Abstract
Only 50 years ago obstetric care providers and women had many concerns regarding whether exercise during pregnancy created a harmful competition for substrate resources between the fetus and the mother. Animal and human research in the past 50 years, which includes acute and chronic aerobic exercise during pregnancy, has a reassuring margin of safety throughout gestation in women. Maternal physiology adapts to pregnancy changes involving the cardiorespiratory and glucometabolic alterations. Due to these changes, pregnant women have slight differences in response to acute exercise sessions. Chronic exposure to aerobic exercise before and during pregnancy is associated with numerous maternal and neonatal adaptations which may have short- and long-term benefits to maternal and child health. On the basis of the consistent evidence of safety of exercise during pregnancy, multiple nations and health care organizations, including the American College of Obstetrics and Gynecology, recommend moderate exercise for 20 to 30 minutes most days of the week. Despite the 15 to 20 years since the first recommendations were made, only 10% to 15% of pregnant women meet this recommendation. It seems there may be 2 foci for failure to achieve these exercise recommendations: patient specific and culturally driven and/or obstetric provider not recommending regular exercise due to lack of knowledge or motivation. This article addresses the provider knowledge by a review of the normal (at rest) physiologic adaptation to pregnancy. Then, we provide a detailed description of the type and intensity of controlled experiments that document the safety of exercise during pregnancy. The short- and long-term benefits are reviewed, including the safety in moderate-risk women.
Keywords: Pregnancy, maternal, fetal, physical activity/exercise, health outcomes
Introduction
The ability of pregnant women to tolerate physical exertion with minimal impact on maternal and fetal health has been the focus of a plethora of studies for at least a half century. The reason for the studies was a perceived risk to the vulnerable fetus by exhaustive exercise through competition for oxygen and substrates. Basic maternal physiologic adaptations required to have a healthy fetus and newborn have remained the same; the growing fetus always needs oxygen, sufficient substrates for rapid development and growth, euthermic environment, and efficient removal of by-products, ie, CO2 and urea. During pregnancy, physical exertion creates a natural challenge to the maternal capacity to meet the fetal needs by increased oxygen, greater substrate utilization, heat generation, and excess by-product production. Despite the perceived challenges of exercise on fetal substrate delivery, studies have shown exercise to be safe for the developing fetus.
Over the past 15 to 20 years, numerous world health institutions, including the American College of Obstetricians and Gynecologists,1 recommend moderate-intensity exercise for 20 to 30 minutes most days of the week (see section “International Recommendations for Exercise in Pregnancy”) for healthy women. In addition, there is a growing body of literature that indicates moderate intensity, duration, and frequency of physical exertion strongly support optimal fetal growth and development in the current generation and future generations, ie, an epigenetic impact.
Unfortunately, the recommendation for regular moderate exercise has been poorly applied among pregnant women. Between 1996 and 2006, only 7.8% to 11% of Scandinavian pregnant women met the international goals for exercise.2,3 Between 1994 and 2000, only 10% of pregnant women in the United States met the recommendation for regular, moderate-intensity exercise for at least 5 days a week.4 It seems the reasons for the failure to meet minimum exercise recommendations during pregnancy are 2-fold; one reason is patient specific and culturally driven. The second is the lack of obstetric provider knowledge, which prevents them from being able to educate pregnant patients. This article will address the second issue by reviewing the sentinel studies of maternal physiologic adaptations to moderate and strenuous exercise during pregnancy. This review is not exhaustive, but it will provide a more realistic obstetric provider understanding of the safety of acute exercise on normal pregnant women.
Subsequently, we will address the health of the modern pregnant women who are often overweight and who consume a “high-fructose” diet and reflect the health uncertainties in the international recommendations for exercise in pregnancy. Finally, we will briefly review how moderate-intensity exercise can modify the development of excessive gestational weight gain, gestational diabetes, and preeclampsia and reduce cesarean sections. The discussion will end with an incomplete review of the epigenetic effects of diet and exercise during pregnancy on adult health and future generations.
Framework of Studies in Exercise Physiology in Pregnancy
A review of human pregnancy physiology must be placed in a cultural and historical framework. The pregnant woman and her fetus are considered a vulnerable dyad where great caution and judgment are needed in the approval and application of experimental protocols. Today, inclusion of pregnant women in research requires prior animal studies demonstrating minimal risk, and human studies must have no more than everyday life (minimal risk) and informed consent.5 As a result, human studies are limited to relatively few numbers of highly selected volunteers with mostly noninvasive techniques for measurements.
Initial animal studies, mainly ovine or rodent, in exercise physiology allowed for more invasive and precise measurements of exercise tolerance for future study of human volunteers. If moderate exercise throughout pregnancy in the ovine model showed more than minimal maternal or fetal risk, then human research on the acute risk of moderate exercise on humans would have been unethical. Fortunately, animal studies showed little adverse effects. However, there is greater concern for applicability to human physiology and specifically the fetoplacental structure and function. One example might be the animal’s stress, steroid, and catecholamine response to experimentation. This perceived stress has led to an appropriate concern for animal welfare and applicability to human exercise. In addition, the prohibitive cost of animal experimentation has limited focused study of pregnant animals.
In the past 50 years, the tremendous growth of interest and research monies in exercise physiology has been fostered by the aerospace and professional sports industries. The interest in sports physiology and women in space has led to improved analysis and safer, more compact technology in indirect physiologic measurement. These advances have greatly benefited the study of maternal adaptation to exercise in pregnancy. These sources of interest and funding may have led to selection biases in human pregnancy exercise studies—normal-weight (ie, body mass index [BMI]), healthy, and active or athletic volunteers.
Maternal and Fetal Physiology
Maternal physiologic adaptation to pregnancy (at rest) varies by gestational age, reflects the growth rate of the fetus, and changes with the gestational weight gain. The growth rate relative to somatic (body) weight is higher in fetal development than any other time in human life. The fetal weight change during gestation is 5 g/d at 15 weeks, 10 g/d at 20 weeks, 25 g/d at 30 weeks, 35 g/d at 35 weeks, and 15 g/d at 40 weeks. The greatest velocity of linear growth occurs at 22 to 25 weeks, whereas the greatest velocity of overall weight (somatic growth) occurs at 32 to 35 weeks. These weight and growth patterns indicate that the greatest need for maternal adaption to exercise is after 20 weeks of gestation. The oxygen and/or substrate challenges by exercise in the late third trimester might manifest a decrease in the weight-to-length ratio, measured by head circumference/abdominal circumference at greater than the 95th percentile for gestational age, and asymmetric fetal growth restriction. At birth, a neonatal ponderal index = (weight [g])/(length [cm3]) × 100 measures similar fetal challenges to oxygen and substrate delivery. A ponderal index less than 2.0 is considered asymmetric small for gestational age (SGA) and usually reflects the failure to store adequate substrates in the liver and fat. Stress, which might include sustained anaerobic exercise, after 28 weeks results in an abnormally high head circumference/abdominal circumference and a ponderal index less than 2.0 at birth. Stress early in pregnancy, such as genetic or environmental (ie, aneuploidy, smoking, alcohol exposure), may manifest as a neonate born at less than 10% of the birth weight, but with a normal ponderal index ⩾2.0.
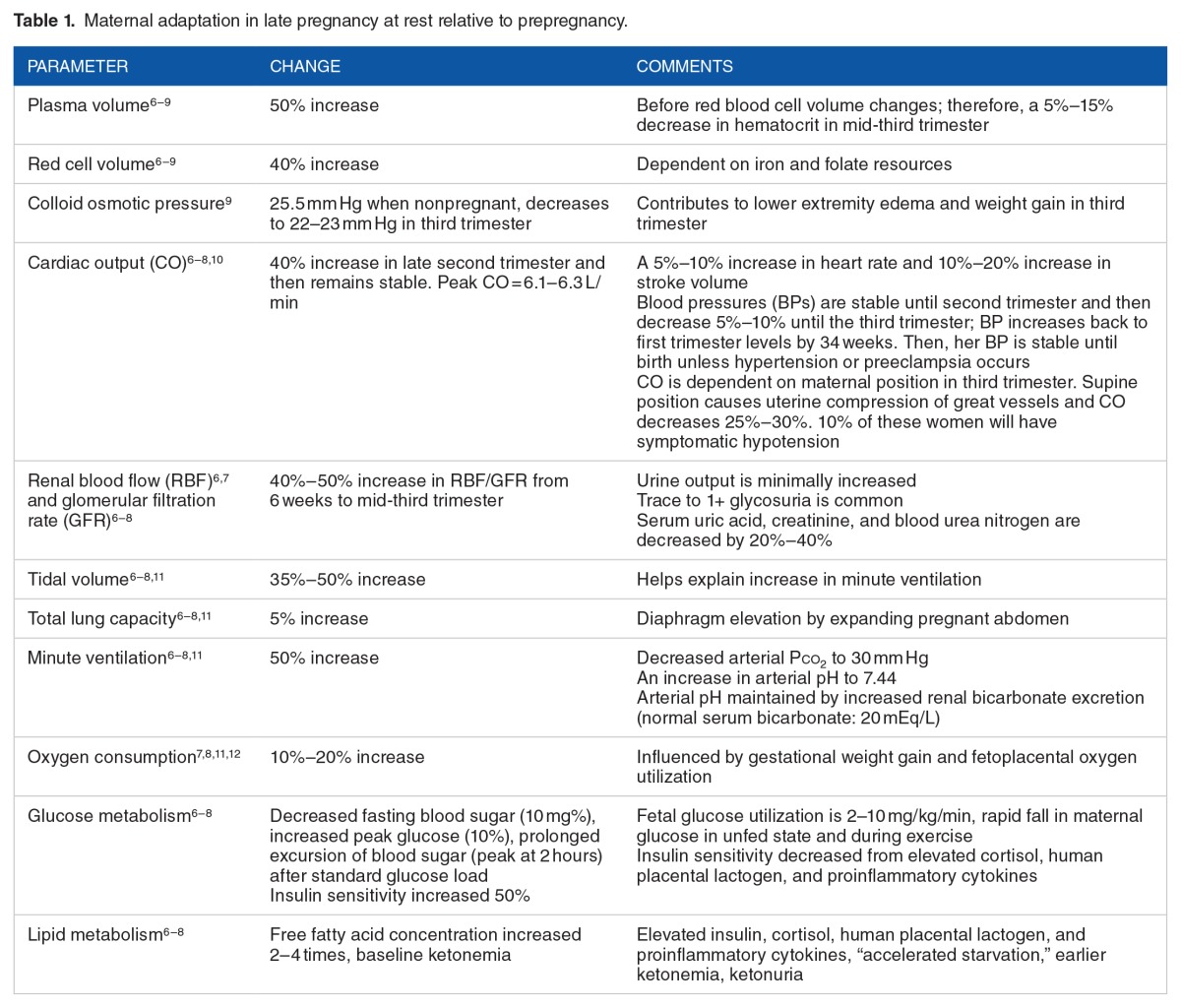
The changes in maternal weight reflect the rate of fetal somatic growth, a curvilinear pattern. In pregnant women with a normal prepregnancy BMI of 20 to 26, about one-third of weight (4.5 kg) is gained in the first 20 weeks of gestation and two-thirds (9 kg) of weight is gained in the last 20 weeks. Many of the maternal adaptations follow this pattern of change. Table 1 describes selected, resting maternal adaptations at about 32 to 36 weeks compared with prepregnancy levels. The selection of maternal adaptations reflects those pertinent to maternal response to exercise challenge. In broad terms, these include oxygen delivery, glucose and substrate delivery, heat dispersal, and metabolic by-product disposal.
Table 1.
Maternal adaptation in late pregnancy at rest relative to prepregnancy.
The dynamics of uterine blood flow determines the amount of oxygen (assuming normal O2-carrying capacity) and substrates delivered to the uteroplacental unit.6–8,10,12–14 The uterine blood flow increases 10-fold from nonpregnancy to peak resting cardiac output in pregnancy—a shift from 2% to 17% (500–800 mL/min at term) of the total cardiac output. Approximately 80% of uterine blood flow is directed to the maternal placental interface to supply the intervillous spaces. The major factors determining oxygen delivery to the fetoplacental unit include cardiac output, maternal hemoglobin (O2-carrying capacity), maternal PO2/PCO2, and maternal arterial/venous pH differences. When moderate maternal stress from exercise causes blood flow redistribution away from the gut, the blood flow to the myometrium is reduced, but maternal placental perfusion is maintained until maternal cardiac output is decreased with exhaustion. Maternal hemoconcentration during moderate exercise results in a small loss of maternal plasma (250–450 cm3) and a 5% to 15% drop in total protein, albumin, and colloid osmotic pressure within 5 minutes of the onset of cycle exercise at 75 W.15 However, the maternal hemoglobin concentration increases by 0.4 to 0.7 g/dL or a 0.7–mL O2-carrying capacity of maternal blood, thus leading to more O2 delivered to the fetoplacental unit.
Fetal physiology is also critical in the evaluation of the influence of maternal exercise on pregnancy outcome.8,12,13,16 First and foremost, the fetus survives and grows in a hypoxic environment relative to his or her mother—Po2 of 20 to 32 mm Hg with an O2 saturation of 55% to 82% (arterial versus venous). Based on an ovine model, the human fetus has an oxygen consumption rate of 300 μmol/min/kg (twice the adult values), which must be met by adequate umbilical blood flow. Appropriate fetal tissue oxygenation is met by high fetal cardiac output of 460 mL/min/kg (more than twice the adult levels) and an umbilical flow rate of 110 mL/min/kg. The major portion of the increased cardiac output results from an increased fetal heart rate of 140 beats per minute (bpm), rather than increased stroke volume. Fetal blood has a higher oxygen-carrying capacity than adult blood, about 6.3 mM. The higher oxygen capacity of 6.7 to 8.1 mM results from a greater affinity of fetal hemoglobin to oxygen and higher concentrations of red cells in fetal blood (14.5–15.5 g/L). Certain conditions, such as carboxyhemoglobinemia (ie, smoking) or hemoglobinopathies (ie, sickle cell disease), alter the release of oxygen to the fetus within the intervillous space. Maternal anemia reduces oxygen-carrying capacity, subsequently reducing oxygen delivery to the fetoplacental unit.
During the physiologic stress of exercise, in the ovine model, the placental-fetal blood flow appears to be redistributed preferentially to the fetus rather than the placental mass.13,16 Due to the invasive nature of these techniques in animal models, the local control of fetoplacental blood flow and distribution has not been demonstrated in pregnant women. However, in pregnant women, 30 to 31 weeks of gestation, undergoing treadmill exercise to volitional fatigue, the predicted peak Vo2 is 21 mL/kg/min in nonexercisers, 24 mL/kg/min in regular exercisers, and 28 mL/kg/min in highly active women.14 Indirect measurement of placental blood flow using umbilical Doppler interrogation demonstrates a slight decrease in placental blood resistance (umbilical artery systolic to diastolic ratio—S/D ratio) and an increased fetal heart rate (+10 bpm) in nonexercisers and regular exercisers.14 Interestingly, highly active exercisers had fetal bradycardias and no changes in placental resistance. The active exercisers spent almost twice as long on the treadmill (22 minutes) relative to nonexercisers (12 minutes). The maximum maternal heart rates (163–172 bpm) were 87% to 92% of predicted maximum heart rate across the 3 exercise intensities.14
The influences of placental performance in exercise are important but are poorly understood. The framework of understanding the placenta and fetal growth (in ovine model) is elegantly reviewed by Clapp.16 The trophoblast serves as a sensor of oxygen and substrate delivery, which causes a release of vasoactive substances, such as vascular endothelial growth factors, decidual renin, and cytokine growth hormones (ie, insulinlike growth factors). In addition, compounds (ie, tumor necrosis factor-α [TNF-α], human placental lactogen, and cortisol) are released into the maternal system, which further modulate substrate delivery and ultimately fetoplacental growth. Chronic physiologic effects of moderate to vigorous exercise during pregnancy are increased maternal plasma volume, blood volume, cardiac output, vascular compliance, and placental volume.16
Sentinel Human Studies in Exercise During Pregnancy
One of the sentinel studies by Clapp et al17 illustrates human placental adaptation to moderate exercise as measured by fetoplacental growth. Nonexercising women with confirmed normal gestation underwent a physical fitness assessment (ie, treadmill evaluation of maximum aerobic capacity). Women were randomly assigned to a control (nonexercise) group or weight-bearing exercise program during pregnancy. The intervention was a weight-bearing exercise for 20 minutes 3 to 5 times a week at 55% to 65% intensity of their preconception aerobic capacity from 8 to 9 weeks until delivery. All measurements of interval exercise, diet, morphometric change, and oxygen consumption were performed every 2 weeks. Placental volume was measured by standard ultrasound techniques at 16, 20, and 24 weeks of gestation. After delivery, the newborn was measured extensively, including fat mass. The placenta underwent a standardized gross and histologic examination. In all, 46 (24 control/22 exercise) women completed the protocol. The women were matched in age (31 years), education (16+ years), prepregnancy weight (62 kg), gestational weight gain (16 kg), average daily caloric intake (44 kcal/kg lean body mass), and gestational age at delivery (278 days). The findings included the following:
Exercise increased fetoplacental growth rate and ultimately size at birth.
Increased placental growth was documented as early as 20 weeks and persisted through the pregnancy.
The exercise group had placentas with significantly greater functional volume, nonfunctional volume, villous volume, and terminal villi.
The increased fetal growth was symmetric with the intervention group having larger newborns with greater lean body mass than the control group.
Another research focus is the changes in maternal physiology with acute exercise sessions. Most of the early studies of the effects of acute exercise in pregnancy were conducted in ovine and rat models.16,18 These studies provided the framework for understanding the effects of acute and chronic exercise in the human model. Many of the findings have translated to the human model well when the exercise has been moderate to strenuous, 50% to 75% maximum Vo2. Exercise at 95% to 100% of maximum Vo2 to the point of exhaustion creates an elevated maternal sympathetic response, reduces maternal placental blood flow, elevates maternal lactic acid, reduces glucose delivery, and lowers maternal pH. Uterine contractility increases and fetal oxygenation is reduced. Repetitive episodes of maximal exercise in the ewe significantly reduced fetal growth.
For ethical reasons, exercising pregnant humans have not been studied with the same levels of invasive monitoring. For more than 50 years, pregnant women have volunteered in numerous studies that have provided essential physiologic data related to exercise exposure. Data have been collected throughout gestation up to 12 weeks postpartum from pregnant women exposed to stationary exercise modalities (ie, stationary bicycle, stair step, and treadmill) and at progressive increments of workloads to 55% to 90% maximum of their individualized maximum Vo2. The next series of paragraphs illustrate various experimental conditions.
A well-designed study by Mottola et al19 used the latest technology to measure blood flow, oxygen delivery, and substrate delivery/utilization in pregnant and similar nonpregnant women. In all, 24 of 39 (62%) pregnant and 16 of 29 (55%) nonpregnant women completed the study. The average pregnant woman was 33 ± 2 (SE) years old, weighed 67 ± 2.3 kg (SE) pregnancy weight at 16 to 20 weeks, and were 168 ± 3.4 cm (SE) tall. All participants underwent a physical activity readiness survey, and all women had a similar level of physical activity at baseline. Nonpregnant women were tested during the luteal phase of their menstrual cycle. Women on oral contraceptives were excluded. All women underwent a peak exercise treadmill test to determine the participant’s 95% ventilatory threshold (VT), aka anaerobic threshold.20
Nonpregnant and pregnant women at 16 to 20 weeks of gestation performed similarly in work rate (W ± SD), 192 ± 4.4 versus 205.4 ± 5.9, respectively; peak heart rate (BPM ± SD), 146 ± 3.1 versus 149.4 ± 2.5, respectively; and minute ventilation (VE [L/min] ± SD), 43.5 ± 2.1 versus 45.3 ± 3.6, respectively. Pregnant women had significantly lower oxygen consumption (Vo2 [mL/kg/min] ± SD) than nonpregnant women, 21.6 ± 0.8 versus 24.3 ± 0.9, respectively. In addition, pregnant women had significantly lower carbon dioxide output (Vco2[mL/kg/min] ± SD) than nonpregnant women, 22.7 ± 0.8 versus 35.1 ± 0.8, respectively.19
Nonpregnant and pregnant women continued to exercise 3 to 4 times a week (confirmed by exercise logs and heart rate monitor) at their individualized target heart rate intensity (95% of VT20 from their peak test). At 35 to 38 weeks, pregnant women completed a steady-state treadmill test at 95% of their VT for 40 minutes. The cardiopulmonary responses in well-trained 35- to 38-week pregnant women were similar to well-trained nonpregnant women at baseline; they had a maternal heart rate of 73 bpm, oxygen consumption (Vo2) of 4.4 mL/kg/min, and carbon dioxide output (Vco2) of 4.1 mL/kg/min. After 40 minutes, at 95% of their individual VT, pregnant women versus nonpregnant women had significantly lower maternal heart rate of 147 versus 156 bpm, respectively, oxygen consumption of 18.4 versus 23.4 mL/kg/min, respectively, and carbon dioxide output of 18.8 versus 23.3 mL/kg/min, respectively. At the end of 15 minutes of active recovery (slow, level walk), pregnant women versus nonpregnant women had significantly higher maternal heart rate of 118 versus 103 bpm, respectively. Oxygen consumption of about 5.2 mL/kg/min and carbon dioxide output of about 5.1 mL/kg/min were similar in pregnant and nonpregnant women.19
Glucose, insulin, lipids, and cholesterol, ie, substrate compounds, were measured at baseline, 20, 30, and 40 minutes during the steady-state treadmill test. In addition, at the end of the 15-minute recovery period, subjects ingested a 75-g glucose load and substrate compounds were taken every 30 minutes for 2 hours after the glucose challenge. Glucose, insulin, lipid, and cholesterol patterns after the glucose test have a pattern normal for pregnant women; glucose and insulin rise was higher and for longer duration in pregnant versus nonpregnant women. Lipids and cholesterol were higher at baseline and from recovery through 120 minutes after glucose ingestion in pregnant versus nonpregnant women. In both pregnant and nonpregnant women, the lipids and cholesterol peaked at the same levels in the immediate recovery phase. All births and neonatal outcomes were normal at a mean of 39.6 weeks. Birth weights were within normal limits for a Canadian population.
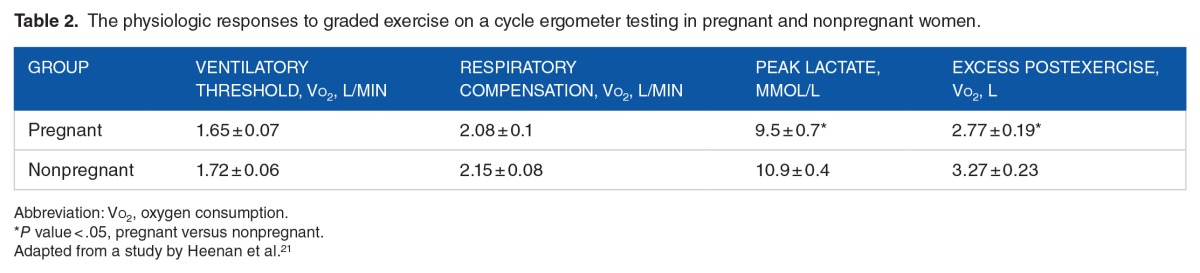
Although the latter study examined vigorous steady-state weight-bearing exercise at 35 to 38 weeks, another study focused on the response of pregnant women to non–weight bearing cycle exercise. Heenan et al21 looked at 14 healthy, nonsmoking pregnant women (34.7 ± 0.4 weeks) whose age (30.8 years), height (162.5 cm), and parity were matched with 14 nonpregnant women (controls) with similar physical and demographic characteristics. Pregnant women had a higher BMI at gestational age of testing than nonpregnant controls (27.7 ± 2.0 versus 23.9 ± 0.6, respectively). All women were involved in regular moderate physical activity 3 to 6 sessions per week. The exercise intervention was a progressive maximal exercise test on a constant work rate cycle ergometer. The protocol involved 5 minutes of rest, 4 minutes of warm-up at 20 W, followed by a progressive increase in work rate of 20 W/minute until volitional fatigue. Data were collected during the 15-minute postexercise recovery period. Respiratory responses were measured on a breath-to-breath basis during the exercise intervention, and blood samples were drawn at baseline, 1, and 3 minutes during exercise, as well as 3 minutes after exercise.
Ventilatory responses during exercise in pregnant and nonpregnant women were similar; however, pregnant women had higher minute ventilation at rest than nonpregnant women at rest (10.9 ± 0.4 versus 9.2 ± 0.4 L/min, respectively). At maximal exercise, the Vo2 and heart rates were similar in pregnant and nonpregnant women (2.25 ± 0.1 L/min versus 2.28 ± 0.08 L/min and 178 ± 2 bpm versus 179 ± 2 bpm, respectively). Table 2 depicts the physiologic responses to graded exercise testing in pregnant and nonpregnant women. The VT (anaerobic threshold),20 an index of the onset of blood lactate accumulation, reflects the work intensity an individual can sustain without progressive accumulation of lactic acid and muscular fatigue. Ventilatory compensation is the increase in minute ventilation disproportionate to the increase in Vco2. Excess postexercise oxygen consumption is the excess oxygen consumption above baseline required to restore baseline energy and substrate needs in the recovery period.
Table 2.
The physiologic responses to graded exercise on a cycle ergometer testing in pregnant and nonpregnant women.
Does exercise change uterine activity? Spinnewijn et al22 examined this question in 30 healthy women at gestational ages of 38 to 42 weeks during an uncomplicated pregnancy. The mean age was 31 (23–37) years. All women were admitted for elective induction of labor with an inducible cervix and all fetuses were in the vertex position. Fetal heart rates from a scalp electrode were scored in a standard fashion—Fischer Score23 by 3 independent observers and an intrauterine pressure catheter measured intrauterine pressure. After 20 minutes of baseline measurements, women began cycling with an initial workload of 50 W. The workload was increased 10 W every 30 seconds until a maternal heart rate of 140 bpm. This workload and heart rate were continued for 20 minutes. Measurements were continued during the 20-minute recovery period in semirecumbent position. Of the 30 subjects, 26 completed the protocol. The peak maternal heart rate was 140 (130–152) bpm. The strenuous exercise stimulated significantly more contractions (median [range]) at 20 minutes during the exercise period (median: 11; range: 6–18) than at baseline (median: 2; range: 0–7) and during recovery in semirecumbent position (median: 3; range: 0–10). The contractions had more intensity with a higher time pressure integral during exercise (median: 94; range: 46–343 mm Hg/20 minutes) and recovery (median: 38; range: 0–188 mm Hg/20 minutes) than at baseline (median: 25; range: 0–136 mm Hg/20 minutes). Overall, there were no significant changes in the Fischer Score (fetal heart rate pattern scoring) between baseline, exercise, and recovery. There was little change in fetal sleep cycles during exercise. All babies were delivered vaginally with normal Apgar scores at 1 and 5 minutes and had a median birth weight of 3620 g (2690–4800 g).
The increased uterine contractility during vigorous exercise when the cervix and uterus have prepared for labor, “cervix was inducible,” provides caution for women who have cervical maturity, ie, cervical dilatation greater than 1 cm and effacement greater than 50%, especially prior to 37 weeks of gestation. The reader is cautioned that the experimental protocol does not indicate that exercise initiated labor only in those in whom contractions occurred. In addition, the findings validate that the fetus tolerates vigorous exercise at term.
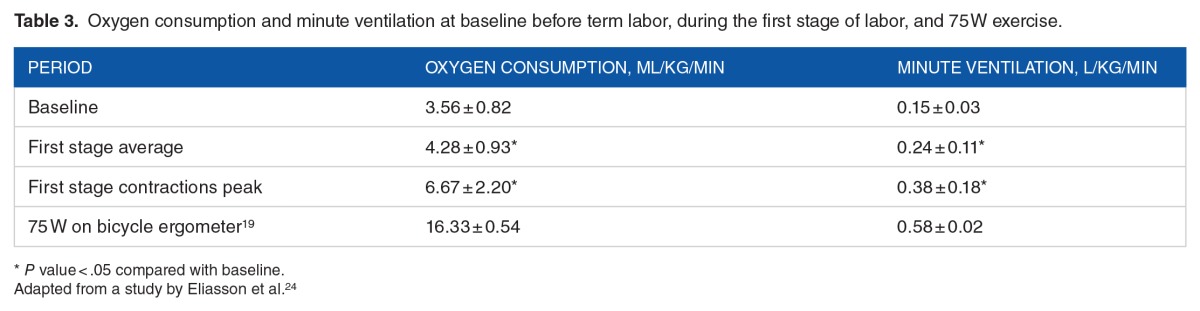
The one “exercise” every woman anticipates (except those planning an elective cesarean birth) is labor. There has been relatively little study of the cardiopulmonary physiology of human labor and it is not known how the O2 consumption and minute ventilation during labor compare with measurements during exercise. Eliasson et al24 enrolled 20 pregnant women to have pulmonary function tests and a 10-minute breath-to-breath blood gas analysis in late term and repeated in the first stage of labor. Data collection was complete on 8 patients. Table 3 depicts the oxygen consumption and minute ventilation Vo2 at baseline before term labor and during the first stage of labor.
Table 3.
Oxygen consumption and minute ventilation at baseline before term labor, during the first stage of labor, and 75 W exercise.
When labor is clearly energy using, if not “exercise,” the overall energy expenditure is 10% to 20% of moderate-vigorous exercise during pregnancy per each 30 minutes of activity. When labor is not normal, ie, long (>20 hours), hypertonic (high-dose oxytocin), with relative starvation (“NPO”), or complicated by chorioamnionitis, the latter oxygen consumption and minute ventilation estimates may not be reliable.
Another natural experiment is the effects of altitude (relative hypoxemia) on maternal physiology during exercise. At 25°C to 26°C, the barometric pressure at sea level (754 mm Hg) falls 18% (617 mm Hg) at 6000-ft altitude. This corresponds to an 18% fall in Po2 inhaled by the exercising woman. Artal et al25 performed an experiment to examine the effect of altitude on exercise performance. Seven healthy sedentary women exercised at 33 to 34 weeks of gestation. The average pregnant participant was 25 years old, was 74 kg, and had 34% body fat. After medical clearance, subjects underwent testing on a bicycle ergometer at sea level then at 6000-ft altitude. The bicycle protocol was 5 minutes resting at baseline measurements; 5 minutes at 25, 50, and 75 W; and then increased 25 W every 2 minutes until volitional fatigue.
At altitude, resting cardiac output was significantly higher (6.41 ± 0.18) than at sea level (5.85 ± 0.23 L/min). There was no difference at 50 W of exercise. At symptom-limited maximal exercise in the same women, measures (maximal work, oxygen consumption, and maximal CO2 ventilation) were lower at altitude than at sea level. Maximum heart rate (164 bpm) and ventilatory frequency (40 breaths/minute) were similar at maximal exercise between sea level and altitude. At maximal volitional exercise levels, glucose, lactate, norepinephrine, and epinephrine were not different at sea level than at altitude. One of 7 subjects developed self-limited fetal heart rate decelerations, and another demonstrated a self-limited episode of contractility during maximal exercise. All subjects had normal obstetric and newborn outcomes at term. In summary, pregnant women tolerate less workload at altitude but seem to respond normally to acute exercise stress. Although the sample size is small and more studies are needed, women at altitude who exercise at moderate intensity should monitor contractility and maintain a 50% to 60% of maximum predicted heart rate intensity for shorter duration, ie, 20 minutes. Anaerobic exercise should be avoided. More studies of higher intensity and longer duration are needed.
Fetal temperature is approximately 0.5°C higher than maternal temperature. Efficient heat dispersion by the exercising mother is important in reducing excess fetal oxygen consumption by hyperthermia. O’Neill26 performed upright cycling tests in 11 healthy, low-risk women at 34 to 37 weeks to measure maternal rectal temperature and correlate the temperature change with fetal heart rate changes. The exercise protocol used an upright bicycle ergometer. On day 1, participants exercised at 62.5 W for 15 minutes, followed by 1 hour of recovery, and finished with 15 minutes at 87.5 W. On day 2, they cycled at 62.5 W for 15 minutes, rested for 1.5 minutes, and then continued to cycle at 62.5 W for another 15 minutes. An active recovery of gentle peddling concluded each test period. The maximum increase in maternal rectal temperature was 0.3°C to 0.6°C. Increased intensity and longer duration independently increased rectal temperature and, by inference, increased fetal temperature similarly. High intensity (87.5 W) and longer duration (30 minutes) increased the fetal heart rate by 13 bpm. Higher fetal heart rates correlated with higher rectal temperatures (r2 = 58%–68%, P < .02). The fetal heart rate returned to baseline by 15 minutes after the exercise session. Although the sample size is small, these findings begin to set guidelines for duration and intensity of exercise in pregnant women: 15 minutes for intense exercise, 30 to 45 minutes for moderate exercise, followed by an active recovery period of 15 minutes. The hyperthermic effect of consecutive and similar sets of maternal exercise has not been studied.
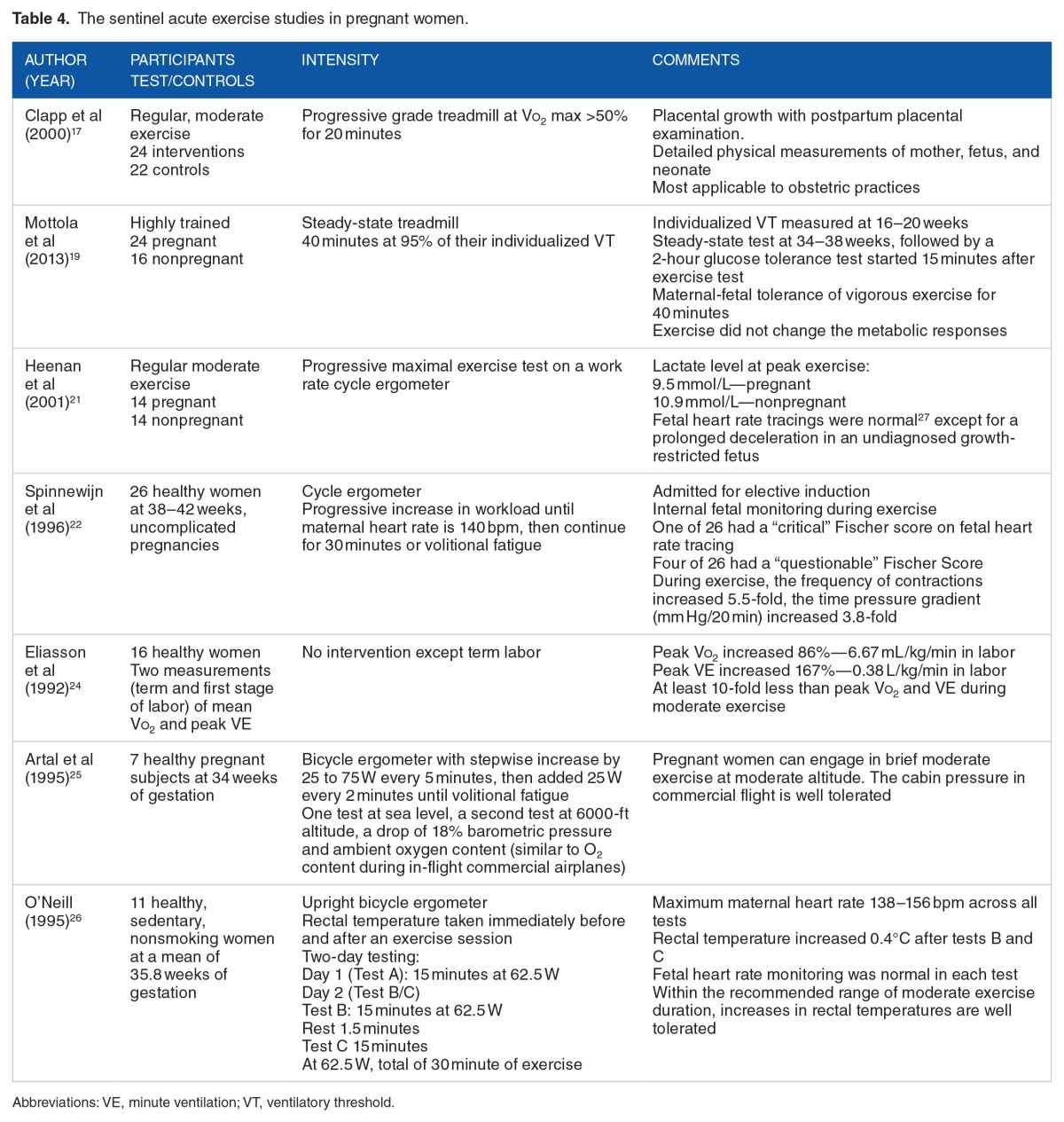
In summary of the acute exercise studies, acute moderately vigorous (Vo2 up to 70%–75% of predicted maximum) exercise is well tolerated by healthy, sedentary, or active pregnant women and her fetus. Healthy trained pregnant women and her fetus can tolerate somewhat higher exercise intensity (85%–90% of maximum Vo2). Pregnant women can increase their aerobic capacity and exercise efficiency through training similar to nonpregnant women. As shown in a study by Clapp et al17, modest aerobic exercise training improves fetal outcomes. However, prolonged exercise of more than 45 minutes results in an elevated core maternal and fetal temperature, a potential fetal risk. When pushed into anaerobic metabolism during prolonged maximal exercise, fetal compromise and increased uterine contractions occur in 15% to 20% of pregnant women. Table 4 summarizes the sentinel acute exercise studies. Over the breath of studies, the findings of these sentinel studies are confirmed.13
Table 4.
The sentinel acute exercise studies in pregnant women.
The optimal exercise session in healthy normal-weight pregnant women is 30 to 45 minutes at 50% to 75% maximum Vo2, followed by at least a 15-minute recovery period, ie, gentle walking or cycling. There are 2 practical ways for women to estimate Vo2 consumption: heart rate and ventilatory rate. Maximum heart rate is a proxy to maximum exercise tolerance and can be approximated by subtracting age from 220, ie, a 30-year-old has a maximum predicted heart rate of 190 bpm. Seventy percent of maximum heart rate or maximum Vo2 would be exercising at heart rate of 130 to 135 bpm. Recent advances in portable heart rate monitors make this method much easier. Another way to practically estimate the target range (50%–75% maximum Vo2) is to exercise at level where the woman can talk complete sentences, <75% maximum Vo2, but not sing, >50% maximum Vo2. A more complex method is to use metabolic equivalents (METs). An MET is the Vo2 at rest or about 3.5 mL O2/kg/min. Moderate exercise uses 3 to 4 METs, ie, brisk walking. An excellent resource for METs for types of exercise, work activities, and everyday living activities was published by Jetté et al28 and Ainsworth et al.29
International Recommendations for Exercise in Pregnancy
In 2002 (reaffirmed in 2009 and in 2015), the American College of Obstetricians and Gynecologists1,30 published guidelines for exercise during pregnancy. In the absence of medical or obstetric complications, pregnant women should exercise at a moderate level for 30 or more minutes per day on most, if not all, days of the week, ie, 150 to 180 minutes a week.1,30 Pregnant women who habitually engage in vigorous-intensity aerobic activity or are highly active can continue this activity during pregnancy.1,30 Numerous other national and international governmental agencies and societies support this basic guideline31: US Department of Health and Human Services, American College of Sports Medicine, Centers for Disease Control and Prevention, Canadian Society for Exercise Physiology, Royal College of Obstetricians and Gynecologists, and National Institute for Health and Clinical Excellence (United Kingdom), Denmark, and Norway. Most of these groups recommend muscle- and bone-strengthening activities using major muscle groups twice a week as beneficial.
Cautions and Concerns for Exercise During Pregnancy
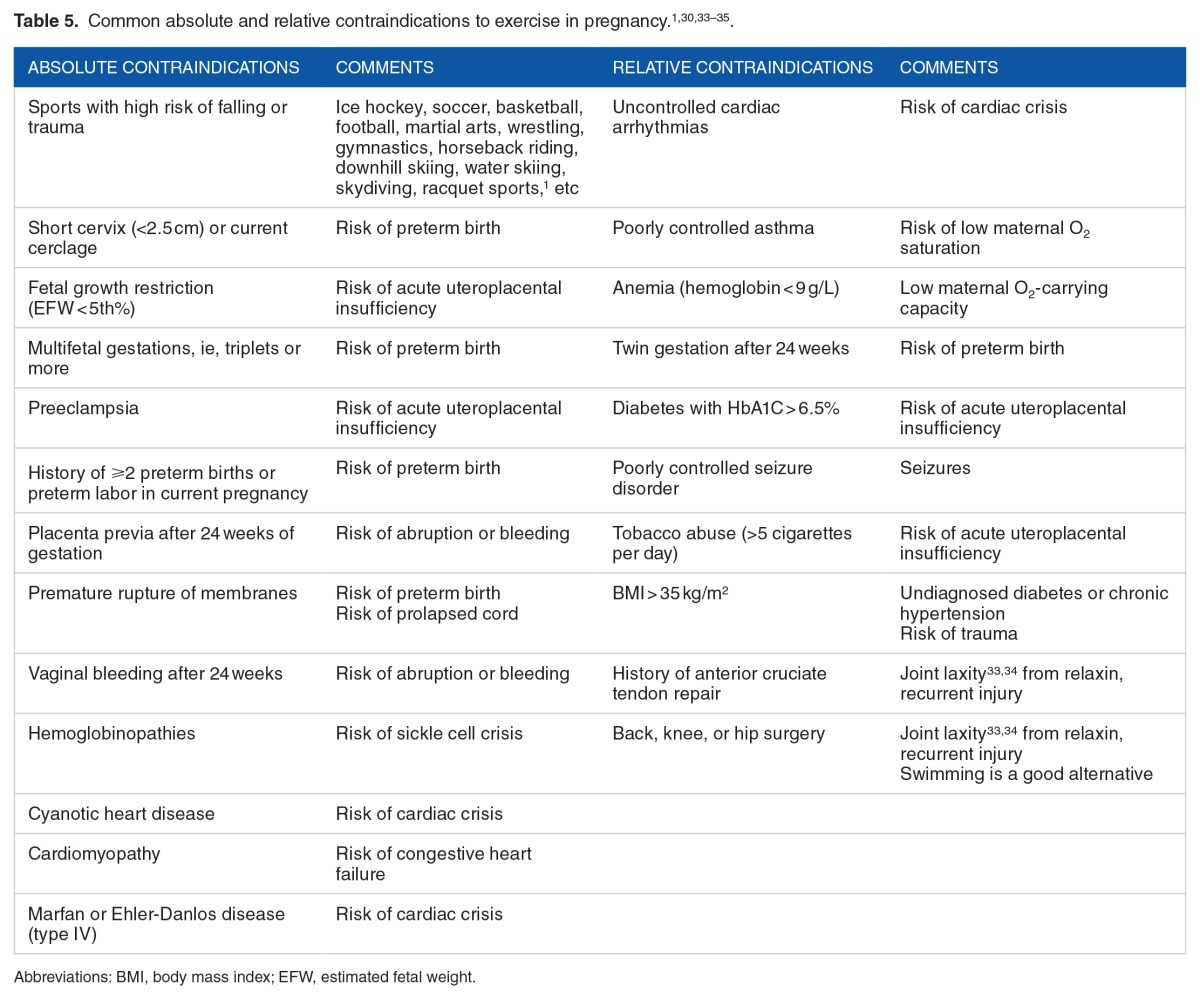
The key caveat of the aforementioned recommendations is the phrase “in the absence of medical or obstetric complications.” However, most of the US women of reproductive age (20–39 years) are unhealthy prior to conception, for instance, 30% are obese (BMI > 30 kg/m2), 7% have chronic hypertension, 2% to 3% are diabetic, 15% to 20% smoke, 10% to 15% are anemic (hemoglobin < 10.0 g/L), and 10% have cardiovascular disease.32 In addition to being unhealthy prior to conception, many develop conditions during pregnancy, such as 10% to 15% develop hypertension in pregnancy, 5% to 10% develop gestational diabetes, and 50% gain excessive gestational weight. Some of these percentages could be doubled because 30% to 50% of nonpregnant young women with chronic hypertension or carbohydrate intolerance/type 2 diabetes are unaware that they have these conditions. Of patients with known chronic hypertension or diabetes, only 30% to 50% are optimally controlled. Because the studies of exercise in pregnancy involve healthy volunteers without underlying disease and the obstetrician often worries about the presence, visible or invisible, of these complications, their motivation to recommend regular, moderate exercise most days of the week is diminished. The obstetric care provider is strongly recommended to reinforce the benefits of physical activity in pregnancy, yet review and identify risks for exercise and advise women accordingly. In certain cases, consultation from a specialist and/or exercise testing is warranted prior to starting regular, moderate exercise most days of the week. Table 5 reviews common absolute and relative contraindications to exercise in pregnancy. Relative contraindications might allow monitored low-intensity exercise.
Table 5.
Women who were sedentary prior to pregnancy but desire to gain the benefits of aerobic exercise should start at low intensity (50% Vo2) with 10-minute stretches with 10-minute breaks between sets. Over the next 2 to 4 weeks, the duration and intensity of sets are increased to 30 to 45 minutes and a higher Vo2 (75% maximum Vo2), respectively. Dehydration, hypoglycemia, and exercise hyperthermia are of special concern for the fetus; all exercise should be performed in a fed, well-hydrated state. When the ambient temperature and humidity are high, a special focus on hydration and shortened duration of each set is prudent. Indoor exercise and swimming are better exercise choices in a hot and humid environment. All pregnant women should be advised to do a light 5-minute warm-up before exercise and to cool down after exercise, ie, gentle walking or cycling. It is suggested the best exercises are non–weight bearing; of these exercises, swimming is a safe, full body exercise. However, scuba diving deeper than 30 ft is contraindicated as the fetus is at risk of decompression sickness. In general, the warning signs to terminate exercise immediately include vaginal bleeding, amniotic fluid leakage, contractions more frequent than every 6 minutes, chest pain, irregular heartbeat, shortness of breath, dizziness, syncope, calf pain, calf swelling, or decreased fetal movement.
Neonatal and Maternal Effects of Chronic Exercise in Pregnancy
Leisure time physical activity (LTPA), ie, chronic exercise, during pregnancy at the durations and intensities described by international guidelines for exercise in pregnancy,1,30,31 has few risks and many benefits for the newborns and mothers. The major immediate neonatal outcomes of concern are miscarriage, birth defects, preterm delivery, and birth weight extremes, ie, SGA or large for gestational age (LGA). From the Danish National Birth Cohort (DNBC), Madsen et al36 analyzed the prospective risk of miscarriage up to 22 weeks of gestation in 92 671 pregnant women reporting physical activity. They found that there is no dose effect with miscarriage among all levels of activity compared with sedentary women. A recent review of studies examining the risk of birth defects in exercising women could not identify any consistent increase in birth defects.37 Two large prospective studies have demonstrated a reduced risk of preterm birth in exercising women. The Norwegian Mother and Child Cohort Study (MoBa) with 61 098 pregnant women demonstrated lower rates of preterm birth in women exercising 3 to 5 times per week at 30 weeks of gestational age: adjusted odds ratio = 0.74 and 95% confidence interval = 0.65 to 0.83.2 Similar protective effects of LTPA on preterm births were demonstrated in the DNBC.38
The likelihood of small and LGA newborns is an important variable in analysis as exercise competes with the placental-fetal unit for oxygen and substrates. Moderate LTPA in pregnancy reduces the risk of LGA neonates by 30% with no increase in the risk of SGA neonates. The 2 large prospective studies,2,38 DNBC and MoBa, confirmed a 23% to 28% reduction in LGA newborns with no significant increase in SGA neonates. Clapp and colleagues16,17 demonstrated that the newborns of exercising women are leaner and have decreased body fat percent for their weight than newborns of nonexercising women.
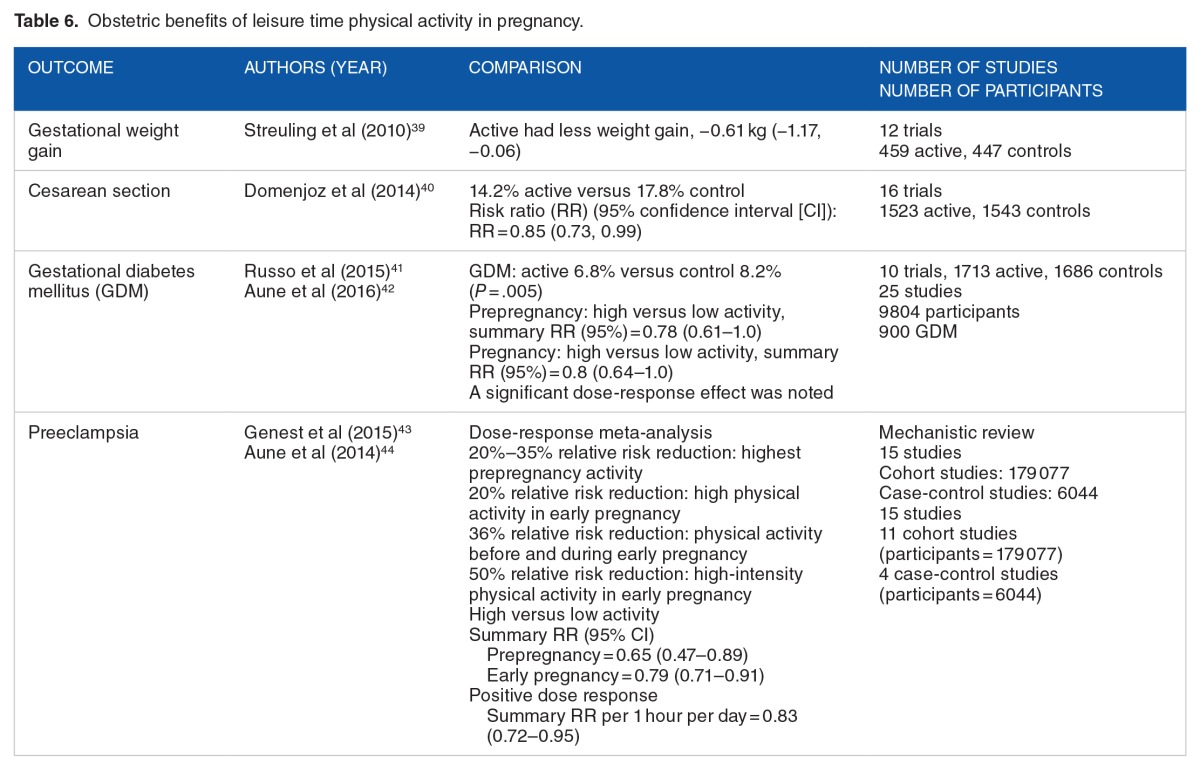
Normalized birth weight outcomes may be the result of higher blood volume, which can increase nutrient delivery, seen in active pregnant women versus sedentary pregnant women. Similarly, red cell volume, ie, better oxygen delivery, is greater in active pregnant women versus sedentary pregnant women.16,17 Leisure time physical activity during pregnancy has multiple obstetric benefits apart from the exercise benefits seen in nonpregnant women. Table 6 reports the results of several recent meta-analyses on obstetric outcomes.
Table 6.
Obstetric benefits of leisure time physical activity in pregnancy.
Exercise and diet during pregnancy have profound effects on the newborn into adulthood. There is a rapidly expanding literature to support the impact of in utero environment on adult health and the health of future generations.45–48 The extremes in fetal growth increase the risks of metabolic disorders in the child and adult, ie, “fetal origin hypothesis,” “early life programming,” and “thrifty phenotype hypothesis.” Fetal growth restriction is associated with adult metabolic syndrome, chronic hypertension, and type 2 diabetes mellitus. Maternal obesity, diabetes, and subsequent fetal hyperalimentation, aka macrosomia, predict 2- to 3-fold risk in childhood and adult obesity and type 2 diabetes. The hyperalimented female offspring have a 2- to 3-fold increase in gestational obesity and gestational diabetes in their future pregnancies. The metabolic dysfunction crosses generations, perhaps by an epigenetic mechanism.
Epigenetics is defined as heritable changes in gene expression that are not due to any alteration in the primary DNA sequence. Epigenetic mechanisms include DNA methylation, histone modification, and regulation by noncoding RNAs. Early epigenetic perturbations in the fetal environment program a dysfunctional developmental response. Micronutrients (vitamins, minerals), macronutrients (glucose, lipids, protein), and oxygen environment, ie, exercise, modulate the early in utero environment to create a potentially permanent pattern of metabolic performance, response to oxidative stress, immunologic response, and neurologic interaction, ie, sympathetic/autonomic response and fat deposition/utilization. Diet and exercise during pregnancy act in combination to direct normal epigenetic homeostasis. A high-fat, high-fructose diet coupled with a sedentary lifestyle before and during pregnancy creates an in utero environment that sets the stage for future metabolic syndrome.
Summary
Maternal and fetal physiology at rest provides support for maternal-fetal adaptation to acute and chronic exercise during pregnancy. Sentinel animal and human studies demonstrate a wide margin of safety across wide variety of intensity, duration, and altitude for aerobic treadmill and cycling exercise. Prolonged anaerobic exercise creates an environment of hyperthermia, dehydration, and enhanced uterine activity which may have fetal threats. Population data with self-reported exercise frequency and intensity have shown improved pregnancy outcomes, less gestational weight gain, fewer cesarean deliveries, less gestational diabetes, and less preeclampsia. The observations from acute exercise experiments and the population studies buttress the international recommendations for moderate exercise most days of the week in normal women throughout pregnancy.
Obstetric care providers should use their knowledge of the reviewed sentinel articles to better inform their normal patients as to the evidence-based benefits of regular exercise. The intensity of the acute exercise studies suggests an important margin of safety in many patients who are less healthy, ie, overweight, unfit, or have mild hypertension or gestational diabetes. The available evidence suggests a monitored, stepwise increase in physical activity will decrease adverse pregnancy outcomes.
Future Studies
Because many modern, pregnant women have conditions which may caution the obstetric care provider and the patient, there is a profound and urgent need to design and publish studies that randomly assign patients with obesity, diabetes, or hypertension and smoking to a well-monitored exercise program of regular, moderate intensity most days of the week versus no intervention.
The recommendation by multiple national and international organizations is that every neonate should breastfeed exclusively for 6 months and continue breastfeeding for 12 months or more.49 Although an understanding of lactation physiology and the consistency of breastmilk content suggest few concerns in lactating women who perform moderate-intensity exercise most days of the week, there is little no research on the success of breastfeeding and breastmilk volume and content in exercising, well-fed women.
Footnotes
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1706 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
Both authors have written and edited the article.
REFERENCES
- 1.American College of Obstetricians and Gynecologists Physical activity and exercise during pregnancy and the postpartum period. Committee Opinion Number 650 December 2015. Obstet Gynecol. 2015;126:e135–e142. doi: 10.1097/AOG.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 2.Owe KM, Nystad W, Skjaerven R, Stigum H, Bo K. Exercise during pregnancy and the gestational age distribution: a cohort study. Med Sci Sports Exerc. 2012;44:1067–1074. doi: 10.1249/MSS.0b013e3182442fc9. [DOI] [PubMed] [Google Scholar]
- 3.Juhl M, Olsen J, Andersen PK. Physical exercise during pregnancy and fetal growth measures: a study within the Danish National Birth Cohort. Am J Obstet Gynecol. 2002;202:63.e1–63.e8. doi: 10.1016/j.ajog.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Peterson AM, Leet TL, Brownson RC. Correlates of physical activity among pregnant women in the United States. Med Sci Sports Exerc. 2005;37:1748–1753. doi: 10.1249/01.mss.0000181302.97948.90. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. Title 45 CFR 46, Sub-Part B: additional protections for pregnant women, human fetuses, and neonates involved in research. Section 46.204: research involving pregnant women or fetuses.
- 6.Gorski J. Exercise during pregnancy: maternal and fetal responses. A brief review. Med Sci Sports Exerc. 1985;17:407–416. doi: 10.1249/00005768-198508000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Flick AA, Kahn DA. Chapter 8. Maternal Physiology during Pregnancy & Fetal & Early Neonatal Physiology. In: DeCherney AH, Nathan L, Laufer N, Roman AS, editors. CURRENT Diagnosis & Treatment: Obstetrics & Gynecology, 11e. New York, NY: McGraw-Hill; 2013. [Accessed February 13, 2017]. http://accessmedicine.mhmedical.com/content.aspx?bookid=498§ionid=41008597. [Google Scholar]
- 8.Meschia G. Chapter 14. Placental respiratory gas exchange and fetal oxygenation. In: Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore TR, Greene MF, editors. Creasy and Resnik’s Maternal-Fetal Medicine. 7th ed. Philadelphia, PA: Elsevier Saunders; 2014. pp. 181–192. [Google Scholar]
- 9.Pivarnik JM, Lee W, Miller J, Werch J. Alterations in plasma volume and protein during cycle exercise throughout pregnancy. Med Sci Sports Exerc. 2003;22:751–755. doi: 10.1249/00005768-199012000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Clark SL, Cotton DB, Lee W. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161:1439–1449. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 11.Borrowski RA. Pulmonary physiology in pregnancy. Clin Obstet Gynecol. 2010;53:285–300. doi: 10.1097/GRF.0b013e3181e04776. [DOI] [PubMed] [Google Scholar]
- 12.Pivarnik JM, Stein AD, Rivera JM. Effect of pregnancy on heart rate/oxygen consumption calibration curves. Med Sci Sports Exerc. 2002;34:750–755. doi: 10.1097/00005768-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Riemann MK, Kanstrup Hansen IL. Effects on the fetus of exercise in pregnancy. Scand J Med Sci Sport. 2000;10:12–19. doi: 10.1034/j.1600-0838.2000.010001012.x. [DOI] [PubMed] [Google Scholar]
- 14.Szymanski LM, Satin AJ. Strenuous exercise during pregnancy: is there a limit? Am J Obstet Gynecol. 2012;207:179.e1–179.e6. doi: 10.1016/j.ajog.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pivarnik JM, Malier M, Ayres A, Kirshon B, Dildy GA, Cotton DB. Effects of chronic exercise on blood volume expansion and hematologic indices during pregnancy. Obstet Gynecol. 1994;83:265–269. [PubMed] [Google Scholar]
- 16.Clapp JF. Influence of endurance exercise and diet on human placental development and fetal growth. Placenta. 2006;27:527–534. doi: 10.1016/j.placenta.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Clapp JF, Kim H, Burciu B, Lopez B. Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol. 2000;183:1484–1488. doi: 10.1067/mob.2000.107096. [DOI] [PubMed] [Google Scholar]
- 18.Lotgering FK, Gilbert GD, Longo LD. Interaction of exercise and pregnancy. Am J Obstet Gynecol. 1984;149:560–569. doi: 10.1016/0002-9378(84)90036-x. [DOI] [PubMed] [Google Scholar]
- 19.Mottola MF, Inglis S, Brun CR, Hammond JA. Physiological and metabolic responses of late pregnant women to 40 min of steady-state exercise followed by an oral glucose tolerance perturbation. J App Physiol. 2013;115:597–604. doi: 10.1152/japplphysiol.00487.2013. [DOI] [PubMed] [Google Scholar]
- 20.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 21.Heenan AP, Wolfe LA, Davies GAL. Maximal exercise testing in late gestation: maternal response. Obstet Gynecol. 2001;97:127–134. doi: 10.1016/s0029-7844(00)01089-9. [DOI] [PubMed] [Google Scholar]
- 22.Spinnewijn WEM, Lotgering FK, Struijk PC, Wallenburg HC. Fetal heart rate and uterine contractility during maternal exercise at term. Am J Obstet Gynecol. 1996;174:43–48. doi: 10.1016/s0002-9378(96)70371-x. [DOI] [PubMed] [Google Scholar]
- 23.Fischer WM, Stube I, Brandt H. Ein Vorschlog zur Beuteilung des antepartualen Kardiotokogramms. Z Geburtshilfe Perinat. 1976;180:117–123. [PubMed] [Google Scholar]
- 24.Eliasson AH, Phillips YY, Stajduhar KC, Carome MA, Cowsar JD., Jr Oxygen consumption and ventilation during normal labor. Chest. 1992;102:467–471. doi: 10.1378/chest.102.2.467. [DOI] [PubMed] [Google Scholar]
- 25.Artal R, Fortunato V, Welton A, et al. A comparison of cardiopulmonary adaptations to exercise in pregnancy at sea level and altitude. Am J Obstet Gynecol. 1995;174:1170–1180. doi: 10.1016/0002-9378(95)91475-7. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill ME. Maternal rectal temperature and fetal heart rate responses to upright cycling in late pregnancy. Brit J Sport Med. 1996;30:32–35. doi: 10.1136/bjsm.30.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacPhail A, Davies GAL, Victory R, Wolfe LA. Maximal exercise testing in late gestation: fetal responses. Obstet Gynecol. 2000;96:565–570. doi: 10.1016/s0029-7844(00)00940-6. [DOI] [PubMed] [Google Scholar]
- 28.Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 29.Ainsworth BE, Haskell WL, Herrmann SD, et al. Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 30.American College of Obstetrics and Gynecology Exercise during pregnancy and the postpartum period. Committee Opinion number 267. Obstet Gynecol. 2009;99:171–173. doi: 10.1016/s0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- 31.Mudd LM, Owe KM, Mottola MF, Pivarnik JM. Health benefits of physical activity during pregnancy: an international perspective. Med Sci Sports Exerc. 2013;45:268–277. doi: 10.1249/MSS.0b013e31826cebcb. [DOI] [PubMed] [Google Scholar]
- 32.Nickens MA, Long RC, Geraci SA. Cardiovascular disease in pregnancy. South Med J. 2013;106:624–630. doi: 10.1097/SMJ.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 33.Olson D, Sikka RS, Hayman J, Novak M, Stavig C. Exercise in pregnancy. Curr Sport Med Rep. 2009;8:147–153. doi: 10.1249/JSR.0b013e3181a61d51. [DOI] [PubMed] [Google Scholar]
- 34.Borg-Stein J, Dugan SA, Gruber J. Musculoskeletal aspects of pregnancy. Am J Phys Med Rehab. 2005;84:180–192. doi: 10.1097/01.phm.0000156970.96219.48. [DOI] [PubMed] [Google Scholar]
- 35.Gavard JA, Artal R. Effect of exercise on pregnancy outcome. Clin Obstet Gynecol. 2008;51:467–480. doi: 10.1097/GRF.0b013e31816feb1d. [DOI] [PubMed] [Google Scholar]
- 36.Madsen M, Jorgensen T, Jensen ML, et al. Leisure time physical exercise during pregnancy and the risk of miscarriage: a study within the Danish National Birth Cohort. BJOG. 2007;114:1419–1426. doi: 10.1111/j.1471-0528.2007.01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flak AL, Yun Tark J, Tinker SC, Correa A, Cogswell ME. Major, non-chromosomal, birth defects and maternal physical activity: a systematic review. Birth Def Res A Clin Mol Teratol. 2012;94:521–531. doi: 10.1002/bdra.23017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juhl M, Andersen PK, Olsen J, et al. Physical exercise during pregnancy and the risk of preterm birth: a study within the Danish National Birth Cohort. Am J Epidemiol. 2008;167:859–866. doi: 10.1093/aje/kwm364. [DOI] [PubMed] [Google Scholar]
- 39.Streuling I, Beyerlein A, Rosenfeld E, Hofmann H, Schulz T, von Kries R. Physical activity and gestational weight gain: a meta-analysis of intervention trials. BJOG. 2011:278–284. doi: 10.1111/j.1471-0528.2010.02801.x. [DOI] [PubMed] [Google Scholar]
- 40.Domenjoz I, Kayser B, Boulvain M. Effect of physical activity during pregnancy on mode of delivery. Am J Obstet Gynecol. 2014;211:401.e1–401.e11. doi: 10.1016/j.ajog.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 41.Russo LM, Nobles C, Ertel KA, Chasan-Taber L, Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol. 2015;125:576–582. doi: 10.1097/AOG.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 42.Aune D, Sen A, Henriksen T, Saugstad OD. Physical activity and the risk of gestational diabetes mellitus: a systematic review and dose-response meta-analysis of epidemiological studies. Eur J Epidemiol. 2016;31:967–997. doi: 10.1007/s10654-016-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genest DS, Falcao S, Gutkowska J, Lavoie JL. Impact of exercise training on preeclampsia: potential preventive mechanisms. Hypertension. 2015;60:1104–1109. doi: 10.1161/HYPERTENSIONAHA.112.194050. [DOI] [PubMed] [Google Scholar]
- 44.Aune D, Saugstad OD, Henriksen T, Tonstad S. Physical activity and the risk of preeclampsia: a systematic review and meta-analysis. Epidemiology. 2014;25:331–343. doi: 10.1097/EDE.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 45.Mathias PC, Elmhiri G, de Oliveria JC, et al. Maternal diet, bioactive molecules, and exercising as reprogramming tools of metabolic programming. Euro J Nutr. 2014;53:711–722. doi: 10.1007/s00394-014-0654-7. [DOI] [PubMed] [Google Scholar]
- 46.Fetita LS, Sobngwi E, Serradas P, Calvo F, Gautier JF. Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endo Metab. 2006;91:3718–3724. doi: 10.1210/jc.2006-0624. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins SA, Cutfield WS. Exercise in pregnancy: weighing up the long-term impact on the next generation. Exerc Sport Sci Rev. 2011;39:120–127. doi: 10.1097/JES.0b013e31821a5527. [DOI] [PubMed] [Google Scholar]
- 48.Lind JM, Hennessey A, McLean M. Cardiovascular disease in women: the significance of hypertension and gestational diabetes during pregnancy. Curr Opin Cardiology. 2014;29:447–453. doi: 10.1097/HCO.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 49.Newton ER. Chapter 23. Lactation and breastfeeding. In: Gabbe SG, Niebyl JR, Simpson JL, et al., editors. Obstetrics: Normal and Problem Pregnancies. New York, NY: W.B. Saunders; 2016. pp. 533–566. [Google Scholar]