Abstract
The advent of cranial implants revolutionized primate neurophysiological research because they allow researchers to stably record neural activity from monkeys during active behavior. Cranial implants have improved over the years since their introduction, but chronic implants still increase the risk for medical complications including bacterial contamination and resultant infection, chronic inflammation, bone and tissue loss and complications related to the use of dental acrylic. These complications can lead to implant failure and early termination of study protocols. In an effort to reduce complications, we describe several refinements that have helped us improve cranial implants and the wellbeing of implanted primates.
The rhesus macaque is a commonly used animal model in the field of neurophysiological research, and one important advancement in this field was the development of cranial implants, including head-holding devices and recording chambers. This equipment allows investigators to record the activity of individual neurons in an unanesthetized animal, permitting scientists to observe and test the correlation of electric activity in a single cell with perception and behavior. In 1966, Evarts described a technique that allowed extracellular recording of individual neurons in awake, unrestrained ani-mals1. This technique began with researchers aseptically preparing the skull and performing a craniotomy under general anesthesia. A rigid tube was then attached at the margins of the craniotomy site to support a microelec-trode carrier for advancing an electrode into the brain for neural recordings. This method allowed researchers to correlate the electrical activity of a single cell with the perception and behavior of an awake animal, and it completely transformed the field of neuroscience research during that time. Since its publication, many labs have adopted Evart's technique, and various refinements have been developed and introduced over time2–11. These methods continue to provide investigators with the necessary tools to collect data in neurophysiological studies, but there remains considerable potential for complications. It is imperative that researchers pursue further refinements that can reduce the occurrence of these complications, to uphold both animal welfare as well as research integrity. To this end we describe several recent refinements that have helped us improve the use of cranial implants and avoid common complications during primate research at the University of Pennsylvania.
Complications from bacterial contamination
Contamination is one of the foremost causes of deleterious side-effects when implanting abiotic materials, and bacterial and fungal infections have been reported in cephalic implants12–17. In one study at the University of Pennsylvania, researchers cultured the interiors of nine chronic cranial chambers for evidence of microbial contamination and identified 13 different pathogens, with coagulase-positive Staphylococcus spp., Corynebacterium spp., Enterococcus, coagulase-negative Staphylococcus spp. and Providencia rettgeri being the most common isolates (unpublished observations). Three of the chambers grew methicillin-resistant Staphylococcus aureus, which can lead to widespread infection owing to its resistance to treatment. Moreover, S. aureus is zoonotic and can be transmitted to humans that work with the infected animal.
In addition to the risk of localized microbial contamination, cranial implants can also allow infections to spread to deeper tissues of the cranium, including the calvarium and the dura. Infection of the calvarium can produce painful periosteal damage and bone degeneration that results in bone loss. Over the long term, infection of the calvarium can cause the skull to become thin, soft and spongy; this can allow the entire implant to become loose or dislodged and can even lead to failure of the implant12,18. Failure of the implant can lead to additional surgeries to repair or replace the implant, and even to euthanasia if the monkey can no longer participate in neurophysiological experiments. The spread of infection to the dura is also a serious concern because it can endanger the health of experimental animals, delay experiments, promote the formation of scar tissue and cause pain as the electrode penetrates the inflamed dura6,12,19. Other rare but possible complications that arise from the spread of a local infection include meningitis and brain abscesses20.
Avoiding bacterial contamination is not a simple matter of ensuring aseptic technique during the initial surgery, routine chamber maintenance, electrode placement and neural recordings. Indeed, there are problems inherent to current methods of cranial implantation that increase the risk of bacterial contamination. For example, the craniotomy procedure itself can be a source of contamination. When the dura is exposed during surgery and, subsequently, after placement of the chamber, it is normal for a small amount of extravas-cular fluid to leak into the craniotomy site from the surrounding tissues. This transudate provides an ideal environment for bacterial growth as it contains sugars, amino acids and other nutrients19. If bacteria successfully colonize the recording chamber, penetration of the electrode can further introduce organisms into the cortex17. Microbial colonization can also arise from the use of dental acrylic, which is traditionally used to anchor cranial implants, cover bone screws and create a base for the implants. This material often creates a gap between the cranial implants and the surface of the skull, and this gap is an ideal environment for bacterial colonization owing to the presence of heavily vascular-ized granulation tissue. Finally, a large base of dental acrylic creates a proportionally large border where the skin margin and the implant meet, which also increases the risk of bacterial colonization18.
Complications from dental acrylic
The use of traditional dental acrylic can cause additional complications beyond increasing the risk of bacterial contamination. Most commercially available dental acrylics are formulated using methyl methacrylate. As this material cures, the reaction that occurs is strongly exothermic, and the resulting heat can damage the bone18 as well as the underlying brain tissue21. The use of dental acrylic also promotes bleeding by causing dilation of superficial blood vessels; this can interfere with adherence of the acrylic to the bone screws12. Evidence also suggests that a monomer form of the acrylic material can leak from the base of cranial implants and cause toxic effects in the bone, including disturbances and standstill of blood-flow and intravascular hemoly-sis22. Lastly, researchers often score the bone surface to encourage bonding of the acrylic. This procedure improves binding even though dental acrylic is not very biocompatible with surrounding tissues; however it also compromises the bone and can prevent natural heal-ing18. The practice of bone scoring can lead to chronic inflammation, rejection of the implant and systemic disease sequelae23. In turn, chronic inflammation can cause amyloidosis with subsequent amyloid deposition in viscera, connective tissue and blood vessel walls that cause damage to the tissues24.
Complications of multiple surgeries
When failure or complications occur during the placement of cranial implants, additional surgeries might be necessary to correct or repair the implantation process. This presents another possible complication that accompanies current methods for placing cranial implants in macaques, as additional major surgeries entail significant risk to animal subjects and potential burdens for researchers. According to the Guide for the Care and Use of Laboratory Animals25, a major surgery is one that “penetrates and exposes a body cavity, produces substantial impairment of physical or physiologic function, or involves extensive tissue dissection or transection.” Multiple major survival surgeries increase the risk that an animal subject experiences anesthetic and post-surgical complications and post-surgical pain. In addition, federal regulations might restrict additional surgeries outright. The USDA Animal Welfare Act and Regulations and the Guide for the Care and Use of Laboratory Animals both stipulate that multiple major survival surgeries may be performed in animals only when they are required to meet essential components of a single research project or protocol. Even when that requirement is met, the principal investigator must provide a very detailed written scientific justification, and it must be approved by the institution's animal care and use committee25,26. If the complications cannot be corrected with an additional surgery, the animal can no longer be used for data collection and often the experiment must be terminated early.
At our institution, a craniotomy is classified as a major surgery because it involves opening the calvarium and exposing the dura. The placement of a head-holding device is also classified as a major surgery because it includes extensive dissection and transection of tissues. Implantation of head-holding devices and recording chambers in rhesus macaques entails potential complications that vary in severity from minor to significant, but all of these potential complications can adversely affect animal health and welfare as well as the integrity of their contextual research. Also, if during the initial surgery the recording chamber is placed incorrectly, a new craniotomy might be necessary to place a new chamber correctly. For these reasons, current methods for placing cranial implants can be complicated by the need for multiple major survival surgeries. It is therefore important that the research and laboratory animal medicine communities continue to develop and improve refinements to these techniques, to minimize or eliminate the likelihood of such complications.
Refinements to cranial implants
All of the procedures described below were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and were performed within a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. We have combined several techniques to refine the current practices in our facility by which cranial implants are placed. These refinements include integrating several technologies offered by an MRI-guidance system (Brainsight 2 Vet, Rogue Research, Montreal, Canada), using a surgical chair and C-clamp (Rogue Research, Montreal, Canada), using a piezoelectric drill (Synthes Piezoelectric System, Acteon, Mt. Laurel, NJ) to make the craniotomy and using a specific biocompatible bonding material (Geristore, DenMat, Lompoc, CA) to seal cranial implants to the skull.
MRI-guided surgeries
We have adopted an MRI-guided surgical procedure using the Brainsight 2 Vet guidance system to help reduce the risks associated with cranial implants and generally minimize the risk of multiple invasive surgeries, as might be required owing to poor chamber placement, for example. This system has introduced several technological advancements that contribute to refinements in the process of placing chronic cranial implants in macaques. The primary purpose of this system is to facilitate MRI-guided surgery and the design of customized cranial implants.
A primary benefit of using an MRI-guided system is the ability to plan the placement of the recording chambers before surgery. With the MRI-guided system, the surgeon can use MRI images that have been imported into the associated software to simulate the type of recording chamber and place it anywhere on the skull. Using this simulation, it is possible to view the trajectory of each grid mark into the brain (Fig. 1). This ensures that the surgeon can reach the correct recording targets during neurosurgery with an electrode placement error of 1.2-3.3 mm27,28. Once determined, the placement of the chamber can be recorded and saved in the software. The surgeon can then refer to this saved location during surgery and can use it to simulate electrode insertion in the MRI images. This simplifies data collection and reduces the time needed for electrode placement during surgery because the surgeon can determine the optimal location on the grid and depth of electrode placement before the first recording session. To further aid in surgical planning, a three-dimensional (3D) rendered image of the macaques skull can be used to 3D-print a full-size model of the skull (Fig. 2). These technologies substantially refine the processes of neurosurgery as they allow quick and accurate placement of electrodes and help to eliminate the need for additional surgeries due to incorrect placement of the recording chamber.
FIGURE 1.
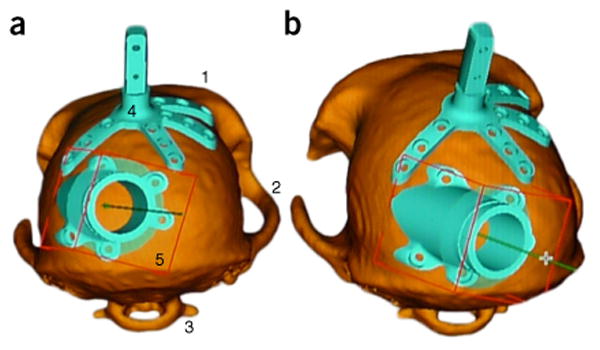
MRI-based 3D images for implants. (a) Posterior view and (b) slightly oblique view of a macaque's skull as simulated with the software of the Brainsight Vet 2 system. The 3D images were reconstructed from MRI images, and the surgeon has planned where to place the recording chamber in order to ensure that correct recording targets can be reached. Easily discernible features include (1) the brow ridge, (2) the zygomatic arches, (3) the C1 vertebra, (4) the head-holding device and (5) the recording chamber.
FIGURE 2.
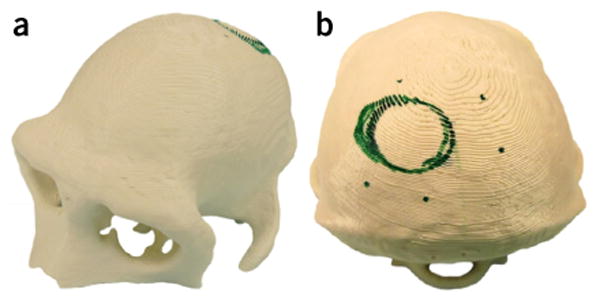
Example 3D-printed model, made from MRI images of a macaque's skull and used for realistic surgical planning. (a) Oblique anterior view and (b) posterior view of the model. Green marker shows the planned location of the recording chamber (circle) and screws (dots).
Dental imprint platform for fiducial markers
During MRI-guided surgery, researchers use fiducial markers when obtaining MRI images to help determine the location of targeted brain regions; traditionally, markers are implanted in bone for this calibration process. Such bone-implanted markers offer the best accuracy for neurosurgery, but they must be permanently implanted, which requires an additional and invasive surgical procedure27. As an alternative to bone-implanted markers, in the adopted system an array of fiducial markers is mounted to a dental imprint platform, allowing satisfactory calibration without requiring an additional invasive surgery. The dental imprint platform incorporates the maxillary teeth and hard palate, as this is the optimal impression technique for reducing positional and rotational errors29. The platform is made of thermoplastic dental material that is softened in hot water and placed on a T-shaped mouthpiece to make the impression (Fig. 3; ref. 27). Once the dental mold has hardened, it fits firmly into the animal's mouth and nothing further is needed to secure it. The mold can be placed into the mouth after intubation, and it does not interfere with the endotracheal tube because it fits snugly against the maxillary teeth and hard palate. This technique saves time for the research team and contributes to the welfare of our macaques by eliminating the need for an additional surgery to implant fiducial markers.
FIGURE 3.
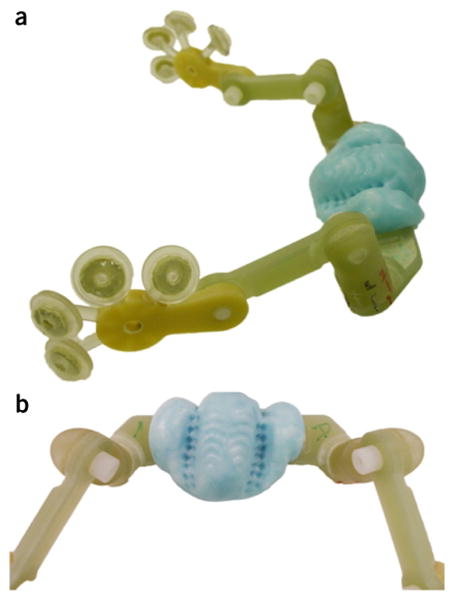
Fiducial marker array mounted to a dental imprint platform (Rogue Research, Montreal, Canada). (a) Side view and (b) a closer view of the dental imprint that is molded to the macaque's mouth, incorporating maxillary teeth and hard palate, before surgery. This platform allows researchers to attach a full array of fiducial markers for MRI-guidance without surgical implantation.
Frameless C-clamp and adjustable surgical chair
Using the MRI-guided system, we carry out the surgical procedure of cranial implantation with a frameless stereotaxic device. During the surgery the fiducial array is placed in the animals mouth to calibrate equipment to the location of the target brain region. Because the fiducial markers are targeted using MRI images and not standard stereotaxic coordinates, the macaque does not need to be placed in a classic stereotaxic frame. This eliminates the need for traditional stereotaxic ear-bars and eye-pieces, which improves the comfort and welfare of our macaques. Instead, we place macaques in a unique surgical chair and head holding apparatus (Figs. 4 and 5). This system allows for the flexible positioning of the animal and the ability to reach areas of the brain that are difficult to access using traditional stereotaxic frames28. The chair and frame can be adjusted to optimize the animals position for surgical procedures as well as anesthesia. The chair features an adjustable locking mechanism that allows the seat and backrest assembly to be adjusted independently28 and provides thick cushioning (Fig. 4). This provides the experimenter better access for procedures and minimizes the post-operative discomfort of the macaque. The animal's head is held in place using a C-clamp, which is a rigid semicircular head-holding apparatus designed specifically to fix the animal's head during all surgical procedures. The C-clamp assembly is attached to the animal's head using adjustable skull screws, which are tipped with removable, sterilized skull pins. The C-clamp is then carefully attached to the upright surgical chair with an adjustable mounting system and can then be locked into the appropriate position (Fig. 5; ref. 29). The arm that holds the C-clamp allows researchers to fix the head with a high degree of customizability. Together, the chair and C-clamp provide better access for the surgeon and for anesthesiology equipment, simplifying both anesthetic monitoring and the surgical procedure.
FIGURE 4.
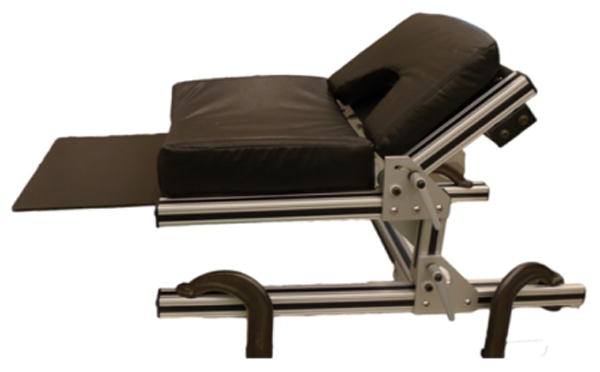
Adjustable surgical chair (Rogue Research, Montreal, Canada) that provides accessibility for the surgeon and for anesthesiology equipment. During surgery the macaque sits on the seat of the chair facing forward and reclines on the back of the chair.
FIGURE 5.

C-clamp (Rogue Research, Montreal, Canada) used to fix the head in position for MRI-guided surgery without a stereotaxic frame. Several pins fix the clamp to the skull and securely hold the animal's head in place. Three sensors attached to the C-clamp (left) can be picked up by a position sensor in the software of the Brainsight Vet 2 system to accurately determine the locations of different components of the cranial implant.
Custom-made chambers and head-holders
The previous generation of implant hardware needed to be attached to the skull through screws on attachment arms. Typically, with this equipment, researchers would bend the attachment arms during surgery so that they fit against the skull; but it is very difficult to create a flush fit between the skull and these titanium arms. Typically, a flush fit was not wholly accomplished, and considerable amounts of acrylic were needed to fix the implant securely. Using a current MRI-guided surgery system, we now order custom-made chambers and head-holding devices that are machined to fit, based on the MRI images of an individual macaque's skull. Consequently, these custom-made implants are designed to fit perfectly on the macaque's skull (Fig. 6). Additionally, because the head-holding devices and recording chambers fit flush against the skull, more of the screws that are used to secure the implant are actually seated into the skull. This greatly diminishes the need for bone cement or acrylic, reducing the overall size of the implant and lowering the risk of various complications that are associated with these materials. Furthermore, this minimizes the gap between the implant and the skull, so there is less space for granulation tissue to form and bacterial colonization to occur.
FIGURE 6.
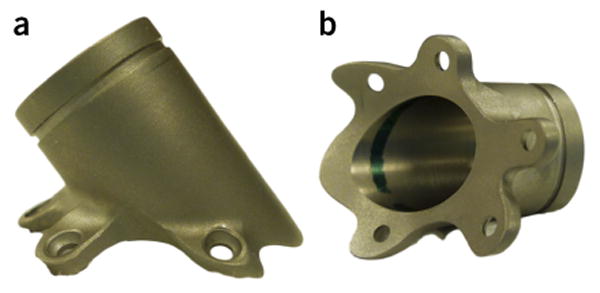
Example of a custom-fitted titanium recording chamber (Rogue Research, Montreal, Canada). (a) Side view and (b) bottom view of the contact surface of the chamber. Chambers are custom-made using a computer-aided design to fit the skull of each individual macaque.
Choice of bonding material
Traditionally, the dental acrylic methyl methacrylate has been widely used for sealing the implant chamber to prevent leaks and for covering bone screws. Given the aforementioned complications that can accompany this material, we decided to investigate an alternative bonding material that could improve our procedures. Geristore is a material that is commonly used as a restorative in dental procedures, and it is now being used in the place of methyl methacrylate. This material is a dual-cure, hydrophilic Bis-GMA hybrid ionomer composite. Because it is dual-cure, it will harden on its own after a period of time or it can be fixed using a handheld ultraviolet light. Neither reaction is exothermic, thereby avoiding the complications that arise from the highly exothermic reaction that takes place as methyl meth-acrylate cures. Importantly, Geristore is hydrophilic, so this dental composite bonds in the presence of moisture and blood, both of which are encountered in surgery. Additionally, owing to its hybrid ionomer composition, this bonding material is both strong and biocompatible. In studies that used Geristore with human periodon-tal ligament cells and human gingival fibroblasts, the bonding material showed superior biocompatibility, minimal cytotoxicity and excellent cellular attachment in comparison to other bonding materials, and the bonded tissue showed increased cell viability and proliferation30–33. Additionally, Geristore has a higher shear bond strength compared to more traditional dental restorative materials34,35. In our experience all of these properties make Geristore superior to traditional methyl methacrylate dental acrylics for securing cranial implants to bone screws and providing a sealant layer.
Piezoelectric drill
We have taken one last measure to refine our implant procedure by using a piezoelectric drill to make the craniotomy under the recording chamber. Traditionally, this was done using a trephine, but this method depends heavily on the skill and experience of the surgeon who is using the drill, and the shape of the resultant crani-otomy might not be perfectly round because it is difficult to make a circle on a rounded skull using a rigid tool. For this reason the use of a trephine increases the risk of damaging the underlying dura. Furthermore, trephines are available only in limited sizes, but with a piezoelectric drill a user can make a craniotomy of any size and shape allowing more flexibility and precision. Additionally, a piezoelectric drill cuts only bone tissue, without damaging soft tissue on contact36,37. For these reasons, the use of a piezoelectric drill is both faster for the surgeon and safer for the macaque.
Conclusions
The scientific community has greatly benefitted from the use of cranial implants in rhesus macaques for neurophysiological research. However, there are complications associated with the implantation and maintenance of cranial implants, and these can negatively affect for both animal welfare and research outcomes. Therefore, it is important to explore and employ advancements that can refine the processes of cranial implantation. We recommend that researchers consider the refinements that we have implemented in our procedures by combining technologies from multiple sources, which, in our experience, conserve time for the research team and improve the welfare of our animal subjects. By using an MRI-guided platform for cranial implants, we have greatly reduced the risk of incorrect chamber placement and the need for multiple invasive surgeries. By mapping out the exact location of the recording chamber before surgery, we have made the process of electrode placement quicker and more accurate. We have reduced the risks of implant instability and bacterial colonization by using custom-made implants that fit perfectly on the skull, which also reduces the need to use copious amounts of dental acrylic. During the surgical procedure, the surgical chair and C-clamp provide a more comfortable surgical and post-operative experience for the macaque, and using a piezoelectric drill is both faster for the surgeon and safer for the animal. Lastly, the use of Geristore instead of traditional dental acrylic reduces the incidence of complications caused by traditional acrylic because Geristore is more biocompatible and cures without producing heat. The combination of these refinements has allowed us to use smaller implants with fewer complications during neurophysiological research with our macaque colony. While these refinements are certainly a step in the right direction, it is important that the research and veterinary communities continue to explore and develop further refinements to this system in order to improve and protect both the welfare of animals and the integrity of research.
Footnotes
Competing Interests Statement: The authors declare no competing financial interests.
References
- 1.Evarts EV. Methods for recording activity of individual neurons in moving animals. In: Rushmer RF, editor. Methods in Medical Resesarch. Year Book Medical Publishers; Chicago, IL: 1966. pp. 241–250. [Google Scholar]
- 2.Adams DL, Economides JR, Jocson CM, Parker JM, Horton JC. A watertight acrylic-free titanium recording chamber for electrophysiology in behaving monkeys. J Neurophysiol. 2011;106:1581–1590. doi: 10.1152/jn.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betelak KF, Margiotti EA, Wohlford ME, Suzuki DA. The use of titanium implants and prosthodontic techniques in the preparation of non-human primates for long-term neuronal recording studies. J Neurosci Methods. 2001;112:9–20. doi: 10.1016/s0165-0270(01)00442-3. [DOI] [PubMed] [Google Scholar]
- 4.Foeller P, Tychsen L. Eye movement training and recording in alert macaque monkeys: 1. Operant visual conditioning; 2. Magnetic search coil and head restraint surgical implantation; 3. Calibration and recording. Strabismus. 2002;10:5–22. doi: 10.1076/stra.10.1.5.8154. [DOI] [PubMed] [Google Scholar]
- 5.Isoda M, et al. Design of a head fxation device for experiments in behaving monkeys. J Neurosci Methods. 2005;141:277–282. doi: 10.1016/j.jneumeth.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Kalwani RM, Bloy L, Elliott MA, Gold JI. A method for localizing microelectrode trajectories in the macaque brain using MRI. J Neurosci Methods. 2009;176:104–111. doi: 10.1016/j.jneumeth.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAndrew RM, Lingo VanGilder JL, Naufel SN, Helms Tillery SI. Individualized recording chambers for non-human primate neurophysiology. J Neurosci Methods. 2012;207:86–90. doi: 10.1016/j.jneumeth.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miocinovic S, et al. Stereotactic neurosurgical planning, recording, and visualization for deep brain stimulation in non-human primates. J Neurosci Methods. 2007;162:32–41. doi: 10.1016/j.jneumeth.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pigarev IN, Nothdurft HC, Kastner S. A reversible system for chronic recordings in macaque monkeys. J Neurosci Methods. 1997;77:157–162. doi: 10.1016/s0165-0270(97)00124-6. [DOI] [PubMed] [Google Scholar]
- 10.Rebert CS, Hurd RE, Matteucci MJ, De LaPaz R, Enzmann DR. A procedure for using proton magnetic resonance imaging to determine stereotaxic coordinates of the monkey's brain. J Neurosci Methods. 1991;39:109–113. doi: 10.1016/0165-0270(91)90076-c. [DOI] [PubMed] [Google Scholar]
- 11.Yakushin SB, Reisine H, Buttner-Ennever J, Raphan T, Cohen B. Functions of the nucleus of the optic tract (NOT). I. Adaptation of the gain of the horizontal vestibulo-ocular refex. Exp Brain Res. 2000;131:416–432. doi: 10.1007/s002219900303. [DOI] [PubMed] [Google Scholar]
- 12.Gardiner TW, Toth LA. Stereotactic surgery and long-term maintenance of cranial implants in research animals. Contemp Top Lab Anim Sci. 1999;38:52–63. [PubMed] [Google Scholar]
- 13.Dannemiller SD, Watson JR, Rozmiarek H. Fluconazole therapy in a rhesus monkey (Macaca mulatta) with epidural Trichosporon beigelii in a cephalic recording cylinder. Lab Anim Sci. 1995;45:31–35. [PubMed] [Google Scholar]
- 14.Lee G, Danneman P, Rufo R, Kalesnykas R, Eng V. Use of chlorine dioxide for antimicrobial prophylactic maintenance of cephalic recording devices in rhesus macaques (Macaca mulatta) Contemp Top Lab Anim Sci. 1998;37:59–63. [PubMed] [Google Scholar]
- 15.Bergin IL, Chien CC, Marini RP, Fox JG. Isolation and characterization of Corynebacterium ulcerans from cephalic implants in macaques. Comp Med. 2000;50:530–535. [PubMed] [Google Scholar]
- 16.Venezia J, et al. Characterization of Corynebacterium species in macaques. J Med Microbiol. 2012;61:1401–1408. doi: 10.1099/jmm.0.045377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newsome JT, Portnoy LG. Neuromuscular weakness in a baboon. Lab Anim Sci. 1999;49:349–357. [PubMed] [Google Scholar]
- 18.Adams DL, Economides JR, Jocson CM, Horton JC. A biocompatible titanium headpost for stabilizing behaving monkeys. J Neurophysiol. 2007;98:993–1001. doi: 10.1152/jn.00102.2007. [DOI] [PubMed] [Google Scholar]
- 19.Spitler KM, Gothard KM. A removable silicone elastomer seal reduces granulation tissue growth and maintains the sterility of recording chambers for primate neurophysiology. J Neurosci Methods. 2008;169:23–26. doi: 10.1016/j.jneumeth.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leblanc M, Berry K, McCort H, Reuter JD. Brain abscess in a rhesus macaque (Macaca mulatta) with a cephalic implant. Comp Med. 2013;63:367–372. [PMC free article] [PubMed] [Google Scholar]
- 21.Hamlen HJ, Olson E. Methylmethacrylate-associated thermal injury during cranioplasty in a nonhuman primate. Contemp Top Lab Anim Sci. 1995;34:73–75. [PubMed] [Google Scholar]
- 22.Albrektsson T, Linder L. Bone injury caused by curing bone cement. A vital microscopic study in the rabbit tibia. Clin Orthop Relat Res. 1984;183:280–287. [PubMed] [Google Scholar]
- 23.Straub RH. Systemic disease sequelae in chronic infammatory diseases and chronic psychological stress: comparison and pathophysiological model. Ann N Y Acad Sci. 2014;1318:7–17. doi: 10.1111/nyas.12409. [DOI] [PubMed] [Google Scholar]
- 24.Simons JP, et al. Pathogenetic mechanisms of amyloid A amyloidosis. Proc Natl Acad Sci USA. 2013;110:16115–20. doi: 10.1073/pnas.1306621110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. 8. National Academies Press; Washington, DC: 2011. [Google Scholar]
- 26.Animal Welfare Act Regulations. Code of Federal Regulations. Title 9, Part 3, Subpart D. [Google Scholar]
- 27.Chen AV, et al. Description and validation of a magnetic resonance imaging-guided stereotactic brain biopsy device in the dog. Vet Radiol Ultrasound. 2012;53:150–156. doi: 10.1111/j.1740-8261.2011.01889.x. [DOI] [PubMed] [Google Scholar]
- 28.Frey S, Comeau R, Hynes B, Mackey S, Petrides M. Frameless stereotaxy in the nonhuman primate. Neuroimage. 2004;23:1226–1234. doi: 10.1016/j.neuroimage.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Herbert CE, et al. Effect of bite tray impression technique on relocation accuracy in frameless stereotactic radiotherapy. Med Dosim. 2003;28:27–30. doi: 10.1016/S0958-3947(02)00142-5. [DOI] [PubMed] [Google Scholar]
- 30.Gupta SK, Saxena P, Pant VA, Pant AB. Adhesion and biologic behavior of human periodontal fibroblast cells to resin ionomer Geristore: a comparative analysis. Dent Traumatol. 2013;29:389–393. doi: 10.1111/edt.12016. [DOI] [PubMed] [Google Scholar]
- 31.Al-Sabek F, Shostad S, Kirkwood KL. Preferential attachment of human gingival fbroblasts to the resin ionomer Geristore. J Endod. 2005;31:205–208. doi: 10.1097/01.don.0000137650.61607.25. [DOI] [PubMed] [Google Scholar]
- 32.Al-Sa'eed OR, Al-Hiyasat AS, Darmani H. The effects of six root-end flling materials and their leachable components on cell viability. J Endod. 2008;34:1410–1414. doi: 10.1016/j.joen.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Al-Hiyasat AS, Al-Sa'eed OR, Darmani H. Quality of cellular attachment to various root-end flling materials. J Appl Oral Sci. 2010;20:82–88. doi: 10.1590/S1678-77572012000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arora R, Deshpande SD. Shear bond strength of resin-modifed restorative glass-ionomer cements to dentin—an in vitro study. J Indian Soc Pedod Prev Dent. 1998;16:130–133. [PubMed] [Google Scholar]
- 35.Chung CH, Brendlinger EJ, Brendlinger DL, Bernal V, Mante FK. Shear bond strengths of two resin-modifed glass ionomer cements to porcelain. Am J Orthod Dentofacial Orthop. 1999;115:533–535. doi: 10.1016/s0889-5406(99)70275-1. [DOI] [PubMed] [Google Scholar]
- 36.Eggers G, Klein J, Blank J, Hassfeld S. Piezosurgery: an ultrasound device for cutting bone and its use and limitations in maxillofacial surgery. Br J Oral Maxillofac Surg. 2004;42:451–453. doi: 10.1016/j.bjoms.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Leclercq P, Zenati C, Amr S, Dohan DM. Ultrasonic bone cut part 1: State-of-the-art technologies and common applications. J Oral Maxillofac Surg. 2008;66:177–182. doi: 10.1016/j.joms.2005.12.054. [DOI] [PubMed] [Google Scholar]


