Abstract
Taking into consideration that glucocorticoid (GC) hormones have been used clinically for over half a century and that more than 20 yr have passed since the cloning of the GC receptor (GR), it is hard to imagine that novel aspects in the molecular mechanism by which GCs mediate their antiinflammatory actions are still being unveiled today. Partly, this is because almost on a daily basis, novel insights arise from parallel fields, e.g. nuclear receptor cofactor and chromatin regulation and their concomitant impact on gene transcription events, eventually leading to a revisitation or refinement of old hypotheses. On the other hand, it does remain striking and puzzling why GCs use different mechanisms in so many different cell types and on many different target genes to elicit an antiinflammatory effect. Meanwhile, the obvious question for the clinic remains: is the separation of GR functionalities through differential ligand design the strategy of choice to avoid most GC-mediated side effects? This minireview aims to highlight some of the latest findings on aspects of the antiinflammatory working mechanisms of GCs.
A number of recent advances are discussed regarding various anti-inflammatory mechanisms that are mediated by the glucocorticoid receptor.
Most effects of glucocorticoids (GCs) are mediated by the intracellular receptor, GC receptor (GR). This nuclear hormone receptor is involved in the general regulation of homeostasis and controls stress pathways of diverse origin. GR can be found in almost all tissues of the human body. Nevertheless, the levels of GR protein, of which different splice and translation variants have been identified, are regulated in a tissue- and cell cycle-specific manner (1, 2, 3, 4, 5, 6).
Three key elements were originally described that affect the functionality and transcriptional regulation by GCs: first, the availability of ligand; second, the receptor itself; and third, the recruitment of cofactors and other proteins (3). Evidence is growing to support that neither ligand availability nor GR-interacting protein levels suffice to explain the observed tissue-specific gene regulation via GR. Therefore, the unique transcriptional activities and distinct tissue-specific distribution patterns of GRα isoforms may well provide a novel mechanism that helps to explain tissue-specific GC responses. Tissue-selective targeting of various mutants of GR or tissue-selective knockout of GR in mice are momentarily among the most elegant ways to find out more about the mechanism of action of GR in diverse functional programs (7, 8, 9). For new antiinflammatory drug design purposes, however, an important question to resolve is which nonexclusive mechanisms different GR isoforms may use in various cell types to combat inflammation.
Although it may appear from static immunofluorescence analyses that unliganded GR is mainly being kept inactive in the cytoplasm of the cell, complexed by chaperone proteins, such as heat-shock proteins (e.g. Hsp90, Hsp70, and Hsp23) and immunophilins (e.g. FKBP51, FKBP52, Cyp44, and PP5) (10), in fact, it was found instead that a continuous shuttling of the receptor between the two cellular compartments occurs (11). Also, chaperoning proteins are not restricted to the cytosol. Apart from ensuring ligand accessibility to the ligand-binding pocket of GR, Hsp90 interaction with ligand-loaded GR mimics the interaction of GR with transcriptional coactivators (12).
Upon ligand binding, GR undergoes a conformational change (13) causing exposure of a nuclear localization signal, subsequently allowing the receptor to translocate to the nucleus, to recruit regulatory cofactor complexes, and to influence target gene transcription as a genuine transcription factor.
In general, the segregation of nuclear receptors in different subcellular compartments is believed to act as an important regulatory checkpoint. Recently, Carrigan and co-workers (14) have defined a nuclear retention signal in the hinge region of GR. Active nuclear retention of GR was subsequently correlated with a strong inducibility of the transcriptional activity of GR.
GR target genes that are positively regulated through the transactivation mechanism, carry GC response elements (GREs), typically consisting of two conserved six-nucleotide halves separated by three nonconserved bases (5′-GGTACAnnnTGTTCT-3′), onto which GR can directly bind as a homodimer (15). Although the core GR-binding sequences are highly variable, the precise sequence of an individual GRE is highly conserved across mammalian species (16). GR-binding sites are now rather regarded as distinct GR ligands themselves. Indeed, different GR-binding sites exert different cofactor requirements, different receptor domain utilization, different levels of transactivation, and a different conformation of the GR DNA-binding domain. As such, one base pair difference in a GR-binding site can result in distinct transcriptional programs (Meijsing, S., M. Pufall, and K. Yamamoto, personal communication). GR/GRE complexes undergo a continuous assembly/disassembly; this exchange is regulated via the ligand-binding domain of GR, but the dissociation has been found to happen independently of ligand release (17). GR binding further invariably occurs at inducible or constitutive deoxyribonuclease I hypersensitive sites, involving different remodeling complexes (18).
Interestingly, only a small proportion of the directly up-regulated target genes of GR have been identified to carry a conventional GRE within 10 kb of transcribed genes (19, 20), suggesting that the vast majority of genes up-regulated by GCs are subject to other types of regulatory mechanisms. GC-induced gene expression is often enhanced via composite GC-responsive regions, in which binding of additional transcription factors allows an efficient induction of GC-mediated gene expression. Another level of regulation of transactivation is imposed by receptor modifications (21, 22). For example, GR phospho-isoforms have recently been found to selectively occupy promoters of some GR target genes but not of others (23).
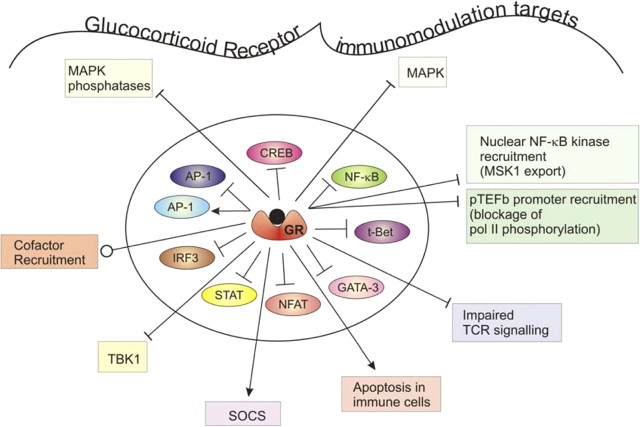
Target genes that are negatively regulated by GR, via the transrepression mechanism or so-called tethering mechanism, most often involve the negative interference of GR with the activity of other DNA-bound transcription factors, such as nuclear factor (NF)-κB, cAMP response element-binding protein (CREB), interferon regulatory factor 3 (IRF3), nuclear factor of activated T cells (NFAT), signal transducer and activator of transcription (STAT), T-box expressed in T cells (T-Bet), GATA-3, and activating protein (AP)-1 (see below) (see Fig. 2). Typical target genes include a vast number of inflammatory proteins including IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-12, IL-18, cyclooxygenase (COX)-2, E-selectin, inducible NO synthase (iNOS), interferon (IFN) γ, TNFα, and intercellular adhesion molecule (ICAM), monocyte chemoattractant protein 1 (MCP-1) [chemokine (C-C motif), and ligand 2] vascular cell adhesion molecule (VCAM).
Fig. 2.
Targets of GR for immunomodulation. Hormone-activated GR is able to negatively regulate the activity of various other DNA-bound transcription factors, including among others NF-κB, CREB, IRF3, NFAT, STAT, T-Bet, GATA-3, and AP-1, via the transrepression mechanism or so-called tethering mechanism (factors inside circle). For AP-1, it has been described that GR can either negatively or positively influence its activity, depending on the composition of this dimer (see text for details). The pleiotropic GR is further capable of exerting its immune system modulatory effects through additional mechanisms (events depicted outside the circle). pol II, RNA polymerase II; TCR, T cell receptor. Arrow, Activating signal; blocked arrow, inhibitory signal; round arrow, modulatory signal, which can be either activating or inhibitory depending on the context, e.g. the loss of a coactivator or the recruitment of a corepressor molecule.
Transactivation vs. Transrepression: Separable Entities with a Clinical Benefit?
Exogenous GCs are used in the clinic to treat inflammatory, autoimmune, and allergic disorders; to attenuate organ rejection after transplantation; to treat brain edema, shock, and various blood cancers; and to balance out adrenal cortex insufficiencies. A number of synthetic analogs of the natural human GC, cortisol, have been developed by the pharmaceutical industry and include among others dexamethasone (DEX), betamethasone, triamcinolone, prednisone, prednisolone, and methylprednisolone (15). As stated above, their clinical success as effective antiinflammatory agents is largely attributed to their ability to reduce the expression of proinflammatory genes, via activation of the GR and the concomitant inhibition of the activity of proinflammatory transcription factors, including NF-κB and AP-1, through a mechanism called transrepression (24, 25, 26). Despite this, their use in the clinic is nevertheless compromised by the appearance of a range of side effects, which mainly arise from the ability of the steroid-activated GR to activate target genes involved in the metabolism of sugar, protein, fat, muscle, and bone via a mechanism called transactivation (27). Bona fide functional GR target genes in this respect include tyrosine aminotransferase (involved in amino acid catabolism), glutamine synthetase (involved in muscle catabolism), and glucose-6-phosphatase and phosphoenol pyruvate carboxy kinase (involved in gluconeogenesis) (27, 28, 29).
The therapeutic usage of GCs for the treatment of inflammatory and autoimmune disorders or, rather, the quest for ways how to specifically modulate the respective GR, has not been given up yet, at least not when judging from the plethora of recent work dealing with the characterization of novel selective GR modulators (28, 30, 31, 32, 33, 34, 35, 36, 37, 38). Furthermore, ongoing studies are carefully comparing the nuclear receptor selectivity profiles and benefit to side effect ratio of newer-generation GCs (39) (Fig. 1).
Fig. 1.

What steroid pharmacologists are aiming for, based on GR. Classical GCs, e.g. DEX and triamcinolone, elicit transrepression and transactivation mechanisms equally well. The latter event is deemed responsible for many unwanted effects, the so-called metabolic side effects. Dissociated steroidal ligands have been developed and are still being developed, which should mainly focus on the transrepression mechanism and stimulate the side effect pathway to a lesser extent, at least in specific tissues (smaller picture on the right), e.g. RU24858 and AL-438 (26 31 40 41 42 43 44 ). A newer generation of antiinflammatory drugs includes the nonsteroidal dissociated GR modulators. So far, they have shown promising benefit to side effect ratios, e.g. ZK216348 (27 ) and CpdA (24 29 ). The X means that in this category, some of them do not support transactivation. For example, for the plant-derived GR modulator CpdA, no GRE-dependent gene stimulation has been found so far, in vitro and in vivo (24 29 ). Note that the different ligands are able to impose different receptor conformations, partially explaining their differential effects on gene regulation.
Selective GR modulators are usually also termed as so-called dissociating ligands, e.g. displaying limited transactivation from a simple GRE but still able to transrepress transcription factors, of which AP-1 and/or NF-κB represent predominant targets. The initial belief that such dissociating compounds could be developed came from studies using a GR mutant with a defect in its dimerization capacity (A458T) and also in its subsequent DNA-binding and GRE-mediated transactivation ability (40) yet allowing transrepression to proceed normally. Upon the replacement in mice of wild-type GR with this dimerization-defective GR (creating a so-called knock-in strain called GRdim), it was found that endogenous GRE-dependent TAT and PEPCK expression was hampered, whereas transrepression mechanisms remained largely unaffected (41, 42, 43). In macrophages or T cells from GRdim mice, DEX still reduced the expression of TNF, IL-1β, IL-6, IL-2, and COX-2 (42). These findings, which supported the hypothesis that gene-activating and gene-repressing properties from GR can be uncoupled from one another, spurred the quest for dissociating ligands even more vigorously than before (Fig. 1). However, this viewpoint soon proved to be too optimistic, because GR functionalities, either beneficial or detrimental, do not necessarily display a similar degree of uncoupling. After establishing a dissociating profile, the need for a more extensive profiling of candidate compounds is nicely illustrated with the following example. RU24848 was characterized as a compound fulfilling the abovementioned criteria in vitro and shown to display antiinflammatory activities in vivo (44, 45, 46). However, this compound still elicited losses of weight and bone mass and was able to induce GRE-mediated lipocortin-1 expression in human eosinophils, gravely compromising its earlier described advantageous status over DEX or prednisolone (46, 47). Tanigawa and co-workers (48) proposed that the in vivo potency of the compound might be modulated by additional metabolism pathways and therefore may explain why the in vivo data do not necessarily correlate with in vitro data. Upon feeding GCs to mice that had been inoculated with various GRE-dependent reporter genes in mouse abdominal skin by means of a gene gun, a higher reporter gene activity was noted for RU24848 than for prednisolone (48). Also in liver cells, RU24848 was able to induce TAT gene expression, albeit to a different extent for different hepatoma cells, perhaps due to a differential ability between these cell lines to metabolize this compound (48). By contrast, in osteoblastic cells, this steroidal compound was a poor inducer of receptor-activator of NF-κB ligand (RANKL), of which the gene product is involved in stimulating bone resorption (49). From these examples, it can be concluded that the transactivation vs. transrepression characteristics are highly cell type and gene specific and, therefore, highly context dependent. Caution must therefore be taken when predicting the behavior of these compounds on a whole animal level, and more information should become available on what set of parameters are minimally required to make the most accurate prediction, especially concerning the clinically most important side effects, i.e. diabetes, osteoporosis, and growth retardation in children.
Different GC-inducible genes are in need of different aspects of GR functionalities. The most striking evidence for this assumption came from studying the expression of the phenylethanolamine N-methyltransferase (PNMT) gene, which is involved in adrenalin biosynthesis and which harbors a complex GRE promoter. Strikingly, whereas the dimerization-defective GR is unable to transactivate simple GRE-driven genes, the PNMT gene remains highly GC-inducible (50). The same goes for the expression of the antiinflammatory gene MAPK phosphatase 1 (MKP-1) (51). From a drug development perspective, the fact that a dimerization mutant is still competent for the activation of a subset of GR-dependent genes is an important complication. Again, the study of complex GRE-driven genes may be more indicative when investigating a novel dissociated compound for GR. In support and as already mentioned above, in a study investigating in depth the cell- and gene-specific determinants of transcriptional regulation by GR, the majority of GC-responsive genes was found to contain GRE sites that diverge from the simple GRE palindrome but are leaning more toward so-called composite elements (20).
Recently, more and more studies are questioning the benefit of ending up with a compound that would completely eradicate all GRE-driven gene expression (reviewed in Refs. 24 and 52). The reason for this is that there are actually quite a number of recently characterized genes that are being up-regulated by GR and that have distinct antiinflammatory roles. These include, besides the already known IκBα, the genes coding for MKP-1, lipocortin-1, secretory leukoprotease inhibitor (SLPI), type II IL-1 (decoy), annexin A1, IL-10, and GC-induced leucine zipper (GILZ), also some newly characterized proteins, including docking protein 1 (DOK-1), Dexras, p11/calpactin-binding protein, and tristetraprolin (TTP), which inhibit various stages of cytokine signaling, synthesis, secretion, and activity (24, 52). Recent studies using a phytomodulator ligand of GR, CpdA, exhibiting clear dissociated properties on GR signaling, may shed some light on this concern. It was found that CpdA could mediate transrepression of NF-κB-driven genes but did not support the transactivation properties of GR through GRE-driven gene expression, neither from simple nor from more complex promoters, exemplified by a study of the gene regulation of TAT, glucose-6-phosphatase, and PNMT (28, 33) (our unpublished results) (Fig. 1). Yet, CpdA was able to inhibit the progression of rheumatoid arthritis in a CIA mouse model to a quite reasonable extent as compared with DEX, one of the strongest agonists of GR (28).
Leaving the dogma behind that only molecules with modified steroidal scaffolds can activate GR through binding in the ligand-binding domain pocket, a novel and broad way has been paved for the development of various classes of safer GR modulators. Because a steroidal backbone may still allow binding to other steroid receptors, e.g. mineralocorticoid receptor and progesterone receptor, thereby mediating side effects through the activation of other hormonal pathways, an additional advantage of nonsteroidal ligands may be an increase in target specificity. For example, the recently characterized antiinflammatory compound benzylidene LGD5552, which binds GR ligand-binding domain and displays antagonistic activities on the mineralocorticoid receptor, has no effect on the mean arterial blood pressure in rats (38). A reduced impact on blood pressure in patients would certainly be advantageous over the standard GC treatment.
The quest for novel GR modulators displaying a specific gene expression profile may, however, be complicated by the fact that a number of genes may contribute both to antiinflammatory and side effects, depending on the target tissue, e.g. MKP-1 (or DUSP1), MIF and AnxA1, or even in a single cell type, as is e.g. demonstrated for GILZ. GILZ is often used as a paradigm for GC-induced gene expression, because it displays a very good GC inducibility and because it has well-characterized functional GREs in its promoter sequence (53). Indeed, knockdown experiments demonstrated a contribution of GILZ to the inhibition of IL-8 gene expression in endothelial cells (54), whereas GILZ-induced stimulation of the expression and activity of ENaCα implicates a role for this protein in GC-induced hypertension (55, 56). Likewise, the fact that AnxA1−/− mice demonstrated impaired responses to GCs in carrageenan-induced edema, antigen-induced arthritis, and zymosan-induced peritonitis supported their antiinflammatory role, but AnxA1 has also been negatively implicated in mediating suppression of the HPA axis, which leads to the undesirable effect of adrenal insufficiency (57). A plethora of evidence supports a clear antiinflammatory role for MKP-1, but recent reports suggest that MKP-1 may also be involved in GC-induced osteoporosis as well as metabolic dysregulation (52). It is noteworthy that in fact all three of these putative antiinflammatory mediators were strongly up-regulated by the dissociated steroidal compound RU24858 (47, 58), putting a large question behind the truly dissociated character, depending on which cell types are studied. As a final example, macrophage migration inhibitory factor (MIF), a GC-inducible proinflammatory cytokine, has been implicated in the pathogenesis of both rheumatoid arthritis and atherosclerosis (59).
An important clue toward novel ligand-screening approaches is given by the work of Coghlan et al. (60). A modified progestin, AL-438, retained its competence for transcriptional repression of NF-κB-driven genes, yet exhibited reduced side effects. AL-438 differentially affects gene expression by reducing the interaction between GR and PPARγ coactivator 1 (PGC-1) but maintaining the interaction between GR and GR interacting protein 1 (GRIP1)/transcription intermediary factor 2 (TIF2).
Consequently, because PGC-1 seems preferentially used in steroid-mediated glucose up-regulation (60) and because GRIP1 is implicated in the suppression of inflammatory gene expression (61), a better side effect profile is being generated. It would be interesting to explore gene expression patterns differentially affected by these two and also other relevant GR-associated cofactors in different tissues.
A number of genes exist that are transrepressed by GC and that reside in the osteoporosis side-effect circuitry, e.g. osteocalcin and osteoprotegerin. Considering the complexity of pathways regulated by GR, including cell proliferation, differentiation, and apoptosis at the cellular level or immune cell homeostasis, metabolism, and responses to stress pathways at the level of the organism, it is clearly too naive to assume that an ideal exogenous GR modulator, only eliciting the beneficial antiinflammatory effects without any trace of side effects, will ever be found. Life is all about balances. Having said that, it is nevertheless highly recommended not to give in and to try and relieve the suffering of many patients dealing with various inflammatory disorders, via solving the puzzle as to how GR mediates its antiinflammatory effects. With this information, better GR ligands may be able to replace the ones we commonly use now.
Mechanisms of Cross Talk between GR and Proinflammatory Transcription Factors
The way a cell responds to GCs is determined not only by the nature and amount of the ligand but also by a modulation of the signaling capacity of GR through interaction with other signaling pathways. This interaction can occur in the cytoplasm by interference with the activity of various signaling proteins (kinases, phosphatases, etc.) or in the nucleus by interfering with the DNA-binding or transactivation capacity, transcription factor-cofactor interactions, or transcription factor-kinase interactions or through inhibiting contacts with the general transcription machinery by the targeted transcription factors.
Transcription factor-transcription factor interactions
The most-studied cross talk mechanisms are the ones between GR and NF-κB or GR and AP-1 because they form a clear basis for the GC-mediated inhibition of various inflammatory cytokines (e.g. IL-6, IL-1β, and TNFα), enzymes (e.g. iNOS, COX-2, and MMPs), and adhesion molecules (e.g. ICAM-1, VCAM, and E-selectin), which all have one or more NF-κB and/or AP-1 elements in their gene promoters (reviewed in Refs. 15 , 62 , and 63). The cross talk mechanism is not restricted to these well known transcription factors, but has in recent years been expanded to other factors as well, including CREB, NFAT, STAT, and T-Bet. Recently, it was found that GCs are able to block the activity of T-Bet, a transcription factor with a role in T-cell differentiation and inflammation and that can drive expression of e.g. the IFNγ target gene. The molecular mechanism involves a direct interaction between T-Bet and GR and a diminished DNA binding of T-Bet as well as a down-regulation of T-Bet mRNA and protein expression in T cells (64).
A simple direct physical protein-protein interaction was indeed also among the first described mechanisms, explaining cross talk between GR and NF-κB (65, 66).
In general, in terms of the mechanism GR is deploying for protein-protein interactions, ample evidence exists that GR would block the activity of DNA-bound transcription factors in its monomeric form rather than as a homodimer and without contacting the DNA itself (28, 41). As described above, antiinflammatory effects have already been ascribed to GILZ in immune cells (67). Recently, it was shown that the effects of GILZ are not restricted to T cells but are also apparent for airway epithelial cells, in which the knockdown of GILZ leads to a desensitization of GC-mediated chemokine repression (54). The mechanism by which GILZ acts is dual; besides binding to NF-κB and AP-1 family members, it can also associate with Raf-1, blocking its potential to activate downstream ERK MAPKs (68, 69) and through this way effectively blocking inflammatory gene transcription. Intriguingly, only the interaction between GILZ and NF-κB requires GILZ homodimerization through their leucine zipper domains (70). Recently, multiple isoforms of GILZ have been described, GILZ1 to GILZ4, explaining previously reported distinct roles of GILZ in cellular proliferation and ion transport mechanisms (71).
Interactions between GR and c-Jun have long been known to modulate AP-1 activity. In most cases, the outcome is a down-regulatory effect (72, 73, 74), exemplified in fibroblasts for the c-jun gene promoter (75). However, in T lymphoblasts, the cross talk between GR and AP-1 rather results in an enhanced transcription of the c-jun gene (76, 77) (Fig. 2). Thus, although the underlying mechanism governing the immunosuppressive effect of activated GR with NF-κB or of activated GR with AP-1 seems highly similar, important differences exist. This is further documented by the fact that a mutation in the first zinc finger of GR can affect NF-κB but not AP-1 inhibition (78). Vice versa, a GR point mutant exists, namely GRR488Q, that is unable to repress NF-κB yet does repress AP-1 (79).
Although it was generally assumed and shown before that GR is able to repress AP-1-dependent transcription independent of the composition of AP-1 subunits (80), the group of Kassel recently presented evidence that only Fos-containing dimers are transrepressed by GR, additionally involving a role for nTrip6, a nuclear isoform of the LIM-domain protein Trip6 (81). The underlying reason for this discrepancy has so far not been studied; one explanation may be that different cell lines were used in the two different studies.
At any rate, cross talk between NF-κB and GR also does not necessarily lead to a mutual inhibition. For some genes, activation of both NF-κB and GR results in a cooperative environment. In fact, gene profiling studies demonstrated both enhancing and suppressive effects of GCs on immune cells (82). For example, the promoter for Toll-like receptor 2 (TLR2) is cooperatively stimulated by GCs and TNFα, through the necessary presence of a functional NF-κB, a 3′-GRE, and a STAT-binding element (83). In terms of functionality, TLR2 is a transmembrane receptor, acting as a so-called pattern recognition receptor for diverse bacteria and thus making part of the innate immunity defense system. It is in this respect important to note that in the complex transcriptional networks stimulated by both TLRs and GCs various other cross talk mechanisms have been identified that cosupport the emerging role of GR in the regulation of innate immunity (84). Transrepression mechanisms by GR in macrophages further displayed a highly signal- and gene-dependent character, allowing the receptor to differentially modulate pathogen-specific gene expression pathways. As such, activated GR could affect TLR4- and TLR9-driven gene activation but not TLR3-dependent gene activation. Other nuclear receptors with antiinflammatory activities, including peroxisome proliferator-activated receptor γ (PPARγ) and liver X receptor (LXR), even demonstrate a synergy with GR to transrepress a specific subset of TLR-dependent genes (85). Finally, very recent data indicate that a cross talk mechanism exists between GR and PPARα, whereby PPARα cooperates with the activated GR for transrepression on NF-κB but was found to block GR-mediated transactivation (our unpublished results). Taken together, these recent findings not only illustrate the combinatorial control mechanisms used by nuclear receptors to restore immune homeostasis but may also hold the key for the development of future therapeutics.
Transcription factor-cofactor interactions
The family of p160 coactivators, including, e.g. steroid receptor coactivator 1 (SRC-1) and GRIP1, has been described to act as adaptor proteins bridging the GR with other cofactors, e.g. p300 and CREB-binding protein (CBP) (which are histone acetyl transferases), and protein arginine methyltransferase 1 and coactivator-associated arginine methyltransferase (which are histone methyl transferases). These cofactors thus modulate the activity of GR and undoubtedly also of its diverse isoforms in a tissue-specific manner (reviewed in Ref. 86).
Because of their newly discovered role in alternative splicing processes, it recently became clear that cofactors not only influence the abundance but also the nature of their products (87).
Furthermore, the spatial and temporal mode in which the process of cofactor recruitment occurs varies not only for different nuclear receptors but also, using the same nuclear receptor, for different promoters (87). With this new information, the picture once again becomes blurry on how exactly coregulators are involved in the regulation of cytokine gene repression mechanisms by GR or how redundant the functionalities of the plethora of GR-interacting cofactors may be.
Before, the differential gene regulation induced by nonsteroidal GR ligands has largely been attributed to selective interaction with a number of coactivator or corepressor molecules. GC-mediated transcriptional repression of critical inflammatory genes has been linked to interaction with corepressors in some cases (88) and coactivators in other cases (61). Also, cofactor competition events between GR and proinflammatory transcription factors have been described (reviewed in Ref. 62). Clearly, in light of the recent findings on cofactor functionality, the research area studying their impact on GR-mediated gene regulation mechanisms will benefit from novel investigations. One important strategy is to define, through peptide array analyses, which cofactors are crucial determinants in governing the behavior of GR as a gene repressor by using dissociated ligands.
Histone deacetylases (HDACs) are another category of molecules capable of modulating GC sensitivity. They form the counterpart of histone acetyltransferases, because they reverse histone acetylation events. Some time ago, HDAC2 was described to be recruited to inflammatory promoters upon GC treatment (89), helping to explain gene inhibitory effects. However, their territory has recently been expanded with their ability to deacetylate nonhistone proteins. Indeed, by targeting acetylated GR, HDAC2 is capable of potentiating the inhibitory effect of GCs (90). Moreover, the deacetylation of GR by HDAC2 seems critical for the interaction between p65 and the receptor (91). Not only (de)acetylation events are important, a complex interplay between histone-modifying HDACs, phosphatases, methylases, and heterochromatin protein 1α culminates at the SP-A gene promoter in response to DEX and contributes to a closure of the chromatin structure, concomitant with the GR-mediated inhibition of gene activation (92).
Another level of control on GR signaling is imposed by the orphan receptor dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X-chromosome, gene 1 (DAX-1) (93), which can function as a corepressor and has been described to negatively regulate GC production. Recently, it became clear that DAX-1 selectively inhibits GR-mediated transactivation but not GR-mediated transrepression through a mechanism involving competition for GRIP1 coactivator binding to the GR (94).
Interference with upstream signaling components
Under physiological conditions, gene induction of, e.g. AP-1 or NF-κB target genes, is the sum of a simultaneous and parallel activation of multiple kinases (e.g. MAPK and IκB kinases), the phosphorylation of more than one transcription factor, and the transmission of signals to a given gene through multiple sequence elements (95). Taking into account that kinases are not the only signal-stimulated and transcription factor-modifying enzymes but that also histone and factor (de)acetylases can play a role, a plethora of possible combinatorial events further adds to the complexity of the studied system of transcriptional initiation. Adding on top of this an extra inhibitory signaling pathway, i.e. through the activated GR, functional interference can take place at many different key points, ultimately resulting in an efficient transcriptional inhibition of target genes.
Inflammatory signaling is propagated via a kinase-activating cascade, including at the almost distal end the MAPKs p38, ERK, and c-Jun N-terminal kinase (JNK). All of the MAPKs have been identified as potential targets for the antiinflammatory actions of GCs through blockage of their activating phosphorylations (Fig. 2). Which MAPKs are preferentially targeted seems to be cell type and GR ligand dependent (52, 96, 97, 98, 99). Still in terms of the immunosuppressive action of GCs, but dependent on novel protein synthesis, it was found for some cell types that GCs inhibit the cytokine-induced phosphorylation and activation of MAPKs p38 and JNK via the up-regulation of MKP-1, a dual-specificity phosphatase, which has been characterized as a negative feedback mechanism restricting inflammatory and innate immune responses (51) (Fig. 2). In MKP-1−/− murine macrophages, GC treatment could no longer inhibit lipopolysaccharide-induced JNK and p38 activation or repress the synthesis of typical inflammatory mediators COX-2, TNFα, and IL-1β (51). MKP-1 absence was further also associated with an increased lethality in response to endotoxin (100) and with a marked increase in the frequency and severity of the murine collagen-induced arthritis model (101). The precise mechanism by which GCs up-regulate MKP-1 is as yet unknown, because the proximal promoter region lacks obvious classical palindromic GRE sequences. However, from studies employing primary macrophages from GR dimer mutant mice, it was found that endogenous MKP-1 expression still responded to GCs (51), suggesting a mechanism similar as for the PNMT gene, namely that GR monomers can still transactivate on promoters bearing concerted GREs (50). Alternatively, in endothelial cells, the rapid DEX-induced stimulation of both ERK and JNK MAPK (the latter activated through DEX-mediated generation of reactive oxygen species), leading to activation of CREB and AP-1, was suggested to be involved in the DEX-mediated up-regulation of MKP-1 (102).
New kinase targets of GCs are still being discovered. The synthetic GC DEX was reported to inhibit phosphorylation of TNF receptor-associated factor (TRAF) family member-associated NF-κB activator (TANK)-binding kinase 1 (TBK1) and subsequent TBK1 kinase activity. Because TBK1 is required for the activation of IRF3 downstream of stimulation of both TLR3 [responsive to the double-stranded RNA mimic poly(inosine-cytosine)] and TLR4 (activation in response to lipopolysaccharide) (103, 104), these data illustrate a novel level of antiinflammatory regulation by GCs (Fig. 2).
Vice versa, cytokine-activated MAPK signaling can phosphorylate the GR protein itself, thereby modulating its turnover and its transcriptional activity (105, 106), imposing an extra layer of regulation on the functionality of GR. This mutually antagonistic cross talk mechanism may well contribute to the occurrence of steroid insensitivity (107, 108), further illustrating the need for new GR modulators. The detrimental effects of MAPK in GC resistance are proposed to be attributed to an altered phosphorylation status of the receptor, affecting GR ligand binding, Hsp90 interactions, subcellular localization, and transactivation potential of the receptor (91). The phosphorylation status of GR can further also affect the magnitude of repression by GR in a gene-selective manner (109). At the level of diverse pathway integrations, a cross talk between JNK and small ubiquitin-like modifier (SUMO) pathways was recently found to modulate GR transcriptional activity, albeit again in a target-gene-specific manner (110).
Not less important to the inflammatory cascade are second-stage activated cytokines such as IL-6 that signal through the Janus kinase (JAK)/STAT pathways, which in turn are subject to a negative feedback by suppressors of cytokine signaling (SOCS) proteins. The latter proteins target the signaling Janus kinases for degradation (111). SOCS-1 has not only been reported to interfere with TLR signaling, but its mRNA levels are also up-regulated by GCs in hematopoietic cells and immune cell cancers, via a presently unknown mechanism (84) (Fig. 2). A responsiveness of the multiple GRE elements in the promoter region of SOCS-1 remains to be explored. A cross talk between GR and SOCS-1 also manifests itself at an entirely different level: SOCS-1 was recently reported to negatively influence transcription of FKBP5 and MKP-1, two GR-regulated target genes, possibly via a direct interaction between GR and SOCS-1 (112).
In a number of cell types, including immune cells, GCs block the activity of transcriptional activity of NF-κB via the up-regulation of the cytoplasmic inhibitor IκBα, leading to the retention of NF-κB in the cytoplasm (113, 114). For years, the underlying mechanism represented a puzzle, because no GRE could be found in the IκBα promoter. Later on, the group of Archer (115) found that the mechanism involves a higher accessibility and stability of transcription factor binding at the promoter (that already bears an open chromatin structure in absence of hormone), leading to effective gene activation.
Recently, a completely novel aspect within the action mechanism of GC transrepression, specifically for NF-κB-driven gene expression, was unveiled. It was found that GCs are able to modulate the chromatin environment and the functionality of the inflammatory enhanceosome via targeting the nuclear kinase mitogen- and stress-activated protein kinase-1 (MSK1). GC-mediated gene repression was found to involve a loss of MSK1 recruitment at inflammatory gene promoters, causing inhibition of NF-κB transactivation and H3 S10 phosphorylation (116). Earlier reports had identified MSK1 as the targeting kinase of H3 S10 (117, 118, 119). Together with histone acetylation, histone H3 S10 phosphorylation contributes to the conformational change of chromatin from a so-called closed to a more open configuration, allowing transcription factor access and formation of the preinitiation transcription machinery. MSK1, a downstream target of ERK and p38 MAPK, was also characterized before as an essential NF-κB p65 S276 kinase (119). Phosphorylation of this serine residue mediates CBP histone acetylase effects, ensuring an optimal expression of NF-κB target genes (120). MSK1 was further found to associate with GR, and interestingly, a substantial amount of activated MSK1 is observed to be exported to the cytoplasm in a GR- and chromosomal region maintenance 1 (CRM1)-dependent manner, revealing a completely novel aspect within the molecular mechanism of GC-dependent inhibition of NF-κB (116) (Fig. 2). It is still unclear whether this specific fraction of MSK1 is merely exported to the cytoplasm and being targeted for degradation or, alternatively, is subject to a continuous shuttling mechanism, perhaps via a tightly regulated (de)phosphorylation event.
Notwithstanding some exceptions (121), GR activation in general does not block promoter recruitment of the targeted transcription factors NF-κB or AP-1, as demonstrated by chromatin immunoprecipitation analysis and genomic footprinting experiments (33, 121, 122, 123). A couple of years ago, Luecke and Yamamoto (124) found that the IL-8 promoter-bound GR competes with the Cdk9 and cyclinT complex, positive transcription elongation factor b (P-TEFb), for recruitment at the IL-8 promoter, thus impeding the P-TEFb-mediated phosphorylation of the RNA polymerase II C-terminal domain on the S2 residue. The combined data possibly entail a dual mechanism in which GR may inhibit both the transcription-facilitating phosphorylation of H3 S10 and of RNA polymerase II C-terminal domain (CTD) S2, by blocking the recruitment of both MSK1 and P-TEFb, respectively (Fig. 2). These results also still fit in the framework of a protein-protein interference work model in which GC-mediated inhibition of NF-κB-activated genes involves the association of GR and p65 NF-κB.
Other mechanisms
Another level at which GCs contribute in eliciting immunosuppression is by promoting apoptosis of a subset of immune cells. In accordance with the finding that in GRdim mice, thymocyte apoptosis was compromised, GCs are mainly believed to induce proapoptotic genes in thymocytes and T cells, e.g. thioredoxin-interacting protein (Txnip) (125), a mechanism supplemented with GC-mediated repression of antiapoptotic factors or by GC-mediated posttranscriptional mRNA destabilization of positive cell cycle genes, e.g. cyclin D3 (126, 127).
The GC-mediated immunosuppressive effect on T cell activation is recently explained via an alternative mechanism: after ligand activation of GR, a physical interaction between GR and the T cell receptor (TCR) complex is disturbed, leading to impaired T cell signaling (128) (Fig. 2).
Furthermore, in the rapidly evolving field of nongenomic actions of GCs, Buttgereit and colleagues (129) showed that high concentrations of GCs can intercalate into the plasma membrane of immune cells, thereby interfering with calcium and sodium cycling across the membranes. Although the mechanistic details of these rapid actions are still lacking, steroids do seem to be able to increase several second messengers such as inositol 1,4,5-trisphosphate, cAMP, and Ca2+ (130).
Conclusion
This review has aimed to highlight the most recent findings on the molecular mechanisms of GR and has tried to put these findings in context of the ongoing quest for novel selective GR modulators, which will aid in the fight against various inflammatory disorders. We have discussed the complexity and difficulties researchers are facing when developing novel strategies to combat chronic inflammatory disorders, when choosing GR as a target molecule. Simple cellular experimental models investigating the dissociated character of novel GR modulators do not suffice to accurately predict the therapeutic index. Complementing genome-wide gene profiling studies and transcription factor/DNA-binding patterns on various target tissues at once will become an adamant strategy for the future.
Acknowledgments
We apologize to those colleagues whose relevant work may unwillingly have escaped our attention.
NURSA Molecule Pages:
Coregulators: AR | DAX1 | LXRα | LXRβ | PPARα | PPARγ.
Footnotes
K.D.B. is a postdoctoral researcher at the FWO-Vlaanderen. Financial support was provided by Interuniversity Attraction Poles (IAP) P5/12 and by GOA from Ghent University.
Disclosure Statement: K.D.B. and G.H. are inventors on WO2006EP0012520, published June 28, entitled Synephrine derivatives useful as anti-inflammatory agents.
First Published Online December 18, 2008
Abbreviations: AP-1, Activating protein 1; COX, cyclooxygenase; CREB, cAMP response element-binding protein; DEX, dexamethasone; GC, glucocorticoid; GILZ, GC-induced leucine zipper; GR, GC receptor; GRE, GC response element; GRIP1, GR-interacting protein 1; HDAC, histone deacetylase; Hsp, heat-shock protein; IRF3, interferon regulatory factor 3; JNK, c-Jun N-terminal kinase; MKP-1, MAPK phosphatase 1; MSK1, mitogen- and stress-activated protein kinase-1; NF, nuclear factor; NFAT, nuclear factor of activated T cells; PNMT, phenylethanolamine N-methyltransferase; PPARγ, peroxisome proliferator-activated receptor γ; P-TEFb, positive transcription elongation factor b; TBK1, TNF receptor-associated factor (TRAF) family member-associated NF-κB activator (TANK)-binding kinase 1; TLR2, Toll-like receptor 2; SOCS, suppressors of cytokine signaling; STAT, signal transducer and activator of transcription; T-Bet, T-box expressed in T cells.
References
- 1.Duma D, Jewell CM, Cidlowski JA2006. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol 102:11–21 [DOI] [PubMed] [Google Scholar]
- 2.Turner JD, Schote AB, Macedo JA, Pelascini LP, Muller CP2006. Tissue specific glucocorticoid receptor expression, a role for alternative first exon usage? Biochem Pharmacol 72:1529–1537 [DOI] [PubMed] [Google Scholar]
- 3.Lu NZ, Cidlowski JA2006. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol 16:301–307 [DOI] [PubMed] [Google Scholar]
- 4.Lu NZ, Cidlowski JA2005. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell 18:331–342 [DOI] [PubMed] [Google Scholar]
- 5.Yudt MR, Cidlowski JA2001. Molecular identification and characterization of A and B forms of the glucocorticoid receptor. Mol Endocrinol 15:1093–1103 [DOI] [PubMed] [Google Scholar]
- 6.Oakley RH, Sar M, Cidlowski JA1996. The human glucocorticoid receptor β-isoform. Expression, biochemical properties, and putative function. J Biol Chem 271:9550–9559 [DOI] [PubMed] [Google Scholar]
- 7.Donet E, Bosch P, Sanchis A, Bayo P, Ramirez A, Cascallana JL, Bravo A, Perez P2008. Transrepression function of the glucocorticoid receptor regulates eyelid development and keratinocyte proliferation but is not sufficient to prevent skin chronic inflammation. Mol Endocrinol 22:799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdmann G, Berger S, Schütz G2008. Genetic dissection of glucocorticoid receptor function in the mouse brain. J Neuroendocrinol 20:655–659 [DOI] [PubMed] [Google Scholar]
- 9.Reichardt HM, Tuckermann JP, Bauer A, Schütz G2000. Molecular genetic dissection of glucocorticoid receptor function in vivo. Z Rheumatol 59(Suppl 2): II1–II5 [DOI] [PubMed]
- 10.Pratt WB, Toft DO2003. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med 228:111–133 [DOI] [PubMed] [Google Scholar]
- 11.Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Hache RJ, Lefebvre YA1999. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol 19:1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricketson D, Hostick U, Fang L, Yamamoto KR, Darimont BD2007. A conformational switch in the ligand-binding domain regulates the dependence of the glucocorticoid receptor on Hsp90. J Mol Biol 368:729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bledsoe RK, Montana VG, Stanley TB, Delves CJ, Apolito CJ, McKee DD, Consler TG, Parks DJ, Stewart EL, Willson TM, Lambert MH, Moore JT, Pearce KH, Xu HE2002. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110:93–105 [DOI] [PubMed] [Google Scholar]
- 14.Carrigan A, Walther RF, Salem HA, Wu D, Atlas E, Lefebvre YA, Hache RJ2007. An active nuclear retention signal in the glucocorticoid receptor functions as a strong inducer of transcriptional activation. J Biol Chem 282:10963–10971 [DOI] [PubMed] [Google Scholar]
- 15.Liberman AC, Druker J, Perone MJ, Arzt E2007. Glucocorticoids in the regulation of transcription factors that control cytokine synthesis. Cytokine Growth Factor Rev 18:45–56 [DOI] [PubMed] [Google Scholar]
- 16.So AY, Cooper SB, Feldman BJ, Manuchehri M, Yamamoto KR2008. Conservation analysis predicts in vivo occupancy of glucocorticoid receptor-binding sequences at glucocorticoid-induced genes. Proc Natl Acad Sci USA 105:5745–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meijsing SH, Elbi C, Luecke HF, Hager GL, Yamamoto KR2007. The ligand binding domain controls glucocorticoid receptor dynamics independent of ligand release. Mol Cell Biol 27:2442–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John S, Sabo PJ, Johnson TA, Sung MH, Biddie SC, Lightman SL, Voss TC, Davis SR, Meltzer PS, Stamatoyannopoulos JA, Hager GL2008. Interaction of the glucocorticoid receptor with the chromatin landscape. Mol Cell 29:611–624 [DOI] [PubMed] [Google Scholar]
- 19.Horie-Inoue K, Takayama K, Bono HU, Ouchi Y, Okazaki Y, Inoue S2006. Identification of novel steroid target genes through the combination of bioinformatics and functional analysis of hormone response elements. Biochem Biophys Res Commun 339:99–106 [DOI] [PubMed] [Google Scholar]
- 20.So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR2007. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet 3:e94 [DOI] [PMC free article] [PubMed]
- 21.Ito K2007. Impact of post-translational modifications of proteins on the inflammatory process. Biochem Soc Trans 35:281–283 [DOI] [PubMed] [Google Scholar]
- 22.Weigel NL, Moore NL2007. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol 21:2311–2319 [DOI] [PubMed] [Google Scholar]
- 23.Blind RD, Garabedian MJ2008. Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. J Steroid Biochem Mol Biol 109:150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton R, Holden NS2007. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol Pharmacol 72:799–809 [DOI] [PubMed] [Google Scholar]
- 25.Rhen T, Cidlowski JA2005. Antiinflammatory action of glucocorticoids: new mechanisms for old drugs. N Engl J Med 353:1711–1723 [DOI] [PubMed] [Google Scholar]
- 26.Barnes PJ2006. Corticosteroids: the drugs to beat. Eur J Pharmacol 533:2–14 [DOI] [PubMed] [Google Scholar]
- 27.Schäcke H, Docke WD, Asadullah K2002. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96:23–43 [DOI] [PubMed] [Google Scholar]
- 28.Dewint P, Gossye V, De Bosscher K, Vanden Berghe W, Van Beneden K, Deforce D, Van Calenbergh S, Muller-Ladner U, Vander Cruyssen B, Verbruggen G, Haegeman G, Elewaut D2008. A plant-derived ligand favoring monomeric glucocorticoid receptor conformation with impaired transactivation potential attenuates collagen-induced arthritis. J Immunol 180:2608–2615 [DOI] [PubMed] [Google Scholar]
- 29.Carballo-Jane E, Pandit S, Santoro JC, Freund C, Luell S, Harris G, Forrest MJ, Sitlani A2004. Skeletal muscle: a dual system to measure glucocorticoid-dependent transactivation and transrepression of gene regulation. J Steroid Biochem Mol Biol 88:191–201 [DOI] [PubMed] [Google Scholar]
- 30.Miner JN, Hong MH, Negro-Vilar A2005. New and improved glucocorticoid receptor ligands. Expert Opin Investig Drugs 14:1527–1545 [DOI] [PubMed] [Google Scholar]
- 31.Schäcke H, Berger M, Rehwinkel H, Asadullah K2007. Selective glucocorticoid receptor agonists (SEGRAs): novel ligands with an improved therapeutic index. Mol Cell Endocrinol 275:109–117 [DOI] [PubMed] [Google Scholar]
- 32.Wang JC, Shah N, Pantoja C, Meijsing SH, Ho JD, Scanlan TS, Yamamoto KR2006. Novel arylpyrazole compounds selectively modulate glucocorticoid receptor regulatory activity. Genes Dev 20:689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Bosscher K, Vanden Berghe W, Beck IM, Van Molle W, Hennuyer N, Hapgood J, Libert C, Staels B, Louw A, Haegeman G2005. A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci USA 102:15827–15832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Bosscher K, Van Craenenbroeck K, Meijer OC, Haegeman G2008. Selective transrepression versus transactivation mechanisms by glucocorticoid receptor modulators in stress and immune systems. Eur J Pharmacol 583:290–302 [DOI] [PubMed] [Google Scholar]
- 35.Owen HC, Miner JN, Ahmed SF, Farquharson C2007. The growth plate sparing effects of the selective glucocorticoid receptor modulator, AL-438. Mol Cell Endocrinol 264:164–170 [DOI] [PubMed] [Google Scholar]
- 36.Uings IJ, Farrow SN2005. A pharmacological approach to enhancing the therapeutic index of corticosteroids in airway inflammatory disease. Curr Opin Pharmacol 5:221–226 [DOI] [PubMed] [Google Scholar]
- 37.Roach SL, Higuchi RI, Adams ME, Liu Y, Karanewsky DS, Marschke KB, Mais DE, Miner JN, Zhi L2008. Discovery of nonsteroidal glucocorticoid receptor ligands based on 6-indole-1,2,3,4-tetrahydroquinolines. Bioorg Med Chem Lett 18:3504–3508 [DOI] [PubMed] [Google Scholar]
- 38.López FJ, Ardecky RJ, Bebo B, Benbatoul K, De Grandpre L, Liu S, Leibowitz MD, Marschke K, Rosen J, Rungta D, Viveros HO, Yen WC, Zhi L, Negro-Vilar A, Miner JN2008. LGD-5552, an antiinflammatory glucocorticoid receptor ligand with reduced side effects, in vivo Endocrinology 149:2080–2089 [DOI] [PubMed] [Google Scholar]
- 39.Mirshahpanah P, Docke WD, Merbold U, Asadullah K, Rose L, Schacke H, Zollner TM2007. Superior nuclear receptor selectivity and therapeutic index of methylprednisolone aceponate versus mometasone furoate. Exp Dermatol 16:753–761 [DOI] [PubMed] [Google Scholar]
- 40.Heck S, Kullmann M, Gast A, Ponta H, Rahmsdorf HJ, Herrlich P, Cato AC1994. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J 13:4087–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schütz G1998. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93:531–541 [DOI] [PubMed] [Google Scholar]
- 42.Reichardt HM, Tuckermann JP, Gottlicher M, Vujic M, Weih F, Angel P, Herrlich P, Schütz G2001. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J 20:7168–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuckermann JP, Reichardt HM, Arribas R, Richter KH, Schütz G, Angel P1999. The DNA binding-independent function of the glucocorticoid receptor mediates repression of AP-1-dependent genes in skin. J Cell Biol 147:1365–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vayssière BM, Dupont S, Choquart A, Petit F, Garcia T, Marchandeau C, Gronemeyer H, Resche-Rigon M1997. Synthetic glucocorticoids that dissociate transactivation and AP-1 transrepression exhibit antiinflammatory activity in vivo Mol Endocrinol 11:1245–1255 [DOI] [PubMed] [Google Scholar]
- 45.Vanden Berghe W, Francesconi E, De Bosscher K, Rèsche-Rigon M, Haegeman G1999. Dissociated glucocorticoids with anti-inflammatory potential repress interleukin-6 gene expression by a nuclear factor-κB-dependent mechanism. Mol Pharmacol 56:797–806 [PubMed] [Google Scholar]
- 46.Belvisi MG, Wicks SL, Battram CH, Bottoms SE, Redford JE, Woodman P, Brown TJ, Webber SE, Foster ML2001. Therapeutic benefit of a dissociated glucocorticoid and the relevance of in vitro separation of transrepression from transactivation activity. J Immunol 166:1975–1982 [DOI] [PubMed] [Google Scholar]
- 47.Janka-Junttila M, Moilanen E, Hasala H, Zhang X, Adcock I, Kankaanranta H2006. The glucocorticoid RU24858 does not distinguish between transrepression and transactivation in primary human eosinophils. J Inflamm (Lond) 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanigawa K, Tanaka K, Nagase H, Miyake H, Kiniwa M, Ikizawa K2002. Cell type-dependent divergence of transactivation by glucocorticoid receptor ligand. Biol Pharm Bull 25:1619–1622 [DOI] [PubMed] [Google Scholar]
- 49.Humphrey EL, Williams JH, Davie MW, Marshall MJ2006. Effects of dissociated glucocorticoids on OPG and RANKL in osteoblastic cells. Bone 38:652–661 [DOI] [PubMed] [Google Scholar]
- 50.Adams M, Meijer OC, Wang J, Bhargava A, Pearce D2003. Homodimerization of the glucocorticoid receptor is not essential for response element binding: activation of the phenylethanolamine N-methyltransferase gene by dimerization-defective mutants. Mol Endocrinol 17:2583–2592 [DOI] [PubMed] [Google Scholar]
- 51.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR2006. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med 203:1883–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark AR2007. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol 275:79–97 [DOI] [PubMed] [Google Scholar]
- 53.Wang JC, Derynck MK, Nonaka DF, Khodabakhsh DB, Ha C, Yamamoto KR2004. Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc Natl Acad Sci USA 101:15603–15608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eddleston J, Herschbach J, Wagelie-Steffen AL, Christiansen SC, Zuraw BL2007. The anti-inflammatory effect of glucocorticoids is mediated by glucocorticoid-induced leucine zipper in epithelial cells. J Allergy Clin Immunol 119:115–122 [DOI] [PubMed] [Google Scholar]
- 55.Bhalla V, Soundararajan R, Pao AC, Li H, Pearce D2006. Disinhibitory pathways for control of sodium transport: regulation of ENaC by SGK1 and GILZ. Am J Physiol Renal Physiol 291:F714–F721 [DOI] [PubMed]
- 56.Itani OA, Auerbach SD, Husted RF, Volk KA, Ageloff S, Knepper MA, Stokes JB, Thomas CP2002. Glucocorticoid-stimulated lung epithelial Na+ transport is associated with regulated ENaC and sgk1 expression. Am J Physiol Lung Cell Mol Physiol 282:L631–L641 [DOI] [PubMed]
- 57.Buckingham JC, John CD, Solito E, Tierney T, Flower RJ, Christian H, Morris J2006. Annexin 1, glucocorticoids, and the neuroendocrine-immune interface. Ann NY Acad Sci 1088:396–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chivers JE, Gong W, King EM, Seybold J, Mak JC, Donnelly LE, Holden NS, Newton R2006. Analysis of the dissociated steroid RU24858 does not exclude a role for inducible genes in the anti-inflammatory actions of glucocorticoids. Mol Pharmacol 70:2084–2095 [DOI] [PubMed] [Google Scholar]
- 59.Morand EF, Leech M, Bernhagen J2006. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov 5:399–410 [DOI] [PubMed] [Google Scholar]
- 60.Coghlan MJ, Jacobson PB, Lane B, Nakane M, Lin CW, Elmore SW, Kym PR, Luly JR, Carter GW, Turner R, Tyree CM, Hu J, Elgort M, Rosen J, Miner JN2003. A novel antiinflammatory maintains glucocorticoid efficacy with reduced side effects. Mol Endocrinol 17:860–869 [DOI] [PubMed] [Google Scholar]
- 61.Rogatsky I, Luecke HF, Leitman DC, Yamamoto KR2002. Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proc Natl Acad Sci USA 99:16701–16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Bosscher K, Vanden Berghe W, Haegeman G2003. The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev 24:488–522 [DOI] [PubMed] [Google Scholar]
- 63.De Bosscher K, Vanden Berghe W, Haegeman G2006. Cross-talk between nuclear receptors and nuclear factor κB. Oncogene 25:6868–6886 [DOI] [PubMed] [Google Scholar]
- 64.Liberman AC, Refojo D, Druker J, Toscano M, Rein T, Holsboer F, Arzt E2007. The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein-protein interaction. FASEB J 21:1177–1188 [DOI] [PubMed] [Google Scholar]
- 65.Ray A, Prefontaine KE1994. Physical association and functional antagonism between the p65 subunit of transcription factor NF-κB and the glucocorticoid receptor. Proc Natl Acad Sci USA 91:752–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin Jr AS1995. Characterization of mechanisms involved in transrepression of NF-κB by activated glucocorticoid receptors. Mol Cell Biol 15:943–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riccardi C, Bruscoli S, Ayroldi E, Agostini M, Migliorati G2001. GILZ, a glucocorticoid hormone induced gene, modulates T lymphocytes activation and death through interaction with NF-κB. Adv Exp Med Biol 495:31–39 [DOI] [PubMed] [Google Scholar]
- 68.Ayroldi E, Zollo O, Macchiarulo A, Di Marco B, Marchetti C, Riccardi C2002. Glucocorticoid-induced leucine zipper inhibits the Raf-extracellular signal-regulated kinase pathway by binding to Raf-1. Mol Cell Biol 22:7929–7941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mittelstadt PR, Ashwell JD2001. Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. J Biol Chem 276:29603–29610 [DOI] [PubMed] [Google Scholar]
- 70.Di Marco B, Massetti M, Bruscoli S, Macchiarulo A, Di Virgilio R, Velardi E, Donato V, Migliorati G, Riccardi C2007. Glucocorticoid-induced leucine zipper (GILZ)/NF-κB interaction: role of GILZ homo-dimerization and C-terminal domain. Nucleic Acids Res 35:517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soundararajan R, Wang J, Melters D, Pearce D2007. Differential activities of glucocorticoid-induced leucine zipper protein isoforms. J Biol Chem 282:36303–36313 [DOI] [PubMed] [Google Scholar]
- 72.Jonat C, Rahmsdorf HJ, Park KK, Cato AC, Gebel S, Ponta H, Herrlich P1990. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 62:1189–1204 [DOI] [PubMed] [Google Scholar]
- 73.Schüle R, Rangarajan P, Kliewer S, Ransone LJ, Bolado J, Yang N, Verma IM, Evans RM1990. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell 62:1217–1226 [DOI] [PubMed] [Google Scholar]
- 74.Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, Karin M1990. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 62:1205–1215 [DOI] [PubMed] [Google Scholar]
- 75.Wei P, Inamdar N, Vedeckis WV1998. Transrepression of c-jun gene expression by the glucocorticoid receptor requires both AP-1 sites in the c-jun promoter. Mol Endocrinol 12:1322–1333 [DOI] [PubMed] [Google Scholar]
- 76.Barrett TJ, Vig E, Vedeckis WV1996. Coordinate regulation of glucocorticoid receptor and c-jun gene expression is cell type-specific and exhibits differential hormonal sensitivity for down- and up-regulation. Biochemistry 35:9746–9753 [DOI] [PubMed] [Google Scholar]
- 77.Zhou F, Thompson EB1996. Role of c-jun induction in the glucocorticoid-evoked apoptotic pathway in human leukemic lymphoblasts. Mol Endocrinol 10:306–316 [DOI] [PubMed] [Google Scholar]
- 78.Tao Y, Williams-Skipp C, Scheinman RI2001. Mapping of glucocorticoid receptor DNA binding domain surfaces contributing to transrepression of NF-κB and induction of apoptosis. J Biol Chem 276:2329–2332 [DOI] [PubMed] [Google Scholar]
- 79.Bladh LG, Lidén J, Dahlman-Wright K, Reimers M, Nilsson S, Okret S2005. Identification of endogenous glucocorticoid repressed genes differentially regulated by a glucocorticoid receptor mutant able to separate between nuclear factor-κB and activator protein-1 repression. Mol Pharmacol 67:815–826 [DOI] [PubMed] [Google Scholar]
- 80.Rogatsky I, Zarember KA, Yamamoto KR2001. Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J 20:6071–6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diefenbacher M, Sekula S, Heilbock C, Maier JV, Litfin M, van Dam H, Castellazzi M, Herrlich P, Kassel O2008. Restriction to Fos family members of Trip6-dependent coactivation and glucocorticoid receptor-dependent trans-repression of activator protein-1. Mol Endocrinol 22:1767–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'Shea JJ, Chrousos GP, Bornstein SR2002. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J 16:61–71 [DOI] [PubMed] [Google Scholar]
- 83.Hermoso MA, Matsuguchi T, Smoak K, Cidlowski JA2004. Glucocorticoids and tumor necrosis factor α cooperatively regulate toll-like receptor 2 gene expression. Mol Cell Biol 24:4743–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chinenov Y, Rogatsky I2007. Glucocorticoids and the innate immune system: crosstalk with the toll-like receptor signaling network. Mol Cell Endocrinol 275:30–42 [DOI] [PubMed] [Google Scholar]
- 85.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK2005. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 122:707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lonard DM, O'Malley BW2007. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700 [DOI] [PubMed] [Google Scholar]
- 87.Auboeuf D, Batsche E, Dutertre M, Muchardt C, O'Malley BW2007. Coregulators: transducing signal from transcription to alternative splicing. Trends Endocrinol Metab 18:122–129 [DOI] [PubMed] [Google Scholar]
- 88.Jee YK, Gilmour J, Kelly A, Bowen H, Richards D, Soh C, Smith P, Hawrylowicz C, Cousins D, Lee T, Lavender P2005. Repression of interleukin-5 transcription by the glucocorticoid receptor targets GATA3 signaling and involves histone deacetylase recruitment. J Biol Chem 280:23243–23250 [DOI] [PubMed] [Google Scholar]
- 89.Ito K, Barnes PJ, Adcock IM2000. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1β-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol 20:6891–6903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM2006. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-κB suppression. J Exp Med 203:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ito K, Chung KF, Adcock IM2006. Update on glucocorticoid action and resistance. J Allergy Clin Immunol 117:522–543 [DOI] [PubMed] [Google Scholar]
- 92.Islam KN, Mendelson CR2008. Glucocorticoid/glucocorticoid receptor inhibition of surfactant protein-A (SP-A) gene expression in lung type II cells is mediated by repressive changes in histone modification at the SP-A promoter. Mol Endocrinol 22:585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCabe ER2007. DAX1: increasing complexity in the roles of this novel nuclear receptor. Mol Cell Endocrinol 265–266:179–182 [DOI] [PMC free article] [PubMed]
- 94.Zhou J, Oakley RH, Cidlowski JA2008. DAX-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X-chromosome, gene 1) selectively inhibits transactivation but not transrepression mediated by the glucocorticoid receptor in a LXXLL-dependent manner. Mol Endocrinol 22:1521–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hazzalin CA, Mahadevan LC2002. MAPK-regulated transcription: a continuously variable gene switch? Nat Rev Mol Cell Biol 3:30–40 [DOI] [PubMed] [Google Scholar]
- 96.Rider LG, Hirasawa N, Santini F, Beaven MA1996. Activation of the mitogen-activated protein kinase cascade is suppressed by low concentrations of dexamethasone in mast cells. J Immunol 157:2374–2380 [PubMed] [Google Scholar]
- 97.Caelles C, Gonzalez-Sancho JM, Muñoz A1997. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev 11:3351–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Bosscher K, Vanden Berghe W, Haegeman G2001. Glucocorticoid repression of AP-1 is not mediated by competition for nuclear coactivators. Mol Endocrinol 15:219–227 [DOI] [PubMed] [Google Scholar]
- 99.Gossye V, Haegeman G, De Bosscher K2008. Therapeutic implications of the nuclear factor-κB/nuclear receptor cross-talk. Front Biosci 13:4122–4143 [DOI] [PubMed] [Google Scholar]
- 100.Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y2006. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med 203:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T2006. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol 176:1899–1907 [DOI] [PubMed] [Google Scholar]
- 102.Fürst R, Schroeder T, Eilken HM, Bubik MF, Kiemer AK, Zahler S, Vollmar AM2007. MAPK phosphatase-1 represents a novel anti-inflammatory target of glucocorticoids in the human endothelium. FASEB J 21:74–80 [DOI] [PubMed] [Google Scholar]
- 103.McCoy CE, Carpenter S, Palsson-McDermott EM, Gearing LJ, O'Neill LA2008. Glucocorticoids inhibit IRF3 phosphorylation in response to Toll-like receptor-3 and -4 by targeting TBK1 activation. J Biol Chem 283:14277–14285 [DOI] [PubMed] [Google Scholar]
- 104.Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R2008. TLR-4, IL-1R and TNF-R signaling to NF-κB: variations on a common theme. Cell Mol Life Sci 65:2964–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Webster JC, Jewell CM, Bodwell JE, Munck A, Sar M, Cidlowski JA1997. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J Biol Chem 272:9287–9293 [DOI] [PubMed] [Google Scholar]
- 106.Hittelman AB, Burakov D, Iniguez-Lluhi JA, Freedman LP, Garabedian MJ1999. Differential regulation of glucocorticoid receptor transcriptional activation via AF-1-associated proteins. EMBO J 18:5380–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schaaf MJ, Cidlowski JA2002. Molecular mechanisms of glucocorticoid action and resistance. J Steroid Biochem Mol Biol 83:37–48 [DOI] [PubMed] [Google Scholar]
- 108.Chikanza IC, Kozaci DL2004. Corticosteroid resistance in rheumatoid arthritis: molecular and cellular perspectives. Rheumatology (Oxford) 43:1337–1345 [DOI] [PubMed] [Google Scholar]
- 109.Chen W, Dang T, Blind RD, Wang Z, Cavasotto CN, Hittelman AB, Rogatsky I, Logan SK, Garabedian MJ2008. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol Endocrinol 22:1754–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davies L, Karthikeyan N, Lynch JT, Sial EA, Gkourtsa A, Demonacos C, Krstic-Demonacos M2008. Cross talk of signaling pathways in the regulation of the glucocorticoid receptor function. Mol Endocrinol 22:1331–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alexander WS, Hilton DJ2004. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol 22:503–529 [DOI] [PubMed] [Google Scholar]
- 112.Haffner MC, Jurgeit A, Berlato C, Geley S, Parajuli N, Yoshimura A, Doppler W2008. Interaction and functional interference of glucocorticoid receptor and SOCS1. J Biol Chem 283:22089–22096 [DOI] [PubMed] [Google Scholar]
- 113.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin Jr AS1995. Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science 270:283–286 [DOI] [PubMed] [Google Scholar]
- 114.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M1995. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science 270:286–290 [DOI] [PubMed] [Google Scholar]
- 115.Deroo BJ, Archer TK2001. Glucocorticoid receptor activation of the IκBα promoter within chromatin. Mol Biol Cell 12:3365–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beck IM, Vanden Berghe W, Vermeulen L, Bougarne N, Vander Cruyssen B, Haegeman G, De Bosscher K2008. Altered subcellular distribution of MSK1 induced by glucocorticoids contributes to NF-κB inhibition. EMBO J 27:1682–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saccani S, Pantano S, Natoli G2002. p38-Dependent marking of inflammatory genes for increased NF-κB recruitment. Nat Immunol 3:69–75 [DOI] [PubMed] [Google Scholar]
- 118.Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JS2003. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J 22:2788–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G2003. Transcriptional activation of the NF-κB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J 22:1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhong H, Voll RE, Ghosh S1998. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1:661–671 [DOI] [PubMed] [Google Scholar]
- 121.Bhavsar PK, Sukkar MB, Khorasani N, Lee KY, Chung KF2008. Glucocorticoid suppression of CX3CL1 (fractalkine) by reduced gene promoter recruitment of NF-κB. FASEB J 22:1807–1816 [DOI] [PubMed] [Google Scholar]
- 122.Nissen RM, Yamamoto KR2000. The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev 14:2314–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lidén J, Rafter I, Truss M, Gustafsson JA, Okret S2000. Glucocorticoid effects on NF-κB binding in the transcription of the ICAM-1 gene. Biochem Biophys Res Commun 273:1008–1014 [DOI] [PubMed] [Google Scholar]
- 124.Luecke HF, Yamamoto KR2005. The glucocorticoid receptor blocks P-TEFb recruitment by NFκB to effect promoter-specific transcriptional repression. Genes Dev 19:1116–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Z, Rong YP, Malone MH, Davis MC, Zhong F, Distelhorst CW2006. Thioredoxin-interacting protein (txnip) is a glucocorticoid-regulated primary response gene involved in mediating glucocorticoid-induced apoptosis. Oncogene 25:1903–1913 [DOI] [PubMed] [Google Scholar]
- 126.Newton R2000. Molecular mechanisms of glucocorticoid action: what is important? Thorax 55:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reisman D, Thompson EA1995. Glucocorticoid regulation of cyclin D3 gene transcription and mRNA stability in lymphoid cells. Mol Endocrinol 9:1500–1509 [DOI] [PubMed] [Google Scholar]
- 128.Löwenberg M, Verhaar AP, van den Brink GR, Hommes DW2007. Glucocorticoid signaling: a nongenomic mechanism for T-cell immunosuppression. Trends Mol Med 13:158–163 [DOI] [PubMed] [Google Scholar]
- 129.Stahn C, Löwenberg M, Hommes DW, Buttgereit F2007. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol 275:71–78 [DOI] [PubMed] [Google Scholar]
- 130.Lösel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M2003. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev 83:965–1016 [DOI] [PubMed] [Google Scholar]