Abstract
The present study investigated whether erythromycin (ERY) reduces cigarette smoke (CS)-induced emphysema in rats and aimed to determine the anti-inflammatory effect of ERY, which may identify potential treatments for chronic obstructive pulmonary disease. Furthermore, the current study focused on the potential effects on the imbalance between matrix metalloprotease (MMP) and anti-MMP activity, the phosphorylation of mitogen-activated protein kinases (MAPKs) and the nuclear factor-κB (NF-κB) signaling pathway. Wistar rats were divided into the following three groups (n=12 each): control (ERY vehicle only, without any CS exposure), CS (animals were exposed to CS for 12 weeks) and CS + ERY (animals were exposed to CS for 12 weeks and received 100 mg/kg/day ERY). The recruitment of inflammatory cells into the bronchoalveolar lavage fluid (BALF) and the histopathology of lung tissue from all groups was evaluated to grade the severity of the emphysema. The expression of MMP-2, MMP-9 and tissue inhibitor of metalloproteinase-1 was evaluated by immunohistochemistry and western blotting. The activation of MAPKs, NF-κB and inhibitor of NF-κB (IκBα), in lung tissues was examined by western blotting. Treatment with ERY resulted in fewer inflammatory cells and cytokines in the BALF, and fewer emphysema-associated changes in the lungs compared with control. The stimulus of CS promoted the phosphorylation of extracellular signal-regulated kinase (ERK)1/2 and p38, but not c-Jun NH2-terminal kinase, thereby inducing the activation of the ERK/MAPK signaling pathway in rats. Furthermore, CS exposure increased the expression of NF-κB and decreased the expression of IκBα. The levels of phosphorylated ERK1/2 and p38 were significantly reduced in rats with CS-induced emphysema when treated with ERY compared with the CS group. The results of the present study therefore indicate that oral administration of ERY may suppress CS-induced emphysema by regulating inflammatory cytokines and the MMP/anti-MMP imbalance via the MAPK/NF-κB pathway.
Keywords: erythromycin, chronic obstructive pulmonary disease, metalloprotease, anti-metalloprotease, mitogen-activated protein kinase, emphysema
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most significant chronic conditions and is now the fourth most common cause of death worldwide, which has an increasing economic and social burden (1,2). COPD affects >300 million people worldwide and accounts for 4 million deaths every year (3). However, unlike heart disease and malignancy, mortality due to COPD has increased progressively; without effective intervention, the number of deaths caused by COPD is predicted to increase by >30% between 2010 and 2020 (4). Smoking, the major risk factor for COPD (5), is a powerful inducer of inflammatory mediators, including oxidants and proteases (6). Emphysema is a characteristic of COPD, which is caused by chronic inflammation in the lung that contributes to lung tissue destruction and is mediated by a multistep pathway, which involves an imbalance of metalloproteases, including matrix metalloproteases (MMPs) and ADAM metallopeptidases, and anti-metalloproteases, which include tissue inhibitor of metalloproteinases (TIMPs) and α-2 macroglobulin. This imbalance leads to MMP overproduction that is not sufficiently counteracted by TIMPs (7,8). The imbalance of the metalloproteases and anti-metalloproteases may be a potential target for the treatment of the chronic inflammation and emphysema caused by cigarette smoke (CS). The term ‘macrolide’ is used to describe drugs with a macrocyclic lactone ring of ≥12 elements (9,10). In addition to antimicrobial properties, other properties of macrolides were suggested in the 1960s, particularly in chronic inflammatory diseases, and these actions are observed only for treatment with 14- and 15-membered macrolides, including erythromycin (ERY), clarithromycin, roxithromycin and azithromycin (11). The effects of macrolides in patients with chronic inflammatory airway disease appear to be independent of antimicrobial properties. A previous report demonstrated the anti-inflammatory effect of macrolides in CS-induced lung inflammation and emphysema, and also reported the effect of ERY on the imbalance of metalloproteases/anti-metalloproteases (12). However, the potential mechanism of ERY in the inhibition of the inflammation in CS-induced emphysema has not been clarified. Central to the inflammatory response induced by CS is an increased level of chemotactic cytokines, particularly interleukin (IL)-8. It is associated with an increase in the activation of intracellular signaling molecules, including mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) (13,14). MAPKs, together with NF-κB activation, are the most activated kinases in airway epithelial cells and macrophages exposed to CS extracts, and these kinases are important in inducing the expression of various pro-inflammatory chemokines and cytokines, and inducing the activation of cytosolic phospholipase A2 (15). The present study evaluated the imbalance of metalloproteases/anti-metalloproteases in CS-induced emphysema and investigated the upstream signaling that altered metalloprotease/anti-metalloprotease levels, via phosphorylation of the MAPK family and NF-κB overactivation. The current study also investigated whether ERY affects the metalloprotease/anti-metalloprotease imbalance in a dose-dependent manner, and if so, whether it does so by affecting signaling pathways dependent on MAPKs or NF-κB.
Materials and methods
Animal model for cigarette smoke exposure
All of the experiments were conducted in accordance with the guidelines of the Research Ethics Committee of China Medical University (Shenyang, China). Male Wistar rat weanlings (6–7-weeks-old;170-210 g) were obtained from the Laboratory Animal Center of China Medical University and were maintained in a pathogen-free environment. They were fed laboratory rodent chow and water ad libitum and were kept on a 12 h light/dark cycle. Rats were randomly assigned into the following three groups: control (n=12), CS exposure (n=12) and CS+ERY (n=12). Rats in the CS group were exposed to smoke from 16 commercial cigarettes (Marlboro; Philip Morris USA, New York, NY, USA; each cigarette contains 0.9 mg nicotine, 14 mg carbon monoxide and 12 mg tar oil; the smokescope v/V% was 8%) with the filters removed for 30 min twice a day, 6 days per week for 12 weeks. The rats in the ERY group were also exposed to CS for 12 weeks and treated with an orally administered injection of ERY (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 100 mg/kg, once daily. The drug treatments were performed 0.5 h prior to CS exposure from day 1of the 12-week exposure period. The control group received a daily orally administered injection of saline, which was the vehicle for ERY, without any smoke exposure. At the end of week 12, rats were sacrificed by exsanguination under anesthetic (40 mg/kg pentobarbital intraperitoneally; Sigma-Aldrich; Merck KGaA) and lungs were excised. The right upper lobes were taken and stored at −80°C. The middle lobes of the right rat lungs, which were not lavaged, were briefly washed in ice-cold saline containing 5 mM CaCl2+1 mM MgCl2, fixed in ExCell Plus™ fixative (American MasterTech Scientific, Inc., Lodi, CA, USA) at room temperature for 24 h. Subsequently, the middle lobes of the right rat lungs were embedded in paraffin blocks and sectioned (4 µm) for conventional hematoxylin and eosin staining. The left lungs were infused with 2 ml PBS four times. The bronchoalveolar lavage fluid (BALF) was centrifuged for 10 min at 900 × g and 4°C. The cell-free supernatants were stored at −80°C.
Morphologic and morphometric analyses of lung tissue
Hematoxylin and eosin staining was performed on paraffin-embedded lung tissue sections (4 µm). The measure of lung tissue morphology was determined by light microscopy at a magnification of ×100. The mean linear intercept (MLI) (16), a measure of inter-alveolar wall distance, was defined by the total length of the cross-line/the numbers of the alveolar walls intersecting the test lines. The mean alveolar number (MAN) (16), an indicator of alveolar density, was calculated by counting the numbers of alveoli in each field. Five sections were analyzed per animal, and four images were acquired from a randomly selected location in each slide.
Measurement of IL-8 and leukotriene B4 (LTB4)
The protein expression of IL-8 and LTB4-α in the BALF supernatant of rats in each group was determined by ELISA kits purchased from EnzoLife Sciences, Inc. (Farmingdale, NY, USA; cat nos. ADI-900-074 and ADI-900-068, respectively), according to the manufacturer's instructions.
Characterization of inflammatory cells in BALF
The collected BALF samples from the left lung tissues were centrifuged at 1,400 × g for 10 min at 4°C, and their supernatants were stored at −80°Cuntil ELISA analysis. The pelleted cells were resuspended in PBS, and 1×105 cells were subjected to cytospin centrifugation on glass slides and fixed with 100% methanol for 15 min, followed by staining with May-Grünwald-Giemsa solution for 15 min. A differential cell count of BALF cell pellets was performed under a light microscope at a magnification of ×200 according to morphological characteristics.
Measurement of MMP-2, MMP-9 and TIMP-1
The right upper lobes of lungs were lysed by RIPA buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) at a ratio of 100 mg of tissue to 1 ml of lysis buffer. This was followed by centrifugation at 1,500 × g for 20 min at 4°C. Total protein concentration was determined using the Bradford assay (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and equal amounts of protein (30 µg) were separated by SDS-PAGE (4% stacking gel and 10% separating gel). Protein was subsequently transferred onto a nitrocellulose membrane by electroblotting. The membrane was blocked for 1 h at room temperature with blocking buffer [3% non-fat milk in TBS (pH 7.4) containing 0.1% Tween-20] and was subsequently incubated overnight at 4°Cwith mouse antibodies against MMP-2 (1:500; cat. no. ab7033;), MMP-9 (1:500; cat. no. ab38898), TIMP-1 (1:500; cat. no. ab61224) and β-actin (1:2,000; cat. no. ab6276; all from Abcam, Cambridge, UK). The membrane was washed three times and incubated for 2 h at room temperature with horseradish peroxidase-conjugated anti-mouse and anti-rabbit immunoglobulin G (IgG; 1:5,000; cat. nos. sc-516102 and sc-2357, respectively; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The bound secondary antibody was detected using the Enhanced Chemiluminescence Plus kit (GE Healthcare Life Sciences, Chalfont, UK) and analyzed using the Las-3000 Luminescent Image Analyzer (Fujifilm, Tokyo, Japan). The band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The fold changes in protein expression were calculated relative to the levels in the control group. The average values for each group were determined by 3 independent experiments. Lung tissues were sectioned (4 µm), cleared of paraffin and endogenous peroxidases were blocked by incubation with 3% H2O2 for 10 min at room temperature, followed by three washes with PBS for 3 min. Tissue sections were subsequently incubated with rabbit serum (Sigma-Aldrich; Merck KGaA) for 10 min at ambient temperature. Sections were incubated overnight with mouse MMP-2 (1:50; cat. no. ab7033; Abcam), mouse MMP-9 (1:50; cat. no. ab38898; Abcam) or rabbit TIMP-1 (1:50; cat. no. ab61224; Abcam) antibodies at 4°C, which was followed by the addition of biotinylated rabbit anti-mouse (1:2,000; cat. PA1-28567; Thermo Fisher Scientific, Inc.) or mouse anti-rabbit and anti-mouse IgG secondary antibody (1:2,000; cat. nos. sc-2357 and sc-516102; Santa Cruz Biotechnology, Inc.). To verify the binding specificity for MMP-2, MMP-9 and TIMP-1, certain sections were incubated with only a primary or secondary antibody. In these situations, no positive staining was identified in the sections, indicating that the immunoreactions were positive in all of the experiments. Immunohistochemistry staining was performed in accordance with the manufacturer's instructions and visualized by the use of diaminobenzidine staining. Digital photos were analyzed with Image-Pro Plus 4.1 (MediaCybernetics, Inc., Rockville, MD, USA) by an observer who was blind to the group classification.
Measurement of phosphorylated (p)-MAPK and NF-κB
Total protein was extracted from the upper right lobes of the lungs by lysis with RIPA buffer (100 mg of tissue to 1 ml of lysis buffer). This was followed by centrifugation at 1,500 × g for 20 min at 4°C. The protein concentration was determined using the Bradford assay and equal amounts of protein (50 µg) were separated by SDS-PAGE (4% stacking gel and 10% separating gel) for detection of extracellular signal-regulated kinase (ERK)1/2, c-Jun NH2-terminal kinase (JNK) and p38. Following electrophoresis and electrophoretic transfer of proteins to nitrocellulose membranes, membranes were blocked with 3% non-fat milk in TBS (pH 7.4) containing 0.1% Tween-20 for 1 h at room temperature. The membranes were then rinsed three times in TBST and incubated at room temperature with mouse monoclonal antibodies against activated diphosphorylated ERK1/2 (1:500; cat. no. sc-81492; Santa Cruz Biotechnology, Inc.), p-JNK (1:500; cat. no. sc-293137; Santa Cruz Biotechnology, Inc.) and p-p38 (1:500; cat. no. sc-17852-R; Santa Cruz Biotechnology, Inc.) for 1 h. β-actin levels were also assessed as a loading control using a mouse β-actin antibody (1:2,000; cat. no. ab6276; Abcam). The blots were processed as described for the measurement of MMP-2, MMP-9 and TIMP-1. To assess the activation of NF-κB, nuclear extracts were analyzed by western blot analysis for the p65 fraction of NF-κB and the NF-κB inhibitor α (IκBα). Nuclear extracts were isolated as previously described (17). The protein concentration was determined using the Bradford assay and equal amounts of protein (30 µg) were separated by SDS-PAGE (4% stacking gel and 10% separating gel). NF-κB was detected with NF-κB p65 rabbit polyclonal antibody (1:500; cat. no. ab16502; Abcam) and IκBα rabbit polyclonal antibody (1:500; cat. no. ab7217; Abcam). β-actin (1:2,000) served as the control. The blots were processed as described for the measurement of MMP-2, MMP-9 and TIMP-1.
Statistical analysis
Statistical analyses were performed with SPSS 11.5 (SPSS, Inc., Chicago, IL, USA). All data were expressed as the mean ± standard deviation. Differences between multiple groups were compared with a one-way analysis of variance. Differences between two groups were tested for significance by the least significant difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of ERY on CS-induced lung damage
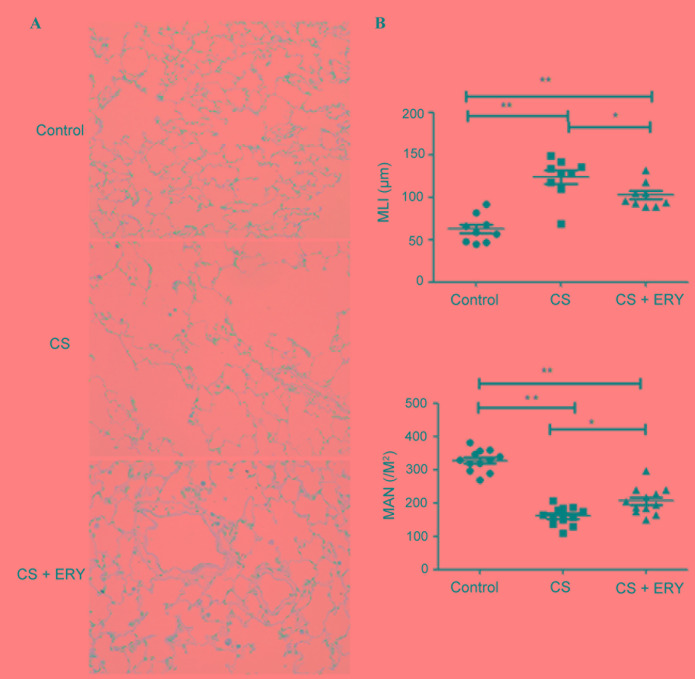
Histopathological studies were performed to assess the morphological damage caused by CS and the effect of ERY treatment. To investigate the effect of ERY on the emphysema induced by CS, MLI and MAN were measured. The control group demonstrated no evident histological changes; the structure of the alveoli was intact with regular ciliary arrangement in the control group (Fig. 1A). Compared with the morphology of the control group, CS induced significant morphological changes characterized by conspicuous inflammation accompanied with focal emphysema; the alveolar walls were broken and merged, and the cavities of the alveoli were enlarged (Fig. 1A). MLI was significantly increased (P<0.01; Fig. 1B), and MAN was significantly lower (P<0.01; Fig. 1B) in the CS group compared with the control group. ERY demonstrated a protective effect on emphysematous changes induced by CS, with a significantly reduced MLI (P<0.05 vs. the CS group; Fig. 1B) and a significantly higher MAN (P<0.05 vs. the CS group; Fig. 1B) in the CS + ERY group compared with the CS group. Compared with the morphological changes in the CS group, the structural damage induced by CS was partially recovered in the CS + ERY group (Fig. 1A); however, the MLI remained higher than in the control group (P<0.01; Fig. 1B) and MAN remained lower that the control group (P<0.01; Fig. 1B).
Figure 1.
ERY has a protective effect on the histological changes observed in CS-induced emphysema in rats. (A) Lung tissue sections from different groups of rats were subjected to hematoxylin and eosin staining (original magnification, ×400). (B) Quantitative analysis of alveolar airspace by MLI and MAN. *P<0.05 and **P<0.01, comparisons indicated by brackets; n=12 per group. Control rats were exposed to normal air and received daily saline injections. CS rats were exposed to CS for 12 weeks. CS + ERY rats were exposed to CS for 12 weeks and received daily injections of 100 mg/kg ERY. CS, cigarette smoke; ERY, erythromycin; MLI, mean linear intercept; MAN, mean alveolar number.
ERY reduces the number of inflammatory cells, and the levels of IL-8 and LTB4 present in the BALF following CS exposure
As presented in Table I, CS significantly increased the number of neutrophils (P<0.01) and lymphocytes (P<0.01), and significantly decreased the number of macrophages (P<0.01) compared with the control group. Additionally, as presented in Fig. 2, rats in the CS group had significantly increased levels of IL-8 (P<0.01) and LTB4 (P<0.01) compared with the control group. Compared with the CS group, ERY treatment led to a significant reduction in the total cell count (P<0.05; Table I), the relative count of neutrophils (P<0.01; Table I) and lymphocytes (P<0.01; Table I), and a significant reduction in the levels of IL-8 (P<0.01) and LTB4 (P<0.01; Fig. 2), and increased the macrophage count (P<0.05; Table I).
Table I.
Cytological count in bronchoalveolar lavage fluid of rats from different groups.
| Group | Total cell count (x106/l) | Neutrophil (%) | Lymphocyte (%) | Macrophage (%) |
|---|---|---|---|---|
| Control | 4.20±2.41 | 3.02±1.96 | 3.96±2.02 | 86.52±12.49 |
| CS | 14.6±4.37b | 21.64±10.73b | 8.38±4.82b | 63.73±12.55b |
| ERY | 10.3±3.67b,c | 12.74±5.16b,d | 10.54±5.52a,c | 73.65±15.69a,c |
P<0.05
P<0.01 vs. control group
P<0.05
P<0.01 vs. the CS group. Data are presented as the mean ± standard deviation (n=12). Control rats were exposed to normal air and received daily saline injections. CS rats were exposed to CS for 12 weeks. CS + ERY rats were exposed to CS for 12 weeks and received daily injections of 100 mg/kg ERY. CS, cigarette smoke; ERY, erythromycin.
Figure 2.
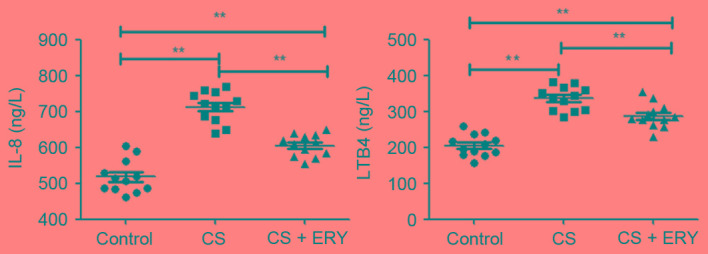
ERY has a protective role in the changes to inflammatory cytokinesIL-8 and LTB4 in the BALF from rats with CS-induced emphysema. The levels of IL-8 and LTB4 in BALF of individual rats were analyzed by ELISA. Data are presented as the mean + standard deviation of individual samples from three separate experiments. **P<0.01, comparisons indicated by brackets; n=12 per group. Control rats were exposed to normal air and received daily saline injections. CS rats were exposed to CS for 12 weeks. CS + ERY rats were exposed to CS for 12 weeks and received daily injections of 100 mg/kg ERY. BALF, bronchoalveolar lavage fluid; IL, interleukin; CS, cigarette smoke; ERY, erythromycin; LTB4, leukotriene B4.
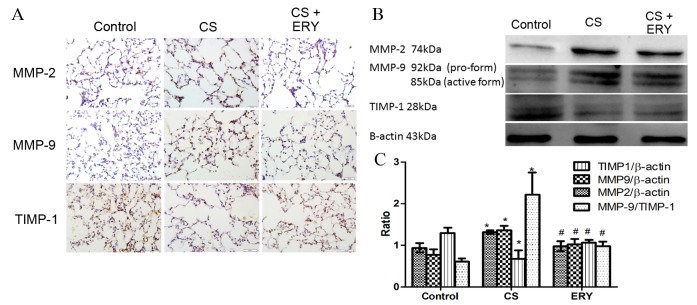
Effects of ERY on lung MMP-2, MMP-9 and TIMP-1 protein expression
To evaluate the effect of ERY on the regulation of the imbalance of metalloproteases/anti-metalloproteases, the present study performed immunohistochemical staining (Fig. 3A) and western blotting for MMP-2, MMP-9 and TIMP-1 (Fig. 3B). The protein expression levels of MMP-2 and MMP-9 in the CS group were significantly increased compared with the control group (P<0.05 and P<0.05, respectively; Fig. 3B), while the level of TIMP-1 was significantly reduced compared with the control group (P<0.05; Fig. 3B). While the expression of MMP-2 and MMP-9 were reduced significantly by ERY treatment compared with the CS group (P<0.05 and P<0.05, respectively; Fig. 3B), the expression of TIMP-1 was significantly increased by ERY treatment compared with the CS group (P<0.05; Fig. 3B). The ratio of MMP-9/TIMP-1 was increased significantly following CS exposure compared with the control group (P<0.05; Fig. 3B) but significantly reduced in the CS + ERY group compared with the CS group (P<0.05; Fig. 3B).
Figure 3.
ERY has a protective role in the disturbance of MMP and anti-MMP activity in CS-induced emphysema in rats. (A) The protein expression of MMP-2, MMP-9 and TIMP-1 as measured by immunohistochemical staining (original magnification, ×400). (B) Protein expression levels of MMP-2, MMP-9 and TIMP-1 as measured by western blotting, with quantification relative to β-actin. For TIMP-1, the upper band was considered to be non-specific binding. Data are presented normalized to the control and are the mean ± standard deviation. Control rats were exposed to normal air and received daily saline injections. CS rats were exposed to CS for 12 weeks. CS + ERY rats were exposed to CS for 12 weeks and received daily injections of 100 mg/kg ERY. *P<0.05 vs. control group and #P<0.05 vs. CS group; n=12 per group. CS, cigarette smoke; ERY, erythromycin; MMP, metalloprotease; TIMP-1, tissue inhibitor of metalloproteinases.
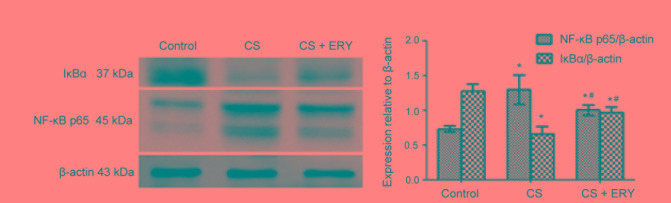
Effect of ERY on NF-κB activation
To investigate the mechanism of ERY-mediated regulation of NF-κB in vivo, the present study investigated the effect of ERY on IκBα (Fig. 4). Compared with the control group, the level of IκBα was significantly decreased in the CS group (P<0.05; Fig. 4), while treatment with ERY increased the expression of IκBα compared with the CS group (P<0.05; Fig. 4). Thus, this indicated that CS-induced IκBα degradation was significantly blocked by treatment with ERY. Additionally, the activation of NF-κB p65 was decreased significantly in the CS + ERY group compared with the CS group (P<0.05; Fig. 4).
Figure 4.
Effects of ERY on the expression of NF-κB, p65 and IκBα in CS-induced emphysema in rats by western blotting. Protein expression levels of NF-κB, p65 and IκBα as measured by western blotting, with quantification relative to β-actin. The upper band on the blot for NF-κB p65 was quantified and the lower band was considered to be non-specific binding and was not quantified. Data are presented as the mean ± standard deviation. Control rats were exposed to normal air and received daily saline injections. CS rats were exposed to CS for 12 weeks. CS + ERY rats were exposed to CS for 12 weeks and received daily injections of 100 mg/kg ERY. *P<0.05 vs. control group and #P<0.05 vs. CS group; n=12 per group. CS, cigarette smoke; ERY, erythromycin; IκBα, inhibitor of NF-κB; NF-κB, nuclear factor-κB.
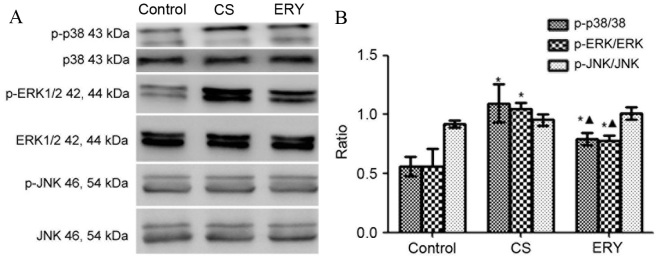
Effect of ERY on MAPK pathway activation
The levels of phosphorylated MAPKs were measured by western blot analysis (Fig. 5). Total protein levels of MAPKs were used as controls for potential fluctuations in the overall protein levels. ERK1/2 and p38 phosphorylation was significantly increased in the CS group compared with the control group (P<0.05 and P<0.05, respectively; Fig. 5), butp38 and ERK1/2phosphorylationwas significantly reduced in the CS + ERY group compared with the CS group (P<0.05; Fig. 5). No significant changes in JNK phosphorylation levels in the CS group and CS + ERY group compared with those in the control group (Fig. 5).
Figure 5.
Effects of ERY on the expression of MAPK phosphorylation in CS-induced emphysema in rats by western blotting. (A) The protein expression of MAPKs and p-MAPKs as measured by western blotting. (B) For p-p38, p-ERK1/2, ERK1/2, p-JNK and JNK, both bands were quantified in the densitometry. Densitometrically analyzed target p-MAPKs bands normalized to MAPKs. Data are presented as the mean ± standard deviation. Control rats were exposed to normal air and received daily saline injections. CS rats were exposed to CS for 12 weeks. CS + ERY rats were exposed to CS for 12 weeks and received daily injections of 100 mg/kg ERY. *P<0.05 vs. control group and ΔP<0.05 vs. CS group; n=12 per group. MAPK, mitogen-activated protein kinase; p-, phospho-; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; CS, cigarette smoke; ERY, erythromycin.
Discussion
COPD is a lung disease characterized by progressive airflow limitation due to the inflammation-driven destruction of the alveolar walls (18). The increased expression of MMPs is considered to be a key factor in the development of COPD and emphysema (8,19). Because CS exposure is the biggest risk factor for the development of COPD, the present study aimed to investigate the effect of CS on the imbalance of metalloproteases/anti-metalloproteases, and the effect of ERY on the destruction of lung tissue and the inhibition of the imbalance of metalloproteases/anti-metalloproteases. In the present study, the expression of MMP-2 and MMP-9 was demonstrated to be increased by stimulation with CS in a rat model of CS-induced emphysema, while TIMP-1 expression was decreased. Previous studies have demonstrated that the inhibition of MMPs by macrolides may reduce the extracellular spread of inflammation (9,20). In the current study, the imbalance of metalloproteases/anti-metalloproteases was accompanied by altered levels of inflammatory cells and cytokines in BALF, while ERY reduced the imbalance of metalloproteases/anti-metalloproteases and the changes in the inflammatory cells and cytokines in BALF to some extent, which was also associated with the amelioration of the emphysema. The potential signaling pathway of ERY in the inhibition of the inflammation in CS-induced emphysema has not been previously clarified. One previous study demonstrated the protective role of ERY on CS-induced emphysema and its involvement in the reduction of inflammation, the imbalance of MMP-9/TIMP-1 and the apoptosis of lung structural cells (7). The results of the present study provided novel evidence for the protective effect of ERY on the development of emphysema induced by CS via regulation of the expression of MMP-2, MMP-9 and TIMP-1, and improvement of the inflammation of the lungs via MAPK/NF-κB activation, thus indicating its potential use as a therapeutic agent for the treatment of COPD. Similar studies on the potential signaling pathway of the macrolides were performed in an asthma model in vivo (21) or in inflammation-stimulated cells in vitro (22). MAPKs, together with NF-κB activation, are the kinases that are activated in airway epithelial cells and macrophages exposed to CS extracts, which induces the expression of various pro-inflammatory chemokines and cytokines (17). A study on a second-generation p38 MAPK inhibitor, SB681323, with the greatest selectivity for p38α, demonstrated that a single oral dose inhibited p-heat shock protein 27 and tumor necrosis factor-α (TNF-α) production in whole blood obtained from patients with COPD (23). In a 4-week treatment regime using this p38 inhibitor in patients with COPD who were not receiving inhaled corticosteroid therapy, a reduction in sputum neutrophils and in serum fibrinogen, but not in serum C-reactive protein, IL-8, IL-1β or IL-6 levels, was reported. This result was associated with an improvement in forced vital capacity but not in forced expiratory volume during the first second of a forced breath (23). ERK1/2 and JNK inhibition abrogated CS effects on heme oxygenase-1 expression and nuclear factor erythroid 2 like 2/BTB domain and CNC homolog 1 translocation to the nucleus (24). Therefore, it is thought that MAPKs are involved in the inflammatory responses induced by CS exposure, endotoxins and oxidative stress through the activation and release of pro-inflammatory cytokines/chemokines, post-translational regulation of these genes and activation of inflammatory cell migration (25). Inflammation in COPD is amplified by increased oxidative stress, which activates NF-κB, a transcription factor that orchestrates the expression of multiple inflammatory genes, including TNF-α, C-X-C motif chemokine ligand 8 (CXCL-8) and MMP-9 (26). The current study demonstrated that CS induced MAPK phosphorylation. The phosphorylation of p38 and ERK1/2 were increased in the CS-induced emphysema model in vivo compared with the control, while the oral administration of ERY suppressed CS-induced emphysema by regulating inflammatory cytokines and the MMP/anti-MMP imbalance that was associated with the changes in MAPKs.
A number of studies have indicated that NF-κB is regulated by macrolides (27–29). For example, azithromycin inhibits pro-inflammatory mediators, including CXCL-8, via inhibition of NF-κB in in vitro models (28,29). Solithromycin, another macrolide, inhibits activation of NF-κB under oxidative stress (30). Macrolides suppress the degradation of IκBα, an inhibitor of NF-κB, and/or affect the downstream dissociation from IκBα in the NF-κB signaling pathway (31). In the present study, CS activated the expression of NF-κB p65 and lower expression of IκBα was detected, while ERY inhibited the activation of NF-κB and promoted the expression of IκBα.
The results of the present study do not conclusively demonstrate whether MAPK and NF-κB are upstream or downstream of the signaling pathway. However, the changes identified in the present study indicated that there may be an association between the amelioration of emphysema when the emphysema model was treated with ERY and the changes in MAPK and NF-κB. In a previous study of acute and chronic CS exposure, the activation and expression of p38 MAPK in the lungs were compared between the following two mouse strains: C57BL/6 emphysema-susceptible mice and NZW emphysema-resistant mice: the selective p38 MAPK inhibitor, SB203580, ameliorated CS-induced lung inflammation and injury (32). ERY and clarithromycin inhibited CXCL-8 with inactivation of NF-κB and/or activator protein-1 in human bronchial epithelial cells in vitro (33,34). In one in vitro experiment, CS extract significantly induced ERK1/2 phosphorylation. PD98059, a specific inhibitor of ERK-MAPK, significantly blocked the effect of CS extract on ERK1/2 phosphorylation. Furthermore, PD98059 significantly inhibited the effect of CS extract on MMP-1 production and mRNA expression (35). These results indicate that CS may stimulate the production of and potentially activates MMP-1 through activation of the ERK1/2 signal transduction pathway. By inducing MMP-1, CS may result in excessive tissue destruction and may contribute to the development of emphysema (35).
In conclusion, CS may induce emphysema by simultaneously disturbing the balance of metalloproteases/anti-metalloproteases, increasing airway inflammation and upregulating MAPKs together with increased NF-κB activation signaling. Treatment with ERY reduced the development of emphysema via inhibition of the phosphorylation of MAPKs and the activation of NF-κB. These results revealed the anti-inflammatory role of ERY in CS-induced emphysema and airway inflammation, indicating that macrolides may have therapeutic potential for chronic airway inflammation and the associated emphysema caused by exposure to CS.
Acknowledgements
This research was supported by project 2012BAI05B01 from the Ministry of Science and Technology of the People's Republic of China, project 2014021031 from the Education Department of Liaoning Province and project 201409 from the Department of Liaoning Province.
Glossary
Abbreviations
- MAPK
mitogen-activated protein kinase
- ERK1/2
extracellular signal-regulated kinase-1/2
- JNK
c-Jun NH2-terminal kinase
- CS
cigarette smoke
- ERY
erythromycin
- MMP
matrix metalloprotease
- TIMP
tissue inhibitor of metalloprotease
References
- 1.Celli BR, MacNee W. ATS/ERS Task Force: Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 2.Nowak D, Berger K, Lippert B, Kilgert K, Caeser M, Sandtmann R. Epidemiology and health economics of COPD across Europe: A critical analysis. Treat Respir Med. 2005;4:381–395. doi: 10.2165/00151829-200504060-00003. [DOI] [PubMed] [Google Scholar]
- 3.Fragoso CA. Epidemiology of chronic obstructive pulmonary disease (COPD) in aging populations. COPD. 2016;13:125–129. doi: 10.3109/15412555.2015.1077506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, Connell C, Jemal A, Lee TA, Miravitlles M, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 5.Stockley RA, O'Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638–1645. doi: 10.1378/chest.117.6.1638. [DOI] [PubMed] [Google Scholar]
- 6.Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med. 2005;26:142–153. doi: 10.1055/s-2005-869535. [DOI] [PubMed] [Google Scholar]
- 7.Kwiatkowska S, Noweta K, Zieba M, Nowak D, Bialasiewicz P. Enhanced exhalation of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in patients with COPD exacerbation: A prospective study. Respiration. 2012;84:231–241. doi: 10.1159/000339417. [DOI] [PubMed] [Google Scholar]
- 8.Mocchegiani E, Giacconi R, Costarelli L. Metalloproteases/anti-metalloproteases imbalance in chronic obstructive pulmonary disease: Genetic factors and treatment implications. Curr Opin Pulm Med. 2011;17:S11–S19. doi: 10.1097/01.mcp.0000410743.98087.12. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 9.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzei T, Mini E, Novelli A, Periti P. Chemistry and mode of action of macrolides. J Antimicrob Chemother. 1993;31:S1–S9. doi: 10.1093/jac/31.suppl_C.1. (Suppl C) [DOI] [PubMed] [Google Scholar]
- 11.Perry DK, Hand WL, Edmondson DE, Lambeth JD. Role of phospholipase D-derived diradylglycerol in the activation of the human neutrophil respiratory burst oxidase. Inhibition by phosphatidic acid phosphohydrolase inhibitors. J Immunol. 1992;149:2749–2758. [PubMed] [Google Scholar]
- 12.Zhou Y, Tan X, Kuang W, Liu L, Wan L. Erythromycin ameliorates cigarette-smoke-induced emphysema and inflammation in rats. Transl Res. 2012;159:464–472. doi: 10.1016/j.trsl.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Birrell MA, Wong S, Catley MC, Belvisi MG. Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages. J Cell Physiol. 2008;214:27–37. doi: 10.1002/jcp.21158. [DOI] [PubMed] [Google Scholar]
- 14.Moretto N, Facchinetti F, Southworth T, Civelli M, Singh D, Patacchini R. alpha, beta-Unsaturated aldehydes contained in cigarette smoke elicit IL-8 release in pulmonary cells through mitogen-activated protein kinases. Am J Physiol Lung Cell Mol Physiol. 2009;296:L839–L848. doi: 10.1152/ajplung.90570.2008. [DOI] [PubMed] [Google Scholar]
- 15.Cheng SE, Luo SF, Jou MJ, Lin CC, Kou YR, Lee IT, Hsieh HL, Yang CM. Cigarette smoke extract induces cytosolic phospholipase A2 expression via NADPH oxidase, MAPKs, AP-1, and NF-kappaB in human tracheal smooth muscle cells. Free Radic Biol Med. 2009;46:948–960. doi: 10.1016/j.freeradbiomed.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Zheng H, Liu Y, Huang T, Fang Z, Li G, He S. Development and characterization of a rat model of chronic obstructive pulmonary disease (COPD) induced by sidestream cigarette smoke. Toxicol Lett. 2009;189:225–234. doi: 10.1016/j.toxlet.2009.06.850. [DOI] [PubMed] [Google Scholar]
- 17.Yin Y, Hou G, Li E, Wang Q, Kang J. PPARγ agonists regulate tobacco smoke-induced Toll like receptor 4 expression in alveolar macrophages. Respir Res. 2014;15:28. doi: 10.1186/1465-9921-15-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson JJ, Lutey BA, Suzuki Y, Toennies HM, Kelley DG, Kobayashi DK, Ijem WG, Deslee G, Moore CH, Jacobs ME, et al. The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema. Am J Respir Crit Care Med. 2011;183:876–884. doi: 10.1164/rccm.201005-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanai K, Asano K, Hisamitsu T, Suzaki H. Suppression of matrix metalloproteinase production from nasal fibroblasts by macrolide antibiotics in vitro. Eur Respir J. 2004;23:671–678. doi: 10.1183/09031936.04.00057104. [DOI] [PubMed] [Google Scholar]
- 21.Ci X, Chu X, Xu X, Li H, Deng X. Short-term roxithromycin treatment attenuates airway inflammation via MAPK/NF-κB activation in a mouse model of allergic asthma. Inflamm Res. 2012;61:749–758. doi: 10.1007/s00011-012-0470-6. [DOI] [PubMed] [Google Scholar]
- 22.Willems-Widyastuti A, Vanaudenaerde BM, Vos R, Dilisen E, Verleden SE, De Vleeschauwer SI, Vaneylen A, Mooi WJ, de Boer WI, Sharma HS, Verleden GM. Azithromycin attenuates fibroblast growth factors induced vascular endothelial growth factor via p38(MAPK) signaling in human airway smooth muscle cells. Cell Biochem Biophys. 2013;67:331–339. doi: 10.1007/s12013-011-9331-0. [DOI] [PubMed] [Google Scholar]
- 23.Singh D, Smyth L, Borrill Z, Sweeney L, Tal-Singer R. A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J Clin Pharmacol. 2010;50:94–100. doi: 10.1177/0091270009347873. [DOI] [PubMed] [Google Scholar]
- 24.Goven D, Boutten A, Leçon-Malas V, Boczkowski J, Bonay M. Prolonged cigarette smoke exposure decreases heme oxygenase-1 and alters Nrf2 and Bach1 expression in human macrophages: Roles of the MAP kinases ERK(1/2) and JNK. FEBS Lett. 2009;583:3508–3518. doi: 10.1016/j.febslet.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Chung KF. p38 mitogen-activated protein kinase pathways in asthma and COPD. Chest. 2011;139:1470–1479. doi: 10.1378/chest.10-1914. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Zeng S, Zou J, Chen Y, Yue Z, Gao Y, Zhang L, Cao W, Liu P. Rapamycin attenuated cardiac hypertrophy induced by isoproterenol and maintained energy homeostasis via inhibiting NF-κB activation. Mediators Inflamm. 2014;2014:868753. doi: 10.1155/2014/868753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aghai ZH, Kode A, Saslow JG, Nakhla T, Farhath S, Stahl GE, Eydelman R, Strande L, Leone P, Rahman I. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res. 2007;62:483–488. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura Y, Mitani A, Suga T, Kamiya Y, Kikuchi T, Tanaka S, Aino M, Noguchi T. Azithromycin may inhibit interleukin-8 through suppression of Rac1 and a nuclear factor-kappa B pathway in KB cells stimulated with lipopolysaccharide. J Periodontol. 2011;82:1623–1631. doi: 10.1902/jop.2011.100721. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y, Wada H, Rossios C, Takagi D, Higaki M, Mikura S, Goto H, Barnes PJ, Ito K. A novel macrolide solithromycin exerts superior anti-inflammatory effect via NF-κB inhibition. J Pharmacol Exp Ther. 2013;345:76–84. doi: 10.1124/jpet.112.200733. [DOI] [PubMed] [Google Scholar]
- 31.Aghai ZH, Kode A, Saslow JG, Nakhla T, Farhath S, Stahl GE, Eydelman R, Strande L, Leone P, Rahman I. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res. 2007;62:483–488. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 32.Marumo S, Hoshino Y, Kiyokawa H, Tanabe N, Sato A, Ogawa E, Muro S, Hirai T, Mishima M. p38 mitogen-activated protein kinase determines the susceptibility to cigarette smoke-induced emphysema in mice. BMC Pulm Med. 2014;14:79. doi: 10.1186/1471-2466-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe S, Nakamura H, Inoue S, Takeda H, Saito H, Kato S, Mukaida N, Matsushima K, Tomoike H. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding site in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2000;22:51–60. doi: 10.1165/ajrcmb.22.1.3400. [DOI] [PubMed] [Google Scholar]
- 34.Desaki M, Takizawa H, Ohtoshi T, Kasama T, Kobayashi K, Sunazuka T, Omura S, Yamamoto K, Ito K. Erythromycin suppresses nuclear factor-kappaB and activator protein-1 activation in human bronchial epithelial cells. Biochem Biophys Res Commun. 2000;267:124–128. doi: 10.1006/bbrc.1999.1917. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Liu X, Kohyama T, Kobayashi T, Conner H, Abe S, Fang Q, Wen FQ, Rennard SI. Cigarette smoke stimulates MMP-1 production by human lung fibroblasts through the ERK1/2 pathway. COPD. 2004;1:13–23. doi: 10.1081/COPD-120030164. [DOI] [PubMed] [Google Scholar]