Abstract
G protein-coupled receptor 119 (GPR119) is largely restricted to pancreatic insulin-producing β-cells and intestinal glucagon-like peptide-1-producing L-cells. Synthetic agonists of this receptor elicit glucose-dependent release of these endocrine factors, thereby enhancing glycemic control. Oleoylethanolamide also activates GPR119, but it remains unclear whether endogenous production of this lipid modulates GPR119 activity under normal or dysglycemic conditions. We show here that a relatively diverse set of lipid amides activate GPR119. Among these, the endovallinoid N-oleoyldopamine (OLDA) stimulated cAMP accumulation in GPR119-transfected cells as effectively as oleoylethanolamide and the previously described synthetic agonist AR231453. None of these lipid amides increased cAMP in control-transfected cells or in cells transfected with a number of other G protein-coupled receptors. OLDA stimulated both cAMP accumulation and insulin release in HIT-T15 cells, which express GPR119 endogenously, and in GPR119-transfected RIN-5F cells. Oral administration of OLDA to C57bl/6 mice elicited significant improvement in glucose tolerance, whereas GPR119-deficient mice were essentially unresponsive. OLDA also acutely elevated plasma gastric inhibitory peptide levels, a known hallmark of GPR119 activation. OLDA represents a possible paracrine modulator of GPR119 in pancreatic islets, where markers of dopamine synthesis correlated well with GPR119 expression. However, no such correlation was seen in the colon. Collectively, these studies indicate that multiple, distinct classes of lipid amides, acting via GPR119, may be important modulators of glucose homeostasis.
Structurally novel endogenous activators of GPR119 are identified, suggesting that diverse lipid amides may contribute to receptor activation in vivo.
G protein-coupled receptor 119 (GPR119) is a Gαs-coupled receptor selectively expressed in insulin-producing cells of the pancreas and glucagon-like peptide-1-producing cells of the ileum and colon. Synthetic GPR119 agonists elicit marked improvements in glucose tolerance largely by enhancing the release of hormones from these cells (1, 2, 3, 4). GPR119 agonists also substantially increase plasma gastric inhibitory peptide (GIP) levels, although it is currently uncertain whether this effect is via receptors present on duodenal K-cells (2). Thus, GPR119 is a component of an important endocrine or paracrine system that regulates multiple endocrine cell types involved in glucose homeostasis.
Further understanding of this novel system would benefit from knowledge of the endogenous substance(s) that are bona fide regulators of GPR119 activity in vivo. In this regard, oleoylethanolamide (OEA), lysophosphatidylcholine (LPC), and retinoic acid have all been suggested as possible activators of GPR119 (5, 6, 7). Among these, OEA may be the best candidate as an endogenous ligand, based on its significantly superior efficacy and potency [(6) and data shown herein]. However, nutrient-regulated production of OEA appears to be specifically restricted to the intestinal jejunem (8) and thus does not correlate with sites of GPR119 expression. Moreover, the anorectic action of OEA is not mediated by GPR119 (9) but rather by peroxisome proliferator-activated receptor α (10). Thus, the nature of the physiologically relevant endogenous GPR119 modulator(s) remains uncertain.
N-oleoyldopamine (OLDA) was originally identified as one of several lipid amides detected in the striatum of the central nervous system (11). OLDA and OEA both modulate the activity of vanilloid-responsive transient receptor potential vanilloid 1 (TRPV1) channels (10, 12, 13). However, the relevance of these lipid amides to TRPV1 function is somewhat complicated by the observations that OLDA elicits hyperalgesia (11), whereas OEA is analgesic (14). Furthermore, the pleiotropic nature of many lipid amides further complicates their assignment to specific molecular targets (15). So far, peripheral sites of OLDA synthesis have not been described. Nevertheless, mesenteric tissues are a major source of circulating dopamine, which is of nonneuronal origin (16), suggesting the possibility that OLDA could potentially be synthesized in the gut. Plasma dopamine levels rise postprandially and fall during fasting (17, 18) but for physiological reasons that remain largely unknown. Here we show that OLDA and other hydroxybenzyl lipid amides are robust activators of GPR119 and that OLDA elicits robust effects on insulin release and glucose homeostasis in vitro and in vivo, respectively. These data suggest a possible functional role for the substantial levels of postprandial dopamine observed in the mesenteric circulation. Moreover, they implicate OLDA and its structural relatives as possible endogenous modulators of GPR119 function.
Results
GPR119 is activated by fatty acid amides
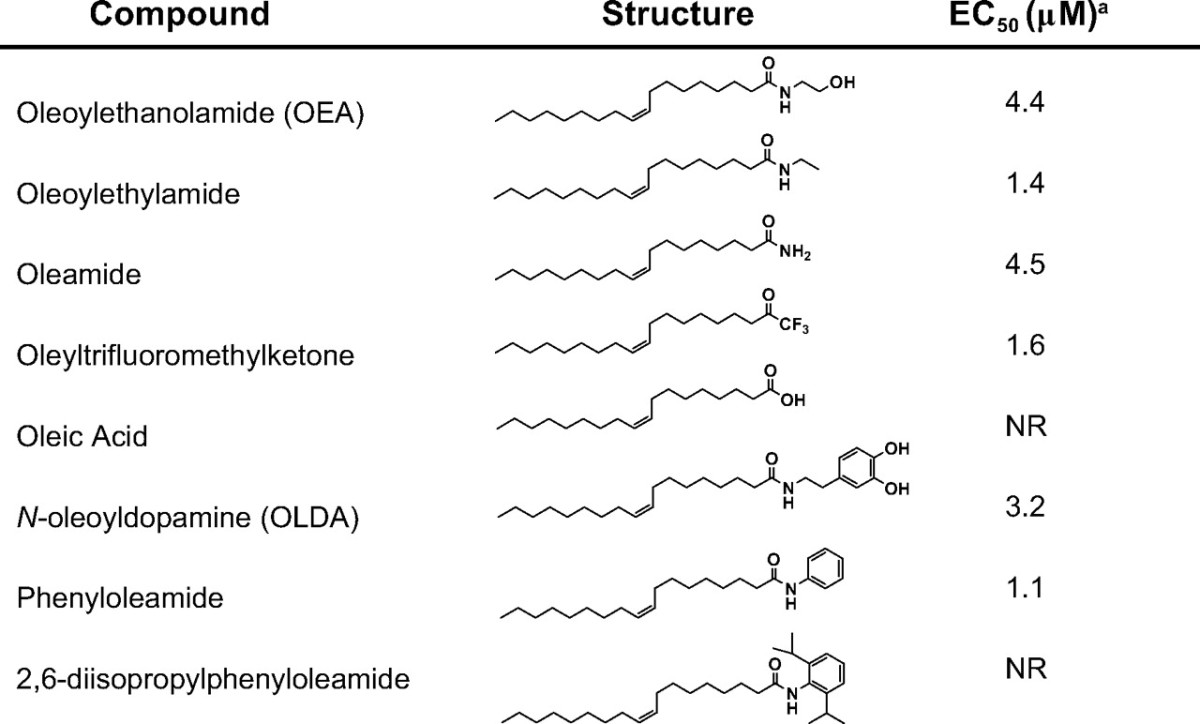
To identify potential physiological modulators of GPR119, more than 3000 endogenously produced substances were tested for their ability to modulate cAMP levels in GPR119-transfected cells. In addition, a library of more than 200 bioactive lipids (Biomol) was screened against the receptor. None of the peptides, biogenic amines, trace amines, free fatty acids, retinoids, lipid phosphates, amino acids, nucleotides, sugars, or other components of intermediary metabolism in this diverse substance collection was able to elicit GPR119-mediated increases in cAMP. However, several fatty acid amides were able to activate the receptor (Table 1). Among these, the previously identified GPR119 activator OEA (6) increased cAMP in GPR119-transfected HEK293 cells with a potency similar to published data (EC50, 4.4 μm). However, in contrast to previous reports (5, 7), all-trans-retinoic acid and C18:1 LPC were inactive.
Table 1.
Activity of representative compounds in human GPR119-transfected cells

NR, No response.
a Measured as induction of cAMP in GPR119-transfected HEK293 cells.
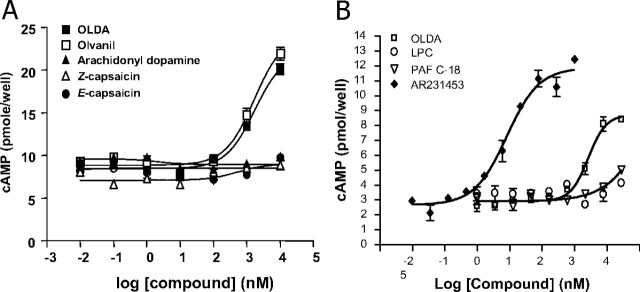
Interestingly, the endogenous TRPV1 ligand, OLDA, also activated GPR119 with similar potency to OEA (Table 1 and Fig. 1). Other TRPV1 ligands elicited varied responses. Olvanil, a structurally similar long-chain lipid amide, behaved similarly to OLDA. However, E- and Z-capsaicin were inactive, suggesting that lipid chain lengths more than C10 were essential. N-arachidonyldopamine (NADA) was also inactive, suggesting that the saturation state of the lipid chain can influence activity. With regard to the amine-derived “head group,” considerable permissiveness was observed (Table 2). Nonhydroxylated aliphatic or aromatic amides (e.g. OEA and phenyloleamide) were as active as hydroxylated relatives. Moreover, the endogenously produced free amide, oleamide, was fully active. Even the amide moiety per se was not essential for activity, as illustrated by oleyltrifluoromethylketone.
Fig. 1.
GPR119 is responsive to lipid amides. A, Adenylyl cyclase assay in human GPR119-transfected HEK293 cells. HEK293 cells were transfected with 5 μg of plasmid DNA encoding haemmaglutinin (HA)-tagged human GPR119 in a 10-cm dish. Twenty-four hours after transfection, cells were collected, and 1 × 105 cells per well were used for cAMP assay. Data were shown as picomole cAMP production by cells plated in one well of a 96-well plate (mean ± sem, n = 3) and plotted using Prism software program. B, Comparison between GPR119 synthetic agonist AR231453, OLDA, C18:1 LPC, and platelet-activating factor (PAF) on human GPR119-mediated cAMP production in transfected 293 cells.
Table 2.
Head group permissiveness in human GPR119-transfected cells
NR, No response.
a Measured as induction of cAMP in GPR119-transfected HEK293 cells.
The most potent lipid amide GPR119 agonists identified were the N-oleoyltyrosinol stereoisomers (Table 1), which have previously been characterized as inhibitors of anandamide uptake (19, 20). Both stereoisomers exhibited a modest left shift in potency relative to OLDA or OEA. Acid derivatives of lipid amides, such as N-oleoyltyrosine (Table 2) and oleoyltaurine (not shown), were invariably inactive.
None of the lipids described here were active in vector-transfected cells. Moreover, all lipids were independently tested in a Xenopus melanophore dispersion assay, which measures dispersion of melanosome organelles in response to elevations in cAMP (21). All lipids active in cAMP assays were also active in dispersion assays using GPR119-transfected melanophores but not mock-transfected melanophores (data not shown).
OLDA stimulates insulin release in a GPR119-dependent manner
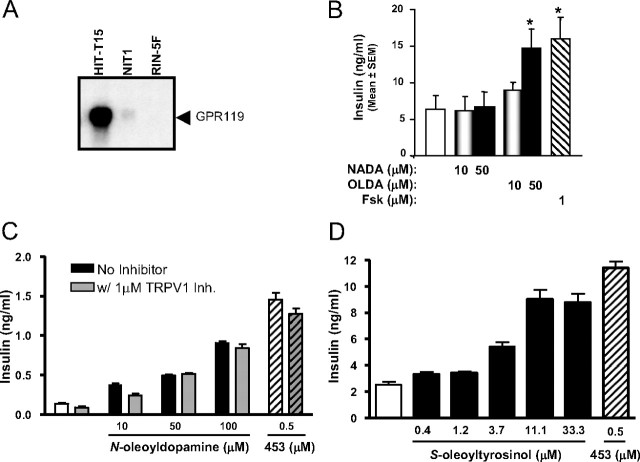
Synthetic agonists of GPR119 elicit insulin release from pancreatic β-cells (1). OLDA was chosen to explore the possible physiologic role of endogenous lipid amides in GPR119-mediated glycemic control, in part because OEA and oleamide trigger significant metabolic effects via other molecular targets (10, 22). OLDA stimulated insulin release in the hamster insulinoma cell line HIT-T15 (Fig. 2B), which express GPR119 at high levels (1). By contrast, NADA, which is inactive on GPR119 (see Fig. 1A), had no effect.
Fig. 2.
OLDA stimulates insulin release from HIT-T15 cells. A, Northern blot evaluation of GPR119 expression in HIT-T15, NIT-1, and RIN-5Fcells. B, Insulin release in HIT-T15 cells treated with the indicated concentrations of OLDA, NADA, or forskolin (fsk) for 1 h. C, Effect of 6′-iodononivamide [TRPV1 inhibitor (Inh.)] on the ability of OLDA or AR231453 (453) to stimulate insulin release from HIT-T15 cells. D, Insulin release from S-oleoyltyrosinol-stimulated HIT-T15 cells. Cell culture supernatants were collected and assayed for insulin content as described in Materials and Methods. Results are shown as means of insulin concentration of three independent experiments (mean ± sem, n = 3). fsk, Forskolin. *, Statistically significant difference (P < 0.05) between compound-treated group (filled bar) vs. buffer-treated control (open bar) determined via t test statistics.
Both OLDA and NADA are potent agonists of TRPV1 (11, 12, 13), which has also been implicated in insulin release (23). Because NADA did not stimulate insulin release from HIT-T15 cells, it would seem unlikely that the insulin-releasing effects of OLDA are the result of activation of TRPV1. Nevertheless, to rule out this possibility, we assessed the ability of the potent TRPV1 antagonist 6′-iodononivamide (24) to block OLDA action in HIT-T15 cells. Both OLDA and the GPR119 agonist AR231453 retained full insulinotropic activity in the presence of 6′-iodononivamide (Fig. 2C). Additionally, the related lipid amide S-oleoyltyrosinol, which is inactive on TRPV1 (19), stimulated insulin release from HIT-T15 cells (Fig. 2D). Collectively, these data indicate that TRPV1 does not mediate enhanced insulin release in response to OLDA or related lipid amides.
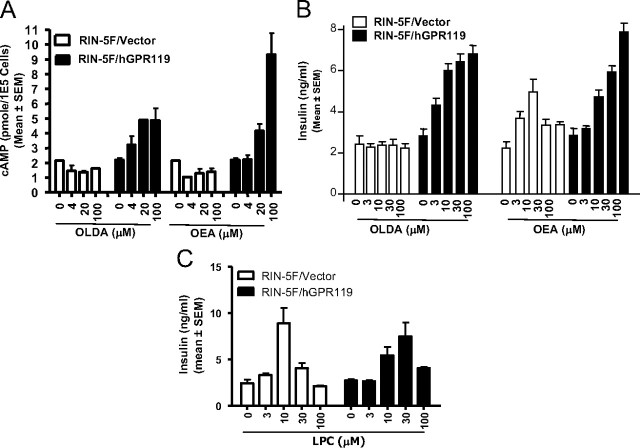
To provide additional evidence that OLDA stimulates insulin release via activation of GPR119, we generated isogenic sublines of RIN-5F-containing vector DNA alone (RIN-5F/vector) or a human GPR119 expression plasmid (RIN-5F/hGPR119). RIN-5F cells do not normally express endogenous GPR119 (see Fig. 2A). In GPR119-expressing RIN-5F cells, OLDA dose dependently triggered both cAMP accumulation and insulin release (Fig. 3, A and B). By contrast, in parental RIN-5F cells (not shown) or RIN-5F/vector control stables, OLDA did not stimulate cAMP accumulation or insulin release (Fig. 3, A and B). Thus, the insulinotropic effect of OLDA is GPR119 dependent. The effects of OEA were more complex. OEA stimulated insulin release in RIN-5F/hGPR119 cells and to a lesser extent in control cells. OEA may therefore mediate both GPR119-dependent and GPR119-independent effects on β cells, consistent with recently published data in MIN6c4 insulinoma cells (25).
Fig. 3.
GPR119-dependent insulinotropic effect of OLDA and OEA. RIN-5F cells were stably transfected with human GPR119 expression plasmid (RIN-5F/hGPR119) or with empty plasmid (RIN-5F/vector). Cells were treated with the indicated concentrations of OLDA or OEA and subsequently evaluated for cAMP production (A) or insulin release (B). C, LPC-stimulated insulin release in both vector-transfected and GPR119-expressing RIN-5F stables.
LPC also stimulates insulin release, potentially via a GPR119-based mechanism (7). Although LPC was inactive when tested in GPR119-transfected Chinese hamster ovary cells (Table 1), we wished to rule out that its activity might be cell-type dependent. LPC was therefore evaluated in RIN-5F/vector and RIN-5F/hGPR119 cells. In both cells, LPC stimulated insulin release robustly at low μm concentration (Fig. 3C). Therefore, although LPC is insulinotropic, its effect is apparently GPR119 independent.
OLDA induces GIP release and improvesglucose tolerance
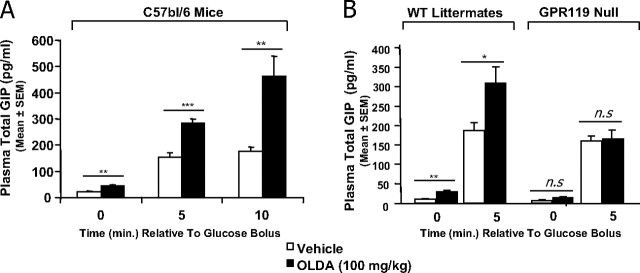
In addition to its role in pancreatic β cells, GPR119 mediates GIP and glucagon-like peptide-1 release from intestinal endocrine cells (2). We therefore examined whether OLDA can elicit similar physiologic responses in mice. In C57bl/6 mice, OLDA (100 mg/kg, per oral) increased GIP levels approximately 2-fold within 30 min (Fig. 4A, at time 0 relative to glucose challenge). Then, after challenge with oral glucose, OLDA-treated mice continued to exhibit a significant increase in plasma total GIP relative to vehicle-treated mice (Fig. 4A). These data are similar to those seen with the GPR119-selective synthetic agonist AR231453 (2).
Fig. 4.
OLDA increases plasma total GIP levels in mice. A, GIP pharmacodynamic analysis in C57bl/6 mice. Overnight-fasted male mice were dosed with either vehicle or 100 mg/kg OLDA via oral gavage. An oral glucose bolus (3 g/kg) was then administered 30 min after vehicle or OLDA treatment. Plasma was collected for total GIP levels at the indicated time point (time in minutes relative to glucose bolus). Results were given as the mean GIP levels from six mice in each group (mean ± sem, n = 6). B, OLDA does not increase plasma GIP levels in GPR119-deficient mice. GIP pharmacodynamic analysis was conducted in GPR119-deficient mice and their wild-type littermates as described in A. WT, Wild type. *, **, ***, statistically significant difference (P < 0.05, 0.01, and 0.001 respectively) between vehicle-treated and OLDA-treated animals at each time point using t test statistical method. n.s., Not significant.
To confirm that GIP release by OLDA is mediated via GPR119, we used GPR119-deficient mice and their wild-type littermates. As shown in Fig. 4B, OLDA-induced GIP release was readily seen in wild-type littermates both before glucose challenge and 5 min after glucose challenge. However, in GPR119-deficient mice, glucose stimulated GIP release as expected, but OLDA had no effect on basal or glucose-stimulated GIP release. Thus, the induction of GIP by OLDA requires the presence of GPR119 in vivo.
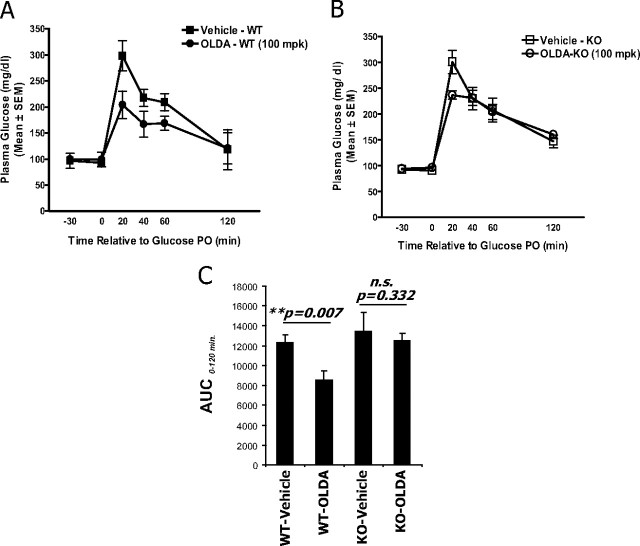
Next, OLDA was evaluated for its ability to improve glucose handling in mice. In wild-type littermates, delivery of OLDA (100 mg/kg, per oral) improved oral glucose tolerance significantly (Fig. 5A). By contrast, OLDA had virtually no effect on glucose tolerance in GPR119-deficient mice (Fig. 5B). When areas under the curve were evaluated, there was no statistically significant difference between vehicle-treated vs. OLDA-treated mice (Fig. 5C). OLDA therefore improves glucose handling in mice in a GPR119-dependent manner.
Fig. 5.
OLDA improves oral glucose tolerance in mice in a GPR119-dependent manner. Overnight-fasted male mice were dosed with either vehicle or 100 mg/kg OLDA via oral gavage. An oral glucose bolus (3 g/kg) was then administered 30 min after vehicle or OLDA treatment. Plasma glucose levels (mg/dl) were determined using a OneTouch Ultra Glucose meter and blood (∼5 μl) collected from tail nick at indicated time points (time in minutes relative to glucose bolus). Glucose excursion curve was shown for wild-type littermates (A) and for GPR119-deficient mice (B) and from data collected from six (n = 6) in each treatment group and genotype (mean ± sem, n = 6). C, The area under the curve (AUC) of the glucose excursion curve from time 0 to 120 min after glucose bolus was shown for each treatment group, as indicated. Statistical significance (P value) was assessed by t test between vehicle-treated and OLDA treated-groups. KO, Knockout; PO, per oral; WT, wild type; n.s., not significant.
Tyrosine hydroxylase (TH), dopa decarboxylase (DDC), and fatty acid amide hydrolase (FAAH) are expressed in mouse islets
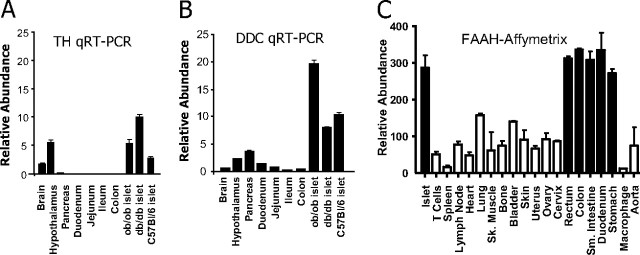
If OLDA functions as an endogenous GPR119 agonist, it is likely to be produced in a paracrine fashion, as is generally the case for endocannabinoids and endovanilloids (15). Thus, the synthetic machinery for OLDA should be detectable in islets and colon, where GPR119 is mainly expressed. Although the synthetic pathway for OLDA has not been characterized, it is very likely to include TH and DDC, which are essential for the synthesis of dopamine. We isolated islets from several mouse strains including C57bl/6, ob/ob, and db/db and analyzed TH and DDC expression by quantitative RT-PCR. As shown in Fig. 6 A and B, both TH and DDC were highly expressed in mouse islets. However, TH and DDC were poorly expressed in colon.
Fig. 6.
Expression of TH, DDC, and FAAH in mouse islets and intestines. RNA was extracted from whole brain, hypothalamus, indicated regions of the gut, and pancreas of C57bl/6 mice (n = 3) and from isolated pancreatic islets of C57bl/6, ob/ob, and db/db mice. Expression of TH and DDC was analyzed by Taqman QPCR (qRT-PCR). Expression levels were normalized with internal β-actin control and shown for TH (A) and for DDC (B). FAAH expression in the comparable sets of mouse tissues (C) was determined using a custom Affymetrix gene expression chip. QRT-PCR, Quantitative real-time RT-PCR; SK, skeletal; Sm, small.
FAAH, which is responsible for the degradation of endocannabinoids, may also be an important component of OLDA synthesis. The striatal levels of the close OLDA relative, NADA, are greatly reduced in mice lacking FAAH or in mice treated with a FAAH inhibitor (26). Moreover, FAAH efficiently catalyzes the formation of NADA in vitro. Relative to most other tissues, FAAH was found at remarkably high levels in islets and in various intestinal subregions (Fig. 6C). The regional tissue distribution of FAAH therefore significantly overlaps with known sites of GPR119 expression (1, 2).
Discussion
We show here that OLDA and structurally related hydroxybenzyl amides are robust activators of GPR119. In vitro, OLDA is essentially equipotent to OEA, which has previously been proposed to be the endogenous ligand for this receptor (6). OLDA also stimulates insulin release from islet cell lines that endogenously express GPR119, as well as from GPR119-transfected insulinoma lines. In vivo, OLDA both enhances GIP release and improves glucose tolerance in a GPR119-dependent fashion. These data collectively demonstrate that OLDA is a robust, potentially physiologically relevant modulator of GPR119.
In principle, the effects of OLDA on insulin release and glucose homeostasis might also depend partly on the vallinoid-responsive channel TRPV1, given its well-characterized agonist activity at this channel (12). TRPV1 has been detected in both rat pancreatic β cells and in RINm5F cells (23). Additionally, the TRPV1 agonist capsaicin stimulates both calcium mobilization and insulin release in RINm5F cells (23, 27). In the present study, however, the insulinotropic effects of OLDA in HIT-T15 cells are almost certainly not mediated via TRPV1 channels because the potent TRPV1 antagonist 6′-iodononivamide does not reverse the activity seen with OLDA. Furthermore, the insulin-releasing effects of hydroxybenzyl lipid amides correlates with their GPR119 activity but not with their TRPV1 activity. The TRPV1 agonist NADA does not elicit insulin release from HIT-T15 cells, whereas S-oleoyltyrosinol (a potent GPR119 agonist with no TRPV1 activity) was as effective as the GPR119 agonist AR231453. However, it is not readily apparent why we did not see an insulinotropic effect of OLDA in RIN-5F cells, an activity that might be predicted on the basis of previously published data with capsaicin (23). This could be the result of clonal differences between RIN-5F cells and RINm5F cells or alternatively the result of the rather poor efficacy of TRPV1 activation in RINm5F cells (23) when compared with OLDA-mediated GPR119 activation in RIN-5F cells (Fig. 3).
It is unknown whether OLDA is produced at sites of GPR119 expression, and it is difficult to assess this issue indirectly because the enzymatic machinery responsible for the synthesis of OLDA is currently unknown. At least some lipid amides derived from bioactive amines may be synthesized by direct conjugation to fatty acids, including arachidonyldopamine, oleoylglycine, and arachidonylglycine (26, 28, 29, 30). Thus, at the least, paracrine or regional synthesis of dopamine is likely to be an essential aspect of OLDA synthesis. Our data illustrate that isolated mouse pancreatic islets contain significant levels of transcripts encoding tyrosine hydroxylase and dopa decarboxylase. The levels of these transcripts compare favorably to their corresponding expression in hypothalamus and are maintained in islets isolated from ob/ob and db/db mice. Published immunohistochemical data indicate that TH and DDC are present in embryonic and postnatal rodent pancreatic islets (31, 32, 33, 34, 35), and TH immunoreactivity is elevated in vasoactive-intestinal-peptide-treated mouse islets (36). Moreover, dopamine is detected in rat pancreatic islets at significant levels (37, 38). Collectively, these observations strongly suggest that pancreatic islets from normoglycemic and dysglycemic mice are capable of dopamine production. However, we did not detect robust expression of TH and DDC in colon, where GPR119-containing enteroendocrine cells are found (2). Nevertheless, mesenteric organs in the human gastrointestinal tract are a rich source of plasma dopamine, which is of nonneuronal origin (16). The significance of islet- and gut-derived dopamine production to GPR119 function ultimately will rest on whether this biogenic amine is subsequently conjugated with fatty acids in these tissues.
One interesting possibility for such a conjugating enzyme is FAAH. We show here that FAAH is preferentially expressed in pancreatic islets at significant levels, consistent with published immunohistochemical data (39, 40). FAAH is a well-known catabolic enzyme for endocannabinoids, but it can also act in reverse to mediate the synthesis of anandamide from arachidonic acid and ethanolamine (41). The latter reaction is likely insignificant in vivo because anandamide is an excellent substrate for FAAH, and the rate of hydrolysis far exceeds the rate of synthesis. However, NADA and OLDA are poor substrates for FAAH (11, 26, 28). Additionally, recent data have indicated that FAAH plays an essential role in the synthesis of NADA via a reaction involving the conjugation of arachidonic acid with dopamine (26). It is therefore likely that FAAH is an important mediator of NADA synthesis. OLDA synthesis has not been similarly evaluated to date but at least with regard to catalysis of ethanolamides, FAAH does not appear to discriminate between arachidonyl and oleoyl amides (42).
Although OLDA was chosen to investigate the functional properties of GPR119-activating lipid amides more broadly, it is important to recognize that numerous endogenous and synthetic lipid amide agonists are active at this receptor. There is a strikingly broad permissiveness in the amine-derived moieties of lipid amides with agonist activity at GPR119. For example, oleoyl amides containing a wide range of bulk are active, as exemplified by oleamide, OEA, and OLDA. In addition, hydroxylation of the head group is not required for activity because both phenyloleamide and ethyloleamide retain full efficacy at GPR119. The requirements of the fatty acyl chain likewise demonstrate some flexibility. C16-18 fatty acyl chains with 0–2 unsaturated bonds display similar low micromolar potency and full efficacy. However, linolenyl amides are weakly active (data not shown), and arachidonyl amides are inactive. Furthermore, the inactivity seen with capsaicin suggests that significant truncation of the fatty acyl chain abrogates activity. These data are essentially consistent with the previously reported fatty acyl requirements of ethanolamides (6). Finally, note that even the amide linkage is not essential for activity, as shown by the active trifluoromethyl-C18 ketone. The minimal lipid pharmacophore for GPR119 therefore appears to consist of linear long-chain lipids containing limited unsaturation and a carbonyl motif near the chain terminus.
The number of endogenous lipids with agonist activity at GPR119 is shown in Table 3. All of these compounds activate GPR119 at low micromolar potencies and thus their relative physiological significance cannot be distinguished on the basis of this criterion. Moreover, most of these substances interact with other targets (10, 11, 13, 22, 43, 44), complicating their utility as pharmacological indicators of GPR119 function. Conversely, caution must be applied when evaluating the previously described functions of some lipid amides. For example, oleoyltyrosinols, termed OMDM-1 and OMDM-2 in the literature, have been viewed as selective inhibitors of anandamide uptake and therefore tools to elucidate functions of the endocannabinoid system (19, 20). However, their potency as GPR119 agonists is at least equivalent, and perhaps better, in comparison to inhibition of anandamide uptake. In sum, it currently is unknown whether physiological activation of GPR119 is mediated by 1) multiple lipid amides acting in cooperative fashion, 2) a particular low-potency lipid amide among those described here that is synthesized and regulated in regions in which GPR119 is expressed, or 3) an undiscovered lipid with significantly greater potency toward GPR119. Given the widespread current interest in GPR119 as a therapeutic target for metabolic diseases, there is likely significant value in future efforts to obtain a fuller understanding of the endogenous signals that govern the activation of this receptor.
Table 3.
Endogenous lipids with activity at GPR119
| Compound | Othertargets | References |
|---|---|---|
| OEA | PPARa, TRPV1 | 10 13 43 |
| Palmitoylethanolamide1 | TRPV1 | 43 |
| Stearoylethanolamide1 | ||
| Oleamide | CB1 | 22 |
| N-oleoyldopamine | TRPV1 | 11 12 |
CB1, Cannabinoid receptor type 1; PPAR, peroxisome proliferator-activated receptor; TRPV1, transient receptor potential vanilloid 1.
See Ref. 6 .
Materials and Methods
Cell lines and GPR119 expression analysis
The insulinoma cell lines HIT-T15 (hamster), NIT-1 (mouse), and RIN-5F (rat) were obtained from American Type Culture Collection. GPR119-expressing RIN-5F stable lines were generated by cotransfection of pCDNA3.1 (Invitrogen, Carlsbad, CA) and a human GPR119 expression plasmid encoding amino acids 2–335 of the receptor with an hemagglutinin epitope tag at the N-terminus, as previously reported (1). Expression of GPR119 in insulinoma cell lines was examined by Northern hybridization using a 32P-labeled cDNA probe corresponding to the full coding sequence of mouse GPR119. Expression of human GPR119 in stably transfected RIN-5F cells was confirmed both by Northern hybridization and by immunofluorescence with an anti-hemagglutinin antibody.
Adenylyl cyclase assay
Adenylyl cyclase activity was measured in GPR119-expressing cells by FlashPlate Assay, as previously described (1), or by HTRF Assay. For FlashPlate studies, cells were harvested in GIBCO cell dissociation buffer (Invitrogen; catalog no. 13151-014), pelleted by centrifugation for 5 min at 1100 rpm and carefully resuspended into an appropriate volume of Assay Buffer (50% 1× PBS and 50% Stimulation Buffer provided in the FlashPlate Adenylyl Cyclase kit, New England Nuclear, Newton, MA) to give a final cell count of 2 × 106 cells/ml. Compounds were first dissolved in dimethylsulfoxide at 1000× of final concentration, transferred to 50 μl Assay Buffer to yield the indicated final assay concentration, and pipetted into wells of the 96-well Flash Plate. The cell suspension prepared above was then added (50 μl per well). After incubation for 60 min at room temperature, 100 μl of Detection Mix containing tracer [125I]-cAMP was then added to the wells. Plates were incubated for an additional 2 h followed by counting in a Wallac MicroBeta scintillation counter. Values of cAMP/well were extrapolated from a standard cAMP curve that was included on each assay plate.
HTRF assays were performed using reagents supplied by Cisbio (HTRF Dynamic 2 cAMP kit, cat. no. 62AM4PEJ). Cells were harvested with GIBCO cell dissociation buffer (catalog no. 13151-014), pelleted by centrifugation for 5 min at 1100 rpm, and carefully resuspended in an appropriate volume of assay buffer (1× PBS containing 100 μm IBMX and 0.1% fatty acid free BSA) to give a final cell count of 2 × 105 cells/ml. Cells were then plated into 384-well assay plates (Perkin Elmer Proxiplate 384-Plus, catalog no. 6008280) at 5 μl (1000 cells) per well. Compounds were dissolved and serially diluted in dimethylsulfoxide. After a further 100-fold dilution in assay buffer, compounds were added to the assay plates at 5 μl per well and incubated for 1 h at room temperature. Stock solutions of HTRF assay reagents (cAMP-D2 and Europium cryptate anti-cAMP) were prepared in lysis buffer according to the manufacturer’s instructions and added sequentially, at 5 μl per well, to the assay plates. After a 1-h incubation, plates were read on a Pherastar (BMG Labtech, Cary, NC) or Envision (PerkinElmer, Waltham, MA) HTRF microplate reader. Values of cAMP/well were extrapolated from a standard cAMP curve that was included on each assay plate.
Insulin secretion assay
HIT-T15 insulinoma cells were maintained in F-12K medium supplemented with 10% dialyzed horse serum, 2.5% fetal bovine serum, and antibiotics. Cells were plated in 24-well plates at 2.5 × 105 cells/well for insulin release assays. One day before the assay, culture media were changed to DMEM (3 mm glucose) with 10% dialyzed horse serum and 2.5% fetal bovine serum. The next day, cells were washed twice with PBS and incubated for 1 h with the desired dose of test compounds in DMEM in the presence of 15 mm glucose in 0.25 ml HEPES-buffered Krebs-Ringer buffer. Supernatants were collected and clarified via centrifugation, and insulin levels were determined using an Ultra Sensitive Insulin ELISA kit (Crystal Chem Inc., Downers Grove, IL). Insulin assays with RIN-5F cells and transfected derivatives were performed in the same way, except that the culture media was RPMI 1640 supplemented with 10% fetal bovine serum, 2 mm L-glutamine, 10 mm HEPES, 1 mm sodium pyruvate, and antibiotics.
In vivo experiments
C57Bl/6 male mice were obtained from Harlan (Indianapolis, IN). GPR119-deficient mice were described previously (1). All in vivo animal protocols were approved by the Animal Welfare Committee of Arena Pharmaceuticals, Inc., and in adherence with government regulations. For the oral glucose tolerance test, overnight fasted mice (n = 6 mice/treatment) were administered either vehicle or test compounds at desired doses via oral gavage. A glucose bolus (3 g/kg) was then delivered per orally. Plasma glucose levels were determined at desired time points over a 2-h period using blood (∼5 μl) collected from tail nick and a glucose meter. For GIP (total) pharmacodynamic studies, vehicle or test compounds were administered orally to fasted animals, and after 30 min a glucose bolus of 3 g/kg was administered orally. Blood was collected in Eppendorf tubes containing EDTA and a dipeptidyl peptidase IV inhibitor (Millipore, Billerica, MA; catalog no. DPP4-010) at desired time points. Plasma samples were obtained via centrifugation at 500 × g for 20 min. Total plasma GIP levels were determined using an ELISA kit from Linco (catalog no. EZRMGIP-55K).
Expression analysis for TH, DDC, and FAAH
Expression of TH and DDC in mouse tissues including isolated islets were analyzed by Taqman real-time RT-PCR (Applied Biosystems, Carlsbad, CA). For TH mRNA detection, a fluorescence (6FAM)-labeled probe (5′-CATGTTGGCTGACCGCA-3′) and amplification primers (forward, 5′-CGA GCTGCTGGGACACGTA-3′; reverse, 5′-CCTGGGAGAACTGGGCAAA-3′) were used. For DDC messenger RNA detection, a fluorescence (6FAM)-labeled probe (5′-ACGTGGAGCTGTCTCA-3′) and amplification primers (forward, 5′-CTGCAGGCTTACATCCGAAAG-3′; reverse, 5′-TGGCGTACCAGTGACTCAAACT-3′) were used. A β-actin probe (fluorescent VIC dye-labeled) was used as internal control. Total RNA was prepared with RNA-Bee, DNAse-treated (DNA-free kit, Ambion, Austin, TX), and converted to cDNA (iScript cDNA Synthesis Kit, Bio-Rad, Hercules, CA). Relative expression levels of genes of interest were determined by using Taqman 7900 HT Sequence Detection System (ABI Prism) and normalized against β-actin internal control.
Statistical analysis
All data are presented as mean ± sem. Statistical analysis (t test) was performed using either Microsoft Excel or Prism statistical methods.
Footnotes
Disclosure Summary: All authors conducted this work while employed by Arena Pharmaceuticals and have equity interests therein. D.P.B. is also on the Arena Board of Directors. R.C. and J.L. are inventors on U.S. Patent No. 7108991.
First Published Online November 9, 2009
Abbreviations: DDC, Dopa decarboxylase; FAAH, fatty acid amide hydrolase; GPR119, G protein-coupled receptor 119; GIP, gastric inhibitory peptide; HTRF, homogeneous time-resolved fluorescence; LPC, lysophospatidylcholine; NADA, N-arachidonyldopamine; OLDA, N-oleoyldopamine; OEA, oleoylethanolamide; TH, tyrosine hydroxylase.
References
- 1.Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, Moloney M, Gao H, Mondala H, Bagnol D, Unett D, Liang Y, Demarest K, Semple G, Behan DP, Leonard J2007. A role for β-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology 148:2601–2609 [DOI] [PubMed] [Google Scholar]
- 2.Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D, Chen R, Jones RM, Behan DP, Leonard J2008. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology 149:2038–2047 [DOI] [PubMed] [Google Scholar]
- 3.Lauffer LM, Iakoubov R, Brubaker PL2009. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 58:1058–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semple G, Fioravanti B, Pereira G, Calderon I, Uy J, Choi K, Xiong Y, Ren A, Morgan M, Dave V, Thomsen W, Unett DJ, Xing C, Bossie S, Carroll C, Chu ZL, Grottick AJ, Hauser EK, Leonard J, Jones RM2008. Discovery of the first potent and orally efficacious agonist of the orphan G-protein coupled receptor 119. J Med Chem 51:5172–5175 [DOI] [PubMed] [Google Scholar]
- 5.Bonini J, Borowsky B, Adham N, Boyle N, Thompson T2002. Methods of identifying compounds that bind to SNORF25 receptors. U.S. Patent No. 6468756
- 6.Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C2006. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 3:167–175 [DOI] [PubMed] [Google Scholar]
- 7.Soga T, Ohishi T, Matsui T, Saito T, Matsumoto M, Takasaki J, Matsumoto S, Kamohara M, Hiyama H, Yoshida S, Momose K, Ueda Y, Matsushime H, Kobori M, Furuichi K2005. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun 326:744–751 [DOI] [PubMed] [Google Scholar]
- 8.Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, Piomelli D2007. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem 282:1518–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan H, Vassileva G, Corona A, Liu L, Baker H, Golovko A, Abbondanzo SJ, Hu W, Yang S, Ning Y, Del Vecchio RA, Poulet F, Laverty M, Gustafson EL, Hedrick JA, Kowalski TJ2009. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J Endocrinol 201:219–230 [DOI] [PubMed] [Google Scholar]
- 10.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodríguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D2003. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 425:90–93 [DOI] [PubMed] [Google Scholar]
- 11.Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, Walker JM2003N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem 278:13633–13639 [DOI] [PubMed] [Google Scholar]
- 12.De Petrocellis L, Chu CJ, Moriello AS, Kellner JC, Walker JM, Di Marzo V2004. Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels. Br J Pharmacol 143:251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahern GP2003. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem 278:30429–30434 [DOI] [PubMed] [Google Scholar]
- 14.Suardíaz M, Estivill-Torrús G, Goicoechea C, Bilbao A, Rodríguez de Fonseca F2007. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain 133:99–110 [DOI] [PubMed] [Google Scholar]
- 15.Pacher P, Bátkai S, Kunos G2006. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhofer G, Aneman A, Friberg P, Hooper D, Fåndriks L, Lonroth H, Hunyady B, Mezey E1997. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab 82:3864–3871 [DOI] [PubMed] [Google Scholar]
- 17.Eldrup E, Moller SE, Andreasen J, Christensen NJ1997. Effects of ordinary meals on plasma concentrations of 3,4-dihydroxyphenylalanine, dopamine sulphate and 3,4-dihydroxyphenylacetic acid. Clin Sci (Lond) 92:423–430 [DOI] [PubMed] [Google Scholar]
- 18.Eldrup E, Richter EA2000. DOPA, dopamine, and DOPAC concentrations in the rat gastrointestinal tract decrease during fasting. Am J Physiol Endocrinol Metab 279:E815–E822 [DOI] [PubMed]
- 19.Ortar G, Ligresti A, De Petrocellis L., Morera E, Di Marzo V2003. Novel selective and metabolically stable inhibitors of anandamide cellular uptake. Biochem Pharmacol 65:1473–1481. [DOI] [PubMed] [Google Scholar]
- 20.de Lago E, Ligresti A, Ortar G, Morera E, Cabranes A, Pryce G, Bifulco M, Baker D, Fernandez-Ruiz J, Di Marzo V2004. In vivo pharmacological actions of two novel inhibitors of anandamide cellular uptake. Eur J Pharmacol 484:249–257 [DOI] [PubMed] [Google Scholar]
- 21.Potenza MN, Graminski GF, Lerner MR1992. A method for evaluating the effects of ligands upon Gs protein-coupled receptors using a recombinant melanophore-based bioassay. Anal Biochem 206:315–322 [DOI] [PubMed] [Google Scholar]
- 22.Leggett JD, Aspley S, Beckett SR, D'Antona AM, Kendall DA, Kendall DA2004. Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br J Pharmacol 141:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T2004. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet β cells modulates insulin secretion in rats. Biochem Biophys Res Commun 321:219–225 [DOI] [PubMed] [Google Scholar]
- 24.Appendino G, Daddario N, Minassi A, Moriello AS, De Petrocellis L, Di Marzo, V2005. The taming of capsaicin. Reversal of the vanilloid activity of N-acylvanillamines by aromatic iodination. J Med Chem 48:4663–4669 [DOI] [PubMed] [Google Scholar]
- 25.Ning Y, O'Neill K, Lan H, Pang L, Shan LX, Hawes BE, Hedrick JA2008. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br J Pharmacol 155:1056–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu SS, Bradshaw HB, Benton VM, Chen JS, Huang SM, Minassi A, Bisogno T, Masuda K, Tan B, Roskoski Jr R, Cravatt BF, Di Marzo, V, Walker JM2009. The biosynthesis of N-arachidonoyl dopamine (NADA), a putative endocannabinoid and endovanilloid, via conjugation of arachidonic acid with dopamine. Prostaglandins Leukot Essent Fatty Acids 89:291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Petrocellis L, Marini P, Matias I, Moriello AS, Starowicz K, Cristino L, Nigam S, Di Marzo, V2007. Mechanisms for the coupling of cannabinoid receptors to intracellular calcium mobilization in rat insulinoma β-cells. Exp Cell Res 313:2993–3004 [DOI] [PubMed] [Google Scholar]
- 28.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V2002. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA 99:8400–8405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller GP, Driscoll WJ2007. In vitro synthesis of oleoylglycine by cytochrome c points to a novel pathway for the production of lipid signaling molecules. J Biol Chem 282:22364–22369 [DOI] [PubMed] [Google Scholar]
- 30.McCue JM, Driscoll WJ, Mueller GP2008. Cytochrome c catalyzes the in vitro synthesis of arachidonoyl glycine. Biochem Biophys Res Commun 365:322–327 [DOI] [PubMed] [Google Scholar]
- 31.Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D1993. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development 118:1031–1039 [DOI] [PubMed] [Google Scholar]
- 32.Iturriza FC, Thibault J1993. Immunohistochemical investigation of tyrosine-hydroxylase in the islets of Langerhans of adult mice, rats and guinea pigs. Neuroendocrinology 57:476–480 [DOI] [PubMed] [Google Scholar]
- 33.Rorsman F, Husebye ES, Winqvist O, Björk E, Karlsson FA, Kämpe O1995. Aromatic-l-amino-acid decarboxylase, a pyridoxal phosphate-dependent enzyme, is a β-cell autoantigen. Proc Natl Acad Sci USA 92:8626–8629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borelli MI, Villar MJ, Orezzoli A, Gagliardino JJ1997. Presence of DOPA decarboxylase and its localisation in adult rat pancreatic islet cells. Diabetes Metab 23:161–163 [PubMed] [Google Scholar]
- 35.Borelli MI, Rubio M, García ME, Flores LE, Gagliardino JJ2003. Tyrosine hydroxylase activity in the endocrine pancreas: changes induced by short-term dietary manipulation. BMC Endocr Disord 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persson-Sjögren S, Forsgren S, Shi CL, Täljedal IB2001. Mouse islets cultured with vasoactive intestinal polypeptide: effects on insulin release and immunoreactivity for tyrosine hydroxylase. Pancreas 22:84–90 [DOI] [PubMed] [Google Scholar]
- 37.Cegrell L1968. The occurrence of biogenic monoamines in the mammalian endocrine pancreas. Acta Physiol Scand Suppl 314:1–60 [PubMed] [Google Scholar]
- 38.Lundquist I, Ahrén B, Hansson C, Håkanson R1989. Monoamines in pancreatic islets of guinea pig, hamster, rat, and mouse determined by high performance liquid chromatography. Pancreas 4:662–667 [DOI] [PubMed] [Google Scholar]
- 39.Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A, Izzo AA, Di Marzo, V2008. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring) 16:553–565 [DOI] [PubMed] [Google Scholar]
- 40.Tharp WG, Lee YH, Maple RL, Pratley RE2008. The cannabinoid CB1 receptor is expressed in pancreatic δ-cells. Biochem Biophys Res Commun 372:595–600 [DOI] [PubMed] [Google Scholar]
- 41.Arreaza G, Devane WA, Omeir RL, Sajnani G, Kunz J, Cravatt BF, Deutsch DG1997. The cloned rat hydrolytic enzyme responsible for the breakdown of anandamide also catalyzes its formation via the condensation of arachidonic acid and ethanolamine. Neurosci Lett 234:59–62 [DOI] [PubMed] [Google Scholar]
- 42.Maurelli S, Bisogno T, De Petrocellis L, Di Luccia A, Marino G, Di Marzo, V1995. Two novel classes of neuroactive fatty acid amides are substrates for mouse neuroblastoma “anandamide amidohydrolase.” FEBS Lett 377:82–86 [DOI] [PubMed] [Google Scholar]
- 43.Ho WS, Barrett DA, Randall MD2008. “Entourage” effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol 155:837–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almási R, Szoke E, Bölcskei K, Varga A, Riedl Z, Sándor Z, Szolcsányi J, Petho G2008. Actions of 3-methyl-N-oleoyldopamine, 4-methyl-N-oleoyldopamine and N-oleoylethanolamide on the rat TRPV1 receptor in vitro and in vivo. Life Sci 82:644–651 [DOI] [PubMed] [Google Scholar]