 | 0.019 |
| P2ry1 | NM_008772.4 | 6040121 | 2.12 | 0.017 |
| Serpina1 | NM_009243 | 106590035 | 3.207 | 0.008 |
| Serpina12 | NM_026535.2 | 1230128 | 14.69 | 0.014 |
| Serpina1e | NM_009247.2 | 2630180 | 145.30 | 0.001 |
| (Continued) |
Table 1A.
Continued
| Common name | GenBank | Gene ID | Fold Change | P value |
|---|---|---|---|---|
| Slco1a1 | NM_013797 | 104230484 | 17.37 | 0.007 |
| Srd5a1 | NM_175283.3 | 5910347 | 4.23 | 0.011 |
| Sult2a1 | XM_001471625.1 | 2650286 | 0.04 | 0.002 |
| Tmprss2 | NM_015775.2 | 2970279 | 0.38 | 0.010 |
| Ugt2b1 | NM_152811.1 | 6650097 | 3.40 | 0.011 |
| Zap70 | NM_009539.2 | 1410494 | 3.53 | 0.012 |
| Zbtb25 | NM_028356.1 | 430500 | 2.37 | 0.017 |
| Zer1 | NM_178694.3 | 580524 | 0.49 | 0.010 |
| GHRBox1−/− vs. 391 (Jak2) | ||||
| 4932415G12Rik | NM_026339 | 6900152 | 0.43 | 0.019 |
| 5033411D12Rik | NM_138654.2 | 2320471 | 0.15 | 0.006 |
| 9630038C08Rik | AK036131 | 103870239 | 3.95 | 0.004 |
| Accn5 | NM_021370.2 | 6220348 | 3.51 | 0.002 |
| BC002216 | AK089229 | 106400035 | 3.47 | 0.016 |
| Ccl9 | NM_011338.2 | 4610725 | 3.37 | 0.019 |
| Clpx | NM_011802.2 | 6220156 | 0.45 | 0.010 |
| Gdi2 | NM_008112.4 | 4780161 | 0.11 | 0.019 |
| Mif4gd | NM_027162.3 | 6130685 | 0.43 | 0.019 |
| Nedd4l | NM_031881.1 | 6380368 | 0.46 | 0.019 |
| Pttg1 | NM_013917.1 | 520463 | 0.37 | 0.001 |
| Sphk2 | NM_020011.4 | 4850114 | 0.48 | 0.016 |
| Ugt1a10 | NM_201641.2 | 100780102 | 4.74 | 0.001 |
Listed are hepatic transcripts with altered expression in liver from 4-month-old male mice, based on whole-genome Illumina array showing transcripts differing by greater than 2-fold, and P < 0.02 by pairwise t test based on normalized data. Three animals per group were fasted for 16 h. Comparisons are of GHRBox1−/− with GHR−/−, WT with 391-GHR truncation, and GHRBox1−/− with 391-GHR truncation mutants. Fold change is presented relative to the numerator (GHRBox1−/− from Src-regulated genes, WT from STAT5-regulated genes, and GHRBox1−/− from JAK2-regulated genes).
Table 2.
Gene ontology and function for Src/ERK and JAK2 specifically regulated genes, based on DAVID (http://david.abcc.ncifcrf.gov/) and the literature
| Ontology category | Gene | Function |
|---|---|---|
| Src only | ||
| Small GTPase | A1cf | Alternative splicing, cytoplasm, endoplasmic reticulum, mRNA processing, nucleus, RNA-binding |
| Dhtkd1 | Catalytic activity, oxoglutarate dehydrogenase (succinyl-transferring) activity | |
| Gfra1 | Glycoprotein, GPI-anchor, lipoprotein, membrane, receptor, signal | |
| H2-Bl | Signal, transmembrane | |
| Fgfr1op2 | Coiled coil, alternative splicing | |
| Epb4.1l2 | Actin-binding, cytoplasm, cytoskeleton, phosphoprotein, structural protein | |
| Enzyme regulator activity | Cdkn1a | Acetylation, cell cycle, cyclin, cytoplasm, kinase, metal-binding, nucleus, phosphoprotein, protein kinase inhibitor |
| Serpina11 | Secreted, serine protease inhibitor, glycoprotein, protease inhibitor, signal | |
| Ngef | Developmental protein, SH3 domain, alternative splicing, cell projection, cytoplasm, differentiation, guanine-nucleotide releasing factor, membrane, neurogenesis | |
| Other | Blm | ATP-binding, DNA replication, DNA-binding, helicase, hydrolase, nucleotide-binding, nucleus, phosphoprotein |
| Ghr | Direct protein sequencing, secreted, alternative splicing, endocytosis, glycoprotein, membrane, phosphoprotein, receptor, signal, transmembrane, transmembrane protein, UBL conjugation | |
| Stip1 | Direct protein sequencing, TPR repeat, acetylation, cytoplasm, nucleus, phosphoprotein | |
| Abhd1 | Glycoprotein, hydrolase, membrane, serine esterase, signal-anchor, transmembrane | |
| Vnn3 | Glycoprotein, GPI-anchor, hydrolase, lipoprotein, membrane, signal | |
| Jak2 only | ||
| Signal transduction | Ccl9 | Secreted, chemotaxis, cytokine, signal |
| Sphk2 | Sphingolipid metabolism, calcium signaling pathway, VEGF signaling pathway | |
| Accn5 | Ionic channel, ion transport, sodium channel, sodium transport, transmembrane, transport | |
| 9630038C08Rik | Pax9, transcription factor, thymic development, skeletal development | |
| Other | Clpx | ATP-binding, chaperone, metal-binding, nucleotide-binding, zinc, zinc-finger |
| Pttg1 | DNA damage, DNA repair, cell cycle, cell division, chromosome partition, cytoplasm, mitosis, nucleus, phosphoprotein, protooncogene, SH3-binding, UBL conjugation | |
| 4932415G12Rik | Unknown | |
| Src and Jak2 | ||
| Mif4gd | Nucleic acid binding, RNA binding | |
| Nedd4l | Protein modification process, ubiquitin cycle, metabolic process, cellular process, post-translational protein modification | |
| Gdi2 | GDP dissociation inhibitor XAP-4 | |
| BC002216 | Glycolipid transfer protein | |
| 5033411D12Rik | Transferase | |
| Ugt1a10 | Pentose and glucuronate interconversions, Androgen and estrogen metabolism, Starch and sucrose metabolism, Porphyrin and chlorophyll metabolism, Metabolism of xenobiotics by cytochrome P450 |
Verification of ERK-regulated transcripts
A number of the putative ERK-regulated genes identified by the array were verified using liver cDNA from GHR−/− and GHRBox1−/− mice and real-time PCR (Fig. 6). Of these genes, Gdi2, Vnn3, and H2-B1 were verified as ERK regulated using an in vitro model of AML12 murine hepatocytes treated with human GH (hGH) and the ERK inhibitor PD98059 (Fig. 7). Both Gdi2 and Vnn3 were suppressed by hGH treatment, correlating with increased expression seen in the GHR−/− liver, and this suppression was lost after pretreatment with the ERK inhibitor. H2-B1 expression was increased by hGH treatment, again correlating with loss of expression seen in the GHR−/− liver, and this stimulation was lost after pretreatment with the ERK inhibitor.
Fig. 6.
Real-time PCR verification of selected transcripts identified as different between GHRBox1−/− and GHR−/− mice (Table 1). Transcript levels, normalized to GAPDH, is expressed as change in cycle threshold CT relative to GHRBox1−/− mice. *, P < 0.05; **, P < 0.01 by t test.
Fig. 7.

Real-time PCR validation of ERK-regulated genes. AML12 murine hepatocytes were treated with PD98059 for 1 h followed by hGH for 6 h. White bars represent vehicle-treated cells; black bars represent GH-treated cells. Transcript levels, normalized to GAPDH, are expressed as change in cycle threshold relative to vehicle-treated cells. Results are the average of three separate experiments. *, P < 0.05 by t test.
Discussion
A number of important findings have emerged from this study of GHRBox1−/− mice. First, JAK2 is responsible for the entirety of hepatic STAT5 activation by the GHR, with SFK playing no separate role. In the absence of GH-dependent STAT5 activation, growth is severely retarded, both in humans and in mice with receptor truncation (11, 24, 25), in accord with its key role in transcriptional up-regulation of the Igf-1 gene (26). A second finding is that the difference in growth rate between GHR−/− mice and mice with 391-GHR truncation (lacking ability to activate STAT5) is abolished when the ability to activate JAK2 is prevented in GHRBox1−/− mice. This implies that the residual growth seen in 391-GHR truncated mice (around 7%, or 13% of GH-dependent growth) is JAK2-dependent rather than STAT5 dependent. This signaling may account for the size difference between IGF-I-deleted mice and those with combined IGF-I and GHR deficiency (27).
The altered body composition we observe in mature GHRBox1−/− mice is similar to that seen with loss of STAT5 signaling in the 391-GHR truncated mice and GHR−/− mice. The major depot affected is sc fat, with peritoneal depots becoming affected after 10 months of age, resulting in an increase in fat content by DEXA from 26 to 51%. There is evidence that STAT5 can act as an antiobesity agent by suppressing FAS, AOX, and PDK4 (28, 29) as well as promoting lipolysis (30), so loss of STAT5 activation by GH would be predicted to lead to obesity, as observed here with the GHRBox1−/− mice and in STAT5b−/− mice, which become obese after 3 months of age (31).
A key finding is that abrogation of ability to activate JAK2 by the GHR does not block its ability to activate SFK or pathways that can be initiated by SFK, notably the ERK pathway in liver. This in vivo observation is supported by our in vitro findings that GH can activate ERK and SFK in JAK2−/− fibrosarcoma cells and that inhibition of JAK2 in FDCP-1 myeloid cells does not prevent GH from activating ERK (7). In these three situations (inability of GHR to activate JAK2 in vivo in GHRBox1−/− mice, inability to activate JAK2 in JAK2−/− cells, and low relative expression of JAK2 in FDCP-1 cells), JAK2 activation is compromised, and the alternate tyrosine kinase, a SFK, is able to carry the ERK signal effectively, although STAT3, STAT5, and Akt signaling from the GHR is abrogated. It is likely that the relative levels of JAK2 and SFK in any particular cell will determine which kinase is the major signaling element, with JAK2 predominating in most cases. The latter is evidently the case for 3T3-F442A preadipocytes and H4IIE hepatoma cells, which have been convincingly shown to use JAK2 for ERK activation (32). Both JAK2 and Src kinase are able to bind constitutively to the upper cytoplasmic portion of the GHR (4, 7), so it is plausible that there is competition for GHR binding between these two tyrosine kinases. Alternatively, phospholipase-Cγ may act as a switch between them, promoting signaling to ERK from SFK (7) but inhibiting signaling from JAK2 to ERK (33).
To assess the relative physiological roles of these two pathways, we have undertaken transcript profiling by microarray to identify genes whose expression is regulated by SFK, rather than by JAK2. Through comparing hepatic transcript profiles for GHRBox1−/− mice with GHR−/− mice, we have identified those GH-dependent transcripts regulated by the alternate (non-JAK2) pathway, because this is operative in the GHRBox1−/− mice but not in the GHR−/− mice. We could also quantify transcripts directly regulated by JAK2 by comparing mice with the GHR truncated below Box1 (391-GHR truncation), which do have functional JAK2 and SFK signaling, with the GHRBox1−/− mice, which have no JAK2 signaling. Moreover, comparison of 391-GHR mice lacking the ability to activate STAT5 with WT mice provides an estimate of transcripts regulated by STAT5a/b and any other signals in the distal 260 residues of the receptor cytoplasmic domain. However, comparing our 391-GHR gene set with the published study on liver-specific STAT5a/b deletion in 28-d-old fed mice using the Affymetrix platform (17), less than 10% of listed genes are common. The correlation can be improved marginally to 14% by reducing the stringency of our data to P < 0.05, which increases our gene list to approximately 200. This discrepancy is presumably due to the very different age groups in the two studies. Day 28 falls within the peak GH-induced growth phase, suggesting that many genes detected at this time may have developmental functions. The present study uses 4-month-old mice, which are developmentally mature. This is supported by comparison with Clodfelter et al. (16), which compares STATb−/− mice at 6–8 wk of age, showing 55% gene similarity. This is relatively high, given that these mice are on different genetic backgrounds, and these authors use the Agilent platform. We can discount IGF-I-regulated genes from our analysis of Box1-regulated genes, because IGF-I is equally decreased in GHRBox1−/− and GHR−/− mice. However, loss of IGF-I presumably contributes to our estimate of STAT5-regulated genes, because loss of STAT5 activation ability correlates with loss of IGF-I (11). This would be relevant for Kupffer cells, stellate cells, and endothelial cells in liver, i.e. those cells capable of responding to IGF-I.
The contributions by these separate signaling pathways to hepatic gene expression are summarized in Fig. 5, based on a greater than 2-fold change criteria with significance of P < 0.02 by t test. To our knowledge, this is the first time such an in vivo analysis has been reported. The proportion of genes regulated by the STAT5a/b-generating part of the cytoplasmic domain is large in the liver, in accord with the important role of the GH pulse pattern in sexually dimorphic gene expression (16, 34, 35). The estimate of 52% of GH-regulated genes as STAT5 dependent in liver is higher than our previous estimate based on 2-month-old fed mice analyzed with a smaller Affymetrix array representing 12,400 transcripts reported in Rowland et al. (11), whereas the Illumina bead array used here covered 46,643 transcripts in the NCBI RefSeq database. It is likely that a significant number of genes in categories involving loss of STAT5 signaling are altered as a result of concomitant up-regulation of STAT1 and -3 related to loss of CIS and SOCS3 expression, in response to cytokines such as IL-6 (34). For example, basal STAT3 level in GHRBox1−/− mice was almost significantly elevated (P = 0.06) (34), whereas reductions in transcripts for CIS (3.3-fold), SOCS2 (6.6-fold), and SOCS3 (1.7-fold) are evident in these mice (see supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
One would predict that hepatic genes whose expression relates to the SFK pathway possess regulatory elements responsive to ERK, and this is the case for many genes in this group. However, over 40 transcription factors are regulated by ERK alone, including ATF2, CBP, C/EBPb, Elk1, c-fos, estrogen receptor, Fra1, c-Jun, N-myc, NFAT, Sap1, Smads, SP1, sterol response element-binding protein-1/2, and steroid receptor coactivator-1 as well as inhibitory phosphorylation of STAT1, -3, and -5 (21), so it is not a simple task to predict which of the non-JAK2-regulated hepatic genes are regulated by ERK through these intermediaries. Nevertheless, we have shown that altered expression of three transcripts identified by the array analysis as Src/ERK dependent is reversed by a specific MAPK kinase inhibitor in a GH-stimulated murine hepatocyte line (Fig. 7). Furthermore, literature searching has revealed that expression of Cdkn1a, Ugt1a10, and A1cf are also dependent on Src or ERK signaling via Sp1 or AP1 activation (36, 37, 38). Finally, we have recently identified a physiological outcome of SFK/ERK signaling deficit in that GHR−/− mice challenged with 70% partial hepatectomy; all die after the operation, whereas the GHRBox1−/−, 391-GHR, and WT mice all survive (Ishikawa, M., T.McPhee, M. Fernandez-Rojas, J. Barclay, and M. J. Waters, in preparation).
The use of both SFK and JAK2 by the GHR as demonstrated here is not restricted to this receptor alone, because other homodimeric class 1 cytokine receptors have been reported to use both tyrosine kinases in vitro, based on dominant-negative Src expression and SFK inhibitors. Thus, Acosta et al. (39) found that in breast cancer lines, the ability of prolactin to activate ERK, focal adhesion kinase, and Akt was abrogated by SFK inhibition, whereas JAK2 activation was not affected. Importantly, Fresno Vara et al. (8) reported that prolactin activation of Src is evident even when the prolactin receptor Box1 sequence is mutated (hence ability to activate JAK2 is lost), as we have observed with the GHR. Miura and colleagues (9) have also provided evidence that the erythropoietin receptor uses a receptor-associated SFK (Lyn), in this case to tyrosine phosphorylate CrkL, leading to activation of ERK and Elk-1.
In conclusion, we have corroborated our in vitro study showing that the GHR can use SFK in addition to JAK2 through the creation of targeted knock-in mice that lack the ability to activate JAK2 in response to GH. These GHRBox1−/− mice demonstrate the key role of JAK2 in regulating postnatal growth and IGF-I expression through STAT5 and potentially other pathways, but they also demonstrate a set of hepatic transcripts that can be regulated by GH independently of JAK2. These are presumably regulated by SFK/ERK signaling and include genes coding for proteins involved in mRNA transcription and metabolism, including the GHR itself. We have also identified genes regulated directly by JAK2 in vivo in the absence of STAT5 activation, including those involved with metabolic processes and DNA-protein/chromatin interactions. These multiple signaling pathways presumably provide the necessary flexibility for the undertaking the many roles of this ubiquitous pleiotropic receptor.
Materials and Methods
Gene replacement strategy and construction of targeting vector
The GHR cytoplasmic domain was modified by homologous recombination with the GHR locus encompassing exon 9, which codes for the Box1 sequence. A targeting construct was generated with a short homology arm of 1.4 kb upstream of exon 9 and a long homology arm of 4 kb including exon 10 and downstream intronic sequence (see Fig. 1A).
A 16-kb Sau3A1 fragment containing exons 9 and 10 of the mGHR gene was isolated from a 129/SVJ mouse genomic λ-phage library (Stratagene, La Jolla, CA). A 6.4-kb portion of this that contained exons 9 and 10 with flanking intronic sequence was cloned to pBluescript by XbaI to use for targeting to ES cells (Genbank accession number AY271378). Probes for the exon 9 and 10 portions of the GHR were generated by synthetic oligonucleotide creation (Genset Oligos, Lismore, Australia) or by PCR, respectively (exon 10 forward primer; 10F, 5′-CCTGGGTCGAGTTCATTGAGC3-′; exon 10 reverse primer; 10R, 5′-GCCCACTTACACCACC CAGC-3′; 1-kb exon 10 product). The 6.4-kb fragment was subjected to QuikChange mutagenesis (Stratagene) to substitute all four prolines of the PPVPVP Box1 sequence with alanines. The mutagenic oligonucleotide primer used was 4P-A forward 5′-GATGCTGATTTTAGCCGCGGTCGCAGTTGCAAAGATTAAAGGG-3′. Correct mutation was confirmed by automated sequence analysis [Australian Genome Research Facility (AGRF), University of Queensland, Australia]. A floxed selection cassette (PGKneopA) (40) was then inserted between exons 9 and 10 by BamHI and XbaI engineered restriction sites.
Production of gene-targeted mice
Targeting vector plasmids were linearized by NotI digestion and purified by Geneclean II (Qbiogene, Carlsbad, CA) before electroporation into low passage 129/SVJ agouti ES cells, and homologous recombinants were selected for by G418 resistance followed by genotyping. Positive ES cells were then injected into C57black blastocysts and implanted to pseudopregnant foster mothers. Germline/chimeric pups were assessed by coat color and then confirmed by genotyping. Chimeric offspring were mated with C57B6/J mice to give rise to germline heterozygote offspring. They were then mated with Cre deletor transgenic mice, which ubiquitously express Cre recombinase enzyme to remove the PGKneoNTRtkpA selection cassette (40). All studies with all mutants reported here were undertaken with mice at least eight generations into C57BL/6.
Animals
Animals were housed in an approved facility and treated as per university guidelines and had ethics approval from the University of Queensland Animal Ethics Committee and the Australian Office of the Gene Technology Regulator. Water and feed pellets were available ad libitum under a 12-h light, 12-h dark cycle at 20–22°C. All animals passed standard virus screens quarterly throughout. DEXA measurements were carried out with a PIXImus densitometer (Lunar Corp., Madison, WI).
IGF-I measurements
Acid ethanol-extracted serum IGF-I was measured using an IGF-I RIA kit (Bioclone, Sydney, Australia).
RNA extraction and Northern blot analysis
Liver RNA samples from 42-d-old mice for Northern blots were isolated using RNA-Bee reagent (Tel-Test Inc., Friendswood, TX). Samples were separated on a denaturing gel in MOPS buffer (41) and transferred onto MSI membrane (Micron Separations, Westborough, MA). Hybridizations were performed using Northern Max (Ambion, Austin, TX) and 32P-labeled (Megaprime labeling systems; Amersham Pharmacia, Piscataway, NJ) cDNA probes. A rat IGF-I cDNA was kindly provided by Dr. Adrian Herington (QUT, Brisbane, Australia), and the mouse GHR cDNA was kindly provided by Dr. Frank Talamantes (University of California, Santa Cruz, CA) A probe encompassing exons 2–7 was prepared from this cDNA by restriction digest with HindIII and EcoRV. The loading control hybridization was performed with an 18S-specific oligomer, 5′-CATGGTAGGCACGGCGACTACCATC-3′ (Genset Pacific, Lismore, Australia) or 150-bp fragment of 28S cDNA.
Illumina analysis of transcript profile
Liver tissue was collected from three 4-month-old male mice of each genotype starved overnight. RNA was extracted using RNeasy Mini kits (QIAGEN, Valencia, CA).
Total RNA was analyzed for quality and quantity using the Agilent bioanalyzer and nanodrop ND-1000 spectrophotometer (Biolab, Clayton, Victoria, Australia), respectively. Biotin-labeled antisense RNA was synthesized with the Illumina RNA amplification kit (Ambion) as per the manufacturer’s instructions. Briefly, 500 ng total RNA was reverse transcribed to synthesize first- and second-strand cDNA, purified, and then in vitro transcribed to synthesize biotin-labeled antisense RNA. A total of 1.5 ng biotin-labeled cRNA was hybridized to each Illumina mouse WG6 version 2 array (Illumina) at 55 C for 18 h. The hybridized BeadChip was washed and labeled with streptavidin-Cy3 (GE Healthcare, Piscataway, NJ) and then scanned with the Illumina BeadStation 500 System (Illumina). The scanned image was imported into BeadStudio version 3 software (Illumina), and raw probe expression values were extracted. Expression analysis was undertaken with GeneSpring GX software (Agilent Technologies, Forest Hill, Victoria, Australia), and t test (P < 0.02) was used to calculate significance for all transcripts with 2-fold or greater difference. DAVID was used for Gene Ontology analysis. Raw data are deposited at GEO GSE11396 (Gene expression of transgenic knock-in mutant GH receptor mice, released February 1, 2009).
Contributions of GHR signaling pathways to transcript expression were determined as follows: STAT5-regulated (GHR distal portion below 391) transcripts = WT vs. 391-GHR transcripts; JAK2-regulated transcripts = 391-GHR vs. GHRBox1−/− transcripts; and Src-regulated transcripts = GHRBox1−/− vs. GHR−/− transcripts.
Real-time PCR verification of Illumina array targets
Deoxyribonuclease-treated RNA was reverse transcribed using Superscript III (Invitrogen, Carlsbad, CA), and used for real-time PCR with Sybr Green Technology (Applied Biosystems, Foster City, CA). Primer sequences are detailed in Table 3. Analysis was performed by calculating the change in cycle threshold between glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and the gene of interest and expressed as fold change relative to GHRBox1−/− animals.
Table 3.
Full details of the primers used for real-time PCR verification of putative Src/ERK-regulated genes identified from Illumina arrays
| Gene | Primer or sequence source | Primer sequence |
|---|---|---|
| H2-B1 | NM_008199 | Fwd TGGTGGCTTTTGTGTTGAAGAG |
| Rev TCAGGATGCAGATGATCCCTTA | ||
| Serpina11 | NM_199314 | Fwd AGGACGCCACTCCGAATTC |
| Rev GACGCCTCCTGGTCGTACAG | ||
| Adhd1 | PrimerBank ID 15011864a1 | Fwd AAGATGCTGAGTTCCTCCCTG |
| Rev ACATGCCCAGTAGTAGCCCAA | ||
| Cdkn1a | PrimerBank ID 6671726a1 | Fwd CCTGGTGATGTCCGACCTG |
| Rev CCATGAGCGCATCGCAATC | ||
| Gdi2 | NM_008112 | Fwd AGCCCTGGCATCTAGCTTAATG |
| Rev TGGCAACATAAACCAGGAACTTC | ||
| Mif4gd | PrimerBank ID 34328402a1 | Fwd ATGAGCGAGGCCAGTAGAGAT |
| Rev TCTAAGTCCACAGCACTTGGAT | ||
| Vnn3 | NM_011979 | Fwd GCCACCCCTGCAGAGGTTA |
| Rev TGCCTGCCCCGATGAG |
Fwd, Forward; Rev, reverse.
In vitro inhibitor studies
AML12 murine hepatocytes were cultured in DMEM/F12 containing 10% Serum Supreme, ITS, and dexamethasone (0.4 μg/ml). Cells were washed in PBS and cultured for 5 h in DMEM-F12 media without additives. Cells were treated with either dimethylsulfoxide vehicle or ERK inhibitor PD98059 (20 μm) (Merck) for 1 h, followed by hGH (50 ng/ml) for 6 h. Cells were washed with PBS and harvested into Trizol reagent (Invitrogen, CA). RNA was prepared as per manufacturer’s instructions, reverse transcribed, and analyzed by real-time PCR.
Western ligand blot
Recombinant rat IGF-I (GroPep, Adelaide, Australia) was iodinated using the iodogen method and purified using Sephadex G-50 chromatography (11). Western ligand blotting was performed as described previously, and blots were analyzed using densitometry (11). Relative [125I]IGF-I bound to IGFBP was calculated from the integrated OD value per sample. Bands at 38.5–41.5, 32–34, and 30 kDa correspond to IGFBP-3; IGFBP-1, -2, and -5; and IGFBP-4, respectively (42).
Signaling immunoblots
Immunoblot analysis for signaling protein phosphorylation was carried out on hepatic homogenates obtained from 19-d-old mice injected ip with 8 μg/g body weight of bGH (Monsanto Co., St. Louis, MO) and killed 5–15 min later, according to Choi and Waxman (13). For phospho-Src and phospho-Akt detection, mice were withdrawn from food for 6 h before bGH injection. Antibodies used were JAK2 (sc-278), STAT5 (sc-835), and ERK1 (sc-93) antibodies from Santa Cruz Biotechnology (Santa Cruz, CA), anti-phosphotyrosine 4G10 (Upstate), phospho-p44/42 MAPK (Thr202/Tyr204 no. 9101), phospho-Src (Y416 no. 2101), and phospho-Akt (S473 no. 9270) antibodies from Cell Signaling Technology (Beverly, MA) and anti-Src clone GD11 mouse antibody from Chemicon (Temecula, CA). All primary antibodies were used at 1:1000, except for the phospho-Src (1:500). Immunoprecipitation was performed as per Rowland et al. (11). After transfer immunoblots were blocked in 2% BSA (fraction V; Sigma) or 5% skim milk powder in TBS/Tween 20 and then incubated with primary and secondary antibodies. After autoradiography, the bands were quantified with Bio-Rad imaging densitometer GS-700 and software. All estimates were normalized by second-round immunoblotting with the immunoprecipitation antibody (JAK2, STAT5, and Src) or a total ERK1 antibody (sc-93; Santa Cruz Biotechnology) or Akt antibody no. 610860 from BD Biosciences (San Diego, CA).
GHR measurements
Immunoblot analysis for GHR in liver membranes from 42- to 60-d-old mice was carried out using a polyclonal antibody raised against a GST fusion of the rabbit GHR cytoplasmic domain as the immunoprecipitating antibody (43) and mGHR antibody (R&D Systems, Minneapolis, MN; catalog item AF1360) for the blot.
Statistical analysis
Multiple comparisons were undertaken by ANOVA, with Tukeys post hoc test. Comparisons of two groups only used unpaired Student’s t test.
Acknowledgments
We thank John Kopchick and Karen Coschigano for providing us with the GHR−/− mice. We also thank Edith Gardiner for her expertise with the PIXImus.
Footnotes
This work was supported by the National Health and Medical Research Council (Australia), both as project grants to M.J.W. and P.G.N. and as a Senior Principal Research fellowship to M.J.W.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 2, 2009
J.L.B. and L.M.K. are joint first authors.
Abbreviations: bGH, Bovine GH; DEXA, dual-energy x-ray absorptiometry; ES, embryonic stem; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GHR, GH receptor; hGH, human GH; IGFBP3, IGF-binding protein-3; JAK, Janus tyrosine kinase; mGH, murine GH; MUP, major urinary proteins; SFK, Src family kinases; STAT5, signal transducer and activator of transcription 5; WT, wild type.
References
- 1.Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244 [DOI] [PubMed] [Google Scholar]
- 2.Frank SJ, Gilliland G, Kraft AS, Arnold CS1994. Interaction of the GH receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology 135:2228–2239 [DOI] [PubMed] [Google Scholar]
- 3.Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ2006. New insights into growth hormone action. J Mol Endocrinol 36:1–7 [DOI] [PubMed] [Google Scholar]
- 4.Smit L, Meyer D, Argetsinger L, Schwartz J, Carter-Su C1999. Molecular events in GH-receptor interaction and signaling. In: Kostyo JL, ed. Handbook of physiology, section 7: the endocrine system. New York: Oxford University Press; 445–480
- 5.Clayton PE, Freeth JS, Whatmore AJ, Ayling RM, Norman MR, Silva CM1999. Signal transduction defects in growth hormone insensitivity. Acta Paediatr Suppl 88:174–178; discussion 179 [DOI] [PubMed] [Google Scholar]
- 6.Zhu T, Ling L, Lobie PE2002. Identification of a JAK2-independent pathway regulating growth hormone (GH)-stimulated p44/42 mitogen-activated protein kinase activity. GH activation of Ral and phospholipase D is Src-dependent. J Biol Chem 277:45592–45603 [DOI] [PubMed] [Google Scholar]
- 7.Rowlinson SW, Yoshizato H, Barclay JL, Brooks AJ, Behncken SN, Kerr LM, Millard K, Palethorpe K, Nielsen K, Clyde-Smith J, Hancock JF, Waters MJ2008. An agonist-induced conformational change in the growth hormone receptor determines the choice of signaling pathway. Nat Cell Biol 10:740–747 [DOI] [PubMed] [Google Scholar]
- 8.Fresno Vara JA, Carretero MV, Gerónimo H, Ballmer-Hofer K, Martín-Pérez J2000. Stimulation of c-Src by prolactin is independent of Jak2. Biochem J 345:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai A, Kanda E, Nosaka Y, Miyasaka N, Miura O2001. CrkL is recruited through its SH2 domain to the erythropoietin receptor and plays a role in Lyn-mediated receptor signaling. J Biol Chem 276:33282–33290 [DOI] [PubMed] [Google Scholar]
- 10.Rowland JE, Kerr LM, White M, Noakes PG, Waters MJ2005. Heterozygote effects in mice with partial truncations in the growth hormone receptor cytoplasmic domain: assessment of growth parameters and phenotype. Endocrinology 146:5278–5286 [DOI] [PubMed] [Google Scholar]
- 11.Rowland JE, Lichanska AM, Kerr LM, White M, d'Aniello EM, Maher SL, Brown R, Teasdale RD, Noakes PG, Waters MJ2005. In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol Cell Biol 25:66–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ1997. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA 94:13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi HK, Waxman DJ2000. Plasma growth hormone pulse activation of hepatic JAK-STAT5 signaling: developmental regulation and role in male-specific liver gene expression. Endocrinology 141:3245–3255 [DOI] [PubMed] [Google Scholar]
- 14.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R1998. STAT3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol 18:2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson BJ, Shang CA, Waters MJ2000. Identification of genes induced by GH in rat liver using cDNA arrays. Endocrinology 141:4321–4324 [DOI] [PubMed] [Google Scholar]
- 16.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ2006. Sex-dependent liver gene expression is extensive and largely dependent on STAT5b: STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20:1333–1351 [DOI] [PubMed] [Google Scholar]
- 17.Engblom D, Kornfeld JW, Schwake L, Tronche F, Reimann A, Beug H, Hennighausen L, Moriggl R, Schütz G2007. Direct glucocorticoid receptor-STAT5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev 21:1157–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, Waxman DJ2007. Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology 148:1977–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodyer CG, Rhani Z, Zheng H2008. Expression of the hepatic specific V1 messenger ribonucleic acid of the human growth hormone receptor gene is regulated by hepatic nuclear factor (HNF)-4α2 and HNF-4α8. Mol Endocrinol 2008 22:485–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q, Walther N, Jiang H2004. Chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) and hepatocyte nuclear factor 4γ (HNF-4γ) and HNF-4α regulate the bovine growth hormone receptor 1A promoter through a common DNA element. J Mol Endocrinol 32:947–961 [DOI] [PubMed] [Google Scholar]
- 21.Yoon S, Seger R2006. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24:21–44 [DOI] [PubMed] [Google Scholar]
- 22.Cakmakci NG, Lerner RS, Wagner EJ, Zheng L, Marzluff WF2008. SLIP1, a factor required for activation of histone mRNA translation by the stem-loop binding protein. Mol Cell Biol 28:1182–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu R, Melmed S2004. Pituitary tumor transforming gene: an update. Front Horm Res 32:175–185 [DOI] [PubMed] [Google Scholar]
- 24.Rosenfeld RG, Belgorosky A, Camacho-Hubner C, Savage MO, Wit JM, Hwa V2007. Defects in growth hormone receptor signaling. Trends Endocrinol Metab 18:134–141 [DOI] [PubMed] [Google Scholar]
- 25.Tiulpakov A, Rubtsov P, Dedov I, Peterkova V, Bezlepkina O, Chrousos GP, Hochberg Z2005. A novel C-terminal growth hormone receptor (GHR) mutation results in impaired GHR-STAT5 but normal STAT-3 signaling. J Clin Endocrinol Metab 90:542–547 [DOI] [PubMed] [Google Scholar]
- 26.Chia DJ, Ono M, Woelfle J, Schlesinger-Massart M, Jiang H, Rotwein P2006. Characterization of distinct Stat5b binding sites that mediate growth hormone-stimulated IGF-I gene transcription. J Biol Chem 281:3190–3197 [DOI] [PubMed] [Google Scholar]
- 27.Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A2001. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 229:141–162 [DOI] [PubMed] [Google Scholar]
- 28.Hogan JC, Stephens JM2005. The regulation of fatty acid synthase by STAT5A. Diabetes 54:1968–1975 [DOI] [PubMed] [Google Scholar]
- 29.White UA, Coulter AA, Miles TK, Stephens JM2007. The STAT5A-mediated induction of pyruvate dehydrogenase kinase 4 expression by prolactin or growth hormone in adipocytes. Diabetes 56:1623–1629 [DOI] [PubMed] [Google Scholar]
- 30.Fain JN, Ihle JN, Bahouth SW1999. Stimulation of lipolysis but not of leptin release by growth hormone is abolished in adipose tissue from Stat5a and b knockout mice. Biochem Biophys Res Commun 263:201–205 [DOI] [PubMed] [Google Scholar]
- 31.Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW1997. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94:7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin H, Lanning NJ, Carter-Su C2008. JAK2, but not Src family kinases, is required for STAT, ERK, and Akt signaling in response to growth hormone in preadipocytes and hepatoma cells. Mol Endocrinol 22:1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi JH, Kim HS, Kim SH, Yang YR, Bae YS, Chang JS, Kwon HM, Ryu SH, Suh PG2006. Phospholipase Cγ1 negatively regulates GH signaling by forming a ternary complex with JAK2 and protein tyrosine phosphatase-1B. Nat Cell Biol 8:1389–1397 [DOI] [PubMed] [Google Scholar]
- 34.Hosui A, Hennighausen L2008. Genomic dissection of the cytokine-controlled STAT5 signaling network in liver. Physiol Genomics 34:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lichanska AM, Waters MJ2008. How GH controls growth, obesity and sexual dimorphism. Trends Genet 24:41–47 [DOI] [PubMed] [Google Scholar]
- 36.Vigneron A, Roninson IB, Gamelin E, Coqueret O2005. Src inhibits adriamycin-induced senescence and G2 checkpoint arrest by blocking the induction of p21waf1. Cancer Res 65:8927–8935 [DOI] [PubMed] [Google Scholar]
- 37.Gregory PA, Gardner-Stephen DA, Lewinsky RH, Duncliffe KN, Mackenzie PI2003. Cloning and characterization of the human UDP-glucuronosyltransferase 1A8, 1A9 and 1A10 gene promoters: differential regulation through an interior-like region. J Biol Chem 278:3601–3614 [DOI] [PubMed] [Google Scholar]
- 38.Hirano K, Min J, Funahashi T, Davidson NO1997. Cloning and characterization of the rat apobec-1 gene: a comparative analysis of gene structure and promoter usage in the mouse and rat. J Lipid Res 38:1103–1119 [PubMed] [Google Scholar]
- 39.Acosta JJ, Muñoz RM, González L, Subtil-Rodríguez A, Dominguez-Caceres MA, García-Martínez JM, Calcabrini A, Lazaro-Trueba I, Martín-Pérez J2003. Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/Erk1/2 and phosphatidylinositol 3-kinase pathways. Mol Endocrinol 17:2268–2282 [DOI] [PubMed] [Google Scholar]
- 40.Wu H, Liu X, Jaenisch R1994. Double replacement: strategy for efficient introduction of subtle mutations into the murine Col1a-1 gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci USA 91:2819–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E, Maniatis T1989. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press
- 42.Hossenlopp P, Seurin D, Segovia-Quinson B, Hardouin S, Binoux M1986. Analysis of serum insulin-like growth factor binding proteins using Western blotting: use of the method for titration of the binding proteins and competitive binding studies. Anal Biochem 154:138–143 [DOI] [PubMed] [Google Scholar]
- 43.Lobie PE, Wood TJ, Chen CM, Waters MJ, Norstedt G1994. Nuclear translocation and anchorage of the GH receptor. J Biol Chem 269:31735–31746 [PubMed] [Google Scholar]