Abstract
Biomarkers of chronic kidney disease-mineral and bone disorder (CKD-MBD) correlate with morbidity and mortality in dialysis patients. However, the comparative roles of each CKD-MBD biomarker remained undetermined on long-term peritoneal dialysis (PD) patients. This retrospective study, employing a population-based database, aimed to evaluate the performance and provide the best evidence of each biomarker of CKD-MBD as predictor of all-cause mortality. Throughout the 8-year study period, total 12,116 PD patients were included in this study. Cox proportional regression and Kaplan-Meier method were used for survival analysis. For Cox regression model, baseline measurements and time-varying covariates were used for analysis. In Cox regression model using time-dependent covariates, serum calcium level of ≧9.5 mg/dL was associated with increased mortality. For phosphorus, serum levels of either ≧6.5 mg/dL or <3.5 mg/dL were associated with increased mortality. For parathyroid hormone (PTH), higher serum levels were not associated increased mortality. For alkaline phosphatase (ALP), mortality increased at levels ≧100 IU/L. Our findings suggested that the detrimental effect of ALP on survival was more consistent, while serum calcium, phosphorus and PTH may have a less prominent effect on mortality. This study provided additional information for manipulating CKD-MBD biomarkers in PD patients.
Introduction
In the last two decades, numerous studies have provided evidence that biochemical changes in chronic kidney disease-mineral and bone disorder (CKD-MBD) are associated with survival outcomes in dialysis patients. Observational studies have demonstrated that adverse cardiovascular outcomes and mortality in dialysis patients are associated with high levels of serum phosphorus1–8 and calcium1–5, parathyroid hormone (PTH) levels either higher3–7 or lower9, 10 than the target range, and elevated alkaline phosphatase (ALP) levels11–13. These findings have contributed significantly to the widely accepted Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines for CKD-MBD14, 15.
While the majority of studies focusing on CKD-MBD in dialysis patients involved haemodialysis (HD) patients, relatively few studies involving peritoneal dialysis (PD) patients have been published16, 17. Recently, several studies have revealed that an association between ALP level and mortality also exists in PD patients18–20. These studies form the basis for the current management of CKD-MBD in PD patients. Nonetheless, the amount of evidence supporting the management of CKD-MBD in PD patients remains relatively small compared to that for HD patients. Thus, a comprehensive, large-scaled study focusing on CKD-MBD in PD patients would be help to improve our understanding in this field.
In practice, adherence to every recommendation for CKD-MBD in a single dialysis patient is not always feasible. For example, the use of calcitriol to suppress the PTH level may result in an adverse elevation of serum calcium and phosphorus levels. In addition, use of a calcium-based phosphate binder may inappropriately increase calcium load and serum calcium level. As such, providing optimal treatment for each dialysis patient is difficult, particularly as the relative role of each CKD-MBD biomarker has not yet been determined.
Therefore, the aims of the present study were to evaluate the effect of each CKD-MBD biomarker, namely, serum calcium, phosphorus, PTH and ALP, on all-cause mortality, and to compare the performance of these biomarkers in predicting mortality in a Taiwanese PD population. To that end, we conducted a nationwide, population-based analysis using a database established by the Taiwan Renal Registry Data System (TWRDS).
Subjects and Methods
This study was approved by the ethics committee of Taipei Medical University-Institutional Review Board (Number: 20141017) and was carried out in accordance with the Declaration of Helsinki of 1975, as revised in 2000. The requirement for written informed consent was waived by the Review Board.
The TWRDS was established in 1987 for the purpose of the accreditation and quality-monitoring of dialysis units. All dialysis units in Taiwan are obligated to provide the TWRDS with appropriate information on a quarterly basis to obtain reimbursement from the National Health Insurance. In 1996, data collection was computerized, and in 1997, additional information, including comorbidities, rehabilitation status, indices of dialysis adequacy and mineral and bone metabolism, and serologic markers of viral hepatitis were included. The data maintained by the TWRDS provide not only a robust foundation for comprehensive monitoring of the dialysis quality at a national level, but are also an important resource for population-based epidemiologic research21–24.
Study population
The investigated population consisted of 12,966 patients on PD from all 117 PD units across Taiwan, who were registered in the TWRDS between 2005 and 2012. Among the total 12,966 patients in the registry data, 8,514 patients remained on PD, while 3,577 patients shifted to HD and 875 patients received kidney transplantation by the end of 2012. Of these three groups, patients who remained on PD were older; patients who shifted to HD included more diabetics, and had longer PD vintage, higher serum ALP concentration, and renal creatinine clearance; and patients who received a kidney transplant included more men and had higher serum albumin, calcium, phosphorus, and PTH concentrations (Table 1). Among the entire PD population in the registry data, patients with missing PTH values were excluded from analysis as logical imputation was not feasible (n = 850). Finally, 12,116 patients were included in the current statistical analysis. Patients who remained on PD were followed until mortality or the end of the study, while those who switched to HD or received kidney transplantation were censored.
Table 1.
Demographic characteristics and time-averaged laboratory data of the entire PD population in Taiwan from 2005 to 2012.
| Total | Remained PD | Shifted to HD | Kidney Transplant | p value | |
|---|---|---|---|---|---|
| Number | 12966 | 8514 | 3577 | 875 | — |
| Age (year) | 53 ± 16 | 54 ± 16 | 52 ± 16 | 39 ± 13 | <0.0001 |
| Male (%) | 6022(46) | 3880(46) | 1679(47) | 463(53) | <0.0001 |
| DM (%) | 4743(37) | 3156(37) | 1494(42) | 93(11) | <0.0001 |
| PD Vintage (year)† | 3.8 ± 2.6 | 3.8 ± 2.6 | 5.4 ± 2.3 | 3.3 ± 2.5 | <0.0001 |
| Time-averaged laboratory data | |||||
| Albumin (g/dL) | 3.7 ± 0.4 | 3.6 ± 0.4 | 3.7 ± 0.4 | 3.9 ± 0.3 | <0.0001 |
| Hb (g/dL) | 10.1 ± 1.2 | 10.2 ± 1.2 | 10.0 ± 1.1 | 10.0 ± 1.2 | <0.0001 |
| Ca (mg/dL) | 9.2 ± 0.7 | 9.2 ± 0.7 | 9.3 ± 0.6 | 9.4 ± 0.7 | <0.0001 |
| P (mg/dL) | 5.2 ± 1.1 | 5.1 ± 1.0 | 5.2 ± 1.0 | 5.6 ± 1.1 | <0.0001 |
| PTH (pg/mL) | 271.6 ± 181.0 | 270.8 ± 184.7 | 266.2 ± 168.9 | 302.0 ± 192.2 | <0.0001 |
| ALP (IU/L) | 126.7 ± 98.1 | 126.1 ± 100.7 | 130.7 ± 93.5 | 115.7 ± 90.3 | 0.0002 |
| Kt/V‡ | 2.1 ± 0.32 | 2.1 ± 0.31 | 2.1 ± 0.32 | 2.1 ± 0.34 | <0.0001 |
| Renal CCr (mL/min) | 5.0 ± 1.7 | 4.9 ± 1.8 | 5.3 ± 1.7 | 4.5 ± 1.4 | <0.0001 |
PD, peritoneal dialysis; HD, hemodialysis; DM, diabetes mellitus; Hb, Hemoglobin; Ca, serum total calicium; P, serum phosphorus; PTH, parathyroid hormone; ALP, total alkaline phosphatase; Renal CCr, renal creatinine clearance.
†PD vintage of each patient was calculated from the start of 2005 to termination of PD or the end of 2012. ‡Weekly Kt/V, including renal CCr.
Study outcome and predictors
The main outcome was all-cause mortality, which was defined as loss from registration in the TWRDS. This definition was based on the complete, nationwide coverage of renal replacement treatment expenditure provided by the National Health Insurance policy.
Serum concentrations for calcium, phosphorus, PTH, and ALP were analysed as potential predictors of mortality. The measuring and monitoring of these laboratory data were specified strictly by the TWRDS. Serum calcium and phosphorus levels were measured monthly and reported to the TWRDS every 3 months. Serum total calcium was corrected using the following formula: corrected calcium = (0.8 × [normal albumin level – exact albumin level]) + measured serum calcium. PTH was measured and reported to the TWRDS every 3 months. The majority of PTH tests across hospitals in Taiwan (97.9%) were performed by the Union Clinical Laboratory, where the second-generation PTH Chemiluminescence assay ADVIA Centaur (Siemens Healthcare Diagnostics, Tarrytown, NY) has been employed since 2007 (reference range, 14–72 pg/mL). Compared with the second-generation intact PTH Allegro Nichols IRMA suggested by the KDIGO guidelines in 2009, this commercial assay exhibited variation of 60–152%25. Total ALP was reported every 3 months as specified by the TWRDS, which was tested using the modified International Federation of Clinical Chemistry ADVIA 1800 (Siemens Healthcare Diagnostics, Tarrytown, NY).
Statistical analysis
Descriptive statistics are expressed as mean ± standard deviation (SD) or as frequencies (percentages). Time-averaged values of biochemical and hematologic parameters were used for descriptive statistics. The time-averaged values were defined as the average values across the entire registry. Significance was evaluated using one-way analyses of variance (ANOVA) or chi-square tests, as appropriate.
A Cox proportional regression model and Kaplan-Meier method were performed with mortality as the outcome, censoring patients who shifted to HD or received a kidney transplant. For the Kaplan-Meier analyses, baseline data were used and the significance of survival differences was evaluated using the log-rank test. Multiple comparisons of Kaplan-Meier curves were conducted with Sidak correction. The baseline values were defined as the average value in the first year in the registry. To build the multivariate Cox regression model, all potential predictor variables were evaluated for association to mortality using the univariate Cox regression method. The serum concentrations of calcium, phosphorus, PTH, and ALP were considered as potential predictors. The pre-defined criteria for inclusion in the multivariate Cox regression model consisted of a univariate p-value < 0.2. In addition, all continuous variables were evaluated for collinearity by calculating the Pearson correlation coefficient. To reveal a potential non-linear association of predictors with mortality, a multivariate Cox regression model with the potential predictors represented as categorical variables was performed. Considering the U-shape association between PTH and mortality recently reported by Rhee et al.20 and the reference range provided in the KDIGO guidelines14, PTH values were categorized into 5 groups (<50, 50–150, 150–300, 300–600, and ≧600 pg/mL). Similarly, calcium values were categorized into 4 groups (<8.5, 8.5–9.5, 9.5–10.5, and ≧10.5 mg/dL) and phosphorus values were categorized into 6 groups (<3.5, 3.5–5.5, 5.5–6.5, 6.5–7.5, 7.5–8.5, and ≧8.5 mg/dL). In a large-scale study conducted in patients on PD by Rhee et al.20, the risk of mortality increased at an ALP level ≧150 IU/L. Therefore, ALP values were classified into 4 groups (<50, 50–100, 100–150, and ≧150 IU/L). As described previously, patients with missing PTH values were excluded from the study population. For missing values in descriptive statistics, the available data were used.
For survival analysis, patients with missing values for variables used in the regression model were excluded from the respective analysis. In addition to the Cox regression model using baseline data, time-dependent analyses were performed. In time-dependent analyses, calcium, phosphorus, PTH, and ALP were used as time-dependent covariates. Each time-dependent covariate was updated every 3 months. Missing values for time-dependent covariates were imputed by the last value carried forward method. However, patients with missing baseline values were excluded from time-dependent analyses as logical imputation was not feasible.
All statistical analyses were performed using SPSS, version 17.0. (SPSS Inc., Chicago, IL, USA) and SAS, version 9.1 (SAS Institute, Cary, NC, USA).
Results
Cohort description
For the 12,116 included patients, the mean age was 52 ± 16 years and the mean PD vintage was 3.9 ± 2.5 years. At the end of the 8-year study period, 3,036 (25%) patients were deceased. Among the included patients, higher PTH concentration was associated with younger age, and fewer male and diabetic patients. Mortality and PD vintage were significantly different among PTH groups. The PTH < 150 pg/mL group showed the highest mortality while the PTH 300–600 pg/mL group showed the longest PD vintage. The PTH ≧600 pg/mL group was associated with lower haemoglobin, higher phosphorus, higher ALP, and higher Kt/V. According to the KDIGO practice guidelines26, the haemoglobin level of patients in the PTH ≧600 pg/mL group was lower than normal, while the serum phosphorus level in all groups was higher than normal (>5.0 mg/dL)14. All groups had weekly Kt/V values that met the recommended dose (>1.7 per week) (Table 2).
Table 2.
Demographic characteristics and time-averaged laboratory data of the Included PD patients.
| PTH (pg/mL) | p value | |||||
|---|---|---|---|---|---|---|
| Total | <150 | 150–300 | 300–600 | ≧600 | ||
| Number | 12,116 | 3653 | 3829 | 3978 | 656 | — |
| Age (year) | 52 ± 16 | 57 ± 16 | 53 ± 15 | 48 ± 15 | 45 ± 15 | <0.0001 |
| Male (%) | 5632(46) | 1752(48) | 1850(48) | 1765(44) | 265(40) | <0.0001 |
| DM (%) | 4435(37) | 1785(49) | 1487(39) | 1025(26) | 138(21) | <0.0001 |
| PD vintage (year) | 3.9 ± 2.5 | 3.4 ± 2.4 | 3.8 ± 2.4 | 4.4 ± 2.6 | 3.5 ± 2.7 | <0.0001 |
| Mortality (%) | 3036(25) | 1225(34) | 942(25) | 721(18) | 148(23) | <0.0001 |
| Time-averaged laboratory data | ||||||
| Albumin (g/dL) | 3.7 ± 0.4 | 3.7 ± 0.4 | 3.7 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.4 | <0.0001 |
| Hb (g/dL) | 10.1 ± 1.2 | 10.2 ± 1.2 | 10.2 ± 1.2 | 10.1 ± 1.1 | 9.9 ± 1.20 | <0.0001 |
| Ca (mg/dL) | 9.3 ± 0.7 | 9.3 ± 0.6 | 9.2 ± 0.6 | 9.3 ± 0.7 | 9.3 ± 0.8 | <0.0001 |
| P (mg/dL) | 5.2 ± 1.0 | 5.2 ± 1.0 | 5.1 ± 1.0 | 5.4 ± 1.0 | 5.8 ± 1.1 | <0.0001 |
| ALP (IU/L) | 125.3 ± 94.1 | 125.3 ± 94.1 | 120.0 ± 89.6 | 132.5 ± 94.6 | 166.2 ± 120.0 | <0.0001 |
| Kt/V | 2.07 ± 0.31 | 2.04 ± 0.33 | 2.06 ± 0.31 | 2.09 ± 0.31 | 2.10 ± 0.31 | <0.0001 |
| Renal CCr | 5.0 ± 1.7 | 5.0 ± 1.7 | 5.0 ± 1.7 | 4.7 ± 1.5 | 4.7 ± 1.5 | <0.0001 |
PD, peritoneal dialysis; DM, diabetes mellitus; Hb, Hemoglobin; Ca, serum total calicium; P, serum phosphorus; PTH, parathyroid hormone; ALP, total alkaline phosphatase; Renal CCr, renal creatinine clearance.
†PD vintage of each patient was calculated from the start of 2005 to termination of PD or the end of 2012. ‡Weekl Kt/V, including renal CCr.
Survival analysis
Univariate Cox proportional regression analyses showed that age, sex, diabetic status, weekly Kt/V, renal creatinine clearance, albumin, and haemoglobin, along with our presumed predictors (calcium, phosphorus, PTH, and ALP), were significantly associated with mortality (p < 0.2, Supplementary Table 1). All continuous variables were found to be weakly correlated (0.2 < |r| ≦ 0.5) or uncorrelated (|r| ≦ 0.2) with each other and were included in the multivariate Cox regression model (Supplementary Table 2).
In the multivariate Cox regression model, increased age was associated with increased mortality while higher weekly Kt/V and albumin level were associated reduced mortality. Diabetes status and haemoglobin concentration were not associated with the hazard ratio of mortality. In contrast to the general expectation, higher renal creatinine clearance was associated with increased mortality. Of note, renal creatinine clearance was not included in the time-dependent analysis due to a large number of missing baseline values (n = 1,879).
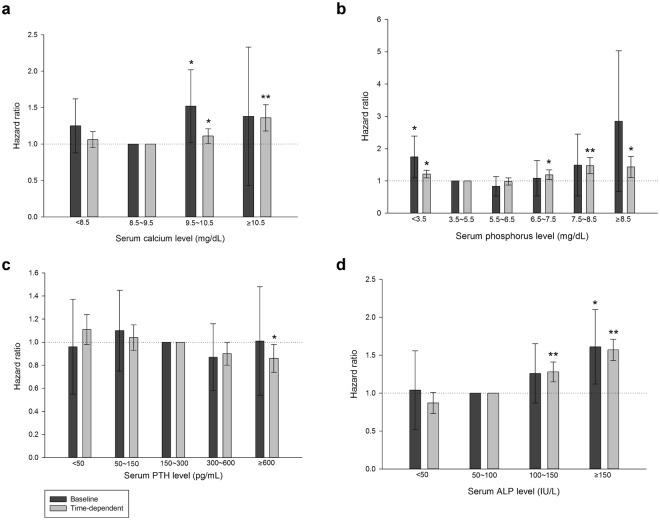
The majority of the findings were consistent whether using baseline data or time-dependent covariates; however, male sex was associated with increased mortality only in the time-dependent analysis (Table 3). Additionally, in the time-dependent analysis, serum calcium levels ≧9.5 mg/dL exhibited higher mortality. In contrast, in the analysis using baseline data, calcium levels either higher or lower than the normal range (8.5–10.5 mg/dL) were not consistently associated with increased mortality (Fig. 1a). Furthermore, in the time-dependent analysis, higher serum phosphorus was found to be positively associated with mortality at ≧7.5 mg/dL, while higher serum phosphorus levels were not associated with increased mortality in the analysis using baseline data. In contrast, serum phosphorus levels <3.5 mg/dL were consistently associated higher mortality in both baseline and time-dependent analyses (Fig. 1b).
Table 3.
Multivariate Cox proportional hazard ratio of mortality in which PTH, ALP, calcium and phosphorus were considered as categorical variables.
| Baseline | Time-dependent | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (per 10 year increments) | 1.21 | 1.08~1.34 | 0.0007 | 1.13 | 1.10~1.16 | <0.0001 |
| Male | 1.05 | 0.77~1.43 | 0.7498 | 1.11 | 1.02~1.20 | 0.0153 |
| DM | 1.06 | 0.78~1.45 | 0.6964 | 1.02 | 0.94~1.11 | 0.6073 |
| Kt/V (per 1 increment) | 0.52 | 0.33~0.83 | 0.0061 | 0.68 | 0.61~0.75 | <0.0001 |
| Albumin (per g/dL increment) | 0.56 | 0.39~0.82 | 0.0028 | 0.31 | 0.29~0.34 | <0.0001 |
| Hb (per g/dL increment) | 0.89 | 0.78~1.01 | 0.0809 | 1 | 0.98~1.03 | 0.8929 |
| Ca (mg/dL) | ||||||
| <8.5 | 1.25 | 0.88~1.79 | 0.2148 | 1.06 | 0.95~1.18 | 0.2962 |
| 8.5~9.5 | Ref | — | — | Ref | — | — |
| 9.5~10.5 | 1.52 | 1.02~2.27 | 0.0276 | 1.11 | 1.01~1.22 | 0.0353 |
| ≧10.5 | 1.38 | 0.43~4.44 | 0.8687 | 1.36 | 1.18~1.56 | <0.0001 |
| P (mg/dL) | ||||||
| <3.5 | 1.74 | 1.09~2.27 | 0.0198 | 1.21 | 1.09~1.35 | 0.0005 |
| 3.5~5.5 | Ref. | — | — | Ref | — | — |
| 5.5~6.5 | 0.83 | 0.53~1.31 | 0.4333 | 0.98 | 0.87~1.09 | 0.6490 |
| 6.5~7.5 | 1.08 | 0.53~2.22 | 0.8261 | 1.19 | 1.04~1.37 | 0.0127 |
| 7.5~8.5 | 1.49 | 0.53~4.20 | 0.4461 | 1.47 | 1.22~1.77 | <0.0001 |
| ≧8.5 | 2.85 | 0.67~12.09 | 0.1551 | 1.43 | 1.10~1.86 | 0.0079 |
| PTH (pg/mL) | ||||||
| <50 | 0.96 | 0.55~1.66 | 0.8834 | 1.11 | 0.98~1.24 | 0.0985 |
| 50~150 | 1.10 | 0.75~1.63 | 0.6225 | 1.04 | 0.93~1.16 | 0.4726 |
| 150~300 | Ref. | — | — | Ref | — | — |
| 300~600 | 0.87 | 0.58~1.31 | 0.5130 | 0.90 | 0.80~1.01 | 0.0806 |
| ≧600 | 1.01 | 0.54~1.89 | 0.9832 | 0.86 | 0.74~0.99 | 0.0377 |
| ALP (IU/L) | ||||||
| <50 | 1.04 | 0.52~2.09 | 0.9216 | 0.87 | 0.73~1.03 | 0.1072 |
| 50~100 | Ref | — | — | Ref | — | — |
| 100~150 | 1.26 | 0.87~1.83 | 0.2185 | 1.28 | 1.15~1.41 | <0.0001 |
| ≧150 | 1.61 | 1.12~2.31 | 0.0100 | 1.57 | 1.43~1.72 | <0.0001 |
| Renal CCr (per mL/min increment)† | 1.13 | 1.06~1.19 | <0.0001 | — | — | — |
PTH, parathyroid hormone; ALP, total alkaline phosphatase; HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus; Kt/V, weekly Kt/V; Hb, hemoglobin, Ca, total calcium; P, phosphorus; renal CCr, renal creatinine clearance.
†Renal creatinine clearance was not included in the time-dependent analysis due to large number of missing baseline values (n = 1879).
Figure 1.
Cox proportional model showing the hazard ratios of CKD-MBD biomarkers to all-cause mortality in patients on peritoneal dialysis in Taiwan. Cox proportional regression model was adjusted for age, gender, diabetes mellitus, Kt/V, albumin, haemoglobin and renal creatinine clearance. Renal creatinine clearance was not included in the time-dependent analysis due to large number of missing baseline values (n = 1879). (a) Grouped by serum calcium levels (b) Grouped by serum phosphorus levels (c) Grouped by serum PTH levels (d) Grouped by serum total ALP levels. CKD-MBD, chronic kidney disease-mineral bone disorder; PTH, parathyroid hormone; ALP, total alkaline phosphatase. **p < 0.0001; *p < 0.05.
In the time-dependent analysis, PTH levels ≧600 pg/mL were associated with lower mortality compared to reference range (150–600 pg/mL), while PTH levels lower than reference range (<150 pg/mL) showed an insignificant trend of increasing mortality. In the analysis using baseline data, PTH levels either lower or higher than the reference range were not associated with higher mortality (Fig. 1c). In the time-dependent analysis, serum ALP levels ≧100 IU/L were associated with increased mortality. In the analysis using baseline data, higher ALP levels was associated higher mortality at ≧150 IU/L. In both analyses, lower ALP levels (<50 IU/L) were not associated with mortality (Fig. 1d).
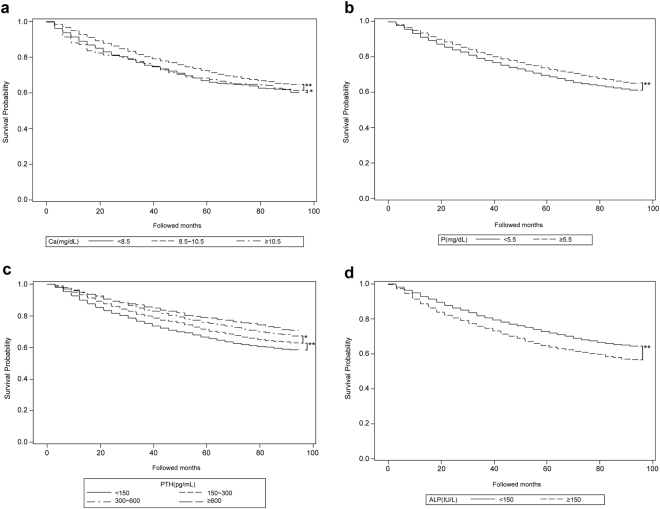
In the Kaplan-Meier analyses, serum calcium levels of 8.5–10.5 mg/dL showed superior survival probability compared to that for either higher or lower levels. The survival probabilities of the three groups stratified by serum calcium levels were significantly different by a Log-rank test (p < 0.0001, Fig. 2a). The survival probabilities among calcium levels significantly differed from each other (Table 4). Phosphorus levels ≧5.5 mg/dL showed a significantly higher survival probability compared to that for levels <5.5 mg/dL, contrary to our findings in the Cox regression model (p < 0.0001, Fig. 2b). PTH levels ≧600 pg/mL showed the highest survival and PTH levels <150 pg/mL showed the lowest survival. The survival was significantly different among PTH groups (p < 0.0001, Fig. 2c). The survival for PTH levels <150 pg/mL was significantly lower compared to that for all other PTH levels, while the survival for PTH levels ≧600 pg/mL was not significantly higher compared to that for PTH levels 150–300 or 300–600 pg/mL. The survival for PTH levels 300–600 pg/mL was significantly higher compared to that for PTH levels 150–300 pg/mL (Table 4). ALP levels <150 U/L showed a significantly higher survival probability compared to that for levels ≧150 U/L (p < 0.0001, Fig. 2d).
Figure 2.
Kaplan-Meier curves showing the survival of peritoneal dialysis patients in Taiwan categorized by CKD-MBD biomarkers. Significance was tested by log-rank test. (a) Grouped by serum calcium (b) Grouped by serum phosphorus (c) Grouped by serum PTH (d) Grouped by serum total ALP. CKD-MBD, chronic kidney disease-mineral bone disorder; PTH, parathyroid hormone; ALP, alkaline phosphatase. Multiple comparison was performed by Sidak correction. **p < 0.0001; *p < 0.05.
Table 4.
Multiple comparisons of Kaplan-Meier curves conducted by Sidak correction.
| Serum calcium concentrations (mg/dL) | p value | ||
|---|---|---|---|
| <8.5 | To | 8.5~10.5 | <0.0001 |
| <8.5 | ≧10.5 | 0.0134 | |
| 8.5~10.5 | ≧10.5 | <0.0001 | |
| Serum PTH concentrations (pg/mL) | |||
| <150 | To | 150~300 | <0.0001 |
| <150 | 300~600 | <0.0001 | |
| <150 | ≧600 | <0.0001 | |
| 150~300 | 300~600 | 0.0021 | |
| 150~300 | ≧600 | 0.1231 | |
| 300~600 | ≧600 | 0.1223 | |
PTH, parathyroid hormone.
Discussion
In the present study, the Kaplan-Meier curves and Cox proportional models using baseline data and time-dependent covariates demonstrated inconsistent effects of serum calcium and phosphorus on mortality in PD patients. However, since models using time-varying explanatory variables are in general more robust, several trends can be summarized from our findings as follows: 1) A higher serum calcium level was associated with increased mortality. 2) Serum phosphorus level ≧6.5 mg/dL or <3.5 mg/dL were associated with increased mortality. 3) A higher serum PTH level was not associated with increased mortality. 4) A higher serum ALP level was associated with increased mortality with a cut-off value of 100–150 IU/L.
In the present study, the association between CKD-MBD biomarkers and all-cause mortality was inconsistent between statistical models using baseline measurements or time-varying covariates. This suggests that in a cohort study with a long follow-up period, the use of baseline measurements as predictors in statistical models overlooks important fluctuations in the predictor variables across the study period. Accordingly, in a registration-based study with repeated measurements, time-dependent analyses theoretically provide a more appropriate evaluation of the association between the time-varying covariates and outcomes.
The majority of observational studies conducted in HD patients have shown that serum phosphorus and calcium levels either higher or lower than the normal limits are associated with a higher risk of mortality, depicting U-shaped relationships27–31. Noordij et al.17, in a prospective cohort study involving 586 PD patients, demonstrated that serum phosphorus levels higher than the normal upper limit, but not abnormal calcium or PTH levels, were associated with increased mortality. Similarly, in another prospective cohort study involving 515 dialysis patients (including 158 PD patients) conducted by Stevens et al.16, only serum phosphorus (not calcium or PTH) showed significant association with mortality. In the present study, conducted exclusively in PD patients, time-dependent analyses showed that serum calcium levels ≧9.5 mg/dL, but not hypocalcemia, was significantly associated with increased mortality. This suggests a deleterious effect of higher serum calcium levels on the survival of patients on PD. In addition, the results of the present study showed that the effect of serum phosphorus on mortality depicted a U-shaped relationship. While the time-dependent analysis showed increased mortality with serum phosphorus levels ≧6.5 mg/dL, phosphorus levels <3.5 mg/dL were consistently associated with increased mortality in statistical models using either baseline or time-varying variables. This may suggest that malnutrition has a strong detrimental effect in patients on PD, regardless of its duration.
The relationship between PTH and mortality in dialysis patients has also been inconsistent across multiple studies. While some studies have shown that higher PTH is associated with decreased risk of mortality3–7, 9, others have conversely suggested an increased risk of mortality10, 32. In addition, several studies have shown a U-shaped relationship5, 27–29, 31 or no significant association1, 2, 8, 33 between PTH and mortality. In a prospective study, which included 277 PD patients, Avram et al.32 demonstrated that lower PTH is associated with increased mortality. However, they considered PTH as a continuous variable; thus, a non-linear association may have been overlooked. More recently, Rhee et al.20 conducted a retrospective cohort study enrolling 9,244 patients on PD which evaluated the impact of PTH and ALP on mortality. In their study, PTH had a U-shaped association with mortality, with a concentration of 200–700 pg/mL exhibiting the lowest mortality and a concentration <100 pg/mL exhibiting the highest mortality. Partly in line with these results, the present study showed that a higher PTH level was not associated with increased mortality in PD patients. However, a U-shaped association of PTH to mortality in patients on PD was not demonstrated in the present study.
Recently, evidence that the serum ALP levels are associated with adverse outcomes for dialysis patients, including cardiac failure11, 12, renal osteodystrophy34–36, and mortality has been accumulating20, 37, 38. These findings suggest that ALP might be a valuable parameter in the treatment of CKD-MBD in dialysis patients. In Rhee et al.20, higher ALP in patients on PD was associated with increased mortality in a binary fashion, with a cut-off value of ≧150 IU/L. The results of the present study showed a similar trend, supporting the role of ALP as a predictor of mortality in patients on PD.
Considering the results for PTH and ALP, our study revealed similar findings to the work of Rhee et al.20, which was another large-scaled, retrospective cohort study in patients on PD. However, the race and ethnicity of the subjects in this previous study consisted mainly of white, black, and Hispanic. In contrast, the present study is comprised of exclusively Asian people, and therefore, the present study extends the generalisation of these findings to a wider racial spectrum.
The multivariate Cox regression model paradoxically demonstrated that renal creatinine clearance was associated with increased mortality. One explanation to this phenomenon is that this study started with a cross-sectional cohort; thus, the status and duration of dialysis before the onset of the registry was not available. Possibly, the patients with lower renal creatinine clearance represented those who had longer dialysis vintage and who were more stable patients. This may have led to a bias. Another explanation is that, as reported recently, urinary creatinine clearance is prone to falsely overestimate glomerular filtration rate in patients with renal impairment and is less reliable in patients with end-stage kidney disease39. In addition, since renal creatinine clearance was calculated as the filtered creatinine per minute divided by serum creatinine concentration, patients with malnutrition and lower muscle mass may have exhibited lower serum creatinine levels and therefore, higher renal creatinine clearance. This may also be an explanation for the unexpected finding in the present study.
There were a number of limitations in the present study. One limitation is that the study started with a cross-sectional cohort; therefore, patients who switched from HD to PD before the beginning of the registry were also included, which may have affected the results. The outcome of the current study, all-cause mortality, was defined as loss from registration in TWRDS. However, this definition was not cross-referenced with national vital statistics and might over-estimate the actual mortality. Other limitations include that only total ALP, not bone-specific ALP was available, and that the PTH assay used was a second generation assay with high cross-reactivity to inactive PTH fragments. In addition, the actual medical records of each individual were not available; therefore, information about cardiovascular diseases and other comorbidities, medication, dialysate calcium concentration, and the cause of mortality were absent from our data. However, the main strengths of this study include the use of a large, population-based database with nationwide coverage of PD patients in Taiwan with laboratory data that was strictly specified and measured, and the use of time-dependent variables in the statistical analyses.
It has been argued that features of CKD-MBD differ between patients undergoing PD and HD in that a dynamic bone disease was more frequent, and bone microarchitecture was less affected, in patients on PD40, 41; nevertheless, studies comparing the effects of CKD-MBD biomarkers to mortality in patients on PD and HD remain limited. Currently, the KDIGO does not recommend specific guidelines on the management of CKD-MBD for patients on PD or HD. A recent nationwide, registry-based study conducted with patients on HD in Taiwan found the following: 1) higher ALP level was associated with increased mortality in a binary fashion, with a cut-off value of ≧100 IU/L; 2) a PTH level <100 pg/mL was associated increased mortality, while higher PTH levels not being associated with increased mortality; and 3) both serum calcium and phosphorus were associated with mortality in a U-shaped fashion, with the nadir located at 9.5–10.5 mg/dL and 5.5–6.5 mg/dL, respectively42. The U-shaped associations for serum calcium and phosphorus with regard to mortality reported in the work by Lin et al., are in line with most observational studies conducted with patients on HD27–31. While an association between serum phosphorus and mortality has been inconsistently reported across multiple studies conducted with patients on PD, several studies have reported no effects of serum calcium and PTH on mortality16, 17. In another large-scaled retrospective cohort study conducted with HD patients in the U.S.A., higher ALP level was associated with increased mortality in a binary fashion with a cut-off at ≧120 IU/L43. Comparing these HD-related findings to the present study, it is possible that in the PD population, serum calcium and phosphorus may have a less prominent effect on mortality, while ALP has a more consistent effect on mortality. In addition, several recent studies have shown that in both patients on HD and PD, higher PTH levels did not increase mortality. However, the evidence to date remains insufficient to provide recommendations specifically for PD patients. We expect that further research on CKD-MBD in PD patients could contribute to a practice guideline being developed specifically for this population in the future.
Electronic supplementary material
Acknowledgements
This study is granted by Taipei Medical University-Wan Fang Hospital (102-wf-eva-32).
Competing Interests
The authors declare no competing financial interests.
Author Contributions
C.L. wrote the main manuscript text and Y.L. (Yi-Chun Lin) designed the experiments. C.L. and Y.L. (Yen-Chung Lin) analyzed the data. M.W. contributed reagents and materials. All authors reviewed the manuscript.
Footnotes
Yen-Chung Lin and Mai-Szu Wu contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00080-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yen-Chung Lin, Email: yclin0229@tmu.edu.tw.
Mai-Szu Wu, Email: maiszuwu@gmail.com.
References
- 1.Tentori F, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Kimata N, et al. Association of mineral metabolism factors with all-cause and cardiovascular mortality in haemodialysis patients: the Japan dialysis outcomes and practice patterns study. Hemodial Int. 2007;11:340–348. doi: 10.1111/j.1542-4758.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 3.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in haemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16:1788–1793. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 4.Young EW, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 5.Block GA, et al. Mineral metabolism, mortality, and morbidity in maintenance haemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 6.Marco, M. P. et al. Higher impact of mineral metabolism on cardiovascular mortality in a European haemodialysis population. Kidney Int Suppl, S111–114, doi:10.1046/j.1523-1755.63.s85.26.x (2003). [DOI] [PubMed]
- 7.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic haemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 8.Block, G. A., Hulbert-Shearon, T. E., Levin, N. W. & Port, F. K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic haemodialysis patients: a national study. Am J Kidney Dis31, 607–617. [DOI] [PubMed]
- 9.Melamed ML, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70:351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 10.Jean G, et al. Association between very low PTH levels and poor survival rates in haemodialysis patients: results from the French ARNOS cohort. Nephron Clin Pract. 2011;118:c211–216. doi: 10.1159/000321642. [DOI] [PubMed] [Google Scholar]
- 11.Salgueira M, et al. [Cardiac failure and diastolic disfunction in haemodialysis patients: associated factors] Nefrologia. 2005;25:668–677. [PubMed] [Google Scholar]
- 12.Nasri H, Baradaran A, Naderi AS. Close association between parathyroid hormone and left ventricular function and structure in end-stage renal failure patients under maintenance haemodialysis. Acta Med Austriaca. 2004;31:67–72. [PubMed] [Google Scholar]
- 13.Regidor DL, et al. Serum alkaline phosphatase predicts mortality among maintenance haemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. S1–130, doi:10.1038/ki.2009.188 (2009). [DOI] [PubMed]
- 15.Uhlig K, et al. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD) Am J Kidney Dis. 2010;55:773–799. doi: 10.1053/j.ajkd.2010.02.340. [DOI] [PubMed] [Google Scholar]
- 16.Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol. 2004;15:770–779. doi: 10.1097/01.ASN.0000113243.24155.2F. [DOI] [PubMed] [Google Scholar]
- 17.Noordzij M, et al. The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis. 2005;46:925–932. doi: 10.1053/j.ajkd.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Fein PA, et al. Relationship between alkaline phosphatase and all-cause mortality in peritoneal dialysis patients. Adv Perit Dial. 2013;29:61–63. [PubMed] [Google Scholar]
- 19.Liu X, et al. Alkaline phosphatase and mortality in patients on peritoneal dialysis. Clin J Am Soc Nephrol. 2014;9:771–778. doi: 10.2215/CJN.08280813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhee CM, et al. Comparative mortality-predictability using alkaline phosphatase and parathyroid hormone in patients on peritoneal dialysis and haemodialysis. Perit Dial Int. 2014;34:732–748. doi: 10.3747/pdi.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang WC, Hwang SJ. Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: the impact of national health insurance. Nephrol Dial Transplant. 2008;23:3977–3982. doi: 10.1093/ndt/gfn406. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SJ, et al. Increased risk of mortality in the elderly population with late-stage chronic kidney disease: a cohort study in Taiwan. Nephrol Dial Transplant. 2008;23:3192–3198. doi: 10.1093/ndt/gfn222. [DOI] [PubMed] [Google Scholar]
- 23.Huang, C. C., Cheng, K. F. & Wu, H. D. Survival analysis: comparing peritoneal dialysis and haemodialysis in Taiwan. Perit Dial Int28 Suppl 3 (2008). [PubMed]
- 24.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant. 2010;25:2616–2624. doi: 10.1093/ndt/gfq308. [DOI] [PubMed] [Google Scholar]
- 25.Cantor T, Yang Z, Caraiani N, Ilamathi E. Lack of comparability of intact parathyroid hormone measurements among commercial assays for end-stage renal disease patients: implication for treatment decisions. Clin Chem. 2006;52:1771–1776. doi: 10.1373/clinchem.2006.071589. [DOI] [PubMed] [Google Scholar]
- 26.Chapter 3: Use of ESAs and other agents to treat anemia in CKD. Kidney Int Suppl (2011) 2, 299–310, doi:10.1038/kisup.2012.35 (2012). [DOI] [PMC free article] [PubMed]
- 27.Fernandez-Martin JL, et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant. 2015;30:1542–1551. doi: 10.1093/ndt/gfv099. [DOI] [PubMed] [Google Scholar]
- 28.Floege J, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naves-Diaz M, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant. 2011;26:1938–1947. doi: 10.1093/ndt/gfq304. [DOI] [PubMed] [Google Scholar]
- 30.Miller JE, et al. Association of cumulatively low or high serum calcium levels with mortality in long-term haemodialysis patients. Am J Nephrol. 2010;32:403–413. doi: 10.1159/000319861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, et al. Survival predictability of time-varying indicators of bone disease in maintenance haemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 32.Avram MM, Mittman N, Myint MM, Fein P. Importance of low serum intact parathyroid hormone as a predictor of mortality in haemodialysis and peritoneal dialysis patients: 14 years of prospective observation. Am J Kidney Dis. 2001;38:1351–1357. doi: 10.1053/ajkd.2001.29254. [DOI] [PubMed] [Google Scholar]
- 33.Tangri N, et al. Effect of bone mineral guideline target achievement on mortality in incident dialysis patients: an analysis of the United Kingdom Renal Registry. Am J Kidney Dis. 2011;57:415–421. doi: 10.1053/j.ajkd.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 34.Jorna FH, et al. Early identification of risk factors for refractory secondary hyperparathyroidism in patients with long-term renal replacement therapy. Nephrol Dial Transplant. 2004;19:1168–1173. doi: 10.1093/ndt/gfh018. [DOI] [PubMed] [Google Scholar]
- 35.Jarava C, Armas JR, Palma A. [Study of renal osteodystrophy by bone biopsy. Age as an independent factor. Diagnostic value of bone remodeling markers] Nefrologia. 2000;20:362–372. [PubMed] [Google Scholar]
- 36.Fletcher S, et al. Assessment of renal osteodystrophy in dialysis patients: use of bone alkaline phosphatase, bone mineral density and parathyroid ultrasound in comparison with bone histology. Nephron. 1997;75:412–419. doi: 10.1159/000189578. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama Y, et al. A higher serum alkaline phosphatase is associated with the incidence of hip fracture and mortality among patients receiving haemodialysis in Japan. Nephrol Dial Transplant. 2014;29:1532–1538. doi: 10.1093/ndt/gfu055. [DOI] [PubMed] [Google Scholar]
- 38.Blayney MJ, et al. High alkaline phosphatase levels in haemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int. 2008;74:655–663. doi: 10.1038/ki.2008.248. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, et al. Measurement error as alternative explanation for the observation that CrCl/GFR ratio is higher at lower GFR. Clin J Am Soc Nephrol. 2016;11:1574–1582. doi: 10.2215/CJN.12821215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Oliveira RA, et al. Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamic bone disease. Kidney Int. 2015;87:1039–1045. doi: 10.1038/ki.2014.372. [DOI] [PubMed] [Google Scholar]
- 41.Pelletier S, et al. Bone microarchitecture is more severely affected in patients on haemodialysis than in those receiving peritoneal dialysis. Kidney Int. 2012;82:581–588. doi: 10.1038/ki.2012.166. [DOI] [PubMed] [Google Scholar]
- 42.Lin YC, et al. Effect modifying role of serum calcium on mortatlity-perdictability of PTH and alkaline phosphatase in haemodialysis patients: An investigation using data from the Taiwan Renal Registry Data Dystem from 2005 to 2012. PLoS ONE. 2015;10(6):e0129737. doi: 10.1371/journal.pone.0129737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regidor DL, et al. Serum alkaline phosphatase predicts mortality among maintenance haemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.