Abstract
People with severe mental illness (SMI) – schizophrenia, bipolar disorder and major depressive disorder – appear at risk for cardiovascular disease (CVD), but a comprehensive meta‐analysis is lacking. We conducted a large‐scale meta‐analysis assessing the prevalence and incidence of CVD; coronary heart disease; stroke, transient ischemic attack or cerebrovascular disease; congestive heart failure; peripheral vascular disease; and CVD‐related death in SMI patients (N=3,211,768) versus controls (N=113,383,368) (92 studies). The pooled CVD prevalence in SMI patients (mean age 50 years) was 9.9% (95% CI: 7.4‐13.3). Adjusting for a median of seven confounders, patients had significantly higher odds of CVD versus controls in cross‐sectional studies (odds ratio, OR=1.53, 95% CI: 1.27‐1.83; 11 studies), and higher odds of coronary heart disease (OR=1.51, 95% CI: 1.47‐1.55) and cerebrovascular disease (OR=1.42, 95% CI: 1.21‐1.66). People with major depressive disorder were at increased risk for coronary heart disease, while those with schizophrenia were at increased risk for coronary heart disease, cerebrovascular disease and congestive heart failure. Cumulative CVD incidence in SMI patients was 3.6% (95% CI: 2.7‐5.3) during a median follow‐up of 8.4 years (range 1.8‐30.0). Adjusting for a median of six confounders, SMI patients had significantly higher CVD incidence than controls in longitudinal studies (hazard ratio, HR=1.78, 95% CI: 1.60‐1.98; 31 studies). The incidence was also higher for coronary heart disease (HR=1.54, 95% CI: 1.30‐1.82), cerebrovascular disease (HR=1.64, 95% CI: 1.26‐2.14), congestive heart failure (HR=2.10, 95% CI: 1.64‐2.70), and CVD‐related death (HR=1.85, 95% CI: 1.53‐2.24). People with major depressive disorder, bipolar disorder and schizophrenia were all at increased risk of CVD‐related death versus controls. CVD incidence increased with antipsychotic use (p=0.008), higher body mass index (p=0.008) and higher baseline CVD prevalence (p=0.03) in patients vs. controls. Moreover, CVD prevalence (p=0.007), but not CVD incidence (p=0.21), increased in more recently conducted studies. This large‐scale meta‐analysis confirms that SMI patients have significantly increased risk of CVD and CVD‐related mortality, and that elevated body mass index, antipsychotic use, and CVD screening and management require urgent clinical attention.
Keywords: Cardiovascular disease, severe mental illness, schizophrenia, bipolar disorder, major depression, coronary heart disease, cerebrovascular disease, congestive heart failure, premature mortality
People with severe mental illness (SMI) – including schizophrenia, bipolar disorder, major depressive disorder, and their related spectrum disorders – have a life expectancy shortened of 10‐17.5 years compared to the general population1, 2. While suicide explains some of this reduced life expectancy3, it is now established that physical diseases account for the overwhelming majority of premature mortality4, 5. Among physical conditions, cardiovascular disease (CVD) is the main potentially avoidable contributor to early deaths in patients with SMI4.
Given the importance of understanding the magnitude, contributors to and relative distribution of CVD risk in people with SMI, a number of disease‐specific meta‐analyses investigated if people with major depressive disorder, bipolar disorder or schizophrenia are at an increased risk of CVD compared to controls. These meta‐analyses reported that people with depression (defined by the presence of depressive symptoms or a diagnosis of major depressive disorder) are at increased CVD risk6, 7, including stroke (risk ratio, RR=1.34, 95% CI: 1.17‐1.54), myocardial infarction (hazard ratio, HR=1.31, 95% CI: 1.09‐1.57), coronary heart disease (RR=1.36, 95% CI: 1.24‐1.49) and coronary heart disease‐related death (HR=1.36, 95% CI: 1.14‐1.63)6, 7, 8. While clearly informative, results concerning CVD were not specific for major depressive disorder defined according to established diagnostic criteria, possibly biasing such observed association towards a lower risk9. Another meta‐analysis of longitudinal studies, which utilized standardized criteria to define bipolar disorder, reported mixed results, since people with that disorder were actually not at increased risk of myocardial infarction (RR=1.09, 95% CI: 0.96‐1.24), whereas the risk of stroke was higher compared to controls (RR=1.74, 95% CI: 1.29‐2.35)10. Among individuals with schizophrenia, previous meta‐analyses11, 12 reported an overall increased risk of CVD compared to controls (RR=1.53, 95% CI: 1.27‐1.86). This risk increase included stroke (up to RR=1.71, 95% CI: 1.19‐2.46) and heart failure (RR=1.81, 95% CI: 1.42‐2.29), but not coronary heart disease (RR=1.20, 95% CI: 0.93‐1.53).
While the existing literature has provided relevant insights, several limitations are to be highlighted and important questions remain unanswered. First, some of the previous meta‐analyses did not use standardized clinical assessments to identify and categorize SMI and/or cardiovascular events. Second, the exact prevalence and incidence of each type of CVD among people with SMI, both within and across major diagnostic SMI subgroups, remains unclear. Third, the magnitude of premature CVD‐related mortality risk in people with SMI versus controls is to be specified. Fourth, potential risk factors for increased CVD and related mortality risk across the SMI groups have not been elucidated via meta‐analytic techniques, which could help identify targets for treatment guidelines, clinical standards and development of preventive and therapeutic programs. In this regard, large‐scale pooled analyses in the SMI population can provide relevant information, allowing the investigation of potentially shared risk factors across many studies and participants, thus dissecting CVD risk factors associated with SMI and/or treatments for these disorders from factors which are non‐specific or shared with the general population13. Additionally, pooling of data allows for the investigation of demographic, regional and treatment variables, both within and across major diagnostic categories.
Given the caveats mentioned above, the current gaps within the literature and the need to better understand CVD risk among people with SMI, we conducted a large scale meta‐analysis investigating the prevalence, incidence and mortality attributed to CVD and their correlates among people with SMI, both within and across major diagnostic groups.
METHODS
This systematic review and meta‐analysis adhered to the PRISMA statement14, following a predetermined, but unpublished protocol.
Search strategy
An electronic literature search was conducted in PubMed, Embase and Scopus from database inception until August 2, 2016 by two independent reviewers, using the search terms (“bipolar disorder” OR mania OR schizophrenia OR schizoaffective OR psychosis OR “major depression” OR “serious mental illness”) AND (cardiovascular OR stroke OR cerebrovascular OR “transient ischemic attack” OR “transient ischaemic attack” OR “peripheral vascular” OR “myocardial infarction” OR “coronary heart disease” OR” coronary artery disease” OR “ischemic heart disease” OR “ischaemic heart disease” OR “hypertensive heart disease” OR angina OR “cardiac failure” OR “heart failure” OR “congestive heart failure” OR “atrial fibrillation” OR “pulmonary embolism” OR “cardiovascular mortality”). Furthermore, bibliographies of included papers were reviewed.
Inclusion and exclusion criteria
We included studies with the following characteristics: a) reporting on patients with schizophrenia, schizophrenia spectrum or schizoaffective disorder, bipolar disorder or bipolar spectrum disorders, major depressive disorder or depressive episodes, or SMI (defined as at least two among major depressive spectrum, bipolar spectrum and schizophrenia spectrum disorders) according to DSM‐III, DSM‐IV, DSM‐5, ICD‐8, ICD‐9 or ICD‐10, or a medical record diagnosis based on a clinical interview; b) having a cross‐sectional or a retrospective/prospective longitudinal design, either with or without a control group; c) using a standardized definition of CVD; d) reporting RR, HR or odds ratio (OR) comparing patients with region‐specific controls, percentage or number of events at baseline (data used for cross‐sectional analysis = prevalence) and/or follow‐up (data used for longitudinal analysis = cumulative incidence).
We excluded studies that investigated cardiovascular risk estimates and/or factors, subclinical CVD, or SMI rates in populations with CVD. In case of multiple publications from the same study, only the most recent paper or the article with the longest follow‐up was included. When required, we contacted the primary/corresponding authors of potential studies to confirm eligibility or acquire unpublished variables of interest.
Data extraction
Seven authors divided in four pairs independently extracted data in a standardized Microsoft Excel sheet, with reciprocal validation of data extraction results. The extracted data included: authors, year and country; geographic region; study design; data source; period of data collection; SMI diagnostic criteria; CVD diagnostic criteria; specific SMI and CVD diagnosis; case and control inclusion criteria; number of cases and controls; percentage or number with CVD, coronary heart disease, cerebrovascular disease and congestive heart failure at baseline; number of events at follow‐up; follow‐up duration; number and type of covariates considered in the analyses; OR, RR, rate ratio and HR with their respective 95% upper and lower CIs; mean age with standard deviation; mean body mass index with standard deviation; proportion of males; co‐occurring obesity, alcohol and substance related disorders, diabetes, hypertension, and hyperlipidemia; married status; employment status; percentage of patients with poorest income and least urbanized; and percentage of patients taking antipsychotics. Rate ratios calculated with Cox regression models were included in HR analyses. When authors did not specify whether or not a rate ratio had been calculated with Cox regression models, we contacted them seeking clarification.
Outcomes
Primary outcomes were CVD prevalence and cumulative incidence plus CVD‐related mortality in people with SMI, as well as adjusted OR for prevalence and HR for incidence rates in SMI versus controls. Secondary outcomes were the same measures for specific CVDs (i.e., coronary heart disease, cerebrovascular disease, congestive heart failure) in SMI patients, as well as adjusted OR and HR versus controls.
Prevalence and OR were calculated from cross‐sectional studies and from baseline results of longitudinal studies. Where available, incidence, RR and HR were calculated from longitudinal studies.
Quality assessment
For the purpose of this meta‐analysis, a checklist (yes versus no) was used to assess the methodological quality of included studies. The evaluation of methodological quality across studies was based on the following factors: clear diagnostic criteria, presence of a control group, matching of the control group, covariate‐adjusted outcomes, reported cardiovascular risk factors at baseline, and follow‐up ≥5 years.
Data analysis
This meta‐analysis was performed using Comprehensive Meta‐Analysis V315. All outcomes were meta‐analyzed when at least two studies provided data. A random effects model16, 17 was used to account for between‐study heterogeneity. We calculated pooled CVD prevalences and pooled CVD cumulative incidences, each with SMI subgrouping. For dichotomous primary and secondary outcomes comparing pooled SMI and SMI subgroups with controls, we calculated unadjusted as well as adjusted pooled OR for cross‐sectional data, and unadjusted pooled RR, as well as adjusted pooled HR, for longitudinal data. Funnel plots were visually inspected, and Egger's test18 and Begg‐Mazumdar Kendall's tau19 were used to determine if publication bias was likely. When publication bias was present, the trim and fill20 procedure was run to evaluate if the results changed after imputing potentially missing studies.
Between‐study heterogeneity was measured using the chi‐squared and I‐squared statistics, with chi‐squared p<0.05 and I‐squared ≥50% indicating significant heterogeneity21. To identify potential moderators, meta‐regression was run with Comprehensive Meta‐Analysis V3 for unadjusted outcomes where heterogeneity was significant.
Since CVD rates in the general population vary across the world, we also performed a stratified analysis across geographic regions (Asia, Europe, North America, Oceania) regarding raw CVD prevalence and incidence in SMI populations, and compared patients to their respective region‐specific general population controls (calculating RRs as well as adjusted ORs and HRs for the four regional strata and comparing them across the different regions whenever at least two studies provided data per each region).
The following study and patient characteristics were explored as potential moderators and mediators in addition to SMI status: geographical region of the sample; time of data collection; percentage of patients taking antipsychotics; and the difference between patient and control samples regarding age, body mass index, proportion of males and of those with married status, unemployed, with poorest income, least urbanized, and having co‐occurring obesity, alcohol and substance‐related disorders, diabetes, hypertension or hyperlipidemia.
RESULTS
Search results
Out of 18,064 initial hits across the searched electronic databases, 11,878 unduplicated hits were screened, and 11,576 were excluded through title/abstract reading. Altogether, 302 full texts were reviewed, and 210 were excluded with specific reasons. Among 92 studies meeting inclusion criteria, 27 had a cross‐sectional design22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 and 65 studies had a retrospective or prospective longitudinal design49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113 (Figure 1).
Figure 1.
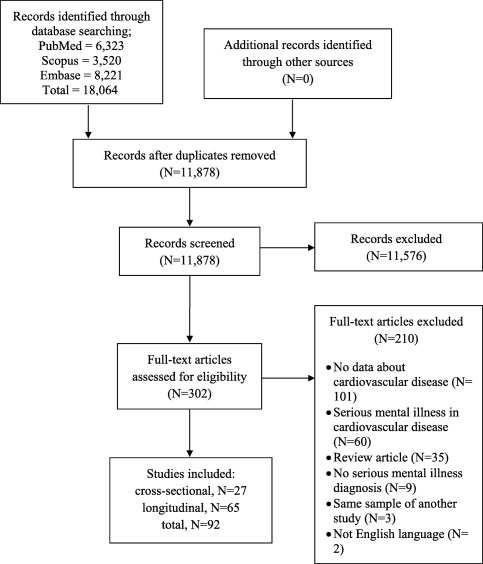
PRISMA flow chart
Characteristics of included studies
We included 92 studies, with a total population of 3,211,768 patients (mean age 50 years, 49% male) with SMI and 113,383,368 controls (mean age 51 years, 49% male), with a total of 116,595,136 subjects when summing those studies where patient and control sample sizes were not separately reported. Altogether, 27 studies (N=27,037,943) were cross‐sectional and 65 studies (N=89,557,193) were longitudinal. Overall, 38 studies included patients with schizophrenia (of which 29 were longitudinal), 30 with bipolar disorder (21 longitudinal), 30 with major depressive disorder (22 longitudinal), and 14 with SMI (8 longitudinal). Taken together, six studies included only patients with SMI (N=884,412), 16 studies included only patients with bipolar disorder (N=71,832), 20 studies included only patients with major depressive disorder (N=111,360), and 29 studies included only patients with schizophrenia (N=1,591,106), while 19 studies included different subgroups of SMI, providing data for each of them separately (some studies included more than one diagnostic group, see Tables 1 and 2 for details).
Table 1.
Cross‐sectional studies: characteristics of included studies and samples
| Study | Country | No. cases | No. controls | Period of data collection | SMI definition | Inclusion criteria for cases |
No. covariates |
|---|---|---|---|---|---|---|---|
| Beyer et al22 | USA | 1,379 | ‐ | 2001‐2002 | Medical records | Bipolar disorder | ‐ |
| Bresee et al23 | Canada | 28,775 | 2,281,636 | 1995‐2006 | ICD‐9,10 | Schizophrenia | 4 |
| Bresee et al24 | Canada | 399 | 120,044 | 2005 | Medical records | Schizophrenia | 11 |
| Chen et al25 | Taiwan | 80 | ‐ | 2015 | DSM‐IV | Bipolar disorder, >60 years | ‐ |
| Curkendall et al26 | Canada | 3,022 | 12,088 | 1994‐1999 | ICD‐9 | Schizophrenia | 7 |
| Devantier et al27 | Denmark | 28 | 27 | 2009‐2011 | ICD‐10 | Major depressive disorder, late onset | ‐ |
| Hagg et al28 | Sweden | 269 | ‐ | 2000‐2003 | DSM‐IV | Schizophrenia, 20‐69 years | ‐ |
| Herbst et al29 | USA | 10,573 total population | 2001‐2002 | DSM‐IV | Major depressive disorder, >60 years | 11 | |
| Huang et al30 | Taiwan | 117,987 | 21,356,304 | 2000‐2003 | ICD‐9 | Bipolar disorder or major depressive disorder | 1 |
| Hyde et al31 | Australia | 355 | ‐ | 2008‐2012 | Medical records | Severe mental illness, prescribed clozapine | ‐ |
| Kilbourne et al32 | USA | 8,083 | ‐ | 2001 | ICD‐9 | Severe mental illness, >60 years | ‐ |
| Kilbourne et al33 | USA | 9,705 | 5,353 | 2000‐2001 | ICD‐9 | Bipolar disorder or severe mental illness, male | 3 |
| Lindegard34 | Sweden | 368 | 87,176 | 1966‐1979 | ICD‐9, DSM‐III | Major depressive disorder or bipolar disorder | ‐ |
| Maina et al35 | Italy | 185 | ‐ | 2006‐2008 | DSM‐IV | Severe mental illness | ‐ |
| Morden et al36 | Canada | 65,362 | 65,362 | 2000‐2007 | ICD‐9 | Schizophrenia | 4 |
| Munoli et al37 | India | 120 | ‐ | 2011 | ICD‐10 | Bipolar disorder | ‐ |
| Nielsen et al38 | Denmark | 937 | ‐ | 1969‐2014 | ICD‐10 | Schizophrenia | ‐ |
| Niranjan et al39 | USA | 5,695 | 34,979 | 2007 | DSM‐IV | Major depressive disorder | 6 |
| Oreski et al40 | Croatia | 289 | 192 | 2011 | ICD‐10 | Bipolar disorder or schizophrenia | ‐ |
| Prieto et al41 | USA | 988 | ‐ | 2009‐2013 | DSM‐IV | Severe mental illness | ‐ |
| Scherrer et al42 | USA | 628 | 6,903 | 1990‐1992 | DSM‐III | Major depressive disorder, male twins | ‐ |
| Scott et al43 | Multicenter | 52,095 total population | 2001‐2011 | DSM‐IV | Bipolar disorder or major depressive disorder | 6 | |
| Shen et al44 | Taiwan | 203 | 2,036 | 2005‐2007 | ICD‐9 | Schizophrenia, in intensive care unit | 6 |
| Smith et al45 | UK | 9,677 | 1,414,701 | 2007 | Medical records | Schizophrenia | 3 |
| Smith et al46 | UK | 2,582 | 1,421,796 | 2007 | Medical records | Bipolar disorder | 2 |
| Swain et al47 | Multicenter | 45,288 total population | 2001‐2011 | DSM‐IV | Bipolar disorder or major depressive disorder | 7 | |
| Zilkens et al48 | Australia | 656 | 349 | 2000‐2009 | ICD‐8,9,10 | Major depressive disorder, 65‐84 years, developing dementia | ‐ |
SMI – severe mental illness
Table 2.
Longitudinal studies: characteristics of included studies and samples
| Study | Country | No. cases | No. controls | Period of data collection | SMI definition | Inclusion criteria for cases | No. covariates |
|---|---|---|---|---|---|---|---|
| Almeida et al49 | Australia | 1,503 | 35,691 | 1996‐2010 | ICD‐9 | Schizophrenia, bipolar disorder or major depressive disorder, 65‐85 years, male | 8 |
| Bremmer et al50 | The Netherlands | 41 | 2,080 | 1992‐2000 | DSM‐III | Major depressive disorder, >55 years | 13 |
| Butnoriene et al51 | Lithuania | 184 | 369 | 2003‐2004 | DSM‐IV | Major depressive disorder, >45 years | 4 |
| Callaghan et al52 | Canada | 5,999 | 5,999 | 2002‐2006 | Medical records | Bipolar disorder | 6 |
| Callaghan et al53 | Canada | 9,815 | 9,815 | 2002‐2006 | ICD‐10 | Bipolar disorder | 8 |
| Carney et al54 | USA | 1,074 | 726,262 | 1996‐2001 | ICD‐9 | Schizophrenia or schizoaffective disorder | 4 |
| Chen et al55 | Taiwan | 63,913 | 63,913 | 2002‐2008 | ICD‐9 | Schizophrenia | 8 |
| Clouse et al56 | USA | 16 | 60 | 1982‐1992 | DSM‐III | Major depressive disorder with diabetes | 7 |
| Coryell et al57 | USA | 903 | ‐ | 1998‐1999 | RDC | Severe mental illness | ‐ |
| Crump et al58 | Sweden | 6,618 | 6,580,418 | 2003‐2009 | ICD‐10 | Bipolar disorder | 6 |
| Crump et al59 | Sweden | 8,277 | 6,097,834 | 2003‐2009 | ICD‐10 | Schizophrenia, >25 years | 6 |
| Davis et al60 | Hawaii | 280 | 39,000 | 1999‐2005 | Medical records | Major depressive disorder | 5 |
| Davydow et al61 | Denmark | 68,137 | 5,912,158 | 1999‐2013 | ICD‐9 | Schizophrenia, schizoaffective disorder or bipolar disorder | 5 |
| Enger et al62 | USA | 1,920 | 9,600 | 1995‐1999 | ICD‐9 | Schizophrenia, on antipsychotic treatment, 15‐64 years | ‐ |
| Fiedorowicz et al63 | USA | 288 | 147 | 1978‐1981 | RDC | Bipolar disorder | 8 |
| Filik et al64 | UK | 482 | 1,998 | 1999‐2002 | DSM‐IV | Schizophrenia, schizophreniform or schizoaffective disorder | 6 |
| Fors et al65 | Sweden | 255 | 1,275 | 1981‐1991 | DSM‐II | Schizophrenia | 3 |
| Gasse et al66 | Denmark | 873,898 | 52,693,301 | 1995‐2009 | ICD‐8,10 | Severe mental illness (affective psychoses) | 22 |
| Goldstein et al67 | USA | 5,835 | 26,266 | 2001‐2005 | DSM‐IV | Bipolar disorder or major depressive disorder | 8 |
| Healy et al68 | UK | 1,429 | ‐ | 1875‐1924; 1994‐2010 | Medical records | Schizophrenia | ‐ |
| Hendrie et al69 | USA | 757 | 30,831 | 1999‐2008 | ICD‐9 | Schizophrenia, >65 years | ‐ |
| Hou et al70 | Taiwan | 8,264 | ‐ | 1985‐2008 | DSM‐III or IV, ICD‐9 | Schizophrenia | ‐ |
| Hsieh et al71 | Taiwan | 9,715 | ‐ | 2001‐2009 | ICD‐9 | Schizophrenia | 10 |
| Huang et al72 | Taiwan | 7,937 | 31,748 | 1996‐2006 | ICD‐9 | Major depressive disorder | 9 |
| Ifteni et al73 | Romania | 7,189 | ‐ | 1989‐2011 | DSM‐IV | Schizophrenia, inpatients | ‐ |
| Jakobsen et al74 | Denmark | 74,759 | 338,747 | 1977‐2000 | ICD‐8,10 | Schizophrenia or major depressive disorder | 2 |
| Janszky et al75 | Sweden | 646 | 48,675 | 1969‐2007 | ICD‐8 | Major depressive disorder, 18‐20 years | 7 |
| Jokinen & Nordstrom76 | Sweden | 346 | ‐ | 1980‐2005 | DSM‐IV | Major depressive disorder or bipolar disorder | ‐ |
| Joukamaa et al77 | Finland | 606 | 8,000 | 1977‐1994 | Medical records | Schizophrenia, mood disorder or severe mental illness | 1 |
| Kendler et al78 | Sweden | 5,647 | 24,727 | 1998‐2003 | ICD‐10 | Major depressive disorder, twins | ‐ |
| Kiviniemi et al79 | Finland | 6,987 | ‐ | 1998‐2003 | ICD‐9 | Schizophrenia, first onset | ‐ |
| Lahti et al80 | Finland | 204 | 11,880 | 1969‐2004 | ICD‐8,9,10 | Schizophrenia | 5 |
| Lan et al81 | Taiwan | 3,681 | ‐ | 2001‐2006 | ICD‐9 | Bipolar disorder | ‐ |
| Laursen et al82 | Denmark | 22,294 | 2,411,852 | 1995‐2007 | ICD‐8,10 | Schizophrenia or bipolar disorder, 15‐52 years | 3 |
| Laursen et al83 | Denmark | 1,454 | 59,256 | 1995‐2006 | ICD‐8,10 | Schizophrenia or bipolar disorder | 4 |
| Lemogne et al84 | France | 4,336 | 16,621 | 1990‐2010 | ICD‐9,10 | Depression or severe mental illness (bipolar disorder, psychosis) | 6 |
| Li et al85 | Taiwan | 1,003 | 4,012 | 1996‐2009 | ICD‐9 | Major depressive disorder | 6 |
| Lin et al86 | Taiwan | 7,353 | 22,059 | 2000‐2006 | ICD‐9 | Schizophrenia | 8 |
| Lin et al87 | Taiwan | 2,289 | 16,413 | 1998‐2003 | ICD‐9 | Bipolar disorder | 10 |
| Lin et al88 | Taiwan | 5,001 | 10,002 | 1998‐2003 | ICD‐9 | Schizophrenia, <45 years | 9 |
| Maina et al89 | Italy | 309 | ‐ | 2003‐2011 | DSM‐IV | Bipolar disorder | ‐ |
| McDermott et al90 | USA | 503 | 2,083 | 1990‐2003 | ICD‐9 | Schizophrenia or severe mental illness | 9 |
| Murray‐Thomas et al91 | UK | 232,132 | 193,920 | 1997‐2001 | ICD‐10 | Schizophrenia, bipolar disorder or major depressive disorder | 2 |
| Olfson et al92 | USA | 1,138,853 | ‐ | 2001‐2007 | ICD‐10 | Schizophrenia, 20‐64 years | 4 |
| Osborn et al93 | UK | 38,824 | ‐ | 1995‐2010 | Medical records | Bipolar disorder or severe mental illness, 30‐90 years | ‐ |
| Pratt et al94 | USA | 73 | 1,107 | 1981‐1994 | DSM‐III | Major depressive disorder | 11 |
| Prieto et al95 | USA | 334 | 334 | 1966‐1996 | DSM‐IV | Bipolar disorder | 4 |
| Rahman et al96 | Sweden | 6,822 | 29,832 | 1998‐2002 | ICD‐7,8,9,10 | Major depressive disorder, twin population study | 7 |
| Ramsey et al97 | USA | 129 | 1,339 | 1981‐1982 | DSM‐III | Bipolar disorder or major depressive disorder | 6 |
| Saint Onge et al98 | USA | 548 | 10,821 | 1999‐2006 | ICD | Major depressive disorder | 11 |
| Scherrer et al99 | USA | 77,568 | 214,749 | 1999‐2007 | ICD‐9 | Major depressive disorder, 25‐80 years | 4 |
| Schoepf & Heun100 | UK | 1,418 | 14,180 | 2000‐2012 | ICD‐10 | Schizophrenia, inpatients | ‐ |
| Schoepf et al101 | UK | 621 | 6,210 | 2000‐2012 | ICD‐10 | Bipolar disorder | ‐ |
| Shah et al102 | USA | 538 | 7,103 | 1988‐2006 | DSM‐III | Major depressive disorder or bipolar disorder, 17‐39 years | 14 |
| Stewart et al103 | USA | 235 | ‐ | NA | ICD‐9 | Major depressive disorder | ‐ |
| Surtees et al104 | UK | 3,057 | 16,592 | 1996‐2008 | DSM‐IV | Major depressive disorder, 45‐80 years | 11 |
| Ting et al105 | China | 153 | 7,682 | 1996‐2008 | DSM‐IV | Major depressive disorder with diabetes | 18 |
| Torniainen et al106 | Sweden | 21,492 | 214,920 | 2006‐2015 | ICD‐10 | Schizophrenia, 17‐65 years | 2 |
| Tsai et al107 | Taiwan | 80,569 | 241,707 | 1999‐2003 | ICD‐9 | Schizophrenia | 8 |
| Tsan et al108 | USA | 49,173 | ‐ | 2002‐2009 | ICD‐9 | Schizophrenia | ‐ |
| van Marwijk et al109 | The Netherlands | 143 | 139 | 2002‐2003 | DSM‐IV | Major depressive disorder, >55 years | ‐ |
| Weeke et al110 | Denmark | 3,795 | ‐ | 1950‐1957; 1969‐1977 | ICD‐8 | Bipolar disorder | ‐ |
| Westman et al111 | Sweden | 17,101 | 10,631,208 | 1987‐2006 | ICD‐10 | Bipolar disorder | 3 |
| Wu et al112 | Taiwan | 16,821 | 67,284 | 1999‐2010 | ICD‐9 | Bipolar disorder | 9 |
| Wu et al113 | Taiwan | 70,225 | 207,592 | 1996‐2007 | ICD‐9 | Schizophrenia or bipolar disorder | 8 |
SMI – severe mental illness, RDC – Research Diagnostic Criteria
Meta‐analysis: cross‐sectional results
The pooled CVD prevalence in SMI was 9.9% (95% CI: 7.4‐13.3; 38 studies). Individual rates were 8.4% for people with bipolar disorder (95% CI: 5.4‐12.6, 12 studies, N=66,911); 11.7% for those with major depressive disorder (95% CI: 3.6‐32.2, 7 studies, N=83,965); 11.8% for those with schizophrenia (95% CI: 7.1‐19.0, 13 studies, N=191,982), and 11.8% for those with SMI (95% CI: 4.1‐29.4, 6 studies, N=17,286) (p<0.001 for SMI diagnostic subgroup comparisons).
Adjusting for a median of seven potential confounders, the adjusted pooled OR for CVD in SMI compared to controls was 1.53 (95% CI: 1.27‐1.83, p<0.001, 11 studies). For specific CVDs, pooled together, people with SMI had an increased risk of coronary heart disease (OR=1.51, 95% CI: 1.47‐1.55, p<0.001, 5 studies) and cerebrovascular disease (OR=1.42, 95% CI: 1.21‐1.66, p<0.001, 6 studies), with a strong statistical trend for congestive heart failure (OR=1.28, 95% CI: 0.99‐1.65, p=0.06, 4 studies). Considering separately single types of SMI and CVD, in adjusted OR analyses, bipolar disorder was not significantly associated with CVD or its subtypes; major depressive disorder was significantly associated with CVD and coronary heart disease; and schizophrenia was significantly associated with coronary heart disease, cerebrovascular disease and congestive heart failure (Table 3). No adjusted ORs were available for mixed SMI groups.
Table 3.
Meta‐analysis of cross‐sectional studies: unadjusted and adjusted odds ratios
| Meta‐analysis of unadjusted odds ratios | Meta‐analysis of covariate adjusted odds ratios | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. studies | No. participants | Unadjusted odds ratios | Hetero‐geneity | No. studies | No. participants | Adjusted odds ratios | Hetero‐geneity | |||||||||
| Disorder | Patients | Controls | OR | 95% CI | p | I2 | Patients | Controls | OR | 95% CI | p | I2 | ||||
| Cardiovascular disease | ||||||||||||||||
| Bipolar disorder | 4 | 19,562 | 1,526,110 | 1.73 | 1.11 | 2.71 | 0.02 | 91 | 4 | 2,640 | 1,423,135 | 1.28 | 0.90 | 1.80 | 0.17 | 52 |
| Major depressive disorder | 3 | 1,577 | 47,851 | 2.08 | 1.51 | 2.88 | <0.001 | 58 | 7 | 7,050 | 43,570 | 1.75 | 1.36 | 2.26 | <0.001 | 69 |
| Schizophrenia | 10 | 190,584 | 4,100,315 | 1.23 | 0.92 | 1.65 | 0.16 | 99 | 5 | 42,076 | 3,860,505 | 1.38 | 0.93 | 2.05 | 0.11 | 96 |
| Severe mental illnesses | 1 | 146 | 2,083 | 1.59 | 0.87 | 2.88 | 0.13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pooled | 14 | 211,869 | 7,808,603 | 1.59a | 1.32 | 1.91 | <0.001 | 99 | 11 | 51,766 | 5,325,871 | 1.53e | 1.27 | 1.83 | <0.001 | 94 |
| Coronary heart disease | ||||||||||||||||
| Bipolar disorder | 3 | 19,504 | 1,524,771 | 1.75 | 1.11 | 2.77 | 0.02 | 94 | 1 | 2,582 | 1,421,796 | 0.94 | 0.79 | 1.11 | 0.49 | ‐ |
| Major depressive disorder | 1 | 958 | 35,691 | 2.44 | 2.13 | 2.79 | <0.0001 | ‐ | 3 | 6,323 | 41,882 | 2.52 | 1.81 | 3.52 | <0.001 | 93 |
| Schizophrenia | 8 | 187,359 | 4,086,191 | 1.03 | 0.85 | 1.25 | 0.76 | 98 | 1 | 399 | 120,044 | 1.52 | 1.48 | 1.56 | <0.001 | ‐ |
| Severe mental illnesses | 1 | 146 | 2,083 | 1.02 | 0.56 | 1.83 | 0.96 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Pooled | 8 | 207,967 | 4,160,030 | 1.80b | 1.62 | 2.00 | <0.001 | 98 | 5 | 9,304 | 1,583,722 | 1.51f | 1.47 | 1.55 | <0.001 | 90 |
| Cerebrovascular disease | ||||||||||||||||
| Bipolar disorder | 3 | 2,741 | 1,458,826 | 1.68 | 1.07 | 2.63 | 0.03 | 47 | 2 | 2,582 | 1,421,796 | 1.06 | 0.85 | 1.31 | 0.62 | 0 |
| Major depressive disorder | 3 | 1,577 | 47,851 | 2.24 | 1.33 | 3.79 | 0.003 | 81 | 2 | 656 | 349 | 1.64 | 0.96 | 2.78 | 0.07 | 72 |
| Schizophrenia | 5 | 41,071 | 37,77,039 | 1.63 | 1.19 | 2.24 | 0.003 | 96 | 3 | 32,196 | 2,413,768 | 2.05 | 1.59 | 2.64 | <0.001 | 61 |
| Severe mental illnesses | 1 | 146 | 2,083 | 1.02 | 0.56 | 1.83 | 0.96 | ‐ | ‐ | ‐ | ‐ | ‐ | – | ‐ | ‐ | ‐ |
| Pooled | 10 | 45,535 | 5,454,785 | 1.63c | 1.31 | 2.02 | <0.0001 | 93 | 6 | 35,434 | 3,835,913 | 1.42g | 1.21 | 1.66 | <0.001 | 90 |
| Congestive heart failure | ||||||||||||||||
| Bipolar disorder | 1 | 2,582 | 1,421,796 | 1.38 | 1.03 | 1.84 | 0.03 | ‐ | 1 | 2,582 | 1,421,796 | 1.11 | 0.80 | 1.54 | 0.53 | 0 |
| Major depressive disorder | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Schizophrenia | 5 | 40,984 | 3,743,431 | 1.71 | 1.36 | 2.15 | <0.001 | 92 | 3 | 41,474 | 5,708,425 | 1.60 | 1.06 | 2.40 | 0.02 | 97 |
| Severe mental illnesses | 1 | 146 | 2,083 | 1.59 | 0.87 | 2.88 | 0.13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pooled | 6 | 43,712 | 5,167,189 | 1.57d | 1.32 | 1.87 | <0.001 | 88 | 4 | 44,056 | 7,130,221 | 1.28h | 0.99 | 1.65 | 0.06 | 96 |
Bold values represent significant results
Egger test for bias: a2.24, p=0.53; b6.41, p=0.03 (Duval & Tweedie trim‐and‐fill procedure adjusted OR: 1.35, 95% CI: 0.98‐1.83); c−1.69, p=0.24; d−2.39, p=0.15; e−1.73, p=0.18; f0.17, p=0.93; g−2.59, p=0.07; h−5.26, p=0.20
All significant results were significantly heterogeneous. After adjusting for publication bias with the trim‐and‐fill method, all pooled previously significant ORs remained statistically significant, confirming the association of CVD, coronary heart disease and cerebrovascular disease with SMI, while the OR for congestive heart failure became marginally significant (p=0.05).
Meta‐analysis: longitudinal adjusted results
Among patients with SMI, 3.6% (95% CI: 2.7‐5.3%) experienced a CVD event during a median follow‐up period of 8.4 years (range 1.8‐30.0) (65 studies). After adjusting for a median of six potential confounders, people with SMI were at significantly increased risk across longitudinal studies for CVD (HR=1.78, 95% CI: 1.60‐1.98) (31 studies, N=671,384 cases vs. N=14,335,203 controls) as well as for specific CVDs, including coronary heart disease (HR=1.54, 95% CI: 1.30‐1.82, 18 studies, N=194,017 cases vs. N=13,530,858 controls), cerebrovascular disease (HR=1.64, 95% CI: 1.26‐2.14, 11 studies, N=188,841 cases vs. N=13,113,564 controls), congestive heart failure (HR=2.10, 95% CI: 1.64‐2.70, 2 studies, N=409 cases vs. N=41,678 controls), peripheral vascular disease (only unadjusted RR=3.11, 95% CI: 2.46‐3.91, three studies), and CVD‐related death (HR=1.85, 95% CI: 1.53‐2.24, 16 studies, N=353,407 cases vs. N=7,317,053 controls).
According to adjusted HRs, schizophrenia was significantly associated with CVD in longitudinal studies (HR=1.95, 95% CI: 1.41‐2.70, 14 studies), as well as with coronary heart disease (HR=1.59, 95% CI: 1.08‐2.35, 5 studies), cerebrovascular disease (HR=1.57, 95% CI: 1.09‐2.25, 5 studies), and CVD‐related death (HR=2.45, 95% CI: 1.64‐3.65, 9 studies).
According to adjusted HRs, bipolar disorder was significantly associated with CVD in longitudinal studies (HR=1.57, 95% CI: 1.28‐1.93, 10 studies) as well as with CVD‐related death (HR=1.65, 95% CI: 1.10‐2.47, 3 studies), with a trend toward a significant association with cerebrovascular disease (HR=1.60, 95% CI: 0.99‐2.57, 4 studies), but no significant association with coronary heart disease (HR=1.16, 95% CI: 0.76‐1.78, 4 studies). One study reported a significant association with congestive heart failure (HR = 2.27, 95% CI: 1.49‐3.45).
According to adjusted HRs, major depressive disorder was significantly associated with CVD in longitudinal studies (HR=1.72, 95% CI: 1.48‐2.00, 18 studies) as well as with coronary heart disease (HR=1.63, 95% CI: 1.33‐2.00, 9 studies), cerebrovascular disease (HR=2.04, 95% CI: 1.05‐3.96, 3 studies), congestive heart failure (HR=2.02, 95% CI: 1.48‐2.75, 2 studies), and CVD‐related death (HR=1.63, 95% CI: 1.25‐2.13, 7 studies).
According to adjusted HRs, mixed SMIs were significantly associated with CVD in longitudinal studies (HR=3.24, 95% CI: 2.15‐4.88, 3 studies) as well as with CVD‐related death (HR=2.75, 95% CI: 1.32‐5.73, 3 studies).
All significant results were significantly heterogeneous, except for mixed SMI and CVD risk, as well as all the congestive heart failure results. After trim and fill procedure, all results remained unchanged, and Egger test did not show any evidence of publication bias influencing the results (see Table 4 for details).
Table 4.
Meta‐analysis of longitudinal studies with publication bias assessment
| Meta‐analysis of unadjusted relative risk | Meta‐analysis of covariate adjusted hazard ratio | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. studies | No. participants | Unadjusted relative risk | Hetero‐geneity | No. studies | No. participants | Adjusted hazard ratio | Hetero‐geneity | |||||||||
| Disorder | Patients | Controls | RR | 95% CI | p | I2 | Patients | Controls | HR | 95% CI | p | I2 | ||||
| Cardiovascular disease | ||||||||||||||||
| Bipolar disorder | 12 | 66,549 | 9,606,575 | 1.50 | 1.28 | 1.75 | <0.0001 | 76 | 10 | 91,187 | 6,967,728 | 1.57 | 1.28 | 1.93 | <0.0001 | 91 |
| Major depressive disorder | 13 | 328,431 | 800,718 | 1.29 | 0.92 | 1.81 | 0.14 | 99 | 18 | 282,621 | 682,045 | 1.72 | 1.48 | 2.00 | <0.0001 | 67 |
| Schizophrenia | 16 | 361294 | 16,096,125 | 1.21 | 1.006 | 1.45 | 0.04 | 98 | 14 | 296,778 | 7,176,374 | 1.95 | 1.41 | 2.70 | <0.0001 | 99 |
| Severe mental illnesses | 2 | 874022 | 52,709,922 | 2.44 | 1.13 | 5.25 | 0.02 | 74 | 3 | 798 | 31,724 | 3.24 | 2.15 | 4.88 | <0.0001 | 0 |
| Pooled | 33 | 1,630,296 | 76,031,192 | 1.38a | 1.23 | 1.54 | <0.0001 | 98 | 31 | 671,384 | 14,335,203 | 1.78g | 1.60 | 1.98 | <0.0001 | 95 |
| Coronary heart disease | ||||||||||||||||
| Bipolar disorder | 4 | 25,286 | 9,200,196 | 1.95 | 1.20 | 3.17 | 0.007 | 96 | 4 | 19,129 | 6,789,683 | 1.16 | 0.76 | 1.78 | 0.49 | 87 |
| Major depressive disorder | 6 | 14,3671 | 515,187 | 1.15 | 0.71 | 1.85 | 0.57 | 98 | 9 | 99,028 | 392,210 | 1.63 | 1.33 | 2.00 | <0.0001 | 80 |
| Schizophrenia | 8 | 169,507 | 15,446,625 | 0.93 | 0.81 | 1.08 | 0.33 | 87 | 5 | 75,860 | 6,348,965 | 1.59 | 1.08 | 2.35 | 0.02 | 95 |
| Severe mental illnesses | 1 | 873,898 | 52,693,301 | 1.80 | 1.74 | 1.86 | <0.0001 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pooled | 17 | 1,212,362 | 75,235,865 | 1.75b | 1.69 | 1.80 | <0.0001 | 99 | 18 | 194,017 | 13,530,858 | 1.54h | 1.30 | 1.82 | <0.0001 | 92 |
| Cerebrovascular disease | ||||||||||||||||
| Bipolar disorder | 6 | 32,898 | 9,082,511 | 1.92 | 1.13 | 3.26 | 0.02 | 97 | 4 | 23,831 | 6,649,375 | 1.60 | 0.99 | 2.57 | 0.05 | 85 |
| Major depressive disorder | 4 | 8,121 | 41,665 | 1.55 | 1.02 | 2.35 | 0.04 | 77 | 3 | 7,046 | 38,853 | 2.04 | 1.05 | 3.96 | 0.04 | 74 |
| Schizophrenia | 8 | 243,254 | 15,475,608 | 1.48 | 1.21 | 1.81 | <0.0001 | 96 | 5 | 157,964 | 6,425,336 | 1.57 | 1.09 | 2.25 | 0.02 | 95 |
| Severe mental illnesses | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pooled | 17 | 284,273 | 22,187,932 | 1.53c | 1.29 | 1.82 | <0.0001 | 96 | 11 | 188,841 | 13,113,564 | 1.64i | 1.26 | 2.14 | <0.0001 | 90 |
| Congestive heart failure | ||||||||||||||||
| Bipolar disorder | 1 | 6,215 | 2,411,852 | 11.52 | 9.37 | 23.14 | <0.0001 | ‐ | 1 | 58 | 1,339 | 2.27 | 1.49 | 3.45 | <0.0001 | 0 |
| Major depressive disorder | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 2 | 351 | 40,339 | 2.02 | 1.48 | 2.75 | <0.0001 | 0 |
| Schizophrenia | 3 | 85,290 | 9,050,272 | 1.80 | 1.15 | 2.79 | 0.009 | 84 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Severe mental illnesses | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pooled | 4 | 91,505 | 11,459,059 | 8.24d | 6.84 | 9.94 | <0.0001 | 99 | 2 | 409 | 41,678 | 2.10 | 1.64 | 2.70 | <0.0001 | 0 |
| Peripheral vascular disease | ||||||||||||||||
| Bipolar disorder | 1 | 6,215 | 2,411,852 | 3.44 | 2.70 | 4.38 | <0.0001 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major depressive disorder | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Schizophrenia | 3 | 85,290 | 9,050,272 | 0.96 | 0.43 | 2.17 | 0.92 | 93 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Severe mental illnesses | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pooled | 3 | 91,505 | 11,402,868 | 3.11e | 2.46 | 3.91 | <0.0001 | 98 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Death due to cardiovascular disease | ||||||||||||||||
| Bipolar disorder | 5 | 37,144 | 356,298 | 1.31 | 0.94 | 1.83 | 0.11 | 75 | 3 | 17,420 | 162,231 | 1.65 | 1.10 | 2.47 | 0.02 | 88 |
| Major depressive disorder | 5 | 18,112 | 283,746 | 1.30 | 0.59 | 2.86 | 0.51 | 99 | 7 | 183,297 | 282,014 | 1.63 | 1.25 | 2.13 | <0.0001 | 81 |
| Schizophrenia | 9 | 53,779 | 7,179,454 | 1.26 | 0.84 | 1.90 | 0.27 | 96 | 9 | 152,690 | 6,872,808 | 2.45 | 1.64 | 3.65 | <0.0001 | 96 |
| Severe mental illnesses | 3 | 874,146 | 52,714,134 | 2.99 | 2.84 | 3.13 | <0.0001 | 0 | 3 | 798 | 31,724 | 2.75 | 1.32 | 5.73 | 0.007 | 75 |
| Pooled | 18 | 1,151,181 | 60,287,400 | 2.89f | 2.75 | 3.03 | <0.0001 | 99 | 16 | 353,407 | 7,317,053 | 1.85j | 1.53 | 2.24 | <0.0001 | 95 |
Egger test for bias: a−0.44, p=0.80; b−1.24, p=0.71; c0.03, p=0.96; d8.07, p=0.37; e3.08, p=0.60; f−3.66, p=0.20; g1.16, p=0.31; h−0.13, p=0.92; i2.57, p=0.07; j−1.19, p=0.43
Quality assessment of included studies
Quality ratings of single studies are presented in Table 5. All studies used clear diagnostic criteria, by design. Among the 27 cross‐sectional studies, all except 9 studies had a control group, 5 studies used a matched control sample, 13 studies adjusted analyses for relevant covariates, and all except 6 studies reported cardiovascular risk factors. Among the 65 longitudinal studies, all had a control group, which was matched in all but 12 studies, only 19 studies adjusted for covariates, 38 studies reported on cardiovascular risk factors, and all except 12 studies had a follow‐up of at least 5 years.
Table 5.
Quality assessment of included studies
| Study | Clear diagnostic criteria | Control group | Matched controls | Coavariate adjusted analyses | Reported cardiovascular risk factors at baseline | Follow‐up at least 5 years |
|---|---|---|---|---|---|---|
| Cross‐sectional studies | ||||||
| Beyer et al22 | Y | N | N | N | Y | N |
| Bresee et al23 | Y | Y | N | Y | Y | N |
| Bresee et al24 | Y | Y | N | Y | Y | N |
| Chen et al25 | Y | N | N | N | Y | N |
| Curkendall et al26 | Y | Y | Y | Y | Y | N |
| Devantier et al27 | Y | Y | Y | N | Y | N |
| Hagg et al28 | Y | N | N | N | Y | N |
| Herbst et al29 | Y | Y | N | Y | N | N |
| Huang et al30 | Y | Y | N | Y | Y | N |
| Hyde et al31 | Y | N | N | N | Y | N |
| Kilbourne et al32 | Y | N | N | N | Y | N |
| Kilbourne et al33 | Y | Y | N | Y | Y | N |
| Lindegard34 | Y | Y | N | N | N | N |
| Maina et al35 | Y | N | N | N | Y | N |
| Morden et al36 | Y | Y | Y | Y | Y | N |
| Munoli et al37 | Y | N | N | N | Y | N |
| Nielsen et al38 | Y | N | N | N | Y | N |
| Niranjan et al39 | Y | Y | N | Y | Y | N |
| Oreski et al40 | Y | Y | N | N | Y | N |
| Prieto et al41 | Y | N | N | N | Y | N |
| Scherrer et al42 | Y | Y | N | N | N | N |
| Scott et al43 | Y | Y | N | Y | N | N |
| Shen et al44 | Y | Y | Y | Y | Y | N |
| Smith et al45 | Y | Y | N | Y | Y | N |
| Smith et al46 | Y | Y | N | Y | Y | N |
| Swain et al47 | Y | Y | N | Y | N | N |
| Zilkens et al48 | Y | Y | Y | N | N | N |
| Longitudinal studies | ||||||
| Almeida et al49 | Y | Y | Y | N | Y | Y |
| Bremmer et al50 | Y | Y | Y | N | Y | Y |
| Butnoriene et al51 | Y | Y | Y | N | N | Y |
| Callaghan et al52 | Y | Y | Y | Y | Y | N |
| Callaghan et al53 | Y | Y | Y | Y | Y | N |
| Carney et al54 | Y | Y | Y | N | Y | N |
| Chen et al55 | Y | Y | Y | Y | Y | Y |
| Clouse et al56 | Y | Y | Y | N | Y | Y |
| Coryell et al57 | Y | Y | N | N | N | Y |
| Crump et al58 | Y | Y | Y | N | N | Y |
| Crump et al59 | Y | Y | Y | N | Y | Y |
| Davis et al60 | Y | Y | Y | N | Y | N |
| Davydow et al61 | Y | Y | Y | N | N | Y |
| Enger et al62 | Y | Y | Y | Y | Y | N |
| Fiedorowicz et al63 | Y | Y | Y | N | Y | Y |
| Filik et al64 | Y | Y | Y | N | Y | N |
| Fors et al65 | Y | Y | Y | Y | N | Y |
| Gasse et al66 | Y | Y | Y | N | N | Y |
| Goldstein et al67 | Y | Y | Y | N | Y | N |
| Healy et al68 | Y | Y | N | N | N | Y |
| Hendrie et al69 | Y | Y | Y | N | Y | Y |
| Hou et al70 | Y | Y | N | N | N | Y |
| Hsieh et al71 | Y | Y | N | N | N | N |
| Huang et al72 | Y | Y | Y | Y | Y | Y |
| Ifteni et al73 | Y | Y | N | N | N | Y |
| Jakobsen et al74 | Y | Y | Y | Y | N | Y |
| Janszky et al75 | Y | Y | Y | N | Y | Y |
| Jokinen & Nordstrom et al76 | Y | Y | N | N | N | Y |
| Joukamaa et al77 | Y | Y | Y | N | N | Y |
| Kendler et al78 | Y | Y | Y | N | N | Y |
| Kiviniemi et al79 | Y | Y | N | N | N | Y |
| Lahti et al80 | Y | Y | Y | N | Y | Y |
| Lan et al81 | Y | Y | N | N | Y | Y |
| Laursen et al82 | Y | Y | Y | N | N | Y |
| Laursen et al83 | Y | Y | Y | N | N | Y |
| Lemogne et al84 | Y | Y | Y | N | Y | Y |
| Li et al85 | Y | Y | Y | Y | Y | Y |
| Lin et al86 | Y | Y | Y | Y | Y | Y |
| Lin et al87 | Y | Y | Y | Y | Y | Y |
| Lin et al88 | Y | Y | Y | Y | Y | Y |
| Maina et al89 | Y | Y | N | N | N | Y |
| McDermott et al90 | Y | Y | Y | N | N | Y |
| Murray‐Thomas et al91 | Y | Y | Y | N | N | N |
| Olfson et al92 | Y | Y | N | N | N | Y |
| Osborn et al93 | Y | Y | Y | N | Y | Y |
| Pratt et al94 | Y | Y | Y | N | Y | Y |
| Prieto et al95 | Y | Y | Y | Y | Y | Y |
| Rahman et al96 | Y | Y | Y | Y | Y | N |
| Ramsey et al97 | Y | Y | Y | N | Y | Y |
| Saint Onge et al98 | Y | Y | Y | N | Y | Y |
| Scherrer et al99 | Y | Y | Y | N | Y | Y |
| Schoepf & Heun100 | Y | Y | Y | Y | N | Y |
| Schoepf et al101 | Y | Y | Y | Y | Y | Y |
| Shah et al102 | Y | Y | Y | N | Y | Y |
| Stewart et al103 | Y | Y | N | N | N | Y |
| Surtees et al104 | Y | Y | Y | N | N | Y |
| Ting et al105 | Y | Y | Y | N | Y | Y |
| Torniainen et al106 | Y | Y | Y | Y | N | Y |
| Tsai et al107 | Y | Y | Y | Y | Y | Y |
| Tsan et al108 | Y | Y | N | N | Y | Y |
| van Marwijk et al109 | Y | Y | Y | Y | Y | N |
| Weeke et al110 | Y | Y | N | N | N | N |
| Westman et al111 | Y | Y | Y | N | N | Y |
| Wu et al112 | Y | Y | Y | Y | Y | Y |
| Wu et al113 | Y | Y | Y | N | Y | Y |
N – no, Y – yes
Regional CVD prevalence, incidence and longitudinal risk
Raw CVD prevalence and incidence rates consistently increased from Asia, through Europe and North America, to Oceania (Asia: 5.4% and 2.6%; Europe: 9.7% and 3.4%; North America: 14.6% and 4.6%; Oceania: 20.6% and 26.3%; p<0.0001 for both prevalence and incidence). However, when comparing CVD risk in SMI patients in each region with their respective control groups, there was no statistically significant difference anymore across regions, with both RRs and adjusted HRs showing comparably increased CVD incidence risk in the SMI population (RRs ranging from 1.17 in Europe to 1.63 in Asia, p=0.08; and HRs ranging from 1.58 in Oceania to 1.88 in both Europe and North America, p=0.29) (Table 6). There were insufficient numbers of studies to perform this analysis for adjusted ORs regarding prevalence rates across regions, or for adjusted ORs, RRs or HRs pertaining to specific CVD subgroups.
Table 6.
Prevalence and incidence of cardiovascular disease (CVD) in severe mental illness stratified by region
| Regional strata | Analysis details | Prevalence of CVD | Incidence of CVD | Risk ratios for incident CVD | Adjusted hazard ratios for incident CVD |
|---|---|---|---|---|---|
| Asia | Pooled estimate, % (95% CI) | 5.4 (4.3‐6.7) | 2.6 (1.9‐3.6) | 1.63 (1.31‐2.04) | 1.75 (1.38‐2.22) |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Heterogeneity, I2 (p value) | 98 (<0.0001) | 100 (<0.0001) | 99 (<0.0001) | 96 (<0.0001) | |
| No. comparisons | 8 | 12 | 9 | 10 | |
| Europe | Pooled estimate, % (95% CI) | 9.7 (6.5‐14.2) | 3.4 (2.2‐5.3) | 1.17 (0.96‐1.42) | 1.88 (1.44‐2.46) |
| p value | <0.0001 | <0.0001 | 0.11 | <0.0001 | |
| Heterogeneity, I2 (p value) | 97 (<0.0001) | 100 (<0.0001) | 97 (<0.0001) | 96 (<0.0001) | |
| No. comparisons | 9 | 35 | 20 | 22 | |
| North America | Pooled estimate, % (95% CI) | 14.6 (12.0‐17.7) | 4.6 (3.4‐6.2) | 1.39 (0.91‐2.12) | 1.88 (1.62‐2.19) |
| p value | <0.0001 | <0.0001 | 0.13 | <0.0001 | |
| Heterogeneity, I2 (p value) | 97 (<0.0001) | 100 (<0.0001) | 97 (<0.0001) | 62 (0.003) | |
| No. comparisons | 17 | 15 | 11 | 11 | |
| Oceania | Pooled estimate, % (95% CI) | 20.6 (10.9‐35.4) | 26.3 (24.1‐28.6) | 1.52 (1.40‐1.66) | 1.58 (1.41‐1.78) |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Heterogeneity, I2 (p value) | 97 (<0.0001) | 100 (<0.0001) | 0 (0.72) | 0 (0.84) | |
| No. comparisons | 4 | 3 | 3 | 3 | |
| p (difference between regions) | <0.0001 | <0.0001 | 0.08 | 0.29 |
Meta‐regression
Due to heterogeneous or partial reporting of possible moderator variables in the included studies, all meta‐regression analyses were based on a much reduced number of studies. Hence, all analyses were less powered in comparison with the large sets of data used for cross‐sectional prevalence and longitudinal incidence analyses. Nonetheless, CVD incidence increased significantly with a higher percentage of patients using antipsychotics (12 studies; β=0.04, 95% CI: 0.01‐0.08, p=0.008), higher baseline body mass index in patients vs. controls (6 studies; β=0.24, 95% CI: 0.06‐0.42, p=0.008), and higher CVD prevalence at baseline in patients vs. controls (7 studies; β=0.07, 95% CI: 0.01‐0.14, p=0.03). CVD prevalence increased in more recent studies (38 studies; β=0.07, 95% CI: 0.02‐0.12, p=0.007), whereas the same was not true for CVD incidence (65 studies; β=–0.02, 95% CI=–0.07 to 0.01, p=0.21).
DISCUSSION
To our knowledge, this is the first large scale meta‐analysis providing comprehensive quantitative data on the prevalence and incidence of CVD in people with SMI, including both pooled data and comparisons across CVD and SMI diagnostic subgroups. Our results establish that approximately 10% of people with SMI with a mean age of 50 years have at least one comorbid CVD. Moreover, our longitudinal analysis documents a 3.6% incidence rate of CVD during a median of 8.4 years of follow‐up. Patients with SMI show a 53% higher risk for having CVD, a 78% higher risk for developing CVD, and an 85% higher risk of death from CVD compared to the regionally matched general population.
This study provides a worldwide epidemiologic representation of CVD prevalence and incidence rates in SMI, reporting the lowest absolute prevalence and incidence in Asia, increasing through Europe and North America, and reaching the highest levels in Oceania. However, in analyses with sufficient numbers of available studies, neither RRs nor adjusted HRs indicated significantly different CVD incidence risk across regions, meaning that SMI patients are at an increased risk across the world and that CVD risk‐reducing interventions in SMI are needed with the same urgency across all regions of the world. Moreover, while the prevalence and incidence of each CVD in people with SMI show some minor variations, people with major depressive disorder, bipolar disorder and schizophrenia are clearly all at an increased risk of CVD‐related deaths compared to population‐stratified controls, calling for urgent action.
We were able to identify some important and actionable moderators of increased CVD risk, including antipsychotic use, elevated body mass index and elevated baseline CVD. Based on these results, it is imperative that clinicians: a) only utilize antipsychotics, particularly for non‐psychotic conditions, when alternative treatment options with lower CVD risk potential have been tried sufficiently; and b) screen for and manage emerging and existing CVDs as well as their risk factors, including weight gain and elevated body mass index. Our data, adding to research demonstrating a significantly higher prevalence of metabolic syndrome in people with SMI compared to controls114, clearly suggest there is an urgent need to prevent and manage CVD risk in this population.
Our results demonstrating a higher CVD prevalence in SMI populations versus controls in more recent studies are also concerning, as they support accumulating data indicating that secondary prevention has been much less successful in the SMI population that in the general population, leading to a widening of the mortality gap in recent years49, 115, 116. Our findings confirm prior reports that antipsychotic medication use is associated with higher CVD risk13, 117, 118. However, due to limitations in the published data, we were unable to explore variations in CVD risk profiles between different antipsychotic medications13, 117, 118, 119, 120. Previous research has suggested that the highest cardio‐metabolic risks are associated with clozapine and olanzapine, whilst the lowest risk is with aripiprazole, ziprasidone, lurasidone, amisulpride and high potency typical antipsychotics13, 117, 118, 119, 120, 121, 122. However, in this context it is also important to note that antipsychotic medications can decrease CVD‐related mortality, as reported for example in Finnish79 and Swedish123 national database studies, that are highly generalizable. These data underscore that symptom control and functional improvement benefit both psychiatric and overall health, as severe psychiatric illness negatively affects lifestyle behaviors, medical care seeking and adherence to medical treatments. Thus, benefits of improved psychiatric status with antipsychotics and other psychotropic agents need to be carefully weighed against their potential for elevated cardiometabolic risk, which differs across available agents13, 117.
Since antipsychotic medication use moderates CVD risk and since antipsychotics are increasingly used as first line treatments for much more prevalent non‐psychotic conditions, including bipolar disorder124 and major depressive disorder with suboptimal response to antidepressant treatment125, the pool of people at an increased CVD risk is greatly enlarged. Therefore, research on the underlying mechanisms for the increased CVD risk after pharmacotherapy initiation is even more urgently needed to develop more effective and targeted preventive and interventional treatments. Studies should also examine whether different clinical subtypes of depression (i.e., melancholic, psychotic, atypical or undifferentiated) and bipolar disorder (e.g., type 1 or 2, cyclothymic disorder), certain mood states (manic, depressive, mixed or euthymic), or different antipsychotics, antidepressants or mood stabilizers13 significantly moderate CVD risk.
Furthermore, the pathophysiology underlying the association between SMI and CVD risk is complex and not well understood, clearly requiring further investigation. Emerging evidence suggests that SMI and CVD share pathophysiological features, including hypothalamic‐pituitary‐adrenal and mitochondrial dysfunction, peripheral immune activation, neuro‐inflammation, oxidative and nitrosative stress, as well as common genetic links and epigenetic interactions126. However, since these different mechanisms probably interact, research that integrates these pathways is urgently needed. Beyond mechanistic evaluations, such studies also need to investigate the general and specific effects of physical health improvements on SMI outcomes.
Future research should also investigate optimal monitoring regimens across stratified patient subgroups as well as the most effective timing and efficacy of primary, secondary and tertiary preventive interventions120, 127. In this regard, studies should comprehensively assess relevant moderator and mediator variables of CVD risk, including type and duration of specific psychotropic medications use, physical activity (including using passive monitoring via actimetry or mobile phone technology), diet, smoking, body mass index, personal and family history of CVD, in order to identify subgroups of patients who may require different monitoring and or interventions schemes. Long‐term follow‐up studies are also required to accurately document the emergence of more distal physical and mental health as well as health economic outcomes in relationship to the early identification and management of CVD risk factors and manifest CVD conditions in people with SMI.
Finally, since people with SMI engage in unhealthy lifestyle and often take psychotropic medication for extensive periods, long‐term follow‐up studies are needed that assess whether current predictor models based on the magnitude of traditional CVD risk factor effects observed in the general population apply or need to be adjusted for the SMI population93, in whom CVD risk factors also emerge at a far earlier age117, 128.
While this is the most comprehensive meta‐analysis of CVD risk in people with SMI conducted to date, we acknowledge several limitations that are largely related to factors in the primary data. First, lifestyle behavior information (e.g., physical activity) was inadequately reported, precluding meta‐analytic assessment of these important factors as moderating or mediating variables. People with SMI are less likely than the general population to engage in physical activity and have higher levels of sedentary behaviour129, smoke more130, consume diets that are high in saturated fats and refined sugars, while being low in fruit and vegetables131, all factors relevant for CVD risk. Second, variables such as clinical subtypes of major depressive disorder and bipolar disorder, negative symptom severity in people with schizophrenia, and concomitant or previous use of specific antipsychotics, antidepressants and mood stabilizers were not reported or were insufficiently reported or controlled for in most available studies. Third, as expected when combining observational data132, many of the results were moderately to highly heterogeneous. However, in accordance with the MOOSE guidelines133, we conducted meta‐regression analyses and were able to explain some of the observed heterogeneity. In addition, all of our results remained robust after adjustment for potential publication bias with the trim and fill analysis.
In conclusion, SMIs pooled together were significantly associated in cross‐sectional studies with CVD, coronary heart disease, cerebrovascular disease and CVD‐related death. Additionally, in longitudinal studies, each specific diagnostic SMI group was significantly associated with CVD and CVD‐related death. Furthermore, schizophrenia was associated with coronary heart disease and cerebrovascular disease, while bipolar disorder was associated with congestive heart failure, and major depressive disorder was associated with coronary heart disease, cerebrovascular disease, and congestive heart failure.
Importantly, our data confirm that CVDs are associated with an increased risk of mortality in people with SMI, which to a large part explains the shortened life expectancy of people with SMI compared to the general population2, 4, 5. Furthermore, we showed geographical variations in raw CVD prevalence and incidence risk in SMI populations, but no significant regional variance in the difference in CVD risk compared to the region‐specific general population. Finally, the fact that antipsychotic use, higher body mass index and baseline CVD significantly increased the risk for CVD morbidity and mortality underscores the urgent need to limit antipsychotic use to those populations truly requiring them, choosing the lowest risk antipsychotic agents first in the treatment algorithm, screening all SMI patients regularly for CVD risk factors and conditions, and addressing any identified abnormalities aggressively.
ACKNOWLEDGEMENTS
B. Stubbs and F. Gaughran receive support from the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King's College Hospital NHS Foundation Trust. F. Gaughran is also funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care Funding scheme and by the Stanley Medical Research Institute. The views expressed in this publication are those of the authors and not necessarily those of the funding institutions. C.U. Correll, M. Solmi and N. Veronese are joint first authors of the paper.
REFERENCES
- 1. Chang CK, Hayes RD, Perera G et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One 2011;6:e19590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ 2013;346:f2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Popovic D, Benabarre A, Crespo JM et al. Risk factors for suicide in schizophrenia: systematic review and clinical recommendations. Acta Psychiatr Scand 2014;130:418‐26. [DOI] [PubMed] [Google Scholar]
- 4. Hoang U, Goldacre MJ, Stewart R. Avoidable mortality in people with schizophrenia or bipolar disorder in England. Acta Psychiatr Scand 2013;127:195‐201. [DOI] [PubMed] [Google Scholar]
- 5. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta‐analysis. JAMA Psychiatry 2015;72:334‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Q, Kling JM. Depression and the risk of myocardial infarction and coronary death: a meta‐analysis of prospective cohort studies. Medicine 2016;95:e2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gan Y, Gong Y, Tong X et al. Depression and the risk of coronary heart disease: a meta‐analysis of prospective cohort studies. BMC Psychiatry 2014;14:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong JY, Zhang YH, Tong J et al. Depression and risk of stroke: a meta‐analysis of prospective studies. Stroke 2012;43:32‐7. [DOI] [PubMed] [Google Scholar]
- 9. Van der Kooy K, van Hout H, Marwijk H et al. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007;22:613‐26. [DOI] [PubMed] [Google Scholar]
- 10. Prieto ML, Cuellar‐Barboza AB, Bobo WV et al. Risk of myocardial infarction and stroke in bipolar disorder: a systematic review and exploratory meta‐analysis. Acta Psychiatr Scand 2014;130:342‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan Z, Wu Y, Shen J et al. Schizophrenia and the risk of cardiovascular diseases: a meta‐analysis of thirteen cohort studies. J Psychiatr Res 2013;47:1549‐56. [DOI] [PubMed] [Google Scholar]
- 12. Li M, Fan YL, Tang ZY et al. Schizophrenia and risk of stroke: a meta‐analysis of cohort studies. Int J Cardiol 2014;173:588‐90. [DOI] [PubMed] [Google Scholar]
- 13. Correll CU, Detraux J, De Lepeleire J et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015;14:119‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg 2010;8:336‐41. [DOI] [PubMed] [Google Scholar]
- 15.Biostat. Comprehensive Meta‐Analysis. https://www.meta-analysis.com.
- 16. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105‐14. [DOI] [PubMed] [Google Scholar]
- 17. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
- 18. Egger M, Davey Smith G, Schneider M et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088‐101. [PubMed] [Google Scholar]
- 20. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56:455‐63. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beyer J, Kuchibhatla M, Gersing K et al. Medical comorbidity in a bipolar outpatient clinical population. Neuropsychopharmacology 2005;30:401‐4. [DOI] [PubMed] [Google Scholar]
- 23. Bresee LC, Majumdar SR, Patten SB et al. Prevalence of cardiovascular risk factors and disease in people with schizophrenia: a population‐based study. Schizophr Res 2010;117:75‐82. [DOI] [PubMed] [Google Scholar]
- 24. Bresee LC, Majumdar SR, Patten SB et al. Diabetes, cardiovascular disease, and health care use in people with and without schizophrenia. Eur Psychiatry 2011;26:327‐32. [DOI] [PubMed] [Google Scholar]
- 25. Chen PH, Gildengers AG, Lee CH et al. High serum sodium level in affective episode associated with coronary heart disease in old adults with bipolar disorder. Int J Psychiatry Med 2015;50:422‐33. [DOI] [PubMed] [Google Scholar]
- 26. Curkendall SM, Mo J, Glasser DB et al. Cardiovascular disease in patients with schizophrenia in Saskatchewan, Canada. J Clin Psychiatry 2004;65:715‐20. [DOI] [PubMed] [Google Scholar]
- 27. Devantier TA, Norgaard BL, Ovrehus KA et al. Coronary plaque volume and composition assessed by computed tomography angiography in patients with late‐onset major depression. Psychosomatics 2014;55:243‐51. [DOI] [PubMed] [Google Scholar]
- 28. Hagg S, Lindblom Y, Mjorndal T et al. High prevalence of the metabolic syndrome among a Swedish cohort of patients with schizophrenia. Int Clin Psychopharmacol 2006;21:93‐8. [DOI] [PubMed] [Google Scholar]
- 29. Herbst S, Pietrzak RH, Wagner J et al. Lifetime major depression is associated with coronary heart disease in older adults: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med 2007;69:729‐34. [DOI] [PubMed] [Google Scholar]
- 30. Huang KL, Su TP, Chen TJ et al. Comorbidity of cardiovascular diseases with mood and anxiety disorder: a population based 4‐year study. Psychiatry Clin Neurosci 2009;63:401‐9. [DOI] [PubMed] [Google Scholar]
- 31. Hyde N, Dodd S, Venugopal K et al. Prevalence of cardiovascular and metabolic events in patients prescribed clozapine: a retrospective observational, clinical cohort study. Curr Drug Saf 2015;10:125‐31. [DOI] [PubMed] [Google Scholar]
- 32. Kilbourne AM, Cornelius JR, Han X et al. General‐medical conditions in older patients with serious mental illness. Am J Geriatr Psychiatry 2005;13:250‐4. [DOI] [PubMed] [Google Scholar]
- 33. Kilbourne AM, Post EP, Bauer MS et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord 2007;102:145‐51. [DOI] [PubMed] [Google Scholar]
- 34. Lindegard B. Physical illness in severe depressives and psychiatric alcoholics in Gothenburg, Sweden. J Affect Disord 1982;4:383‐93. [DOI] [PubMed] [Google Scholar]
- 35. Maina G, D'Ambrosio V, Aguglia A et al. Bipolar disorders and metabolic syndrome: a clinical study in 185 patients. Riv Psichiatr 2010;45:34‐40. [PubMed] [Google Scholar]
- 36. Morden NE, Lai Z, Goodrich DE et al. Eight‐year trends of cardiometabolic morbidity and mortality in patients with schizophrenia. Gen Hosp Psychiatry 2012;34:368‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Munoli RN, Praharaj SK, Sharma PS. Co‐morbidity in bipolar disorder: a retrospective study. Indian J Psychol Med 2014;36:270‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nielsen J, Juel J, Alzuhairi KS et al. Unrecognised myocardial infarction in patients with schizophrenia. Acta Neuropsychiatr 2015;27:106‐12. [DOI] [PubMed] [Google Scholar]
- 39. Niranjan A, Corujo A, Ziegelstein RC et al. Depression and heart disease in US adults. Gen Hosp Psychiatry 2012;34:254‐61. [DOI] [PubMed] [Google Scholar]
- 40. Oreski I, Jakovljevic M, Aukst‐Margetic B et al. Comorbidity and multimorbidity in patients with schizophrenia and bipolar disorder: similarities and differencies. Psychiatr Danub 2012;24:80‐5. [PubMed] [Google Scholar]
- 41. Prieto ML, McElroy SL, Hayes SN et al. Association between history of psychosis and cardiovascular disease in bipolar disorder. Bipolar Disord 2015;17:518‐27. [DOI] [PubMed] [Google Scholar]
- 42. Scherrer JF, Xian H, Bucholz KK et al. A twin study of depression symptoms, hypertension, and heart disease in middle‐aged men. Psychosom Med 2003;65:548‐57. [DOI] [PubMed] [Google Scholar]
- 43. Scott KM, de Jonge P, Alonso J et al. Associations between DSM‐IV mental disorders and subsequent heart disease onset: beyond depression. Int J Cardiol 2013;168:5293‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shen HN, Lu CL, Yang HH. Increased risks of acute organ dysfunction and mortality in intensive care unit patients with schizophrenia: a nationwide population‐based study. Psychosom Med 2011;73:620‐6. [DOI] [PubMed] [Google Scholar]
- 45. Smith DJ, Langan J, McLean G et al. Schizophrenia is associated with excess multiple physical‐health comorbidities but low levels of recorded cardiovascular disease in primary care: cross‐sectional study. BMJ Open 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith DJ, Martin D, McLean G et al. Multimorbidity in bipolar disorder and undertreatment of cardiovascular disease: a cross sectional study. BMC Med 2013;11:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Swain NR, Lim CC, Levinson D et al. Associations between DSM‐IV mental disorders and subsequent non‐fatal, self‐reported stroke. J Psychosom Res 2015;79:130‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zilkens RR, Bruce DG, Duke J et al. Severe psychiatric disorders in mid‐life and risk of dementia in late‐life (age 65‐84 years): a population based case‐control study. Curr Alzheimer Res 2014;11:681‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Almeida OP, Hankey GJ, Yeap BB et al. Mortality among people with severe mental disorders who reach old age: a longitudinal study of a community‐representative sample of 37,892 men. PLoS One 2014;9:e111882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bremmer MA, Hoogendijk WJ, Deeg DJ et al. Depression in older age is a risk factor for first ischemic cardiac events. Am J Geriatr Psychiatry 2006;14:523‐30. [DOI] [PubMed] [Google Scholar]
- 51. Butnoriene J, Bunevicius A, Saudargiene A et al. Metabolic syndrome, major depression, generalized anxiety disorder, and ten‐year all‐cause and cardiovascular mortality in middle aged and elderly patients. Int J Cardiol 2015;190:360‐6. [DOI] [PubMed] [Google Scholar]
- 52. Callaghan RC, Boire MD, Lazo RG et al. Schizophrenia and the incidence of cardiovascular morbidity: a population‐based longitudinal study in Ontario, Canada. Schizophr Res 2009;115:325‐32. [DOI] [PubMed] [Google Scholar]
- 53. Callaghan RC, Khizar A. The incidence of cardiovascular morbidity among patients with bipolar disorder: a population‐based longitudinal study in Ontario, Canada. J Affect Disord 2010;122:118‐23. [DOI] [PubMed] [Google Scholar]
- 54. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population‐based controlled study. J Gen Intern Med 2006;21:1133‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen MH, Li CT, Hsu JW et al. Atopic diseases and subsequent ischemic stroke among patients with schizophrenia: a nationwide longitudinal study. Psychosom Med 2015;77:1031‐8. [DOI] [PubMed] [Google Scholar]
- 56. Clouse RE, Lustman PJ, Freedland KE et al. Depression and coronary heart disease in women with diabetes. Psychosom Med 2003;65:376‐83. [DOI] [PubMed] [Google Scholar]
- 57. Coryell W, Turvey C, Leon A et al. Persistence of depressive symptoms and cardiovascular death among patients with affective disorder. Psychosom Med 1999;61:755‐61. [DOI] [PubMed] [Google Scholar]
- 58. Crump C, Sundquist K, Winkleby MA et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry 2013;70:931‐9. [DOI] [PubMed] [Google Scholar]
- 59. Crump C, Winkleby MA, Sundquist K et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry 2013;170:324‐33. [DOI] [PubMed] [Google Scholar]
- 60. Davis J, Fujimoto RY, Juarez DT et al. Major depression associated with rates of cardiovascular disease state transitions. Am J Manag Care 2008;14:125‐8. [PubMed] [Google Scholar]
- 61. Davydow DS, Ribe AR, Pedersen HS et al. Serious mental illness and risk for hospitalizations and rehospitalizations for ambulatory care‐sensitive conditions in Denmark: a nationwide population‐based cohort study. Med Care 2016;54:90‐7. [DOI] [PubMed] [Google Scholar]
- 62. Enger C, Weatherby L, Reynolds RF et al. Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis 2004;192:19‐27. [DOI] [PubMed] [Google Scholar]
- 63. Fiedorowicz JG, Solomon DA, Endicott J et al. Manic/hypomanic symptom burden and cardiovascular mortality in bipolar disorder. Psychosom Med 2009;71:598‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Filik R, Sipos A, Kehoe PG et al. The cardiovascular and respiratory health of people with schizophrenia. Acta Psychiatr Scand 2006;113:298‐305. [DOI] [PubMed] [Google Scholar]
- 65. Fors BM, Isacson D, Bingefors K et al. Mortality among persons with schizophrenia in Sweden: an epidemiological study. Nord J Psychiatry 2007;61:252‐9. [DOI] [PubMed] [Google Scholar]
- 66. Gasse C, Laursen TM, Baune BT. Major depression and first‐time hospitalization with ischemic heart disease, cardiac procedures and mortality in the general population: a retrospective Danish population‐based cohort study. Eur J Prev Cardiol 2014;21:532‐40. [DOI] [PubMed] [Google Scholar]
- 67. Goldstein BI, Schaffer A, Wang S et al. Excessive and premature new‐onset cardiovascular disease among adults with bipolar disorder in the US NESARC cohort. J Clin Psychiatry 2015;76:163‐9. [DOI] [PubMed] [Google Scholar]
- 68. Healy D, Le Noury J, Harris M et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875‐1924 and 1994‐2010. BMJ Open 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hendrie HC, Tu W, Tabbey R et al. Health outcomes and cost of care among older adults with schizophrenia: a 10‐year study using medical records across the continuum of care. Am J Geriatr Psychiatry 2014;22:427‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hou PY, Hung GC, Jhong JR et al. Risk factors for sudden cardiac death among patients with schizophrenia. Schizophr Res 2015;168:395‐401. [DOI] [PubMed] [Google Scholar]
- 71. Hsieh PH, Hsiao FY, Gau SS et al. Use of antipsychotics and risk of cerebrovascular events in schizophrenic patients: a nested case‐control study. J Clin Psychopharmacol 2013;33:299‐305. [DOI] [PubMed] [Google Scholar]
- 72. Huang CJ, Hsieh MH, Hou WH et al. Depression, antidepressants, and the risk of coronary heart disease: a population‐based cohort study. Int J Cardiol 2013;168:4711‐6. [DOI] [PubMed] [Google Scholar]
- 73. Ifteni P, Correll CU, Burtea V et al. Sudden unexpected death in schizophrenia: autopsy findings in psychiatric inpatients. Schizophr Res 2014;155:72‐6. [DOI] [PubMed] [Google Scholar]
- 74. Jakobsen AH, Foldager L, Parker G et al. Quantifying links between acute myocardial infarction and depression, anxiety and schizophrenia using case register databases. J Affect Disord 2008;109:177‐81. [DOI] [PubMed] [Google Scholar]
- 75. Janszky I, Ahnve S, Lundberg I et al. Early‐onset depression, anxiety, and risk of subsequent coronary heart disease: 37‐year follow‐up of 49,321 young Swedish men. J Am Coll Cardiol 2010;56:31‐7. [DOI] [PubMed] [Google Scholar]
- 76. Jokinen J, Nordstrom P. HPA axis hyperactivity and cardiovascular mortality in mood disorder inpatients. J Affect Disord 2009;116:88‐92. [DOI] [PubMed] [Google Scholar]
- 77. Joukamaa M, Heliovaara M, Knekt P et al. Mental disorders and cause‐specific mortality. Br J Psychiatry 2001;179:498‐502. [DOI] [PubMed] [Google Scholar]
- 78. Kendler KS, Gardner CO, Fiske A et al. Major depression and coronary artery disease in the Swedish twin registry: phenotypic, genetic, and environmental sources of comorbidity. Arch Gen Psychiatry 2009;66:857‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kiviniemi M, Suvisaari J, Koivumaa‐Honkanen H et al. Antipsychotics and mortality in first‐onset schizophrenia: prospective Finnish register study with 5‐year follow‐up. Schizophr Res 2013;150:274‐80. [DOI] [PubMed] [Google Scholar]
- 80. Lahti M, Tiihonen J, Wildgust H et al. Cardiovascular morbidity, mortality and pharmacotherapy in patients with schizophrenia. Psychol Med 2012;42:2275‐85. [DOI] [PubMed] [Google Scholar]
- 81. Lan CC, Liu CC, Lin CH et al. A reduced risk of stroke with lithium exposure in bipolar disorder: a population‐based retrospective cohort study. Bipolar Disord 2015;17:705‐14. [DOI] [PubMed] [Google Scholar]
- 82. Laursen TM, Munk‐Olsen T, Gasse C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS One 2011;6:e24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Laursen TM, Mortensen PB, MacCabe JH et al. Cardiovascular drug use and mortality in patients with schizophrenia or bipolar disorder: a Danish population‐based study. Psychol Med 2014;44:1625‐37. [DOI] [PubMed] [Google Scholar]
- 84. Lemogne C, Nabi H, Melchior M et al. Mortality associated with depression as compared with other severe mental disorders: a 20‐year follow‐up study of the GAZEL cohort. J Psychiatr Res 2013;47:851‐7. [DOI] [PubMed] [Google Scholar]
- 85. Li CT, Bai YM, Tu PC et al. Major depressive disorder and stroke risks: a 9‐year follow‐up population‐based, matched cohort study. PLoS One 2012;7:e46818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lin HC, Chen YH, Lee HC et al. Increased risk of acute myocardial infarction after acute episode of schizophrenia: 6 year follow‐up study. Aust N Z J Psychiatry 2010;44:273‐9. [DOI] [PubMed] [Google Scholar]
- 87. Lin HC, Tsai SY, Lee HC. Increased risk of developing stroke among patients with bipolar disorder after an acute mood episode: a six‐year follow‐up study. J Affect Disord 2007;100:49‐54. [DOI] [PubMed] [Google Scholar]
- 88. Lin HC, Hsiao FH, Pfeiffer S et al. An increased risk of stroke among young schizophrenia patients. Schizophr Res 2008;101:234‐41. [DOI] [PubMed] [Google Scholar]
- 89. Maina G, Bechon E, Rigardetto S et al. General medical conditions are associated with delay to treatment in patients with bipolar disorder. Psychosomatics 2013;54:437‐42. [DOI] [PubMed] [Google Scholar]
- 90. McDermott S, Moran R, Platt T et al. Heart disease, schizophrenia, and affective psychoses: epidemiology of risk in primary care. Community Ment Health J 2005;41:747‐55. [DOI] [PubMed] [Google Scholar]
- 91. Murray‐Thomas T, Jones ME, Patel D et al. Risk of mortality (including sudden cardiac death) and major cardiovascular events in atypical and typical antipsychotic users: a study with the general practice research database. Cardiovasc Psychiatry Neurol 2013;2013:247486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Olfson M, Gerhard T, Huang C et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015;72:1172‐81. [DOI] [PubMed] [Google Scholar]
- 93. Osborn DP, Hardoon S, Omar RZ et al. Cardiovascular risk prediction models for people with severe mental illness: results from the prediction and management of cardiovascular risk in people with severe mental illnesses (PRIMROSE) research program. JAMA Psychiatry 2015;72:143‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pratt LA, Ford DE, Crum RM et al. Depression, psychotropic medication, and risk of myocardial infarction. Prospective data from the Baltimore ECA follow‐up. Circulation 1996;94:3123‐9. [DOI] [PubMed] [Google Scholar]
- 95. Prieto ML, Schenck LA, Kruse JL et al. Long‐term risk of myocardial infarction and stroke in bipolar I disorder: a population‐based Cohort Study. J Affect Disord 2016;194:120‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rahman I, Humphreys K, Bennet AM et al. Clinical depression, antidepressant use and risk of future cardiovascular disease. Eur J Epidemiol 2013;28:589‐95. [DOI] [PubMed] [Google Scholar]
- 97. Ramsey CM, Leoutsakos JM, Mayer LS et al. History of manic and hypomanic episodes and risk of incident cardiovascular disease: 11.5 year follow‐up from the Baltimore Epidemiologic Catchment Area Study. J Affect Disord 2010;125:35‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saint Onge JM, Krueger PM, Rogers RG. The relationship between major depression and nonsuicide mortality for U.S. adults: the importance of health behaviors. J Gerontol B Psychol Sci Soc Sci 2014;69:622‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Scherrer JF, Garfield LD, Chrusciel T et al. Increased risk of myocardial infarction in depressed patients with type 2 diabetes. Diabetes Care 2011;34:1729‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schoepf D, Heun R. Bipolar disorder and comorbidity: increased prevalence and increased relevance of comorbidity for hospital‐based mortality during a 12.5‐year observation period in general hospital admissions. J Affect Disord 2014;169:170‐8. [DOI] [PubMed] [Google Scholar]
- 101. Schoepf D, Uppal H, Potluri R et al. Physical comorbidity and its relevance on mortality in schizophrenia: a naturalistic 12‐year follow‐up in general hospital admissions. Eur Arch Psychiatry Clin Neurosci 2014;264:3‐28. [DOI] [PubMed] [Google Scholar]
- 102. Shah AJ, Veledar E, Hong Y et al. Depression and history of attempted suicide as risk factors for heart disease mortality in young individuals. Arch Gen Psychiatry 2011;68:1135‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Stewart JC, Perkins AJ, Callahan CM. Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial. Psychosom Med 2014;76:29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Surtees PG, Wainwright NW, Luben RN et al. Depression and ischemic heart disease mortality: evidence from the EPIC‐Norfolk United Kingdom prospective cohort study. Am J Psychiatry 2008;165:515‐23. [DOI] [PubMed] [Google Scholar]
- 105. Ting RZ, Lau ES, Ozaki R et al. High risk for cardiovascular disease in Chinese type 2 diabetic patients with major depression–a 7‐year prospective analysis of the Hong Kong Diabetes Registry. J Affect Disord 2013;149:129‐35. [DOI] [PubMed] [Google Scholar]
- 106. Torniainen M, Mittendorfer‐Rutz E, Tanskanen A et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull 2015;41:656‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tsai KY, Lee CC, Chou YM et al. The incidence and relative risk of stroke in patients with schizophrenia: a five‐year follow‐up study. Schizophr Res 2012;138:41‐7. [DOI] [PubMed] [Google Scholar]
- 108. Tsan JY, Stock EM, Gonzalez JM et al. Mortality and guideline‐concordant care for older patients with schizophrenia: a retrospective longitudinal study. BMC Med 2012;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. van Marwijk HW, van der Kooy KG, Stehouwer CD et al. Depression increases the onset of cardiovascular disease over and above other determinants in older primary care patients, a cohort study. BMC Cardiovasc Disord 2015;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Weeke A, Juel K, Vaeth M. Cardiovascular death and manic‐depressive psychosis. J Affect Disord 1987;13:287‐92. [DOI] [PubMed] [Google Scholar]
- 111. Westman J, Hallgren J, Wahlbeck K et al. Cardiovascular mortality in bipolar disorder: a population‐based cohort study in Sweden. BMJ Open 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wu HC, Chou FH, Tsai KY et al. The incidence and relative risk of stroke among patients with bipolar disorder: a seven‐year follow‐up study. PLoS One 2013;8:e73037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wu SI, Chen SC, Liu SI et al. Relative risk of acute myocardial infarction in people with schizophrenia and bipolar disorder: a population‐based cohort study. PLoS One 2015;10:e0134763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Vancampfort D, Stubbs B, Mitchell AJ et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol 2014;10:425‐48. [DOI] [PubMed] [Google Scholar]
- 116. Nielsen RE, Uggerby AS, Jensen SO et al. Increasing mortality gap for patients diagnosed with schizophrenia over the last three decades – a Danish nationwide study from 1980 to 2010. Schizophr Res 2013;146:22‐7. [DOI] [PubMed] [Google Scholar]
- 117. De Hert M, Detraux J, van Winkel R et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011;8:114‐26. [DOI] [PubMed] [Google Scholar]
- 118. Correll CU, Joffe BI, Rosen LM et al. Cardiovascular and cerebrovascular risk factors and events associated with second‐generation antipsychotic compared to antidepressant use in a non‐elderly adult sample: results from a claims‐based inception cohort study. World Psychiatry 2015;14:56‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vancampfort D, Correll CU, Wampers M et al. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta‐analysis of prevalences and moderating variables. Psychol Med 2014;44:2017‐28. [DOI] [PubMed] [Google Scholar]
- 120. De Hert M, Correll CU, Bobes J et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Correll CU, Robinson DG, Schooler NR et al. Cardiometabolic risk in patients with first‐episode schizophrenia spectrum disorders: baseline results from the RAISE‐ETP study. JAMA Psychiatry 2014;71:1350‐63. [DOI] [PubMed] [Google Scholar]
- 122. Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic‐naive schizophrenia patients. Neuropsychopharmacology 2010;35:1997‐2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tiihonen J, Mittendorfer‐Rutz E, Torniainen M et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow‐up study. Am J Psychiatry 2016;173:600‐6. [DOI] [PubMed] [Google Scholar]
- 124. Pillarella J, Higashi A, Alexander GC et al. Trends in use of second‐generation antipsychotics for treatment of bipolar disorder in the United States, 1998‐2009. Psychiatr Serv 2012;63:83‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry 2010;71(Suppl. E1):e04. [DOI] [PubMed] [Google Scholar]
- 126. Manu P, Correll CU, Wampers M et al. Markers of inflammation in schizophrenia: association vs. causation. World Psychiatry 2014;13:189‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. De Hert M, Cohen D, Bobes J et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry 2011;10:138‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med 2011;17:97‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Stubbs B, Firth J, Berry A et al. How much physical activity do people with schizophrenia engage in? A systematic review, comparative meta‐analysis and meta‐regression. Schizophr Res 2016;176:431‐40. [DOI] [PubMed] [Google Scholar]
- 130. Dickerson F, Stallings CR, Origoni AE et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999‐2011. Psychiatr Serv 2013;64:44‐50. [DOI] [PubMed] [Google Scholar]
- 131. Bly MJ, Taylor SF, Dalack G et al. Metabolic syndrome in bipolar disorder and schizophrenia: dietary and lifestyle factors compared to the general population. Bipolar Disord 2014;16:277‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Speyer H, Norgaard HCB, Birk M et al. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry 2016;15:155‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Stroup DF, Berlin JA, Morton SC et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008‐12. [DOI] [PubMed] [Google Scholar]


