Abstract
Anthracyclines play an important role in the management of patients with cancer but the development of anthracycline-induced cardiotoxicity (ACT) remains a significant concern for most clinicians. Recently, genetic approach has been used to identify patients at increased risk of ACT. This systematic review assessed the association between genomic markers and ACT. A systematic literature search was performed in Medline, PubMed, Cochrane Central Register of Controlled Studies, CINAHL Plus, AMED, EMBASE and HuGE Navigator from inception until May 2016. Twenty-eight studies examining the association of genetic variants and ACT were identified. These studies examined 84 different genes and 147 single nucleotide polymorphisms. Meta-analyses showed 3 risk variants significantly increased the risk for ACT; namely ABCC2 rs8187710 (pooled odds ratio: 2.20; 95% CI: 1.36–3.54), CYBA rs4673 (1.55; 1.05–2.30) and RAC2 rs13058338 (1.79; 1.27–2.52). The current evidence remains unclear on the potential role of pharmacogenomic screening prior to anthracycline therapy. Further research is needed to improve the diagnostic and prognostic role in predicting ACT.
Introduction
Anthracycline antibiotics are among the most potent chemotherapeutic agents since their introduction 50 years ago. Agents in this pharmacological group of antineoplastic drugs include doxorubicin, daunorubicin, epirubicin, and idarubicin. They are the backbone for many chemotherapy regimens in the treatment of breast cancer1, 2, lymphoma3–7, leukaemia8, 9 and sarcomas10, 11. This may be due to the wide range of mechanisms which anthracyclines are thought to act on including: (i) initiation of apoptosis via inhibition of topoisomerase II, (ii) DNA synthesis inhibition, (iii) DNA binding and alkylation, (iv) DNA cross-linking, (v) interference with DNA strand separation and helicase activity, and (vi) free radical formation and lipid peroxidation12. While anthracyclines have revolutionised the management of both early and advance-stage diseases, the clinical usefulness of anthracyclines is compromised by the adverse effects of cardiac toxicity. Regimens using anthracyclines were reported to increase the risk of clinical and subclinical cardiac toxicity as well as death by more than 5-fold13–15.
Thus, the early identification of patients at risk of cardiotoxicity is a primary goal for many cardiologist and oncologist. Research over the past few decades have identified several risk factors associated with ACT including: aged ≥ 65 years old or less than 4 years old, female gender, pre-existing hypertension and/or cardiac disease, mediastinal radiation, high doses of anthracycline as well as concurrent treatment with cyclophosphamide, paclitaxel and trastuzumab16, 17. Nevertheless, most of these approaches have low diagnostic sensitivity and predictive power to detect subclinical myocardial injury18, 19. Several studies have recently reported the use of genetic variants as prognostic biomarkers for early detection of ACT20–23. The aim of the current study was to provide an overview on studies using genetic markers for identification of patients at risk of ACT and summarise these associations.
Methods
Search strategy
We searched OVID Medline, PubMed, Cochrane Central Register of Controlled Studies, CINAHL Plus, AMED, EMBASE and HuGE Navigator from inception until May 2016. The search terms include anthracycline, cardiotoxicity and genetic (The full search term can be found in Supplementary Information: Search Strategies). This was supplemented with a manual search of cited references from retrieved articles.
Study selection
Studies that met the following criteria were included: (i) primary studies that determined an association between genetic polymorphism (including single nucleotide polymorphism (SNPs), deletions, duplication and copy-number variants) and cardiotoxicity; (ii) anthracycline was used and (iii) conducted in human population. Articles titles and abstracts were screened for relevancy by two independent reviewers (SWHL and SLL) and full text retrieved in accordance to the inclusion criteria. Any disagreement was resolved through adjudication with input by a third reviewer.
Data extraction
Two reviewers (SWHL and SLL) independently extracted data from identified studies using standardised data extraction form. Reviewers compared the results and resolved any differences by discussion. Information extracted include: geographic location, ethnic group, study design, participant demographics and clinical characteristics, genotyping technique, and definition of cardiotoxicity. The study was conducted following the process specified in the PRISMA statement.
Quality assessment
The reviewers independently assessed the quality of the included studies using quality of genetic association studies (Q-Genie) tool developed by Sohani et al. 24. This validated tool consisting of nine categories was developed based on the Strengthening the Reporting of Genetic Association Studies (STREGA)25 and Strengthening the Reporting of Genetic Risk Prediction Studies (GRIPS)26 guidelines.
Statistical analysis
In studies which had assessed for polymorphisms of the same genotype (minimum 2 studies), we conducted a meta-analysis using a random effects model27. Study heterogeneity was assessed using the Cochran Q and the I 2 statistics. We also calculated the departure from Hardy-Weinberg equilibrium (HWE), which if violated, may bias the estimates and replication of postulated gene-disease associations across different studies28. All analyses were performed using Stata 13.0 (StataCorp, College Station, TX) and Review Manager 5.3 packages (http://comunity.cochrane.org/tools/review-production-tools/revman-5)29.
Result
Study and patient characteristics
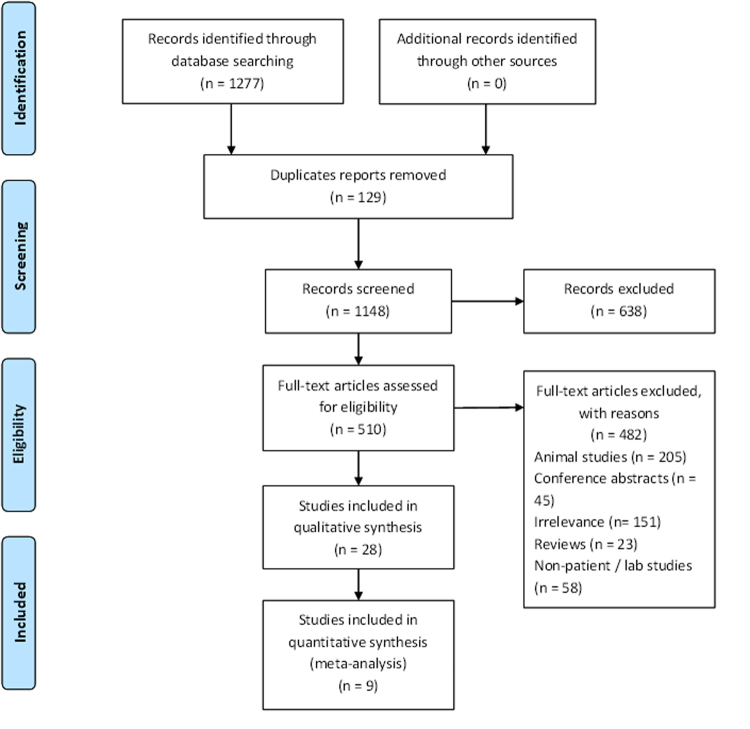
Our search identified 1,277 studies and 510 underwent assessment. A total of twenty-eight studies involving 7,082 patients were included in the current review (Fig. 1). The characteristics of the included studies are presented in Table 1. Eighteen of the studies were case control studies20–23, 30–43, of which eight were nested case-control studies22, 23, 32, 34–37, 42. Another seven were prospective cohort studies44–50 while two were retrospective cohort study51, 52. The remaining one was a case report53. These studies were conducted in the North America (n = 16)20, 21, 23, 30, 31, 33–37, 42–44, 46, 47, 52, Europe (n = 9)22, 32, 38–41, 45, 48, 51, and Asia (n = 1)50 while two did not report the study location49, 53. Almost equal number of studies were conducted in children (n = 10) and adults (n = 13) population. Five studies included both children and adults in their report36–39, 44. Nineteen studies described the ethnicity of their participants20, 23, 30–40, 43–45, 47, 50, 51 but, there were inconsistencies in reporting of race/ethnicity. For example, Weiss et al. 33 described their participants either as Caucasian or not while Blanco et al. 34 described their participants as White, Black and others.
Figure 1.
PRISMA flow diagram showing the selection process and criteria of the included studies.
Table 1.
Descriptions of included studies.
| Study: Author (year) | Geographic location, ethnic group | Study design; number of participants | Age (years) | Gender: male/female | Type of Cancer examined | Anthracycline used/Cumulative dose (mg/m2) | Source of DNA sample | Genotyping | Definition of cardiotoxicity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | ||||||||
| Wojnowski (2005)32 | Germany; 98% Germans | NCC; 550 | Mean = 62.0 ± 10.9 | Mean = 61.3 ± 11.0 | 50/37 | 212/151 | NHL | Doxorubicin/Cases: Med = 504 mg IQR = 160.5 mg Controls: Med = 540 mg IQR = 90 mg | Peripheral blood | i) Pyrosequencing ii) RFLP | i) arrhythmia in the absence of arrhythmia before treatment ii) myocarditis-pericarditis iii) acute heart failure iv) LVEF <50% or SF <25% |
| Weiss(2006)33 | USA; 85% Caucasian | CC; 197 | Med = 68 (56–88) | Approx. 98/99 | AML | Daunorubicin/NR | BM/peripheral blood | i) Multiplex PCR ii) Sequenom’s high-throughput matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) | i) SWOG toxicity criteria for SWOG 9031 ii) CTCAEv2.0 for SWOG-9333. | ||
| Blanco (2008)34 | USA; Whites, Blacks & Others | NCC; 145 | Mean = 10.3 ± 6.5 | Mean = 9.1 ± 5.8 | 10/20 | 57/58 | Leukaemia, brain tumour, HL, NHL, Wilms tumour, bone tumour neuroblastoma, soft tissue sarcoma, | Doxorubicin/<100 = 1 (2)* 100–350 = 13 (46) 350–500 = 7 (31)>500 = 9 (36) | Buccal cells/saliva | i) PCR-RFLP ii) Allelic discrimination with specific fluorescent probes | Self-reporting of signs and symptoms of CHF and use of medication for CHF management. |
| Rajic (2009)40 | Slovenia; Caucasian | CC; 76 | Mean = 25.8 ± 5.3 | 32/44 | ALL | Not specified/Mean = 199 ± 108 Range = 24–540 | Bone marrow smears | i) qPCR ii) Custom TaqMan® genotyping assay | i) Clear conduction disturbances, depolarization and repolarization changes in ECG ii) SF < 30%, LVEF <54% iii) Derangement of (reference range) E (0.75 ± 0.13), A (0.51 ± 0.11), E/A (1.53 ± 0.4), IVRT (67 ± 8), PV-A (0.21 ± 0.08), PV-D (0.47 ± 0.11) PV-S (0.44 ± 0.1) | ||
| Rossi (2009)41 | Italy; NR | CC; 106 | Med = 66 (56–75) | 55/51 | 55/51 | DLBCL | Doxorubicin/15 mg/m2/week | Peripheral blood | SNP minisequencing | Grade 2–4 cardiotoxicity according to CTCAEv 0.3 | |
| Blanco (2012)35 | USA; Non-Hispanic whites, Hispanics, Blacks & Others | NCC; 487 | Mean = 8.3 ± 6 | Mean = 8.2 ± 6 | 76/94 | 162/155 | HL, NHL, bone tumours, soft tissue sarcoma, ALL, AML, other. | Not specified/Cases: Med = 300 (0–575) Controls: Med = 140 (0–1050) | Peripheral blood/buccal cells/saliva | Allelic discrimination with specific fluorescent probes | i) signs and symptoms of cardiac compromise based on American Heart Association criteria 2005 ii) Absence of symptoms/signs with echo evidence of left ventricular dysfunction (EF ≤ 40% and/or SF ≤ 28%). |
| Kitagawa (2012)50 | Japan; Japanese | PC; 34 | Med = 49 (21–71) | 0/34 | Breast cancer | Epirubicin/NR | Whole blood | TaqMan® genotyping assay | i) QTc interval prolongation ii) other toxic effects based on CTCAEv3 | ||
| Lubieniecka (2012)44 | Canada; Caucasian | PC; 185 | Med = 46 (14–74) | 86/99 | AML | Daunorubicin/NR | Blood | Sequenom genotyping assay | Percentage drops in LVEF. | ||
| Sachida-nandam (2012)53 | NR | CS; 2 | Adult | −/2 | Breast cancer | Doxorubicin | Blood | PCR | NR | ||
| Semsei (2012)51 | Hungary; Hungarian | RC; 235 | Mean = 5.7 ± 3.8 | 126/109 | ALL | Daunorubicin, doxorubicin/NR | Peripheral blood | i) Mini-sequencing ii) GenomeLab SNPstream genotyping assay | Changes in LVFS | ||
| Visscher (2012)30 | Canada; 78% Canadian, 22% Dutch | CC; 440 | Discovery Med = 5.5 (0.04–17.0) Replication Med = 6.2 (0.4–17.6) Dutch-EKZ Med = 9.0 (0.5–16.8) | Discovery Med = 3.9 (0.5–16.5) Replication Med = 3.7 (0.05–16.9) Dutch-EKZ Med = 10.6 (2.1–17.1) | Discovery = 17/21 Replication = 22/18 Dutch-EKZ = 22/21 | Discovery = 66/52 Replication = 82/66 Dutch-EKZ = 27/26 | ALL, AML, other leukemia, HL, NHL Osteosarcoma, Rhabdomy-osarcoma, Ewing’s sarcoma, Other sarcoma, Nephroblastoma, Hepatoblastoma, Neuroblastoma, Carcinoma | Doxorubicin, Daunorubicin/Discovery Cases: Med = 300 (36–540) Controls: Med = 175 (60–600) Replication Cases: Med = 270 (45–840) Controls: Med = 250 (25–600) Dutch-EKZ Cases: Med = 360 (100–720) Controls: Med = 300 (50–720) | NR | Custom Illumina GoldenGate SNP genotyping assay | i) SF ≤ 26% ii) sign and symptoms requiring for cardiac compromise intervention based on CTCAEv3 |
| Volkan-Salanci (2012)45 | Turkey; Turkish | PC; 70 | Mean = 49.1 ± 13.6 | 7/63 | Breast cancer, lymphoma, mesenchymal tumour, nasopharyngeal cancer, duodenal cancer, sarcoma | Doxorubicin, epirubicin/Mean = 317.1 ± 94.9 | NR | TaqMan® genotyping assay | i) LVEF decrease > 10% ii) LVEF ≤ 50% | ||
| Windsor (2012)39 | UK, Caucasian, Afro-Caribbean, Indian/Asian | CC, 58 | Med = 18 (10–51) | 34/24 | Osteosarcoma | Doxorubicin/NR | Peripheral blood | i) Standard PCR, ii) PRC- RFLP, iii) Multiplex PCR, iv) Illumina microarray | Decrease in LVEF by ≥ 1 CTCAEv3 grade. | ||
| Armenian (2013)36 | USA; Non-Hispanic whites, Hispanics, Blacks & Others | NCC; 255 | Med = 49.2 (16–68.8) | Med = 51.0 (6.4–72.6) | 34/43 | 119/59 | Haematology malignancy + haematopoietic cell transplant | Not specified/Cases: Med = 300 (60–650) Controls: Med = 300 (40–600) | Peripheral blood stem cells, FFPE BM core biopsies, unstained slides of BM smears | Sequenom MassARRAY | Sign and symptoms of cardiac compromise requiring intervention based American Heart Association criteria 2005 |
| Lipshultz (2013)46 | USA; NR | PC; 184 | Med = 15.2 (3.1–31.4) | 101/83 | ALL | Doxorubicin/Med = 300 (204–420) | Peripheral blood | i) Pyrosequencing ii) Sequenom genotyping assay iii) TaqMan® genotyping assay | i) cTnT > 0.01 ng/mL ii) NT-proBNP > 150 pg/mL (< 1 year old) iii) NT-proBNP > 100 pg/mL (≥ 1 year old) | ||
| Lubieniecka (2013)52 | Canada; NR | RC; 91 | Mean = 48.4 Range = 19–74 | 48/43 | AML | Daunorubicin | Blood | Sequenom genotyping assay | Percentage drop in LVEF | ||
| Visscher (2013)31 | Canada; 41% Canadian, 69% Dutch | CC; 218 | Canadian-CPNDS Med = 12.6 (0.9–17.0) Dutch-EKZ Med = 9.1 (0.5–16.8) | Canadian-CPNDS Med = 4.9 (0.5–16.0) Dutch-EKZ Med = 11.2 (1.8–17.7) | Canadian-CPNDS = 8/4 Dutch-EKZ = 23/21 | Canadian-CPNDS = 31/47 Dutch-EKZ = 44/40 | ALL, AML, other leukemia, HL, NHL Osteosarcoma, Rhabdomyosarcoma, Ewing’s sarcoma, Other sarcoma, Nephroblastoma, Hepatoblastoma, Neuroblastoma, Carcinoma, Germ cell tumour | Doxorubicin, daunorubicin/Canadian CPDNS Cases: Med = 300 (175–550) Controls: Med = 150 (50–540) Dutch-EKZ Cases: Med = 360 (100–720) Controls: Med = 280 (50–720) | Blood/saliva/buccal swab | Custom Illumina GoldenGate SNP genotyping assay | i) SF ≤ 26% ii) sign and symptoms of cardiac compromise requiring intervention based on CTCAEv3 |
| Vivenza (2013)49 | NR | PC; 48 | 57.5 (28–73) | 1/47 | Breast cancer | Epirubcin/540 | Blood | i) Allelic discrimination using Applera SNP assay ii) TaqMan® genotyping assay | i) overt CHF (grade III) based on CTCAEv2 ii) LVEF < 50% (grade II) based on CTCAEv2 | ||
| Wang (2014)37 | USA; Non-Hispanic whites | NCC; 363 | Discovery cohort Med = 19.4 (0.4–41.7) | Discovery cohort Med = 18.5 (3.5–49.2) | 40/53 | 94/100 | HL, NHL bone tumours, soft tissue sarcoma, ALL, AML, other. | Not specified/Discovery Cases: Med = 300 (0–630) Controls:Med = 152 (0–825) Replication Med = 300 (60–649) | Peripheral blood, buccal cells/saliva | Illumina IBC cardiovascular SNP array | American Heart Association criteria for cardiac compromise: i) symptoms and/or signs of cardiac compromise and echo evidence of LV dysfunction. ii) absence of symptoms/signs with echo evidence of LV dysfunction (LVEF ≤ 40% and/or SF ≤ 28%). |
| Wasielewski (2014)38 | The Netherlands; Dutch | CC; 21 (Cohort I = 5; Cohort II = 13, Cohort III = 3) | Cohort I Med = 49 (2–57) Cohort II Med = 46 (34–61) Cohort III Med = 4 (4–9) | NR | Breast cancer, ALL, neuroblastoma, Wilm’s tumour, primary neuroectodermal tumour | Epirubicin, Doxorubicin, Daunorubicin/Range = 175–600 | NR | Targeted next-generation DNA sequencing | i) signs and symptoms of cardiac compromise based on American Heart Association criteria (ii) echo evidence of LV dysfunction. iii) absence of symptoms/signs with echo evidence of LV dysfunction (LVEF ≤ 40% and/or SF ≤ 28%). | ||
| Aminkeng (2015)47 | Canada; European, African, East Asia, Aboriginal Canadian | PC; Discovery = 280 Replication = 96 | Discovery Med = 9.0 (2.5–14) Replication Med = 7.5 (5–12) | Discovery Med = 4.0 (2–7.5) Replication Med = 11 (6–14) | Discovery 15/17 Replication 12/10 | Discovery 136/112 Replication 38/36 | ALL, AML, other leukaemia, HL, NHL, osteosarcoma, rhabdomyosarcoma, Ewing’s sarcoma, other sarcoma, hepatoblastoma, neuroblastoma, Wilms tumour | Doxorubicin, Daunorubicin, Epirubicin/Discovery Cases: Med = 260 (177.5–365) Controls: Med = 175 (140–295) Replication Cases: Med = 407.5 (270–480) Controls: Med = 277.5 (180–364) | NR | Illumina HumanOmniExp-ress assay | i) LVEF < 45% ii) Dilation of LV-end-diastolic dimension >117%. |
| Krajinovic (2015)43 | Canada, French-Canadian | CC; 295 | QcALL cohort Mean = 6.16 DFCI cohort Mean = 5.27 | QcALL cohort = 134/117 DFCI cohort = 21/23 | ALL | Doxorubicin/300–360 | Blood, buccal swabs | PCR allele-specific-oligonucleotide hybridization assays. | Reduction in SF and EF | ||
| Reichwagen (2015)22 | Germany, Czech Republic & Switzerland; NR | NCC; 520 | Med = 68(61–80) | Med = 67(62–79) | 25/31 | 46/48 | NHL | Doxorubicin/Cases: Med = 309 Controls: Med = 318 | Blood | i) Pyrosequencing ii) TaqMan® genotyping assays | Grade >0 based on CTCAEv2 |
| Visscher (2015)21 | Canada & The Netherlands; NR | CC; 536 | Med = 7.4(0.04–17.6) | Med = 4.9(0.1–17.7) | 64/58 | 211/187 | Leukaemia, lymphoma, sarcoma, blastoma and others | Doxorubicin, Daunorubicin/Cases: Med = 300 (36–840) Controls: Med = 200 (25–740) | Blood, saliva, buccal swabs | Custom Illumina GoldenGate SNP genotyping assay | i) Shortening fractions <26% ii) Echo and/or symptoms of cardiac compromise requiring intervention based on CTCAEv3 |
| Vulsteke (2015)48 | Belgium; NR | PC; 877 | Mean = 50.3 | NR | Breast cancer | Epirubicin/NR | Blood | Sequenom MassARRAY | (ii)asymptomatic decrease of LVEF>10% | ||
| Hertz (2016)20 | USA; White, Black, Other | CC, 166 | Med = 50 (35–64) | Med = 50 (24–80) | 0/19 | 0/147 | Breast cancer | Doxorubicin/Cases: Med = 240 (240–350) Controls: Med = 240 (120–366) | Blood | i) Sequenom MassARRAY ii) TaqMan® allelic discrimination assay | EF<55% |
| Reinbolt (2016)42 | USA; NR | NCC, 162 | Mean = 51.9 ± 11.9 | Mean = 50.1 ± 9.3 | 0/52 | 0/110 | Breast cancer | NR | NR | i) TaqMan® allelic discrimination assay ii) | i) EF <50% ii) decrease of LVEF>15% iii) new arrhythmia iv) new myocardial infarction |
| Wang (2016)23 | USA; Non-Hispanic white, Hispanic, others | NCC; 385 (Discovery = 331, Replication = 54) | Discovery Set Mean = 8.4 ± 5.7 Med = 7.5 (0–20) ReplicationSet Mean = 7.7 ± 5.0 Med = 7.7 (0.02–20.6) | Discovery Set Mean = 8.3 ± 5.8 Med = 7.9 (0–21) | Discovery Set: 46/66 Replication Set: 30/24 | Discovery Set:106/113 | HL, NHL, Sarcoma, AML, ALLand others | NR/Discovery Cases: Med = 319 (0–760) Controls: Med = 180 (0–825) Replication Cases: Med = 350 (0–668) Controls: Med = 301 (0–668) | Blood, buccal cells, saliva | i) Illumina HumanOmniExp-ress assay ii) Sequenom MassARRAY | i) signs and symptoms of cardiac compromise based on American Heart Association criteria 2009 ii) absence of symptoms/signs with echo evidence of LV dysfunction (LVEF ≤ 40% and/or SF ≤ 28%). |
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia, BM, bone marrow; CC, case-control; EF, ejection fraction; FFPE, Formalin-fixed, paraffin-embedded; HL, Hodgkin’s lymphoma; LVEF, left ventricular ejection fraction; LVFS, left ventricular shortening fraction; Med, median; CTCAE, National Cancer Institute Common Toxicity Criteria; NCC, nested case control; NHL, non-Hodgkin’s lymphoma, NR, not reported, PC, prospective cohort; RC, retrospective cohort; RFLP, restriction fragment length polymorphism; SF, shortening fraction.
The most common type of cancer examined were leukaemia (n = 7), breast cancer (n = 6), lymphoma (n = 3) and osteosarcoma (n = 1). In the other eleven studies, the authors examined a mix types of cancer. Doxorubicin (n = 8), daunorubicin (n = 4) and epirubicin (n = 3) were the common anthracyclines examined. Only eight studies reported the cumulative anthracycline dose in doxorubicin isotoxic equivalent doses21, 23, 30, 31, 35–37, 47. The median cumulative doses in doxorubicin isotoxic equivalent dose ranged from 240 to 504 mg/m2 for cases and 175 to 540 mg/m2 for controls. These conversions were mainly derived based upon the guidelines of the Children’s Oncology Group54, 55.
The definition of cardiotoxicity varied across studies, with most studies using either a subjective outcome (n = 5), objective outcome (n = 8) or both (n = 14) while one study did not define cardiotoxicity53 (Supplementary Table 1). Most studies using subjective outcomes defined cardiotoxicity as the presence of signs and symptoms requiring intervention21–23, 30, 31, 33, 35–37, 41, 47–49. In addition, some studies have used the left ventricular ejection fraction (LVEF) or shortening fraction (SF) as an objective measure, but the cut-off points varies. For example, the cut-off values of less than 40% to 55% of LVEF20 or decrease of more than 10–15% have been used. Three studies also included electrocardiogram changes in the definition of cardiotoxicity i.e. arrhythmia22, 32, 42 and abnormalities in ECG40 while one study solely examined the effect of anthracycline on QT interval and arrhythmia50.
Blood and buccal cells were the most common bio-specimen used for genotyping. Fifteen studies used single bio-specimen of either, blood20, 22, 32, 39, 41, 44, 46, 48–53, buccal swab34 or bone marrow smear40 while seven studies used more than one type of bio-specimens21, 23, 31, 33, 35–37. Six studies did not report the bio-specimen used for genotyping30, 38, 42, 43, 45, 47. Seventeen studies used single genotyping assay21, 30, 31, 35–38, 41–45, 47, 48, 50, 52, 53 while the remaining eleven studies use multiple genotyping assays20, 22, 23, 32–34, 39, 40, 46, 49, 51. The most commonly used assay technique were TaqMan® genotyping assay (n = 7), Sequenom MassARRAY (n = 4), Sequenom genotyping assay (n = 3), custom Illumina GoldenGate SNP genotyping assay (n = 3) and pyrosequencing (n = 3). Twenty-one studies assessed their cohort or control group for compliance with the HWE20–23, 30–32, 34–39, 41, 44, 45, 47–49, 51, 52.
The quality of the reporting in the studies
Among the reviewed studies, twenty-six studies were rated to have high quality (mean score of 45 for studies with control group and 40 for studies without control group) except for one study44, which was rated to be of moderate quality (Supplementary Table 2). On average, included studies were rated as good for most of the items on the Q-Genie tool except for the domain: sample size and power as studies had not described or determined the sample size required for their studies. In most cases, these were either retrospectively analyses of a research datasets/cohort assembled for different purposes.
Anthracycline-induced cardiotoxicity and genotype
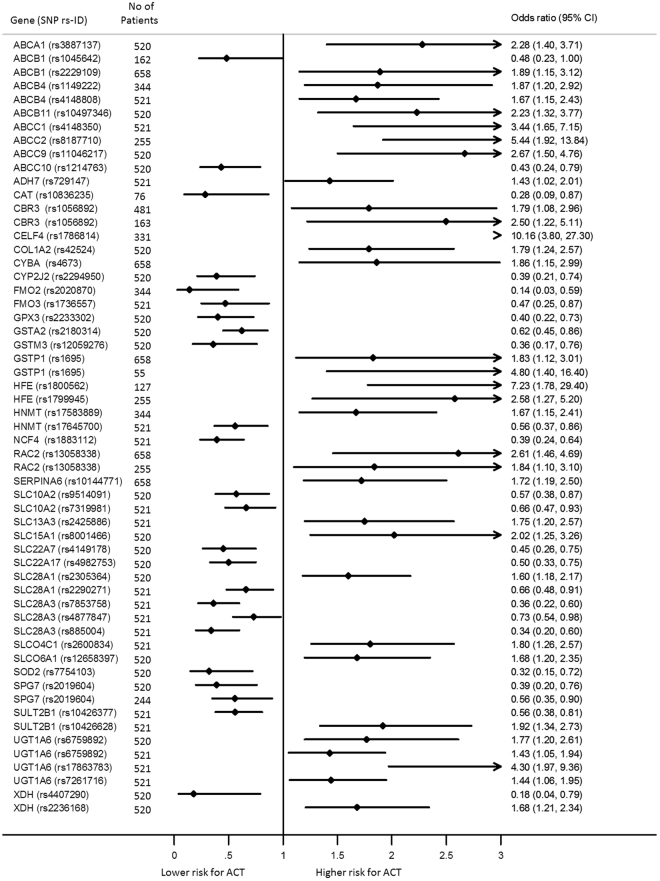
A total of 147 SNPs involving eighty-four genes were reported by the studies (Supplementary Table 3). Three genome-wide association studies23, 39, 47 were identified, and the remaining studies involved using a candidate gene approach. Most of the studies focused on variation in genes implicated in biosynthesis of anthracyclines or cardiac function. Eighty-seven of the SNPs were reported to be significantly associated with ACT by at least one study (Fig. 2). Quantitative analysis was possible for twelve polymorphs in eleven genes (Fig. 3). Most of the SNPs were from genes which encode transporter proteins; of which twenty-eight SNPs were from eleven ATP-binding cassette (ABC) transporters gene while nineteen SNPs were eleven genes encode solute carriers (SLC). The most studied genes encoding metabolising proteins were genes encode aldo/keto reductase (AKR) superfamily and carbonyl reductase (CBR). A discussion on the genes included in meta-analysis follows below.
Figure 2.
Forest plot of SNPs which examined the association of developing anthracycline-induced cardiotoxicity. SNPs significantly associated with ACT with no odds ratio or confidence interval reported are ABCC1 (rs3743527, rs246221, rs45511401), ABCC5 (rs7627754), AKR1C4 (rs7083869, rs2151896), CBR3 (rs10483032), CYP1A2 (rs2069522, rs2069526, rs4646427), CYP2B6 (rs7255904, rs1709115), CYP4B1 (rs837400, rs4646495), CYP4F11 (rs8112732, rs12610962, rs2072270), HSD17B2 (rs16956248, rs13333826, rs7196087, rs2955159, rs2966245), HSD17B4 (rs257970, rs2636968), KCNH2 (rs3807375), POR (rs2868177, rs13240755, rs4732513), SLC22A17 (rs11625724, rs12882406, rs12896494). The diamond in each line represents the effect estimate and weight of each study. The width of the line across the diamond shows the 95% confidence interval of the effect estimate of individual studies. ACT, Anthracycline-induced cardiotoxicity; CI, confidence interval.
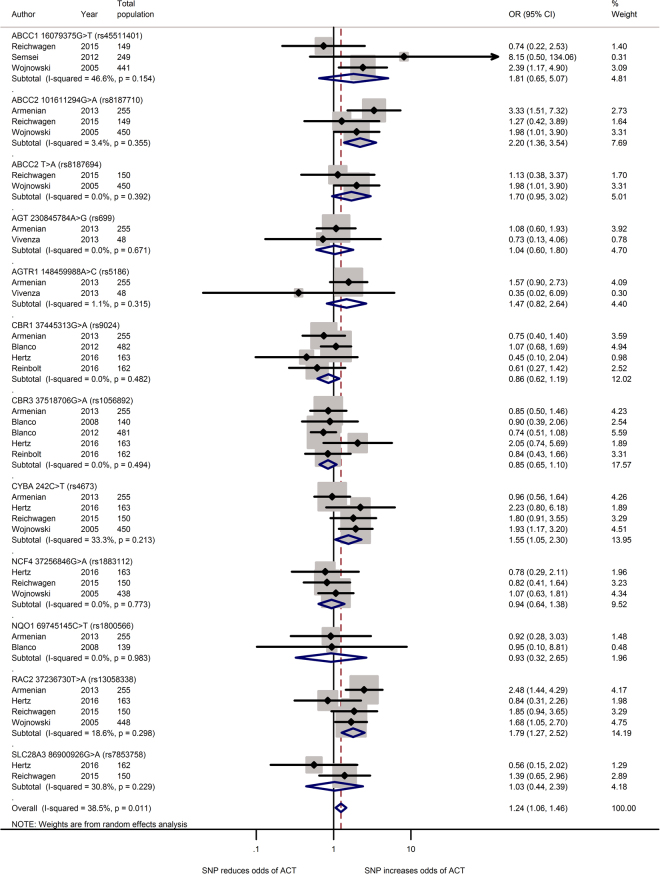
Figure 3.
Forest plot of meta-analysis for 12 SNPs. Three variants, ABCC2 rs8187710, CYBA rs4673 and RAC2 rs13058338, are significantly increased the odds for ACT.
ATP Binding Cassette (ABC) gene
ABC transporters genes encode a superfamily of transmembrane proteins that actively transport substrates including doxorubicin across membranes using adenosine triphosphate56. Fourteen of the twenty-eight variants in ABC transporters were found to significantly increase the risk for ACT20, 21, 30–32, 36, 41, 43, 48, 51 (Supplementary Table 3). ABCC1 is the most studied gene with nine SNPs followed by ABCB1 (5 SNPs) and ABCC2 (3 SNPs). The rs246221 polymorphism of ABCC1 gene was found to significantly deteriorate cardiac function in both studies48, 51. Seven SNPs, rs104564220, 41, rs114922220, 30, 31, 53, rs414880820, 31, rs4551140122, 32, 51, rs414835020, 30, rs818771022, 32, 36 and rs818769422, 30, 32 were found to increase the risk in only one of the studies assessing their association with ACT.
Armenian et al. recruited 77 cases and 178 controls from a population of haematological patients that underwent haematopoietic cell transplantation reported that rs8187710 increased ACT risk (OR: 5.22; 95% CI: 1.92–13.84; false discovery rate-adjusted p = 0.02)36. Using similar study design and a larger sample size (87 cases and 363 controls) of only non-Hodgkin lymphoma survivors, Wojnowski et al. reported the heterozygous or homozygous genotypes risk of acute ACT was statistically significant (OR: 2.3; 95% CI: 1.0–5.4; Fisher exact test p = 0.06)32. In contrast, Reichewagen et al. did not find significant association between the mutation and risk for ACT (OR: 1.3; 95% CI: 0.4–3.9; p = 0.67)22. When combined, the missense mutation was associated with a large increase in risk (pooled OR: 2.20; 95% CI: 1.36–3.54; p = 0.001).
Meta-analysis of three studies in European22, 32, 51 populations revealed that the missense mutation of rs45511401 increased the risk for ACT (pooled OR: 1.81; 95% CI: 0.65–5.07; p = 0.26) with moderate heterogeneity (I 2 = 47%). Similarly the combined effect of ABCC2 rs8187694 from two studies in European22, 32 populations showed no significant association (pooled OR: 1.70; 95% CI: 0.95–3.02; p = 0.07).
Carbonyl reductases (CBR) gene
Carbonyl reductases (CBR) genes encode enzymes that catalyse the reduction of endogenous aliphatic aldehydes and ketones and various xenobiotic, thus offering cardio-protective role against ACT. Four SNPs on carbonyl reductases (CBR) were studied, one on carbonyl reductase 1 gene (CBR1) and three on carbonyl reductase 3 gene (CBR3). However, two SNPs, rs9024 of CBR1 and rs1056892 of CBR3 were associated with cardio-protection, but this did not reach statistical significance (pooled OR: 0.86; 95% CI: 0.62–1.19 and 0.85; 0.65–1.10 respectively, Fig. 3).
Cytochrome b-245, alpha polypeptide (CYBA) gene
Cytochrome B-245, alpha polypeptide gene (CYBA, NC_000016.10) encodes the primary component of the microbicidal oxidase system of phagocytes. We identified six studies which assessed associations of the rs4673 missense SNP of CYBA with ACT, three studies20, 22, 32 are included in qualitative analysis due to unavailability of required information in the other two studies30, 41. Among the samples, the SNP was found to increase the odds of developing ACT (pooled OR: 1.55; 95% CI: 1.05–2.30; p = 0.03) with moderate heterogeneity (I 2 = 33%).
Neutrophil cytosolic factor 4 (NCF4) gene
Neutrophil cytosolic factor 4 gene (NCF4, NC_00002.10) encodes the p40phox subunit of the NAD(P)H oxidase57. The rs1883112 polymorphism at the putative promoter of NCF4 blocks oxidase activation of the enzyme thus reduces the formation of reactive oxidant intermediates58. Two of the six studies examined the effect of SNP rs1883112 found that SNP was significantly associated with cardiac toxicity32, 36. The combined effect of this synonymous substitution from two studies in North America20, 36 and European22, 32 populations showed no significant association (pooled OR: 0.94; 95% CI: 0.64–1.38; p = 0.75).
Ras-Related C3 Botulinum Toxin Substrate 2 (RAC2) gene
Ras-Related C3 Botulinum Toxin Substrate 2 gene (RAC2, NC_000022.11) encodes the protein regulating diverse processes including secretion, phagocytosis, cell polarisation and generation of reactive oxygen species. Three of six studies reported SNP rs13058338 on RAC2 significantly increase risk for ACT32, 36, 41. Analysis of this intron variant in four studies showed that RAC mutation increased the risk of cardiotoxicity by nearly two times (pooled OR: 1.79; 95% CI: 1.27–2.52; p < 0.001).
Discussion
To our knowledge, this is the first and only systematic review which examined the role of genetic polymorphisms with ACT induced cardiotoxicity. We found a total of twenty-eight studies, examining eighty-four different genes. Most of the genes studied were linked to the biochemical pathway of anthracycline, oxidative stress or cardiac function (Fig. 4). As such, it is not surprising that all but one47 genetic studies described in this article have included these candidate genes in their study. Results from our meta-analyses revealed that polymorphism in three (3.6%) of the eight-four genes were significantly associated with an increased odds of cardiotoxicity in individuals treated with anthracyclines. However, the individual risk provided by any of these candidate genes were moderate only (OR: 1.55–2.20), in agreement with previous studies which have examined other complex diseases, such as stroke59 and ischaemic heart diseases60, 61.
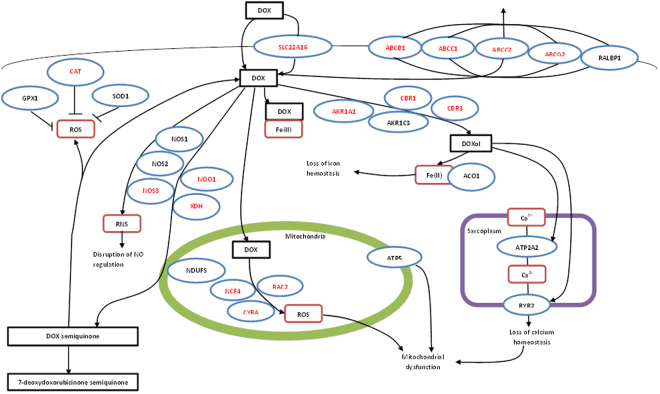
Figure 4.
Diagrammatic representative of the candidate genes involved in transport and metabolism of doxorubicin and doxorubicin induced cardiotoxicity. ABCB1, ATP-Binding Cassette Subfamily B Member 1; ABCC1, ATP-Binding Cassette Subfamily C Member 1; ABCC2, ATP-Binding Cassette Subfamily C Member 2; ABCG2, ATP-Binding Cassette Subfamily G Member 2, ACO1, Aconitase 1; AKR1A1, Aldo-Keto Reductase Family 1 Member A1, AKR1C3, Aldo-Keto Reductase Family 1 Member C3; ATP2A2, ATPase Sarcoplasmic/Endoplasmic Reticulum Ca2+ Transporting 2; ATP5E, ATP synthase H+ Transporting, mitochondrial F1 Complex, Epsilum Subunit; CAT, Catalase gene; CBR1, Carbonyl Reductase 1; CBR3, Carbonyl Reductase 3; CYBA, Cytochrome B-245 Alpha Chain; GPX1, Glutathione Peroxidase 1; NCF4, Neutrophil Cytosolic Factor 4; NDUFS, NADH: Ubiquinone Oxidoreductase Subunit; NOS1, Nitric Oxide Synthase 1; NOS2, Nitric Oxide Synthase 2; NOS3, Nitric Oxide Synthase 3; NQO1, NAD(P)H Quinone Dehydrogenase 1; RAC2, Ras-related C3 Botulinum Toxin Substrate 2; RALBP1, RalA Binding Protein 1; RYR2, Ryanodine Receptor 2; SLC22A16, Solute Carrier Family 22 Member 16; SOD1, Superoxide Dismutase 2, mitochondrial; XDH, Xanthine Dehydrogenase.
For the genes that were found to have a positive association, animal and mechanistic studies have shown that these alleles alter the expression or activity of the encoded protein and thus contribute to disease pathogenesis. ABCC2 gene encodes for proteins that are involved the efflux of substances from cells, and mutation of ABCC2 significantly reduces ATPase activity, resulting in a decrease in efflux activity leading to intracellular accumulation of anthracycline62. Similarly, the Rac2 (Ras-related C3 botulinum toxin substrate 2) encoded by RAC2 gene is a mitochondrial protein that is required in electron transfer reaction of NADPH oxidase63 during the formation of reactive oxygen species (ROS)64. Alteration of the gene results in mitochondrial dysfunction and thus an increase ROS production, which ultimately leads to myocytes damages. Taken together, mutations in these genes are thought to result in cardiomyopathy due to accumulation of anthracycline and excessive ROS in myocytes.
We also observed that some of these genes were not only related to cardiotoxicity, but also other adverse drug reactions (ADRs) of chemotherapy such as myelosuppression and infection as well as overall survival. The SNPs ABCG2 rs223114241, NCF4 rs188311241, GSTP1 rs169539, 41, CYBA rs467339 and GSTM1 null allele39 significantly increased odds for grade 3–4 hematologic toxicity in patients treated with anthracycline-based chemotherapy regimen. Similarly, ABCB1 rs1045642, ABCG2 rs2231137 and NCF4 rs1883112 significantly increased odds for grade 2–4 infection41. In addition, rs1695 of GSTP139, rs17222723 of ABCC241 and rs4673 of CYBA41 were significantly related to progression-free survival or event-free survival.
This study has some limitations which warrant discussion. Firstly, we found a total of 147 SNPs which were examined for the possible association with ACT. Most of the SNPs have only been examined once; which limited our ability to perform a meta-analysis. In addition, there were inconsistencies in reporting of results between studies. As such, our meta-analyses only included between two to five studies, which restricted subgroup analyses. The included studies were also heterogeneous and had not adjusted for confounders, which further limits the precision of overall estimates. We also selectively discussed the roles of genes included in the meta-analysis. It should be noted that the SNPs discussed in this review does not imply that they are superior in any aspect to other SNPs identified. Many of the studies were not prospectively designed but had used a convenience sampling, which is reinforced by the fact that none of the studies had adequately reported the sample size calculations. Similarly, nearly all of the studies (96%) of the studies were carried out in Western populations, thus limiting the generalisability to other populations. Furthermore, most of the studies had not reported the demographics of their population. Finally, only a handful studies had adjusted for some confounding factors in their analysis, although these have been shown to increase the risk factor for AIC.
Over the past few decades, the development in molecular biology has increased our understanding on the role of genetic variation underlying adverse drug reactions (ADRs). Currently, genetic testing is recommended for identifying patients at risk for ADRs. Examples include testing of thiopurine methyltansferase (TMPT) gene variation prior to thiopurine therapy in inflammatory bowel disease and human leukocyte antigen (HLA)-B*1502 for treatment of seizures with carbamazepine. Polymorphisms of TMPT gene have been known to cause lowered TPMT activity, and thus a reduced dose is recommended for heterozygous patients to prevent hematopoietic toxicity65. Meanwhile, HLA-B*15:02 screening is recommended for Asian populations to identify patients at risk for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis66.
However, results from this study suggest that unlike examples listed above, several polymorphs may be involved in ACT. As such, a genome-wide association studies which could examine SNPs across the whole genome should be conducted. In order to ensure that study findings can be more effective to influence the development of personalised medicine for addressing drug toxicities in general and ACT in specific, future studies should ideally be conducted in a prospective large cohort. Multicentre studies including patients from other continents especially Africa, Asia, South America, Australia and Oceania, are encouraged. In addition, the use of an objective definition of cardiotoxicity and reporting the frequency of events for each genotype should be considered.
Conclusions
Results of this study indicate that several polymorphisms of pharmacogenetics candidates across the anthracyclines biochemistry and cardiomyopathy pathways are potentially a predictor for ACT. However, the evidences are limited and too heterogeneous for a significant quantitative analysis. Further studies are needed to generate robust genetic predictor(s) for ACT to achieve the goal of individualising anthracycline therapy.
Electronic supplementary material
Acknowledgements
Siew Lian Leong is funded by the Research Degrees Scholarships from Monash University Malaysia.
Competing Interests
The authors declare no competing financial interests.
Author Contributions
S.L.L. and S.W.H.L. are joint first authors who take responsibility for the planning of the methods and preparation of the manuscript. Leong had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. N.C. is senior author who takes responsibility for final editing of the manuscript.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00075-1
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Comprehensive Cancer Network. Breast Cancer (Version 1.2016).
- 2.Senkus E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. Hodgkin Lymphoma (Version 2.2015). Vol. 2015. [DOI] [PMC free article] [PubMed]
- 4.Eichenauer DA, et al. Hodgkin’s lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii70–5. doi: 10.1093/annonc/mdu181. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Non-Hodgkin’s Lymphomas (Version 1.2016). Vol. 2015. [DOI] [PubMed]
- 6.Tilly H, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–25. doi: 10.1093/annonc/mdv304. [DOI] [PubMed] [Google Scholar]
- 7.Dreyling M, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii83–92. doi: 10.1093/annonc/mdu264. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Acute Myeloid Leukemia (Version 1.2015).
- 9.Fey MF, Buske C, Group EGW. Acute myeloblastic leukaemias in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi138–43. doi: 10.1093/annonc/mdt320. [DOI] [PubMed] [Google Scholar]
- 10.Young RJ, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178–86. doi: 10.1016/j.ejca.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Group, T. E. E. S. N. W. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology23, vii92–vii99 (2012). [DOI] [PubMed]
- 12.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–41. doi: 10.1016/S0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 13.Von Hoff DD, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–7. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin RS, Wiernik PH, Bachur NR. Adriamycin chemotherapy—efficacy, safety, and pharmacologic basis of an intermittent single high-dosage schedule. Cancer. 1974;33:19–27. doi: 10.1002/1097-0142(197401)33:1<19::AID-CNCR2820330107>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Smith LA, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotrionte M, et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112:1980–4. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Kremer LC, et al. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13:819–29. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 18.Jensen BC, McLeod HL. Pharmacogenomics as a risk mitigation strategy for chemotherapeutic cardiotoxicity. Pharmacogenomics. 2013;14:205–13. doi: 10.2217/pgs.12.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershman DL, Shao T. Anthracycline cardiotoxicity after breast cancer treatment. Oncology (Williston Park) 2009;23:227–34. [PubMed] [Google Scholar]
- 20.Hertz DL, et al. Evidence for association of SNPs in ABCB1 and CBR3, but not RAC2, NCF4, SLC28A3 or TOP2B, with chronic cardiotoxicity in a cohort of breast cancer patients treated with anthracyclines. Pharmacogenomics. 2016;17:231–40. doi: 10.2217/pgs.15.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visscher H, et al. Genetic variants in SLC22A17 and SLC22A7 are associated with anthracycline-induced cardiotoxicity in children. Pharmacogenomics. 2015;16:1065–76. doi: 10.2217/pgs.15.61. [DOI] [PubMed] [Google Scholar]
- 22.Reichwagen A, et al. Association of NADPH oxidase polymorphisms with anthracycline-induced cardiotoxicity in the RICOVER-60 trial of patients with aggressive CD20(+) B-cell lymphoma. Pharmacogenomics. 2015;16:361–72. doi: 10.2217/pgs.14.179. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, et al. CELF4 Variant and Anthracycline-Related Cardiomyopathy: A Children’s Oncology Group Genome-Wide Association Study. J Clin Oncol. 2016;34:863–70. doi: 10.1200/JCO.2015.63.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sohani ZN, et al. Assessing the quality of published genetic association studies in meta-analyses: the quality of genetic studies (Q-Genie) tool. BMC Genet. 2015;16:50. doi: 10.1186/s12863-015-0211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little J, et al. Strengthening the reporting of genetic association studies (STREGA)—an extension of the strengthening the reporting of observational studies in epidemiology (STROBE) statement. Journal of Clinical Epidemiology. 2009;62:597–608.e4. doi: 10.1016/j.jclinepi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Janssens AC, et al. Strengthening the reporting of genetic risk prediction studies: the GRIPS statement. Bmj. 2011;342:d631. doi: 10.1136/bmj.d631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Trikalinos TA, Salanti G, Khoury MJ, Ioannidis JP. Impact of violations and deviations in Hardy-Weinberg equilibrium on postulated gene-disease associations. Am J Epidemiol. 2006;163:300–9. doi: 10.1093/aje/kwj046. [DOI] [PubMed] [Google Scholar]
- 29.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration (2014).
- 30.Visscher H, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–8. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 31.Visscher H, et al. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer. 2013;60:1375–81. doi: 10.1002/pbc.24505. [DOI] [PubMed] [Google Scholar]
- 32.Wojnowski L, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–62. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 33.Weiss JR, et al. Glutathione S-transferase (GSTM1, GSTT1 and GSTA1) polymorphisms and outcomes after treatment for acute myeloid leukemia: pharmacogenetics in Southwest Oncology Group (SWOG) clinical trials. Leukemia. 2006;20:2169–71. doi: 10.1038/sj.leu.2404421. [DOI] [PubMed] [Google Scholar]
- 34.Blanco JG, et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112:2789–95. doi: 10.1002/cncr.23534. [DOI] [PubMed] [Google Scholar]
- 35.Blanco JG, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes-a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:1415–21. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armenian SH, et al. Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation. Br J Haematol. 2013;163:205–13. doi: 10.1111/bjh.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, et al. Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: a report from the children’s oncology group. J Clin Oncol. 2014;32:647–53. doi: 10.1200/JCO.2013.50.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wasielewski M, et al. Potential genetic predisposition for anthracycline-associated cardiomyopathy in families with dilated cardiomyopathy. Open Heart. 2014;1:e000116. doi: 10.1136/openhrt-2014-000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Windsor RE, et al. Germline genetic polymorphisms may influence chemotherapy response and disease outcome in osteosarcoma. Cancer. 2012;118:1856–1867. doi: 10.1002/cncr.26472. [DOI] [PubMed] [Google Scholar]
- 40.Rajic V, et al. Influence of the polymorphism in candidate genes on late cardiac damage in patients treated due to acute leukemia in childhood. Leuk Lymphoma. 2009;50:1693–8. doi: 10.1080/10428190903177212. [DOI] [PubMed] [Google Scholar]
- 41.Rossi D, et al. Analysis of the host pharmacogenetic background for prediction of outcome and toxicity in diffuse large B-cell lymphoma treated with R-CHOP21. Leukemia. 2009;23:1118–26. doi: 10.1038/leu.2008.398. [DOI] [PubMed] [Google Scholar]
- 42.Reinbolt RE, et al. Risk factors for anthracycline-associated cardiotoxicity. Support Care Cancer. 2016;24:2173–80. doi: 10.1007/s00520-015-3008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krajinovic, M. et al. Polymorphisms of ABCC5 and NOS3 genes influence doxorubicin cardiotoxicity in survivors of childhood acute lymphoblastic leukemia. Pharmacogenomics J (2015). [DOI] [PubMed]
- 44.Lubieniecka JM, et al. Single-nucleotide polymorphisms in aldo-keto and carbonyl reductase genes are not associated with acute cardiotoxicity after daunorubicin chemotherapy. Cancer Epidemiol Biomarkers Prev. 2012;21:2118–20. doi: 10.1158/1055-9965.EPI-12-1037. [DOI] [PubMed] [Google Scholar]
- 45.Volkan-Salanci B, et al. The relationship between changes in functional cardiac parameters following anthracycline therapy and carbonyl reductase 3 and glutathione S transferase Pi polymorphisms. J Chemother. 2012;24:285–91. doi: 10.1179/1973947812Y.0000000037. [DOI] [PubMed] [Google Scholar]
- 46.Lipshultz SE, et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer. 2013;119:3555–62. doi: 10.1002/cncr.28256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aminkeng F, et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet. 2015;47:1079–84. doi: 10.1038/ng.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vulsteke C, et al. Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients. Breast Cancer Res Treat. 2015;152:67–76. doi: 10.1007/s10549-015-3437-9. [DOI] [PubMed] [Google Scholar]
- 49.Vivenza D, et al. Role of the renin-angiotensin-aldosterone system and the glutathione S-transferase Mu, Pi and Theta gene polymorphisms in cardiotoxicity after anthracycline chemotherapy for breast carcinoma. Int J Biol Markers. 2013;28:e336–47. doi: 10.5301/jbm.5000041. [DOI] [PubMed] [Google Scholar]
- 50.Kitagawa K, et al. Prospective evaluation of corrected QT intervals and arrhythmias after exposure to epirubicin, cyclophosphamide, and 5-fluorouracil in women with breast cancer. Ann Oncol. 2012;23:743–7. doi: 10.1093/annonc/mdr296. [DOI] [PubMed] [Google Scholar]
- 51.Semsei AF, et al. ABCC1 polymorphisms in anthracycline-induced cardiotoxicity in childhood acute lymphoblastic leukaemia. Cell Biol Int. 2012;36:79–86. doi: 10.1042/CBI20110264. [DOI] [PubMed] [Google Scholar]
- 52.Lubieniecka JM, et al. A discovery study of daunorubicin induced cardiotoxicity in a sample of acute myeloid leukemia patients prioritizes P450 oxidoreductase polymorphisms as a potential risk factor. Front Genet. 2013;4:231. doi: 10.3389/fgene.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sachidanandam K, Gayle AA, Robins HI, Kolesar JM. Unexpected doxorubicin-mediated cardiotoxicity in sisters: possible role of polymorphisms in histamine n-methyl transferase. J Oncol Pharm Pract. 2013;19:269–72. doi: 10.1177/1078155212461022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Supportive care of children with cancer: current therapy and guidelines from the Children’s Oncology Group (Baltimore, Md.: Johns Hopkins University Press, Baltimore, Md., 2004).
- 55.Shankar SM, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121:e387–96. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 56.Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112:457–73. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Lopes LR, et al. Phosphorylated p40PHOX as a negative regulator of NADPH oxidase. Biochemistry. 2004;43:3723–30. doi: 10.1021/bi035636s. [DOI] [PubMed] [Google Scholar]
- 58.Vulsteke C, et al. Genetic variability in the multidrug resistance associated protein-1 (ABCC1/MRP1) predicts hematological toxicity in breast cancer patients receiving (neo-)adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide (FEC) Ann Oncol. 2013;24:1513–25. doi: 10.1093/annonc/mdt008. [DOI] [PubMed] [Google Scholar]
- 59.Biffi A, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68:934–43. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schurks M, Zee RY, Buring JE, Kurth T. ACE D/I polymorphism, migraine, and cardiovascular disease in women. Neurology. 2009;72:650–6. doi: 10.1212/01.wnl.0000342517.97178.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tzourio C, et al. APOE genotype, ethnicity, and the risk of cerebral hemorrhage. Neurology. 2008;70:1322–8. doi: 10.1212/01.wnl.0000308819.43401.87. [DOI] [PubMed] [Google Scholar]
- 62.Elens L, et al. Functional defect caused by the 4544G>A SNP in ABCC2: potential impact for drug cellular disposition. Pharmacogenet Genomics. 2011;21:884–93. doi: 10.1097/FPC.0b013e32834d672b. [DOI] [PubMed] [Google Scholar]
- 63.Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol. 2001;2:211–5. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- 64.Dorseuil O, et al. Inhibition of superoxide production in B lymphocytes by rac antisense oligonucleotides. J Biol Chem. 1992;267:20540–2. [PubMed] [Google Scholar]
- 65.Thomas FJ, McLeod HL, Watters JW. Pharmacogenomics: the influence of genomic variation on drug response. Curr Top Med Chem. 2004;4:1399–409. doi: 10.2174/1568026043387638. [DOI] [PubMed] [Google Scholar]
- 66.Tangamornsuksan W, et al. Relationship between the HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:1025–32. doi: 10.1001/jamadermatol.2013.4114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.