Abstract
In this article, the cause, histology, imaging characteristics, clinical presentation, and treatment of these lesions are thoroughly discussed. Bone marrow edema is the generic term classically used to describe the high-signal-intensity alterations detected on magnetic resonance fluid-sensitive sequences. The significance of bone marrow edema for the patient’s clinical condition and the prognosis of the affected joint is being increasingly investigated and discussed, and situations characterized by subchondral insufficiency are receiving increasing attention. More recent studies found some important correlations between bone marrow lesions and patient’s pain and osteoarthritis progression. Conservative treatment is based on anti-inflammatory and analgesic uses according to the patient’s pain, combined with reduced load on the affected limb. Regarding surgical treatment, subchondroplasty is an option still in development, albeit with promising initial results.
Keywords: Bone marrow edema, bone marrow lesion, insufficiency fracture
Introduction
Bone marrow edema (BME) is the generic term classically used to describe the high-signal-intensity alterations detected on magnetic resonance (MR) fluid-sensitive sequences.1–3 These imaging abnormalities may occur in several pathological conditions associated with a broad range of symptoms.2
The term BME was first used in 1988 in an assessment of 10 patients with transient osteoporosis of the hip or knee, wherein bone marrow showed decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images, and ischemic necrosis or metastasis was excluded by biopsy. Interpreting these MR abnormalities as representing a transient increase in the bone marrow water content (edema), for lack of a better term and to emphasize the generic nature of the condition, the authors suggested naming such findings “transient bone marrow edema syndrome.”4
In 2000, Zanetti et al conducted a study in which they subjected 16 patients who underwent total knee arthroplasty to MR imaging (MRI) 1 to 4 days before surgery and correlated the imaging findings with the histopathology. The results showed that the osteoarthritic knee signal abnormalities on MRI, then known as BME, actually represented a series of histologic findings, with 11% of the area of bone marrow necrosis, 8% of abnormal trabeculae, 4% of bone marrow fibrosis, 4% of BME, and 2% of bone marrow bleeding. However, the percentage of BME was not significantly different from the areas of normal appearance on MRI.5
The condition most often associated with BME is trauma, namely, the occurrence of bone contusion manifested on MRI as high signal intensity on T2-weighted images. The histologic features indicate that such lesions represent areas of bone impaction and bleeding caused by trauma. These lesions are reversible and resolve in approximately 2 to 4 months.2 Some of the most prevalent conditions associated with BME include bone contusion with rupture of the anterior cruciate ligament and impaction of the lateral femoral condyle into the posterolateral tibial plateau (pivot shift) and lateral contusion of the lateral femoral condyle by the patella in patellar luxation.2
Bone marrow edema also occurs in the so-called transient BME syndromes, which is a generic term used for conditions characterized by diffuse periarticular high signal intensity on MRI and favorable clinical progression with complete resolution of symptoms. Some of these conditions include transient osteoporosis, regional migratory osteoporosis, and complex regional pain syndrome. All these disorders exhibit diffuse and poorly delimited periarticular edema with preservation of the articular surface. The differential diagnosis is based on the clinical history and the patient’s age, sex, and symptoms. Transient osteoporosis mainly affects the proximal end of the femur of pregnant or perinatal women. Regional migratory osteoporosis affects the hip, knee, and metatarsus, mainly among middle-aged men. Complex regional pain syndrome usually follows trauma, often presenting pain greater than expected for the causative injury.2,3 Currently, transient osteoporosis of the hip, regional migratory osteoporosis, and spontaneous osteonecrosis of the knee (SONK) are believed to be indicative of subchondral fractures whose fracture lines are not always detected on MRI.6
Bone marrow edema is also found on MR images of patients with SONK, which is currently considered a subchondral insufficiency fracture.7,8 Therefore, the high signal intensity detected in this situation can be called bone marrow lesion (BML), reflecting this structural lesion of subchondral bone rather than regional edema.
The significance of BML for the patient’s clinical condition and the prognosis of the affected joint are being increasingly investigated and discussed. In this article, the cause, histology, imaging characteristics, clinical presentation, and treatment of insufficiency fractures are thoroughly discussed.
BML/Insufficiency Fracture
Despite some proposals for classification of BME, there is still no consensus in the literature. However, in this section and throughout this article, we will try to address a type of injury that we believe is central to the growing interest in this subject, the fracture due to bone insufficiency.
Situations characterized by subchondral insufficiency are receiving increasing attention; they tend to occur among older patients, mostly in women. Spontaneous osteonecrosis of the knee, described by Ahlback et al9 in 1968, is currently believed to represent subchondral insufficiency fracture.8 Meniscal lesions are frequently associated, particularly large radial and root lesions, which may result in meniscus extrusion and loss of function.1 Chondral lesions are also common, such as cartilage thinning in the affected compartment or focal chondral injury.10
We consider that a meniscus-cartilage-subchondral bone unit exists in the knee, wherein failure of any 1 component unavoidably affects the other 2. Thus, any abnormality in this functional unit caused by meniscal and/or chondral injury increases the load conveyed to the subchondral bone, for which it is not equipped, thus leading to subchondral insufficiency and consequent fracture.
Histologic examination of the failed subchondral bone reveals microfractures of the bony trabeculae and cell and vessel abnormalities that progress to focal necrosis after a few days. The necrotic tissue is then replaced by new trabecular bone. In some cases, this regeneration process is insufficient and the lesions are not reversible, resulting in collapse of the articular surface.2,5
Bone marrow lesion resulting from subchondral bone insufficiency may be detected in previously asymptomatic patients and in patients with previous pain due to earlier degenerative osteoarthritis. In the latter situation, patients complain of an acute increase in pain and manifest associated findings of effusion, functional limitation, and pain localized to the BML topography.
Distinguishing between insufficiency fracture and avascular necrosis is important because their respective causes are quite different; consequently, the principles underpinning their treatment also differ. Avascular necrosis is caused by interruption of the vascular input, usually due to certain risk factors such as alcoholism, steroid therapy, clotting disorders, and chemotherapy. Patients usually exhibit multiple foci of necrosis affecting more than 1 compartment; involvement of metaphyseal and/or diaphyseal bone is common, and the signal characteristics are entirely different from those of SONK.1,11
Due to the still poor literature on the classification and characterization of different types of BME, it is difficult to find studies that accurately describe the natural history of insufficiency fracture.
Imaging
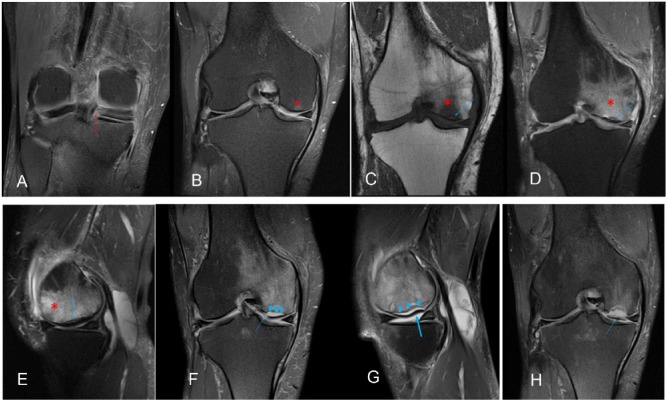
Magnetic resonance imaging findings in SONK/insufficiency fracture are relatively reproducible and specific. The main abnormality is bone edema, characterized by low signal intensity on T1-weighted images and high signal intensity on T2-weighted images in the subchondral region of the high-load areas of the femorotibial compartments, frequently associated with a low-signal-intensity line in the subchondral region, which corresponds to the impaction of trabecular bone (Figure 1). Bone edema appears first, whereas the fracture line is undetectable, possibly in association with microfractures of trabecular bone not evident on MRI. With progression of the causative mechanism of injury, these microfractures coalesce to form the typical subchondral image characterized by low signal intensity on T1-weighted and T2-weighted images, although often with better delimitation on T2-weighted imaging (Figure 2C to E). When the mechanism of injury persists, impaction/collapse of subchondral cortical bone and/or cortical bone discontinuity may occur, whereby the fracture line extends into the joint cavity, which allows the intra-articular fluid to pass into the fracture, thus generating a subchondral image with signal intensity similar to that of fluid on the fracture topography. This phenomenon hinders consolidation and favors the development of pseudarthrosis (Figure 2F to H). Moreover, this series of findings is frequently associated with the development and/or worsening of chondral lesions (Figure 2). Although the medial femoral condyle is the area most frequently affected, lesions may also appear on the lateral femoral condyle and tibial plateau.
Figure 1.
Findings typical of subchondral fracture: (A) coronal T1-weighted image, (B) coronal T2-weighted fat, and (C) sagittal T2-weighted fat. Extensive bone marrow edema characterized by low signal intensity on T1-weighted and high signal intensity on T2-weighted magnetic resonance images (asterisk) with linear low-signal-intensity imaging in the subchondral area (arrow).
Figure 2.
A 56-year-old woman with pain on the medial interline for 3 months. (A) Coronal T2-weighted fat shows a radial tear of the posterior root of the medial meniscus (red arrow). (B) Coronal T2-weighted fat shows discrete subchondral edema of the condyle load-bearing area and medial tibial plateau, without detection of the subchondral fracture (asterisk). (C, D, E, F). Imaging performed 6 months later showing the classic findings of subchondral fracture, including edema (asterisk) and subchondral fracture (blue arrow). (F) and (G). After 12 months, signs of impaction/collapse (thick arrow) and subchondral cortical bone discontinuity (arrow) are evident; intra-articular fluid seeped into the fracture, which now appears as a line with high signal intensity on T2-weighted imaging (arrowhead). (H) After 18 months, the osteochondral fragment is fully detached.
In osteoarthritic knees, the presentation of BML is disproportionate relative to the degenerative abnormalities, extending to the intercondylar area of the femur or anterior tuberosity of the tibia.12
The thickness and dimensions of the subchondral low-signal-intensity image are believed to correlate with the lesion prognosis. Lecouvet et al13 found that low-signal-intensity lines on T2-weighted images with length greater than 14 mm or thickness of more than 4 mm are risk factors for lesion progression and subchondral bone collapse.
As indicated previously, BML is commonly associated with meniscal injury, extrusion, or resection, in some cases showing overlap on imaging with degenerative osteoarthritis (Figure 2A and B).
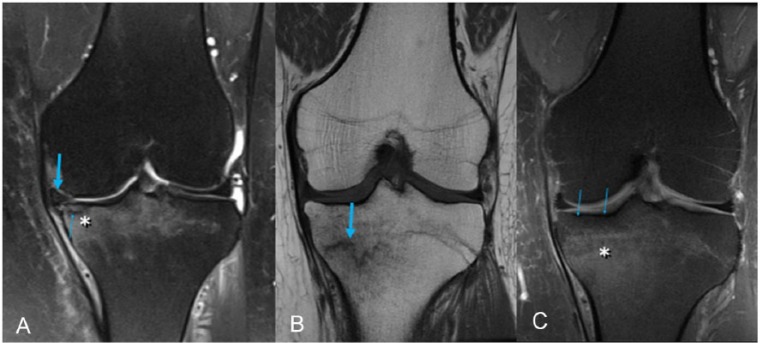
We have observed that abnormalities in SONK/insufficiency fracture are quite different from those corresponding to secondary osteonecrosis, which is characterized by an internal signal similar to that of fat and delimited by a peripheral serpiginous line of granulation tissue. Therefore, secondary osteonecrosis exhibits a central area with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images with saturation (Figure 3). When the bone edema does not extend into the subchondral cortical bone, other diagnostic alternatives should be considered because, as maintained previously, failure of the meniscus-cartilage-subchondral bone unit occurs in subchondral fracture (Figure 3).
Figure 3.
Typical secondary osteoarthritis. (A) Sagittal T1-weighted image. (B) Sagittal T2-weighted fat-saturated image. Central area (asterisk) with high signal intensity on T1-weighted and low signal intensity on T2-weighted (fat signal) imaging, the opposite of the usual findings in subchondral insufficiency fracture, delimited by a line with low signal intensity on T1-weighted and high signal intensity on T2-weighted imaging, which is not found in subchondral fracture. Note an additional infarction focus on the metaphyseal area (circle) exhibiting the same signal characteristics.
Diffusion-weighted imaging (DWI), chemical shift imaging (CSI), and single-voxel or multivoxel spectroscopy (MRS) are not usually necessary in the diagnosis of insufficiency fracture but may be used in case of diagnostic doubt. Usually, in the insufficiency fracture, there is no diffusion restriction in DWI, more than 20% signal intensity loss on out-of-phase imaging in CSI, and no choline peak in MRS. Differently in neoplastic lesions, there is usually diffusion restriction, less than 20% signal intensity loss, or increased signal intensity on out-of-phase imaging and choline peak in MRS.14
Figure 4.
(A) Typical subchondral fracture in the load-bearing area of the medial tibial plateau. Edema (asterisk) extends into the subchondral cortical bone. This image further shows the subchondral fracture line (arrow) and medial meniscus extrusion (thick arrow). (B) and (C) Typical fatigue stress fracture. Notably, edema (asterisk) does not extend into the subchondral cortical bone (arrows) because the meniscus-cartilage-subchondral bone unit is preserved. The incomplete fracture line is better characterized on T1-weighted imaging (thick arrow).
Clinical Presentation
Although SONK is not a very rare condition, few studies have analyzed its epidemiology. One study reported an incidence of 3.4% among patients older than 50 years and ⩽9.4% among patients older than 65 years. Women are typically more affected, although in some studies that performed MRI, the incidence among men and women was similar.15 A higher incidence is also found among patients who are overweight and those with poor bone quality.16
Clinically, patients generally present with localized acute pain, more often on the medial side of the knee, without a previous history of trauma that might account for the symptoms.
More recent studies found some important correlations between BME and pain and progression in patients. Felson et al17 reported a significant correlation between BML and pain in patients with osteoarthritis. In that study, patients with pain had a 3.31-fold higher likelihood of having BME compared with patients with the same radiological degree of arthrosis but without pain.
In another study, Felson et al found greater progression of articular space narrowing among patients with osteoarthritis and BML. The presence of BME in the medial and lateral knee compartments was associated with 6.5-fold and 7-fold greater probabilities, respectively, of osteoarthritis progression.18 In addition, Roemer et al10 detected a higher risk of cartilage loss on the articular surface in the presence of BML through MRI assessment over a 30-month period.
Corroborating those findings, the likelihood of joint replacement and the need for total knee arthroplasty are higher among patients with BML. This correlation is even more significant in cases of global lesions affecting one full femoral condyle or tibial plateau, which are typical of insufficiency fracture.19,20
It is important to understand that current studies evaluating the clinical progression of BME do not have any division as to the different types or patterns of BME. Also, it is not yet clear in the literature the best nomenclature for the image alterations found in MRI with spinal cord edema, using different terms such as BME, BML, and insufficiency fracture. However, there is no clear distinction about the true difference between terms. Therefore, it is difficult to correlate the clinical findings of the studies with a specific type of BME such as failure due to insufficiency.
Treatment
Insufficiency fracture treatment may be surgical or nonsurgical. Generally, surgery is the first choice for patients with large lesions (>5 cm2 or >50% of the condyle) or the aforementioned risk factors on MRI.2
Conservative treatment is based on anti-inflammatory and analgesic use according to the patient’s pain, combined with reduced load on the affected limb for 3 to 8 months according to the patient’s symptoms and radiological findings.2,21 Two additional medications that have been suggested for BML treatment are prostacyclin and the bisphosphonates.22–25
Prostacyclin is a vasodilator; its usage aims to improve the perfusion of tissues with impaired blood flow. It also reduces capillary permeability, inhibits platelet aggregation, and decreases free radical concentration. However, the mechanism that accounts for pain improvement in BML is unknown. Some studies demonstrated the efficacy of prostacyclin for BML treatment over short follow-up periods,22,23,26 with significant improvement in pain over the period of 3 to 6 months of follow-up. However, its results are poor in more advanced disease stages.26
The other drugs suggested for BML treatment are the bisphosphonates, a class of drugs that inhibit osteoclast activity, thus reducing bone resorption. Their usage aims to avoid the collapse of subchondral bone arising from the local bone resorption caused by the reaction to insufficiency fracture. Therefore, bisphosphonates may provide improved structural support until the local regeneration process creates a new bone structure sufficient to support load. The results of clinical studies are controversial. Meier et al25 did not observe significant differences between ibandronate and placebo in the only randomized, double-blind clinical trial reported in the literature. However, Kraenzlin et al24 in another study found clinical and radiological benefits with the use of this drug class. Laslett et al27 tested the use of zoledronic acid for the treatment of BML in patients with osteoarthritis in a randomized clinical study. Pain improvement was observed in patients with medication with 6 months of follow-up but not with 3 and 12 months of follow-up. No significant difference in Knee Injury and Osteoarthritis Outcome Score (KOOS) score was also observed.
Regarding surgical treatment, core decompression is used for the treatment of BME located mainly in the hip. Studies have shown a reduction in the period of pain in patients with BME in the hip, but more benign and transient characteristics.
Another surgical technique, subchondroplasty, is an option still in development, albeit with promising initial results. This method stabilizes microfractures affecting the subchondral trabecular bone by filling the spaces between the trabeculae with a calcium phosphate–based bone substitute.28 Although initial studies demonstrated pain improvement and delay of total knee arthroplasty in some situations, the results cannot yet be generalized for all patients.29 The bone substitute used must possess certain characteristics for treatment to succeed: it must be injectable and capable of filling the spaces between trabeculae, it must exhibit an endothermic crystallization reaction to avoid overheating and necrosis of the local tissue, and it must have mechanical resistance similar to that of bone to support the local load without creating a locus of excessive pressure (Figure 5).30
Figure 5.
Magnetic resonance (MR) image (A) before subchondroplasty and (B) 6 months after subchondroplasty. Note reduced edema (asterisks) and joint effusion (circles). The material used for subchondroplasty has low signal intensity on T2-weighted sequences (arrow).
Conclusions
There is still great controversy regarding the cause and clinical impact of BME images on MRI. However, it becomes increasingly clear the relevance of these alterations in the image, with correlations with pain and progression of joint degeneration. With this, different forms of treatment have been proposed with promising results. However, a more detailed study is still needed to better understand the patterns of bone edema to improve the knowledge of the natural history of each type of lesion and thus determine the indication of treatments.
Footnotes
Peer review:Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1306 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Wrote the first draft of the manuscript: MB, AGOF. Contributed to the writing of the manuscript: MB, AGOP, CPH, XMGRGS, MKD. Made critical revisions and approved final version: CPH, XMGRGS, MKD. All authors reviewed and approved the final manuscript.
References
- 1. Gil HC, Levine SM, Zoga AC. MRI findings in the subchondral bone marrow: a discussion of conditions including transient osteoporosis, transient bone marrow edema syndrome, SONK, and shifting bone marrow edema of the knee. Semin Musculoskelet Radiol. 2006;10:177–186. [DOI] [PubMed] [Google Scholar]
- 2. Kon E, Ronga M, Filardo G, et al. Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1797–1814. [DOI] [PubMed] [Google Scholar]
- 3. Manara M, Varenna M. A clinical overview of bone marrow edema. Reumatismo. 2014;66:184–196. [DOI] [PubMed] [Google Scholar]
- 4. Wilson AJ, Murphy WA, Hardy DC, Totty WG. Transient osteoporosis: transient bone marrow edema? Radiology. 1988;167:757–760. [DOI] [PubMed] [Google Scholar]
- 5. Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–840. [DOI] [PubMed] [Google Scholar]
- 6. Gil HC, Levine SM, Zoga AC. MRI findings in the subchondral bone marrow. a discussion of conditions including transient osteoporosis, transient bone marrow edema syndrome, SONK, and shifting bone marrow edema of the knee. Semin Musculoskelet Radiol. 2006;10:177–186. [DOI] [PubMed] [Google Scholar]
- 7. Plett SK, Hackney LA, Heilmeier U, et al. Femoral condyle insufficiency fractures: associated clinical and morphological findings and impact on outcome. Skeletal Radiol. 2015;44:1785–1794. [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82:858–866. [DOI] [PubMed] [Google Scholar]
- 9. Ahlback S, Bauer GC, Bohne WH. Spontaneous osteonecrosis of the knee. Arthritis Rheum. 1968;11:705–733. [DOI] [PubMed] [Google Scholar]
- 10. Roemer FW, Guermazi A, Javaid MK, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009;68:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roemer FW, Frobell R, Hunter DJ, et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage. 2009;17:1115–1131. [DOI] [PubMed] [Google Scholar]
- 12. Nevalainen MT, Sharkey PF, Cohen SB, Roedl JB, Zoga AC, Morrison WB. MRI findings of subchondroplasty of the knee: a two-case report. Clin Imaging. 2016;40:241–243. [DOI] [PubMed] [Google Scholar]
- 13. Lecouvet FE, van de Berg BC, Maldague BE, et al. Early irreversible osteonecrosis versus transient lesions of the femoral condyles: prognostic value of subchondral bone and marrow changes on MR imaging. AJR Am J Roentgenol. 1998;170:71–77. [DOI] [PubMed] [Google Scholar]
- 14. Del Grande F, Farahani SJ, Carrino JA, Chhabra A. Bone marrow lesions: a systematic diagnostic approach. Indian J Radiol Imaging. 2014;24:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lotke PA, Abend JA, Ecker ML. The treatment of osteonecrosis of the medial femoral condyle. Clin Orthop Relat Res. 1982;171:109–116. [PubMed] [Google Scholar]
- 16. Davies-Tuck ML, Wluka AE, Wang Y, English DR, Giles GG, Cicuttini F. The natural history of bone marrow lesions in community-based adults with no clinical knee osteoarthritis. Ann Rheum Dis. 2009;68:904–908. [DOI] [PubMed] [Google Scholar]
- 17. Felson DT, Chaisson CE, Hill CL, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–549. [DOI] [PubMed] [Google Scholar]
- 18. Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–336. [DOI] [PubMed] [Google Scholar]
- 19. Scher C, Craig J, Nelson F. Bone marrow edema in the knee in osteoarthrosis and association with total knee arthroplasty within a three-year follow-up. Skeletal Radiol. 2008;37:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanamas SK, Wluka AE, Pelletier J-P, et al. Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: a longitudinal study. Rheumatology (Oxford). 2010;49:2413–2419. [DOI] [PubMed] [Google Scholar]
- 21. Yates PJ, Calder JD, Stranks GJ, Conn KS, Peppercorn D, Thomas NP. Early MRI diagnosis and non-surgical management of spontaneous osteonecrosis of the knee. The Knee. 2007;14:112–116. [DOI] [PubMed] [Google Scholar]
- 22. Mayerhoefer ME, Kramer J, Breitenseher MJ, et al. MRI-demonstrated outcome of subchondral stress fractures of the knee after treatment with iloprost or tramadol: observations in 14 patients. Clin J Sport Med. 2008;18:358–362. [DOI] [PubMed] [Google Scholar]
- 23. Mayerhoefer ME, Kramer J, Breitenseher MJ, et al. Short-term outcome of painful bone marrow oedema of the knee following oral treatment with iloprost or tramadol: results of an exploratory phase II study of 41 patients. Rheumatology (Oxford). 2007;46:1460–1465. [DOI] [PubMed] [Google Scholar]
- 24. Kraenzlin ME, Graf C, Meier C, Kraenzlin C, Friedrich NF. Possible beneficial effect of bisphosphonates in osteonecrosis of the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1638–1644. [DOI] [PubMed] [Google Scholar]
- 25. Meier C, Kraenzlin C, Friederich NF, et al. Effect of ibandronate on spontaneous osteonecrosis of the knee: a randomized, double-blind, placebo-controlled trial. Osteoporos Int. 2014;25:359–366. [DOI] [PubMed] [Google Scholar]
- 26. Jager M, Tillmann FP, Thornhill TS, et al. Rationale for prostaglandin I2 in bone marrow oedema—from theory to application. Arthritis Res Ther. 2008;10:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laslett LL, Dore DA, Quinn SJ, et al. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann Rheum Dis. 2012;71:1322–1328. [DOI] [PubMed] [Google Scholar]
- 28. Sharkey PF, Cohen SB, Leinberry CF, Parvizi J. Subchondral bone marrow lesions associated with knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2012;41:413–417. [PubMed] [Google Scholar]
- 29. Cohen SB, Sharkey PF. Subchondroplasty for treating bone marrow lesions. J Knee Surg. 2015;29:555–563. [DOI] [PubMed] [Google Scholar]
- 30. Colon DA, Yoon BJ, Russell TA, Cammisa FP, Abjornson C. Assessment of the injection behavior of commercially available bone BSMs for Subchondroplasty® procedures. The Knee. 2015;22:597–603. [DOI] [PubMed] [Google Scholar]