Abstract
The V2 transcript is the major ubiquitously expressed human GH receptor (hGHR) mRNA in all tissues examined to date. In a previous investigation, we defined the V2 promoter as TATA-less and exhibiting many characteristics of a housekeeping gene promoter. We also demonstrated that its basal activity is determined by several different cis-regulatory regions within both the promoter and the V2 exon. In the present study, we used luciferase-reporter, site-directed mutagenesis, gel shift, chromatin immunoprecipitation, and quantitative RT-PCR assays to investigate the ability of certain transcription factors to regulate hGHR V2 transcription through these regions in mammalian cells, including human adipocytes. Ets1 was found to transactivate the V2 proximal promoter through specific Ets sites. Two CCAAT/enhancer-binding protein (C/EBP) family members [C/EBP-homologous protein (CHOP) and C/EBPβ] enhanced V2 transcription via different pathways: indirectly, by association with a V2 exon region (CHOP), and directly, using a V2 proximal promoter noncanonical binding site (C/EBPβ). The Notch signaling mediator, Hes1, potently suppressed V2 promoter activity through interaction with two Hes sites within the V2 exon. We propose that these transcriptional factors regulate hGHR V2 expression by acting as downstream nuclear effectors, linking specific signaling cascades (e.g. MAPK and Notch) triggered by different growth factor-, development-, and nutrition- as well as stress-related stimuli. Our data also suggest that these factors are likely to be important in the differentiation-induced increase in V2 mRNA expression in adipocytes, with Ets1 and CHOP functioning at the preadipocyte stage to prepare the cells for differentiation and increasing C/EBPs and decreasing Hes1 levels contributing during adipocyte maturation.
Specific stimulatory and inhibitory cis-elements regulate expression of V2, the major ubiquitously expressed transcript of the human growth hormone receptor gene in all tissues.
GH is a key regulator of postnatal somatic growth and an important metabolic hormone (1, 2, 3, 4, 5, 6, 7). At the cell level, GH actions are mediated via its specific GH receptor (GHR), a class I cytokine receptor (4, 5). Thus, the ability of GH to exert its biological effects is intimately linked to adequate amounts and normal functioning of its receptor in target tissues.
GHR expression is tightly controlled at three levels: transcription, translation, and posttranslation (8). Although our understanding of its translational and posttranslational regulation has advanced significantly in the past two decades (8, 9), our knowledge of its transcriptional regulation remains relatively limited. Studies of the mechanisms modulating GHR gene transcription in humans and several animal species have revealed a common characteristic: the use of alternative 5′-noncoding exons, resulting in the generation of multiple mRNA transcripts that differ in their 5′-untranslated region (5′-UTR) sequences but splice into the same site in exon 2 upstream of the translational start site and, thus, code for the same GHR protein. This 5′-UTR heterogeneity is thought to provide a complex regulatory mechanism for determining development- and tissue-specific as well as ubiquitous GHR expression in different target tissues (10, 11, 12, 13, 14, 15, 16, 17, 18).
The human V2 transcript is the predominant ubiquitously expressed human GHR (hGHR) mRNA in all tissues examined to date (1, 10). To better understand what regulates V2 expression, we recently began to characterize a 2.6-kb 5′-flanking region of the V2 exon. Using different primate cell types (HEK293, Huh7, COS-1, CV-1, and SGBS preadipocytes and adipocytes), we found that its basal activity is determined by several distinct regulatory regions, suggesting the involvement of multiple factors (19). The proximal promoter region of the V2 exon, defined as 211 bp upstream of the major (ovine) transcription start site (TSS) (14, 19), possesses several TATA-less promoter structural features: the lack of a TATA box, the occurrence of an initiator-like element, a high G+C content, and the presence of putative cis-elements for several well-characterized ubiquitously expressed transcription factors, including Ets, CCAAT/enhancer-binding protein (C/EBP), nuclear factor (NF)-Y, and Sp proteins (Fig. 1), which cluster within a small region. Moreover, we showed in all of the cell lines tested that specific V2 exon regions enhanced V2 transcriptional activity. These regions contain several putative transcription factor binding sites, some of which are conserved across different species [specific protein (Sp), sterol regulatory element binding protein (SREBP), and zinc finger binding protein-89 (ZBP-89)], whereas others are unique to the human [hairy and enhancer of split 1 (Hes1)].
Fig. 1.

Sequence comparison of the exon and 5′-flanking sequences of human V2, ovine 1B, bovine 1B, and mouse L2. The human V2 sequence (GenBank AF322014) containing 211 bp of the 5′-upstream promoter region and about 300 bp of V2 exon is aligned with its homologs, including ovine 1B (S78252), bovine 1B (AF046861), and mouse L2 (AF120480). Sequence identity is indicated by asterisks, whereas dashes indicate gaps introduced to maximize alignment. The major TSS of ovine 1B (T, bold) is indicated by +1 with an arrow. This is also used as position +1 for our human V2 promoter construct numeration because it represents the longest V2-like cDNA identified to date. Motifs of potential binding sites for transcription factors are underlined and named. The highly conserved CCAAT box and two overlapped Ets binding sites are boxed. The human-specific CHOP and Hes1 sites are marked with a dashed line and round dots, respectively. Sp sites identified in ovine and bovine 1B studies are indicated (bold and italicized). The SREBP (italicized) and ZBP-89 (arrow) sites overlap with the exon Sp site.
In the present study, we examined several of these putative cis-regulatory elements in the V2 proximal promoter and exon, to determine which were functionally significant. Using transient transfection, gel shift and chromatin immunoprecipitation assays, we demonstrated that there are both positive [Ets1, C/EBP-homologous protein (CHOP), C/EBP] and negative (Hes1) regulatory elements modulating V2 transcription, through direct binding or indirect association of their specific transcription factors. These regulatory factors likely serve as nuclear mediators, regulating hGHR expression in response to several different extra- and intracellular signals.
Results
Ets1 directly transactivates the hGHR V2 promoter
The Ets family of transcription factors plays important roles in regulating gene expression in response to multiple developmental, mitogenic, and tumor-related signals. Ets members are characterized by their highly conserved DNA binding (ETS) domain by which they recognize a core DNA motif, GGA(A/T) (20, 21). In the hGHR V2 proximal promoter, two overlapping putative Ets binding sequences were found (Figs. 1 and 2A). Although both sites are highly conserved across different species (Fig. 1), no studies of Ets regulation of GHR mRNA expression have been reported to date.
Fig. 2.
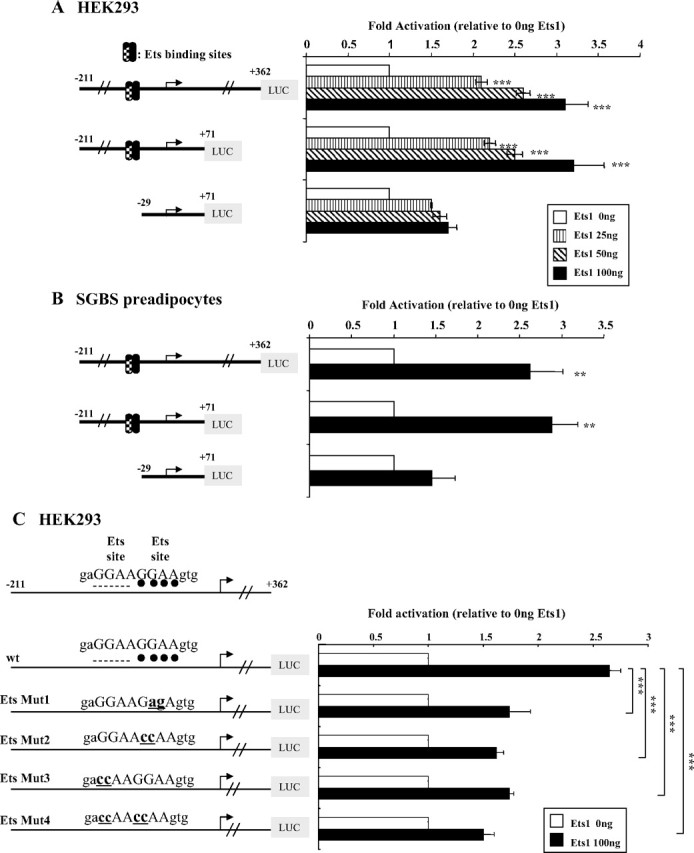
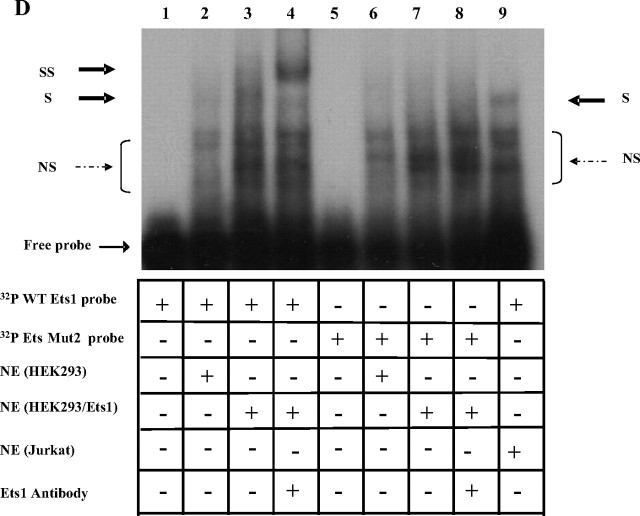
Functional analysis of the Ets binding sites in the hGHR V2 promoter. A, Overexpression of Ets1 stimulates V2 promoter activity in a dose-dependent manner in HEK293 cells. V2 promoter constructs with or without the putative Ets binding sites were cotransfected with increasing amounts of Ets1 expression plasmid (pEVRF-Ets1) in HEK293 cells. Empty control vector (pEVRFO) was added to make equal amounts of total DNA. Data are expressed as fold activation relative to Ets1 at 0 ng, which is arbitrarily set as 1. Results are expressed as mean ± se; n = 3 experiments. ***, P < 0.001 relative to Ets1 at 0 ng. B, Overexpression of Ets1 stimulates V2 transcriptional activity in SGBS preadipocytes. V2 promoter constructs that either contain the putative Ets binding sites or not were cotransfected with 0.1 μg Ets1 expression vector or empty control vector into SGBS preadipocytes at 60–80% confluency. Transcriptional activities are reported as fold induction compared with empty control vector, which is arbitrarily set as 1. Data are presented as mean ± se; n = 3 experiments. **, P < 0.01 relative to Ets1 at 0 ng. C, Impact of mutations in Ets binding sites on transcriptional activation of V2 by Ets1. Wild-type (wt) or Ets site mutant promoter constructs were cotransfected with 0.1 μg pEVRF-Ets1 expression vector or empty control vector (Ets1, 0 ng) in HEK293 cells. All values are expressed as fold induction relative to respective constructs cotransfected with empty control vector, which is arbitrarily set as 1. Data are presented as mean ± se; n = 3–5 experiments. ***, P < 0.001 for differences between wild-type and Ets mutants in response to Ets1 overexpression at 100 ng. D, Ets1 binds directly to the hGHR V2 promoter via the c-Ets1 binding site. Nuclear extracts prepared from HEK293 cells, HEK293 cells cotransfected with pEVRF-Ets1 expression plasmid, or Jurkat cells were incubated with wild-type or Ets_Mut2 oligonucleotide probes and then subjected to EMSA. S, Specific shifted band; NS, nonspecific bands. A supershift assay was performed using an antibody specific for Ets1. SS, Supershifted band. Lane 9, Jurkat cell nuclear extracts were used as a control (high endogenous levels of Ets1).
We first examined whether these two putative Ets binding sites are implicated in V2 constitutional (basal) promoter activity. The V2(−211/+362) promoter reporter construct, which we had found to display maximal V2 promoter activity in previous studies (19), was used as a template for site-directed mutagenesis (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). None of the four mutations resulted in a significant change in luciferase activity (supplemental Fig. 1). Because these data could be due to low levels of endogenous Ets factors in the cells tested (HEK293, SGBS, and COS-1), we then cotransfected an Ets1 expression plasmid together with the V2 promoter constructs. We chose Ets1 because it is the founding member of the Ets family and is also widely expressed. Indeed, overexpression of Ets1 resulted in a marked stimulation of the luciferase activities from both V2(−211/+362) and V2(−211/+71) promoter constructs in a dose-dependent manner in HEK293 cells; in contrast, no significant effects were seen with the control construct, V2(−29/+11), which lacks the putative Ets binding sites (Fig. 2A). Similar effects were observed in the SGBS (Simpson-Golabi-Behmel syndrome) preadipocyte cell line (22) (Fig. 2B) as well as COS-1 and CV-1 cells (data not shown).
To determine whether Ets1 is acting through its putative binding site(s), Ets1 was cotransfected with the four Ets mutant promoter constructs (Fig. 2C). Mutations of either Ets binding site resulted in a significant decrease (∼60%, P < 0.001) in the response of the V2 promoter construct to Ets1 stimulation, whereas mutations of both sites caused a further approximately 10% reduction (Fig. 2C). Furthermore, we performed EMSA to show direct binding of Ets1 to the putative Ets binding sites. When a probe containing the two overlapped Ets sites was incubated with nuclear extracts from HEK293 cells overexpressing Ets1, a protein-DNA complex formed (Fig. 2D, lane 3) that was supershifted by the addition of an anti-Ets1-specific antibody (Fig. 2D, lane 4). In contrast, when the Ets binding site was mutated (Ets_Mut1 or Mut2 probes, supplemental Table 1), the specific complex disappeared (Fig. 2D, lane 7 and data not shown) and no supershift was observed (Fig. 2D, lane 8). Collectively, our results suggest that Ets1 can transactivate the hGHR V2 promoter through direct binding to the Ets binding sites.
CHOP up-regulates V2 transcription by association with a 3′-downstream region
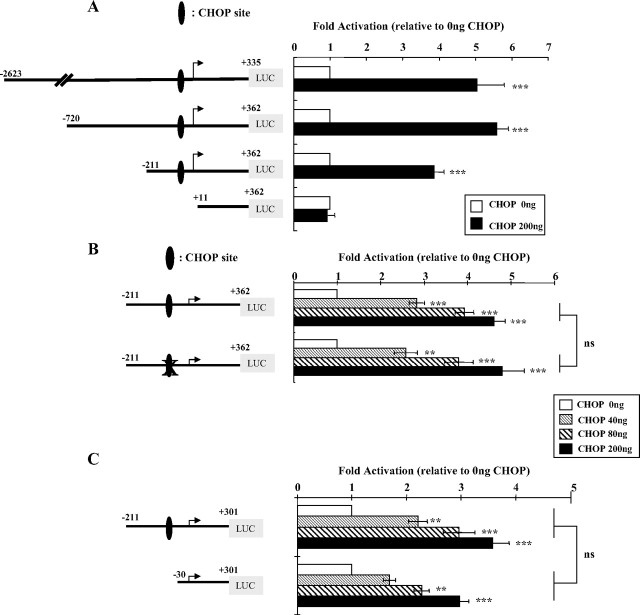
A single putative CHOP-C/EBP heterodimer binding element (CHOP site) was detected in the hGHR V2 promoter immediately downstream of the Ets binding sites (Fig. 1). CHOP, a dominant-negative inhibitor of C/EBPs, is able to form heterodimers with either C/EBPα or C/EBPβ and to activate gene expression through such a site. To address its functional role in regulating the V2 promoter, we cotransfected a human CHOP expression vector with a series of 5′-deletion V2 reporter constructs in COS-1 cells. Overexpression of CHOP markedly up-regulated the activities of all three V2 promoter constructs that contain the putative CHOP site, whereas the negative controls [pGL3-basic and V2(+11/+362)] did not respond (Fig. 3A). Similar stimulatory patterns were observed in other cell lines (HEK293 and CV-1), although the fold activations were lower than in the COS cells (data not shown).
Fig. 3.
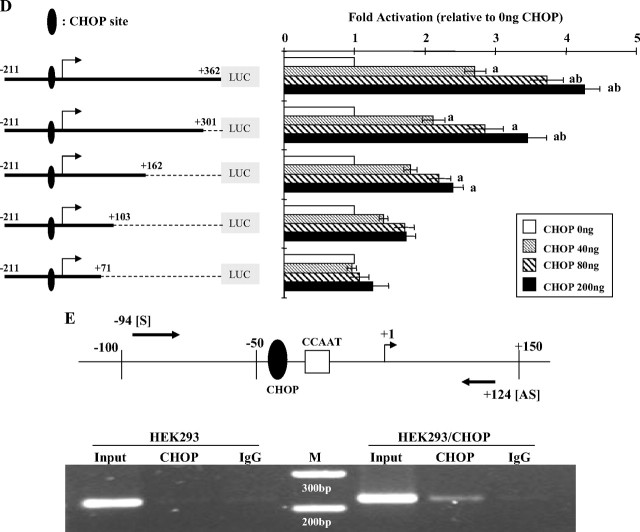
Functional analysis of the role of the CHOP-C/EBP heterodimer site in V2 transcriptional regulation. A, Overexpression of CHOP up-regulates V2 promoter activity. The 5′-deletion V2 promoter constructs were transfected into COS-1 cells together with 0.2 μg expression vector for CHOP (pcDNA3.1-hygro-CHOP) or empty vector (pcDNA3.1-hygro). The fold induction indicates the ratios between CHOP overexpression and CHOP at 0 ng, which is arbitrarily set as 1. Data are expressed as mean ± se; n = 3. ***, P < 0.001 vs. control. Similar stimulation patterns were observed in A549 and HEK293 cells (data not shown). Because COS-1 cells gave the highest stimulation, it was chosen for studying CHOP effects on the V2 promoter. B, Mutation of the CHOP-C/EBP heterodimer site has no effect on dose-dependent response to CHOP stimulation. The sequence of the consensus CHOP-C/EBP heterodimer site is 5′-RRRTGCAATA/CCCC-3′ with the core element bold and underlined. The hGHR V2 CHOP-C/EBP site (5′-AGTTGCAATACAC-3′) was mutated to 5′-AGTggatccACAC-3′, with the mutated sequences shown in lowercase. V2 promoter reporter constructs containing the wild-type or the mutated CHOP site were transfected into COS-1 cells along with CHOP expression vector at increasing amounts. Data are expressed as fold activation relative to CHOP at 0 ng, which is arbitrarily set as 1. C, Deletion of the putative CHOP-C/EBP site has little effect on the dose-dependent response to CHOP stimulation. V2 promoter constructs including the CHOP site or not were transiently transfected into COS-1 cells along with CHOP expression vector at increasing amounts. Data are expressed as fold activation relative to CHOP at 0 ng, which is arbitrarily set as 1. Data from B and C are expressed as mean ± se of n = 4–8 separate experiments. **, P < 0.01; ***, P < 0.001 indicate differences compared with CHOP at 0 ng, whereas nonsignificant (P > 0.05) describes the difference between paired values (wild-type vs. mutant) in response to the same CHOP expression level. D, Progressive 3′-truncations of the V2 exon result in a gradual loss of the dose-dependent response to CHOP stimulation. A series of promoter reporter constructs with sequential 3′-deletions of the V2 exon region were cotransfected into COS-1 cells with increasing amounts of CHOP expression vector. The fold activation is relative to CHOP at 0 ng (set as 1). a, P < 0.05 compared with CHOP at 0 ng; b, P < 0.05 compared with CHOP at 40 ng. Data are presented as mean ± se of n = 3–10 experiments. E, In vivo ChIP assay of the association of CHOP with the human V2. Cross-linked chromatin isolated from HEK293 cells or HEK293 cells transfected with pcDNA3.1-hygro-CHOP (HEK293/CHOP) was immunoprecipitated with an anti-CHOP-specific antibody. Immunoprecipitates were amplified by PCR using primer pairs spanning from bp −94 to +124; this region contains both the proximal promoter region and the V2 exon region, as illustrated on the upper panel. M, 100-bp DNA ladder. PCR assays of a control region 2 kb upstream of V2 were negative (data not shown).
To determine whether CHOP acts directly through this putative response element, a mutant reporter was constructed by replacing the core sequence of the CHOP site with a BamHI restriction site that was previously shown to block CHOP recognition (23). As expected, mutation of this CHOP-C/EBP heterodimer site does not appear to affect V2 constitutional (basal) promoter activity (supplemental Fig. 1), because the endogenous level of CHOP is low in many cell types (24). However, the CHOP site mutant construct also showed a very similar dose-dependent response to CHOP overexpression compared with the wild-type construct (Fig. 3B), implying that CHOP does not act directly via the heterodimer site. This conclusion was supported by our finding that complete deletion of the CHOP site also had little effect on CHOP stimulation of V2 promoter constructs (Fig. 3C).
In contrast, when the reporter construct V2(−211/+362) that responded well to CHOP (Fig. 3, A, B, and D) had its 3′-sequence truncated from +362 to +71, the dose-related stimulation by CHOP was completely abolished (Fig. 3D). To delineate the 3′ critical region essential for the stimulatory effects of CHOP, serial 3′-deletion reporter constructs were cotransfected with CHOP expression plasmid. As shown in Fig. 3D, progressive truncation of the downstream V2 exon region from +362 to +162 resulted in a gradual decrease in the dose-stimulatory effects. And a further deletion to +103 caused a loss of significant CHOP activation. Previous studies of the effects of the 3′ truncations on basal activities of the constructs showed a very different pattern: minimal loss of activity from +362 to +162, an approximately 50% loss of activity with +103 and about 65% loss of activity with +71 (Fig. 3D) (19). Taken together, these data suggest that the approximately 200-bp V2 exon region between +301 and +103 is crucial for CHOP exerting stimulatory effects on V2 promoter activity.
DNA sequence analyses of this region did not reveal any CHOP binding motifs, suggesting that CHOP may function through interacting with other factors. MatInspector detected an Egr-1 site in this region (Fig. 1). Because Zhang et al. (25) had previously shown that Egr-1 can heterodimerize with C/EBPβ, we tested whether mutation of the Egr-1 response element would alter CHOP responsiveness. However, 200 ng/ml of the CHOP expression plasmid had similar effects on the wild-type and mutant Egr-1 V2(−211/+162) constructs (2.4 ± 0.2- vs. 2.2 ± 0.2-fold change; not significant; n = 7). There was also no effect of the Egr-1 mutation on basal activity of the construct (19). Thus, the Egr-1 site does not appear to be directly involved in regulating CHOP effects on the V2 promoter.
We next performed chromatin immunoprecipitation (ChIP) assays to determine whether CHOP can physically associate with the V2 promoter and exon regions. As illustrated in Fig. 3E, using an anti-CHOP antibody, a 220-bp DNA fragment spanning from position −94 to +124 could be specifically immunoprecipitated from HEK293 cells overexpressing CHOP. No recruitment was observed from nontransfected cells or using V2 unrelated control primers for a region about 2 kb upstream of the V2 exon (data not shown). This result suggests that CHOP can associate with this region and that the endogenous level of CHOP in HEK293 cells is low.
Taken together, these experiments indicate that CHOP is able to up-regulate V2 transcription via indirect association with a V2 exon region instead of directly binding to the putative CHOP-C/EBP heterodimer site located at the proximal promoter.
Interactions between CHOP and Ets1
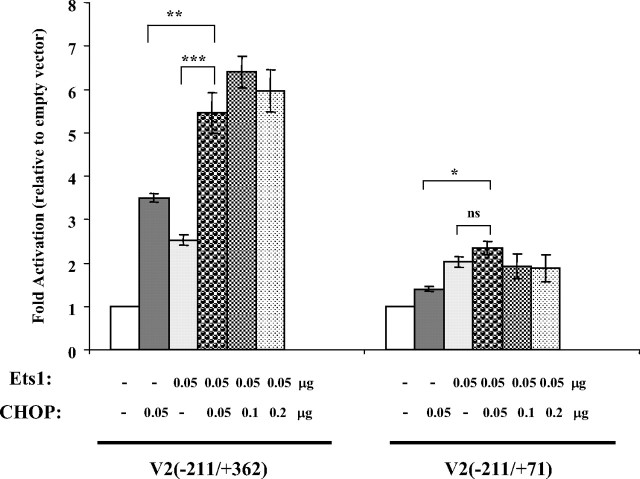
Ets proteins have been shown to interact with several other transcription factors, including members of the basic-leucine zipper family to which CHOP belongs (26). To characterize whether there are functional interactions between Ets1 and CHOP in regulating the V2 promoter, we cotransfected the V2(−211/+362) construct that contains the 5′-Ets sites and the 3′-exon region critical for CHOP with a fixed amount of Ets1 and increasing amounts of CHOP in COS-1 cells (Fig. 4). Overexpression of either Ets1 or CHOP alone enhanced promoter activity about 3-fold. Overexpression of both Ets1 and CHOP at equal amounts gave an approximately 6-fold activation. Further increases in CHOP levels resulted in similar activities. In contrast, the V2(−211/+71) construct, which contains the 5′-Ets sites but lacks the V2 exon region required for the CHOP stimulatory effect, was stimulated only by Ets1. Similarly, the Ets1 mutant construct, V2(−211/+362) Ets Mut2 (Fig. 2C), responded significantly only to CHOP stimulation (data not shown). Collectively, these results indicate that Ets1 and CHOP activate the V2 promoter in independent and additive ways.
Fig. 4.
Interactions between CHOP and Ets1 in regulating V2 promoter activity. COS-1 cells were cotransfected with V2(−211/+362) or V2(−211/+71) and 0.05 μg Ets1 plus increasing amounts (0.05, 0.1, and 0.2 μg) of CHOP and assayed for V2 transcriptional activities. The total amount of plasmid was adjusted to 1 μg/transfection using an empty vector. Data are expressed as fold activation relative to no CHOP or Ets1 overexpression (0 ng CHOP plus 0 ng Ets), which is set as 1. Significant differences between paired values are shown: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P > 0.05. Data are presented as mean ± se from n = 3–5 different experiments.
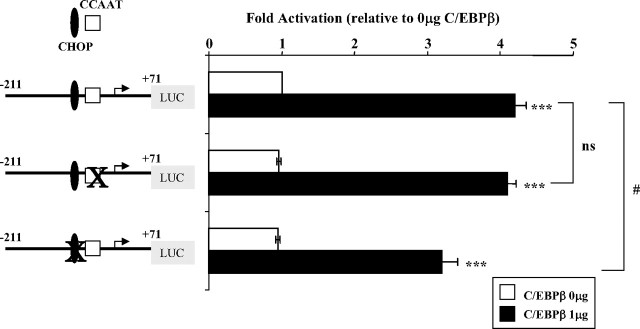
Stimulation of hGHR V2 promoter activity by C/EBPβ
Several studies have reported that C/EBPβ is a critical mediator of GH-regulated gene transcription (27, 28, 29, 30, 31, 32). Although no canonical C/EBP response elements were observed within the V2 proximal promoter region, both the highly conserved CCAAT box (33) and the unique CHOP-C/EBP heterodimer site could be potential binding sites for C/EBPβ homodimers (34, 35).
When the V2 promoter reporter constructs V2(−211/+71) or V2(−211/+362) were transfected together with a rat C/EBPβ expression plasmid, which shares high homology with human C/EBPβ, into either HEK293 or COS-1 cells, a high level of C/EBPβ overexpression (1 μg) significantly stimulated the V2 proximal promoter. The V2(−211/+71) construct showed a greater than 4-fold increase (P < 0.001; Fig. 5), whereas the V2(−211/+362) construct showed a lower, although still highly significant, stimulation (1.5 ± 0.1; P < 0.02; n = 4), likely due to the fact that its basal activity is 3-fold higher than the smaller vector. Mutation of the CCAAT box in V2(−211/+71) did not have an effect on either basal or C/EBPβ-induced activation of V2 promoter activity (Fig. 5). Mutation of the CHOP-C/EBP heterodimer site, however, although not altering basal promoter activity, did result in a moderate (∼25%) but significant (P < 0.05) decrease in response to C/EBPβ stimulation. A double mutant (CCAAT and CHOP sites) resulted in a similar decreased response (36%; P < 0.05; n = 5; no change in basal activity). These data suggest that a high level of C/EBPβ can transactivate the V2 promoter, at least in part by using the CHOP-C/EBP heterodimer site.
Fig. 5.
Functional analysis of transcriptional activation of V2 by C/EBPβ. The V2 promoter construct V2(−211/+71), its CCAAT box mutant, or its CHOP site mutant were transfected into HEK293 cells together with 1 μg C/EBPβ expression plasmid (pSG5-C/EBP LAP) or empty vector and assayed for transcriptional activities in response to C/EBPβ overexpression. Data are expressed as fold activation relative to V2(−211/+71) cotransfected with empty vector which is set as 1. ***, P < 0.001, significant difference observed with C/EBPβ overexpression; ns, P > 0.05 between wild-type and CCAAT box mutant constructs in response to C/EBPβ overexpression; #, statistical difference (P < 0.05) between wild-type and CHOP site mutant reporter constructs in response to C/EBPβ overexpression. Data are presented as mean ± se of n = 3 experiments.
Repression of hGHR V2 promoter activity by Hes1
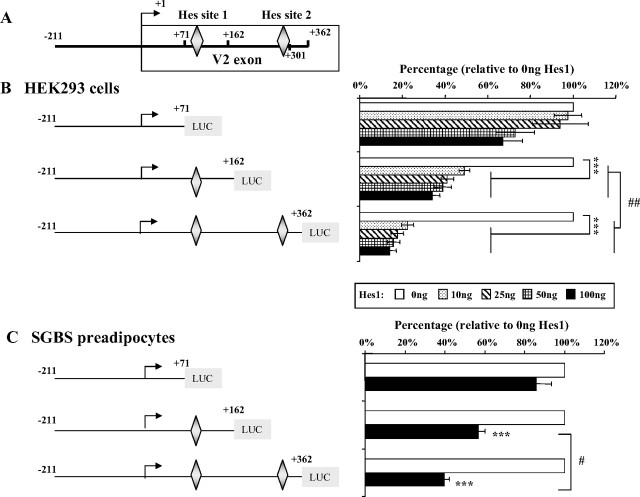
Two putative class C-like Hes1 binding sites were detected within the human V2 exon: the proximal site (no. 1) is at +78/+86, and the distal site (no. 2) is at +296 to +304 (Figs. 1 and 6A) (36, 37, 38). Hes1 was coexpressed with different V2 promoter constructs in HEK293 cells or SGBS preadipocytes. As shown in Fig. 6, B and C, the reporter construct V2(−211/+162), which contains Hes site no. 1, displayed a marked repression (∼50%) after transfection of even a very low amount (10 ng) of Hes1 expression vector. This suppressive effect was almost doubled when both Hes sites 1 and 2 were present [construct V2(−211/+362)]. In contrast, construct V2(−211/+71), which does not contain either Hes site, was minimally affected in response to Hes1 overexpression. Site-directed mutagenesis was used to create a mutant Hes1 site 1 in the V2(−211/+162) vector; transfections in HEK293 cells showed no change in basal activity but a complete loss of Hes1 inhibitory effects at both 10 or 25 ng (data not shown).
Fig. 6.
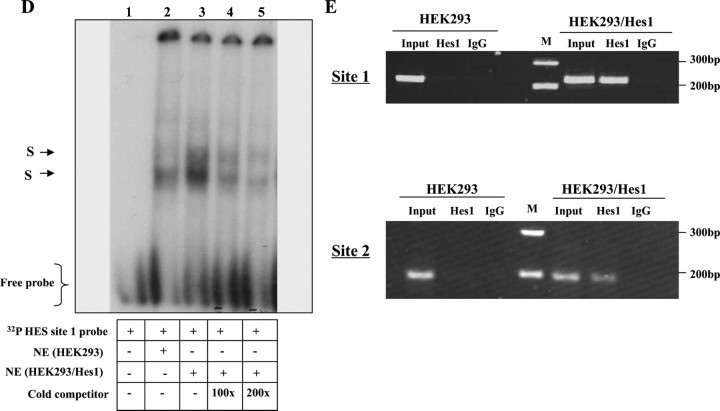
Hes1 expression represses hGHR V2 promoter activity via the HES binding sites. A, Schematic representation of the 362 bp V2 exon region in which two putative HES binding sites were detected. B, Hes1 expression inhibits V2 promoter activity in HEK293 cells. V2 promoter constructs, containing no Hes site (V2(−211/+71)], HES site 1 (V2(−211/+162)] or Hes sites 1 and 2 (V2(−211/+362)], were transfected into HEK293 cells together with increasing amounts of Hes1 expression plasmid. Data are expressed as percentages relative to Hes1 expression at 0 ng, which is set as 100%. ***, P < 0.001 indicates the significant difference between Hes1 overexpression (10, 25, 50, and 100 ng) vs. no Hes1 expression (0 ng); ##, P < 0.01 indicates the significant difference between paired values at the same dose. C, Hes1 suppresses V2 transcriptional activity in SGBS preadipocytes. The V2 promoter constructs V2(−211/+71), V2(−211/+162), and V2(−211 /+362) were transfected into SGBS preadipocytes with 100 ng Hes1 expression vector or 100 ng empty vector (0 ng Hes1). Data are expressed as percentages relative to Hes1 at 0 ng, arbitrarily set as 100%. ***, P < 0.001; #, P < 0.05 indicate the statistically significant differences between paired values. D, In vitro binding of Hes1 protein to Hes site 1 of hGHR V2. The 32P-labeled double-stranded V2 Hes site1-containing oligonucleotide probe (supplemental Table 2) was incubated with nuclear extracts prepared from HEK293 cells (lane 2) or HEK293 cells transfected with Hes1 cDNA (lane 3) for EMSA. For competition experiments, a 100- and 200-fold excess of unlabeled Hes site 1-containing probe was added as a competitor before addition of the labeled probe (lanes 4 and 5). The specific DNA-protein bands are indicated by arrows. E, In vivo binding of Hes1 proteins to the HES sites of hGHR V2. HEK293 cells (HEK293) or HEK293 cells transfected with Hes1 cDNA (HEK293/Hes1) were cross-linked with formaldehyde and immunoprecipitated with anti-Hes1 antibody (Hes1) or control rabbit IgG. DNA was extracted from the immunoprecipitates and PCR amplified by the primer sets surrounding Hes site 1 and Hes site 2. Products were resolved on a 2% agarose gel. PCR assays of a control region 2 kb upstream of V2 were negative (data not shown).
To determine whether Hes1 can bind to the putative Hes sites, we first carried out an EMSA experiment, using a probe containing the Hes site 1. Two specific protein-DNA binding complexes were detected with nuclear extracts of HEK293 cells transfected with Hes1 cDNA (Fig. 6D, lane 3); binding was similar but much weaker with the endogenous nuclear extract (Fig. 6D, lane 2). Both bands could be competitively inhibited by excess cold probe in a dose-related manner (Fig. 6D, lanes 4 and 5). This result indicates that Hes1 can bind to this putative Hes site.
We then performed ChIP assays to determine whether Hes1 interacts with its putative sites in vivo. In HEK293 cells overexpressing Hes1, DNA fragments containing either binding site 1 or 2 could be specifically amplified in the Hes1 antibody-immunoprecipitated complexes, suggesting Hes1 occupancy within these regions (Fig. 6E). No specific associations were observed with a control region about 2 kb upstream or from nontransfected HEK293 cells (data not shown). Collectively, these results indicate that Hes1 can elicit transcriptional repression of the hGHR V2 promoter through interaction with the two Hes sites located within the V2 exon.
Discussion
The hGHR V2 transcript is the major ubiquitously expressed hGHR mRNA in all tissues examined to date (1, 10). However, even though V2 is widely expressed and the V2 promoter is constitutively active in several different cell lines (19), V2 expression is also subject to regulation during development (10) and differentiation (1) and in response to nutritional status (39), suggesting that specific regulatory factors are responsible for mediating these changes. In the present study, we have characterized the regulatory effects of three different types of transcription factors, the helix-turn-helix Ets1, the leucine-zipper C/EBPs (CHOP, C/EBPβ) and the helix-loop-helix Hes1, and their cis-acting elements on the hGHR V2 promoter in different mammalian cells, including human adipocytes.
Ets1 directly transactivates the hGHR V2 promoter
Using cotransfection, site-directed mutagenesis, and EMSA/electrophoretic mobility supershift assays, we have demonstrated that the two highly conserved, overlapping Ets binding sites in the human V2 proximal promoter are functional cis-acting elements for Ets1 to transactivate V2. On the basis of these results, we have concluded that hGHR V2 is a direct target for Ets1.
Ets transcription factors have become increasingly recognized as key regulators of endocrine and other tissues (40). More than 200 genes implicated in a variety of biological pathways regulating cell growth, differentiation, development, hematopoiesis, and angiogenesis have been identified as Ets targets (40, 41). Given the many parallel effects of the hGH/hGHR axis, it is reasonable that the hGHR gene falls into this group.
Ets factors have also been implicated in transforming and tumor-associated events. Many human tumors have been reported to have increased expression of Ets1 (42), and the level of expression is associated with the grade of malignancy and prognosis in several, including breast and colorectal cancers (43). Interestingly, the hGHR gene is also frequently overexpressed in these two types of cancers (44, 45, 46), and its expression level predicts response to radiotherapy treatment (44). Given our present finding that Ets1 can directly transactivate the hGHR V2 promoter, this possible link between the expression of Ets1 and hGHR in cancer-related pathological status should be examined further.
Although Ets1 binding to chromatin in vivo has been shown in some tissues, the mechanisms by which Ets1 interacts with specific promoters to activate transcription are not yet well understood (47). Two distinct mechanisms have been proposed, including 1) via interactions with chromatin-remodeling cofactors (e.g. CBP/p300 or the SRC family) (48, 49, 50) and/or 2) via protein-protein interactions with other transcription factors binding at nearby sites (e.g. USF) (51). These interactions enhance Ets1 DNA binding affinity by relieving its own autoinhibition (51). In the present study, we examined the interaction between Ets1 and CHOP because their putative binding sites were found to be adjacent. We observed additive, but not synergistic, effects when both factors were overexpressed together, suggesting that they act through independent ways on the V2 promoter. This lack of synergy is supported by our later finding that CHOP functions by association with a downstream exon region instead of binding directly to the proximal promoter CHOP site.
In two recent reports, the Ets-binding element has been shown to be one of three sequence motifs frequently found in the proximal promoters of housekeeping genes (52, 53). Considering our recent characterization of the V2 promoter as a housekeeping-like promoter, it is reasonable that the two Ets binding sites are functional as well as being highly conserved across several species. However, we did not observe a significant change in V2 constitutional (basal) promoter activity when the Ets sites were mutated. We assume this is because of low endogenous levels of Ets1 in the cell lines tested (54), because low levels of Ets1 bind weakly to its response element due to autoinhibition (51). It would be of great interest to reinvestigate the role of Ets1 on V2 basal promoter activity using hematopoietic cell lines, where Ets factors, like Ets1, are more abundant (43). These studies might help us to gain insight into V2 transcriptional regulation in the immune system, where hGH is known to be an important modulator.
C/EBP proteins (CHOP and C/EBPβ) transcriptionally activate the V2 promoter via different pathways
CHOP
CHOP, also named as C/EBPζ, is a bZip transcription factor of the C/EBP family (24, 55, 56, 57). Initially identified as a dominant-negative interacting partner with C/EBP proteins at conventional C/EBP-binding sites [(A/G) TTGCG(C/T)AA(C/T)] (57), it was later recognized that CHOP can also transactivate a distinct set of genes by dimerizing with C/EBPα or -β and binding to a unique CHOP-C/EBP heterodimer site [(A/G)(A/G)(A/G)TGCAAT (A/C)CCC] (23, 34).
In the V2 proximal promoter, a putative CHOP-C/EBP binding element was identified that is unique to the hGHR gene. We demonstrated that CHOP overexpression led to a physical association of CHOP with V2 (ChIP) and an up-regulation of V2 transcription (transfection). However, CHOP appears to elicit its effects by indirect interaction with a V2-exon region instead of direct binding to the putative promoter CHOP site. A similar mechanism for CHOP has been previously reported by Ubeda et al. (35), who demonstrated that CHOP tethers to the DNA-bound AP-1 complex by interacting with components of the complex (Jun and Fos) and, in doing so, activates the promoters of somatostatin, JunD, and collagenase genes. They proposed that CHOP acts through this tethering mechanism to mediate endoplasmic reticulum (ER) stress-induced changes in gene expression. In addition to AP-1, CHOP has been shown to dimerize or interact with several different bZIP proteins, including C/EBPα or -β (34), activating transcription factor (ATF) 3 (58), ATF4 (59), and cAMP response element-binding protein-2 (CREB-2) (60). By progressive truncation of the downstream V2 exon region, we were able to narrow down the potential region with which CHOP might associate; the region from +301 to +103 was required for response to CHOP stimulatory effects. However, we did not identify response elements for any of the above-mentioned bZip proteins within this region, suggesting that an as yet unknown interacting partner is involved.
CHOP is expressed at low levels in many cell types but is markedly induced by a variety of stressors, including ER stress and nutrient depletions (24, 55, 56, 61, 62, 63). These stressors can also transcriptionally activate CHOP in a p38-MAPK-dependent manner (64, 65, 66). Flores-Morales et al. (67) showed that cellular stress prolongs the duration of Janus kinase-2 (JAK2)/signal transducer and activator of transcription-5 (STAT5) signaling activated by GH and suggested that this effect was related to inhibition of the GH-induced transcriptional activation of the SOCS genes (68). However, they were unable to correlate the stress-induced effects to GH-dependent expression of SOCS mRNA and protein (67). Our finding that CHOP can up-regulate hGHR V2 transcription suggests an alternate explanation, which is that ER stress induces CHOP expression and enhances its transcriptional ability; activated CHOP can then up-regulate hGHR gene transcription, leading to increased hGHR in the cells, which would amplify the GH signaling.
During glucose deprivation, CHOP expression is increased inversely to the glucose level in the medium and decreased upon addition of d-(+)-glucose (61). Amino acid limitation, due to deficiency of one or more of the essential amino acids or an insufficient intake of protein, can transcriptionally activate CHOP expression (69, 70, 71). Because hGH has such important effects on glucose and protein metabolism, it is logical that hGHR gene expression would be regulated in response to changes in nutrient status. Our studies suggest that CHOP is a likely intracellular mediator of these signals.
Several studies from the Lobie laboratory (65, 66, 72) have demonstrated that hGH, particularly autocrine hGH, working through the hGHR, can up-regulate CHOP expression in mammary carcinoma cells and enhance its transcriptional activity in a p38 MAPK-dependent manner, which results in enhanced protection from apoptosis. Based on our finding that CHOP can up-regulate hGHR transcription, we hypothesize that a positive feedback loop is created; autocrine hGH production enhances CHOP levels and transcriptional activation, including up-regulation of hGHR gene expression, and the increased hGHR levels will produce increased cellular response to autocrine hGH.
Collectively, our data showing that CHOP can up-regulate V2 promoter activity provides a transcriptional link for the regulation of hGHR V2 expression by ER stress, nutrient status, and hGH.
C/EBPβ
A high dose of C/EBPβ stimulated the V2 proximal promoter. It is most likely that, when overexpressed at this level, C/EBPβ acts as a homodimer. Although no consensus C/EBP binding sequences are predicted within the V2 proximal promoter region, our site-directed mutagenesis experiments suggest that C/EBPβ homodimers use, at least in part, the noncanonical CHOP-C/EBP heterodimer site for activation. Indeed, C/EBPβ homodimers have been demonstrated to bind CHOP-C/EBP heterodimer sites but with low affinity (23, 33, 34). Our finding that C/EBPβ transactivates V2 transcription only when expressed at a high level agrees with this scenario.
Several studies have reported that C/EBPβ is a critical mediator of GH-regulated gene transcription (27, 28, 29, 73, 74, 75). GH and other hormones can phosphorylate C/EBPβ through MAPK or phosphatidylinositol 3-kinase signaling pathways and promote its transactivational ability. Our current finding, that C/EBPβ can stimulate V2 promoter activity, provides a mechanism by which hGH can regulate the expression of its own receptor. Except for Adams (76), who also observed that C/EBP members, in particular C/EBPδ, could stimulate the ovine 1B promoter, no other studies of C/EBPs on V2 homologous promoters have been reported. However, because C/EBP proteins regulate a variety of physiological processes including cell proliferation, adipocyte differentiation, energy metabolism, and inflammation (77, 78), it is not surprising that C/EBP members contribute to hGHR V2 transcriptional activation.
Hes1 exerts repressive effects on V2 transcription
Hes1 is a mammalian basic-helix-loop-helix (bHLH) transcription factor that acts as a transcriptional repressor by binding to two specific types of DNA sequences: the N box (CACNAG) or the class C type E box (CACGCG) (36, 38, 79, 80, 81). In the present study, we identified and characterized two class-C-like Hes sites within the V2 exon. Functional analysis and in vitro as well as in vivo binding assays demonstrated that Hes1 overexpression elicits potent transcriptional repression of the hGHR V2 promoter in both HEK293 cells and SGBS preadipocytes by associating with these exon Hes sites. Because both sites are unique to human V2, it provides a mechanism for species-specific regulation of hGHR V2.
Hes1 has been identified as a primary downstream target of Notch signaling, an evolutionarily conserved mechanism controlling cell fate decisions (82); activation of the Notch receptor induces the expression of Hes1 and other Hes family proteins (79, 82, 83, 84, 85). Several recent reports indicate that certain alternative signaling cascades, such as the c-Jun N-terminal kinase pathway (86) or Ras/MAPK signaling (87), can also induce Hes1 expression. Thus, developmental signals and various other stimuli can act through Notch-dependent or -independent pathways to induce Hes1, which subsequently represses target gene transcription. Although Hes1 is essential for several developmental processes, few target genes have been identified, particularly in humans (38). Our present report is the first evidence that hGHR V2 transcription is negatively regulated by Hes1. Regulation by Hes1 may help to explain changes in hGHR expression during development or differentiation, but whether the hGHR gene is a target of the Notch signaling pathway via Hes1 needs to be further investigated.
Impact of different transcription factors on up-regulation of hGHR V2 expression during SGBS adipocyte differentiation
Our recent finding that the V2 promoter exhibits higher activity in mature adipocytes than in preadipocytes suggests that the increase in hGHR V2 expression during SGBS adipocyte differentiation is at least partly due to enhanced transcriptional activity (19). To characterize the specific factors that may be involved in this process, we compared the expression and function of several transcription factors in preadipocytes and during adipocyte differentiation.
Although we found that overexpression of Ets1 or CHOP markedly up-regulates V2 promoter activity, our quantitative RT-PCR data show that Ets1 and CHOP expression levels are highest in confluent SGBS preadipocytes, followed by a marked decrease during the initial stage of differentiation and low levels in later stages (supplemental Fig. 2C). The lack of correlation between Ets1 or CHOP expression and V2 transcription suggests that neither factor is responsible for increasing V2 promoter activity during SGBS adipocyte differentiation. However, we cannot exclude the possibility that either Ets1 or CHOP functions at the preadipocyte stage to prepare the cells for increased hGHR V2 expression and differentiation.
Although our study is the first to examine Ets factors in adipocyte differentiation, it is well accepted that the C/EBP proteins play pivotal roles during adipogenesis. Specific C/EBP family members (CHOP, C/EBPβ, C/EBPδ, and C/EBPα) are expressed in a highly regulated cascade to allow for successful differentiation, including of SGBS cells (22, 77, 88, 89). CHOP is transiently induced in growth-arrested confluent preadipocytes where it sequesters C/EBPβ by heterodimerization and, thus, makes it inactive (90). When differentiation begins, CHOP expression is markedly down-regulated, whereas C/EBPβ expression is markedly enhanced as well as its DNA-binding ability (88, 90, 91). After the early stages of differentiation, C/EBPβ levels drop and c/EBPα expression increases and is maintained at a high level until terminal differentiation (77, 88, 92). Our current finding that high levels of C/EBPβ can up-regulate V2 transcription suggests that the C/EBPs are likely to be major effectors of the differentiation-induced increase in hGHR V2 expression during SGBS adipocyte maturation. In addition, both in vitro and in vivo studies have shown that Hes1 expression is down-regulated during murine adipogenesis (93, 94). We, therefore, propose that loss of Hes1 repression of V2 transcription may also contribute to the increase in V2 expression during adipocyte differentiation.
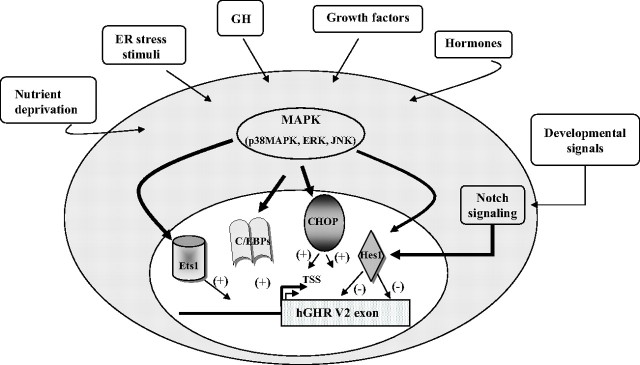
In summary, we have determined that multiple transcription factors act on the hGHR V2 promoter, eliciting positive (Ets1, CHOP, and C/EBPβ) and negative (Hes1) effects. They may serve as downstream nuclear effectors linking specific regulation of hGHR gene expression in response to different signaling cascades triggered by growth factors, development, nutrition, and stress stimuli (Fig. 7).
Fig. 7.
Schematic model of our proposed mechanisms by which distinct transcription factors could be implicated in regulation of hGHR V2 transcription in response to various extra- and intracellular signals. Our data suggest that in different human cells, the transcription factors Ets1, CHOP, C/EBPβ, and Hes1 can serve as downstream nuclear effectors of different signaling cascades to exert positive or negative effects on the hGHR V2 promoter. This allows specific modulation of hGHR gene expression in response to different environmental stimuli, including hormones, growth factors, developmental signals, nutrient status, and stress. (+), Stimulatory effects; (−), suppressive effects.
Materials and Methods
V2 promoter luciferase reporter gene plasmids and expression plasmids
The luciferase fusion plasmids used in the current work were constructed as previously described (19). In our previous (19) as well as the present study, we have chosen to designate the ovine 1B major TSS (T) as +1 for our V2 promoter construct numeration, because it represents the farthest cDNA 5′-end identified among the homologous V2-like transcripts. The cis-elements in the plasmids were mutated using the QuikChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA), as previously described (19) (supplemental Table 1). All mutation constructs were confirmed by DNA sequencing.
The eukaryotic Ets1 expression plasmid pEVRF-Ets1 and its control plasmid pEVRFO were provided by Dr. S. A. Rabbani (McGill University) (95). The human CHOP expression vector pcDNA3.1-hygro-CHOP was obtained from Dr. K. Onazaki (Nagoya City University, Nagoya, Japan) (96), whereas the rat pCMV-C/EBPβ vector was from Dr. E. Holthuizen (University of Urecht, Utrecht, The Netherlands) (97, 98). The Hes1 expression plasmid pCMV2-Hes1 and its control plasmid were provided by Dr. S. Stifani (McGill University) (99).
Cell culture, transient transfections, and luciferase and β-galactosidase assays
HEK293 (human embryonic kidney), COS-1, and CV-1 (African green monkey kidney) cells were obtained from the American Type Culture Collection (Bethesda, MD) and maintained in DMEM supplemented with 10% fetal bovine serum and antibiotics. The human SGBS preadipocytes, derived from the stromal cell fraction of sc adipose tissue from an infant with Simpson-Golabi-Behmel Syndrome (SGBS) (22), were cultured and differentiated into mature adipocytes, as previously described (22). All cells were incubated at 37 C in 5% CO2 in air.
Transient transfections of HEK293, COS-1, and CV-1 cell lines were performed using Polyfect transfection reagent (QIAGEN, Mississauga, Ontario, Canada), according to the manufacturer’s instructions. Briefly, cells were plated in six- or 12-well tissue culture plates and grown in complete medium for 16–20 h to reach 60–80% confluence at the time of transfection. Cells were transfected with a DNA mixture including V2 promoter luciferase reporter constructs (0.5 μg), a β-galactosidase control plasmid (0.04–0.2 μg), and expression vectors (for Ets1, CHOP, C/EBPβ, or Hes1) or their respective empty vectors together with Polyfect reagent at a ratio of 1:3. Transfections were done in triplicate, and the empty pGL3-basic vector was used as a negative control. For the human SGBS preadipocytes, transfection was also carried out using the Polyfect method, except that the amount of reporter construct was increased to 1 μg for maximal luciferase activity.
At 48 h after transfection, cells were harvested in 200 μl 1× passive lysis buffer (Promega, Madison, WI), and the lysate supernatant was quantified for luciferase and β-galactosidase activities (Tropix, ABI, Bedford, MA) using an EG&G Berthold (Oakville, TN) Microlumat Plus bioluminometer (LB 96V). The values of luciferase were normalized to the values of β-galactosidase, an internal control for transfection efficiency, and expressed as fold activation, as specified in the figures and legends.
EMSAs
Nuclear proteins from HEK293 cells or HEK293 cells overexpressing either Ets1 or Hes1 were extracted using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce, Rockford, IL), as described previously (19). Jurkat cell nuclear extract was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Complementary 20- to 35-mer oligonucleotides containing either the Ets binding sequence or the Hes1 site 1 sequence (supplemental Table 1) were synthesized, annealed to form double-stranded DNA probes, end-labeled with γ-32P and T4 polynucleotide kinase, and cleaned up by passing through G-50 spin columns (Amersham, Piscataway, NJ). Labeled probes (50 fmol; ∼50,000 cpm) were incubated with 5–10 μg nuclear extracts at room temperature for 30 min. For competition assays, a 100- to 200-fold molar excess of unlabeled probes was coincubated with the nuclear extracts before the addition of labeled probe. In supershift experiments, 2 μg anti-Ets1 polyclonal antibody (sc-111; Santa Cruz Biotechnology) was added to the reaction mixture and incubated at 4 C for 1 h before the addition of labeled probes. DNA-protein complexes were analyzed by electrophoresis on nondenaturing 5% polyacrylamide gels run at constant 100 V for about 1 h in 0.5× Tris-borate-EDTA (TBE) buffer. The gels were dried and exposed to Kodak Biomax-MR film (Kodak, Rochester, NY).
ChIP assay
To analyze the DNA binding of CHOP or Hes1 protein to the hGHR V2 promoter, ChIP assays were performed based on the Upstate protocol (Upstate Biotechnology, Lake Placid, NY) with certain modifications. Briefly, HEK293 cells were transiently transfected with pcDNA3.1- hygro-CHOP or pCMV3.1-Flag-Hes1. After 48 h, the cells were fixed with 1% formaldehyde at room temperature for 10 min. Next, cells (106) were washed twice with cold PBS, resuspended in 200 μl sodium dodecyl sulfate (SDS) lysis buffer [1% SDS, 10 mm EDTA, 50 mm Tris-HCl (pH 8.1), 1× protease inhibitors cocktail], and incubated on ice for 10 min. The cell lysates were then sonicated on ice six times for 10 sec each (VibraCell Sonicator; Sonics, Betatek Inc., Toronto, Ontario, Canada) to generate genomic fragments from 300–800 bp. After sonication, the suspension was centrifuged at 12,000 × g for 10 min at 4 C. The lysate was diluted 10-fold with ChIP dilution buffer [0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl (pH 8.1), 167 mm NaCl, 1× protease inhibitors]. Equal volumes (1 ml) of diluted samples were subsequently precleared with 45 μl protein A/G-agarose beads (sc-2003; Santa Cruz Biotechnology) for 1 h at 4 C. Ten percent of the cleared supernatant was kept as input, and the remaining volume was immunoprecipitated with 4 μg anti-CHOP/GADD153 (sc-793; Santa Cruz Biotechnology), anti-Hes1 (sc-13844; Santa Cruz Biotechnology), or normal rabbit IgG (sc-2027; Santa Cruz Biotechnology) overnight at 4 C with rotation. Protein A/G-agarose beads (45 μl) supplemented with 2 μg BSA and 4 μg sonicated herring sperm DNA were then added and further incubated for 2 h at 4 C with agitation. The beads were collected and subjected to sequential washes with low-salt buffer, high-salt buffer, LiCl buffer, and Tris-EDTA buffer for 10 min each at 4 C. The immunoprecipitated protein-DNA complex was eluted in elution buffer (1% SDS, 0.1 m NaHCO3). Eluted samples and stored input samples were then reverse cross-linked overnight at 65 C with 200 mm NaCl. DNA was extracted using the QIAquick PCR purification kit (QIAGEN) and analyzed by PCR (supplemental Table 2). PCRs were carried out for a total of 32 cycles and the products were analyzed on 2% agarose gels stained with ethidium bromide.
Real-time quantitative RT-PCR
Quantitative RT-PCR was performed using the Quanti-Tect SYBR Green PCR kit (QIAGEN). Total RNAs were extracted from snap-frozen adipocyte pellets collected at different differentiation time points using the RNeasy Mini kit (QIAGEN), treated with deoxyribonuclease I, and reverse transcribed in a 20-μl reaction using random primers and Superscript II reverse transcriptase (Invitrogen, Burlington, Ontario, Canada). One microliter of each RT reaction was PCR amplified in a 25-μl aliquot containing 2.5 mm MgCl2, 0.4 μm gene-specific primers and 1× SYBR Green master mix. Amplification was performed on an Mx4000 QPCR system (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. Reactions were carried out in duplicate on three independent sets of samples for each differentiation time point. Specificity was assessed by melting-curve analysis and confirmed on 2% agarose gel after each quantitative RT-PCR assay. The abundance of specific mRNAs was determined by comparison with a standard curve generated by serial dilution of the sample and normalized to its corresponding 18S rRNA content. Fold change in expression for a given transcript was calculated relative to the expression of that transcript at d 0 (calibrator). The relative quantities were expressed as fold (hGHR and V2) or percentage (CHOP and Ets1) of the calibrator. The sequences of the primers used are provided in supplemental Table 2.
Statistical analysis
Data are expressed as mean ± se. The significance of observed differences between groups was determined by one-way ANOVA followed by the Tukey multiple comparison test (P < 0.05 significance).
Acknowledgments
We gratefully acknowledge the generous gifts of the Ets1 expression plasmid from Dr. S. A. Rabbani (McGill University), the CHOP expression plasmid from Dr. K. Onazaki (Nagoya City University, Nagoya, Japan), the C/EBPβ expression plasmid from Dr. E. Holthuizen (University of Utrecht, Utrecht, The Netherlands), and the Hes1 expression plasmid from Dr. S. Stifani (McGill University). We also thank Dr. Aimee Ryan for her critical reviews of this paper.
Footnotes
Funding for these studies was from the Canadian Institutes of Health Research (C.G.G.) and studentships from the Fonds de Recherches en Santé du Québec (FRSQ) and the Montreal Children’s Hospital Research Institute (to Y.W.). C.G.G. is a member of the Research Institute of the McGill University Health Centre, which is supported in part by the FRSQ.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 30, 2008
Abbreviations: C/EBP, CCAAT/enhancer-binding protein; ChIP, chromatin immunoprecipitation; CHOP, C/EBP-homologous protein; ER, endoplasmic reticulum; GHR, GH receptor; hGHR, human GHR; NF, nuclear factor; SDS, sodium dodecyl sulfate; TSS, transcription start site; 5′-UTR, 5-′-untranslated region.
References
- 1.Wei Y, Rhani Z, Goodyer CG2006. Characterization of growth hormone receptor messenger ribonucleic acid variants in human adipocytes. J Clin Endocrinol Metab 91:1901–1908 [DOI] [PubMed] [Google Scholar]
- 2.Herrington J, Carter-Su C2001. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab 12:252–257 [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen JO, Moller L, Krag M, Billestrup N, Christiansen JS2007. Effects of growth hormone on glucose and fat metabolism in human subjects. Endocrinol Metab Clin North Am 36:75–87 [DOI] [PubMed] [Google Scholar]
- 4.Conway-Campbell BL, Wooh JW, Brooks AJ, Gordon D, Brown RJ, Lichanska AM, Chin HS, Barton CL, Boyle GM, Parsons PG, Jans DA, Waters MJ2007. Nuclear targeting of the growth hormone receptor results in dysregulation of cell proliferation and tumorigenesis. Proc Natl Acad Sci USA 104:13331–13336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichanska AM, Waters MJ2008. New insights into growth hormone receptor function and clinical implications. Horm Res 69:138–145 [DOI] [PubMed] [Google Scholar]
- 6.Lichanska AM, Waters MJ2008. How growth hormone controls growth, obesity and sexual dimorphism. Trends Genet 24:41–47 [DOI] [PubMed] [Google Scholar]
- 7.Brooks AJ, Wooh JW, Tunny KA, Waters MJ2008. Growth hormone receptor: mechanism of action. Int J Biochem Cell Biol 40:1984–1989 [DOI] [PubMed] [Google Scholar]
- 8.Flores-Morales A, Greenhalgh CJ, Norstedt G, Rico-Bautista E2006. Negative regulation of growth hormone receptor signaling. Mol Endocrinol 20:241–253 [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Lucy MC2001. Variants of the 5′-untranslated region of the bovine growth hormone receptor mRNA: isolation, expression and effects on translational efficiency. Gene 265:45–53 [DOI] [PubMed] [Google Scholar]
- 10.Goodyer CG, Zogopoulos G, Schwartzbauer G, Zheng H, Hendy GN, Menon RK2001. Organization and evolution of the human growth hormone receptor gene 5′-flanking region. Endocrinology 142:1923–1934 [DOI] [PubMed] [Google Scholar]
- 11.Rivers CA, Norman MR2000. The human growth hormone receptor gene: characterization of the liver-specific promoter. Mol Cell Endocrinol 160:51–59 [DOI] [PubMed] [Google Scholar]
- 12.Jiang H, Okamura CS, Boyd CK, Lucy MC2000. Identification of Sp1 as the transcription factor for the alternative promoter P2 of the bovine growth hormone receptor gene. J Mol Endocrinol 24:203–214 [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Okamura CS, Lucy MC1999. Isolation and characterization of a novel promoter for the bovine growth hormone receptor gene. J Biol Chem 274:7893–7900 [DOI] [PubMed] [Google Scholar]
- 14.Adams TE1995. Differential expression of growth hormone receptor messenger RNA from a second promoter. Mol Cell Endocrinol 108:23–33 [DOI] [PubMed] [Google Scholar]
- 15.O'Mahoney JV, Brandon MR, Adams TE1994. Identification of a liver-specific promoter for the ovine growth hormone receptor. Mol Cell Endocrinol 101:129–139 [DOI] [PubMed] [Google Scholar]
- 16.Zou L, Burmeister LA, Sperling MA1997. Isolation of a liver-specific promoter for human growth hormone receptor gene. Endocrinology 138:1771–1774 [DOI] [PubMed] [Google Scholar]
- 17.Zou L, Menon RK1995. A member of the CTF/NF-1 transcription factor family regulates murine growth hormone receptor gene promoter activity. Endocrinology 136:5236–5239 [DOI] [PubMed] [Google Scholar]
- 18.Schwartzbauer G, Menon RK1998. Regulation of growth hormone receptor gene expression. Mol Genet Metab 63:243–253 [DOI] [PubMed] [Google Scholar]
- 19.Wei Y, Puzhko S, Wabitsch M, Goodyer C2009. Structure and activity of the human growth hormone receptor (hGHR) gene V2 promoter. Mol Endocrinol 23:360–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galang CK, Muller WJ, Foos G, Oshima RG, Hauser CA2004. Changes in the expression of many Ets family transcription factors and of potential target genes in normal mammary tissue and tumors. J Biol Chem 279:11281–11292 [DOI] [PubMed] [Google Scholar]
- 21.Nye JA, Petersen JM, Gunther CV, Jonsen MD, Graves BJ1992. Interaction of murine ets-1 with GGA-binding sites establishes the ETS domain as a new DNA-binding motif. Genes Dev 6:975–990 [DOI] [PubMed] [Google Scholar]
- 22.Wabitsch M, Brenner RE, Melzner I, Braun M, Moller P, Heinze E, Debatin KM, Hauner H2001. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord 25:8–15 [DOI] [PubMed] [Google Scholar]
- 23.Sok J, Wang XZ, Batchvarova N, Kuroda M, Harding H, Ron D1999. CHOP-dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol 19:495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oyadomari S, Mori M2004. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389 [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Lin M, Abidi P, Thiel G, Liu J2003. Specific interaction of Egr1 and c/EBPβ leads to the transcriptional activation of the human low density lipoprotein receptor gene. J Biol Chem 278:44246–44254 [DOI] [PubMed] [Google Scholar]
- 26.Li R, Pei H, Watson DK2000. Regulation of Ets function by protein-protein interactions. Oncogene 19:6514–6523 [DOI] [PubMed] [Google Scholar]
- 27.Cesena TI, Cardinaux JR, Kwok R, Schwartz J2007. CCAAT/enhancer-binding protein (C/EBP)β is acetylated at multiple lysines: acetylation of C/EBPβ at lysine 39 modulates its ability to activate transcription. J Biol Chem 282:956–967 [DOI] [PubMed] [Google Scholar]
- 28.Huo JS, McEachin RC, Cui TX, Duggal NK, Hai T, States DJ, Schwartz J2006. Profiles of growth hormone (GH)-regulated genes reveal time-dependent responses and identify a mechanism for regulation of activating transcription factor 3 by GH. J Biol Chem 281:4132–4141 [DOI] [PubMed] [Google Scholar]
- 29.Cui TX, Piwien-Pilipuk G, Huo JS, Kaplani J, Kwok R, Schwartz J2005. Endogenous CCAAT/enhancer binding protein β and p300 are both regulated by growth hormone to mediate transcriptional activation. Mol Endocrinol 19:2175–2186 [DOI] [PubMed] [Google Scholar]
- 30.Schwartz J, Huo JS, Piwien-Pilipuk G2002. Growth hormone regulated gene expression. Minerva Endocrinol 27:231–241 [PubMed] [Google Scholar]
- 31.Liao J, Piwien-Pilipuk G, Ross SE, Hodge CL, Sealy L, MacDougald OA, Schwartz J1999. CCAAT/enhancer-binding protein β (C/EBPβ) and C/EBPδ contribute to growth hormone-regulated transcription of c-fos. J Biol Chem 274:31597–31604 [DOI] [PubMed] [Google Scholar]
- 32.Cesena TI, Cui TX, Piwien-Pilipuk G, Kaplani J, Calinescu AA, Huo JS, Iniguez-Lluhi JA, Kwok R, Schwartz J2007. Multiple mechanisms of growth hormone-regulated gene transcription. Mol Genet Metab 90:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKnight SL2001. McBindall—a better name for CCAAT/enhancer binding proteins? Cell 107:259–261 [DOI] [PubMed] [Google Scholar]
- 34.Ubeda M, Wang XZ, Zinszner H, Wu I, Habener JF, Ron D1996. Stress-induced binding of the transcriptional factor CHOP to a novel DNA control element. Mol Cell Biol 16:1479–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ubeda M, Vallejo M, Habener JF1999. CHOP enhancement of gene transcription by interactions with Jun/Fos AP-1 complex proteins. Mol Cell Biol 19:7589–7599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R2005. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res 306:343–348 [DOI] [PubMed] [Google Scholar]
- 37.Murata K, Hattori M, Hirai N, Shinozuka Y, Hirata H, Kageyama R, Sakai T, Minato N2005. Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1. Mol Cell Biol 25:4262–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan B, Heus J, Lu N, Nichols RC, Raben N, Plotz PH2001. Transcriptional regulation of the human acid α-glucosidase gene. Identification of a repressor element and its transcription factors Hes-1 and YY1. J Biol Chem 276:1789–1793 [DOI] [PubMed] [Google Scholar]
- 39.Hermansson M, Wickelgren RB, Hammarquist F, Bjarneson R, Wennström I, Wernerman J, Carlsson B, Carlsson LMS1997. Measurement of human growth hormone receptor messenger ribonucleic acid by a quantitative polymerase chain reaction-based assay: demonstration of reduced expression after elective surgery. J Clin Endocrinol Metab 82:421–428 [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez-Hartmann A, Duval DL, Bradford AP2007. ETS transcription factors in endocrine systems. Trends Endocrinol Metab 18:150–158 [DOI] [PubMed] [Google Scholar]
- 41.Sementchenko VI, Watson DK2000. Ets target genes: past, present and future. Oncogene 19:6533–6548 [DOI] [PubMed] [Google Scholar]
- 42.Seth A, Watson DK2005. ETS transcription factors and their emerging roles in human cancer. Eur J Cancer 41:2462–2478 [DOI] [PubMed] [Google Scholar]
- 43.Oikawa T2004. ETS transcription factors: possible targets for cancer therapy. Cancer Sci 95:626–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Wan M, Li G, Xu Z, Chen C, Liu F, Li J2006. Growth hormone receptor overexpression predicts response of rectal cancers to pre-operative radiotherapy. Eur J Cancer 42:888–894 [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Liu F, Xu Z, Chen C, Li G, Wu X, Li J2004. Growth hormone receptor expression in human colorectal cancer. Dig Dis Sci 49:1493–1498 [DOI] [PubMed] [Google Scholar]
- 46.Gebre-Medhin M, Kindblom LG, Wennbo H, Tornell J, Meis-Kindblom JM2001. Growth hormone receptor is expressed in human breast cancer. Am J Pathol 158:1217–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu J, Pazin MJ, Ravid K2004. Properties of ets-1 binding to chromatin and its effect on platelet factor 4 gene expression. Mol Cell Biol 24:428–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang C, Shapiro LH, Rivera M, Kumar A, Brindle PK1998. A role for CREB binding protein and p300 transcriptional coactivators in Ets-1 transactivation functions. Mol Cell Biol 18:2218–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jayaraman G, Srinivas R, Duggan C, Ferreira E, Swaminathan S, Somasundaram K, Williams J, Hauser C, Kurkinen M, Dhar R, Weitzman S, Buttice G, Thimmapaya B1999. p300/cAMP-responsive element-binding protein interactions with ets-1 and ets-2 in the transcriptional activation of the human stromelysin promoter. J Biol Chem 274:17342–17352 [DOI] [PubMed] [Google Scholar]
- 50.Petit FG, Salas R, Tsai MJ, Tsai SY2004. The regulation of COUP-TFII gene expression by Ets-1 is enhanced by the steroid receptor co-activators. Mech Ageing Dev 125:719–732 [DOI] [PubMed] [Google Scholar]
- 51.Sun P, Loh HH2001. Transcriptional regulation of mouse δ-opioid receptor gene: role of Ets-1 in the transcriptional activation of mouse δ-opioid receptor gene. J Biol Chem 276:45462–45469 [DOI] [PubMed] [Google Scholar]
- 52.Fitzgerald PC, Shlyakhtenko A, Mir AA, Vinson C2004. Clustering of DNA sequences in human promoters. Genome Res 14:1562–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollenhorst PC, Shah AA, Hopkins C, Graves BJ2007. Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family. Genes Dev 21:1882–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cederberg A, Hulander M, Carlsson P, Enerback S1999. The kidney-expressed winged helix transcription factor FREAC-4 is regulated by Ets-1. A possible role in kidney development. J Biol Chem 274:165–169 [DOI] [PubMed] [Google Scholar]
- 55.Gery S, Park DJ, Vuong PT, Chih DY, Lemp N, Koeffler HP2004. Retinoic acid regulates C/EBP homologous protein expression (CHOP), which negatively regulates myeloid target genes. Blood 104:3911–3917 [DOI] [PubMed] [Google Scholar]
- 56.Ubeda M, Habener JF2003. CHOP transcription factor phosphorylation by casein kinase 2 inhibits transcriptional activation. J Biol Chem 278:40514–40520 [DOI] [PubMed] [Google Scholar]
- 57.Ron D, Habener JF1992. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev 6:439–453 [DOI] [PubMed] [Google Scholar]
- 58.Chen BP, Wolfgang CD, Hai T1996. Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol 16:1157–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H2005. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24:1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gachon F, Gaudray G, Thebault S, Basbous J, Koffi JA, Devaux C, Mesnard J2001. The cAMP response element binding protein-2 (CREB-2) can interact with the C/EBP-homologous protein (CHOP). FEBS Lett 502:57–62 [DOI] [PubMed] [Google Scholar]
- 61.Carlson SG, Fawcett TW, Bartlett JD, Bernier M, Holbrook NJ1993. Regulation of the C/EBP-related gene gadd153 by glucose deprivation. Mol Cell Biol 13:4736–4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bruhat A, Jousse C, Fafournoux P1999. Amino acid limitation regulates gene expression. Proc Nutr Soc 58:625–632 [DOI] [PubMed] [Google Scholar]
- 63.Jousse C, Bruhat A, Fafournoux P1999. Amino acid regulation of gene expression. Curr Opin Clin Nutr Metab Care 2:297–301 [DOI] [PubMed] [Google Scholar]
- 64.Perry JK, Emerald BS, Mertani HC, Lobie PE2006. The oncogenic potential of growth hormone. Growth Horm IGF Res 16:277–289 [DOI] [PubMed] [Google Scholar]
- 65.Mertani HC, Zhu T, Goh EL, Lee KO, Morel G, Lobie PE2001. Autocrine human growth hormone (hGH) regulation of human mammary carcinoma cell gene expression. Identification of CHOP as a mediator of hGH-stimulated human mammary carcinoma cell survival. J Biol Chem 276:21464–21475 [DOI] [PubMed] [Google Scholar]
- 66.Zhu T, Lobie PE2000. Janus kinase 2-dependent activation of p38 mitogen-activated protein kinase by growth hormone. Resultant transcriptional activation of ATF-2 and CHOP, cytoskeletal re-organization and mitogenesis. J Biol Chem 275:2103–2114 [DOI] [PubMed] [Google Scholar]
- 67.Flores-Morales A, Fernandez L, Rico-Bautista E, Umana A, Negrin C, Zhang JG, Norstedt G2001. Endoplasmic reticulum stress prolongs GH-induced Janus kinase (JAK2)/signal transducer and activator of transcription (STAT5) signaling pathway. Mol Endocrinol 15:1471–1483 [DOI] [PubMed] [Google Scholar]
- 68.Fernandez L, Flores-Morales A, Lahuna O, Sliva D, Norstedt G, Haldosen LA, Mode A, Gustafsson JA1998. Desensitization of the growth hormone-induced Janus kinase 2 (Jak 2)/signal transducer and activator of transcription 5 (Stat5)-signaling pathway requires protein synthesis and phospholipase C. Endocrinology 139:1815–1824 [DOI] [PubMed] [Google Scholar]
- 69.Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Fafournoux P2004. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem 279:5288–5297 [DOI] [PubMed] [Google Scholar]
- 70.Bruhat A, Jousse C, Carraro V, Reimold AM, Ferrara M, Fafournoux P2000. Amino acids control mammalian gene transcription: activating transcription factor 2 is essential for the amino acid responsiveness of the CHOP promoter. Mol Cell Biol 20:7192–7204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruhat A, Jousse C, Wang XZ, Ron D, Ferrara M, Fafournoux P1997. Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J Biol Chem 272:17588–17593 [DOI] [PubMed] [Google Scholar]
- 72.Kaulsay KK, Zhu T, Bennett W, Lee KO, Lobie PE2001. The effects of autocrine human growth hormone (hGH) on human mammary carcinoma cell behavior are mediated via the hGH receptor. Endocrinology 142:767–777 [DOI] [PubMed] [Google Scholar]
- 73.Piwien-Pilipuk G, Huo JS, Schwartz J2002. Growth hormone signal transduction. J Pediatr Endocrinol Metab 15:771–786 [DOI] [PubMed] [Google Scholar]
- 74.Piwien-Pilipuk G, MacDougald O, Schwartz J2002. Dual regulation of phosphorylation and dephosphorylation of C/EBPβ modulate its transcriptional activation and DNA binding in response to growth hormone. J Biol Chem 277:44557–44565 [DOI] [PubMed] [Google Scholar]
- 75.Cui TX, Kwok R, Schwartz J2008. Cooperative regulation of endogenous cAMP-response element binding protein and CCAAT/enhancer-binding protein β in GH-stimulated c-fos expression. J Endocrinol 196:89–100 [DOI] [PubMed] [Google Scholar]
- 76.Adams TE1999. Transcription from the P2 promoter of the growth hormone receptor gene involves members of the Sp transcription factor family. Biochem J 344:867–872 [PMC free article] [PubMed] [Google Scholar]
- 77.Lekstrom-Himes J, Xanthopoulos KG1998. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem 273:28545–28548 [DOI] [PubMed] [Google Scholar]
- 78.Ramji DP, Foka P2002. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365:561–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iso T, Kedes L, Hamamori Y2003. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194:237–255 [DOI] [PubMed] [Google Scholar]
- 80.Yan B, Raben N, Plotz PH2002. Hes-1, a known transcriptional repressor, acts as a transcriptional activator for the human acid α-glucosidase gene in human fibroblast cells. Biochem Biophys Res Commun 291:582–587 [DOI] [PubMed] [Google Scholar]
- 81.Fischer A, Gessler M2007. Delta-Notch—and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res 35:4583–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A1995. Signalling downstream of activated mammalian Notch. Nature 377:355–358 [DOI] [PubMed] [Google Scholar]
- 83.Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R1999. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J 18:2196–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kageyama R, Ohtsuka T, Tomita K2000. The bHLH gene Hes1 regulates differentiation of multiple cell types. Mol Cells 10:1–7 [DOI] [PubMed] [Google Scholar]
- 85.Jarriault S, Le BO, Hirsinger E, Pourquie O, Logeat F, Strong CF, Brou C, Seidah NG, Isra l A1998. Delta-1 activation of notch-1 signaling results in HES-1 transactivation. Mol Cell Biol 18:7423–7431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Curry CL, Reed LL, Nickoloff BJ, Miele L, Foreman KE2006. Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest 86:842–852 [DOI] [PubMed] [Google Scholar]
- 87.Stockhausen MT, Sjolund J, Axelson H2005. Regulation of the Notch target gene Hes-1 by TGFα induced Ras/MAPK signaling in human neuroblastoma cells. Exp Cell Res 310:218–228 [DOI] [PubMed] [Google Scholar]
- 88.Otto TC, Lane MD2005. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol 40:229–242 [DOI] [PubMed] [Google Scholar]
- 89.Farmer SR2006. Transcriptional control of adipocyte formation. Cell Metab 4:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang QQ, Lane MD2000. Role of C/EBP homologous protein (CHOP-10) in the programmed activation of CCAAT/enhancer-binding protein-β during adipogenesis. Proc Natl Acad Sci USA 97:12446–12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang QQ, Otto TC, Lane MD2003. CCAAT/enhancer-binding protein β is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci USA 100:850–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD2005. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3β is required for adipogenesis. Proc Natl Acad Sci USA 102:9766–9771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soukas A, Socci ND, Saatkamp BD, Novelli S, Friedman JM2001. Distinct transcriptional profiles of adipogenesis in vivo and in vitro. J Biol Chem 276:34167–34174 [DOI] [PubMed] [Google Scholar]
- 94.Ross DA, Rao PK, Kadesch T2004. Dual roles for the Notch target gene Hes-1 in the differentiation of 3T3-L1 preadipocytes. Mol Cell Biol 24:3505–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo Y, Pakneshan P, Gladu J, Slack A, Szyf M, Rabbani SA2002. Regulation of DNA methylation in human breast cancer. Effect on the urokinase-type plasminogen activator gene production and tumor invasion. J Biol Chem 277:41571–41579 [DOI] [PubMed] [Google Scholar]
- 96.Hattori T, Ohoka N, Hayashi H, Onozaki K2003. C/EBP homologous protein (CHOP) up-regulates IL-6 transcription by trapping negative regulating NF-IL6 isoform. FEBS Lett 541:33–39 [DOI] [PubMed] [Google Scholar]
- 97.Holthuizen P, Van Dijk MA, Rodenburg RJ, Koonen-Reemst AM, Sussenbach JS1993. Transcriptional regulation of the major promoters of the human IGF-II gene. Mol Reprod Dev 35:391–393 [DOI] [PubMed] [Google Scholar]
- 98.Van Dijk MA, Rodenburg RJ, Holthuizen P, Sussenbach JS1992. The liver-specific promoter of the human insulin-like growth factor II gene is activated by CCAAT/enhancer binding protein (C/EBP). Nucleic Acids Res 20:3099–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McLarren KW, Lo R, Grbavec D, Thirunavukkarasu K, Karsenty G, Stifani S2000. The mammalian basic helix loop helix protein HES-1 binds to and modulates the transactivating function of the runt-related factor Cbfa1. J Biol Chem 275:530–538 [DOI] [PubMed] [Google Scholar]