Abstract
There are currently few successful therapies for castration-resistant prostate cancer (CRPC). CRPC is thought to result from augmented activation of the androgen/androgen receptor (AR) signaling pathway, which could be enhanced by AR cofactors. In this study, peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) was found to be an AR cofactor. PGC-1α interacted with the N-terminal domain of AR, was involved in the N- and C-terminal interaction of AR, and enhanced the DNA-binding ability of AR to androgen-responsive elements in the prostate-specific antigen enhancer and promoter regions to increase the transcription of AR target genes. Silencing of PGC-1α suppressed cell growth of AR-expressing prostate cancer (PCa) cells by inducing cell-cycle arrest at the G1 phase, similar to inhibition of androgen/AR signaling. Furthermore, PGC-1α knock-down also suppressed cell growth in the castration-resistant LNCaP-derivatives. These findings indicate that PGC-1α is involved in the proliferation of AR-expressing PCa cells by acting as an AR coactivator. Modulation of PGC-1α expression or function may offer a useful strategy for developing novel therapeutics for PCa, including CRPC, which depends on AR signaling by overexpressing AR and its coactivators.
PGC-1α interacts with and activates the androgen receptor contributing to the growth of prostate cancer cells in which PGC-1α is overexpressed.
Prostate cancer (PCa) is the most common noncutaneous cancer and the second leading cause of cancer-related death in men in developed countries. The incidence of PCa has increased significantly because of the prevalence of high-fat diets and massive increase in the aging population (1, 2). Also, screening using prostate-specific antigen (PSA) has dramatically improved the early detection of PCa. However, 20–30% of patients with localized PCa who received surgical or radiation therapy still suffer from the relapse of the disease (3, 4, 5). Also, many patients with PCa are still only diagnosed at an advanced stage of disease. Most PCas are androgen dependent at diagnosis and, in most patients, androgen-deprivation therapy (ADT) is effective and prevents further growth and often leads to tumor regression. However, most tumors will relapse in a castration-resistant manner after a median of 13 months after ADT, and are, thus, designated as castration-resistant prostate cancer (CRPC) (6). There are currently few successful therapies for CRPC. Therefore, CRPC remains a serious obstacle to overcome.
The androgen/androgen receptor (AR) signaling pathway is thought to have a key role in prostate carcinogenesis and PCa progression. Several studies using PCa cell lines and mouse models have shown that the progression to CRPC could be associated with enhanced AR expression, as indicated by findings from AR down-regulation using dominant-negative AR mutants, small interfering RNA (siRNA), or small molecules, whereas increased AR expression converts androgen-dependent PCa cells to CRPC (7, 8, 9, 10). The AR gene is overexpressed in most CRPCs, 10–20% of which show amplification of the AR gene (11). Also, less than 10% of CRPCs were found to have somatic mutations in the AR gene, which could confer promiscuous activity to the receptor, allowing its activation by nonandrogen steroids and antiandrogens (12). Furthermore, the AR pathway in CRPC was considered to rely on changes in expression of growth factors, such as IGF (13), HER-2 (14), and IL-6 (15), which could modify AR activity. Also, AR signaling could be modulated by AR cofactors such as heat-shock protein 27 (Hsp27) (16), peroxiredoxin1 (17), Tip60, histone deacetylase 1 (HDAC1) (18), ARA 54 (19), ARA55 (20), ARA70 (21), GRIP1 (22), HMGB1, HMGB2 (23), PIAS1, PIAS3 (24), and SRC1 (25), some of which have been reported to be implicated in CRPC. Modification of these growth factors and cofactors in CRPC may cause androgen-dependent PCa to gain castration-resistant status.
Peroxisome proliferator-activated receptor γ (PPARγ) coactivator-1α (PGC-1α) was isolated based on its abilities to interact with PPARγ in a two-hybrid screening system and to enhance glucocorticoid responses in a functional genetic screening system (26, 27). It has previously been shown that PGC-1α has a novel role in adaptive thermogenesis, where it enhances the ability of PPARγ and nuclear respiratory factors (NRF1 and NRF2) to induce the synthesis of the enzymes required for oxidative metabolism (28). PGC-1α has been shown to be expressed and highly regulated in brown adipose tissue and skeletal muscle (26). Also, PGC-1α is expressed in the heart, kidney, and brain, suggesting that it is involved in processes other than thermogenesis (26). Because AR is also expressed in these tissues, we hypothesized that PGC-1α might have a role in various tissues together with AR. PGC-1α is known to interact with and enhance the transactivation of other nuclear receptors such as estrogen receptor α (ERα) (29, 30). Also, PGC-1α interacts with and activates ERα and ERβ in a ligand-independent manner with a particularly high-binding affinity to ERβ (31). In contrast, PGC-1β, also known as PERC, selectively binds to ERα in a ligand-dependent manner and activates its transcriptional ability (29, 32). Similar to a relationship between androgen and AR in carcinogenesis and development of PCa, a relationship between estrogen and ER has also been established, and it was reported that reduced levels of ovarian steroids and ERα significantly decrease the breast cancer risk (33, 34). Furthermore, antiestrogen therapies that inhibit estrogen synthesis or block ER activity are used to treat breast cancer similar to antiandrogen agents to treat PCa. Wirtenberger et al. (35) recently showed that a polymorphism of PGC-1α was associated with familial breast cancer, high-risk familial breast cancer, and bilateral familial breast cancer.
However, the function of PGC-1α in association with AR and in the progression of CRPC currently remains unknown. In this study, we intended to resolve the function of PGC-1α in association with AR and PCa. Our data showed that PGC-1α interacted with AR and was involved in the proliferation of androgen-dependent and CRPC cells. Together, PGC-1α appears to be a key factor involved in the progression to PCa, and is a promising molecular target for treating PCa, even CRPC.
Results
PGC-1α interacts with AR in vitro and in vivo
We intended to research the mechanisms responsible for carcinogenesis and progression of PCa in terms of AR function. Accordingly, we found that PGC-1α might interact with AR. First, the interaction between AR and PGC-1α was investigated by a glutathion S-transferase (GST) pull-down assay using GST-fused AR and Myc-Flag-tagged PGC-1α proteins. As shown in Fig. 1A, Myc-Flag-tagged PGC-1α was found to interact with GST-AR. To confirm this finding, a coimmunoprecipitation assay using the overexpression method was performed. PC-3 cells, which expressed no AR mRNA and protein, were transfected with GFP-tagged AR and Myc-Flag-tagged PGC-1α expression plasmids, and a coimmunoprecipitation assay was performed. Myc-Flag-tagged PGC-1α reproducibly interacted with the GFP-tagged AR protein. Simultaneously, we assayed whether the interaction between PGC-1α and AR could be influenced by dihydrotestosterone (DHT). PC-3 cells were transfected with the GFP-tagged AR and Myc-Flag-tagged PGC-1α expression plasmids, and then cultured under charcoal-stripped medium with or without DHT. The results of the coimmunoprecipitation assay showed that DHT did not influence this interaction (Fig. 1B). Last, we investigated whether endogenous proteins interacted with each other. Using cellular extracts of LNCaP cells expressing AR protein that were cultured under charcoal-stripped medium with or without DHT, the endogenous AR was immunoprecipitated using anti-AR antibody, and the immunoprecipitated samples were blotted with anti-PGC-1α antibody. As expected, endogenous AR interacted with PGC-1α, and reproducibly, this interaction was not affected by DHT (Fig. 1C).
Fig. 1.
PGC-1α interacts with AR in vitro and in vivo. A, Equal amounts of GST and GST-AR fusion proteins were immobilized on glutathione-sepharose 4B and were incubated with nuclear extracts from PC-3 cells transfected with PGC-1α-Myc-Flag plasmid. The bound protein and 10% of the input were subjected to SDS-PAGE, and Western blot analysis was performed using the anti-Flag antibody. Purified GST and GST-AR fusion proteins stained with Coomassie Brilliant Blue (CBB; Wako, Osaka, Japan) are also shown. B, PC-3 cells were cotransfected with 1.0 μg of each of the indicated expression plasmids and incubated in charcoal-stripped medium with or without 10 nm of DHT. Whole-cell extracts (300 μg) were immunoprecipitated with agarose-conjugated anti-GFP antibody. The resulting immunocomplexes and whole-cell extracts (30 μg) were subjected to SDS-PAGE, and Western blot analysis was performed using anti-Flag and anti-GFP antibodies. C, Whole-cell extracts (500 μg) were prepared from LNCaP cells incubated in charcoal-stripped medium with or without 10 nm of DHT and were immunoprecipitated (IP) with 2.0 μg of rabbit IgG or anti-AR antibody (C-19) and 20 μl of protein A/G agarose. The resulting immunocomplexes and whole-cell extracts (50 μg) were subjected to SDS-PAGE, and Western blot analysis was performed using anti-PGC-1α and anti-AR (C-19) antibodies.
PGC-1α is overexpressed in PCa cells and PGC-1α knock-down reduces PSA expression
To investigate a role of PGC-1α in PCa, we examined PGC-1α expressions in human normal prostate epithelial cells (RWPE-1 cells) and a panel of PCa cells (DU145, PC-3, VCaP, 22Rv1, LNCaP, and castration-resistant LNCaP derivatives CxR cells). PGC-1α was overexpressed in PCa cells compared with that in normal prostate epithelial cells. Also, PGC-1α expression level was similar between LNCaP and CxR cells (Fig. 2A). Furthermore, PGC-1α expression was not affected by DHT in LNCaP and CxR cells (Fig. 2B). To confirm the function of PGC-1α on AR, we next examined the expression of a well-known AR target gene, PSA, after knock-down of PGC-1α. After LNCaP and CxR cells were transfected with PGC-1α-specific siRNAs, quantitative real-time PCR and Western blot analysis for PSA were performed. The results showed that the expression of PSA mRNA was decreased by PGC-1α knock-down in the presence of DHT, although basal PSA expression was decreased by androgen starvation, but not in the absence of DHT. In addition, PSA mRNA expression level both in the presence or absence of DHT was decreased after PGC-1α knock-down also in CxR cells (Fig. 2C), in which AR could locate in nucleus and have a potential to transactivate its target genes even without ligand (Shiota, M., A. Yokomizo A., D. Masubichi, Y. Tuda, J. Inokuchi, M.Eto, T. Uchiumi, N. Fujimoto, S. Naito, manuscript submitted). Similar findings in terms of the protein PSA level were obtained when androgen-dependent LNCaP cells and CxR cells were transfected with PGC-1α-specific siRNAs. Also, transfection efficiencies of PGC-1α-specific siRNAs seemed to be equivalent between LNCaP and CxR cells as indicated by decrease of PGC-1α protein expression. Furthermore, as previously reported, the expression of AR was increased in the CxR cells compared with that in the parental LNCaP cells (Fig. 2D) (9, 10, 11).
Fig. 2.
PGC-1α is overexpressed in PCa cells and PGC-1α knock-down reduces PSA expression. A, Whole-cell extracts from the indicated cells were subjected to SDS-PAGE, and Western blot analysis was performed using anti-PGC-1α and anti-β-actin antibodies. B, Whole-cell extracts from LNCaP, CxR cells incubated in charcoal-stripped medium with or without 10 nm of DHT for 72 h were subjected to SDS-PAGE, and Western blot analysis was performed using anti-PGC-1α and anti-β-actin antibodies. C, LNCaP and CxR cells were transfected with 50 nm of control siRNA, PGC-1α siRNA no. 1 or PGC-1α siRNA no. 2, and incubated in charcoal-stripped medium with or without 10 nm of DHT for 72 h. After extraction of total RNA and synthesis of cDNA, quantitative real-time PCR was performed using the primers and probes for AR, PSA, and GAPDH. The transcription levels of AR and PSA were corrected for the corresponding GAPDH transcript level. All values represent at least three independent experiments. The level of each transcript from cells transfected with control siRNA and incubated with DHT corresponds to 1. Boxes, Mean; bars, ± sd. D, Whole-cell extracts from cells prepared in C were subjected to SDS-PAGE, and Western blot analysis was performed using anti-PGC-1α, anti-AR (C-19), anti-PSA, and anti-β-actin antibodies.
PGC-1α activates ARtranscriptional activity
Because PGC-1α was thought to interact with AR and regulate PSA expression, we determined the effect of PGC-1α on AR transcriptional activity using a luciferase assay. First, the PC-3 cells were transfected with a PSA reporter plasmid, pGLPSAp5.8, possessing PSA enhancer and promoter regions (∼5.8 kb) with three putative androgen-responsive elements (AREs) and a PGC-1α expression plasmid in addition to pCMV-AR expressing wild-type AR protein. Without DHT, luciferase activity was hardly detected even with PGC-1α overexpression. However, luciferase activity was significantly increased with DHT. Also, PGC-1α overexpression increased the transcriptional activity of PSA. In PCa, several AR mutations such as T887A in LNCaP cells have been found. Simultaneously, we investigated whether the AR mutation influences the PGC-1α function as a coactivator of AR in PC-3 cells. PGC-1α overexpression enhanced luciferase activity of PSA reporter plasmid even when mutated AR (T887A) was expressed in the PC-3 cells. Next, mouse mammary tumor virus (MMTV)-Luc possessing an MMTV promoter region with a putative ARE was used, and similar results were obtained. PGC-1α overexpression increased the transcriptional activities of MMTV when DHT was applied in addition to AR expression (Fig. 3A). Last, to confirm the above results, a knock-down assay using PGC-1α-specific siRNAs was performed. As expected, PGC-1α knock-down decreased the luciferase activity of the PSA and MMTV reporter plasmids to approximately 10–40% in LNCaP cells with DHT, whereas basal luciferase activity without DHT was not affected by PGC-1α knock-down (Fig. 3B). These results indicate that PGC-1α can affect PSA transcription androgen/AR signaling-dependently.
Fig. 3.
PGC-1α activates transcriptional activity of AR. A, PC-3 cells were transiently transfected with 0.33 μg of the indicated reporter plasmids, 0.33 μg of Myc-Flag or PGC-1α-Myc-Flag, 0.33 μg of the pCMV-AR [wildtype (wt)] or pCMV-ARmut877 [mutant (mt)], and 0.05 μg of pRL-TK, and incubated in charcoal-stripped medium with or without 10 nm of DHT for 48 h. Firefly luciferase activity was corrected for the corresponding Renilla luciferase activity. All values represent at least three independent experiments. The luciferase activity of each reporter plasmid with the pCMV-AR and Myc-Flag expression plasmids transfection incubated in charcoal-stripped medium with DHT corresponds to 1. Boxes, Mean; bars, ± sd. Whole-cell extracts from PC-3 cells transfected with 0.33 μg of each of the indicated plasmids were subjected to SDS-PAGE, and Western blot analysis was performed using anti-Flag, anti-AR (C-19), and anti-β-actin antibodies. B, LNCaP cells were transiently transfected with 20 nm of control siRNA, PGC-1α siRNA no. 1 or PGC-1α siRNA no. 2, followed by transfection with 0.5 μg of the indicated reporter plasmid and 0.05 μg of pRL-TK at intervals of 12 h, and incubated in charcoal-stripped medium with or without 10 nm of DHT. The luciferase assay was performed as described in A. The luciferase activity of each reporter plasmid with control siRNA transfection incubated in charcoal-stripped medium with DHT corresponds to 1. Boxes, mean; bars, ± sd.
N-terminal domain (NTD) of PGC-1α interacts with the N-terminal transactivation domain (TAD) of AR
The finding that PGC-1α interacted with AR and had a functional role with AR prompted us to examine which domains are involved. First, a GST pull-down assay was performed using GST-AR and its series of deletion mutants with Myc-Flag-tagged PGC-1α (Fig. 4A). As shown in Fig. 4B, PGC-1α interacted with the TAD of AR. Next, GST-PGC-1α and its series of deletion mutants were used for the GST pull-down assay with nuclear extracts of LNCaP (Fig. 4C). As shown in Fig. 4D, AR interacted with the NTD of PGC-1α.
Fig. 4.
The NTD of PGC-1α interacts with the TAD of AR. A, Schematic representation of the GST-AR deletion mutants. B, Equal amounts of GST, GST-AR, and various GST-AR deletion mutant fusion proteins, as shown in A, were immobilized on glutathione-sepharose 4B and were incubated with nuclear extracts from PC-3 cells transfected with PGC-1α-Myc-Flag expression plasmid. The bound protein samples and 10% of the input were subjected to SDS-PAGE, and Western blot analysis was performed using an anti-Flag antibody. Purified GST, GST-AR, and GST-AR deletion mutant fusion proteins stained with CBB are also shown. C, Schematic representation of GST-PGC-1α deletion mutants. D, Equal amounts of GST, GST-PGC-1α, and various GST-PGC-1α deletion mutant fusion proteins shown in C, immobilized on glutathione-sepharose 4B, were incubated with nuclear extracts from LNCaP cells. Bound protein samples and 10% of input were subjected to SDS-PAGE, and Western blot analysis was performed using an anti-AR antibody (C-19). Purified GST, GST-PGC-1α, and GST-PGC-1α deletion mutant fusion proteins stained with Coomassie Brilliant Blue (CBB) are also shown. IB, Immunoblots.
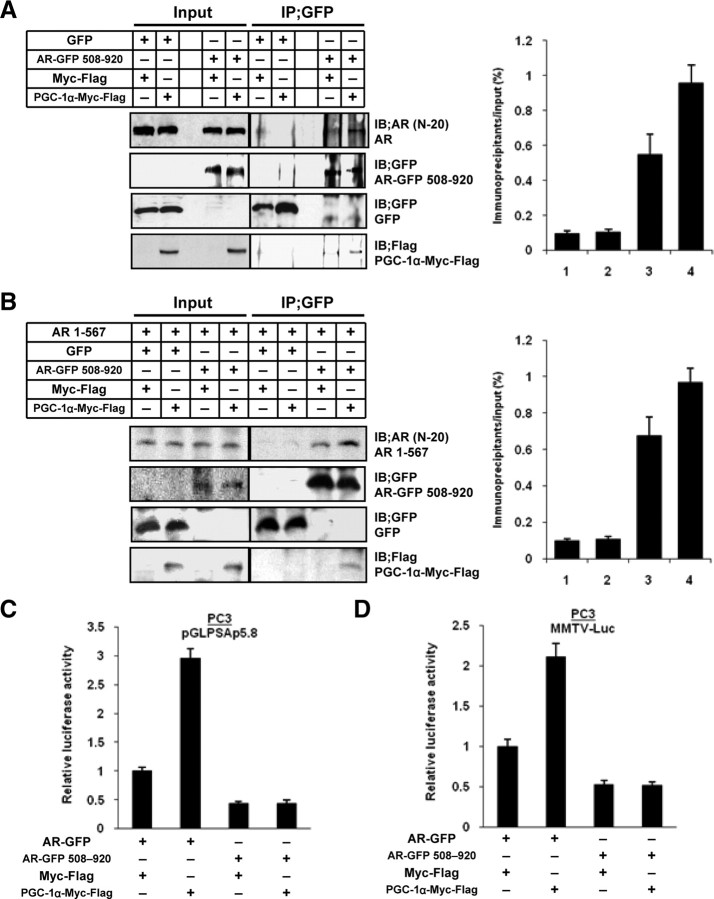
TAD of AR is indispensable for augmentation of AR-transcriptional activity by PGC-1α
Because PGC-1α was found to interact with the TAD of AR, we investigated whether the transcriptional ability of TAD-deleted AR was affected by PGC-1α manipulation. First, we constructed an AR-GFP 508-920 plasmid expressing GFP-tagged TAD-deleted AR protein. It is known that the TAD of AR interacts with a C-terminal ligand-binding domain (LBD) and can form a homodimer in a head-to-tail fashion binding to the ARE drive the expression of its target genes (36). Therefore, a coimmunoprecipitation assay was performed using AR-GFP 508-920 and PGC-1α-Myc-Flag expression plasmids in LNCaP cells. An interaction between AR-GFP 508-920 and endogenous AR protein was found to be augmented by PGC-1α expression, probably through an interaction between the LBD of AR-GFP 508-920 and the TAD of endogenous AR (Fig. 5A). To confirm augmentation of an interaction between TAD and LBD of AR, we constructed pCMV-AR 1-567 plasmid expressing LBD− and most part of DBD-deleted AR protein, and performed coimmunoprecipitation assay using PC-3 cells transfected with AR-GFP 508-920, pCMV-AR 1-567, and PGC-1α-Myc-Flag expression plasmids. The result clearly showed that PGC-1α expression increased an interaction between TAD and LBD of AR (Fig. 5B). Then, a luciferase reporter assay was performed to confirm the effects of PGC-1α against AR-GFP 508-920. Although the transcription of PSA was increased in PC-3 cells transfected with the PGC-1α-Myc-Flag expression plasmid, full-length AR-GFP expression plasmid, and PSA reporter plasmid, the transcriptional ability of AR-GFP 508-920 was not affected by PGC-1α expression (Fig. 5C). Similar results were obtained when the MMTV-reporter plasmid was used (Fig. 5D).
Fig. 5.
The TAD of AR is indispensable for the augmentation of AR transcriptional activity by PGC-1α. A, LNCaP cells were cotransfected with 1.0 μg of each of the indicated expression plasmids and were incubated for 48 h. Whole-cell extracts (300 μg) were immunoprecipitated (IP) with agarose-conjugated anti-GFP antibody. The resulting immunocomplexes and whole-cell extracts (30 μg) were subjected to SDS-PAGE, and Western blot analysis was performed using anti-AR (N-20), anti-GFP, and anti-Flag antibodies. The signal intensities for AR protein of coimmunoprecipitated samples were corrected for the results of the corresponding preimmunoprecipitated samples. All values represent at least three independent experiments. Lane 1, GFP and Myc-Flag; lane 2, GFP and PGC-1α-Myc-Flag; lane 3, AR-GFP 508-920 and Myc-Flag; lane 4, AR-GFP 508-920 and PGC-1α-Myc-Flag. Boxes, mean; bars, ± sd. B, PC-3 cells were cotransfected with 0.65 μg of each of the indicated expression plasmids and were incubated for 48 h. Coimmunoprecipitation assay and Western blot analysis were performed as described in A. The signal intensities for AR 1-567 protein of coimmunoprecipitated samples were corrected for the results of the corresponding preimmunoprecipitated samples. All values represent at least three independent experiments. Lane 1, GFP and Myc-Flag; lane 2, GFP and PGC-1α-Myc-Flag; lane 3, AR-GFP 508-920 and Myc-Flag; lane 4, AR-GFP 508-920 and PGC-1α-Myc-Flag. Boxes, Mean; bars, ± sd. C and D, PC-3 cells were transiently transfected with 0.33 μg of pGLPSAp5.8 (C) or MMTV-Luc (D), 0.33 μg of Myc-Flag or PGC-1α-Myc-Flag, 0.33 μg of the AR-GFP or AR-GFP 508-920, and 0.05 μg of pRL-TK and incubated in charcoal-stripped medium with 10 nm of DHT for 48 h. Firefly luciferase activity was corrected for the corresponding Renilla luciferase activity. All values represent at least three independent experiments. The luciferase activity of each reporter plasmid with the AR-GFP and Myc-Flag expression plasmids transfection corresponds to 1. Boxes, mean; bars, ± sd. IB, Immunoblots.
Knock-down of PGC-1α decreases the DNA-binding ability of AR
Disruption of the interaction between the TAD and LBD of AR has a potential to inhibit the DNA binding ability of AR (37, 38). Because PGC-1α interacted with the TAD of AR and was involved in the interaction between the TAD and the LBD of AR, we investigated whether the DNA binding ability of AR was affected by PGC-1α manipulation. The PSA enhancer and promoter regions contained three AREs known as ARE I, ARE II, and ARE III (Fig. 6A). When the samples from LNCaP cells immunoprecipitated with anti-AR antibody were amplified using ARE-containing primer pairs, PCR products were abundant when the primer pairs A/B, C/D, and G/H were used, but not when the primer pairs E/F and I/J were used. Also, the binding of AR to ARE was decreased by the withdrawal of androgen (39) according to withdrawal duration. Similar to a previous report that AR gradually dissociated with DHT and exported to cytoplasm by androgen withdrawal (40), androgen withdrawal for 4 and 16 h reduced AR binding to PSA A/B, C/D, and G/H by approximately 30 and 60%, respectively. Furthermore, when PGC-1α expression was down-regulated by transfecting LNCaP cells with PGC-1α-specific siRNAs, the binding of AR to ARE within the PSA enhancer and promoter regions was reduced (Fig. 6B). These results were confirmed using conventional PCR method (see supplemental Fig. 1A published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Similar results were obtained also in CxR cells by both quantitative real-time PCR and conventional PCR methods, although AR binding to ARE in CxR cells was less affected by androgen deprivation (Fig. 6C and supplemental Fig. 1B).
Fig. 6.

Knock-down of PGC-1α decreases the DNA-binding ability of AR. A, Black boxes and black arrows indicate the AREs and PCR primer regions, respectively. B and C, LNCaP (B) and CxR (C) cells were transfected with 50 nm of control siRNA, PGC-1α siRNA no. 1 or PGC-1α siRNA no. 2, and incubated for 72 h. The medium was exchanged from charcoal-stripped medium with 10 nm of DHT into medium without DHT at the indicated time before harvest. The nuclear extracts were then immunoprecipitated (IP) with 2.0 μg of rabbit IgG or anti-AR antibody (C-19) and 20 μl of protein A/G agarose. The quantitative real-time PCR was performed using soluble chromatin, immunoprecipitated DNAs, and the indicted primer pairs. The results of immunoprecipitated samples were corrected for the results of the corresponding soluble chromatin samples. All values represent at least three independent experiments. Boxes, Mean; bars, ± sd.
Knock-down of PGC-1α represses cell proliferation in androgen-dependent and CRPC cells
The finding that PGC-1α was involved in a regulation of transcriptional activity of AR prompted us to examine whether PGC-1α might affect the proliferation of PCa cells through modulation of AR function. First, we investigated the proliferation of LNCaP cells transfected with PGC-1α-specific siRNAs in medium containing 1 nm or 10 nm of DHT, or not containing. When PGC-1α was knocked down, cell proliferations in both media were significantly reduced in the presence of DHT, but not in the absence of DHT (Fig. 7A). These results were similar to those with AR knock-down (data not shown). To clarify the mechanism of cell growth retardation by PGC-1α knock-down, we performed flow cytometry analysis for cell-cycle analysis. As shown in Fig. 7B, androgen deprivation induced cell-cycle arrest at G1 phase. Also, PGC-1α knock-down in medium containing 1 nm or 10 nm of DHT induced cell-cycle arrest at the G1 phase, but not in medium not containing DHT similar to that with AR knock-down (data not shown); thus, decreasing cell proliferation. However, PGC-1α knock-down in CRPC cells with no AR expression, PC-3 cells, affected cell proliferation to a lesser extent than that in LNCaP cells. In addition, under androgen starvation, the growth of PC-3 cells was similar to that in medium containing androgens. When PGC-1α was knocked down, PC-3 cell growth in the androgen-deficient medium was similar to that in androgen-containing medium (Fig. 7C). CxR cells are derived from LNCaP cells and exhibit high expression of AR proteins compared with their parental cells, as shown in Fig. 2D. Overexpression of AR has been thought to promote CRPC cell growth, even under androgen starvation by augmentation of AR signaling. Because CxR cells exhibit enhanced AR expression, blockade of AR signaling may be effective to inhibit cell proliferation in CxR cells. As expected, PGC-1α knock-down in CxR delayed cell growth slightly more effectively than that in parental LNCaP cells, most likely by blocking AR signaling both in the absence and presence of DHT (Fig. 7D).
Fig. 7.
Knock-down of PGC-1α suppresses cell proliferation in androgen-dependent and CRPC cells. A, LNCaP cells were transiently transfected with 50 nm of control siRNA, PGC-1α siRNA no. 1 or PGC-1α siRNA no. 2, and incubated in charcoal-stripped medium with 0, 1, or 10 nm of DHT. The cell numbers were counted at the indicated times. The results were normalized to cell numbers at hour 0. All values represent at least three independent experiments. Boxes, mean; bars, ± sd. B, LNCaP cells were transiently transfected with 50 nm of control siRNA, PGC-1α siRNA no. 1 or PGC-1α siRNA no. 2, and incubated in charcoal-stripped medium with 0, 1, or 10 nm of DHT. Seventy-two hours after transfection, the cells were stained with propidium iodide and analyzed by flow cytometry. The cell cycle fraction is shown in the right upper position of each graph. C and D, PC-3 (C) and CxR (D) cells were transiently transfected with 50 nm of control siRNA, PGC-1α siRNA no. 1 or PGC-1α siRNA no. 2, and incubated in charcoal-stripped medium with or without 10 nm of DHT. The cell proliferation assay was performed as described in A. Boxes, Mean; bars, ± sd.
Discussion
Coactivators can interact with and enhance the transcriptional activity of ligand-bound or ligand-unbound AR, and some coactivators are overexpressed in PCa, suggesting that coactivators of AR might be involved in prostate carcinogenesis (41, 42). Various studies have shown that steroid receptors, particularly AR, have pivotal roles in all stages of prostate carcinogenesis (3, 43). Because the activity of steroid receptors is potentiated by a variety of coactivators, it is reasonable to believe that these proteins may also be involved in prostate carcinogenesis (44, 45). Indeed, recent studies have shown that the mRNA of some steroid receptor coactivators is overexpressed in PCa tissues and cell lines (46). The first bona fide steroid hormone receptor coactivator, SRC-1, was identified by virtue of its ability to interact with the hormone binding domain of agonist-activated progesterone receptor (47). Subsequently, it was shown that SRC-1 was able to interact efficiently with most nuclear receptors. SRC-3 was first isolated as a steroid receptor coactivator. Recently, it was reported that SRC-3 was overexpressed in PCa specimens, and its overexpression was correlated with PCa proliferation and is inversely correlated with apoptosis (48). AR coactivators are also involved in castration-resistant progression of PCa, which is critical for advanced PCa patients (49, 50). In this study, PGC-1α was revealed to be overexpressed in PCa cells.
PGC-1α was originally identified as a transcriptional coactivator of PPARγ, and it was determined that PGC-1α also interacts with other nuclear receptors (26, 29). These findings suggest that PGC-1α might also interact with AR and may be involved in carcinogenesis and the progression to CRPC. Therefore, we investigated the interaction between PGC-1α and AR using a GST pull-down assay in vitro and coimmunoprecipitation assay in vivo. As expected, PGC-1α interacted with AR, and enhanced the transcriptional activity of AR target genes, PSA, and MMTV. Also, we determined the regions of PGC-1α and AR that interact with each other. The results indicate that the NTD of PGC-1α interacts with the TAD of AR. The NTD of PGC-1α contains three LXXLL-like motifs resembling an LXXLL motif, which is known to mediate the recruitment of the p160-type of coactivators to nuclear receptors (51). Mutations of LXXLL motif have been shown to disrupt its interactions with nuclear receptors and abolish its coactivator function (51, 52, 53). However, our result showed that deletion mutant of PGC-1α (GST-PGC-1α 1-89) not containing complete LXXLL motif could also interacted with AR. This result indicates that LXXLL motif may be unnecessary for PGC-1α to interact with AR. Otherwise, LL portion (amino acids 88-89) of LXXLL motif may be sufficient to interact with AR.
After determining the region of PGC-1α that interacts with AR, we investigated the region of AR that interacts with PGC-1α. It was shown that PGC-1α interacts with the hinge region or the NTD of nuclear receptors, but the domains of the nuclear receptors that interact with PGC-1α differed between nuclear receptors and between investigators (31, 54, 55). In our experiment, the TAD of AR interacted with PGC-1α. This result is supported by our finding that the interaction between AR and PGC-1α was not affected with or without androgen, suggesting that the LBD of AR was not involved in this interaction. Furthermore, our finding that deletion of the TAD of AR abolished the coactivator function of PGC-1α supported our finding that the TAD of AR is a region that interacts with the PGC-1α. This interaction up-regulated the trans-activating ability of AR through the following mechanism. As indicated in Figs. 5 and 6, the interaction between PGC-1α and AR augmented the formation of a AR homodimer, leading to enhanced AR binding to the ARE and the expression of AR target genes.
Wirtenberger et al. (35) previously reported that the PGC-1α Thr612Met polymorphism was associated with familial breast cancer, high-risk familial breast cancer, and bilateral familial breast cancer risk in patients not carrying the BRCA 1/2 mutation (35). This finding suggests that PGC-1α function is involved in breast cancer carcinogenesis and progression by acting as a coactivator of ER. Therefore, we hypothesized that PGC-1α might also be involved in carcinogenesis and the progression of PCa, and investigated the effect of PGC-1α expression manipulation on androgen-dependent and CRPC cell growth. Our results showed that PGC-1α knock-down suppressed the growth of PCa cells. In particular, PGC-1α knock-down suppressed growth and cell-cycle arrest at the G1 phase in AR-expressing PCa cells more effectively compared with PCa cells with no AR expression. This finding suggests that PGC-1α predominantly acts on PCa cells, at least in part, by interacting with and acting as a coactivator for AR. Although PC-3 cells expressing no AR mRNA and protein were also little affected by PGC-1α knock-down in the presence and absence of androgen, these effects may result from other functions of PGC-1α as other nuclear receptor coactivators, which was confirmed by the finding that cell growth suppression by PGC-1α knock-down was not affected by androgen depletion.
Also, PGC-1α and AR signaling are known to modulate metabolic activity. AR-null mice exhibit metabolic disease-like phenotype (56, 57). Similarly, PGC-1α knock-down reduced lipid metabolism, leading to storage of fat in adipocyte (58). Moreover, SRC-3, an AR coactivator was recently shown to induce PGC-1α acetylation and consequently inhibit its activity. Ablation of SRC-3 was subsequently found to improve insulin sensitivity (59). These findings support our results that PGC-1α interacts with AR and influences AR signaling. In addition, PGC-1α and AR signaling might be a useful therapeutic target for metabolic disease.
In conclusion, PGC-1α interacted with AR and activated the transcriptional function of AR. Also, PGC-1α knock-down delayed cell growth in AR expressing PCa cells. Furthermore, in castration-resistant LNCaP derivatives, CxR cells, PGC-1α knock-down suppressed cellular proliferation. Although PGC-1α expression needs to be investigated in PCa tissues compared with normal prostate glands, these findings indicate that the modulation of PGC-1α expression or function might be a useful strategy for developing novel therapeutics in PCa, which usually depends on androgens. Also, this strategy might be more useful for CRPC cells, which depends on AR signaling.
Materials and Methods
Cell culture
Human normal prostate epithelium RWPE-1 (keratinocyte serum-free medium), Human PCa DU145 (DMEM), PC-3 (Eagle’s MEM), VCaP (DMEM), 22Rv1 (RPMI1640), and LNCaP cells (RPMI1640) were cultured in the indicated media. These media were purchased from Invitrogen (San Diego, CA) and contained 10% fetal bovine serum. LNCaP cells propagated between 10 and 30 times were used. Castration-resistant derivatives of LNCaP cells, LNCaP-CxR cells (referred to as CxR cells), were established and maintained as previously described (60). The cell lines were maintained in a 5% CO2 atmosphere at 37°C.
Antibodies
Antibodies against AR (C-19, sc-815), AR (N-20, sc-7305), PSA (sc-7316), GFP (sc-8334), PGC-1α (sc-13067), and agarose-conjugated anti-GFP antibody (sc-8334 AC) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Flag (M2) and anti-β-actin antibodies were purchased from Sigma (St. Louis, MO).
Plasmid construction
The AR-GFP plasmid expressing C-terminally GFP-tagged AR protein was kindly provided by T. Yanase (Fukuoka University, Fukuoka, Japan) (61). The pCMV-AR plasmid expressing wild-type AR, pCMV-ARmut877 plasmid expressing mutated AR (T877A), and MMTV-Luc were kindly provided by C. Chang (University of Rochester, Rochester, NY). The pGLPSAp5.8 was kindly provided by A. Mizokami (Kanazawa University, Kanazawa, Japan) (62). To construct the AR-GFP 508-920 plasmid expressing GFP-tagged N-terminal deleted AR protein (from 508 to 920 aa), PCR was carried out with AR-GFP as a template using the following primer pairs: 5′-GCTAGCGGTACCCTGGCGGCATGGTGA-3′ and 5′-GGATCCACTGGGTGTGGAAATAGATGG-3′. The PCR product was cloned into the pEGFP vector (Clontech, Mountain View, CA). pCMV-AR 1-567 expressing C-terminal deleted AR protein (1-567 aa) was constructed by deletion of HindIII fragment from pCMV-AR plasmid. To obtain the full-length cDNA for AR, PCR was carried out with pCMV-AR as a template using the following primer pairs: 5′-ATGGAAGTGCAGTTAGGGCTGG-3′ and 5′-TCACTGGGTGTGGAAATAGATG-3′. The PCR product was cloned into the pGEM-T easy vector (Promega, Madison, WI). To construct pGEX-AR expressing GST-AR, a fragment of AR cDNA was ligated into the pGEX plasmid (GE Healthcare Bio-Science, Piscataway, NJ). The GST-AR deletion mutants (GST-AR 1-504, GST-AR 504-920, GST-AR 567-920, GST-AR 723-920, and GST-AR Δ715-844) were constructed from pGEX-AR full-length plasmid by deletion of the SphI-Acc65I, Acc65I-HindIII, BamHI-HindIII, SalI-EcoRI, and BsrGI fragments, respectively. GST-AR 504-721 was created from GST-AR 504-920 by deletion of the StuI-NotI fragment.
The PGC-1α-Myc-Flag plasmid expressing the C-terminally Myc-Flag-tagged PGC-1α protein was purchased from OriGene (Rockville, MD). To obtain the full-length cDNA for PGC-1α, PCR was carried out with the PGC-1α-Myc-Flag plasmid as a template using the following primer pairs: 5′-GATGGCGTGGGACATGTGCAACCA-3′ and 5′-TTACCTGCGCAAGCTTCTCTGAGC-3′. The PCR product was cloned into the pGEM-T easy vector. To construct pGEX-PGC-1α expressing GST-PGC-1α, a fragment of PGC-1α cDNA was ligated into the pGEX plasmid. GST-PGC-1α deletion mutants (GST-PGC-1α 1-409, GST-PGC-1α 1-144, GST-PGC-1α 1-89, and GST-PGC-1α 294-798) were constructed from pGEX-PGC-1α full-length plasmid by deletion of the XbaI-NotI, AflII-NotI, NheI-NotI, and BamHI-StuI fragments, respectively. To construct GST-PGC-1α 1-186, N-terminal EcoRI fragment of cDNA for PGC-1α was ligated into the pGEX plasmid.
Western blot analysis
Whole-cell lysates and nuclear extracts were prepared as previously described (60, 63, 64, 65, 66). The protein concentration of the extracts was quantified using a Protein Assay kit based on the Bradford method (Bio-Rad, Hercules, CA). The indicated amounts of whole-cell lysates and nuclear extracts were separated by 4–20% SDS-PAGE and transferred to polyvinylidene difluoride microporous membranes (GE Healthcare Bio-Science) using a semidry blotter. The blotted membranes were incubated for 1 h at room temperature with the primary antibodies described above. The membranes were then incubated for 40 min at room temperature with a peroxidase-conjugated secondary antibody. The bound antibody was visualized using an ECL kit (GE Healthcare Bio-Science), and the membranes were exposed to Kodak X-OMAT film.
Expression of GST-fusion proteins in Escherichia coli
GST-fusion proteins in E. coli were prepared as previously described (64, 65, 66). To express GST-fusion proteins, bacteria transformed with expression plasmids were incubated with 1 mm isopropyl-β-D-thiogalactopyranoside (Nacalai tesque, Kyoto, Japan) for 2 h at room temperature and collected by centrifugation. The cells were sonicated (TAITEC sonicator, Tokyo, Japan) in buffer X containing 50 mm Tris-HCl (pH 8.0), 1 mm EDTA, 120 mm NaCl, 0.5% (vol/vol) Nonidet P-40 (NP-40), 10% (vol/vol) glycerol, 1 mm phenylmethylsulfonyl fluoride, and 1 mm dithiothreitol, and the cell lysates were collected after centrifugation at 21,000 g for 10 min at 4°C.
GST pull-down assay
The GST pull-down assay was performed as previously described (64, 65, 66). GST-AR, GST-PGC-1α, or their deletion mutants immobilized on glutathione-sepharose 4B (GE Healthcare Bio-Science) were incubated with soluble cell extracts for 2 h at 4 C in buffer X. The bound samples were washed three times with buffer X and subjected to Western blot analysis with the indicated antibodies.
Coimmunoprecipitation assay
The transient transfection and immunoprecipitation assays were performed as previously described (64, 65, 66). Briefly, 1 × 105 LNCaP and PC-3 cells were transfected with the indicated amounts of each of the indicated expression plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions and seeded into six-well plates. After incubation at 37°C for 48 h with the indicated fresh medium, the cells were lysed in buffer X. The lysates were centrifuged at 21,000 × g for 10 min at 4°C, and the supernatants (300 μg) were incubated for 2 h at 4°C with agarose-conjugated anti-GFP antibody. The immunoprecipitated samples were washed three times with buffer X, and the preimmunoprecipitated samples (30 μg) were subjected to Western blot analysis with the indicated antibodies. Signal intensities of preimmunoprecipitated and coimmunoprecipitated AR protein were quantified using the NIH Imaging program (NIH, Bethesda, MD). The intensities of coimmunoprecipitated AR protein were corrected for the corresponding intensities of preimmunoprecipitated AR protein. The results are representative of at least three independent experiments.
For immunoprecipitation assays without transient transfection, 70–80% confluent LNCaP cells were cultured in 100-mm tissue-culture plates with the indicated medium for 48 h and lysed with buffer X. The lysates were centrifuged at 21,000 × g for 10 min at 4 C, and the supernatants (500 μg) were incubated overnight at 4 C with 2.0 μg of rabbit IgG or anti-AR antibody. The immunoprecipitated samples were washed three times with buffer X, and the preimmunoprecipitated samples (50 μg) were subjected to Western blot analysis with the indicated antibodies.
Knock-down analysis using siRNAs
Knock-down analysis using siRNA was performed as previously described (60, 63, 64, 65, 66). The following double-stranded RNA 25-base-pair oligonucleotides were commercially generated (Invitrogen): 5′-AAUCUGUGGAAGAACAAAUCUGCCC-3′ (sense) and 5′-GGGCAGAUUUGUUCUUCCACAGAUU-3′ (antisense) for PGC-1α siRNA no. 1; 5′-UAUUCUUCCCUCUUCAGCCUCUCGU-3′ (sense) and 5′-ACGAGAGGCUGAAGAGGGAAGAAUA-3′ (antisense) for PGC-1α siRNA no. 2. LNCaP, CxR and PC-3 cells were transfected with siRNA using Lipofectamine 2000 according to the manufacturer’s instructions.
RNA isolation and RT-PCR
Total RNA was prepared from cultured cells using RNeasy mini kits (QIAGEN, Valencia, CA). First-strand cDNA was synthesized from 1.0 μg of total RNA using a Transcriptor First-Strand cDNA Synthesis Kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions.
Quantitative real-time PCR
The synthesized cDNA was diluted 1:2, and 2.0 μl of the diluted mixture was used. Quantitative real-time PCR with TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA) and TaqMan Gene Expression Master Mix (Applied Biosystems) was performed using ABI 7900HT (Applied Biosystems). The expression level of AR and PSA mRNA was corrected for the corresponding glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression level. The results are representative of at least three independent experiments.
Luciferase reporter assay
The luciferase reporter assay was performed as previously described (60). Briefly, LNCaP and PC-3 cells (1.5 × 105) were cotransfected with the indicated amounts of reporter plasmids, 0.05 μg of pRL-TK as an internal control and the indicated amounts of expression plasmids or siRNA using Lipofectamine 2000 according to the manufacturer’s instructions and seeded into 12-well plates. After incubation for 48 h, luciferase activity was detected using a Dual-Luciferase Reporter Assay System (Promega). Light intensity was measured using a plate reader (ARVO™ MX; Perkin Elmer Inc., Waltham, MA). Firefly luciferase activity was corrected for the corresponding Renilla luciferase activity. The results are representative of at least three independent experiments.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation assay was performed as previously described (60, 63, 65). Briefly, LNCaP and CxR cells were transfected with control siRNA, PGC-1α siRNA no. 1 or PGC-1α siRNA no. 2, seeded into six-well plates, and incubated for 72 h. Soluble chromatin from 1 × 106 cells was incubated overnight at 4°C with 2.0 μg of antirabbit IgG or anti-AR antibody and 20 μl of protein A/G agarose. Purified DNA was dissolved in 20 μl of dH2O, and 1 μl of the diluted mixture was used for PCR analysis with the following primer pairs: 5′-TCTGCCTTTGTCCCCTAGAT-3′ (forward) and 5′-AACCTTCATTCCCCAGGACT-3′ (reverse) for PSA A/B (−250 bp to −39 bp); 5′-AGGGATCAGGGAGTCTCACA-3′ (forward) and 5′-GCTAGCACTTGCTGTTCTGC-3′ (reverse) for PSA C/D (−406 bp to −164 bp); 5′-CTGTGCTTGGAGTTTACCTGA-3′ (forward) and 5′-GCAGAGGTTGCAGTGAGCC-3′ (reverse) for PSA E/F (−1997 bp to −1846 bp); 5′-CCTCCCAGGTTCAAGTGATT-3′ (forward) and 5′-GCCTGTAATCCCAGCACTTT-3′ (reverse) for PSA G/H (−4170 bp to −3978 bp); 5′-GATGGTGTTTCACCGTGTTG-3′ (forward) and 5′-AGAGTGCAGTGAGCCGAGAT-3′ (reverse) for PSA I/J (−7694 bp to −7484 bp). These primer pairs were described previously (39). The PCR products were separated by electrophoresis on 2% agarose gels and stained with ethidium bromide. The quantitative real-time PCR assay with 1 μl of the diluted DNA, the above primer pairs and SYBR Premix Ex Taq II (Takara Bio, Shiga, Japan) was performed using ABI 7900HT. The results are representative of at least three independent experiments.
Cell proliferation assay
The cell proliferation assay was performed as previously described (60, 63, 64, 66). Briefly, 2.0 × 104 LNCaP, CxR and PC-3 cells were transfected with control siRNA, PGC-1α siRNA no. 1 or PGC-1α siRNA no. 2, as described above and seeded in 12-well plates, and incubated in the indicated medium. Twelve hours after transfection was set as hour 0. The cells were harvested with trypsin and counted daily using a cell counter (Beckman Coulter, Fullerton, CA). The results were normalized to cell counts at h 0, and are representative of at least three independent experiments.
Flow cytometry analysis
The flow cytometry analysis was performed as previously described (60, 63). Briefly, 2.5 × 105 LNCaP cells were transfected with control siRNA, PGC-1α siRNA no. 1 or PGC-1α siRNA no. 2, seeded in six-well plates, and incubated in the indicated medium for 72 h. The cells were harvested, washed twice with ice-cold PBS with 0.1% BSA, and resuspended in 70% ethanol. After washing twice with ice-cold PBS, the cells were resuspended in PBS with 0.1% BSA, incubated with RNase (Roche Molecular Biochemicals, Basel, Switzerland), and stained with propidium iodide (Sigma). Cells were analyzed using a FACS Calibur (BD Biosciences, San Jose, CA).
Acknowledgments
We thank Dr. Toshihiko Yanase, Dr. Chawnshang Chang, and Dr. Atsushi Mizokami for providing the plasmids; Dr. Dongchon Kang (Kyushu University, Fukuoka, Japan) for helping with quantitative real-time PCR and flow cytometry; and Noriko Hakoda, Hitomi Matoba, and Seiko Kamori for their technical assistance.
NURSA Molecule Pages:
Coregulators: PGC-1;
Ligands: Dihydrotestosterone;
Nuclear Receptors: AR.
Footnotes
This work was supported by Health Sciences Research Grants for Clinical Research for Evidenced Based Medicine and Grant-in-Aid for Cancer Research 016 from the Ministry of Health, Labor, and Welfare, Japan; and by a Young Researcher Promotion grant from the Japanese Urological Association, Japan.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 2, 2009
Abbreviations: ADT, Androgen-deprivation therapy; AR, androgen receptor; ARE, androgen-responsive elements; CRPC, castration-resistant prostate cancer; DHT, dihydrotestosterone; GAPDH, glyceraldehyde 3-phosphatase; LBD, ligand-binding domain; MMTV, mouse mammary tumor virus; NTD, N-terminal domain; PCa, prostate cancer; PGC-1α, PPARγ coactivator-1α; PPARγ, peroxisome proliferator-activated receptor γ; PSA, prostate-specific antigen; siRNA, small interfering RNA; TAD, transactivation domain.
References
- 1.Grönberg H2003. Prostate cancer epidemiology. Lancet 361:859–864 [DOI] [PubMed] [Google Scholar]
- 2.Hsing AW, Devesa SS2001. Trends and patterns of prostate cancer: what do they suggest? Epidemiol Rev 23:3–13 [DOI] [PubMed] [Google Scholar]
- 3.Feldman BJ, Feldman D2001. The development of androgen-independent prostate cancer. Nat Rev Cancer 1:34–45 [DOI] [PubMed] [Google Scholar]
- 4.Han M, Partin AW, Piantadosi S, Epstein JI, Walsh PC2001. Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol 186:416–419 [PubMed] [Google Scholar]
- 5.Isaacs W, De Marzo A, Nelson WG2002. Focus on prostate cancer. Cancer Cell 2:113–116 [DOI] [PubMed] [Google Scholar]
- 6.Debes JD, Tindall DJ2002. The role of androgens and the androgen receptor in prostate cancer. Cancer Lett 187:1–7 [DOI] [PubMed] [Google Scholar]
- 7.Scher HI, Sawyers CL2005. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 23:8253–8261 [DOI] [PubMed] [Google Scholar]
- 8.Gregory CW, Hamil KG, Kim D, Hall SH, Pretlow TG, Mohler JL, French FS1998. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res 58:5718–5724 [PubMed] [Google Scholar]
- 9.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL2004. Molecular determinants of resistance to antiandrogen therapy. Nat Med 10:33–39 [DOI] [PubMed] [Google Scholar]
- 10.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ2002. Disruption of androgen function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res 62:1008–1013 [PubMed] [Google Scholar]
- 11.Linja MJ, Savinainen KJ, Saramäki OR, Tammela TL, Vessella RL, Visakorpi T2001. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res 61:3550–3555 [PubMed] [Google Scholar]
- 12.Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, Stadler W, Hayes DF, Kantoff PW, Vogelzang NJ, Small EJ2003. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol 21:2673–2678 [DOI] [PubMed] [Google Scholar]
- 13.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H1994. Androgen receptor activation in prostate tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res 54:5474–5478 [PubMed] [Google Scholar]
- 14.Craft N, Shostak Y, Carey M, Sawyers CL1999. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med 5:280–285 [DOI] [PubMed] [Google Scholar]
- 15.Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z1998. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res 58:4640–4645 [PubMed] [Google Scholar]
- 16.Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, Nelson C, Gleave M2007. Cooperative interaction between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res 67:10455–10465 [DOI] [PubMed] [Google Scholar]
- 17.Park SY, Yu X, Ip C, Mohler JL, Bogner PN, Park YM2007. Peroxiredoxin 1 interacts with androgen receptor and enhances its transactivation. Cancer Res 67:9294–9303 [DOI] [PubMed] [Google Scholar]
- 18.Gaughan L, Logan IR, Cook S, Neal DE, Robson CN2002. Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem 277:25904–25913 [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto H, Rahman M, Takatera H, Kang HY, Yeh S, Chang HC, Nishimura K, Fujimoto N, Chang C2002. A dominant-negative mutant of androgen receptor coregulator ARA-54 inhibits androgen receptor-mediated prostate cancer growth. J Biol Chem 277:4609–4617 [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto N, Yeh S, Kang HY, Inui S, Chang HC, Mizokami A, Chang C1999. Cloning and characterization of androgen receptor coactivator, ARA55, in human prostate. J Biol Chem 274:8316–8321 [DOI] [PubMed] [Google Scholar]
- 21.Rahman MM, Miyamoto H, Taketera H, Yeh S, Altuwaijri S, Chang C2003. Reducing the agonist activity of antiandrogens by a dominant-negative androgen receptor coregulator ARA70 in prostate cancer cells. J Biol Chem 278:19619–19626 [DOI] [PubMed] [Google Scholar]
- 22.Hong H, Kohli K, Garabedian MJ, Stallcup MR1997. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol 17:2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boonyaratanakornkit V, Melvin V, Prendergast P, Altmann M, Ronfani L, Bianchi ME, Taraseviciene L, Nordeen SK, Allegretto EA, Edwards DP1998. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol Cell Biol 18:4471–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross M, Liu B, Tan J, French FS, Carey M, Shuai K2001. Distinct effects of PIAS proteins on androgen-mediated gene activation in prostate cancer cells. Oncogene 20:3880–3887 [DOI] [PubMed] [Google Scholar]
- 25.Bevan CL, Hoare S, Claessens F, Heery DM, Parker MG1999. The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC1. Mol Cell Biol 19:8383–8392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM1998. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839 [DOI] [PubMed] [Google Scholar]
- 27.Knutti D, Kaul A, Kralli A2000. A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Mol Cell Biol 20:2411–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM1999. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124 [DOI] [PubMed] [Google Scholar]
- 29.Kressler D, Schreiber SN, Knutti D, Kralli A2002. The PGC-1-related protein PERC is a selective coactivator of estrogen receptor α. J Biol Chem 277:13918–13925 [DOI] [PubMed] [Google Scholar]
- 30.Tcherepanova I, Puigserver P, Norris JD, Spiegelman BM, McDonnell DP2000. Modulation of estrogen receptor-α transcriptional activity by the coactivator PGC-1. J Biol Chem 275:16302–16308 [DOI] [PubMed] [Google Scholar]
- 31.Bourdoncle A, Labesse G, Margueron R, Castet A, Cavaillès V, Royer CA2005. The nuclear receptor coactivator PGC-1α exhibits modes of interaction with the estrogen receptor distinct from those of SRC-1. J Mol Biol 347:921–934 [DOI] [PubMed] [Google Scholar]
- 32.Castillo G, Brun RP, Rosenfield JK, Hauser S, Park CW, Troy AE, Wright ME, Spiegelman BM1999. An adipogenic cofactor bound by the differentiation domain of PPARγ. EMBO J 18:3676–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson E2002. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4:197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fishman J, Osborne MP, Telang NT1995. The role of estrogen in mammary carcinogenesis. Ann NY Acad Sci 768:91–100 [DOI] [PubMed] [Google Scholar]
- 35.Wirtenberger M, Tchatchou S, Hemminki K, Schmutzhard J, Sutter C, Schmutzler RK, Meindl A, Wappenschmidt B, Kiechle M, Arnold N, Weber BH, Niederacher D, Bartram CR, Burwinkel B2006. Association of genetic variants in the estrogen receptor coactivators PPARGC1A, PPARGC1B and EP300 with familial breast cancer. Carcinogenesis 27:2201–2208 [DOI] [PubMed] [Google Scholar]
- 36.Shenk JL, Fisher CJ, Chen SY, Zhou XF, Tillman K, Shemshedini L2001. p53 Represses androgen-induced transactivation of prostate-specific antigen by disrupting hAR amino- to carboxyl-terminal interaction. J Biol Chem 42:38472–38479 [DOI] [PubMed] [Google Scholar]
- 37.Li J, Fu J, Toumazou C, Yoon HG, Wong J2006. A role of the amino-terminal (N) and carboxyl-terminal (C) interaction in binding of androgen receptor to chromatin. Mol Endocrinol 20:776–785 [DOI] [PubMed] [Google Scholar]
- 38.Need EF, Scher HI, Peters AA, Moore NL, Cheong A, Ryan CJ, Wittert GA, Marshall VR, Tilley WD, Buchanan G2009. A novel androgen receptor amino terminal region reveals two classes of amino/carboxyl interaction-deficient variants with divergent capacity to activate responsive sites in chromatin. Endocrinology 150:2674–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shang Y, Myers M, Brown M2002. Formation of the androgen receptor transcription complex. Mol Cell 9:601–610 [DOI] [PubMed] [Google Scholar]
- 40.Tyagi RK, Lavrovsky Y, Ahn SC, Song CS, Chatterjee B, Roy AK2000. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol Endocrinol 14:1162–1174 [DOI] [PubMed] [Google Scholar]
- 41.Debes JD, Sebo TJ, Lohse CM, Murphy LM, Haugen DA, Tindall DJ2003. p300 In prostate cancer proliferation and progression. Cancer Res 63:7638–7640 [PubMed] [Google Scholar]
- 42.Powell SM, Christiaens V, Voulgaraki D, Waxman J, Claessens F, Bevan CL2004. Mechanisms of androgen receptor signaling via steroid receptor coactivator-1 in prostate. Endocr Relat Cancer 11:117–130 [DOI] [PubMed] [Google Scholar]
- 43.Abate-Shen C, Shen MM2000. Molecular genetics of prostate cancer. Genes Dev 14:2410–2434 [DOI] [PubMed] [Google Scholar]
- 44.McKenna NJ, Lanz RB, O'Malley BW1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 29:321–344 [DOI] [PubMed] [Google Scholar]
- 45.McKenna NJ, O'Malley BW2002. Combined control of gene expression by nuclear receptors and coregulators. Cell 108:465–474 [DOI] [PubMed] [Google Scholar]
- 46.Linja MJ, Porkka KP, Kang Z, Savinainen KJ, Jänne OA, Tammela TL, Vessella RL, Palvimo JJ, Visakorpi T2004. Expression of androgen receptor coregulators in prostate cancer. Clin Cancer Res 10:1032–1040 [DOI] [PubMed] [Google Scholar]
- 47.Oñate SA, Tsai SY, Tsai MJ, O'Malley BW1995. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357 [DOI] [PubMed] [Google Scholar]
- 48.Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H, Ittmann M, Tsai SY, Tsai MJ2005. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res 65:7976–7983 [DOI] [PubMed] [Google Scholar]
- 49.Shi XB, Xue L, Zou JX, Gandour-Edwards R, Chen H, deVere White RW2008. Prolonged androgen receptor loading onto chromatin and the efficient recruitment of p160 coactivators contribute to androgen-independent growth of prostate cancer cells. Prostate 68:1816–1826 [DOI] [PubMed] [Google Scholar]
- 50.Heemers HV, Sebo TJ, Debes JD, Regan KM, Raclaw KA, Murphy LM, Hobisch A, Culig Z, Tindall DJ2007. Androgen deprivation increases p300 expression in prostate cancer cells. Cancer Res 67:3422–3430 [DOI] [PubMed] [Google Scholar]
- 51.Heery DM, Kalkhoven E, Hoare S, Parker MG1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733–736 [DOI] [PubMed] [Google Scholar]
- 52.Voegel JJ, Heine MJ, Tini M, Vivat V, Chambon P, Gronemeyer H1998. The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J 17:507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McInerney EM, Weis KE, Sun J, Mosselman S, Katzenellenbogen BS1998. Transcription activation by the human estrogen receptor subtype β (ERβ) studied with ERβ and ERα receptor chimeras. Endocrinology 139:4513–4522 [DOI] [PubMed] [Google Scholar]
- 54.Greschik H, Althage M, Flaig R, Sato Y, Chavant V, Peluso-Iltis C, Choulier L, Cronet P, Rochel N, Schüle R, Strömstedt PE, Moras D2008. Communication between the ERRα homodimer interface and the PGC-1α binding surface via the helix 8–9 loop. J Biol Chem 283:20220–20230 [DOI] [PubMed] [Google Scholar]
- 55.Delerive P, Wu Y, Burris TP, Chin WW, Suen CS2002. PGC-1 functions as a transcriptional coactivator for the retinoid X receptors. J Biol Chem 277:3913–3917 [DOI] [PubMed] [Google Scholar]
- 56.Sato T, Otaka M, Odashima M, Kato S, Jin M, Konishi N, Matsuhashi T, Watanabe S2006. Specific type IV phosphodiesterase inhibitor ameliorates cerulein-induced pancreatitis in rats. Biochem Biophys Res Commun 346:339–344 [DOI] [PubMed] [Google Scholar]
- 57.Yanase T, Fan W, Kyoya K, Min L, Takayanagi R, Kato S, Nawata H2008. Androgens and metabolic syndrome: lessons from androgen receptor knock out (ARKO) mice. J Steroid Biochem Mol Biol 109:254–257 [DOI] [PubMed] [Google Scholar]
- 58.Sanyal S, Matthews J, Bouton D, Kim HJ, Choi HS, Treuter E, Gustafsson JA2004. Deoxyribonucleic acid response element-dependent regulation of transcription by orphan nuclear receptor estrogen receptor-related receptor γ. Mol Endocrinol 18:312–325 [DOI] [PubMed] [Google Scholar]
- 59.Coste A, Louet JF, Lagouge M, Lerin C, Antal MC, Meziane H, Schoonjans K, Puigserver P, O'Malley BW, Auwerx J2008. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1α. Proc Natl Acad Sci USA 105:17187–17192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shiota M, Yokomizo A, Tada Y, Inokuchi J, Kashiwagi E, Masubuchi D, Eto M, Uchiumi T, Naito S, Castration resistance of prostate cancer cells caused by castration-induced oxidative stress through Twist1 and androgen receptor overexpression. Oncogene 10.1038/onc.2009.322 [DOI] [PubMed]
- 61.Tomura A, Goto K, Morinaga H, Nomura M, Okabe T, Yanase T, Takayanagi R, Nawata H2001. The subnuclear three-dimensional image analysis of androgen receptor fused to green fluorescence protein. J Biol Chem 276:28395–28401 [DOI] [PubMed] [Google Scholar]
- 62.Mizokami A, Gotoh A, Yamada H, Koller ET, Matsumoto T2000. Tumor necrosis factor-α represses androgen sensitivity in the LNCaP prostate cancer cell line. J Urol 164:800–805 [DOI] [PubMed] [Google Scholar]
- 63.Shiota M, Izumi H, Onitsuka T, Miyamoto N, Kashiwagi E, Kidani A, Yokomizo A, Naito S, Kohno K2008. Twist promotes tumor cell growth through YB-1 expression. Cancer Res 68:98–105 [DOI] [PubMed] [Google Scholar]
- 64.Shiota M, Izumi H, Onitsuka T, Miyamoto N, Kashiwagi E, Kidani A, Hirano G, Takahashi M, Naito S, Kohno K2008. Twist and p53 reciprocally regulate target genes via direct interaction. Oncogene 27:5543–5553 [DOI] [PubMed] [Google Scholar]
- 65.Shiota M, Izumi H, Miyamoto N, Onitsuka T, Kashiwagi E, Kidani A, Hirano G, Takahashi M, Ono M, Kuwano M, Naito S, Sasaguri Y, Kohno K2008. Ets regulates peroxiredoxin1 and 5 expressions through their interaction with the high mobility group protein B1. Cancer Sci 99:1950–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shiota M, Izumi H, Tanimoto A, Takahashi M, Miyamoto N, Kashiwagi E, Kidani A, Hirano G, Masubuchi D, Fukunaka Y, Yasuniwa Y, Naito S, Nishizawa S, Sasaguri Y, Kohno K2009. Programmed cell death protein 4 down-regulates Y-box binding protein-1 expression via a direct interaction with Twist1 to suppress cancer cell growth. Cancer Res 69:3148–3156 [DOI] [PubMed] [Google Scholar]