Abstract
Adiponectin is an adipocyte-secreted, insulin-sensitizing hormone the circulating levels of which are reduced in conditions of insulin resistance and diabetes. Previous work has demonstrated the importance of posttranslational modifications, such as proline hydroxylation and lysine hydroxylation/glycosylation, in adiponectin oligomerization, secretion, and function. Here we describe the first functional characterization of adiponectin sialylation. Using a variety of biochemical approaches we demonstrated that sialylation occurs on previously unidentified O-linked glycans on Thr residues of the variable domain in human adiponectin. Enzymatic removal of sialic acid or its underlying O-linked sugars did not affect adiponectin multimer composition. Expression of mutant forms of adiponectin (lacking the modified Thr residues) or of wild-type adiponectin in cells defective in sialylation did not compromise multimer formation or secretion, arguing against a structural role for this modification. Activity of desialylated adiponectin was comparable to control adiponectin in L6 myotubes and acute assays in adiponectin−/− mice. In contrast, plasma clearance of desialylated adiponectin was accelerated compared with that of control adiponectin, implicating a role for this modification in determining the half-life of circulating adiponectin. Uptake of desialylated adiponectin by isolated primary rat hepatocytes was also accelerated, suggesting a role for the hepatic asialoglycoprotein receptor. Finally, after chronic administration in adiponectin−/− mice steady-state levels of desialylated adiponectin were lower than control adiponectin and failed to recapitulate the improvements in glucose and insulin tolerance tests observed with control adiponectin. These data suggest an important role for sialic acid content in the regulation of circulating adiponectin levels and highlight the importance of understanding mechanisms regulating adiponectin sialylation/desialylation.
This report shows that the variable domain of adiponectin is O-glycosylated/sialated and this affects its half-life in the circulation but not multimerisation, secretion or activity.
Adiponectin is an insulin-sensitizing adipokine with antiinflammatory and antiatherogenic properties. Circulating adiponectin levels are reduced in insulin resistance, type 2 diabetes, and associated disease states (1, 2, 3), and adiponectin supplementation by transgenic overexpression ameliorates insulin resistance in diabetic animal models (4). Thus, there is a rationale for exploring therapeutic strategies to reverse hypoadiponectinemia in disease states.
Adiponectin is secreted and circulates as a series of multimers ranging from trimers and hexamers [low molecular weight (LMW) multimers to high-molecular-weight (HMW) multimers (5)]. Of these it appears to be the HMW species that are the most bioactive with respect to insulin sensitivity and regulation of glucose homeostasis (6). Accordingly, it is the HMW forms that are selectively reduced in disease states (3, 7).
Although adiponectin expression is down-regulated in insulin-resistant states (2), other mechanisms may also contribute to the reduction in circulating levels of HMW adiponectin in disease states. For example, we and others have demonstrated the importance of posttranslational modification (PTM) of adiponectin in determining the efficiency of multimer formation and secretion (8, 9). In particular, four hydroxylated and glycosylated lysine residues in the collagenous domain were found to be essential for formation of the HMW forms, selectively (8, 9), and HMW multimers were found to be glycosylated to a greater extent than LMW multimers (9).
Unlike the regulation of adiponectin production, little is understood of the mechanisms regulating adiponectin clearance. Here we characterize additional PTMs of adiponectin O-glycosylation and sialylation of Thr residues in the variable domain and demonstrate a potential role for sialic acid modification of adiponectin in regulating its clearance.
Results
Quantitation of adiponectin sialylation by two-dimensional electrophoresis (2DE)
Sato et al. (10) reported the presence of sialic acid in bovine and murine adiponectin by Western blotting using the S2-566 monoclonal antibody specific for the NeuAcα-2,8-NeuAcα-2,3-Gal disialic acid. Here we demonstrate the use of 2DE to resolve differentially sialylated isoforms and, thus, more accurately measure relative sialic acid content of adiponectin. Human adiponectin secreted from SGBS adipocytes or a human embryonic kidney (HEK) stable cell line was treated with a broad-specificity neuraminidase to remove sialic acid residues. Analysis of the samples by one-dimensional SDS-PAGE and Western blotting revealed that neuraminidase-treated adiponectin migrated with a faster mobility than the untreated protein, consistent with a loss of mass (Fig. 1A). Colabeling of these blots with a sialic acid binding probe, Maackia Amurensis Lectin II (MAL II; specific for α2,3-linked sialic acid) confirmed the loss of sialic acid upon neuraminidase treatment (Fig. 1A). Analysis of the same samples by 2DE revealed a train of up to seven isoelectric point (pI) variants in the untreated samples that were collapsed to a major basic spot after treatment with neuraminidase (Fig. 1B). Thus, it appears that the pI variants of increasing acidity represent states of increasing sialic acid content. 2DE has, therefore, been employed as a semiquantitative means of assessing relative sialic acid content of adiponectin in the subsequent experiments. Hence, the sialic acid content of human adiponectin produced by SGBS adipocytes is greater than that produced by HEK fibroblasts, and this is consistent with greater relative intensity of MAL II signal. Subsequent analysis revealed that human serum adiponectin is similar in sialic acid content to that produced by SGBS adipocytes (see below).
Fig. 1.
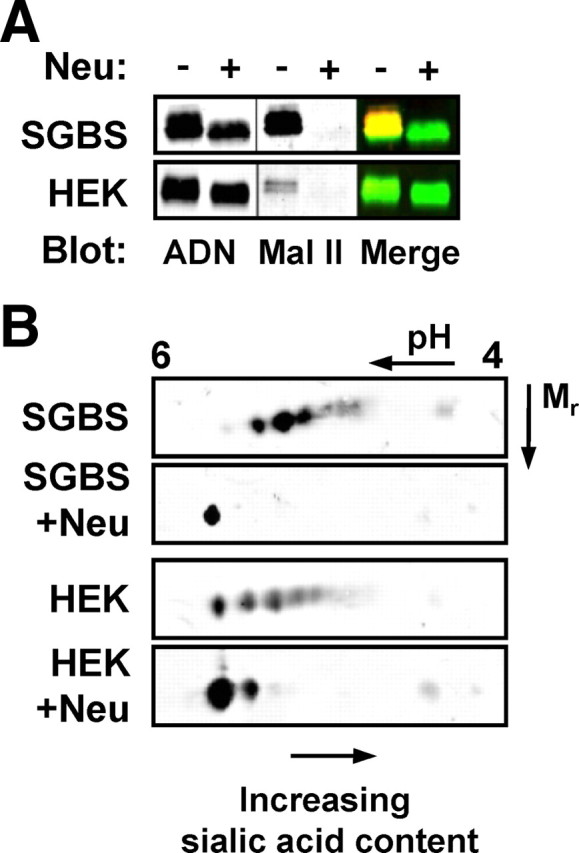
Detection of adiponectin sialylation using MAL II and 2DE. Conditioned serum-free medium from SGBS human adipocytes and HEK cells stably expressing human adiponectin was subjected to neuraminidase treatment (Neu) before analysis by (A) reducing, denaturing SDS-PAGE and dual immunoblotting with anti-adiponectin (ADN, green) and the MAL II lectin (red) or (B) 2DE followed by immunoblotting for adiponectin. Blots are representative of at least three independent experiments.
Sialylation occurs on novel Ser/Thr O-linked sugars
Sialic acid is a common terminal (capping) modification on complex O- and N-linked oligosaccharides of many secreted and cell-surface proteins. Given that the only glycosylations described for adiponectin occur on conserved lysines in the variable and collagenous domains, we set out to determine whether the sialic acids occur on these modified lysines or on other novel O-glycosylated residues in human adiponectin [previous studies suggest adiponectin is not N-glycosylated (10)]. Wild-type (WT) human adiponectin and a mutant lacking the known glycosylated lysines [K1-5R (8)] were transiently expressed in Chinese hamster ovary (CHO) cells, and the secreted proteins were subjected to neuraminidase treatment. One-dimensional SDS-PAGE revealed a similar mobility shift in wild-type (WT) and mutant proteins after treatment, indicating that sialic acids occur on residues other than the glycosylated lysines (Fig. 2A). This is in keeping with our previously published 2DE profile of a K2–5R mutant that exhibits similar pI isoforms as WT adiponectin (8). To determine whether Ser/Thr O-glycosylations may be involved, we also treated WT adiponectin with neuraminidase in combination with an O-glycosidase (endo-α-N-acetylgalactosaminidase) that specifically cleaves the O-GalNAc-Gal sequence of O-linked sugars once sialic acid has been removed. This resulted in a further mobility shift in addition to that observed with neuraminidase alone. As expected O-glycosidase treatment alone (without prior removal of sialic acid) was without effect (Fig. 2B). Thus, sialic acid modification occurs on previously undescribed O-glycosylated Ser/Thr residues.
Fig. 2.
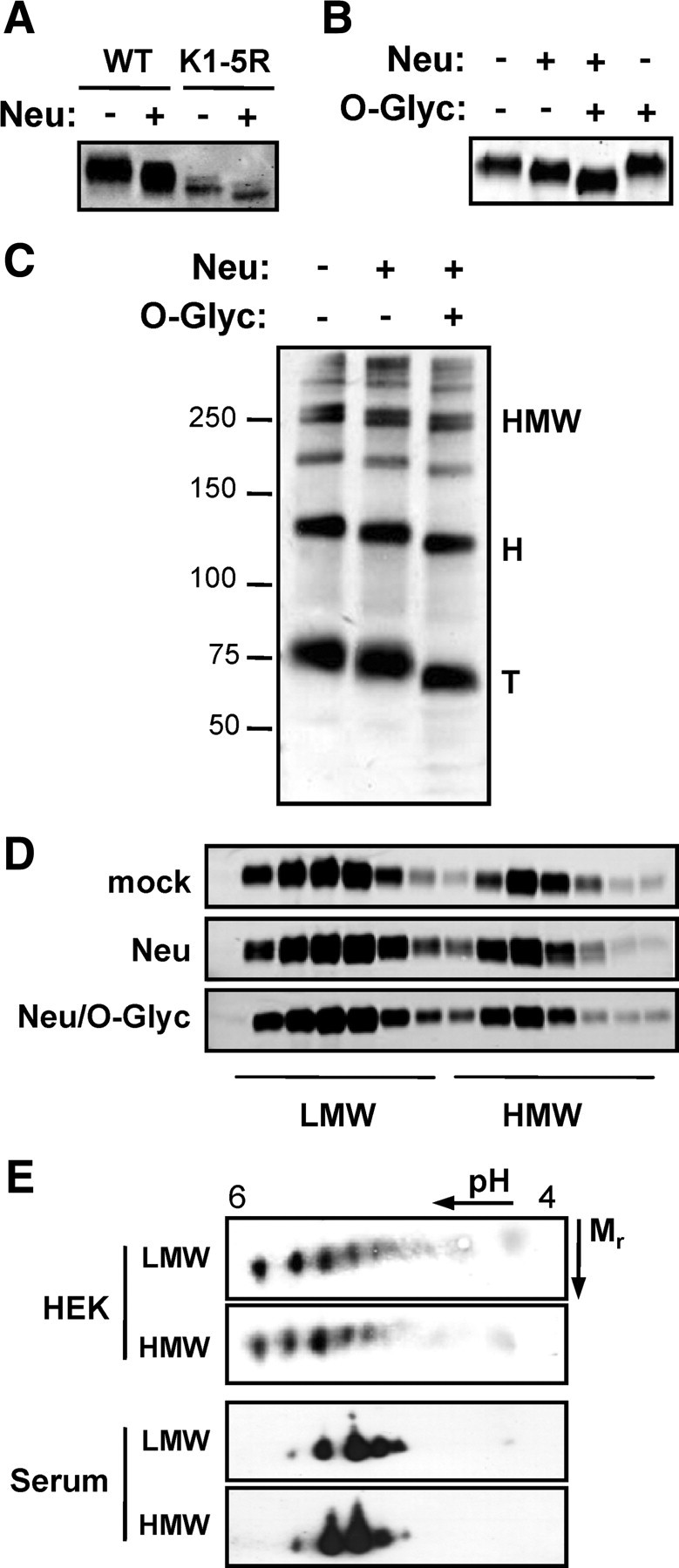
Characterization of adiponectin sialylation. A, WT adiponectin and a mutant (K1-5R; lacking all five glycosylated lysine residues in the collagenous and variable domains) were subjected to neuraminidase treatment before analysis by reducing, denaturing SDS-PAGE, and Western blotting. B, WT adiponectin was either untreated, treated with neuraminidase (Neu), treated with neuraminidase followed by O-glycosidase (O-Glyc), or treated with O-glycosidase alone as indicated before analysis by SDS-PAGE and Western blotting. C, SGBS adiponectin was either untreated or treated with neuraminidase alone (Neu) or with neuraminidase followed by O-glycosidase (O-Glyc) before analysis by SDS-PAGE under nonreducing, non-heat-denaturing conditions and subsequent Western blotting. D, WT adiponectin was treated as above and analyzed by velocity sedimentation on 5–20% sucrose gradients. E, HEK-secreted adiponectin and human serum adiponectin were fractionated on 5–20% sucrose gradients to obtain LMW and HMW multimer fractions and analyzed by 2DE analysis and Western blotting. Data are representative of two to three independent experiments. H, Hexamers; T, trimers.
We next examined the effects of neuraminidase and O-glycosidase treatment on adiponectin multimers by nonreducing, non-heat-denaturing gradient gel electrophoresis. Treatment with neuraminidase revealed no change in multimer composition, although a clear mobility shift was detected for all multimers upon treatment with neuraminidase (Fig. 2C). Similarly, no change was observed even after removal of the underlying O-linked sugars by O-glycosidase (Fig. 2C). Analysis by velocity sedimentation on sucrose gradients provided further evidence that multimer distribution was relatively unaffected by the treatments (Fig. 2D). To determine whether sialic acid modification of HMW multimers is greater than that of LMW multimers [as demonstrated for lysine glycosylation (9)], LMW (which contains trimers and hexamers) and HMW fractions of HEK-secreted adiponectin and human serum adiponectin were enriched by velocity sedimentation and subjected to 2DE and Western blotting. No difference in isoform composition was observed, indicating that sialylation is not a multimer-specific modification (Fig. 2E).
Sialylation is not required for adiponectin multimer formation or secretion
Previously characterized PTMs of adiponectin, including disulfide bond formation, proline hydroxylation, and lysine hydroxylation/glycosylation, have critical roles in multimer formation and secretion. It was therefore surprising that neuraminidase/O-glycosidase treatment had no effect on multimer composition. To further examine the role of sialic acid modification in intracellular multimer formation and secretion, we employed the mutant CHO Lec2 polyclonal cell line, which is defective in sialylation (13). Lec2 cells have defects in the sialic acid transporter responsible for import of CMP-sialic acid, the substrate of sialyltransferases, into the Golgi lumen (13). WT CHO-K1 cells and Lec2 mutant cells were transiently transfected with human or mouse adiponectin, and secreted adiponectin was harvested and analyzed. Human adiponectin secreted by Lec2 cells migrated significantly faster (by SDS-PAGE) than that produced by WT cells (Fig. 3A, Monomer), and 2DE analysis confirmed the loss of sialic acid (Fig. 3C). By comparison, mouse adiponectin produced by Lec2 cells exhibited only a slight mobility shift, suggesting that the mouse protein may be sialylated to a lesser extent than human adiponectin (Fig. 3A). Indeed, 2DE analysis revealed that mouse adiponectin has fewer pI variants than human adiponectin (Fig. 3C), in keeping with previously published data (14). Analysis by SDS-PAGE under nonreducing and non-heat-denaturing conditions revealed no loss of HMW multimers in either human or mouse proteins expressed in Lec2 cells (Fig. 3A, Multimers), and this was confirmed by velocity sedimentation (Fig. 3B). These observations suggest that sialylation is not required for multimerization or secretion.
Fig. 3.
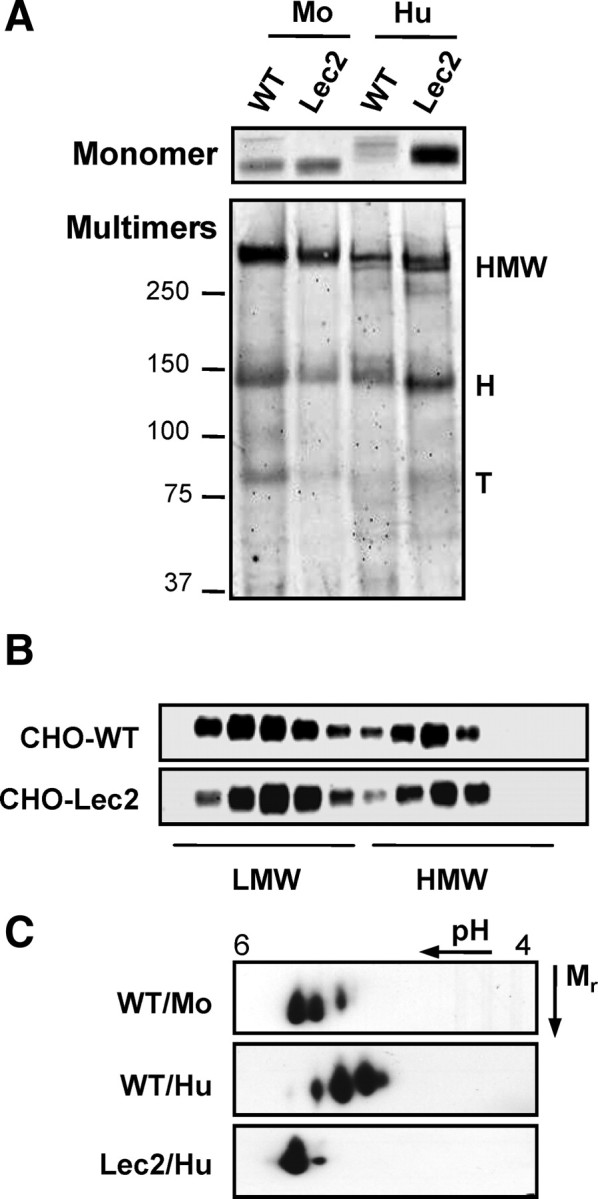
Sialic acid modification is not required for multimer formation or secretion of human or mouse adiponectin. A, WT and Lec2 CHO cells were transiently transfected with human (Hu) or mouse (Mo) adiponectin, and secreted adiponectin was analyzed by SDS-PAGE and Western blotting. B, Velocity sedimentation analysis of human adiponectin secreted from WT and Lec2 CHO. C, 2DE of WT and Lec2 samples. Data are representative of three independent experiments. H, Hexamers; T, trimers.
Sialylation is restricted to the headless domain of human adiponectin
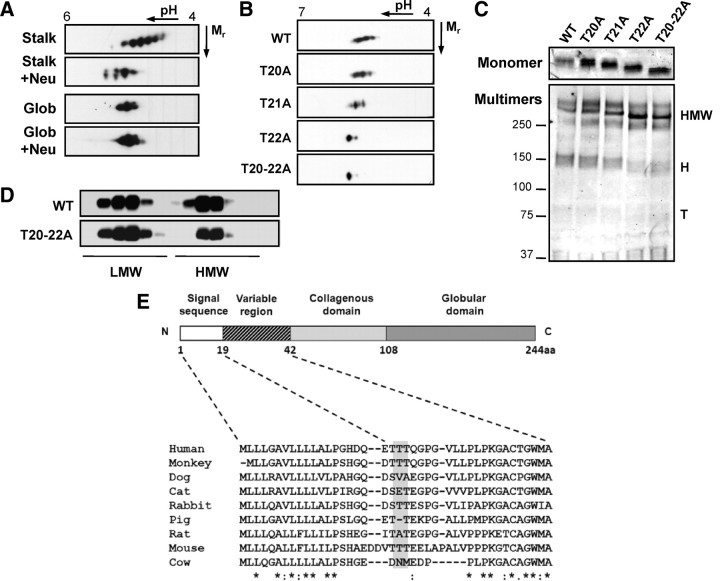
Prediction of Ser/Thr O-glycosylation sites is not straightforward due to a lack of general consensus sequences. Available algorithms are based on mucin-type O-glycosylation sites, which generally occur in clusters in regions of likely amino acid composition and secondary structure (11, 12). In an attempt to identify the protein domain(s) of adiponectin that bare sialic acid, we examined FLAG-tagged globular head and headless domain-truncated proteins secreted from HEK cells. By 2DE, the headless domain alone resembled the full-length protein, displaying a train of six or more pI variants (Fig. 4A). As observed for the full-length protein, neuraminidase treatment of the headless domain produced a dramatic shift in pI consistent with loss of sialic acid. By comparison, neuraminidase treatment was without effect on the globular head domain (Fig. 4A). Thus, the sialylated residues appear to be restricted to the collagenous and/or variable domains of adiponectin.
Fig. 4.
Identification of novel O-glycosylated sialylated Thr residues in human adiponectin. A, FLAG-tagged headless (Stalk) and globular head (Glob) truncated proteins were either untreated or treated with neuraminidase before 2DE and Western blotting for the FLAG tag. B, 2DE of Thr to Ala mutants of human adiponectin secreted from transfected CHO cells. C, SDS-PAGE and Western blotting of the same samples. D, Velocity sedimentation analysis of secreted WT and T20-22A adiponectin. E, Sequence alignment of the adiponectin variable domain from various mammals is shown and reveals limited conservation of the modified sequence/residues (shaded). Data are representative of two to three independent experiments. H, Hexamers; T, trimers.
Mass spectrometric identification of sialylated peptides
To further define the sialylated sites/peptides of human adiponectin, we employed a mass spectrometric approach. Liquid chromatography-matrix-assisted laser desorption ionization mass spectrometry and liquid chromatography-Electrospray ionization tandem mass spectrometric analysis of a tryptic digest of recombinant human adiponectin resulted in high-sequence coverage (>84%), enabling identification of the previously published modified Lys and Pro residues as well as two novel hydroxyprolines (86 and 104). The identification of sialic acid-containing glycopeptides was facilitated by using the extracted ion chromatogram tool within the Analyst software followed by manual interrogation of the tandem mass spectrometry spectra of candidate peptides (for details see supplemental Figs. 1 and 2 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). These analyses allowed unambiguous identification of the modified peptide as E19-R55 containing a carbamidomethyl cysteine, a deamidated asparagine, and hydroxylated residues at Lys33, Pro44, Pro47, and Pro53. Glycopeptide isoforms containing zero, one, and two sialic acid residues (A4–A6) were observed (as listed in supplemental Table I). Although the exact site of glycosylation was unable to be determined from the spectrum, we have previously eliminated Lys33 as a potential site (see Fig. 2A); thus, the likely site of modification is either of three threonine residues (Thr20, Thr21, or Thr22) in the variable region of the peptide. Consistent with this hypothesis, no glycosylated form of the peptide G34-R55 was observed.
Confirmation of sialylation sites by site-directed mutagenesis
Having identified the likely sites of O-glycosylation and sialylation, we performed site-directed mutagenesis to confirm their modification. Thr residues 20, 21, and 22 were substituted with Ala either individually or in concert, and the mutant proteins were found to be expressed and secreted similarly to WT. Analysis of secreted proteins by 2DE revealed a similar train of pI variants for T20A as seen for the WT protein, suggesting no sialylation of this residue, whereas T21A appeared to lack only the most acidic isoform of the train (Fig. 4B). By contrast, mutation of T22 abolished the train of pI variants leaving only one major basic isoform corresponding to that seen after desialylation. A similar profile was observed for the combined mutant, T20-22A (Fig. 4B). Analysis of secreted proteins by SDS-PAGE under reducing, denaturing conditions revealed a striking increase in mobility for the combined mutant (T20-22A), indicative of reduced molecular weight (Fig. 4C). Individual mutants also exhibited partial mobility shifts with T22A>T21A>T20A, indicating increasing degrees of modification (Fig. 4C). Together, these data suggests that T21 and T22 are the major sites of O-glycosylation and that sialylation occurs mainly on T22. Analysis of the mutants by non-heat-denaturing, nonreducing SDS-PAGE revealed a multimer composition similar to that of WT adiponectin (Fig. 4C). Furthermore, sucrose gradient analysis of WT and T20-22A revealed similar multimer profiles (Fig. 4D). Thus, O-glycosylation and sialic acid modification of human adiponectin occur on T21 and T22 of the variable domain, in a sequence that shows only limited conservation between species (Fig. 4E). Taken together with our observations from the Lec2 cells, these findings demonstrate that neither sialylation nor O-glycosylation is required for efficient multimerization or secretion.
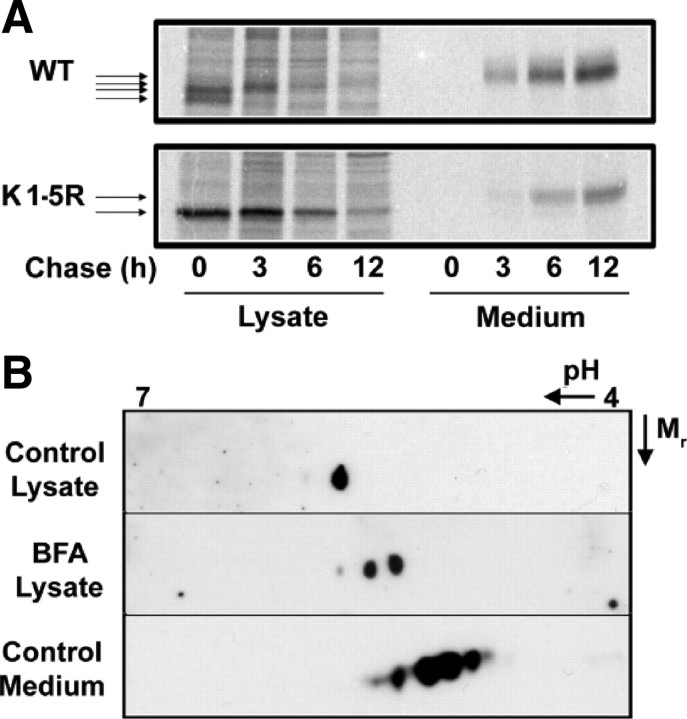
Sialylation is a terminal modification after multimerization and just preceding exocytosis
The finding that sialylation is not required for adiponectin multimer formation or secretion is in keeping with this being a terminal step in the maturation of adiponectin, occurring subsequent to multimer formation and just before secretion. Consistent with this, both the initiation of O-glycosylation (15) and subsequent sialylation (16) (and the enzymes that perform these modifications) are localized in the Golgi. To examine the temporal characteristics of adiponectin sialylation, we performed pulse-chase experiments in stable HEK cell lines expressing hemagglutinin (HA)-tagged human WT or K1-5R adiponectin. Figure 5A shows multiple sequential mobility shifts (indicating serial gains in molecular mass) of the WT protein in lysates, which are absent in the K1-5R mutant, indicating that these shifts represent lysine glycosylations. A final mobility shift is also observed, however, between the largest form in lysate and the secreted form detected in medium samples. This final shift is also detected in the K1-5R mutant, suggesting that it may correspond to O-glycosylation/sialylation. 2DE analyses of lysate and medium samples of CHO stable cell lines expressing human adiponectin were in keeping with the pulse-chase data (Fig. 5B). Adiponectin in lysate was detected as a single basic spot in contrast to the train of spots observed in medium samples. When cells were treated with Brefeldin A to inhibit secretion from the Golgi, however, more acidic isoforms were seen to accumulate in lysate. Similar observations were made in SGBS adipocytes (data not shown).
Fig. 5.
Sialic acid is a terminal modification occurring immediately before secretion. A, HEK cell lines stably expressing HA-tagged WT human adiponectin or the K1–5R mutant (lacking the five known glycosylated lysines in the variable and collagenous domains) were metabolically labeled for 2 h before replacement of the radiolabeled amino acids with an excess of unlabeled amino acids and continued culture for increasing periods of ‘chase’. Lysates and media were harvested and immunoprecipitated with HA antibody before analysis by SDS-PAGE and autoradiography. B, 2DE and Western blotting of lysates, with and without Brefeldin A (BFA) treatment, and medium from CHO cells stably expressing human adiponectin. Data are representative of two independent experiments.
Sialic acid content affects half-life of human adiponectin in the circulation of rats
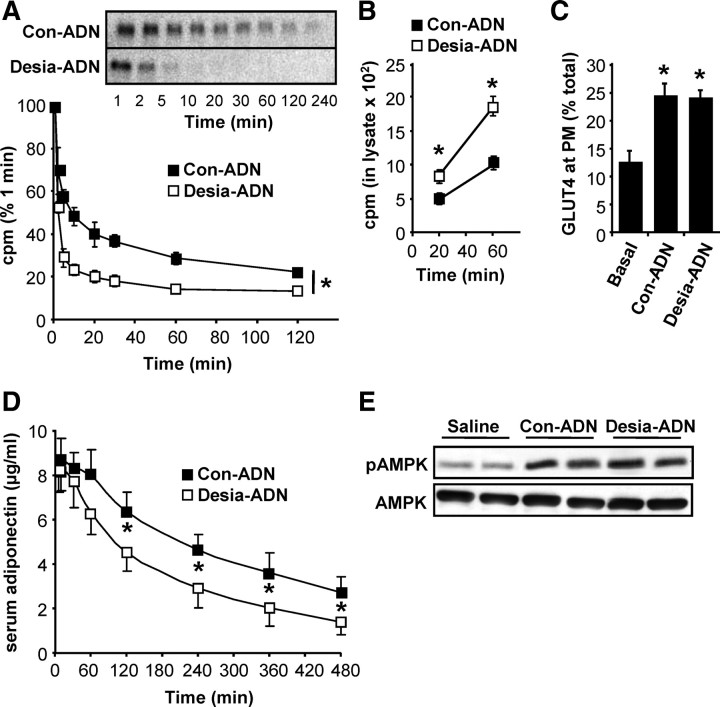
Early studies characterizing the hepatic asialoglycoprotein receptor demonstrated rapid clearance of desialylated serum glycoproteins from the circulation of rodents (17). We, therefore, examined the role of sialic acid in determining the clearance of human adiponectin from the circulation of rats. Purified HEK-produced human adiponectin was radiolabeled by iodination with 125I and desialylated with neuraminidase or mock treated. Male Wistar rats of similar size and age were given an i.v. bolus of either desialylated or control (mock treated) [125I]adiponectin, and blood samples were taken at various time points. Trichloroacetic acid (TCA)-precipitated radioactivity in the serum samples is shown as a percentage of total radioactivity administered and reveals accelerated clearance of the desialylated form (Fig. 6A). Analysis of serum samples by SDS-PAGE and autoradiography confirms that the radioactive counts are reflective of [125I]adiponectin levels. Given the possible role of the hepatic asialogycoprotein receptor in adiponectin clearance, uptake of [125I]adiponectin by primary rat hepatocytes was also assessed. Uptake of desialylated adiponectin was consistently found to occur at a higher rate than that of mock-treated adiponectin (Fig. 6B), and this was confirmed by immunohistochemistry (see supplemental Fig. 3).
Fig. 6.
Acute administration of desialylated adiponectin: accelerated clearance but unaltered activity. A, Male Wistar rats were administered iv with either desialylated (Desia-ADN) or control (Con-ADN) [125I]adiponectin and blood samples were taken from a jugular cannula at various time points after infusion as described in Materials and Methods. TCA-precipitable radioactivity in the serum samples was measured and normalized to the total radioactivity administered (n =5 desialylated and n = 4 control; *, P < 0.001 for the difference in exponential rate constant for disappearance of TCA-precipitable radioactivity from the plasma for control and desialylated adiponectin). Serum samples were also analyzed by SDS-PAGE and autoradiography to confirm the loss of [125I]adiponectin (inset). B, Uptake of control or desialylated [125I]adiponectin by primary rat hepatocytes. Cells were incubated in serum-free culture medium containing the radioligands for various periods of time before washing and harvesting of lysates for measurement of radioactivity (n = 3; *, P < 0.05 Con-ADN vs. Desia-ADN). C, Differentiated L6 rat myotubes expressing HA-GLUT4 were serum starved for 2 h before stimulation with control or desialylated adiponectin for 20 min. Cells were fixed and adiponectin-stimulated GLUT4 translocation to the plasma membrane was assessed (n = 4; *, P < 0.05 Basal vs. Con-ADN or Desia-ADN). D, Male adiponectin−/− mice were starved overnight before administration of control or desialylated recombinant mouse adiponectin-FLAG (50 μg/mouse) or saline by tail vein injection (*, P < 0.05; Con-ADN vs. Desia-ADN). E, Liver lysates from mice killed at 30 min after injection were analyzed by Western blotting for total and phospho-AMPK (pAMPK; Thr172). Note that the circulating concentrations of adiponectin in mice administered with untreated or desialylated adiponectin were 8.3 ± 1.4 mg/liter and 7.7 ± 1.0 mg/liter, respectively (n = 4; P > 0.05).
Sialic acid content has no effect on activity of adiponectin, in vitro or in vivo
The role of the globular domain of adiponectin in receptor binding and activation is well recognized (18). Thus, it seemed unlikely that adiponectin sialylation, a modification of the headless/stalk domain, would influence adiponectin activity, particularly because sialylation does not appear to determine multimer composition. Nevertheless, to address this question, we examined the activity of desialylated vs. mock-treated HEK-produced human adiponectin in an in vitro assay of glucose transporter (GLUT)4 translocation in L6 (rat) myotubes. Human adiponectin from two independent sources consistently induced a 2-fold increase in GLUT4 translocation compared with a 4-fold induction elicited by insulin (data not shown). As expected, desialylated and mock-treated adiponectin preparations also induced a 2-fold induction of GLUT4 translocation, indicating that sialic acid modification of adiponectin is not required for activity (Fig. 5C). This was confirmed by further in vivo experiments in adiponectin−/− mice after tail vein injection of desialylated or untreated preparations of purified mouse adiponectin (50 μg per mouse). Serum sampling for 8 h after injection again revealed a faster rate of clearance of the desialylated adiponectin, however, with slower kinetics than observed for human adiponectin in rats (Fig. 6D). Mice were killed at 30 min after injection when circulating levels of desialylated and mock-treated adiponectin were still comparable, and liver tissues were analyzed by Western blotting for total and phospho-AMP-activated protein kinase (AMPK) (Thr172). Similar activation of AMPK phosphorylation by the desialylated and untreated preparations was observed (Fig. 6E).
Reduced bioavailability of desialylated adiponectin results in reduced metabolic effects
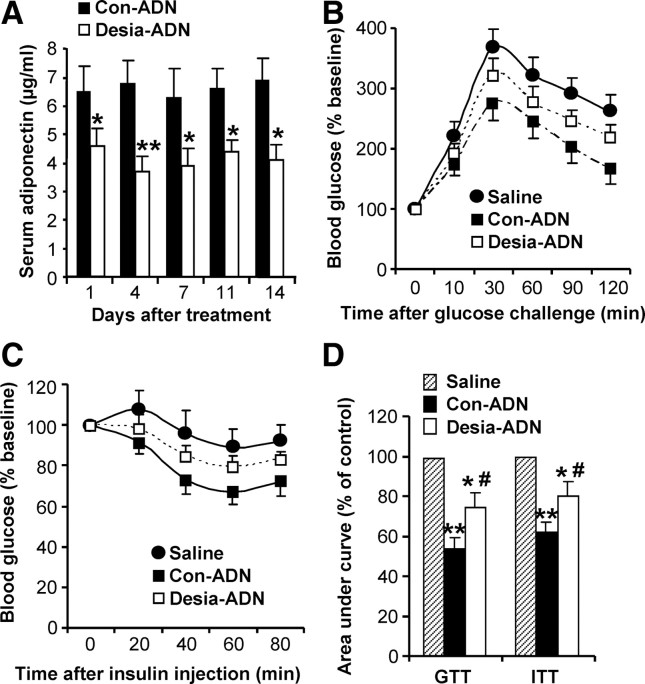
In additional experiments, high-fat-fed male adiponectin−/− mice were chronically administered (by osmotic pump) with either untreated or desialylated adiponectin preparations (1.0 mg/kg body weight/d) for a period of 2 wk. Serum adiponectin levels were measured every 3 d, and a glucose-tolerance test (GTT) and an insulin-tolerance test (ITT) were conducted on d 10 and d 13, respectively. Serum adiponectin levels were found to be consistently lower in mice infused with desialylated adiponectin (Fig. 7A). This was mirrored by impaired ability of desialylated adiponectin to improve glucose tolerance and insulin action (Fig. 7, B–D). These data demonstrate that reduced sialic acid content of adiponectin results in reduced bioavailability and thereby diminished the metabolic benefits of adiponectin.
Fig. 7.
Chronic administration of desialylated adiponectin: reduced steady-state levels and metabolic effects. High-fat-fed male adiponectin−/− mice were chronically administered (by osmotic pump) with control (Con-ADN, n = 6) and desialylated (Desia-ADN, n = 5) adiponectin preparations (1.0 mg/kg body weight · d) or saline for a period of 2 wk. A, Serum adiponectin levels were measured every 3 d (*, P < 0.05; **, P < 0.01). GTT (panel B) and ITT (panel C) were conducted on d 10 and d 13, respectively, and panel D shows the area under the curves (*, P < 0.05; **, P < 0.01 vs. saline; #, P < 0.05 vs. Con-ADN by ANOVA).
Discussion
Sialic acid modification of adiponectin was first identified by Sato and colleagues (10), who demonstrated the presence of NeuAcα-2,8-NeuAcα-2,3-Gal disialic acid in mouse (3T3L1) and bovine (serum) adiponectin using a monoclonal antibody specific for this modification. We have now demonstrated the Thr O-glycosylation and sialylation of human adiponectin using other glycotools (neuraminidase/O-glycosidase treatment and sialic acid-binding lectins) and furthermore, introduce 2DE as a semiquantitative method for analyzing sialic acid content of human adiponectin. Using mass spectrometric approaches coupled with mutagenesis, we have identified the sites of sialic acid modification and begun to elucidate the composition of the O-glycans. Detailed characterization of the glycans is warranted to determine structure and sialic acid linkages. The sialic acid content of sialylated peptides identified by mass spectrometry appears to be incongruous with our 2DE data, because no more than two sialic acid residues were ever observed. However, underrepresentation of sialic acid in matrix-assisted laser desorption ionization mass spectometry data is a common observation due to loss of this sugar upon laser desorption ionization in the absence of derivatization (19). This may be one reason why previous mass spectrometry analyses have failed to detect this modification.
We have also examined the functional relevance/role of O-glycosylation/sialylation in adiponectin multimer formation, secretion, stability, activity, and clearance using a variety of biochemical and cellular assays as well as in vivo experiments in rats and mice. Our finding that O-glycosylation/sialylation plays little or no role in multimer formation or activity of human adiponectin but a much more obvious function in regulating adiponectin clearance sets this PTM apart from those previously characterized for adiponectin. Like those PTMs, O-glycosylation/sialylation occurs in the headless/stalk domain of human adiponectin but does not appear to participate in intermolecular interactions involved in multimer formation, most probably because it occurs late in the maturation process, just before secretion. In keeping with this, sialic acid linkages of intact multimers were found to be fully accessible to neuraminidase activity, resulting in complete collapse of the 2DE train of pI variants.
The location of the O-glycosylation/sialylation sites at the extreme N terminus of human adiponectin (after signal peptide cleavage) follows the common characteristics of mucin-type O-glycosylations, which do not occur within a consensus sequence but rather on secondary structures such as surface-exposed coils/turns positioned at the termini of polypeptides or in linker regions between domains (11, 20). A sequence alignment of adiponectin from multiple species revealed poor conservation of the O-glycosylated/sialylated sequence (Fig. 4E). Indeed, this sequence (E/DTTTQG) is restricted to primate adiponectin, with a similar but shifted sequence in mouse adiponectin (AEDDVTTTEE) and no Thr residues in the corresponding bovine sequence. Thus, it remains to be determined whether the TTT sequence represents the site(s) of O-glycosylation/sialylation in mouse adiponectin, and further work will be required to define the site(s) in bovine adiponectin. Our parallel investigations of mouse adiponectin suggest a lesser extent of sialylation than seen for human adiponectin: fewer and more basic pI variants by 2DE and a modest increase in mobility upon expression in Lec2 cells (Fig. 3). Despite these observations, our in vivo data would suggest a similar effect of desialylation on clearance of mouse adiponectin. Importantly, although the rates of clearance of untreated human and mouse adiponectin differed considerably, desialylation resulted in increased clearance of both proteins in rats and mice, respectively (Fig. 6, A and D).
Sialylation is a common terminal modification on N- and O-linked sugars of glycosylated cell-surface and secreted serum proteins. One class of glycosylated and sialylated serum proteins comprises the acute phase proteins, the production of which is up-regulated in response to inflammation (21). Not only is expression of these proteins up-regulated, sialyltransferases that modify them are also up-regulated (22) resulting in their increased sialylation (23). Taken together with increased expression and activity of neuraminidases that strip sialic acid modifications from glycoproteins (24), these mechanisms result in an elevation of total serum sialic acid levels in inflammatory conditions: both high-grade acute inflammation (e.g. infections, sepsis) and low-grade chronic inflammatory conditions such as obesity/insulin resistance and cardiovascular disease. In fact, total serum sialic acid levels are closely correlated with other inflammatory markers [such as C-reactive peptide (25, 26)] and with disease severity in conditions of insulin resistance/type 2 diabetes (27), atherosclerotic progress (25, 28), and acute myocardial infarction (21, 25).
Adiponectin is an antiinflammatory adipokine, the circulating levels of which are consistently reduced in chronic inflammatory diseases such as those mentioned above. In the current report we have demonstrated that human adiponectin is extensively sialylated and that desialylated adiponectin is rapidly cleared from the circulation. Although the regulation of adiponectin sialylation and desialylation in vivo remains to be elucidated, one obvious possibility is that desialylation of this hormone in inflammatory states may contribute to its accelerated clearance. Interestingly, circulating levels of adiponectin are increased in patients with cirrhosis (29). Evidence suggests that adiponectin is cleared, at least in part, by the liver, and this catabolism is reduced in states of chronic liver disease, possibly as a result of reduced hepatic blood flow (30). Thus, future investigations into the putative role of the hepatic asialoglycoprotein receptor, expression of which is also altered in cirrhosis (31), and mapping of the sialic acid (2DE) profiles of adiponectin in such diseases are warranted.
Materials and Methods
Reagents and antibodies
General reagents were obtained from Sigma-Aldrich (St. Louis, MO). Cell culture reagents were from Invitrogen (Carlsbad, CA). [125I]Radionuclide was from PerkinElmer (Waltham, MA), and Iodogen tubes were from Thermo Fisher Scientific (Rockford, IL). Two rabbit antihuman adiponectin polyclonal antibodies were used for Western blotting and immunofluorescence microscopy, one generated in house (through Auspep, Victoria, Australia) against the globular domain peptide, NH2-QVYGEGRNGLYADNDN-COOH, and the other (AB3784) commercially obtained from Millipore Corp. (Billerica, MA). Antibodies to total and phospho-AMPK were from Cell Signaling Technology (Beverly, MA). Secondary antibodies used were IRDye 800-conjugated antirabbit IgG from Rocklands Immunochemicals (Gilbertsville, PA), horseradish peroxidase-conjugated antirabbit IgG from Pierce Chemical Co. (Rockford, IL), and Alexa Fluor 488-conjugated antirabbit IgG (Molecular Probes, Carlsbad, CA). Biotinylated MAL II was from Vector Laboratories (Burlingame, CA) and was detected with streptavidin-680 (Molecular Probes). Purified recombinant human adiponectin used for mass spectroscopy analysis was from BioVendor Laboratory Medicine (Candler, NC). Purified FLAG-tagged globular head and headless domains of human adiponectin were from Alexis Biochemicals (Lausen, Switzerland). Protease-grade α (2, 3, 6, 8, 9) neuraminidase from Arthrobacter ureafaciens (Sigma-Aldrich) or Clostridium perfringens (Sigma-Aldrich; New England Biolabs, Ipswich, MA) and O-glycosidase (endo-α-N-acetylgalactosaminidase; QA-Bio, San Mateo, CA) were commercially obtained. QuikChange mutagenesis kit was from Stratagene (La Jolla, CA).
Molecular cloning
WT human and K1-5R human adiponectin expression constructs were generated as described previously (8). Mouse cDNA was obtained from the SRC Microarray Facility Fantom 2 cloneset (clone identification: A530090P11, accession no. AK041214). PCR was used to amplify the cDNA and clone it into pcDNA5/FRT-TO. C-terminal hemagglutinin tags were incorporated into WT and K1-5R human cDNAs by PCR before cloning into the pcDNA5/FRT-TO plasmid for generation of stable HEK cell lines. The O-glycosylation-deficient mutants were generated by QuikChange mutagenesis.
Cell culture, transfection, and generation of stable cell lines
WT CHO K1 cells and Lec2 mutant cells were cultured in α-MEM containing l-glutamine, Na pyruvate, deoxyribonucleosides, and ribonucleosides (Invitrogen) supplemented with 10% fetal calf serum. Flp-In HEK cells were cultured in high glucose (25 mm) DMEM supplemented with 2 mm l-glutamine. Both lines were transfected using Lipofectamine Plus. HEK 293 stable cell lines expressing WT or K1-5R human adiponectin constructs were generated using the Flp-In system (8).
Sample preparation and analysis by one-dimensional and 2DE
Cell lysates and medium samples were prepared as described previously (8). SDS-PAGE, Western blotting, and brefeldin A treatment were also as described (8).
GLUT4 translocation in L6 myocytes
GLUT4 translocation assays were performed essentially as described elsewhere (32).
Iodination of recombinant human adiponectin
Human adiponectin (BioVendor) was radiolabeled with 125I by the Chizzonite Indirect Method for Iodination using precoated iodination tubes according to the manufacturer’s instructions (Pierce). [125I]adiponectin was treated with protease-grade neuraminidase (or mock treated) in the reaction buffer provided (pH 6) for 3 h at 37 C with subsequent neutralization with HEPES (pH 7.4) to a final concentration of 100 mm. Mock treatment was followed by addition of an equivalent amount of heat-inactivated neuraminidase. Neuraminidase activity in the desialylated preparation was found to be exhausted by the end of the 3 h incubation at 37 C. Analysis of neuraminidase- and mock-treated preparations confirmed no change in multimer composition (see supplemental Fig. 4), and preparations were subsequently used in in vivo and in vitro rat experiments.
Rat studies
Adult male Wistar rats (∼300 g), supplied from the Animal Resources Centre (Perth, Australia), were communally housed at 22 ± 0.5 C with a controlled 12-h light, 12-h dark cycle (light from 0600 h to 1800 h). They were fed ad libitum a standard chow diet (Rat maintenance diet; Gordons Specialty Feeds, Sydney, Australia). After 1 wk of acclimatization rats were anesthetized with halothane and a cannula implanted into the right jugular vein under aseptic conditions. The catheter was exteriorized at the back of the neck, and rats were allowed 1 wk to recover from surgery. All surgical and experimental procedures performed were approved by the Animal Experimentation Ethics Committee (Garvan Institute/St. Vincent’s Hospital) and were in accordance with the National Health and Medical Research Council of Australia’s guidelines on animal experimentation. On the day of experiment an extension catheter was fitted to the in-dwelling jugular catheter to facilitate blood sampling. Rats received a bolus infusion (>10 sec) of 3 × 106 dpm neuraminidase-treated or mock-treated 125I-labeled human adiponectin (∼300 ng adiponectin), and blood samples (200 μl) were withdrawn at 1, 2, 5, 10, 20, 30, 60, 120, and 240 min. Plasma (50 μl) was diluted to 500 μl with saline and deproteinized by addition of 500 μl of 20% TCA. The samples were centrifuged at 12,000 × g for 5 min, the supernatant was removed, and the radioactivity in the TCA-precipitable pellet was determined as described in Ref. 33 using a γ-counter.
Mouse studies
Preparation of recombinant adiponectin
Recombinant mouse adiponectin was purified as described elsewhere (14, 34). An aliquot of recombinant adiponectin was digested with neuraminidase for 3 h before another round of affinity purification to remove neuraminidase. Protein concentration was determined by BCA.
Chronic experiments
Male C57BL/6J adiponectin KO mice (4 wk old) were fed a high-fat diet for 5 wk and were then surgically implanted with an osmotic pump (DURECT Corp., Cupertino, CA) (14, 34). The pumps were filled with recombinant adiponectin solutions or saline and delivered the protein solutions at a constant rate (1.0 mg/kg body weight · d) for 2 wk. Serum levels of adiponectin were monitored every 3 d. GTT and ITT were conducted at d 10 and d 13 after insertion of the pump after 6 h starvation. There were no obvious differences in blood glucose levels at baseline among the three groups (supplemental Table II).
Acute experiments
Male adiponectin KO mice (10–12 week-old) were starved overnight before treatment with various forms of adiponectin (50 μg/mouse) or saline control by tail vein injection. Mice were killed at 30 min to obtain the liver tissue. Protein (40 μg) from total liver lysates was subjected to immunoblot analysis using anti-total- or anti-phospho-AMPK (Thr172) as indicated.
Primary rat hepatocyte isolation and [125I]adiponectin uptake
Primary rat hepatocytes were isolated using a two-step collagenase perfusion of male Sprague Dawley rat livers, based on the technique of Berry and Friend (35). Hepatocytes were seeded at 125,000 cells per well in collagen-coated 24-well plates and cultured overnight. Cells were washed three times in DMEM before incubation at 37 C for 15 or 60 min in DMEM supplemented with 1% BSA and [125I]adiponectin (neuraminidase treated or mock treated) at 10,000 counts per well. After incubation, the plates were placed on ice and washed three times with ice-cold PBS before solubilization in 1% sodium dodecyl sulfate lysis buffer. Radioactive counts were measured using a Triathler Multilabel Tester (Hidex, Turku, Finland). Empty wells treated identically served as blanks and were subtracted from counts for cell-containing wells.
Acknowledgments
We thank Dr. Joe Tiralongo and Dr. Pamela Stanley for helpful advice and reagents and Ms. Felicity Rose for technical assistance. We also thank Professor Nicki Packer for critical review of the manuscript.
Footnotes
This work was supported by grants from the National Health and Medical Research Council of Australia (to J.P.W., G.A.M., and J.B.P.), the National Heart Foundation (to J.P.W.), and the Diabetes Australia Research Trust (to A.A.R.).
Disclosure summary: The authors have nothing to disclose.
First Published Online October 23, 2009
Abbreviations: AMPK, AMP-activated protein kinase; CHO, Chinese hamster ovary; 2DE, 2-dimensional electrophoresis; GLUT, glucose transporter; GTT, glucose tolerance test; HEK, human embryonic kidney; HMW, high molecular weight; ITT, insulin tolerance test; LMW, Low molecular weight; MAL II, Maackia Amurensis Lectin II; pI, isoelectric point; PTM, posttranslational modification; TCA trichloroacetic acid; WT, wild type.
References
- 1.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA2001. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86:1930–1935 [DOI] [PubMed] [Google Scholar]
- 2.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G2003. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-α expression. Diabetes 52:1779–1785 [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y2004. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res 94:e27–e31 [DOI] [PMC free article] [PubMed]
- 4.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y2002. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8:731–737 [DOI] [PubMed] [Google Scholar]
- 5.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T2003. Impaired multimerization of human adiponectin mutants associated with diabetes: molecular structure and multimer formation of adiponectin. J Biol Chem 278:40352–40363 [DOI] [PubMed] [Google Scholar]
- 6.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE2004. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279:12152–12162 [DOI] [PubMed] [Google Scholar]
- 7.Fisher FF, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, Kumar S2005. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia 48:1084–1087 [DOI] [PubMed] [Google Scholar]
- 8.Richards AA, Stephens T, Charlton HK, Jones A, Macdonald GA, Prins JB, Whitehead JP2006. Adiponectin multimerisation is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerisation by alterations in post-translational modifications. Mol Endocrinol 20:1673–1687 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, Hoo RC, Mak WW, Cooper GJ, Xu A2006. Posttranslational modifications on the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high-molecular-weight oligomeric complex. J Biol Chem 281:16391–16400 [DOI] [PubMed] [Google Scholar]
- 10.Sato C, Yasukawa Z, Honda N, Matsuda T, Kitajima K2001. Identification and adipocyte differentiation-dependent expression of the unique disialic acid residue in an adipose tissue-specific glycoprotein, adipo Q. J Biol Chem 276:28849–28856 [DOI] [PubMed] [Google Scholar]
- 11.Hansen JE, Lund O, Tolstrup N, Gooley AA, Williams KL, Brunak S1998. NetOglyc: prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconj J 15:115–130 [DOI] [PubMed] [Google Scholar]
- 12.Julenius K, Mølgaard A, Gupta R, Brunak S2005. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 15:153–164 [DOI] [PubMed] [Google Scholar]
- 13.Eckhardt M, Gotza B, Gerardy-Schahn R1998. Mutants of the CMP-sialic acid transporter causing the Lec2 phenotype. J Biol Chem 273:20189–20195 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Xu A, Knight C, Xu LY, Cooper GJS2002. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem 277:19521–19529 [DOI] [PubMed] [Google Scholar]
- 15.Röttger S, White J, Wandall HH, Olivo JC, Stark A, Bennett EP, Whitehouse C, Berger EG, Clausen H, Nilsson T1998. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J Cell Sci 111:45–60 [DOI] [PubMed] [Google Scholar]
- 16.Zhao W, Chen TL, Vertel BM, Colley KJ2006. The CMP-sialic acid transporter is localized in the medial-trans Golgi and possesses two specific endoplasmic reticulum export motifs in its carboxyl-terminal cytoplasmic tail. J Biol Chem 281:31106–31118 [DOI] [PubMed] [Google Scholar]
- 17.Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G1971. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem 246:1461–1467 [PubMed] [Google Scholar]
- 18.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, et al.2003. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature [Erratum (2004) 431:1123] 423:762–769 [DOI] [PubMed] [Google Scholar]
- 19.Toyoda M, Ito H, Matsuno YK, Narimatsu H, Kameyama A2008. Quantitative derivatization of sialic acids for the detection of sialoglycans by MALDI MS. Anal Chem 80:5211–5218 [DOI] [PubMed] [Google Scholar]
- 20.Chen YZ, Tang YR, Sheng ZY, Zhang Z2008. Prediction of mucin-type O-glycosylation sites in mammalian proteins using the composition of k-spaced amino acid pairs. BMC Bioinformatics 9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Süer Gökmen S, Kazezođlu C, Sunar B, Ozçelik F, Güngör O, Yorulmaz F, Gülen S2006. Relationship between serum sialic acids, sialic acid-rich inflammation-sensitive proteins and cell damage in patients with acute myocardial infarction. Clin Chem Lab Med 44:199–206 [DOI] [PubMed] [Google Scholar]
- 22.Yasukawa Z, Sato C, Kitajima K2005. Inflammation-dependent changes in α2,3-, α2,6-, and α2,8-sialic acid glycotopes on serum glycoproteins in mice. Glycobiology 15:827–837 [DOI] [PubMed] [Google Scholar]
- 23.Chavan MM, Kawle PD, Mehta NG2005. Increased sialylation and defucosylation of plasma proteins are early events in the acute phase response. Glycobiology 15:838–848 [DOI] [PubMed] [Google Scholar]
- 24.Piagnerelli M, Boudjeltia KZ, Nuyens V, De Backer D, Su F, Wang Z, Vincent JL, Vanhaeverbeek M2005. Rapid alterations in transferrin sialylation during sepsis. Shock 24:48–52 [DOI] [PubMed] [Google Scholar]
- 25.Tseke P, Grapsa E, Stamatelopoulos K, Samouilidou E, Rammos G, Papamichael C, Zakopoulos N2008. Correlations of sialic acid with markers of inflammation, atherosclerosis and cardiovascular events in hemodialysis patients. Blood Purif 26:261–266 [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz FM, Yilmaz G, Erdeve SS, Dallar Y, Topkaya BC, Yücel D2007. Serum sialic acid, hs-CRP and oxidative stress parameters in obese children. J Pediatr Endocrinol Metab 20:205–210 [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz G, Yilmaz FM, Aral Y, Yucel D2007. Levels of serum sialic acid and thiobarbituric acid reactive substances in subjects with impaired glucose tolerance and type 2 diabetes mellitus. J Clin Lab Anal 21:260–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakabayashi I, Masuda H2006. Association of acute-phase reactants with arterial stiffness in patients with type 2 diabetes mellitus. Clin Chim Acta 365:230–235 [DOI] [PubMed] [Google Scholar]
- 29.Kaser S, Moschen A, Kaser A, Ludwiczek O, Ebenbichler CF, Vogel W, Jaschke W, Patsch JR, Tilg H2005. Circulating adiponectin reflects severity of liver disease but not insulin sensitivity in liver cirrhosis. J Intern Med 258:274–280 [DOI] [PubMed] [Google Scholar]
- 30.Tietge UJ, Böker KH, Manns MP, Bahr MJ2004. Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am J Physiol Endocrinol Metab 287:E82–E89 [DOI] [PubMed]
- 31.Burgess JB, Baenziger JU, Brown WR1992. Abnormal surface distribution of the human asialoglycoprotein receptor in cirrhosis. Hepatology 15:702–706 [DOI] [PubMed] [Google Scholar]
- 32.Yip MF, Ramm G, Larance M, Hoehn KL, Wagner MC, Guilhaus M, James DE2008. CaMKII-mediated phosphorylation of the myosin motor Myo1c is required for insulin-stimulated GLUT4 translocation in adipocytes. Cell Metab 8:384–398 [DOI] [PubMed] [Google Scholar]
- 33.Zioncheck TF, Richardson L, DeGuzman GG, Modi NB, Hansen SE, Godowski PJ1994. The pharmacokinetics, tissue localization, and metabolic processing of recombinant human hepatocyte growth factor after intravenous administration in rats. Endocrinology 134:1879–1887 [DOI] [PubMed] [Google Scholar]
- 34.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ2003. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry MN, Friend DS1969. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol 43:506–520 [DOI] [PMC free article] [PubMed] [Google Scholar]