Abstract
Immune microenvironment characterized by T cell clonality as well as expression signatures of immune-related genes in endometrial cancer tissues may play significant roles in clinical outcome of patients. We aimed to investigate the clinical significance of immune-related gene expression and TCR repertoire in endometrial cancer. Using total RNAs extracted from 32 endometrioid endometrial cancer cases, we performed quantitative real-time PCR to measure mRNA expression levels of immune-related genes including TRB, CD8, GZMA, HLA-A, CD11c and PD-L1. Higher mRNA expression levels of CD8 (P=0.039) and CD11c (P=0.046) in the 32 tissue samples were significantly associated with longer progression-free survival (PFS). Expression levels of CD8 (P<0.001) and CD11c (P=0.048) were also significantly associated with longer PFS in 540 cases in TCGA database. We also performed T cell receptor β (TCRβ) sequencing of tumor-infiltrating lymphocytes (TILs) on an Illumina MiSeq platform. To evaluate clonal expansion of TCRβ clonotypes, we adjusted the number of abundant TCRβ clonotypes by TRB mRNA expression levels and examined TCR clonality with the expression levels of immune-related genes and clinicopathological factors. The cases with high clonal T cell expansion along with high PD-L1 expression in cancer tissues was related to higher mRNA expression levels of CD8 (P<0.001), GZMA (P<0.001) and HLA-A (P=0.027), showed a significantly longer PFS (P=0.015), indicating a possibility that these parameters may serve as faborable prognostic factors. Considering clinical stage, mRNA expression of CD8 (P=0.037), GZMA (P=0.027) and HLA-A (P=0.022) was significantly higher in tumors at an early stage. Thus, we identified clinical and prognostic significance of immune microenvironment including the T cell clonality of TILs as well as PD-L1 and CD11c mRNA expression levels in endometrial cancer tissues.
Keywords: endometrial cancer, cancer immunity cycle, TCR repertoire, PD-L1 expression, prognosis
Introduction
Endometrial cancer is the fourth most common malignancy among women in developed countries (1). Abnormal bleeding is one of the useful signs for early diagnosis of endometrial cancer, but 15–20% of patients recur after primary surgery (2) and their 10-year survival rate is still poor at 18% (3). Hence, novel therapeutic options based on better understanding of endometrial cancer, particularly of immune microenvironment, should be developed to improve the poor prognosis.
In the process of antitumor cytotoxic T-cell response, the first event is the capture of cancer-specific antigens by antigen presenting cells (APCs) such as dendritic cells and macrophages (4). APCs process these antigen proteins to peptides, present the antigens on human leukocyte antigen (HLA) class I molecules to CD8+ T cells and activate T-cell responses against specific antigens (4). Then, the activated effector CD8+ T cells infiltrate into tumor tissues and recognize cancer cells through the interaction between T cell receptor (TCR) and its cognate antigen bound to HLA class I molecule (4). Finally, the activated cytotoxic T cells kill cancer cells by the releasing cytotoxic agents such as perforin and granzyme, and then cancer cells killed by T cells further releasing cancer-specific antigens (4). These sequential events are known as ‘cancer immunity cycle’ (5), and many molecules are involved in the present cycle. To escape from the host immune attack, cancer cells frequently downregulate the expression of HLA molecules (6) or overexpress immune checkpoint molecules such as programmed death-ligand 1 (PD-L1) and 2 (PD-L2) (7). Expression levels of PD-L1/L2 are enhanced proportionally according to the amount of tumor-infiltrating T lymphocytes (TILs), and are associated with favorable prognosis in several types of cancer, including melanoma, breast and ovarian cancer (8–11).
Significance of immune microenvironment in prognosis of endometrial cancer patients has been investigated, since the patients with hypermutations by polymerase ε (POLE) gene mutations or microsatellite instability (MSI) were indicated to have better progression-free survival (PFS) (12). These high MSI cases were suggested to possess a higher number of somatic mutations, and considered to generate a higher number of antigenic neo-epitopes and contain significantly higher tumor-infiltrating CD8+ T cells (13,14). High T-cell infiltration into tumor was shown to be associated with favorable prognosis of patients with endometrial cancer (15–17). These results indicate the clinical importance of immune microenvironment in endometrial cancer, but no previous study investigated the possible significance of T cell clonality and expression of genes related to cancer immune responses in endometrial cancer.
TCRs are expressed on the surface of T cells, and 95% of T cells possess TCRs consisting of a heterodimer of α and β chains. Diversity of TCRs is generated by a somatic recombination process of variable (V), diversity (D) (only for β chain), and joining (J) exons, termed the V(D)J recombination (18,19). Rearrangement of these segments generates the highly variable complementarity determining region 3 (CDR3), which is important for the recognition of an antigen on the HLA molecule (20). We recently developed a method to characterize TCR repertoire from mRNA isolated from cancer tissues using a next-generation DNA sequencer (21). In this study, we aimed to investigate the clinical significance of the clonality of TILs and intratumor expression levels of immune-related genes in prognosis of endometrial cancer.
Materials and methods
Patient samples
Surgical samples from 32 patients with endometrioid endometrial carcinoma were obtained at Saitama Medical University International Medical Center. Details of the patient characteristics are summarized in Table I. No patient received any chemotherapy before surgery. The study protocol was approved by the Institutional Review Boards of Saitama Medical University International Medical Center and The University of Chicago. Written informed consent was obtained from each of the study participants.
Table I.
Patient characteristics of 32 endometrial cancer samples.
| Characteristics | Cases | Frequency |
|---|---|---|
| Total | 32 | |
| Histology | ||
| Endometrioid | 32 | (100%) |
| Stage | ||
| Early | 15 | (47%) |
| Advanced | 17 | (53%) |
| Grade | ||
| 1/2 | 22 | (69%) |
| 3 | 10 | (31%) |
| Menopause | ||
| Pre | 6 | (19%) |
| Post | 22 | (69%) |
| Unknown | 4 | (12%) |
| Lymph node metastasis | 8 | (25%) |
| Lymphvascular invasion | 16 | (50%) |
| Muscle invasion | ||
| <1/2 | 27 | (84%) |
| ≥1/2 | 5 | (16%) |
| Recurrence | ||
| Yes | 7 | (22%) |
| No | 25 | (78%) |
| Age (years) | ||
| Median | 61 | (range, 35–78) |
| BMI | ||
| Median | 24 | (range, 17.6–34.7) |
| Tumor size (mm) | ||
| Median | 44.6 | (range, 23.3–108) |
TaqMan gene expression analysis
From the tumor tissues, total RNAs were obtained using an RNeasy Mini kit (Qiagen, Valencia, CA, USA). cDNA with 5-rapid amplification of cDNA ends (5-RACE) adapter was synthesized using SMART cDNA library construction kit (Clontech Laboratories, Inc., Mountain View, CA, USA). Quantitative real-time PCR was conducted using TaqMan gene expression assays (Thermo Fisher Scientific, Carlsbad, CA, USA) on the Applied Biosystems ViiA 7 Real-Time PCR system (Thermo Fisher Scientific) according to the manufacturers instructions. The following TaqMan gene expression assays were used; TCRβ (TRB) (forward, GAGCCATCAGAAGCAGAGATCTC and reverse; GGCCAGGCACACCAGTGT, MGB probe; ACACC AAAAGGC), CD8A (Hs00233520_m1), granzyme A (GZMA) (Hs00989184_m1), HLA-A (Hs01058806_g1), CD11c (ITGAX) (Hs00174217_m1) and PD-L1 (Hs01125301_m1). mRNA expression levels were normalized to GAPDH expression (Hs02758991_g1).
TCGA dataset analysis
We obtained the mRNA expression dataset in endometrial cancer from The Cancer Genome Atlas (TCGA) database (12). The files with IlluminaHiSeq_RNASeqV2 and IlluminaGA_RNASeqV2 platform code were downloaded from the TCGA website (see https://tcga-data.nci.nih.gov/tcga/). Total of 546 cases were obtained from the TCGA website, and we excluded 6 cases from this analysis because of the lack of clinical information we needed.
TCR sequencing
The detailed method of library preparation for TCR sequencing was previously described (21). In brief, we used 957 to 1878 ng of total RNA. cDNAs were synthesized as described above, and performed two step PCRs to obtain sequence libraries. The first PCR was performed to amplify TCRβ cDNA using a forward primer corresponding to the SMART adapter sequence and a reverse primer corresponding to the constant region of TCRβ. The second PCR was done to add the index sequences for Illumina sequencer with barcode sequence using the Nextera Index kit (Illumina, San Diego, CA, USA). The prepared libraries were subjected to sequencing using the MiSeq Reagent v3 600-cycles kit on the MiSeq (Illumina).
TCR repertoire was analyzed using Tcrip software (22)
Briefly, sequencing reads in fastq files were mapped to the TCR reference sequences derived from IMGT/GENE-DB (http://www.imgt.org) using Bowtie2 aligner (version 2.1.0) (23). The V, D and J genes were designated according to the nomenclature provided by the international ImMunoGeneTics information system (IMGT) (24,25). A CDR3 region was defined by identifying the second conserved cysteine encoded in the 3 portion of the V segment and the conserved phenylalanine or tryptophan encoded in the 5 portion of the J segment. TCRβ clonality was defined as the number of abundant TCRβ clonotypes adjusted by TRB mRNA expression, as shown below:
Statistical analysis
Survival analysis was performed using the Kaplan-Meier method and the log-rank test. Differences between two groups were evaluated by the Mann-Whitney U test. All statistical analyses including multivariate analysis were performed using JMP v11 (SAS, Inc., Cary, NC, USA) and GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). In all statistical tests, differences were considered to be statistically significant at P<0.05.
Results
Association of expression levels of cancer immune-related genes with patient prognosis in endometrial cancer
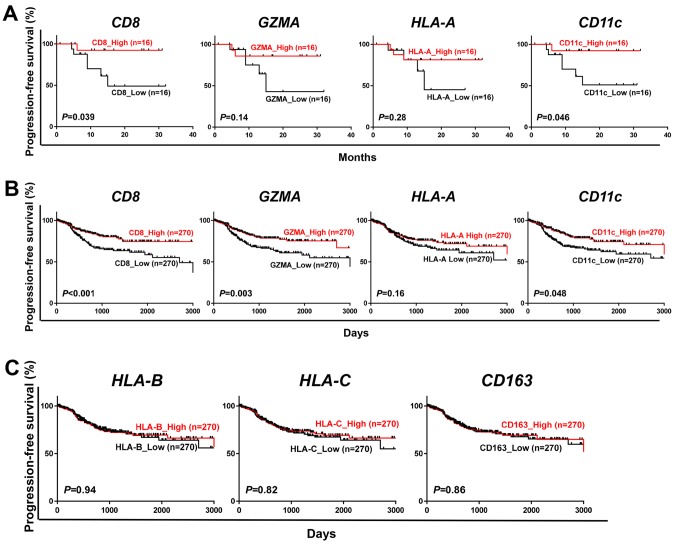
To investigate any effects of expression levels of cancer immune-related genes on the prognosis of endometrial cancer patients, we measured mRNA expression levels of CD8, GZMA (one of molecular markers for cytolytic activity), HLA-A (one of major HLA class I molecules) and CD11c (one of markers for dendritic cells/macrophages) in the surgically-resected cancer tissues from 32 endometrial cancer patients. We classified the cases into two groups by the median expression level of each gene and then compared the progression-free survival (PFS). Higher mRNA expression of CD8 (P=0.039) and CD11c (P=0.046) was significantly associated with longer PFS (Fig. 1A). Expression levels of GZMA (P=0.14) and HLA-A (P=0.28) showed some tendencies although statistically not significant. In 540 cases from the TCGA dataset, we identified significant associations of higher expression level of CD8 (P<0.001), GZMA (P=0.003) and CD11c (P=0.048) with longer PFS (Fig. 1B). In addition, we explored other cancer immune-related genes such as HLA-B (HLA class I molecule), HLA-C (HLA class I molecule) and CD163 (a macrophage marker) using the 540 TCGA cases, but no significant association with prognosis was observed (Fig. 1C). These results imply the importance of the infiltration of CD8+ T cells and APCs into tumor tissues for prognosis in endometrial cancer patients.
Figure 1.
Prognostic significance of cancer immune-related genes in endometrial cancer. (A and B) Kaplan-Meier curves for progression-free survival according to classification of 32 patients by the median expression level of CD8, GZMA, HLA-A and CD11c gene (A) and to that of 540 cases from the TCGA database (B). (C) Kaplan-Meier curves for progression-free survival by the median expression level of HLA-B, HLA-C and CD163 in 540 cases from the TCGA database. P-values were calculated by a log-rank test.
Association of TCRβ clonality in TILs with prognosis of endometrial cancer patients
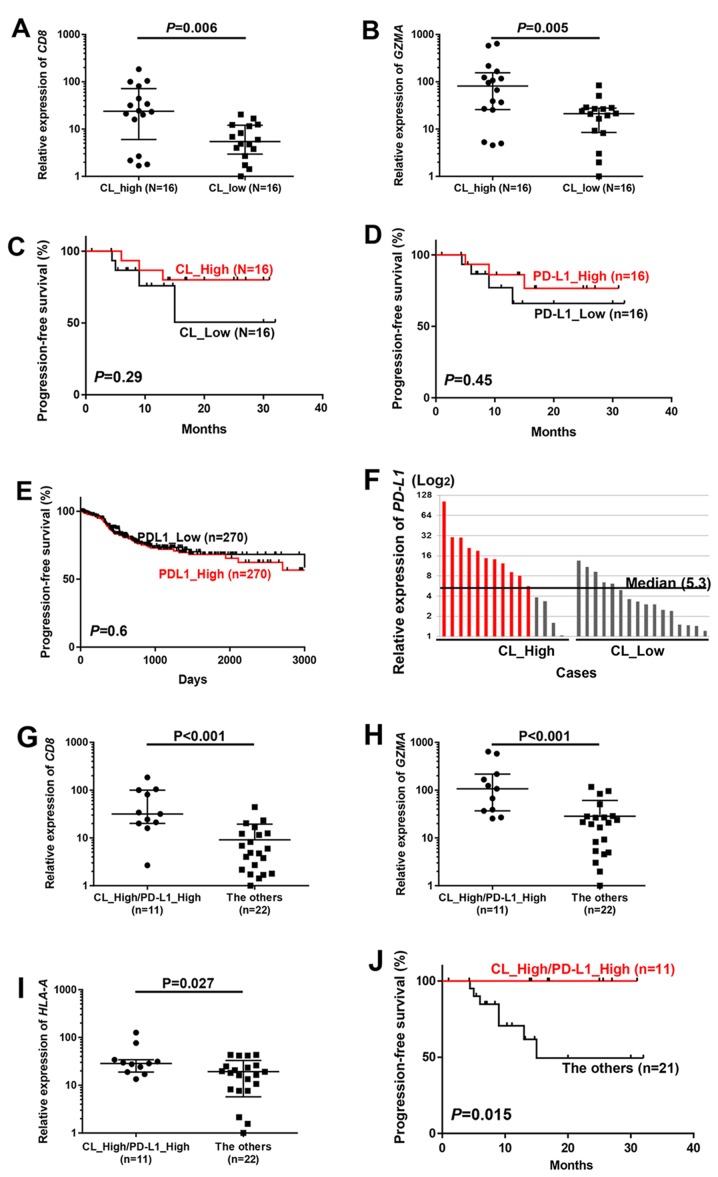
To examine whether clonal expansion of TILs is related to prognosis of patients with endometrial cancer, we explored the TCR repertoire in the 32 endometrial cancer tissues. Through the cDNA sequencing of TCRβ, we obtained total sequence reads of 270,255 to 4,692,690 (average, 1,834,576), and identified 3,765 to 80,739 (average, 23,598) unique TCRβ CDR3 clonotypes (Table II). To evaluate the TCRβ clonality, the numbers of TCRβ clonotypes of >0.1% frequency were adjusted by TRB mRNA expression levels; this value is lower when certain T cells are enriched (defined as ‘CL_High’), and is higher when the number of enriched clones is limited (defined as ‘CL_Low’). In this classification, mRNA expression levels of CD8 (P=0.006) and GZMA (P=0.005) were significantly higher in patients with CL_High, and tend to have longer PFS (Fig. 2A-C).
Table II.
TCRβ sequencing of 32 endometrial cancer samples.
| Samples | RNA (µg) | Total reads | Observed clonotypes | Unique clonotypes |
|---|---|---|---|---|
| 1 | 1256 | 2,799,659 | 2,000,034 | 47,474 |
| 2 | 1515 | 1,706,242 | 1,172,922 | 31,040 |
| 3 | 1248 | 3,483,543 | 1,528,768 | 37,262 |
| 4 | 1032 | 2,014,597 | 1,318,455 | 31,655 |
| 5 | 1356 | 1,259,422 | 887,850 | 39,312 |
| 6 | 1389 | 530,160 | 111,787 | 2,631 |
| 7 | 1203 | 1,244,915 | 791,062 | 9,131 |
| 8 | 1704 | 270,255 | 135,501 | 1,376 |
| 9 | 1383 | 2,088,016 | 1,654,058 | 19,699 |
| 10 | 1095 | 749,670 | 553,644 | 8,055 |
| 11 | 1272 | 349,401 | 206,553 | 3,899 |
| 12 | 1084 | 1,561,372 | 957,506 | 8,705 |
| 13 | 1212 | 4,692,690 | 3,286,445 | 80,739 |
| 14 | 1878 | 2,785,592 | 2,229,815 | 34,177 |
| 15 | 1080 | 841,590 | 710,246 | 8,985 |
| 16 | 957 | 1,884,946 | 1,392,866 | 15,713 |
| 17 | 1026 | 1,661,339 | 858,528 | 5,567 |
| 25 | 1011 | 1,505,156 | 811,544 | 27,736 |
| 26 | 1734 | 760,442 | 313,160 | 6,543 |
| 27 | 1072 | 1,368,263 | 695,285 | 27,130 |
| 28 | 1006 | 2,174,686 | 1,258,224 | 16,901 |
| 29 | 1120 | 800,381 | 223,986 | 4,352 |
| 31 | 1156 | 2,683,768 | 1,483,747 | 38,150 |
| 32 | 1353 | 1,640,097 | 1,047,746 | 23,619 |
| 33 | 1349 | 1,005,383 | 381,802 | 8,887 |
| 34 | 1730 | 1,964,567 | 1,448,467 | 32,797 |
| 35 | 1470 | 1,362,842 | 1,047,478 | 34,492 |
| 36 | 1171 | 3,347,286 | 2,430,597 | 55,515 |
| 37 | 1169 | 3,034,346 | 1,655,574 | 43,294 |
| 38 | 980 | 1,715,642 | 930,467 | 19,235 |
| 39 | 1122 | 4,078,715 | 83,682 | 3,765 |
| 40 | 1267 | 1,341,472 | 992,980 | 27,295 |
Figure 2.
Favorable prognosis in cases with enriched TCRβ clonotypes and high PD-L1 mRNA expression. (A and B) All 32 cases were classified into two groups by the median number of TCRβ clones with >0.1% frequency (Materials and methods), and compared by mRNA expression of (A) CD8 and (B) GZMA. (C-E) Kaplan-Meier curves of progression-free survival for two groups classified by T cell clonal expansion in our 32 cases (C) and PD-L1 expression in our 32 cases (D) and PD-L1 expression in 540 TCGA cases (E) were evaluated by a log-rank test. Cases were classified by the median of each value. (F) All cases were classified into two groups according to high or low TCRβ clonality by the median value of numbers of TCRβ clones with the frequency of >0.1% (Materials and methods), and then individual cases in each group were ordered in the relative PD-L1 expression levels (median, 5.3). (G-I) Comparison of mRNA expression of (G) CD8, (H) GZMA and (I) HLA-A between ‘CL_High/PD-L1_High’ [cases shown in red in (F)] cases and ‘The others’. P-values were calculated by Mann-Whitney test. (J) Kaplan-Meier curves (progression-free survival) of patients by classification of tumors with T cell clonal expansion along with high PD-L1 expression (CL_High/PD-L1_High) and the other cases (The others). P-value was calculated using log-rank test.
Several recent reports indicated that expression of PD-L1 in tumor cells reflect the presence of antigen-induced antitumor immune pressure mediated by TILs (8–11). However, PD-L1 expression itself did not show any prognostic value in our 32 cases (P=0.45) or in 540 cases in TCGA database (P=0.60) (Fig. 2D and E). Among our 16 cases with CL_High, 11 cases showed higher PD-L1 expression than the median PD-L1 expression level of 32 cases, and we defined them as CL_High/PD-L1_High which is considered to have strong immune pressure in their tumor tissues (Fig. 2F). In these 11 CL_High/PD-L1_High cases, we observed significantly higher levels of CD8 (P<0.001), GZMA (P<0.001) and HLA-A (P=0.027) than in the remaining cases (Fig. 2G-I). In prognostic analysis, none of 11 cases in the CL_High/PD-L1_High group had recurrence while the remaining 21 cases showed the significantly higher relapse rate (P=0.015; Fig. 2J).
Association of immune-related gene expression and TCRβ clonality in TILs with patient characteristics
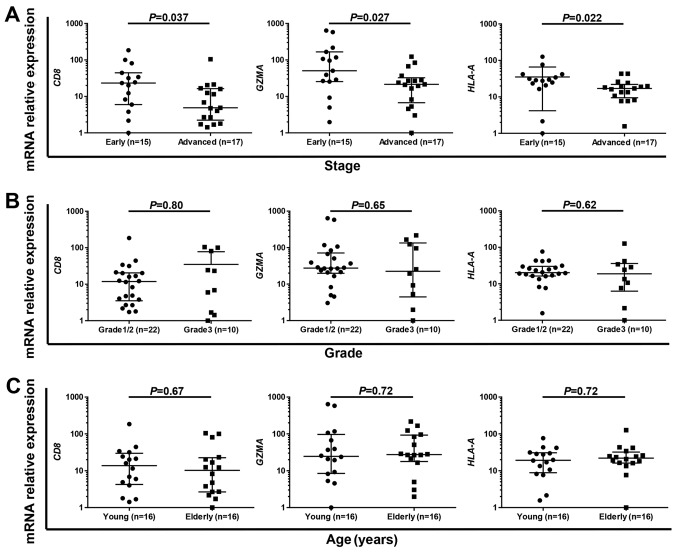
Finally, we examined the association of immune-related gene expression and TCRβ clonality with clinicopathological characteristics such as clinical stage, grade and age. Multivariable analysis revealed that CL_High/PD-L1_High was the only independent favorable prognostic factor in our results (Table III). Therefore, we examined the association between expression level of immune-related genes such as CD8, GZMA and HLA-A with clinicopathological characteristics. We found that the expression levels of CD8 (P=0.037), GZMA (P=0.027) and HLA-A (P=0.022) were significantly higher in early-stage cases than advanced-stage cases (Fig. 3A), while no significant difference was observed in grade (Fig. 3B) or age (young vs. elderly, classified by the median of 61 years; Fig. 3C). These results suggest that early-stage endometrial tumors are immunologically more active compared to advanced-stage tumors.
Table III.
Multivariate analysis of expression levels of immune-related genes.
| Multivariate analysis | |||
|---|---|---|---|
| Characteristics | Hazard ratio | 95% CI | P-value |
| CL_High/PD-L1_High | |||
| Yes | 1.06E-09 | 0-0.43 | 0.01 |
| No (ref) | |||
| Stage | |||
| I and II | 0.14 | 0.01–1.04 | 0.06 |
| III and IV (ref) | |||
| Grade | |||
| 1 and 2 | 0.3 | 0.03–1.88 | 0.2 |
| 3 (ref) | |||
| Age (years) | |||
| <60 | 0.58 | 0.05–6.12 | 0.64 |
| ≥60 (ref) | |||
| CD8 | |||
| >Median | 0.25 | 0.01–1.9 | 0.19 |
| ≤Median (ref) | |||
| Stage | |||
| I and II | 0.19 | 0.01–1.33 | 0.1 |
| III and IV (ref) | |||
| Grade | |||
| 1 and 2 | 0.22 | 0.06–1.85 | 0.22 |
| 3 (ref) | |||
| Age (years) | |||
| 60 | 1.15 | 0.15–9.4 | 0.89 |
| ≥60 (ref) | |||
| CD11c | |||
| >Median | 0.21 | 0.01–2.34 | 0.21 |
| ≤Median (ref) | |||
| Stage | |||
| I and II | 0.16 | 0.01–1.28 | 0.09 |
| III and IV (ref) | |||
| Grade | |||
| 1 and 2 | 0.18 | 0.61–15.4 | 0.18 |
| 3 (ref) | |||
| Age (years) | |||
| <60 | 0.8 | 0.08–7.79 | 0.84 |
| ≥60 (ref) | |||
Figure 3.
Immune-active status was significantly high in early-stage endometrial cancer. (A-C) Comparison of mRNA expression levels of CD8, GZMA and HLA-A in early- (stage I/II) and advanced- (stage III/IV) stages (A), in grade 1/2 and grade 3 tumors (B) and in young (≤61 years) and elderly (>61 years) patients (C) by Mann-Whitney test.
Discussion
In the present study, we analyzed mRNA expression levels of immune-related genes and TCR repertoire of T lymphocytes in tumor tissues of 32 endometrial cancer patients, and demonstrated that: i) the association of higher mRNA expression levels of CD8, GZMA and CD11c with better prognosis; ii) the association of coexistence of higher clonal enrichment of certain T cells and higher PD-L1 expression (CL_High/PD-L1_High) with higher expression levels of CD8, GZMA and HLA-A as well as favorable prognosis; and ii) higher levels of CD8, GZMA and HLA-A expression in early-stage endometrial cancer.
We first explored clinical significance of cancer immunity-related molecules in our 32 endometrial cancer samples as well as the 540 TCGA cases, and identified that CD8 expression level could be an important factor to predict patient prognosis, as reported based on several previous investigations (26,27). In addition, we identified CD11c expression level, which represents the number of infiltrated dendritic cells/macrophages into tumors, was significantly associated with prognosis of endometrial cancer patients. These cells play a crucial role as APCs in defining immune microenvironment, and association of higher CD11c expression level with favorable prognosis was also suggested in previous studies for gastric, colon and cervical cancers (28). This is the first study showing prognostic significance of CD11c expression levels in endometrial cancer.
Since CD11c is a maker of dendritic cells/macrophages, which play an important role for antigen presentation, we also focused on the TCRβ repertoire in endometrial cancer. We previously demonstrated that TCRβ clonality was associated with prognosis in bladder cancer (29). Since TCRβ clonality itself was not significantly associated with prognosis of endometrial cancer patients, we combined T cell clonality and PD-L1 expression for further analysis. PD-L1 expression is associated with prognosis in several types of cancer including breast, ovarian cancer and melanoma (8–11), although our result in endometrial cancer did not clearly show the prognostic significance of PD-L1 expression. However, 11 CL_High/PD-L1_High cases, in which we observed higher T cell clonal expansion along with higher PD-L1 expression in tumor tissues, showed significantly higher expression levels of immune-related genes such as CD8, GZMA and HLA-A than the remaining cases. In addition, none of these 11 cases with CL_High/PD-L1_High experienced recurrence, indicating a prognostic importance of immune microenvironment characterized by expression levels of immune-related genes and the clonality of infiltrated T cells in endometrial cancer.
Finally, we performed subgroup analysis based on the patient clinicopathological characteristics, and identified that CL_high/PD-L1_high was an independent prognostic factor in endometrial cancer. In addition, the anti-immune status of early-stage patients was considered to be more active, although two previous reports were controversial for the association between immune status and clinical stage (26,30). Our results imply that the non-inflamed phenotype is one of the characteristics of tumor progression process in endometrial cancer.
In conclusion, we identified clinical significance of expression levels of cancer immune response-related factors such as CD8 and dendritic cells/macrophages in endometrial tumor tissues. In addition, the clonality of TILs in combination with PD-L1 expression in tumor tissues could be a good prognostic maker in endometrial cancer.
Acknowledgements
The super-computing resource was provided by the Human Genome Center, the Institute of Medical Science, the University of Tokyo (http://sc.hgc.jp/shirokane.html).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012;13:e353–e361. doi: 10.1016/S1470-2045(12)70213-9. [DOI] [PubMed] [Google Scholar]
- 3.Creutzberg CL, Nout RA, Lybeert ML, Wárlám-Rodenhuis CC, Jobsen JJ, Mens JW, Lutgens LC, Pras E, van de Poll-Franse LV, van Putten WL. PORTEC Study Group: Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e631–e638. doi: 10.1016/j.ijrobp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 5.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today. 1999;5:178–186. doi: 10.1016/S1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 7.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–2782. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 9.Harter PN, Bernatz S, Scholz A, Zeiner PS, Zinke J, Kiyose M, Blasel S, Beschorner R, Senft C, Bender B, et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget. 2015;6:40836–40849. doi: 10.18632/oncotarget.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol. 2016;141:293–302. doi: 10.1016/j.ygyno.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Inozume T, Hanada K, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, Yang JC. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother. 2010;33:956–964. doi: 10.1097/CJI.0b013e3181fad2b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, et al. Cancer Genome Atlas Research Network: Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Gool IC, Eggink FA, Freeman-Mills L, Stelloo E, Marchi E, de Bruyn M, Palles C, Nout RA, de Kroon CD, Osse EM, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21:3347–3355. doi: 10.1158/1078-0432.CCR-15-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, Stover E, Strickland KC, DAndrea AD, Wu CJ, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 15.Čermáková P, Melichar B, Tomšová M, Zoul Z, Kalábová H, Spaček J, Doležel M. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in patients with endometrial carcinoma. Anticancer Res. 2014;34:5555–5561. [PubMed] [Google Scholar]
- 16.de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, Nijman HW. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Suemori T, Susumu N, Iwata T, Banno K, Yamagami W, Hirasawa A, Sugano K, Matsumoto E, Aoki D. Intratumoral CD8 lymphocyte infiltration as a prognostic factor and its relationship with cyclooxygenase 2 expression and microsatellite instability in endometrial cancer. Int J Gynecol Cancer. 2015;25:1165–1172. doi: 10.1097/IGC.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 18.Scaviner D, Lefranc MP. The human T cell receptor alpha variable (TRAV) genes. Exp Clin Immunogenet. 2000;17:83–96. doi: 10.1159/000019128. [DOI] [PubMed] [Google Scholar]
- 19.Folch G, Lefranc MP. The human T cell receptor beta variable (TRBV) genes. Exp Clin Immunogenet. 2000;17:42–54. doi: 10.1159/000019130. [DOI] [PubMed] [Google Scholar]
- 20.Danska JS, Livingstone AM, Paragas V, Ishihara T, Fathman CG. The presumptive CDR3 regions of both T cell receptor alpha and beta chains determine T cell specificity for myoglobin peptides. J Exp Med. 1990;172:27–33. doi: 10.1084/jem.172.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang M, Yew P. Deep sequencing of T-cell and B-cell receptors with next-generation DNA sequencers. In: Nakamura Y, editor. Immunopharmacogenomics. Springer; 2015. pp. 3–26. [DOI] [Google Scholar]
- 22.Yamaguchi R, Inoto S, Miyano S. A TCR sequence data analysis pipeline: Tcrip. In: Nakamura Y, editor. Immunopharmacogenomics. Springer; 2015. pp. 27–43. [DOI] [Google Scholar]
- 23.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37:D1006–D1012. doi: 10.1093/nar/gkn838. (Database) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefranc MP. IMGT, the International ImMunoGeneTics Information System. Cold Spring Harb Protoc. 2011;2011:595–603. doi: 10.1101/pdb.top115. [DOI] [PubMed] [Google Scholar]
- 26.Yamagami W, Susumu N, Tanaka H, Hirasawa A, Banno K, Suzuki N, Tsuda H, Tsukazaki K, Aoki D. Immunofluorescence-detected infiltration of CD4+FOXP3+ regulatory T cells is relevant to the prognosis of patients with endometrial cancer. Int J Gynecol Cancer. 2011;21:1628–1634. doi: 10.1097/IGC.0b013e31822c271f. [DOI] [PubMed] [Google Scholar]
- 27.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 28.Origoni M, Parma M, DellAntonio G, Gelardi C, Stefani C, Salvatore S, Candiani M. Prognostic significance of immunohistochemical phenotypes in patients treated for high-grade cervical intraepithelial neoplasia. BioMed Res Int. 2013;2013:831907. doi: 10.1155/2013/831907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhury NJ, Kiyotani K, Yap KL, Campanile A, Antic T, Yew PH, Steinberg G, Park JH, Nakamura Y, ODonnel PH. Low T-cell receptor diversity, high somatic mutation burden, and high neoantigen load as predictors of clinical outcome in muscle-invasive bladder cancer. Eur Urol Focus. 2015;2:445–452. doi: 10.1016/j.euf.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Jung IK, Kim SS, Suh DS, Kim KH, Lee CH, Yoon MS. Tumor-infiltration of T-lymphocytes is inversely correlated with clinicopathologic factors in endometrial adenocarcinoma. Obstet Gynecol Sci. 2014;57:266–273. doi: 10.5468/ogs.2014.57.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]