Abstract
The transdiagnostic expression of psychotic experiences in common mental disorder (anxiety/depression/substance use disorder) is associated with a poorer prognosis, and a small minority of people may indeed develop a clinical picture that meets criteria for schizophrenia. However, it appears neither useful nor valid to observe early states of multidimensional psychopathology in young people through the “schizo”‐prism, and apply misleadingly simple, unnecessary and inefficient binary concepts of “risk” and “transition”. A review of the “ultra‐high risk” (UHR) or “clinical high risk” (CHR) literature indicates that UHR/CHR samples are highly heterogeneous and represent individuals diagnosed with common mental disorder (anxiety/depression/substance use disorder) and a degree of psychotic experiences. Epidemiological research has shown that psychotic experiences are a (possibly non‐causal) marker of the severity of multidimensional psychopathology, driving poor outcome, yet notions of “risk” and “transition” in UHR/CHR research are restrictively defined on the basis of positive psychotic phenomena alone, ignoring how baseline differences in multidimensional psychopathology may differentially impact course and outcome. The concepts of “risk” and “transition” in UHR/CHR research are measured on the same dimensional scale, yet are used to produce artificial diagnostic shifts. In fact, “transition” in UHR/CHR research occurs mainly as a function of variable sample enrichment strategies rather than the UHR/CHR “criteria” themselves. Furthermore, transition rates in UHR/CHR research are inflated as they do not exclude false positives associated with the natural fluctuation of dimensional expression of psychosis. Biological associations with “transition” thus likely represent false positive findings, as was the initial claim of strong effects of omega‐3 polyunsatured fatty acids in UHR samples. A large body of UHR/CHR intervention research has focused on the questionable outcome of “transition”, which shows lack of correlation with functional outcome. It may be more productive to consider the full range of person‐specific psychopathology in all young individuals who seek help for mental health problems, instead of “policing” youngsters for the transdiagnostic dimension of psychosis. Instead of the relatively inefficient medical high‐risk approach, a public health perspective, focusing on improved access to a low‐stigma, high‐hope, small scale and youth‐specific environment with acceptable language and interventions may represent a more useful and efficient strategy.
Keywords: Ultra‐high risk, transition, psychotic experiences, common mental disorder, transdiagnostic expression of psychosis, public health perspective
Over the last two decades, more than 1,500 studies have been published revolving around the concept of “ultra‐high risk” (UHR) or “clinical high risk” (CHR) for “transition” to a psychotic disorder. The basic assumptions behind these studies are as follows: in a group of young people seeking help for mental problems, one can apply criteria for a binary risk diagnosis predicting schizophrenia spectrum disorder, and true positives are people that meet criteria for “transition” at follow‐up.
Reviews of UHR/CHR studies tend to be upbeat, taking the shape of “evidence‐based recommendations” or “guidance”, stating that “the young field of preventive research in psychosis has already resulted in sufficient evidence to formulate recommendations for an early detection of psychosis in the clinical practice”1, and that “psychological, in particular cognitive‐behavioural, as well as pharmacological interventions are able to prevent or at least postpone a first psychotic episode in adult CHR patients”2.
However, the question arises of the degree to which this optimism is based on logical reasoning and scientific evidence. There is a growing literature on the complexities underlying UHR/CHR research, that are not resolved, clouding the interpretation of data3, 4, 5, 6, 7, 8, 9, 10, 11. In this paper, we critically review the assumptions underlying UHR/CHR research. In particular, we focus on outstanding issues to do with sampling variability and basic epidemiological parameters, the fixation on psychosis at the expense of other psychopathology, and the lack of transparency arising from the use of two binary concepts for diagnosis and outcome that lie on the same unidimensional scale, and obscure the temporality and dynamics of multidimensional psychopathological states in young people.
We do not wish to dispute that it is better to intervene early rather than late. Rather, we wish to argue that it is conceptually flawed to frame the treatment of early psychopathology in diagnosed help‐seeking individuals as prevention of psychotic disorder, just because there is some degree of transdiagnostic expression of psychotic experiences.
CLINICAL HIGH RISK SAMPLING IS SELECTIVE AND NON‐EPIDEMIOLOGICAL
In practice, studies that want to apply the UHR/CHR paradigm have to search for young individuals who are slightly‐but‐not‐quite psychotic and have also expressed a wish to receive help. Sampling strategies differ widely from study to study and are based on a mix of advertising, service filters and active searches, thus per definition resulting in selected, non‐representative samples that cannot readily be compared across studies.
For example, in the North‐American multicentre prediction study12, it was stated that “each site recruited potential subjects through clinical referrals as stimulated by talks to school counselors and mental health professionals in community settings”. In the European Prediction of Psychosis Study (EPOS)13, UHR/CHR sampling was described as follows: “knowledge about early warning signs (e.g., concentration and attention disturbances, unexplained functional decline) and inclusion criteria was disseminated (through local workshops, articles in professional journals and newsletters, informational flyers, and web sites) to mental health professionals as well as institutions and persons who might be contacted by at‐risk persons seeking help”.
Of the two largest CHR psychotherapy trials to date, one did not provide details about the sampling procedure – except that it took screening of 5,705 subjects to include 201 patients (3.5%) in the trial14 – and the other described sampling as follows: “our ascertainment strategy was to make services familiar with our entry criteria and to liaise on a regular basis; no systematic screening of service populations was carried out”15.
What becomes clear is that CHR studies have to invest a great deal of resources in detecting and sampling subjects who meet the inclusion criteria. The cost of “finding” rare UHR/CHR subjects is considerable, but not included in cost‐effectiveness analyses of UHR/CHR research. Given the apparent rarity of UHR/CHR states, it becomes a priori unlikely that early intervention along the UHR/CHR paradigm will have public health impact. A recent study, investigating an early intervention service in an inner city area, found that only a tiny proportion (4.1%) of patients with a first‐episode psychotic disorder attending mental health services had been in previous contact with the local prodromal service, indicating that the impact of prodromal services in public health terms may be negligible in relation to their costs16. Such a lack of impact associated with the high‐risk approach is a well‐known phenomenon, referred to as the “prevention paradox”17.
Given the absence of a consistent sampling frame, it is unlikely that CHR samples are readily comparable from study to study. For example, samples differ widely in exclusion criteria regarding previous use of antipsychotics and mood stabilizers, previous episodes of mania, and previous drug‐induced psychotic states. Therefore, referring to CHR patients as if they were a “class” is not warranted. Although many meta‐analyses of UHR/CHR samples have been conducted, the question arises whether these studies are sufficiently similar.
Nevertheless, two issues appear to be consistent across UHR/CHR samples. The first is that these samples in essence consist of individuals with a current diagnosis of mainly anxiety, depression or substance use18, 19. The second is that, of the various CHR criteria, the “attenuated symptom” defines the great majority of individuals20, the others having minimal relevance. In other words, CHR samples are individuals with common mental disorder or a substance use disorder who also present with low‐grade psychotic symptoms.
CLINICAL HIGH RISK = COMMON MENTAL DISORDER WITH SUBTLE PSYCHOSIS ADMIXTURE
The fact that UHR/CHR samples in fact consist of individuals with anxiety/depression/substance use with subtle psychosis admixture is important, as it provides a crucial link to the epidemiological literature with findings derived from representative population‐based samples. Attenuated psychotic symptoms at the population level are closely associated with non‐psychotic diagnoses and/or sub‐diagnostic non‐psychotic psychopathology including anxiety, depression, attention‐deficit/hyperactivity disorder, post‐traumatic stress disorder, substance use disorder, eating disorder and many other forms of psychopathology21. Psychosis can thus be regarded as a transdiagnostic dimension of psychopathology22.
Epidemiological studies show that the presence of attenuated psychotic symptoms in non‐psychotic disorders is associated with greater severity and poorer response to treatment23, 24, 25, 26. In fact, research has shown that more exposure to genetic and environmental risk factors is associated with more severe non‐psychotic psychopathology which in turn is associated with a greater probability of the person also having some degree of expression of psychosis24, 27, 28.
PSYCHOTIC EXPERIENCES IN NON‐PSYCHOTIC DISORDER: MARKER OR CAUSE OF POOR PROGNOSIS?
Psychotic experiences can thus be considered a marker for the severity of non‐psychotic states. However, it may not be valid to see them as causal for a poor prognosis, as the evidence shows that psychosis may also be considered as something that follows passively as a function of the general severity of multidimensional psychopathology22. This is essential with regard to the UHR/CHR framework, where the clinical focus is solely on the binary risk concept of psychosis (“risk” and “transition”, measured on the same dimensional scale), while the multidimensional severity of the psychopathological context is ignored. In the UHR/CHR framework, the binary presence of psychotic experiences, under the implicit assumption of impending, mostly “schizo” outcome29, “trumps” all other dimensional expressions of psychopathology.
A whole generation of UHR/CHR studies has been analyzed from the perspective that outcome of common mental disorder with a degree of psychosis admixture is best predicted on the basis of a binary psychosis “risk” criterion. An alternative hypothesis, however, is that outcome in these states is in fact a consequence of baseline severity of multidimensional psychopathology rather than a binary psychosis risk criterion (Figure 1). Studies that have looked beyond UHR/CHR criteria confirm this prediction13, 30, 31, 32, 33, 34. In other words, what is presented as “risk” may be better summarized as baseline differences in the severity of multidimensional psychopathology.
Figure 1.
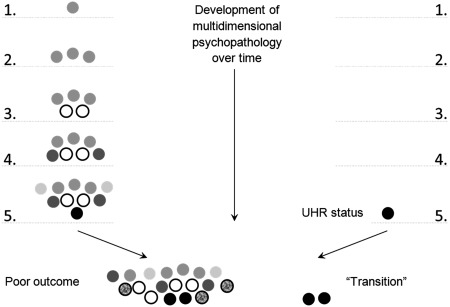
Relative “blindness” of the ultra‐high risk (UHR)/transition paradigm. On the left, the natural development of multidimensional psychopathology over time. Black circles indicate (attenuated) positive psychotic symptoms. Other gray‐scale circles indicate other psychopathology. As the UHR paradigm ignores multidimensional psychopathology, it remains “blind” and only “sees” psychotic phenomena as precursors of schizo‐“transition” (i.e., more severe psychosis; below on the right), while these phenomena are in fact a marker of relative poor outcome of multidimensional psychopathology (below on the left). The restricted focus on positive symptoms in the UHR paradigm means that considerable potential for prevention in phases 1‐4 is missed.
DOES THE CONCEPT OF “TRANSITION” REPRESENT A QUALITATIVE SHIFT?
In UHR/CHR research, “high risk” and “transition” are typically measured on the same dimensional scale rating frequency/duration of attenuated positive psychotic symptoms, usually the Comprehensive Assessment of At‐Risk Mental States (CAARMS)35 or the Scale of Prodromal Symptoms (SOPS)36. These frequency/duration ratings appear either impossibly precise (e.g., “at least once a month to twice a week – more than one hour per occasion, or at least 3 to 6 times a week – less than one hour per occasion”) or rather broad (e.g., “present for at least 1 week and no longer than 5 years”). The scales for positive symptoms range from 0 to 6, where 3‐5, for example, represents “risk for psychosis” and 6 represents “psychosis”. Other symptom domains are ignored, regardless of their severity. “Transition” can be present with a 1‐point shift on the dimensional scale, thus representing a quantitative, not a qualitative shift from “risk” to “transition” status.
While UHR/CHR criteria are generally clearly described in the literature, accounts of “transition” are usually kept vague. For example, in one recent large UHR/CHR trial15, transition was described as “operationally defined on the CAARMS using the recommended criteria of a global rating scale score of 6 on either unusual thought content, non‐bizarre ideas, or disorganised speech, or 5‐6 on perceptual abnormalities, with an associated frequency score of 4‐6, and with these experiences lasting longer than one week”15. In another trial14, it was simply stated that “the primary outcome of this study was the transition to psychosis; the transition is defined by the CAARMS criteria”. Considering the importance of valid outcomes in randomized controlled trials, these descriptions are opaque and appear to rely on small dimensional shifts. These shifts are nevertheless subsequently transformed into a seemingly important qualitative diagnostic change: as the attenuated psychotic symptoms in the UHR/CHR state cannot be counted as a “full” psychotic symptom in the DSM/ICD diagnostic system, the diagnosis in the UHR/CHR “risk” state remains per definition “non‐psychotic”. However, with the dimensional shift in the CAARMS/SIPS towards “transition”, the attenuated psychotic symptom can now be used as a true psychotic symptom, automatically resulting in a diagnosis of psychotic disorder in DSM/ICD. Thus, dimensional shifts are used to evoke the notion that a “diagnosis is born”, creating the suggestion of a qualitative distinction.
IS “TRANSITION” CONFOUNDED BY NATURAL FLUCTUATION OF DIMENSIONAL EXPRESSION OF PSYCHOSIS?
Given the fact that “transition” in fact represents a dimensional shift, false positive ratings of transition are likely to occur given the natural fluctuation in severity of the transdiagnostic psychosis dimension within and between individuals22.
The only study to date that attempted to reduce false positive ratings of transition by serial examination of individuals, excluding individuals rated as UHR that in fact were in a natural “low” of a clinical psychotic syndrome, reported a 2‐year transition rate of 8%15, well below the meta‐analytical estimate of 19% in studies that did not attempt to exclude such false positive ratings2.
IS THE CONCEPT OF “TRANSITION” RELEVANT?
There is a lack of research on the clinical relevance of the “transition” outcome37. However, evidence from long‐term follow‐up studies suggests that the binary “transition” concept is not particularly relevant in terms of predicting clinical and functional outcome, and that other symptom domains (affective, cognitive, negative – but also how mixed and how severe psychopathology is) are more impactful in this respect13, 32, 38, 39.
This observation is supported by the fact that meta‐analyses of UHR/CHR intervention studies, focussing on the prevention of “transition”, fail to show effect on functional outcome1.
THE TRUE TRANSITION RATE OF ATTENUATED PSYCHOTIC SYMPTOMS IS <1%: THE ROLE OF SAMPLING ENRICHMENT
A common and persisting misunderstanding is that the “risk” function in UHR/CHR research is caused by the UHR/CHR criteria themselves. However, already more than a decade ago, it was pointed out that high risk for transition does not so much depend on UHR/CHR criteria themselves, but rather on the way the sampling procedures ensure progressive enrichment in risk4, 40. Thus, the true yearly transition rate of attenuated psychotic symptoms in the general population, established in a meta‐analysis of representative, population‐based samples, is less than 1%41. The fact that the transition rate is much higher in UHR/CHR samples, similarly defined by the presence of attenuated psychotic symptoms20, has to do with the sampling strategies in UHR/CHR research. A recent meta‐analysis showed that the CHR sampling risk enrichment strategy occasioned a 3‐year transition rate of 15%42, thus accounting for half of the most recent meta‐analytical 3‐year transition rate of 29% attributed to CHR criteria2. Other reasons for the inflated transition rates in UHR/CHR research (e.g., natural fluctuation) were discussed earlier.
Direct evidence that the transition rate is caused by sampling enrichment and not CHR criteria came from a study in an early psychosis service for young people, showing that young people presenting to the service meeting UHR criteria had essentially the same 10‐year transition rate (17.3%) as young people presenting to the same service with non‐psychotic disorders (14.6%)43.
DOES BIOLOGICAL RESEARCH OF “TRANSITION” MAKE SENSE?
Given the attractive binary outcome of transition, a range of biological studies have attempted to find differences between those who do and those who do not make a transition, resembling the classical case‐control paradigm that has dominated biological research on the diagnosis of schizophrenia. These studies have reported a range of biological associations with “transition”, published in high‐impact academic journals. For example, studies have reported that transition to psychosis was associated with thalamic dysconnectivity44, progressive reduction of cortical thickness45, and increased glutamate levels in the associative striatum46.
Given the uncertain status of the transition concept, these findings cannot be readily interpreted and appear to be false positives unless true, rather than approximate, replication is attempted47. Analogously, one trial reported an apparently very strong effect of fish oil in reducing transition rates48, which became an informative null finding in the replication study49.
DOES UHR/CHR REPRESENT A VALID AND USEFUL SURROGATE FOR EARLY INTERVENTION?
To lay the groundwork for the current UHR/CHR construct, the architects of the construct started with reviewing the previous literature of the prodromal phase: narratives, early depictions, frequency and pattern of formation of signs and symptoms. This comprehensive review of the prodromal period clearly showed that non‐psychotic symptoms – concentration difficulties, motivational impairment, depressed mood, sleep disturbance, and anxiety – frequently emerge prior to onset of psychotic symptoms50. However, these symptoms were considered not specific enough to target with a therapeutic intervention, because the main driving force was to reproduce successful medical models of indicated prevention for schizophrenia.
This was a hazardous pursuit for several reasons. First, early detection and intervention in psychiatry cannot be easily fit into the framework of preventive medicine, because: a) natural history and underlying biological mechanisms of mental disorders have yet to be understood; b) there are no objective screening tools; c) there is no specific treatment. Second, UHR/CHR is conceptualized after schizophrenia, which is a classic case of the “no true Scotsman fallacy”, as formulated by Robins and Guze51: “good prognosis ‘schizophrenia’ is not mild schizophrenia, but a different illness”. From this perspective, setting the goal of preventing “transition” to schizophrenia by intervening at the level of UHR/CHR creates a paradox, or even a self‐fulfilling prophecy of failure. Third, there is a degree of tautology in the claim that an intervention specific to positive symptoms – the initial research agenda of prodromal research was antipsychotic trials in the UHR/CHR population – shall prevent “transition” to psychosis by reducing positive symptoms in UHR/CHR states that are primarily defined on the basis of milder positive symptoms. This can be likened to saying that increased cholesterol would be reduced by anti‐cholesterol treatment to prevent high cholesterol.
Perhaps not surprisingly, findings of UHR/CHR studies have confirmed what could have been expected: the pragmatic UHR/CHR construct overlooking early expression of nonspecific psychopathology (Figure 1) indeed backfires on early detection and intervention. A retrospective investigation52 of the population of the psychiatric case register in The Hague, the Netherlands, revealed that over half of the patients who developed psychosis had received treatment for non‐psychotic conditions (mood, anxiety and substance use disorders) during the prodromal phase, revealing a lot more prevention potential than the negligible percentage of the prodromal service, that is limited by the prevention paradox16, 17. Similarly, the vast majority of the North American UHR/CHR cohort had received psychosocial or pharmacological treatment long before the onset of subthreshold symptoms53, 54. These findings bring into question the utility of UHR/CHR concept: how early is early intervention?
SHOULD TREATMENT FOCUS ON “PREVENTION” OF “TRANSITION”?
There is no doubt that it is useful to offer early treatment to young individuals with anxiety/depression/substance use and a degree of psychosis admixture as a marker of relatively poor prognosis. It may be expected that non‐specific psychotherapeutic interventions will be beneficial, similar to the non‐specific effects of a range of psychotherapies in anxiety/depression55. For example, there is evidence that simple interventions such as non‐directive listening yield better results than cognitive‐behavioural therapy in UHR/CHR individuals56.
There is a body of intervention research, consisting of mostly small, highly heterogeneous and variably controlled studies, focusing on the outcome of “transition” in UHR/CHR individuals1. However, given the questionable validity and clinical relevance of the “transition” concept, coupled with the fact that these interventions do no impact functioning1, there seems to be an urgent need to reconceptualize and reorient treatment strategies in individuals with anxiety/depression/substance use and a degree of psychosis admixture as a marker of relatively poor prognosis.
The available evidence suggests that the tradition to observe these states through the “schizo”‐prism may be not useful and ethically questionable. Instead, it may be more productive to consider the full range of person‐specific psychopathology in all young individuals with mental health problems and to not become disproportionally fixated on the transdiagnostic manifestation of psychosis. Although psychotic experiences in common mental disorder may be associated with a poorer prognosis, and a small minority of people may indeed develop a clinical picture that meets criteria for schizophrenia, it appears neither useful nor scientifically valid to reduce the transdiagnostic expression of psychosis in early states of multidimensional psychopathology to the misleadingly simple binary concepts of “risk” and “transition”, with the implicit suggestion that all or most psychosis leads to schizophrenia.
CONCLUSIONS
Early intervention is a progressive movement and should be supported. However, the CHR‐cum‐transition concept is overly simplified and uncritically presented as “evidence”. The tools solely rely on positive symptoms and a family history of psychotic disorders. The implicit paradigm is to treat any subthreshold positive symptom as a pathway to schizophrenia. Currently, less emphasis is put on antipsychotic treatment, which is a good point. However, the “transition” concept is not just fuzzy but overreaching, and should not be used as an “outcome” in research or clinical practice.
It may be asked why, if this is the state of the evidence, the CHR‐cum‐transition concept continues to be pushed in research and clinical practice. In two separate articles, Schmidt et al1 and Schultze‐Lutter et al2 appear to provide “guidance” on CHR research and clinical practice on behalf of the European Psychiatric Association. In these days of heightened awareness of the role of not just commercial, but also academic funding, as well as other interests in research57, and the vagaries of research in small and selected samples, the meta‐analysis of which does not resolve the issue of multiple sources of bias58, 59, one would expect guidance by professional bodies to be critical and objective. It may be more useful to reserve journal space for academic debate, rather than uncritically perpetuating fashionable research notions and the academic interests that come with it.
Instead of the medical, relatively inefficient high‐risk approach, a public health perspective, focusing on improved access to a low‐stigma, high‐hope, small‐scale and youth‐specific environment with acceptable language and interventions, as embedded in the recent Headspace initiative60, may represent a more useful and more efficient strategy61.
REFERENCES
- 1. Schmidt SJ, Schultze‐Lutter F, Schimmelmann BG et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur Psychiatry 2015;30:388‐404. [DOI] [PubMed] [Google Scholar]
- 2. Schultze‐Lutter F, Michel C, Schmidt SJ et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry 2015;30:405‐16. [DOI] [PubMed] [Google Scholar]
- 3. Kablinger AS, Freeman AM 3rd. Prodromal schizophrenia and atypical antipsychotic treatment. J Nerv Ment Dis 2000;188:642‐52. [DOI] [PubMed] [Google Scholar]
- 4. Van Os J, Delespaul P. Toward a world consensus on prevention of schizophrenia. Dialogues Clin Neurosci 2005;7:53‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lacluyse K, van Bouwel L, Demunter H et al. Clinical assessment of the ultra high risk of developing a psychotic disorder; review and critical reflection. Tijdschr Psychiatr 2011;53:153‐62. [PubMed] [Google Scholar]
- 6. Amos A. Assessing the cost of early intervention in psychosis: a systematic review. Aust N Z J Psychiatry 2012;46:719‐34. [DOI] [PubMed] [Google Scholar]
- 7. Marshall C, Addington J, Epstein I et al. Treating young individuals at clinical high risk for psychosis. Early Interv Psychiatry 2012;6:60‐8. [DOI] [PubMed] [Google Scholar]
- 8. Fusar‐Poli P, Van Os J. Lost in transition: setting the psychosis threshold in prodromal research. Acta Psychiatr Scand 2013;127:248‐52. [DOI] [PubMed] [Google Scholar]
- 9. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra‐high‐risk patients remains questionable. Schizophr Res 2014;153:240. [DOI] [PubMed] [Google Scholar]
- 10. Simon AE, Umbricht D, Lang UE et al. Declining transition rates to psychosis: the role of diagnostic spectra and symptom overlaps in individuals with attenuated psychosis syndrome. Schizophr Res 2014;159:292‐8. [DOI] [PubMed] [Google Scholar]
- 11. Mittal VA, Dean DJ, Mittal J et al. Ethical, legal, and clinical considerations when disclosing a high‐risk syndrome for psychosis. Bioethics 2015;29:543‐56. [DOI] [PubMed] [Google Scholar]
- 12. Cannon TD, Cadenhead K, Cornblatt B et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry 2008;65:28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salokangas RK, Heinimaa M, From T et al. Short‐term functional outcome and premorbid adjustment in clinical high‐risk patients. Results of the EPOS project. Eur Psychiatry 2014;29:371‐80. [DOI] [PubMed] [Google Scholar]
- 14. van der Gaag M, Nieman DH, Rietdijk J et al. Cognitive behavioral therapy for subjects at ultrahigh risk for developing psychosis: a randomized controlled clinical trial. Schizophr Bull 2012;38:1180‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morrison AP, French P, Stewart SL et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ 2012;344:e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ajnakina O. First episode psychosis: looking backwards and forwards. https://kclpure.kcl.ac.uk.
- 17. Rose G. Strategy of prevention: lessons from cardiovascular disease. BMJ (Clin Res Ed) 1981;282:1847‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Addington J, Case N, Saleem MM et al. Substance use in clinical high risk for psychosis: a review of the literature. Early Interv Psychiatry 2014;8:104‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fusar‐Poli P, Nelson B, Valmaggia L et al. Comorbid depressive and anxiety disorders in 509 individuals with an at‐risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull 2014;40:120‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fusar‐Poli P, Cappucciati M, Borgwardt S et al. Heterogeneity of psychosis risk within individuals at clinical high risk: a meta‐analytical stratification. JAMA Psychiatry 2016;73:113‐20. [DOI] [PubMed] [Google Scholar]
- 21. Linscott RJ, van Os J. An updated and conservative systematic review and meta‐analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med 2013;43:1133‐49. [DOI] [PubMed] [Google Scholar]
- 22. van Os J, Reininghaus U. Psychosis as a transdiagnostic and extended phenotype in the general population. World Psychiatry 2016;15:118‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perlis RH, Uher R, Ostacher M et al. Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Arch Gen Psychiatry 2011;68:351‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelleher I, Keeley H, Corcoran P et al. Clinicopathological significance of psychotic experiences in non‐psychotic young people: evidence from four population‐based studies. Br J Psychiatry 2012;201:26‐32. [DOI] [PubMed] [Google Scholar]
- 25. Wigman JT, van Nierop M, Vollebergh WA et al. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity – implications for diagnosis and ultra‐high risk research. Schizophr Bull 2012;38:247‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wigman JT, van Os J, Abidi L et al. Subclinical psychotic experiences and bipolar spectrum features in depression: association with outcome of psychotherapy. Psychol Med 2014;44:325‐36. [DOI] [PubMed] [Google Scholar]
- 27. Guloksuz S, van Nierop M, Lieb R et al. Evidence that the presence of psychosis in non‐psychotic disorder is environment‐dependent and mediated by severity of non‐psychotic psychopathology. Psychol Med 2015;45:2389‐401. [DOI] [PubMed] [Google Scholar]
- 28. van Nierop M, Viechtbauer W, Gunther N et al. Childhood trauma is associated with a specific admixture of affective, anxiety, and psychosis symptoms cutting across traditional diagnostic boundaries. Psychol Med 2015;45:1277‐88. [DOI] [PubMed] [Google Scholar]
- 29. Woods SW, Addington J, Cadenhead KS et al. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull 2009;35:894‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cannon TD, Cadenhead K, Cornblatt B et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry 2008;65:28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Velthorst E, Nieman DH, Becker HE et al. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr Res 2009;109:60‐5. [DOI] [PubMed] [Google Scholar]
- 32. Carrion RE, McLaughlin D, Goldberg TE et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry 2013;70:1133‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nelson B, Yuen HP, Wood SJ et al. Long‐term follow‐up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry 2013;70:793‐802. [DOI] [PubMed] [Google Scholar]
- 34. Falkenberg I, Valmaggia L, Byrnes M et al. Why are help‐seeking subjects at ultra‐high risk for psychosis help‐seeking? Psychiatry Res 2015;228:808‐15. [DOI] [PubMed] [Google Scholar]
- 35. Yung AR, Yuen HP, McGorry PD et al. Mapping the onset of psychosis: the comprehensive assessment of at‐risk mental states. Aust N Z J Psychiatry 2005;39:964‐71. [DOI] [PubMed] [Google Scholar]
- 36. Miller TJ, McGlashan TH, Rosen JL et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry 2002;159:863‐5. [DOI] [PubMed] [Google Scholar]
- 37. Simon AE, Velthorst E, Nieman DH et al. Ultra high‐risk state for psychosis and non‐transition: a systematic review. Schizophr Res 2011;132:8‐17. [DOI] [PubMed] [Google Scholar]
- 38. Lin A, Wood SJ, Nelson B et al. Neurocognitive predictors of functional outcome two to 13 years after identification as ultra‐high risk for psychosis. Schizophr Res 2011;132:1‐7. [DOI] [PubMed] [Google Scholar]
- 39. Brandizzi M, Valmaggia L, Byrne M et al. Predictors of functional outcome in individuals at high clinical risk for psychosis at six years follow‐up. J Psychiatr Res 2015;65:115‐23. [DOI] [PubMed] [Google Scholar]
- 40. Simon AE, Roth B, Zmilacher S et al. Developing services for the early detection of psychosis: a critical consideration of the current state of the art. Eur Child Adolesc Psychiatry 2007;16:96‐103. [DOI] [PubMed] [Google Scholar]
- 41. Kaymaz N, Drukker M, Lieb R et al. Do subthreshold psychotic experiences predict clinical outcomes in unselected non‐help‐seeking population‐based samples? A systematic review and meta‐analysis, enriched with new results. Psychol Med 2012;42:2239‐53. [DOI] [PubMed] [Google Scholar]
- 42. Fusar‐Poli P, Schultze‐Lutter F, Cappucciati M et al. The dark side of the moon: meta‐analytical impact of recruitment strategies on risk enrichment in the clinical high risk state for psychosis. Schizophr Bull 2016;42:732‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conrad AM, Lewin TJ, Sly KA et al. Utility of risk‐status for predicting psychosis and related outcomes: evaluation of a 10‐year cohort of presenters to a specialised early psychosis community mental health service. Psychiatry Res 2017;247:336‐44. [DOI] [PubMed] [Google Scholar]
- 44. Anticevic A, Haut K, Murray JD et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry 2015;72:882‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cannon TD, Chung Y, He G et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry 2015;77:147‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de la Fuente‐Sandoval C, Leon‐Ortiz P, Azcarraga M et al. Striatal glutamate and the conversion to psychosis: a prospective 1H‐MRS imaging study. Int J Neuropsychopharmacol 2013;16:471‐5. [DOI] [PubMed] [Google Scholar]
- 47. Maxwell SE. The persistence of underpowered studies in psychological research: causes, consequences, and remedies. Psychol Methods 2004;9:147‐63. [DOI] [PubMed] [Google Scholar]
- 48. Amminger GP, Schafer MR, Papageorgiou K et al. Long‐chain omega‐3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo‐controlled trial. Arch Gen Psychiatry 2010;67:146‐54. [DOI] [PubMed] [Google Scholar]
- 49. McGorry PD, Nelson B, Markulev C et al. Effect of omega‐3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: the NEURAPRO randomized clinical trial. JAMA Psychiatry 2017;74:19‐27. [DOI] [PubMed] [Google Scholar]
- 50. Yung AR, McGorry PD. The prodromal phase of first‐episode psychosis: past and current conceptualizations. Schizophr Bull 1996;22:353‐70. [DOI] [PubMed] [Google Scholar]
- 51. Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry 1970;126:983‐7. [DOI] [PubMed] [Google Scholar]
- 52. Rietdijk J, Hogerzeil SJ, van Hemert AM et al. Pathways to psychosis: help‐seeking behavior in the prodromal phase. Schizophr Res 2011;132:213‐9. [DOI] [PubMed] [Google Scholar]
- 53. Woods SW, Addington J, Bearden CE et al. Psychotropic medication use in youth at high risk for psychosis: comparison of baseline data from two research cohorts 1998‐2005 and 2008‐2011. Schizophr Res 2013;148:99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Woodberry KA, Seidman LJ, Bryant C et al. Treatment precedes positive symptoms in North American adolescent and young adult clinical high risk cohort. J Clin Child Adolesc Psychol 2016;5:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cuijpers P, Donker T, van Straten A et al. Is guided self‐help as effective as face‐to‐face psychotherapy for depression and anxiety disorders? A systematic review and meta‐analysis of comparative outcome studies. Psychol Med 2010;40:1943‐57. [DOI] [PubMed] [Google Scholar]
- 56. Stain HJ, Bucci S, Baker AL et al. A randomised controlled trial of cognitive behaviour therapy versus non‐directive reflective listening for young people at ultra high risk of developing psychosis: the detection and evaluation of psychological therapy (DEPTh) trial. Schizophr Res 2016;176:212‐9. [DOI] [PubMed] [Google Scholar]
- 57. Smith R, Feachem R, Feachem NS et al. The fallacy of impartiality: competing interest bias in academic publications. J R Soc Med 2009;102:44‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Open Science Collaboration . An open, large‐scale, collaborative effort to estimate the reproducibility of psychological science. Perspect Psychol Sci 2012;7:657‐60. [DOI] [PubMed] [Google Scholar]
- 59. van Assen MA, van Aert RC, Nuijten MB et al. Why publishing everything is more effective than selective publishing of statistically significant results. PLoS One 2014;9:e84896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McGorry PD, Tanti C, Stokes R et al. Headspace: Australia's National Youth Mental Health Foundation – where young minds come first. Med J Aust 2007;187:S68‐70. [DOI] [PubMed] [Google Scholar]
- 61. Fusar‐Poli P, Yung AR, McGorry P et al. Lessons learned from the psychosis high‐risk state: towards a general staging model of prodromal intervention. Psychol Med 2014;44:17‐24. [DOI] [PubMed] [Google Scholar]


