Abstract
Human GH (hGH) has important effects on growth as well as carbohydrate, fat, and protein metabolism. These actions require the presence of normal levels of a functional hGH receptor (hGHR) on the surface of target cells. hGHR gene expression is characterized by the use of several 5′-noncoding exons and alternative splicing, resulting in the generation of multiple mRNA isoforms. The hGHR V2 transcript is predominant in most tissues, including human fat. However, factors regulating its ubiquitous expression have remained unidentified. The present study was aimed at characterizing the mechanisms regulating hGHR V2 transcription. Two major V2 transcriptional start sites were identified by primer extension assays. The V2 proximal promoter is TATA-less, with several characteristics of a housekeeping gene promoter. Transient transfection analyses of 2.6 kb of the 5′-flanking region of V2 confirmed its promoter activity in multiple primate cell lines. Similar promoter activity patterns were observed in human SGBS preadipocytes and mature adipocytes but with much higher V2 promoter activity in mature adipocytes, suggesting that changes in the availability of specific factors during adipocyte differentiation play a role in V2 promoter regulation. Serial deletion and mutation analyses revealed that transcription of hGHR V2 in different cell types, including adipocytes, is determined by a core promoter and distinct inhibitory and activation domains in the 5′-promoter region as well as within the V2 exon. Our data suggest that V2 transcription is the result of a complex interplay involving multiple factors, to ensure appropriate expression of hGHR in different hGH target cells.
Proximal promoter and 5′UTR exon domains regulate expression of V2, the major ubiquitously expressed transcript of the human growth hormone receptor gene in all tissues.
Human GH (hGH) is one of the most functionally diverse pituitary hormones; it exerts a wide range of biological actions, including regulation of body growth, carbohydrate, protein, and fat metabolism, and immune function as well as cell proliferation and differentiation (1, 2, 3, 4, 5, 6, 7). These pleiotropic effects result either from direct actions of hGH on target cells or from indirect actions by stimulating hepatic or local tissue production of IGF-I (8, 9). To initiate these actions, hGH must bind to its specific cell surface receptor (hGHR) and activate various intracellular signaling cascades (10, 11, 12, 13). Therefore, the ability of hGH to exert its biological effects is intimately linked to the number and functional status of hGHRs in individual target cells. Dysregulation of the hGH/hGHR axis has been implicated in the pathogenesis of growth retardation, hGH insensitivity syndromes, and certain types of tumors as well as the progression of chronic complications of diabetes mellitus (1, 6, 9, 14).
The hGHR is encoded by a single gene on chromosome 5p13.1-p12 that contains several 5′-untranslated exons under the control of different promoters (15, 16, 17, 18, 19, 20, 21). The multiple mRNA variants (V) transcribed from the different 5′-untranslated region (5′-UTR) noncoding exons splice into a common acceptor site in the first coding exon, 11 bp upstream of the ATG translation start codon; thus, all transcripts encode the same hGHR protein. Fourteen hGHR mRNA variants have been identified to date (17, 18, 19, 20); some (V2, V3, V5, V9, and VA-VE) are widely expressed, whereas others (V1, V7, V4, and V8) are developmentally regulated and exclusively expressed in the postnatal liver (19).
Similar 5′-UTR heterogeneity and mRNA expression patterns have been observed for the GHR genes in several other species, suggesting that different promoter usage provides a conserved mechanism for controlling GHR gene expression in individual GH target tissues (reviewed in Refs. 22 and 23). Indeed, several different promoters have been identified (17, 19, 21, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33). The most well known are 1) the liver-specific promoter, which is responsible for generating liver-specific transcripts (e.g. human V1, ovine 1A, bovine 1A, and mouse L1) and whose activity appears to be regulated primarily by liver-enriched transcription factors (21, 24, 30, 34, 35, 36), and 2) the promoter for ubiquitously expressed variants (e.g. human V2, ovine 1B, bovine 1B, and mouse L2), whose regulation seems to involve ubiquitous transcription factors (26, 27, 32, 37, 38, 39, 40).
The hGHR V2 mRNA variant is readily detectable in a variety of fetal and adult tissues, including liver, muscle, fat, kidney, lung, and heart (19, 20). Except in adult liver, where the V1 transcript predominates, the V2 transcript accounts for the majority of the hGHR mRNA pool in all other target tissues (19, 20). Homologs of the hGHR V2 (ovine 1B, bovine 1B, and mouse L2) represent the major GHR transcript in their nonhepatic tissues as well; in mouse, the L2 transcript also predominates in the liver of nonpregnant mice (23). Thus, the V2-like transcript appears to be one of the most important GHR mRNA variants.
The promoter elements and mechanisms implicated in regulation of transcription from ovine 1B, bovine 1B, and mouse L2 have been partially studied (26, 27, 28, 29, 41). Multiple transcription start sites (TSSs) have been observed for all three species. The proximal promoter regions for these V2 homologs lack a consensus TATA element but have a high G+C content and multiple Sp1/Sp3 sites. The similar DNA sequences and tissue mRNA expression patterns between human V2 and its homologs in other species suggest that a common regulatory mechanism may be present.
hGH has major effects on adipose tissue, influencing cell numbers as well as lipid content (3, 4, 6, 20, 42, 43). In a previous study, we demonstrated that V2 is the predominant hGHR transcript in both preadipocytes and mature adipocytes as well as the major variant expressed as the total hGHR mRNA pool increases during human adipocyte differentiation. The proportion is much higher in adipose tissue (∼90%) than in liver (∼30%) or in cardiac muscle (∼40%), indicating that differential transcription of the V2 exon may be a regulatory mechanism to achieve a tissue-specific pattern of hGHR expression (19, 20).
To better understand how this might be achieved, we have begun to characterize the mechanisms regulating V2 transcription. We defined the proximal promoter of the hGHR V2 exon and determined that it is active in several different primate cell types, including human adipocytes. We demonstrated for the first time that the V2 proximal promoter is TATA-less, with characteristics of a housekeeping gene promoter, and that there are several distinct inhibitory and stimulatory domains in the 5′-promoter region as well as within the V2 exon. Similar promoter activity patterns were observed in preadipocytes as well as mature adipocytes, with much higher levels in the mature cells, suggesting that changes in the availability of specific factors during adipocyte differentiation play a role in V2 promoter regulation.
Results
Mapping of the TSSs
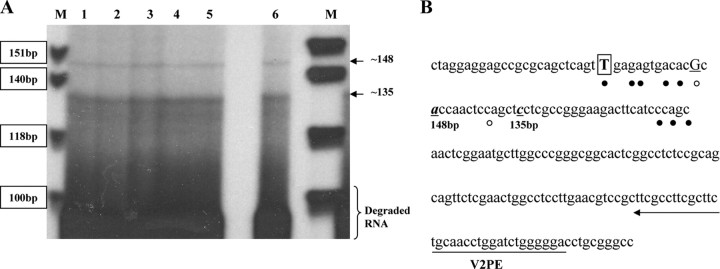
In our previous publications, we reported the location of the hGHR V2 exon on chromosome 5 (19, 20). We have now identified the major V2 TSSs, using primer extension analysis of RNA isolated from three human cell lines [HEK293, Huh7, and SGBS (Simpson-Golabi-Behmel syndrome) preadipocytes] and human fetal as well as postnatal liver. The two longest 5′-cDNA ends in all of the samples corresponded to about 148 and 135 bp relative to the DNA markers (Fig. 1A). Both sites were mapped to a region similar to where the multiple ovine 1B and bovine 1B TSSs have been defined (Fig. 1B). In the present study, we have chosen to designate the ovine 1B major TSS (T) as +1 for our V2 promoter construct numeration, because it represents the farthest cDNA 5′-end identified among the homologous V2-like transcripts.
Fig. 1.
Mapping TSSs for hGHR V2. A, Representative autoradiograph of size-fractionated products of primer extension reactions carried out with a primer (V2PE) complementary to the V2 exon sequence and 20 μg total RNA extracted from three human cell lines and liver tissues. The sizes of two specific products (arrows) were determined by a concurrently electrophoresed 32P-labeled φX174 HinfI DNA ladder (lane M). Lane 1, HEK293 cells; lane 2, HEK293 cells transfected with V2(−2623/+331); lane 3, Huh7 cells; line 4, SGBS preadipocytes; lane 5, human fetal liver; lane 6, human adult liver. This assay was repeated three times with RNAs from at least two different cell pools and tissue samples. B, Positions corresponding to the two longest extended V2 5′-cDNA ends in our primer extension assays are shown in bold italic and underlined. The major (T, bold, boxed) and minor TSSs for ovine 1B are indicated by solid circles. The major (G, bold, underlined) and minor TSSs identified for bovine 1B are marked by open circles. The oligonucleotide primer (V2PE) used for primer extension is underlined by an arrow.
Functional analysis of the hGHR V2 promoter activity
5′-Deletion analysis
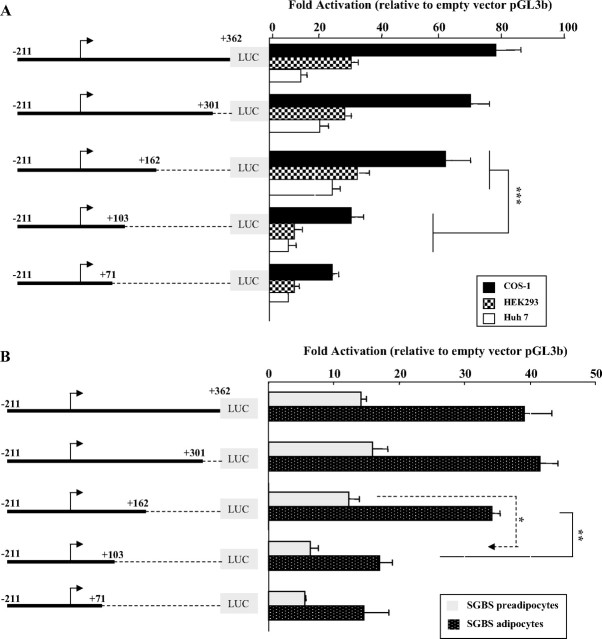
The promoter activity of the 2623-bp region upstream of the hGHR V2 exon was assessed by its ability to direct expression of the pGL3-basic luciferase reporter gene (Fig. 2A). DNA fragments with progressively shorter 5′-ends, ranging from −2623 to +11, were assayed for luciferase activities after transient transfection into five cell types: HEK293 (human embryonic kidney epithelial cells), CV-1 (African green monkey kidney fibroblasts), Huh7 (hepatoma cells), and SGBS human preadipocytes as well as mature adipocytes. In each cell type, promoter constructs V2(−2623/+335), V2(−720/+362), V2(−211/+362), and V2(−29/+301) all showed more than 4-fold higher activity compared with the empty vector, with maximal activity consistently observed with the V2(−211/+362) construct (Fig. 2, B and C). A further 5′-deletion to position +11 resulted in dramatically decreased promoter activity in HEK293 and CV-1 cells (P < 0.001, Fig. 2B) and mature SGBS adipocytes (P < 0.01, Fig. 2C), and almost background levels in both Huh7 cells (Fig. 2B) and SGBS preadipocytes (Fig. 2C), indicating that the fragment between −211 and +11 is essential to direct high-level transcription of V2. Therefore, we have defined the 211-bp region upstream of the designated TSS (+1) as the proximal promoter region of the human V2 exon. The fact that the V2(−211/+362) construct was significantly more active (P < 0.001) than the V2(−720/+362) construct in all of the tested cells (Fig. 2, B and C) also suggests the presence of major negative regulatory elements within the 520-bp region from position −720 to −211.
Fig. 2.
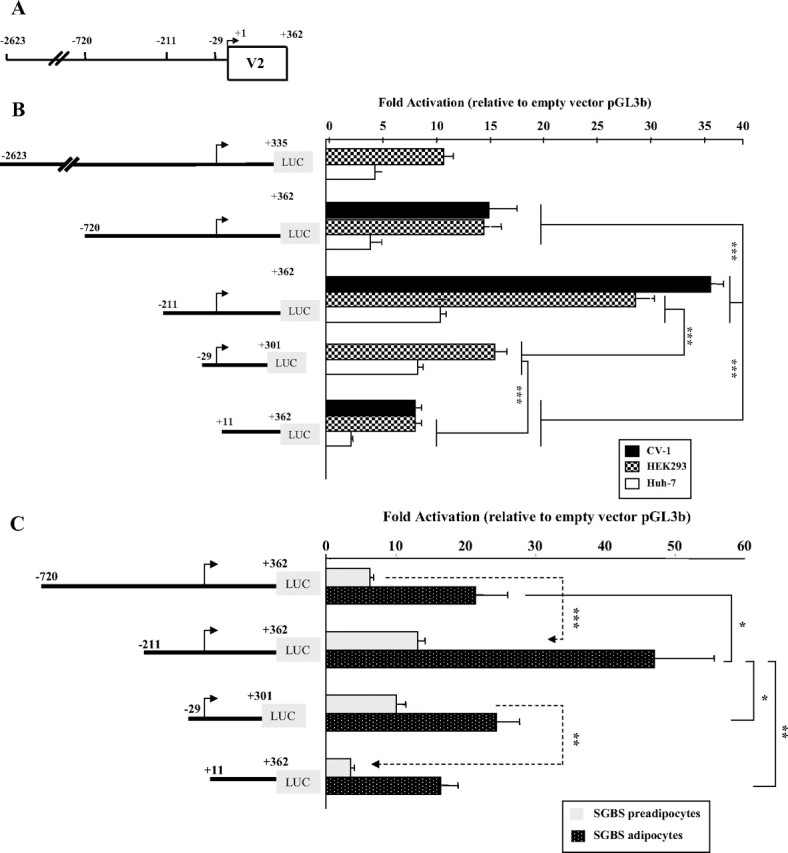
5′-Deletion analysis of the promoter activity of hGHR V2. We chose to designate the ovine 1B major TSS (T) as position +1 for our human V2 promoter construct numeration, because it represents the most 5′-cDNA end identified to date among V2 homologs (ovine 1B, bovine 1B, and mouse L2). A, A schematic diagram representing the first 362 nucleotides of the hGHR V2 exon and 2.623 kb of its 5′-flanking region. The numbering is relative to the designated major TSS (+1, indicated by arrow). 5′-Deletion promoter reporter constructs were prepared by inserting different portions of the 5′-flanking sequence of hGHR V2 into the promoterless luciferase plasmid pGL3-basic (pGL3b). B and C, These expression plasmids were transiently transfected into HEK293, CV-1 and Huh7 cells (B) and SGBS preadipocytes and mature adipocytes (C). Luciferase activity of the transfectants was normalized to β-galactosidase activity and then expressed as relative fold activation compared with the empty pGL3-b vector. The data are expressed as mean ± se; n = 3–9 experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
3′-Deletion analysis
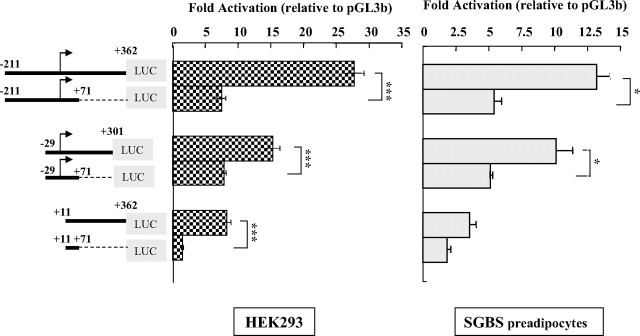
Intriguingly, all three of the initial 3′-truncation constructs [V2(−211/ +71), V2(−29/+71), and V2(+11/+71)] showed an approximately 2–5-fold lower activity than its corresponding longer construct [V2(−211/+362), V2(−29/+301), and V2(+11/+362)] when transfected into either HEK293 cells or SGBS preadipocytes (Fig. 3), suggesting that the presence of a portion of the V2 exon can enhance V2 promoter activity. We therefore carried out a detailed 3′-deletion analysis using reporter plasmids bearing the same 5′-end (−211) but varying lengths of the V2 exon. As shown in Fig. 4, A and B, sequential deletions of the 3′-end from +362 to +162 had minimal effects in HEK293, COS-1, Huh7, and SGBS preadipocytes or adipocytes. However, a deletion from +162 to +103 sharply reduced the promoter activity in all five cell types (∼50%, P < 0.05 to P < 0.001), whereas a further 3′-deletion to +71 had little additional effect. These results demonstrate that 1) at least 162 bp of the V2 exon sequence is required to maintain maximal V2 promoter activity and 2) there are important positive regulatory elements between +103 and +162.
Fig. 3.
Presence of a portion of the V2 exon sequence enhances V2 promoter activity. Pairs of V2 promoter reporter constructs that contain the same 5′-upstream regions, but different downstream V2 exon lengths were transiently transfected into HEK293 cells or SGBS preadipocytes and compared for fold activation relative to the empty vector, pGL3b. The data are presented as mean ± se of n = 3–9 independent experiments. Significantly (*, P < 0.05; ***, P < 0.001) higher luciferase activity was noted for V2 reporter constructs that contain the longer 3′-downstream region sequences.
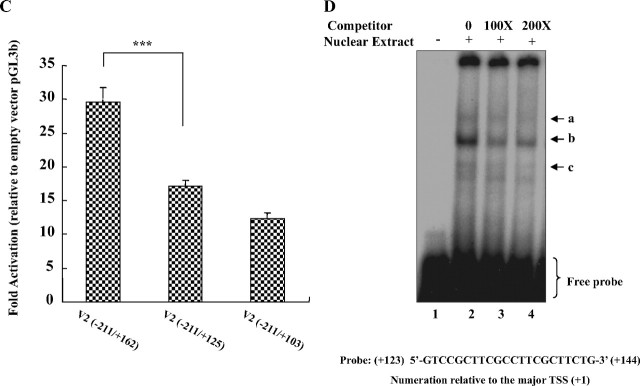
Fig. 4.
Characterization of the V2 exon regions required for achieving maximal V2 promoter activity. A and B, V2 promoter reporter constructs with progressive deletions of the 3′ V2 exon region were prepared and transfected into COS-1, HEK293, and Huh7 cells (A) and SGBS preadipocytes and mature adipocytes (B). Data are presented as mean ± se from n = 3–14 experiments. A significant decrease in promoter activity was observed from +162 to +103 for all five cell types. *, P < 0.05; **, P < 0.01; ***, P < 0.001. C, 3′-Deletion constructs covering the region from +162 to +103 were generated and transfected into HEK293 cells. Deletion of the region from +162 to +125 in the V2 exon caused the reporter activity to decrease significantly (***, P < 0.001). Data are expressed as mean ± se of n = 10 independent experiments. D, A double-stranded oligonucleotide probe containing the +123 to +144 sequence was end-labeled with [γ-32P]ATP and incubated with nuclear extracts prepared from HEK293 cells for EMSA. For oligonucleotide competition experiments, a 100-fold (lane 3) or a 200-fold (lane 4) excess of unlabeled probe was added before the addition of the labeled probe. The three specific DNA-protein complexes are indicated by arrows.
To more closely define these elements, constructs bearing deletions within this region were generated and tested in HEK293 cells (Fig. 4C). A significant decrease in luciferase activity was observed when the V2 exon 3′-sequence was reduced from +162 to +125 (P < 0.001), whereas a deletion from +125 to +103 caused a nonsignificant decrease. These results indicate that the 37-bp region between +162 to +125 is an activation domain that is essential for achieving maximal levels of basal V2 transcription.
To examine whether there are specific factors binding within this region, EMSAs were performed, by incubating nuclear extracts from HEK293 cells with a radioactive-labeled probe corresponding to this region. Three protein-DNA complexes were observed (Fig. 4D). After the addition of unlabeled probe, all three complexes were competed out in a dose-dependent manner, suggesting that they are all sequence specific. Although the MatInspector program (http://www. genomatix.de) predicted a putative Egr-1 site in this region (see Fig. 6 and supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), we did not get a supershift with an Egr-1 antibody (data not shown). We also did not observe a change in basal activity of the −211/+162 construct when the Egr-1 site was mutated (118.3 ± 8.3% of wild type, not significant; n = 7). These data suggest that factors other than Egr-1 are involved.
Fig. 6.
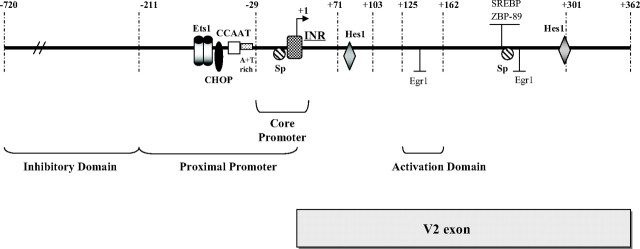
Schematic representation of the hGHR V2 exon and its 5′-upstream promoter. This diagram shows the hGHR V2 and its promoter region spanning from 720 bp upstream to 362 bp downstream of the designated TSS (+1), with the identified proximal promoter, core promoter, and activation as well as inhibitory domains. No TATA box was found within the V2 promoter region, whereas an INR-like element surrounds the TSS (+1). Putative cis-elements, including a CCAAT box, CHOP, and c- Ets1 binding sites, were found within a 40-bp region approximately 50 bp upstream of the TSS. Two putative Hes1 binding sites are present within the V2 exon as well as putative binding sites for Sp, SREBP, ZBP-89, and Egr-1 that are located as a cluster within an approximately 20-bp region.
Identification of the core promoter for V2 basal transcription
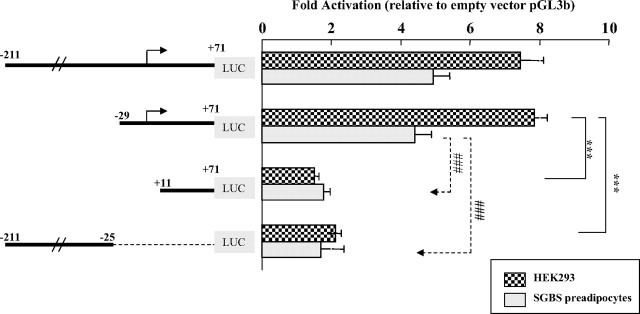
Accurate and efficient transcriptional initiation of eukaryotic genes by RNA polymerase II requires a DNA region known as the core promoter, which includes the TSS and immediate flanking sequences extending from −40 to +40 relative to the TSS (+1) (44). To determine the core promoter for V2 basal transcription, we carried out a deletion analysis based on the V2(−211/+71) construct, because it includes the 5′-proximal promoter region but excludes the influences caused by downstream positive regulatory elements within exon V2. Removal of the 5′-fragment upstream of the TSS from −211 to −29 did not alter luciferase activities (Fig. 5). In contrast, fragments (+11/+71) and (−211/−25) gave only background levels of luciferase activities. Thus, the V2 sequence from −29 to +11 relative to the designated TSS (+1) appears to be the core promoter region.
Fig. 5.
Identification of the core promoter for V2 basal transcription. Using the reporter construct V2(−211/+71) as a primary template, which contains the proximal promoter region but is not influenced by the downstream exon activation region, a set of further 5′- and 3′-deletion constructs were tested in both HEK293 cells and SGBS preadipocytes to define the minimal promoter region required for initiation of V2 transcription. Data are presented as mean ± se of n = 3–9 independent experiments. ***, P < 0.001 for the comparison of V2(−29/+71) with V2(+11/+71) and V2(−211/−25) in HEK293 cells; ###, P < 0.001 for the comparison in SGBS cells.
In summary, our deletional construct assays revealed several distinct domains in the hGHR V2 promoter that are implicated in the maintenance and modulation of V2 basal transcription: the proximal promoter (−211/+1), the core promoter (−29/+11), a 5′-upstream inhibitory domain (−720/−211), and a 3′-downstream activation domain (+125/+162) (Fig. 6).
V2 transcriptional activities in SGBS pre- vs. mature adipocytes
Transcriptional regulation of V2 showed a similar pattern in HEK293 and Huh7 cell lines (Figs. 2B and 4A) as well as SGBS pre- and mature adipocytes (Figs. 2C and 4B). However, a comparison of the promoter profiles of pre- vs. mature adipocytes suggests several interesting differences. First, 5′-deletion from −211 to −29 in mature adipocytes resulted in a significant 40% drop in V2 promoter activity (P < 0.05, Fig. 2C), whereas there was no change in activity in preadipocytes, suggesting that there may be specific transcription factors acting through this 5′-promoter region to positively regulate V2 basal transcription at the mature adipocyte stage. Second, the reporter construct V2(+11/+362), which is missing the major TSS, showed only background activity in preadipocytes but a significant level of activity in mature adipocytes (P < 0.05, Fig. 2C), implying that certain factors induced during adipocyte maturation may function through the V2 exon to promote transcription at minor TSSs. Importantly, the activities of individual 5′- and 3′-deletion V2 promoter constructs were always much higher (∼3- to 4-fold) in adipocytes than in preadipocytes (Figs. 2C and 4B), indicating that V2 promoter activity was stronger in the mature adipocyte. This supports our previous findings that differentiation results in a significant increase in V2 mRNA levels (20).
Promoter structure of the hGHR V2 exon
Using computer-based sequence alignment (ClusterW) and transcription factor binding site search programs [MatInspector (45) and Signal Scan (46)], we analyzed the nucleotide sequence containing the 211-bp V2 proximal promoter region and the complete V2 exon and compared it with homologous regions in the ovine, bovine, and mouse GHR genes (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). High sequence homology was observed between human V2 and ovine 1B (81%), bovine 1B (80%), and mouse L2 (68%), in particular the core promoter sequence from position −40 to +40 [relative to the ovine TSS (+1)]. No TATA-like elements were found within the V2 core promoter region (supplemental Fig. 1). However, an initiator (INR)-like sequence (TCAGTTG) is present, overlapping the ovine TSS (+1) (44, 47); this putative INR sequence is highly conserved across several different species. Immediately upstream of the core promoter (−29/+11), there is a highly conserved CCAAT box followed by an A+T-rich sequence (−28 to −32) (Fig. 6 and supplemental Fig. 1). Multiple putative binding sites for transcription factors, including Ets1, CHOP, Sp, Hes1, Egr-1, ZBP-89, and SREBP as well as several GAGA binding elements, were detected in the proximal promoter region and within the V2 exon itself. Some of them (e.g. the Ets1 and V2 exon Sp sites) are conserved in homologous regions of the ovine, bovine, and mouse GHR genes, whereas others (e.g. CHOP and Hes1 sites) are found only in the hGHR. The upstream Sp sites shown to be highly active in the ovine, and bovine GHR 1B promoters (26, 27, 41) are significantly altered in the hGHR [Fig. 1 of accompanying article (48)].
Functions of important binding motifs within the core promoter on V2 basal transcription
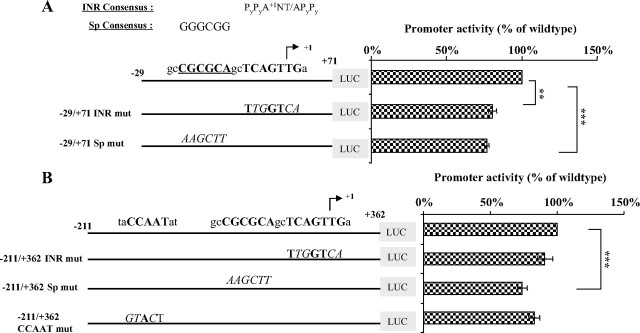
Although the promoter region of hGHR V2 lacks TATA-like sequences, two potentially important binding motifs are present. They are the INR-like element and a putative noncanonical Sp site immediately upstream (Fig. 7 and supplemental Fig. 1). Their importance in regulating V2 basal transcription was examined by mutational analysis. Initially, site-directed mutagenesis was carried out on the −29/+71 V2 promoter segment to create a −29/+71 INR mutant and a −29/+71 Sp mutant (Fig. 7A). Each mutation caused a moderate but significant decrease in promoter activity, suggesting that each motif contributes to V2 basal transcription. Next, the same mutations were introduced into a larger DNA fragment (−211/+362) to investigate whether these mutations would have an effect when both the core promoter and upstream as well as downstream regulatory regions were present, which more closely represents the in vivo situation. As shown in Fig. 7B, mutation of the Sp site resulted in the same reduced luciferase activity as above. In contrast, the INR mutation had little impact on promoter activity, implying that initiation of V2 transcription is not solely dictated by the INR sequence.
Fig. 7.
Impact of important potential binding motifs within the core promoter on V2 basal transcription. A, The reporter construct V2(−29/+71), which includes only the core promoter region, was tested in HEK293 cells. Activity of the wild-type construct was compared with constructs containing mutations in the INR-like or putative Sp elements. The consensus sequences for the INR and Sp elements are provided at the top and mutated sequences are indicated by italic uppercase. Arrow indicates the major TSS (+1). Horizontal bars demonstrate activities of mutated constructs relative to wild type, which is arbitrarily set as 100%. Data are expressed as mean ± se of n = 4 separate assays. **, P < 0.01; ***, P < 0.001. B, Mutations of the INR-like, Sp, or CCAAT box elements were introduced into the larger promoter construct V2(−211/+362), which contains both the core promoter and upstream as well as downstream regulatory elements. Bar graphs on the right demonstrate activities of the mutated constructs in HEK293 cells relative to the wild-type vector, arbitrarily set at 100%. Data are presented as mean ± se of n = 6 experiments. ***, P < 0.001.
Immediately 5′ to the core promoter region, there is a CCAAT box (−37 to −33) that is highly conserved in the ovine, bovine, and mouse GHR genes. To determine whether this element is required for basal expression, a substitution mutation encompassing the entire CCAAT motif was created in the context of the −211 to +362 promoter fragment. Transfection studies demonstrated that inactivation of this CCAAT box failed to affect V2 promoter activities in HEK293 cells (Fig. 7B), SGBS preadipocytes, and adipocytes (data not shown), indicating that V2 basal transcription does not rely on this CCAAT box.
Thus, the V2 core promoter is TATA-less and, despite the presence of an INR-like sequence, it appears to function as a null promoter. Moreover, the putative Sp cis-element within the core promoter seems to play a minor role in V2 transcription.
Discussion
The presence of multiple alternative 5′-UTRs is a common feature of GHR transcripts in a number of species, including humans, sheep, cattle, rabbits, monkeys, mice, and rats (reviewed in Refs. 22 and 23). In humans, 14 hGHR mRNA variants have been isolated to date (18, 19, 20). The V1 and V2 transcripts are the predominantly expressed variants in human liver and extrahepatic tissues, respectively, and they represent two distinct expression patterns: liver-specific and developmentally regulated (V1) vs. ubiquitous (V2) (19, 20). The current work represents the first extensive characterization of the hGHR V2 promoter and the evaluation of cis-regions responsible for V2 transcription. We have mapped the TSSs, demonstrated that the 5′-flanking region of the human V2 exon displays constitutive promoter activity in multiple cell lines, including SGBS pre- and mature adipocytes, identified the core promoter region, and characterized several distinct functional domains that determine V2 basal transcription.
The promoter of the hGHR V2 exon is TATA-less, with several features of a housekeeping gene promoter
Using RNA primer extension, we identified two major initiation sites for V2 transcription in liver and kidney cells as well as human SGBS preadipocytes. Multiple TSSs appear to be a common feature for human V2 and its homologs in other species; two major start sites were mapped for bovine 1B (26) and multiple start sites for ovine 1B (29) and mouse L2 (27, 28). Although the mouse L2 major start site is located approximately 300 nucleotides further downstream, the initiation sites we mapped for human V2 are located within a few nucleotides of the TSSs of ovine 1B and bovine 1B.
A 5′-deletional analysis of the 2.6-kb region upstream of the major (ovine) TSS allowed us to map the V2 proximal promoter to a 211-bp domain. This result is consistent with what Orlovskii et al. (17) have reported; they defined the 165-bp region upstream of the TSS as having maximal promoter activity for hGHR V2. Ovine 1B and bovine 1B were found to have proximal promoters 134 and 191 bp upstream of their respective major TSSs (26, 29). The high degree of homology between human V2, ovine 1B (81.5%), and bovine 1B (82.4%) nucleotide sequences within this proximal promoter region highlights its importance in regulating transcription of V2-like mRNA variants and suggests that their transcriptional regulation is likely achieved through similar mechanisms (22, 23, 26, 28).
Apart from species similarity, the V2 proximal promoter was found to be active in all of the cell types tested in this study, cells derived from different tissues (e.g. liver, kidney and adipose) and at different developmental stages (pre- and postnatal). The basal activities of the promoter constructs in the six cell lines were variable (2- to 4-fold), but the patterns were synchronous. These results correlate well with data from previous in vivo studies showing that V2 mRNA exhibits a ubiquitous expression pattern and indicate that ubiquitous transcription factors are involved in the regulation of V2 transcription.
Sequence analysis of this proximal promoter region reveals several interesting features: the lack of TATA elements, a relatively high GC content (∼60% within the −100- to +100-bp promoter region), and the presence of several putative cis-elements such as two Ets binding sites, a CCAAT box, a noncanonical Sp site (CGCGCA), and an E-box element (CANNTG) that could be bound by ubiquitous transcription factors such as Ets, NF-Y or C/EBPs, Sp1/Sp3 and upstream stimulating factor (USF), respectively (49, 50, 51, 52, 53) (supplemental Fig. 1).
However, our 5′-deletion and mutation analyses within the V2 proximal promoter region showed that loss of these sites did not cause a dramatic change in V2 promoter activity. Although we cannot exclude the possibility that these data may be due to relatively low endogenous levels of certain ubiquitous transcription factors in the cell lines we used, we postulate that transcription of human V2 may not be controlled by one specific transcription factor but is more likely to be the result of input from multiple factors. Indeed, similar concepts have been obtained from studies of both ovine 1B and bovine 1B (26, 41); although the authors reported that deletion of a proximal promoter Sp site resulted in a significant decrease in promoter activities, only partial loss of activity was observed, suggesting that multiple regulatory elements are required to achieve full promoter activity.
The V2-like exon promoters that have been studied to date, human V2, ovine 1B, bovine 1B, and mouse L2, share the common feature of lacking a TATA box but having a high GC content. Such promoters are often associated with Sp binding motifs (44) and are considered to be targets for regulation by Sp family members (27). In fact, studies of V2 homologs in the ovine, bovine, and mouse have demonstrated an important role for Sp1/Sp3 in regulating their promoter activities (26, 27, 41). However, a sequence comparison of the promoter/exon regions revealed that only an atypical Sp site within the V2 core promoter and a consensus Sp site within the V2 exon are conserved in the human; the major promoter Sp sites identified in ovine and bovine studies are significantly altered [Fig. 1 of accompanying manuscript (48)]. In addition, analyses of the V2 promoter and exon showed that either mutation of the atypical site or deletion of the consensus site led to only a moderate decrease in basal promoter activity (Figs. 4, A and B; and 7, A and B), suggesting that the role for Sp transcription factors in hGHR V2 basal transcription is not as critical as it appears to be in other species.
Taken together, our findings of a TATA-less promoter, multiple transcription initiation sites, and constitutively active promoter activity in different cell types support the concept that the V2 promoter functions like the promoter of a housekeeping gene to ensure that there is sufficient basal production of hGHR throughout all tissues in the body.
The V2 core promoter functions like a null core promoter
A core promoter is defined as the minimal DNA region that is sufficient to direct low levels of activator-independent (basal) transcription by RNA polymerase II in vitro. The core promoter typically extends approximately 40 bp up- and downstream of the TSS and can contain distinct core promoter elements, such as a TATA box, an INR, and a downstream promoter element (DPE) (44, 54, 55). These functional DNA motifs help to direct recruitment and interaction with basal transcriptional machinery to allow accurate and efficient initiation of transcription.
From our 5′- and 3′-serial deletional analyses, it appears that the region between positions −29 and +11 is the core promoter for hGHR V2 transcription. This result is similar to the finding of Adams (29) for ovine 1B; removal of the promoter fragment −39 to −5 caused a complete loss of promoter activity. The V2 core promoter is relatively GC rich (∼60%) and is TATA-less. Two types of core promoters lacking a TATA element have been described (44, 56). One is a so-called null promoter that frequently is associated with multiple TSSs because it has neither a TATA box nor an INR. The second contains an INR element within the TSS region that functions as an analog of the TATA box. Within the V2 core promoter region, there is an INR-like element that matches well to the 7-mer INR consensus and encompasses the main TSS. However, functional analysis demonstrated that mutation of this INR-like element resulted in either marginal or almost no change in V2 basal promoter activity. This finding may be, in part, due to the presence of additional structural features, such as an A+T-rich sequence at the −30 region of the V2 promoter, which can have an affinity for TBP and result in an intermediate effect of an INR mutation (47), or an Sp site immediately upstream of the INR element, which can contribute to transcription factor IID interactions with the INR (57) and, thus, preferentially stimulate transcription from INR-containing promoters (47).
Therefore, despite the presence of an INR-like sequence, it is most likely that the V2 core promoter functions like a null promoter, with multiple elements, including the INR-element, acting in a combinatorial fashion to allow for efficient initiation of V2 transcription. Indeed, it has been documented that the INR promoter category contains predominantly genes involved in basic biological processes such as protein synthesis and mRNA processing, whereas the null promoter category includes primarily genes involved in other basic processes such as cell growth and maintenance and protein metabolism (44); the latter matches the major biological consequences initiated after GH/GHR interactions.
hGHR V2 basal promoter activity is subject to a complex form of transcriptional regulation
Although housekeeping genes maintain consistent expression, their promoters are also regulated. For example, the HMGCoA reductase promoter responds to cholesterol levels, and transcription of the mouse dihydrofolate reductase gene increases during periods of DNA synthesis (58). In the present work, in addition to characterizing hGHR V2 promoter as a housekeeping gene promoter, we also showed that the V2 promoter activity is under the regulation of two functionally discrete domains: an inhibitory domain located 5′-upstream of the proximal promoter and an activation domain located within the V2 exon.
Orlovskii et al. (17) also found that insertion of a part of the V2 exon in promoter constructs enhanced V2 transcription. In contrast, inclusion of a 300-bp 1B exon sequence into the bovine 1B promoter constructs was not found to be important for activity (26). Thus, the stimulatory effects of the first exon appear to be specific for the human V2 promoter. Orlovskii et al. (17) hypothesized that this enhancement effect resulted from the additive actions of putative Sp transcription binding sites located in front of and within the V2 exon. Our data suggest an alternate explanation. First, the stimulatory effects arising from the downstream exon region do not depend on the 5′-flanking sequence; promoter constructs containing different 5′-flanking regions, with or without the putative promoter Sp site all showed significantly enhanced promoter activities with the insertion of a portion of the V2 exon (Fig. 3). Second, deletion of the V2 exon sequence from positions +362 to +162 (Fig. 4, B and C), which contains multiple transcription factor binding sites, including the conserved exon Sp site, resulted in unchanged (HEK293 and Huh7 cells) or only slightly reduced (SGBS preadipocytes and adipocytes) promoter activities. Therefore, our data suggest that the putative V2 promoter and exon Sp sites are not responsible for achieving maximal V2 transcription. Instead, we identified a 37-bp activation domain located at position +162 to +125 and demonstrated that this positive regulatory region is required for achieving maximal V2 promoter activity. Proteins in HEK293 nuclear extracts specifically bound to this element, indicating that there are endogenous proteins that can bind to this region. However, the identity of the proteins remains to be characterized.
Stimulation of basal transcription by promoter downstream regions usually involves certain types of downstream core promoter elements within a 30- to 40-bp region, such as a CT signal at +7 (59), a DPE at +30 (60, 61), a motif ten element (MTE) at +18 to +29 (62), and a multiple start site element (MED-1) located downstream of a multiple initiation site window (63). They act as accessory elements to facilitate the formation of a specific transcription factor IID-promoter complex for efficient transcription in many TATA-less/INR-containing promoters (64).
We did not observe any consensus sequences for these motifs in the V2 promoter downstream region. Instead, the 37-bp region that we determined to be responsible for maximal V2 promoter activity is located approximately 100–130 bp further downstream. There have been a few reports of similar downstream elements participating in maximizing transcriptional activities of TATA-less promoters. In the TATA-less rat prolactin receptor gene promoter III, a downstream Sp binding sequence (located between +65 to +76 relative to the TSS) together with an atypical DPE are required for achieving full promoter activity (65). In the mammalian poly(ADP-ribose) polymerase-1 gene, a region between +30 to +52 from the TSS was shown to be necessary for maximal transcriptional activity, through synergizing with an INR element and an upstream Sp site (55). Sheng et al. (64) observed similar complex regulation of the TATA-less promoter of the GR-LACS gene, a member of the fatty acyl coenzyme A synthetase gene family; they found that an Sp1/Sp3 binding element located 115 bp downstream of the TSS associates with the core promoter and exerts significant influence on its transcriptional activity.
In addition to this unusual 3′-downstream positive regulation, we demonstrated that the V2 promoter is also subject to significant repression due to the presence of a specific 5′ region; the −720 to −211 domain caused about 50% inhibition of V2 basal transcriptional activity in all of the cell lines tested. Similar 5′-inhibitory regions have been identified in both ovine 1B and bovine 1B promoter studies (26, 29). It will be important to identify which specific negative regulatory elements are involved in future studies and whether they are conserved across the three species.
Regulation of hGHR V2 transcriptional activity in SGBS preadipocytes and adipocytes
V2 is the predominant hGHR mRNA expressed in human adipocytes and is the major contributor to the increase in hGHR mRNA levels during SGBS preadipocyte differentiation (20). To understand the underlying mechanisms involved, we investigated the transcriptional regulation of V2 in SGBS pre- and mature adipocytes. Primer extension studies of SGBS preadipocyte RNA showed that V2 transcription is initiated at the same major start sites as in liver or kidney cells. The 5′- or 3′-serial deletion analyses of V2 promoter activities demonstrated similar activity profiles in both SGBS preadipocytes and mature adipocytes. These results are consistent with our observations that V2 is highly expressed at both developmental stages.
However, we also witnessed that mature adipocytes always showed approximately 3- to 4-fold higher promoter activities than preadipocytes. These enhanced activities suggest that changes in the availability of positive and/or negative regulatory factors during adipocyte maturation play a role in the regulation of V2 promoter function (66). The enhanced transcriptional ability in mature adipocytes also agrees with our in vivo finding that V2 mRNA levels reach a maximum with maturity (20) and suggests that the increase in V2 mRNA levels takes place at the transcriptional level.
Summary
In summary, we have characterized the promoter of the hGHR V2 exon as TATA-less, with several features of a housekeeping gene promoter. Transcription of human V2 is determined by several distinct functional domains in the promoter region and within the V2 exon itself and probably reflects an interplay between multiple factors. The coordination between the core promoter elements, the 5′-inhibitory region and the 3′-activation domain are likely required to maintain hGHR gene expression at a relatively consistent level to ensure a normal GH response in various target tissues. In addition, multiple cis-elements for distinct transcription factors have been predicted in the proximal region and within the V2 exon. In the accompanying manuscript, we show that, in fact, many of these factors provide a basis for specific transcriptional regulation of hGHR V2 expression in different tissues and in response to different extra- or intracellular signals (48).
Materials and Methods
Tissue collection
Fetal liver tissue samples were obtained after therapeutic abortion (12.5–14 wk fetal age) (67). Postnatal liver specimens (37–60 yr) were collected at the time of surgery for pediatric transplantation within 4–10 h after death. McGill University Health Centre Ethics Committees approved the studies, and informed consent was obtained in each case. Tissues were flash frozen and stored at −70 C until analysis.
Cell culture
HEK293 (human embryonic kidney epithelial), CV-1, and COS-1 (African green monkey kidney fibroblast) cells, obtained from American Type Culture Collection (Manassas, VA), were grown in standard culture medium (DMEM, containing 25 mm HEPES, 100 IU/ml penicillin, 1.6 mg/ml gentamicin sulfate, and 10% fetal bovine serum). Huh7, a human hepatoma cell line (kindly provided by Dr. Ken K. Ho, Garvan Institute of Medical Research, Australia), was cultured in Earle’s salts MEM with 25 mm HEPES, 50 IU/ml penicillin, and 10% fetal bovine serum. The human SGBS preadipocyte cell line, derived from the stromal cell fraction of sc adipose tissue from an infant with Simpson-Golabi-Behmel Syndrome (SGBS), was cultured and differentiated into mature adipocytes, as previously described (68). All of these cells were previously found by RT-PCR assays to express GHR mRNA (total and V2) and/or by Western blot or immunohistochemistry to have immunoreactive GHR, except the COS-1 cells (19, 20, 21, 69, 70) (unpublished data).
Primer extension analysis
Primer extension analysis was used to map the TSSs for hGHR V2. A 28-nucleotide oligonucleotide primer (V2PE: 5′-gtcccccagatccaggttgcagaag-cga-3′) complementary to +162 to +135 of the hGHR V2 sequence (Fig. 1, relative to designated +1) was end-labeled with [γ-32P]ATP (3000 Ci/mmol; Amersham, Piscataway, NJ) and T4 polynucleotide kinase (Invitrogen, Burlington, Ontario, Canada). Total RNAs from multiple cell lines (HEK293, Huh7, SGBS preadipocytes) and human adult as well as fetal livers were isolated using the QIAGEN RNeasy Mini Kit (QIAGEN, Mississauga, Ontario, Canada) or Trizol reagent (Invitrogen). An aliquot (20 μg) of total RNA was mixed with 100 fmol labeled primer (106 cpm) and incubated at 65 C for 5 min. This primer-RNA mixture was then reverse transcribed at 60 C for 1 h with ThermoScript reverse transcriptase (Invitrogen). After termination of the reaction, the extension products were ethanol precipitated and size fractionated on a 6% denaturing polyacrylamide gel together with a 32P-labeled φX174 HinfI DNA marker (Promega, Madison, WI).
Generation of V2 promoter deletion and mutation reporter constructs
To characterize the V2 promoter activity, a series of luciferase reporter gene constructs were engineered by ligation of various portions of the hGHR V2 exon and its 5′-upstream regulatory regions into the promoterless and enhancerless pGL3-basic vector (Promega). All promoter constructs are identified according to the major ovine TSS (TSS) as +1, because it represents the longest V2-like cDNA identified to date. The largest V2(−2623/+335) construct was created by three steps: 1) a 3100-bp fragment was PCR amplified using forward primer V2F(−2623) and reverse primer V2R(−512) (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org) with human genomic DNA as a template; 2) this 3100-bp fragment was cloned into pCR2.1-TOPO vector (Invitrogen); and 3) the MluI-BsrBI fragment containing the V2(−2623/+335) piece was excised and ligated into pGL3-basic using MluI and SmaI sites. The V2(−721/+362) construct was engineered by HindIII digestion of a plasmid containing the hGHR V2 exon and approximately 1 kb of 5′-upstream region (21) and ligated into the HindIII site of pGL3-basic. The V2(−211/+362) and V2(+11/+362) reporter constructs were prepared by PCR amplification and subcloned into pGL3-basic. Using V2(−211/+362) as a template, other 5′- or 3′-deletion V2 promoter constructs were generated by amplifying the relevant promoter pieces with nested sense and antisense primers (supplemental Table 1) and cloning the fragments into pGL3-basic. All of the promoter constructs were sequenced through the vector-insert junctions to ensure nucleotide fidelity.
To obtain the derivatives V2(−211/+71)_INR mut and V2(−211/+362)_ INR mut (mutated in the putative INR sequence), V2(−211/+71)_Sp mut and V2(−211/+362)_Sp mut (mutated in the atypical Sp site) as well as the V2(−211/+362)_CCAAT mut (mutated in the CCAAT box), and the V2(−211/+162)_Egr-1 mut (mutated in the Egr-1 site), the QuikChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) was used according to the manufacturer’s instructions. The reaction mixture was set up in 50 μl final volume containing 1× buffer, 200 nm dNTPs, 20 ng of either V2(−211/+71) or V2(−211/+362) plasmid DNA as template, 200 nm synthetic oligonucleotides (supplemental Table 1), and 2.5 U Pfu Turbo DNA polymerase. The samples were subjected to an initial cycle at 95 C for 30 sec followed by 16 cycles at 95 C for 30 sec, 55 C for 1 min, and 68 C for 10 min. The resulting products were then digested with DpnI (Roche Diagnostics, Laval, Quebec, Canada) at 37 C for 1 h to remove the nonmutated parental DNA template. The digested DNA constructs were subsequently transformed in DH5α competent cells. The DNA insert of each mutant construct was sequenced to verify the authenticity of the desired mutations.
Transient transfection, luciferase, and β-galactosidase assays
Transient transfections of HEK293, CV-1, COS-1, and Huh7 cells were performed using Polyfect transfection reagent (QIAGEN), according to the manufacturer’s instructions. Briefly, cells were plated in 12-well tissue culture plates and grown in complete medium for 16–20 h to reach 60–80% confluence at the time of transfection. A DNA mixture including wild-type or mutant V2 promoter reporter construct (0.5 μg) and a β-galactosidase-control plasmid (0.2 μg) was then added to the cells together with Polyfect reagent at a ratio of 1:3. Transfections were done in triplicate, and the empty pGL3-basic vector was used as a negative control.
Transfection was also carried out on 60–80% confluent human SGBS preadipocytes using the Polyfect method, except that the amount of reporter construct was increased to 1 μg for maximal luciferase activity. For differentiated SGBS adipocytes, transfections were performed as previously described, on d 16–17 after differentiation (71).
At 48 h after transfection, cells were harvested in 200 μl 1× passive lysis buffer (Promega) and the lysate supernatant was quantified for luciferase and β-galactosidase activities (Tropix, ABI, Bedford, MA) using an EG&G Berthold (Oak Ridge, TN) Microlumat Plus bioluminometer (LB 96V). The values of luciferase were normalized to the values of β-galactosidase, an internal control for transfection efficiency, and expressed as fold activation, as specified in the figures and legends.
Nuclear extracts and EMSA
Nuclear proteins were extracted from HEK293 cells using the NE-PER nuclear and cytoplasmic extraction reagents kit (Pierce, Rockford, IL), according to directions of the manufacturer. The nuclear extract was subjected to dialysis in ice-cold PBS using the Slide-A-Lyzer MINI Dialysis Unit (Pierce) and stored in aliquots at −80 C until use. Protein concentration was estimated using the Bradford assay (Bio-Rad Laboratories Inc., Hercules, CA).
Synthesized oligomers including the 3′-activation domain were annealed to form double-stranded DNA, 5′-end-labeled with [γ-32P]ATP (3000 Ci/mmol; Amersham), passed through G-50 spin columns (Amersham) and subsequently used as a labeled probe in EMSA. Ten micrograms of HEK293 nuclear extracts were incubated in 20 μl reaction containing 10 mm Tris-HCl (pH 7.5), 1 mm MgCl2, 50 mm NaCl, 0.5 mm EDTA, 5% glycerol, 1 mm dithiothreitol, and 50 μg/ml polydeoxyinosinic-deoxycytidylic acid (dI-dC), with or without excess unlabeled probe on ice for 20 min, followed by addition of 50 fmol labeled probe (∼50,000 cpm) and further incubated for 30 min at room temperature. DNA-protein complexes were resolved on 5% native polyacrylamide gels and run in 0.5× Tris-borate-EDTA (TBE) buffer. The dried gels were then autoradiographed on Kodak Biomax-MR films (Kodak, Rochester, NY).
Statistical analysis
Data are expressed as mean ± se. The significance of observed differences between groups was determined by one-way ANOVA followed by Tukey-Kramer multiple comparison tests (P < 0.05 significance).
Acknowledgments
We gratefully acknowledge the gift of Huh7 cells from Dr. Ken K. Ho (Garvan Institute of Medical Research, Sydney, New South Wales, Australia). We also thank Dr. Aimee Ryan for her critical reviews of this paper.
Footnotes
Funding for these studies was from the Canadian Institutes of Health Research (C.G.G.) and studentships from the Fonds de recherches en santé du Québec (FRSQ) and the McGill University Health Centre-Montreal Children’s Hospital Research Institute (Y.W.). C.G.G. is a member of the Research Institute of the McGill University Health Centre, which is supported in part by the FRSQ.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 30, 2008
Abbreviations: DPE, Downstream promoter element; hGH, human GH; hGHR, hGH receptor; INR, initiator; TSS, transcription start site; 5′UTR, 5′-untranslated region.
References
- 1.Perry JK, Emerald BS, Mertani HC, Lobie PE2006. The oncogenic potential of growth hormone. Growth Horm IGF Res 16:277–289 [DOI] [PubMed] [Google Scholar]
- 2.Waters MJ, Shang CA, Behncken SN, Tam SP, Li H, Shen B, Lobie PE1999. Growth hormone as a cytokine. Clin Exp Pharmacol Physiol 26:760–764 [DOI] [PubMed] [Google Scholar]
- 3.Wabitsch M, Hauner H, Heinze E, Teller W1994. In vitro effects of growth hormone in adipose tissue. Acta Paediatr Suppl 406:48–53 [DOI] [PubMed] [Google Scholar]
- 4.Wabitsch M, Hauner H, Heinze E, Teller WM1995. The role of growth hormone/insulin-like growth factors in adipocyte differentiation. Metabolism 44:45–49 [DOI] [PubMed] [Google Scholar]
- 5.Wabitsch M, Braun S, Hauner H, Heinze E, Ilondo MM, Shymko R, De Meyts P, Teller WM1996. Mitogenic and antiadipogenic properties of human growth hormone in differentiating human adipocyte precursor cells in primary culture. Pediatr Res 40:450–456 [DOI] [PubMed] [Google Scholar]
- 6.Lichanska AM, Waters MJ2008. How growth hormone controls growth, obesity and sexual dimorphism. Trends Genet 24:41–47 [DOI] [PubMed] [Google Scholar]
- 7.Herrington J, Carter-Su C2001. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab 12:252–257 [DOI] [PubMed] [Google Scholar]
- 8.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A2001. The somatomedin hypothesis: 2001. Endocr Rev 22:53–74 [DOI] [PubMed] [Google Scholar]
- 9.Lichanska AM, Waters MJ2008. New insights into growth hormone receptor function and clinical implications. Horm Res 69:138–145 [DOI] [PubMed] [Google Scholar]
- 10.Pilecka I, Whatmore A, van Huijsduijnen RH, Destenaves B, Clayton P2007. Growth hormone signalling: sprouting links between pathways, human genetics and therapeutic options. Trends Endocrinol Metab 18:12–18 [DOI] [PubMed] [Google Scholar]
- 11.Lanning NJ, Carter-Su C2006. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 7:225–235 [DOI] [PubMed] [Google Scholar]
- 12.Zhu T, Goh EL, Graichen R, Ling L, Lobie PE2001. Signal transduction via the growth hormone receptor. Cell Signal 13:599–616 [DOI] [PubMed] [Google Scholar]
- 13.Brooks AJ, Wooh JW, Tunny KA, Waters MJ2008. Growth hormone receptor: mechanism of action. Int J Biochem Cell Biol 40:1984–1989 [DOI] [PubMed] [Google Scholar]
- 14.Thimmarayappa J, Sun J, Schultz LE, Dejkhamron P, Lu C, Giallongo A, Merchant JL, Menon RK2006. Inhibition of growth hormone receptor gene expression by saturated fatty acids: role of Kruppel-like zinc finger factor, ZBP-89. Mol Endocrinol 20:2747–2760 [DOI] [PubMed] [Google Scholar]
- 15.Leung DW, Spencer SA, Cachianes G, Hammonds RG, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI1987. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature 330:537–543 [DOI] [PubMed] [Google Scholar]
- 16.Godowski PJ, Leung DW, Meacham LR, Galgani JP, Hellmiss R, Keret R, Rotwein PS, Parks JS, Laron Z, Wood WI1989. Characterization of the human growth hormone receptor gene and demonstration of a partial gene deletion in two patients with Laron-type dwarfism. Proc Natl Acad Sci USA 86:8083–8087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlovskii IV, Sverdlova PS, Rubtsov PM2004. [Fine structure, expression and polymorphism of the human growth hormone receptor gene]. Mol Biol (Mosk) 38:29–39 (Russian) [PubMed] [Google Scholar]
- 18.Pekhletsky RI, Chernov BK, Rubtsov PM1992. Variants of the 5′-untranslated sequence of human growth hormone receptor mRNA. Mol Cell Endocrinol 90:103–109 [DOI] [PubMed] [Google Scholar]
- 19.Goodyer CG, Zogopoulos G, Schwartzbauer G, Zheng H, Hendy GN, Menon RK2001. Organization and evolution of the human growth hormone receptor gene 5′-flanking region. Endocrinology 142:1923–1934 [DOI] [PubMed] [Google Scholar]
- 20.Wei Y, Rhani Z, Goodyer CG2006. Characterization of growth hormone receptor messenger ribonucleic acid variants in human adipocytes. J Clin Endocrinol Metab 91:1901–1908 [DOI] [PubMed] [Google Scholar]
- 21.Goodyer CG, Rhani Z, Zheng H2008. Expression of the hepatic specific V1 mRNA of the human growth hormone receptor gene is regulated by HNF4α2 and HNF4α8. Mol Endocrinol 22:485–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edens A, Talamantes F1998. Alternative processing of growth hormone receptor transcripts. Endocr Rev 19:559–582 [DOI] [PubMed] [Google Scholar]
- 23.Schwartzbauer G, Menon RK1998. Regulation of growth hormone receptor gene expression. Mol Genet Metab 63:243–253 [DOI] [PubMed] [Google Scholar]
- 24.O'Mahoney JV, Brandon MR, Adams TE1994. Identification of a liver-specific promoter for the ovine growth hormone receptor. Mol Cell Endocrinol 101:129–139 [DOI] [PubMed] [Google Scholar]
- 25.Rivers CA, Norman MR2000. The human growth hormone receptor gene: characterization of the liver-specific promoter. Mol Cell Endocrinol 160:51–59 [DOI] [PubMed] [Google Scholar]
- 26.Jiang H, Okamura CS, Boyd CK, Lucy MC2000. Identification of Sp1 as the transcription factor for the alternative promoter P2 of the bovine growth hormone receptor gene. J Mol Endocrinol 24:203–214 [DOI] [PubMed] [Google Scholar]
- 27.Yu JH, Schwartzbauer G, Kazlman A, Menon RK1999. Role of the Sp family of transcription factors in the ontogeny of growth hormone receptor gene expression. J Biol Chem 274:34327–34336 [DOI] [PubMed] [Google Scholar]
- 28.Moffat JG, Edens A, Talamantes F1999. Structure and expression of the mouse growth hormone receptor/growth hormone binding protein gene. J Mol Endocrinol 23:33–44 [DOI] [PubMed] [Google Scholar]
- 29.Adams TE1995. Differential expression of growth hormone receptor messenger RNA from a second promoter. Mol Cell Endocrinol 108:23–33 [DOI] [PubMed] [Google Scholar]
- 30.Menon RK, Stephan DA, Singh M, Morris Jr SM, Zou L1995. Cloning of the promoter-regulatory region of the murine growth hormone receptor gene. Identification of a developmentally regulated enhancer element. J Biol Chem 270:8851–8859 [DOI] [PubMed] [Google Scholar]
- 31.Menon RK, Shaufl A, Yu J, Stephan A, Friday R2001. Identification and characterization of a novel transcript of the murine growth hormone receptor gene exhibiting development- and tissue-specific expression. Mol Cell Endocrinol 172:135–146 [DOI] [PubMed] [Google Scholar]
- 32.Jiang H, Okamura CS, Lucy MC1999. Isolation and characterization of a novel promoter for the bovine growth hormone receptor gene. J Biol Chem 274:7893–7900 [DOI] [PubMed] [Google Scholar]
- 33.Zou L, Burmeister LA, Sperling MA1997. Isolation of a liver-specific promoter for human growth hormone receptor gene. Endocrinology 138:1771–1774 [DOI] [PubMed] [Google Scholar]
- 34.Jiang H, Lucy MC2001. Involvement of hepatocyte nuclear factor-4 in the expression of the growth hormone receptor 1A messenger ribonucleic acid in bovine liver. Mol Endocrinol 15:1023–1034 [DOI] [PubMed] [Google Scholar]
- 35.Xu Q, Walther N, Jiang H2004. Chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) and hepatocyte nuclear factor 4γ (HNF-4γ) and HNF-4α regulate the bovine growth hormone receptor 1A promoter through a common DNA element. J Mol Endocrinol 32:947–961 [DOI] [PubMed] [Google Scholar]
- 36.Xu Q, Springer L, Merchant JL, Jiang H2006. Identification of zinc finger binding protein 89 (ZBP-89) as a transcriptional activator for a major bovine growth hormone receptor promoter. Mol Cell Endocrinol 251:88–95 [DOI] [PubMed] [Google Scholar]
- 37.Adams TE, Baker L, Fiddes RJ, Brandon MR1990. The sheep growth hormone receptor: molecular cloning and ontogeny of mRNA expression in the liver. Mol Cell Endocrinol 73:135–145 [DOI] [PubMed] [Google Scholar]
- 38.Heap D, Collier RJ, Boyd CK, Lucy MC1996. Expression of alternate growth hormone receptor messenger RNA in ovary and uterus of cattle. Domest Anim Endocrinol 13:421–430 [DOI] [PubMed] [Google Scholar]
- 39.Heap D, Lucy MC, Collier RJ, Boyd CK, Warren WC1995. Rapid communication: nucleotide sequence of the promoter and first exon of the somatotropin receptor gene in cattle. J Anim Sci 73:1529. [DOI] [PubMed] [Google Scholar]
- 40.Southard JN, Barrett BA, Bikbulatova L, Ilkbahar Y, Wu K, Talamantes F1995. Growth hormone (GH) receptor and GH-binding protein messenger ribonucleic acids with alternative 5′-untranslated regions are differentially expressed in mouse liver and placenta. Endocrinology 136:2913–2921 [DOI] [PubMed] [Google Scholar]
- 41.Adams TE1999. Transcription from the P2 promoter of the growth hormone receptor gene involves members of the Sp transcription factor family. Biochem J 344:867–872 [PMC free article] [PubMed] [Google Scholar]
- 42.Osafo J, Wei Y, Kenth G, Goodyer CG2005. Growth hormone during development. Rev Endocr Metab Disord 6:173–182 [DOI] [PubMed] [Google Scholar]
- 43.Nam SY, Lobie PE2000. The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes Rev 1:73–86 [DOI] [PubMed] [Google Scholar]
- 44.Yang C, Bolotin E, Jiang T, Sladek FM, Martinez E2007. Prevalence of the initiator over the TATA box in human and yeast genes and identification of DNA motifs enriched in human TATA-less core promoters. Gene 389:52–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T2005. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21:2933–2942 [DOI] [PubMed] [Google Scholar]
- 46.Prestridge DS1991. SIGNAL SCAN: a computer program that scans DNA sequences for eukaryotic transcriptional elements. Comput Appl Biosci 7:203–206 [DOI] [PubMed] [Google Scholar]
- 47.Smale ST1997. Transcription initiation from TATA-less promoters within eukaryotic protein-coding genes. Biochim Biophys Acta 1351:73–88 [DOI] [PubMed] [Google Scholar]
- 48.Wei Y, Puzhko S, Wabitsch M, Goodyer CG2009. Transcriptional regulation of the human growth hormone receptor (hGHR) gene V2 promoter by transcriptional activators and repressor. Mol Endocrinol 23:373–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantovani R1999. The molecular biology of the CCAAT-binding factor NF-Y. Gene 239:15–27 [DOI] [PubMed] [Google Scholar]
- 50.Corre S, Galibert MD2005. Upstream stimulating factors: highly versatile stress-responsive transcription factors. Pigment Cell Res 18:337–348 [DOI] [PubMed] [Google Scholar]
- 51.Oikawa T, Yamada T2003. Molecular biology of the Ets family of transcription factors. Gene 303:11–34 [DOI] [PubMed] [Google Scholar]
- 52.Suske G1999. The Sp-family of transcription factors. Gene 238:291–300 [DOI] [PubMed] [Google Scholar]
- 53.Wilson HL, Roesler WJ2002. CCAAT/enhancer binding proteins: do they possess intrinsic cAMP-inducible activity? Mol Cell Endocrinol 188:15–20 [DOI] [PubMed] [Google Scholar]
- 54.Butler JE, Kadonaga JT2002. The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev 16:2583–2592 [DOI] [PubMed] [Google Scholar]
- 55.Laniel MA, Poirier GG, Guerin SL2004. A conserved initiator element on the mammalian poly(ADP-ribose) polymerase-1 promoters, in combination with flanking core elements, is necessary to obtain high transcriptional activity. Biochim Biophys Acta 1679:37–46 [DOI] [PubMed] [Google Scholar]
- 56.Werner T1999. Models for prediction and recognition of eukaryotic promoters. Mamm Genome 10:168–175 [DOI] [PubMed] [Google Scholar]
- 57.Kaufmann J, Smale ST1994. Direct recognition of initiator elements by a component of the transcription factor IID complex. Genes Dev 8:821–829 [DOI] [PubMed] [Google Scholar]
- 58.Dynan WS, Sazer S, Tjian R, Schimke RT1986. Transcription factor Sp1 recognizes a DNA sequence in the mouse dihydrofolate reductase promoter. Nature 319:246–248 [DOI] [PubMed] [Google Scholar]
- 59.Larsen NI, Engelbrecht J, Brunak S1995. Analysis of eukaryotic promoter sequences reveals a systematically occurring CT-signal. Nucleic Acids Res 23:1223–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadonaga JT2002. The DPE, a core promoter element for transcription by RNA polymerase II. Exp Mol Med 34:259–264 [DOI] [PubMed] [Google Scholar]
- 61.Kutach AK, Kadonaga JT2000. The downstream promoter element DPE appears to be as widely used as the TATA box in Drosophila core promoters. Mol Cell Biol 20:4754–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim CY, Santoso B, Boulay T, Dong E, Ohler U, Kadonaga JT2004. The MTE, a new core promoter element for transcription by RNA polymerase II. Genes Dev 18:1606–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ince TA, Scotto KW1995. A conserved downstream element defines a new class of RNA polymerase II promoters. J Biol Chem 270:30249–30252 [DOI] [PubMed] [Google Scholar]
- 64.Sheng Y, Li J, Dufau ML, Tsai-Morris CH2005. The gonadotropin-regulated long-chain acyl CoA synthetase gene: a novel downstream Sp1/Sp3 binding element critical for transcriptional promoter activity. Gene 360:20–26 [DOI] [PubMed] [Google Scholar]
- 65.Hu ZZ, Zhuang L, Meng J, Dufau ML1998. Transcriptional regulation of the generic promoter III of the rat prolactin receptor gene by C/EBPβ and Sp1. J Biol Chem 273:26225–26235 [DOI] [PubMed] [Google Scholar]
- 66.Otto TC, Lane MD2005. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol 40:229–242 [DOI] [PubMed] [Google Scholar]
- 67.Munsick RA1984. Human fetal extremity lengths in the interval from 9 to 21 menstrual weeks of pregnancy. Am J Obstet Gynecol 149:883–887 [DOI] [PubMed] [Google Scholar]
- 68.Wabitsch M, Brenner RE, Melzner I, Braun M, Moller P, Heinze E, Debatin KM, Hauner H2001. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord 25:8–15 [DOI] [PubMed] [Google Scholar]
- 69.Goodyer CG, Figueiredo R, Krackovitch S, DeSouza Li L, Manalo J, Zogopoulos G2001. Characterization of the growth hormone receptor in human dermal fibroblasts and liver during development. Am J Physiol Endocrinol Metab 281:1213–1220 [DOI] [PubMed] [Google Scholar]
- 70.Kenth G, Mergelas J, Goodyer C2008. Developmental changes in the human growth hormone receptor and its signal transduction pathways. J Endocrinol 198:71–82 [DOI] [PubMed] [Google Scholar]
- 71.Dalen KT, Ulven SM, Bamberg K, Gustafsson JA, Nebb HI2003. Expression of the insulin-responsive glucose transporter GLUT4 in adipocytes is dependent on liver X receptor α. J Biol Chem 278:48283–48291 [DOI] [PubMed] [Google Scholar]