Abstract
The DNA content of bacteroids from 22 different Rhizobium-legume associations was compared to that of the corresponding free living Rhizobium species using laser flow microfluorometry. In all 18 effective associations, the bacteroids had either similar or higher DNA content than the free living rhizobia. Bacteroid populations isolated from effective clover (Trifolium repens) and alfalfa (Medicago sativa) nodules had an average DNA content of >1.5-fold higher than free living R. trifolii and R. meliloti. These populations also contained a significant number of bacteroids with more than 3-fold the DNA content of the free living rhizobia. Populations isolated from effective nodules of winged beans (Psophocarpus tetragonolobus), peas (Pisum sativum), and mung beans (Phaseolus aureus) had an average DNA content of 1.1- to 1.5-fold higher than free living R. “cowpeas” and R. leguminosarum. Bacteroids from nodules of lupins (Lupinus angustifolius and L. minaretta), kidney beans (Phaseolus vulgaris), and soybeans (Glycine max), however, had similar DNA content to the free living forms. Two of the four associations which formed ineffective nodules contained bacteroids with lower DNA content than the free living rhizobia. The other two associations contained bacteroids with slightly higher or similar DNA content to the free living rhizobia. Nodules of the ineffective associations also did not contain leghemoglobin.
Full text
PDF

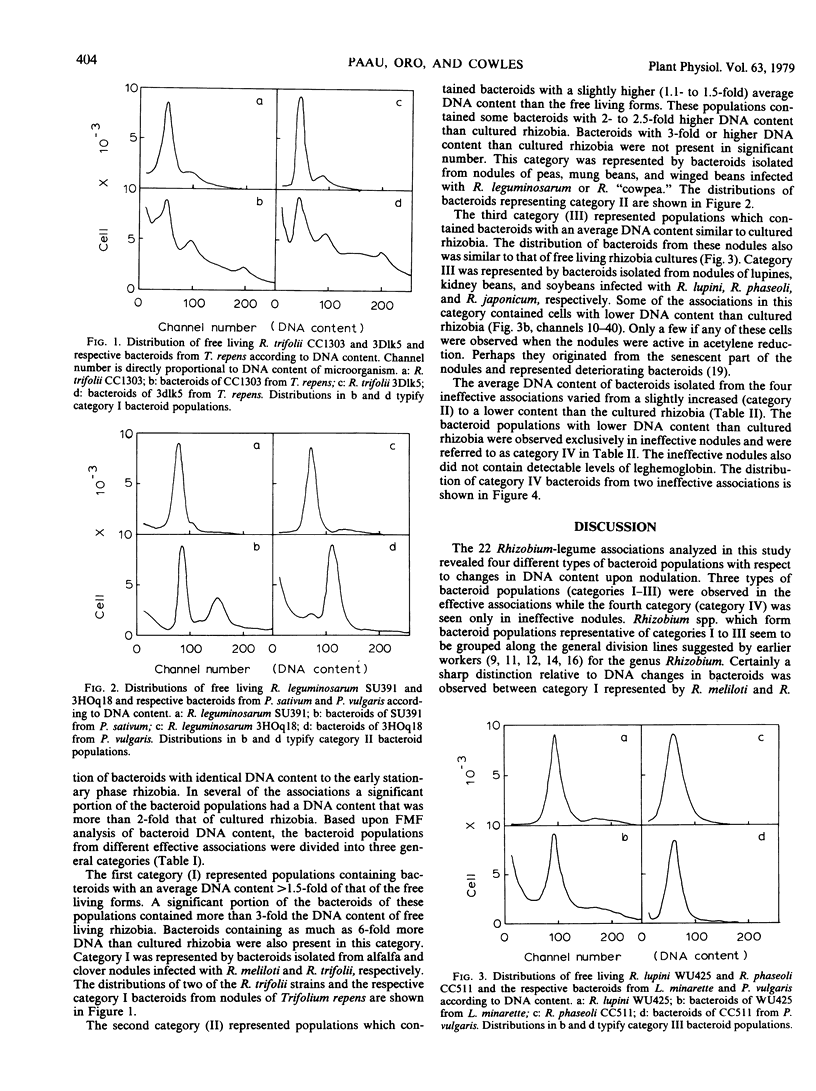
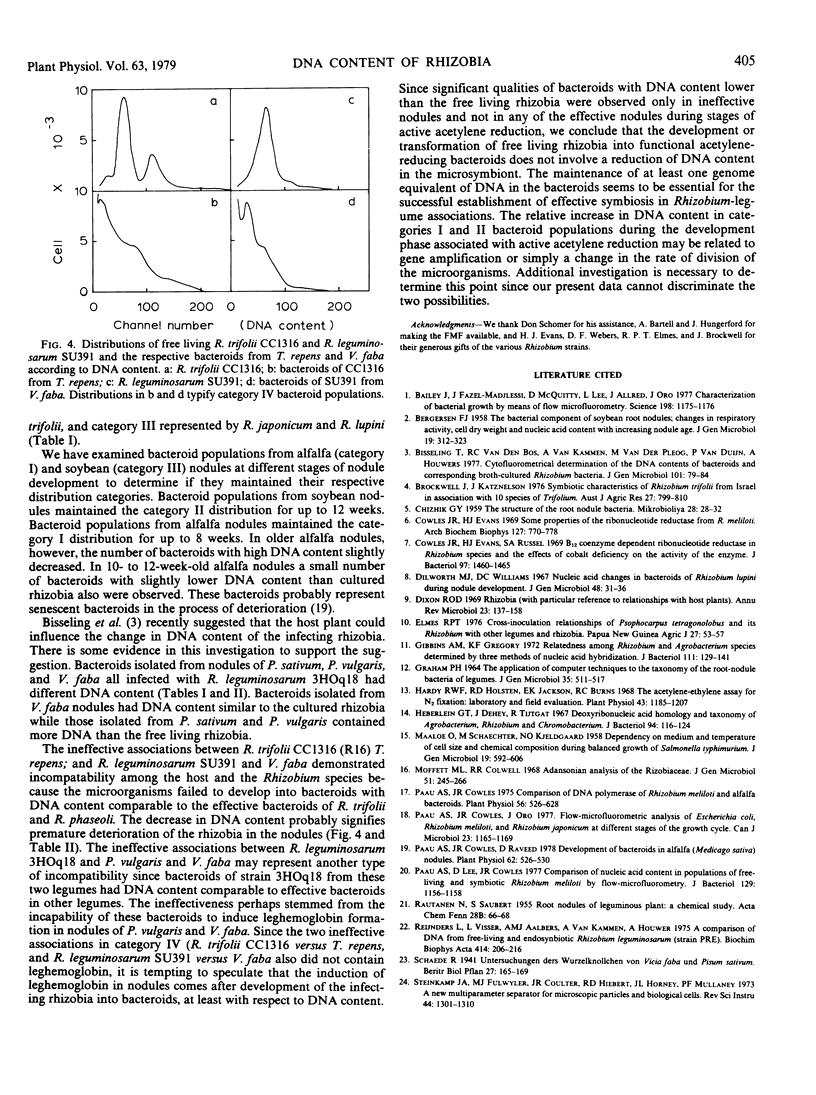
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGERSEN F. J. The bacterial component of soybean root nodules; changes in respiratory activity, cell dry weight and nucleic acid content with increasing nodule age. J Gen Microbiol. 1958 Oct;19(2):312–323. doi: 10.1099/00221287-19-2-312. [DOI] [PubMed] [Google Scholar]
- Bailey J. E., Fazel-Makjlessi J., McQuitty D. N., Lee Y. N., Allred J. C., Oro J. A. Characterization of bacterial growth by means of flow microfluorometry. Science. 1977 Dec 16;198(4322):1175–1176. doi: 10.1126/science.412254. [DOI] [PubMed] [Google Scholar]
- CHIZHIK G. Ia. O stroenii kluben'kovykh bakterii. Mikrobiologiia. 1959 Jan-Feb;28(1):28–33. [PubMed] [Google Scholar]
- Cowles J. R., Evans H. J., Russell S. A. B12 coenzyme-dependent ribonucleotide reductase in Rhizobium species and the effects of cobalt deficiency on the activity of the enzyme. J Bacteriol. 1969 Mar;97(3):1460–1465. doi: 10.1128/jb.97.3.1460-1465.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles J. R., Evans H. J. Some properties of the ribonucleotide reductase from Rhizobium meliloti. Arch Biochem Biophys. 1968 Sep 20;127(1):770–778. doi: 10.1016/0003-9861(68)90288-9. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Rhizobia (with particular reference to relationships with host plants). Annu Rev Microbiol. 1969;23:137–158. doi: 10.1146/annurev.mi.23.100169.001033. [DOI] [PubMed] [Google Scholar]
- Gibbins A. M., Gregory K. F. Relatedness among Rhizobium and Agrobacterium species determined by three methods of nucleic acid hybridization. J Bacteriol. 1972 Jul;111(1):129–141. doi: 10.1128/jb.111.1.129-141.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein G. T., De Ley J., Tijtgat R. Deoxyribonucleic acid homology and taxonomy of Agrobacterium, Rhizobium, and Chromobacterium. J Bacteriol. 1967 Jul;94(1):116–124. doi: 10.1128/jb.94.1.116-124.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett M. L., Colwell R. R. Adansonian analysis of the Rhizobiaceae. J Gen Microbiol. 1968 Apr;51(2):245–266. doi: 10.1099/00221287-51-2-245. [DOI] [PubMed] [Google Scholar]
- Paau A. S., Cowles J. R. Development of Bacteroids in Alfalfa (Medicago sativa) Nodules. Plant Physiol. 1978 Oct;62(4):526–530. doi: 10.1104/pp.62.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S., Cowles J. R., Oro J. Flow-microfluorometric analysis of Escherichia coli, Rhizobium meliloti, and Rhizobium japonicum at different stages of the growth cycle. Can J Microbiol. 1977 Sep;23(9):1165–1169. doi: 10.1139/m77-175. [DOI] [PubMed] [Google Scholar]
- Paau A. S., Lee D., Cowles J. R. Comparison of nucleic acid content in populations of free-living and symbiotic Rhizobium meliloti by flow microfluorometry. J Bacteriol. 1977 Feb;129(2):1156–1158. doi: 10.1128/jb.129.2.1156-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A., Cowles J. R. Comparison of DNA Polymerase of Rhizobium meliloti and Alfalfa Bacteroids. Plant Physiol. 1975 Oct;56(4):526–528. doi: 10.1104/pp.56.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijnders L., Visser L., Aalbers A. M., Van Kammen A., Houwers A. A comparison of DNA from free living and endosymbiotic Rhizobium leguminosarum (strain PRE). Biochim Biophys Acta. 1975 Dec 4;414(2):206–216. doi: 10.1016/0005-2787(75)90224-5. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Steinkamp J. A., Fulwyler M. J., Coulter J. R., Hiebert R. D., Horney J. L., Mullancy P. F. A new multiparameter separator for microscopic particles and biological cells. Rev Sci Instrum. 1973 Sep;44(9):1301–1310. doi: 10.1063/1.1686375. [DOI] [PubMed] [Google Scholar]


