Abstract
New data have been accumulated in the scientific literature in recent years which allow a more adequate risk assessment of selenium with reference to human health. This new evidence comes from environmental studies, carried out in populations characterized by abnormally high or low selenium intakes, and from high-quality and large randomized controlled trials with selenium recently carried out in the US and in other countries. These trials have consistently shown no beneficial effect on cancer and cardiovascular risk, and have yielded indications of unexpected toxic effects of selenium exposure. Overall, these studies indicate that the minimal amount of environmental selenium which is source of risk to human health is much lower than anticipated on the basis of older studies, since toxic effects were shown at levels of intake as low as around 260 µg/day for organic selenium and around 16 µg/day for inorganic selenium. Conversely, populations with average selenium intake of less than 13–19 µg/day appear to be at risk of a severe cardiomyopathy, Keshan disease. Overall, there is the need to reconsider the selenium standards for dietary intake, drinking water, outdoor and indoor air levels, taking into account the recently discovered adverse health effects of low-dose selenium overexposure, and carefully assessing the significance of selenium-induced proteomic changes.
Keywords: selenium, risk assessment, environment, epidemiology, biochemistry
1. Introduction
The health risk assessment of environmental selenium, concerning both abnormally low and high intakes, and the related regulatory guidelines are generally based on ‘old’ evidence, since they have generally been unable to take into consideration the most recent epidemiologic and biochemical evidence, and particularly the recent results of large and well-designed randomized controlled trials (RCTs) (1–3). The results of these trials, in connection with biochemical and toxicological studies, have shed new light on this relevant public health issue. This has happened with reference to both the upper and the lower limits of selenium intake, which have been so far based in all the assessment on observational studies carried out in seleniferous Chinese areas during the 1980s (4,5). The availability of the experimental studies (the trials) is of particular importance, since they allowed to rule out the key issue of (unmeasured) confounding, typically affecting most of observational studies with the possible only exception of the so called ‘natural experiments’ (6). In addition, the recent observational and experimental studies made it possible to investigate different populations with reference to age, genetic background and life-style factors, also allowing to test the health effect of selective exposure to specific selenium compounds, such as inorganic haxavalent selenium (selenate) and an organic form, selenomethionine. This is particularly important since there is growing evidence of the key importance of the specific selenium forms in influencing the biological activity of this element, with reference to both its toxicological and nutritional effects (7–11).
2. The epidemiologic evidence
A very large number of epidemiologic studies assessed the relation between chronic exposure to environmental selenium and human health. The studies on this issue frequently investigated the effect on human health of unusually low or high environmental exposures to selenium, due to an abnormal selenium content in soil, locally produced foods and drinking water, or following combustion of coal with high selenium content (5,12,13). In addition, the scientific literature encompasses a large number of nutritional epidemiology studies on the long-term health effects of selenium carried out in populations living in non-seleniferous regions and countries. These studies include experimental investigations (randomized controlled trials) and observational studies, the latter characterized by case-control, cohort, cross-sectional and ecologic design and being characterized by a far weaker ability compared with trials in addressing the selenium and health relation (1,14,15). While the entire review of this huge literature goes beyond the possibility of this report, we aim at briefly updating the evidence generated by the most recent environmental and nutritional studies on the human health effects of selenium, the biological plausibility of this relation, an overview of the challenges that these studies and their interpretation pose, and finally their implications on the adequacy of current environmental selenium standards. Our update of this issue starts from the comprehensive assessments of selenium exposure carried out by the US Institute of Medicine in 2000 (4) and by a World Health Organization (WHO) working group in 2004 (16).
Studies in populations living in unusually high and low selenium environments
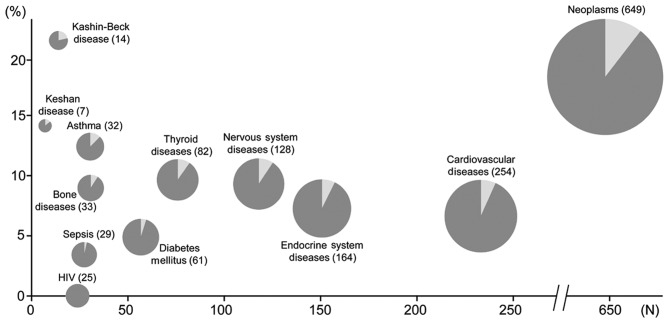
A large number of environmental studies which investigated the health effects of unusually high or low selenium areas have been published, as summarized in Table I. These studies have also substantially contributed to the PubMed-indexed papers on the epidemiology of selenium and human health in addition to the previous papers (Fig. 1), adding relevant data to our understanding of the health effects of selenium in humans. Some of these studies have been published after the 2000 Institute of Medicine selenium assessment (4), considerably extending the limited evidence previously available on the basis of a few ‘old’ Chinese studies. This literature includes the investigation of health effects of high-selenium environment in South and North America, India, China, and Italy. The high content of selenium in these areas, in most cases of geological origin, has induced unusually high levels of selenium in locally grown foodstuffs and occasionally in outdoor air and in drinking water, thus increasing human exposure to the element. However, systematic investigations of the health effects of such exposures are unfortunately limited, and in most cases they came from cross-sectional studies, and very rarely from studies with a more adequate design, such as case-control and particularly cohort studies. In addition, the observational design of these studies induces in most cases a major concern, the potential bias arising from unmeasured (dietary and life-style) confounding, in addition to the potential issue of exposure misclassification. Moreover, health endpoints were generally different in these studies, thus not allowing their systematic analysis (and meta-analysis) in the different populations. Finally, in several cases the small number of exposed subjects made it impossible to compute statistically stable estimates, and this lack of precision hampered the detection of potential health effects of such abnormally low and high exposures to environmental selenium.
Table I.
Overview of studies on the health effects of environmental selenium.
| A, Studies published up to 2000 | |||
|---|---|---|---|
| Area | Population study | Laboratory indications | Health effects |
| Colorado Tsongas 1977 and 1978 (99,100) | A community of 120 households receive tap water containing unusually high Se levels (50–125 µg/l). Eighty-six persons participate in this study. | Higher urinary levels of Se in the exposed group. Much higher urinary Se levels of women with a history of miscarriage. | No significant differences between exposed and unexposed groups. Lack of association between spontaneous abortion and Se exposure through drinking water in women experiencing miscarriages in that community. |
| Mianning, Sichuan Province, China Keshan Disease Research Group of the Chinese Academy of Medical Sciences, Bejing 1979 (18) | This study was carried out among children of susceptible age (1–9 years) in 1974 and 1975. One half of the children were given sodium selenite and the other half placebo. The subjects took sodium selenite once a week, the dosage being 1–5 year old 0.5 mg, 6–9 year old 1.0 mg and above 11 years 2.0 mg. In 1976–1977 all the subjects were given sodium selenite, no controls were used. | Not assessed. | In 1974, there were 13.5% cases of Keshan disease among control group, while only 2.2% subjects fell ill in the Se supplemented group. In 1975, there were 9.5% cases of Keshan disease in the control group, while only 1.0% cases in the treated group. In 1976 and 1977 there were respectively 0.32% and 0 cases of Keshan disease among treated subjects. |
| Milan, New Mexico Valentine 1980, 1988 and 1997 (101–103) | Thirty-three residents in a small community consumed drinking water from personal wells containing very high levels of Se (26–1800 µg/l). | High Se exposure through drinking water was associated with lower blood glutathione peroxidase activity. | Not assessed. |
| Enshi, Hubei Province, China Yang 1983 (55) | Endemic disease in 1961 in parts of the population of Enshi county. During 1961–1964, the morbidity was almost 50% in the 248 inhabitants of the most affected villages. Daily dietary intake of Se of 4.99 mg. | In high Se area of chronic selenosis, hair Se level was 32.2 µg/g and blood Se level was 3.2 µg/ml. | The tissues most affected during the time of heavy prevalence were loss of hair and nails, skin lesions, tooth decay and abnormalities of the nervous system like peripheral anesthesia, acroparaesthesia and pain in the extremities. |
| Red Butte and Jade Hills in Wyoming and Grants in New Mexico Valentine 1987 (104) | Fifty residents in three communities with unusually high Se content in their drinking water supply systems (respectively 494, 194, 327 µg/l) were compared to 99 individuals from Nevada and Wyoming communities which had drinking water with 3 and 2 µg/l Se, respectively. | Blood and hair Se levels were higher in exposed subjects but the differences were small despite the large difference in water Se levels. Differences in urine Se concentrations between exposed and unexposed subjects were much larger, though still less marked than the dif ference in water Se content. | Little evidence of a relation between Se exposure and risk for a number of gastrointestinal, cutaneous and nervous system conditions emerged. Higher prevalence of diarrhea, neurological diseases in the most exposed communities of Grants and Red Butte. |
| South Dakota and Wyoming Longnecker 1991 (105) | Inhabitants (142) of areas with endemic Se overexposure recruited over a 2-year period. About half of the 142 free-living subjects had selenium intake greater than 200 µg/day. | Se levels assessed using whole blood, serum, toenail, urine and dietary intake. | No effect of Se exposure on the risk of paresthesias was found (it actually decreased). By contrast, an increased risk of lethargy emerged since the OR of having this sign more frequently than the median for an increase of one standard deviation of whole blood, toenails or dietary Se was equal to 1.41, 1.41 and 1.43 respectively. |
| Portoguesa, Venezuela Brätter 1996 (106) | Sixty-five mothers living in three regions of different dietary Se intake level were examined. The range of dietary Se range in the Venezuelan seleniferous areas is 250–980 µg/day. | Mean serum TSH, thyroxin and tri-iodo-thyronine were in the normal range. Strong regional differences for serum Se and FT3, but no effect on the TSH and FT4 levels. | Long-term high dietary Se supply on the FT3 level in serum is associated with the depression in the activity of the selenoenzyme iodo-thyronine-deiodinase (5 DI), which catalyzes the production of T3 from T4. |
| Enshi, Hubei Province, China Fordyce 2000 (107) | Fifteen villages from 3 Se environments in Enshi district were investigated. Soil, grain, drinking water and human hair samples were collected from 5 Low-Se-Keshan-Disease villages (LK), 5 High-Se-Notoxicity (HN) villages, and 5 High-Se-Toxicity (HT) villages. | In the 5 HT villages were higher Se levels than NH villages, with geometric mean of Se in soil of 9.46 µg/g, in water of 32.6 µg/l and in the hair of 26.4 µg/g. | Despite the high concentrations of Se found in the population of the HN and HT villages, no incidence of selenosis have been reported in recent years in Enshi District. |
| Yu Tang Ba, Hubei Province, China Zhu 2001 (108) | Se was mainly present in the carbonaceous shale (stone coal) of this area. | In 1999, Se in soil was 4.75±7.43 mg/kg. Se in stream water was 58.4±16.8 µg/l. | Few people living in this area experienced loss of hair and nails from early 1930s to 1961. In 1963, 19 of 23 local inhabitants manifested symptoms of Se poisoning. |
| Punjab, India Hira 2003 (109) | Eighty subjects living in a seleniferous area compared to 80 controls living in non-seleniferous area. Se intake in the endemic area was 632±31.2 µg/day in men and 475±52.8 µg/day in women. | Concentration of Se in the hair, nails and urine samples in the study group were higher than control group. | 17.5% of men and 15% of women showed loss of hair and other Se related symptoms like tooth decay or black teeth. |
| Nunavik, Northern Quebec, Canada Saint-Amour 2006 (27) | One hundred and two Canadian Inuit children aged 5–6 years are involved in this study. The high consumption of fish and marine mammals by this population was associated to an unusually high intake of polychlorinated biphenyls, methyl-Hg, Se and other potentially neurotoxic substances. | The average blood Se concentration observed in the present study was about 5.6 mmol/l. Moreover, close to 20% of the children tested had blood Se concentrations exceeding the maximum safe level recommended for adults, which was from 8 to 10 mmol/l. Se umbilical blood level was 429 µg/l. | Neurotoxic signs, i.e. alterations in visual evoked potentials with the induction of longer latency of some of these parameters. |
| Lower Tapajos River region (Parà state), Brazil Lemire 2011 and 2012 (110,111) | A study on 448 residents aged 15–87 years in 12 communities. High level exposure to Se and Hg in these population derived from the consumption of a Se-rich diet of brazil nuts, fish species, meat and eggs. | Median plasma Se level was 135 µg/l. A direct association between Se plasma levels and motor performance was found. | These results appeared to disprove the detrimental effect of Se exposure on motor exposure on motor functions, but could also be due to confounding such as unmeasured heavy metals and other chemicals. |
| B, Studies published after 2000 | |||
| Shaanxi Province, China Lei 2011 (20) | Seventy-one Keshan disease (KD) patients were compared to 78 controls from the same endemic area and 212 external healthy controls from a non-endemic area. | Blood selenium level was 0.065±0.016 µg/ml in KD patients and 0.086±0.020 µg/ml in controls. GPx-1 activity was 73.002±12.623 U/g Hb) in KD patients and 106.402±24.268 U/g Hb) in controls. | Patients (24, 25, 20 and 2 KD) had NYHA class I, II, III, IV, respectively. All controls had NYHA class I. A large number of KD patients showed abnormal ECG, the most common disorders were conduction disorder and cardiac load. All chronic KD patients showed cardiac dilation on echocardiography. |
| Enshi, Hubei Province, China Qin 2013 (112) | Outbreak of human selenium poisoning in the early 1960s. Se-rich carbonaceous rock was responsible for high-Se content in soils, crops, water and thus human Se poisoning. The calculated daily intake of Se was 2144 µg/day, cereal consumption is the major pathway of Se intake for local residents, followed by vegetables, meats and drinking water. | Blood Se was 3248 µg/l. | Unusual signs and symptoms of Se poisoning were observed in this population. Neurological signs were found in 18 out of 22 rural residents affected by severe selenosis: acroparesthesia and dysestesia, hyperreflexia, convulsions, motor weakness and hemiplegia, polyneuritis. |
| Reggio Emilia, Northern Italy Vinceti 2013 and 2016 (5,26) | From 1974 to 1985, 2065 municipal residents consumed drinking water with high Se content approaching the European standard of 10 µg/l in its inorganic hexavalent form (selenate). | Not assessed. | Inorganic Se seemed to increase mortality from some site-specific cancers (melanoma, multiple myeloma, lymphoid neoplasms as well as colorectal and kidney cancer) and neurodegenerative diseases like Parkinson's disease and ALS, while lower breast cancer mortality was found. |
| Macapà (Amapà state) and Belem (Parà state), Brazil Martens 2015 (113) | Forty-one preschool children from Macapa and 88 preschool from Belem were enrolled. The Brazilian Amazon region is considered to have particularly Se-rich soil. Brazil nuts are often used as a strategy to improve Se status in Se-deficient populations. This study investigated Se intake and Se status of children from Macapa who received a Brazil nut-enriched diet and compared with children from Belem where Brazil nut supplementation did not occur. Mean Se intake in Macapa diet: 155.30 (range: 98.70–195.30) µg/day. | Children from Belem presented adequate plasma and erythrocytes levels, whereas the Macapà group had higher levels. Also nails and hair were more elevated in children from Macapà. | Se intake of children from two cities was adequate but the inclusion of Brazil nuts in Macapà diet resulted in excess Se dietary intake, although children from this city did not present symptoms of selenosis (i.e. changes to and loss of nails and hair, skin lesions, unusual garlic odor on the breath, nervous system defects). |
| Punjab, India Chawla 2016 (114) | Human subjects (650) living in a seleniferous area compared to 50 healty controls from a village in a non-seleniferous area. | Hair Se were 50.9±58.0 µg/g in the exposed group compared to 22.5±10.7 µg/g in controls. Corresponding Se levels (mean-SD) in nail clippings in the study and control groups were 154.0±91.5 µg/g and 117.4±49.8 µg/g. | Chronic exposure to high Se through the soil-plant water continuum could place the human population at risk of developing impaired organ function. |
Figure 1.
Number of hits (on the horizontal axis) generated by PubMed search on January 4, 2017 with the MeSH terms ‘selenium’ and ‘humans’ and (‘epidemiology’ or ‘epidemiologic methods’) linked to specific diseases (dark plus light gray areas). Those additionally linked with the additional MeSH terms ‘environment’ or ‘risk’ or ‘assessment and government regulation’ are in light gray (percentage on the total hits on the vertical axis).
Overall, these studies have yielded an indication that the extremely low selenium intake, in the order of <10-15 µg/day, may increase the risk of a severe cardiomyopathy named ‘Keshan disease’ (17–20), while high selenium intake may have unfavorable effects on the endocrine system and particularly on the thyroid status (21), and increase the risk of type 2 diabetes (3,22,23), some specific cancers such as melanoma and lymphoid cancers (24–26), and nervous system disturbances including alterations in visual evoked potentials (27) and excess risk of amyotrophic lateral sclerosis (26,28).
Studies in populations with ‘intermediate’ selenium status. Several studies have investigated the effects on human health of even limited changes in exposure to environmental selenium, which occurs through different environmental sources (primarily diet, but also air pollution, occupational environment, smoking and drinking water), in populations characterized by exposure levels not considered a priori to be unusually ‘low’ or ‘high’ (14). These studies, generally carried out in Western populations, have investigated a broad number of health outcomes, but in the majority of cases they focused on cancer risk (14,15). However, most of these studies had an observational design, thus suffering from the potential severe bias due to unmeasured confounding and exposure misclassification even in prospective cohort studies, in addition to the other biases typically effecting studies with case-control, cross-sectional and clearly ecologic design (1,14,29). In addition, their results have frequently been conflicting even for the same cancer type, as shown for instance for liver cancer (30,31), lung cancer (32–34) or breast cancer (35–38), though in most cases they supported the occurrence of an inverse relation between selenium status and cancer risk (1). Luckily and rather unexpectedly for a nutrient with also was known to exert a powerful toxicity, the nutritional interest in this metalloid as well as the extremely ‘attractive’ preliminary results of the first selenium trial carried out in Western countries, the Nutrition Prevention of Cancer (NPC) trial (39), a large number of randomized controlled trials have been conducted during the last two decades. The aim of these studies has been to investigate the effects on cancer risk of an increased intake of this element (1,40).
In Table II, we report the main features and results of these human studies with experimental design, including an assessment of the possible or established bias of this study based on our evaluation and the criteria developed within the Cochrane Collaboration network (41). In this overview, ‘old’ selenium trials carried out in China are also reported, but their scientific interest is very limited, if any, due to their very high risk of bias, as reported in detail in a previous assessment (1). Fortunately, these RCTs have generated a clear and consistent pattern of evidence about the effect of selenium on cancer risk, though partially unexpected given the underlying hypothesis which generated the trials, i.e. a beneficial effect of selenium on cancer risk (15). This is even more particularly with reference to the cancer type originally suggested by NPC to be most strongly associated with a beneficial effect of selenium, prostate cancer (39). In addition, these studies contributed in elucidating the relation between selenium and cardiovascular risk, another major issue of interest (42). Moreover, these trials have been fundamental in our understanding of the adverse effects of environmental selenium, rather unexpectedly since they encompassed supplementation of selenium doses considered a priori to be entirely safe (1,15). Therefore, and differently from other elements of comparable toxicity and of less nutritional interest, the risk assessment of environmental selenium has benefitted from the implementation of experimental studies originally designed for a setting of potential selenium deficiency, but later found to be able to show and identify the early signs and symptoms related to the toxicity of this element.
Table II.
Overview and main details of the randomized controlled trials with selenium supplementation in cancer prevention.
| Trial | Region | Populationa | Type of Se supplement | Median follow-up or duration | Risk of bias | Main results |
|---|---|---|---|---|---|---|
| PLC prevention in general population (115,116) | China | 130471 (20847/109624) Subjects at high risk to PLC | Se-salt tablet with 15 ppm anhydrous sodium selenite | Up to 5 years | Very high risk | Reduction of PLC incidence in township treated respect other four placebo treated |
| PLC prevention in HBsAg carriers (116,117) | China | 226 (113/113) Subjects HBsAg carriers in area with high PLC incidence | 200 µg/day selenized yeast | Up to 4 years | Very high risk | 0 and 5 PLC cases in Se supplement and placebo arms, respectively |
| PLC prevention in members of families with high PLC incidence (116) | China | 2474 (1444/1030) Members of families with high incidence of PLC | 200 µg/day selenized yeast | Up to 2 years | Very high risk | PLC: RRb 0.55 (95% CI 0.22–1.35) |
| PLC prevention in members of families with high PLC incidence (117) | China | 3849 (2364/1485) Members of families with high incidence of PLC | 200 µg/day selenized yeast | Up to 2 years | Very high risk | PLC: RRb 0.39 (95% CI 0.10–1.36) |
| PLC prevention in HBsAg carriers (118) | China | 2065 (1112/953) Subjects HBsAg carriers in area with high PLC incidence | 0.5 mg sodium selenite tablet | Up to 3 years | Very high risk | PLC: RRb 0.51 (95% CI 0.32–0.80) |
| NPC - Nutritional Prevention of Cancer study (49,52) | USA | 1250 (621/629) in 2002 Subjects with history of basal or squamous cell skin cancer | 200 µg/day high- high-selenium yeast | Mean 7.9 years up to 13 years (end of blinded period) | High risk | Any cancer: HR 0.75 (95% CI 0.58–0.97) Bladder cancer: HR 1.28 (95% CI 0.50–3.25) Breast cancer: HR 1.89 (95% CI 0.69–5.14) Colorectal cancer: HR 0.46 (95% CI 0.21–1.02) Lung cancer: HR 0.74 (95% CI 0.44–1.24) Melanoma: HR 1.18 (95% CI 0.49–2.85) NMSC: HR 1.17 (95% CI 1.02–1.34) Prostate cancer: HR 0.48 (95% CI 0.28–0.80) |
| Organ transplant recipients (50) | France | 184 (91/93) Organ graft (liver, kidney or heart) recipients aged 18–65 | 200 µg/day selenium enriched yeast | Up to 5 years (3 of treatment and 2 only of follow-up) | High risk | Skin keratoses: RRb 1.09 (95% CI 0.65–1.84) Skin cancers: RRb 3.07 (95% CI 0.55–31.06) |
| SELECT - Selenium and Vitamin E Cancer Prevention Trial (2,23,119,120) | USA, Canada and Puerto Rico | 17448 (8752/8696) Healthy men.50 years, not suspicious for cancer at digital rectal examination | 200 µg/day selenized yeast/ l-selenomethionine | Median 5.46 years | Low risk | Any cancer: HR 1.01 (99% CI 0.89–1.15) Bladder cancer: HR 1.13 (99% CI 0.78–1.63) Colorectal cancer: HR 1.05 (99% CI 0.66–1.67) Lung cancer: HR 1.12 (99% CI 0.73–1.72) Prostate cancer: HR 1.04 (99% CI 0.87–1.24) Cardiovascular events: HR 1.07 (99% CI 0.94–1.22) |
| BRCA1 carriers (51) | Poland | 1,135 Women BRCA1 carriers | 250 µg/day inorganic selenite | Median 2.92 years | Not evaluable | Any cancer: HR 1.4 (95% CI 0.9–2.0) Breast cancer: HR 1.3 (95% CI 0.7–2.5) |
| SWOG (Southwest Oncology Group) Trial S9917 (121) | USA | 423 (212/211) Men aged.40 years with biopsy-confirmed diagnosis of HGPIN but cancer free | 200 µg/day selenium | Up to 3 years | Low risk | Prostate cancer: RR 0.97 (95% CI 0.68–1.39) |
| NBT - Negative Biopsy Trial (44) | USA and New Zealand | 699 (467/232) Men aged <80 years at high risk for prostate cancer, negative for cancer or HGPIN | 234 with 200 µg/day and 233 with 400 µg/day selenized yeast | Median 3 years Up to 5 years | Low risk | Any cancer: RRb 0.98 (95% CI 0.64–1.52) Colon cancer: RRb 0.99 (95% CI 0.05–58.62) Melanoma: RRb 1.24 (95% CI 0.20–13.04) NMSC: RRb 0.57 (95% CI 0.35–0.95) Prostate cancer: RRb 0.98 (95% CI 0.64–1.52) |
| Eastern Cooperative Oncology Group (ECOG) Trial 5597 (45) | USA | 1,561 (1040/521) Adult subjects with resected Stage I Non-small cell lung cancer | 200 µg/day selenized yeast | Up to 4 years | Low risk | Secondary primary tumors: Any cancer: RRb 1.02 (95% CI 0.78–1.34) Bladder cancer: RRb 0.75 (95% CI 0.24–2.67) Colonrectal cancer: RRb 0.50 (95% CI 0.09–2.69) Lung cancer: RRb 1.26 (95% CI 0.77–2.15) Melanoma: RRb 1.25 (95% CI 0.21–13.15) NMSC: RRb 0.70 (95% CI 0.36–1.35) Prostate cancer: RRb 0.89 (95% CI 0.37–2.29) |
| Selenium and Celecoxib (Sel/Cel) Trial (3) | USA | 1,374 (685/689) subjects aged 40–80 years following colonoscopic removal of colorectal adenomas | 200 µg/day selenized yeast | Median 2.96 years | Low risk | Any adenoma: RR 1.03 (95% CI 0.91–1-16) Advanced adenoma: RR 1.02 (95% CI 0.74–1.43) Multiple (.3) adenoma: RR 1.47 (95% CI 1.08–2.02) Squamous cell ca: RR 1.34 (95% CI 0.76–2.37) |
HBsAg, hepatitis B virus surface antigen S; HGPIN, high-grade prostatic intraepithelial neoplasia; PLC, primary liver cancer; NMSC, non-melanoma skin cancer; NR, not reported; SPC, secondary primary cancer.
Number of total subjects in the trial (treatment/placebo) and study description.
Computed using the ‘iri’ routine of STATA 14.1 (Stata Corp., College Station, TX, USA).
Overall, all the recent trials have consistently shown that selenium does not modify risk of overall cancer, prostate cancer and other specific cancers (2,3,23,43–45), while it may even increase risk of cancers such as advanced (46,47) or overall prostate cancer (48), non-melanoma skin cancer (49,50) and possibly breast cancer in high-risk women (51). These results strongly and unexpectedly differ from the results reported in the earliest trial, the NPC (49,52), which however was small and more importantly was later found to be affected by a detection bias (53). As previously mentioned, these trials have also been of fundamental (and unforeseen) importance in identifying the early signs, symptoms and diseases associated with chronic or subchronic selenium toxicity. In fact, they have shown that already at amount of selenium exposure (baseline dietary intake plus supplementation) of around 250–300 µg/day there is an increased risk of type-2 diabetes. Such excess diabetes risk linked to selenium overexposure was first discovered in trial carried out in a population with a ‘low’ baseline selenium status (15,22) and later confirmed in large trials (3,23). Finally, the largest of the selenium RCTs, SELECT (23), whose overall selenium intake in the supplemented group averaged 300 µg/day (15), has shown that such amount of exposure induces ‘minor’ adverse effects such as dermatitis and alopecia [a long-recognized sign of selenium toxicity (12)]. These effects indicate that the selenium lower-observed-adverse-effect-level (LOAEL) is much lower than previously considered by regulatory agencies (5,54), which could base their assessment on the scarce data yielded by a few old Chinese environmental studies (55), calling for an update of the risk assessment of this element (5,15,56,57).
3. Adequacy of environmental standards
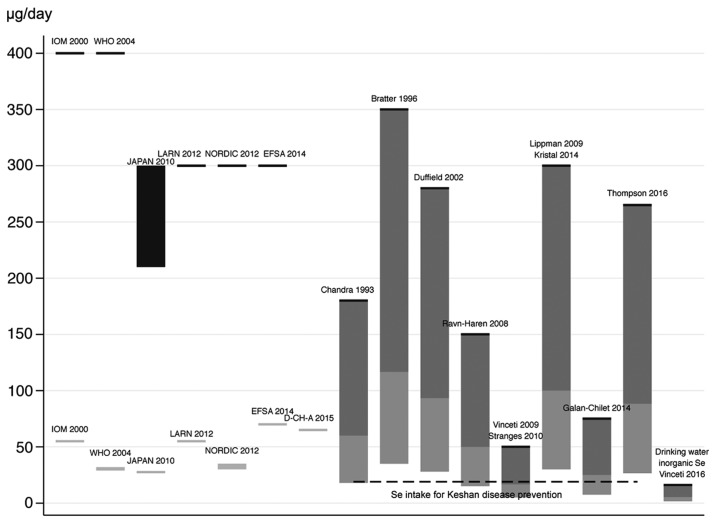
An issue therefore arises about the adequacy of current standards for environmental risk assessment of selenium in the human, for both abnormally low and high exposures. These standards have been defined by a number of agencies since 2000 to 2014, and as summarized in Fig. 2 they encompass minimal recommended values ranging from 30 to 70 µg/day, and upper doses ranging from 300 to 400 µg/day (in adults) for overall selenium exposure (4,16,58–61). On the contrary, specific guidelines for single selenium species have not been unfortunately set, despite the clear evidence that the various chemical forms of selenium have different biological properties, i.e. nutritional and toxicological activities (7,9,10,62).
Figure 2.
Comparison between environmental and dietary upper and lower standards for selenium (left) and the thresholds of adverse health effects of selenium (right) using an uncertain factor of 3 (dark grey) and of 10 (light gray). Data refer to daily overall selenium dietary intake.
So far, the adequacy of the selenium standards has been mainly based on biochemical endpoints (for the lowest recommended intake) and on the occurrence of adverse health outcomes (for the upper level), as identified in old studies carried out in seleniferous areas from China. However, the newly available data from the clinical trials indicate the need of a substantial reassessment of the dose of selenium toxicity, though they unfortunately do not allow to clearly identify a NOAEL and probably also a reliable LOAEL, since only one supplemental dose (200 µg/selenium/day) have been used in these trials and dose-response data are lacking. However, using an uncertainty factor as little as 3, i.e. lower that the uncertainty factors usually adopted in risk assessment (10 or more) also in light of the peculiar nature of this element and its nutritional relevance, selenium intake should not exceed 90 µg/day taking into account the signs of toxicity yielded by the NPC trial (an excess diabetes and skin cancer risk) and by the SELECT trial (an excess incidence of diabetes, advanced prostate cancer, dermatitis and alopecia) (1), as shown in Fig. 2. However, this estimate may be still inadequate to protect human health from chronic selenium toxicity, and in addition it appears to apply only to organic selenium, and to selenomethionine in particular [whose toxicity has bene recently much better elucidated (63–65)].
For inorganic selenium, typically selenate such as those found in underground and drinking waters, the epidemiologic evidence points to a much higher toxicity compared with organic selenium and exactly as expected on the basis of experimental studies (10), therefore suggesting much lower acceptable environmental standards (57), tentatively 1 µg/l for drinking water (5). New standards should also be considered for occupational exposure to selenium, given the limited data available and the potential for toxicity of this source of exposure (12,13,66,67). Finally, air selenium might represent a so far overlooked risk factor for chronic diseases, taking into account that its outdoor air concentrations have been positively associated with cardiovascular mortality (68) and with childhood leukemia risk (69), though more evidence is clearly required to confirm such possible associations mainly due to the inherent risk of unmeasured confounding in these observational studies.
The lowest acceptable amount of selenium exposure is instead much more controversial and uncertain. Two approaches have been used to define such lowest safe level of exposure: the proteomic change induced by the trace element, and the avoidance of adverse health effects. Concerning the latter point (health issues), still limited and inconclusive evidence is available on the large number of diseases tentatively ascribed to a deficiency of environmental selenium (4,70), such as the chronic degenerative osteoarthropathy with unclear etiology named ‘Kashin-Beck’ disease (71,72) and an increased susceptibility to viral infections (73,74).
In addition, the hypothesis of an effect of ‘low’ environmental selenium exposure in increasing cancer risk may now be ruled out, thanks to the consistent evidence yielded by the recent large and well-conducted randomized trials, which ruled out any preventive effect of selenium on cancer risk. On the converse, evidence exists on the involvement of selenium deficiency on the etiology of a rare but severe cardiomyopathy named Keshan disease and endemic in some Chinese areas (17,18,20,75–77), and this observation has played a key role in the identification of the minimal amount of selenium which appears to be required in humans (4,78). Such involvement has been suggested mainly on the basis of observational evidence, i.e. a lower selenium status in the populations more affected by this disease, and following the beneficial effects of a selenium supplementation trial on disease incidence.
However, some epidemiologic features of the disease have since the discovery of the disease suggested alternative etiologic hypotheses (79), particularly a cardiotropic infectious agent such as a Coxsackie virus, selenium deficiency possibly being a cofactor in disease etiology or simply an innocent bystander (19,20,54,77). Under this perspective, the beneficial effect of selenium supplementation in a Chinese trial might be interpreted as an indication of antiviral effects of the selenium compound used (inorganic tetravalent selenium, i.e. selenite), as suggested by laboratory studies (40,80). In any case, while still investigating the cause of Keshan disease and the possible involvement of selenium status, it is prudent to avoid a too low intake of selenium under the hypothesis of a role in Keshan disease etiology, and therefore average population intake must be higher than that shown to be required to avoid disease incidence, i.e. 13.3 µg/day in females and 19.1 in males (16). Finally, recent evidence has suggested adverse health effects of mutations affecting Sec insertion sequence-binding protein 2 or the selenoprotein N1 gene (81–83), though such abnormalities might not be strictly related to a ‘selenium deficiency’ neither were they corrected by its supplementation (84), thus being of limited interest in the setting of minimal dietary selenium requirements.
Alternatively, to the use of health endpoints, and considerably more frequently, the amount of the selenium needed to induce the maximization of selenoprotein synthesis (particularly glutathione-peroxidase and plasma selenoprotein P) has been proposed to set the minimal requirement of selenium in the human. This approach has been based on the assumption that achievement of this biochemical endpoint, i.e. upregulation (frequently defined as ‘optimization’) of selenoprotein synthesis indicates the achievement of an adequate supply of this trace element to the human (40,85). This would point to adequate dietary intake (considering this as only source of selenium exposure) of amount in the order of 70 µg/day (85), thus reaching or even exceeding the upper limit definable on the basis of the SELECT trial results using an uncertainty factor of 3 and clearly even more, of course, when using an uncertainty factor of 10 (Fig. 2). In addition, this ‘biochemical’ approach does not take into account that selenoprotein maximization which follows selenium species administration may derive not just from the ‘correction’ of a nutritional deficiency of the trace element, but as a compensatory response of these proteins (all characterized by antioxidant properties) to the pro-oxidant activity of selenium species (40,54,86–94).
There is also little evidence showing that selenoprotein activity, and particularly its maximization, are beneficial to human health, and therefore (as more generally levels for antioxidant enzymes) this should not be regarded as an objective unless more evidence in humans are provided (40,54). This approach is further strengthened when taking into account that these enzymes are physiologically induced and inducible by oxidative stress (for selenoproteins, even in the absence of any change in selenium supply) (40,54), as long recognized since the discovery of the selenium-containing antioxidant enzyme glutathione-peroxidase (95–97). Overall, it seems therefore prudent to avoid a maximal expression of selenoproteins (54,98), setting as standard a lower amount of their activity, such as proposed by WHO when suggesting a ‘nutritionally adequate’ target (‘recommended nutrient intake’) the achievement of two thirds of the maximal selenoprotein activity, corresponding to a daily selenium intake of 25–34 µg in adults (Fig. 2). However, more research is clearly required to set reliable lower and upper safe selenium levels, though the current standards need to be quickly updated with reference to the upper levels taking into account the above-mentioned recent results of the epidemiologic studies, i.e. the high-quality RCTs and the environmental studies, and also considering the opportunity to set species-specific standards for this element.
References
- 1.Vinceti M, Dennert G, Crespi CM, Zwahlen M, Brinkman M, Zeegers MP, Horneber M, D'Amico R, Del Giovane C. Selenium for preventing cancer. Cochrane Database Syst Rev. 2014;3:CD005195. doi: 10.1002/14651858.CD005195.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lance P, Alberts DS, Thompson PA, Fales L, Wang F, Jose San J, Jacobs ET, Goodman PJ, Darke AK, Yee M, et al. Colorectal adenomas in participants of the SELECT randomized trial of selenium and vitamin E for prostate cancer prevention. Cancer Prev Res (Phila) 2017;10:45–54. doi: 10.1158/1940-6207.CAPR-16-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson PA, Ashbeck EL, Roe DJ, Fales L, Buckmeier J, Wang F, Bhattacharyya A, Hsu CH, Chow HH, Ahnen DJ, et al. Selenium supplementation for prevention of colorectal adenomas and risk of associated type 2 diabetes. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw152. pii: djw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine Food and Nutrition Board: Dietary references intakes for vitamin C, vitamin E, selenium, and carotenoids. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- 5.Vinceti M, Crespi CM, Bonvicini F, Malagoli C, Ferrante M, Marmiroli S, Stranges S. The need for a reassessment of the safe upper limit of selenium in drinking water. Sci Total Environ. 2013;443:633–642. doi: 10.1016/j.scitotenv.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Lippincott Williams & Wilkins; Philadelphia: 2012. [DOI] [Google Scholar]
- 7.Michalke B, Halbach S, Nischwitz V. JEM spotlight: Metal speciation related to neurotoxicity in humans. J Environ Monit. 2009;11:939–954. doi: 10.1039/b817817h. [DOI] [PubMed] [Google Scholar]
- 8.Gammelgaard B, Jackson MI, Gabel-Jensen C. Surveying selenium speciation from soil to cell--forms and transformations. Anal Bioanal Chem. 2011;399:1743–1763. doi: 10.1007/s00216-010-4212-8. [DOI] [PubMed] [Google Scholar]
- 9.Weekley CM, Harris HH. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem Soc Rev. 2013;42:8870–8894. doi: 10.1039/c3cs60272a. [DOI] [PubMed] [Google Scholar]
- 10.Vinceti M, Solovyev N, Mandrioli J, Crespi CM, Bonvicini F, Arcolin E, Georgoulopoulou E, Michalke B. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology. 2013;38:25–32. doi: 10.1016/j.neuro.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinceti M, Grill P, Malagoli C, Filippini T, Storani S, Malavolti M, Michalke B. Selenium speciation in human serum and its implications for epidemiologic research: A cross-sectional study. J Trace Elem Med Biol. 2015;31:1–10. doi: 10.1016/j.jtemb.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Vinceti M, Wei ET, Malagoli C, Bergomi M, Vivoli G. Adverse health effects of selenium in humans. Rev Environ Health. 2001;16:233–251. doi: 10.1515/REVEH.2001.16.4.233. [DOI] [PubMed] [Google Scholar]
- 13.Vinceti M, Mandrioli J, Borella P, Michalke B, Tsatsakis A, Finkelstein Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol Lett. 2014;230:295–303. doi: 10.1016/j.toxlet.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Vinceti M, Crespi CM, Malagoli C, Del Giovane C, Krogh V. Friend or foe? The current epidemiologic evidence on selenium and human cancer risk. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2013;31:305–341. doi: 10.1080/10590501.2013.844757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinceti M, Burlingame B, Fillippini T, Naska A, Bargellini A, Borella P. The epidemiology of selenium and human health. In: Hatfield D, Schweizer U, Gladyshev VN, editors. Selenium: Its Molecular Biology and Role in Human Health. 4th. Springer Science Business Media; New York: 2016. pp. 365–376. [DOI] [Google Scholar]
- 16.Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements: Vitamin and mineral requirements in human nutrition. 2nd. World Health Organization and Food and Agriculture Organization of the United Nations; Geneva: 2004. [Google Scholar]
- 17.Keshan Disease Research Group of the Chinese Academy of Medical Sciences: Epidemiologic studies on the etiologic relationship of selenium and Keshan disease. Chin Med J (Engl) 1979;92:477–482. [PubMed] [Google Scholar]
- 18.Keshan Disease Research Group of the Chinese Academy of Medical Sciences: Observations on effect of sodium selenite in prevention of Keshan disease. Chin Med J (Engl) 1979;92:471–476. [PubMed] [Google Scholar]
- 19.Wang T, Li Q. The 5th International Selenium Seminar. Organizing Committee of the International Selenium Seminar; Moscow: 2015. Interpretation of selenium deficiency and Keshan disease with causal inference of modern epidemiology; pp. 34–35. [Google Scholar]
- 20.Lei C, Niu X, Ma X, Wei J. Is selenium deficiency really the cause of Keshan disease? Environ Geochem Health. 2011;33:183–188. doi: 10.1007/s10653-010-9331-9. [DOI] [PubMed] [Google Scholar]
- 21.Winther KH, Bonnema SJ, Cold F, Debrabant B, Nybo M, Cold S, Hegedüs L. Does selenium supplementation affect thyroid function? Results from a randomized, controlled, double-blinded trial in a Danish population. Eur J Endocrinol. 2015;172:657–667. doi: 10.1530/EJE-15-0069. [DOI] [PubMed] [Google Scholar]
- 22.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: A randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 23.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinceti M, Rovesti S, Gabrielli C, Marchesi C, Bergomi M, Martini M, Vivoli G. Cancer mortality in a residential cohort exposed to environmental selenium through drinking water. J Clin Epidemiol. 1995;48:1091–1097. doi: 10.1016/0895-4356(95)00014-U. [DOI] [PubMed] [Google Scholar]
- 25.Vinceti M, Rothman KJ, Bergomi M, Borciani N, Serra L, Vivoli G. Excess melanoma incidence in a cohort exposed to high levels of environmental selenium. Cancer Epidemiol Biomarkers Prev. 1998;7:853–856. [PubMed] [Google Scholar]
- 26.Vinceti M, Ballotari P, Steinmaus C, Malagoli C, Luberto F, Malavolti M, Rossi Giorgi P. Long-term mortality patterns in a residential cohort exposed to inorganic selenium in drinking water. Environ Res. 2016;150:348–356. doi: 10.1016/j.envres.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Saint-Amour D, Roy MS, Bastien C, Ayotte P, Dewailly E, Després C, Gingras S, Muckle G. Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. Neurotoxicology. 2006;27:567–578. doi: 10.1016/j.neuro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Vinceti M, Bonvicini F, Rothman KJ, Vescovi L, Wang F. The relation between amyotrophic lateral sclerosis and inorganic selenium in drinking water: A population-based case-control study. Environ Health. 2010;9:77. doi: 10.1186/1476-069X-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinceti M, Rothman KJ. More results but no clear conclusion on selenium and cancer. Am J Clin Nutr. 2016;104:245–246. doi: 10.3945/ajcn.116.139469. [DOI] [PubMed] [Google Scholar]
- 30.Hughes DJ, Duarte-Salles T, Hybsier S, Trichopoulou A, Stepien M, Aleksandrova K, Overvad K, Tjønneland A, Olsen A, Affret A, et al. Prediagnostic selenium status and hepatobiliary cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2016;104:406–414. doi: 10.3945/ajcn.116.131672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma X, Yang Y, Li HL, Zheng W, Gao J, Zhang W, Yang G, Shu XO, Xiang YB. Dietary trace element intake and liver cancer risk: Results from two population-based cohorts in China. Int J Cancer. 2017;140:1050–1059. doi: 10.1002/ijc.30522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knekt P, Marniemi J, Teppo L, Heliövaara M, Aromaa A. Is low selenium status a risk factor for lung cancer? Am J Epidemiol. 1998;148:975–982. doi: 10.1093/oxfordjournals.aje.a009574. [DOI] [PubMed] [Google Scholar]
- 33.van den Brandt PA, Goldbohm RA, van't Veer P, Bode P, Dorant E, Hermus RJ, Sturmans F. A prospective cohort study on selenium status and the risk of lung cancer. Cancer Res. 1993;53:4860–4865. [PubMed] [Google Scholar]
- 34.Suadicani P, Hein HO, Gyntelberg F. Serum selenium level and risk of lung cancer mortality: A 16-year follow-up of the Copenhagen Male Study. Eur Respir J. 2012;39:1443–1448. doi: 10.1183/09031936.00102711. [DOI] [PubMed] [Google Scholar]
- 35.Menkes MS, Comstock GW, Vuilleumier JP, Helsing KJ, Rider AA, Brookmeyer R. Serum beta-carotene, vitamins A and E, selenium, and the risk of lung cancer. N Engl J Med. 1986;315:1250–1254. doi: 10.1056/NEJM198611133152003. [DOI] [PubMed] [Google Scholar]
- 36.van Noord PA, Maas MJ, van der Tweel I, Collette C. Selenium and the risk of postmenopausal breast cancer in the DOM cohort. Breast Cancer Res Treat. 1993;25:11–19. doi: 10.1007/BF00662396. [DOI] [PubMed] [Google Scholar]
- 37.van den Brandt PA, Goldbohm RA, van't Veer P, Bode P, Dorant E, Hermus RJ, Sturmans F. Toenail selenium levels and the risk of breast cancer. Am J Epidemiol. 1994;140:20–26. doi: 10.1093/oxfordjournals.aje.a117155. [DOI] [PubMed] [Google Scholar]
- 38.Garland M, Morris JS, Stampfer MJ, Colditz GA, Spate VL, Baskett CK, Rosner B, Speizer FE, Willett WC, Hunter DJ. Prospective study of toenail selenium levels and cancer among women. J Natl Cancer Inst. 1995;87:497–505. doi: 10.1093/jnci/87.7.497. [DOI] [PubMed] [Google Scholar]
- 39.Clark LC, Combs GFJ, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, et al. Nutritional Prevention of Cancer Study Group: Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. JAMA. 1996;276:1957–1963. doi: 10.1001/jama.276.24.1957. [DOI] [PubMed] [Google Scholar]
- 40.Jablonska E, Vinceti M. Selenium and Human Health: Witnessing a Copernican Revolution? J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33:328–368. doi: 10.1080/10590501.2015.1055163. [DOI] [PubMed] [Google Scholar]
- 41.Higgins JP. A revised tool to assess risk of bias in randomized trials. 2016 (RoB 2.0) [Google Scholar]
- 42.Rees K, Hartley L, Day C, Flowers N, Clarke A, Stranges S. Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD009671. doi: 10.1002/14651858.CD009671.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stratton MS, Reid ME, Schwartzberg G, Minter FE, Monroe BK, Alberts DS, Marshall JR, Ahmann FR. Selenium and prevention of prostate cancer in high-risk men: The Negative Biopsy Study. Anticancer Drugs. 2003;14:589–594. doi: 10.1097/00001813-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Algotar AM, Stratton MS, Ahmann FR, Ranger-Moore J, Nagle RB, Thompson PA, Slate E, Hsu CH, Dalkin BL, Sindhwani P, et al. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate. 2013;73:328–335. doi: 10.1002/pros.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karp DD, Lee SJ, Keller SM, Wright GS, Aisner S, Belinsky SA, Johnson DH, Johnston MR, Goodman G, Clamon G, et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: ECOG 5597. J Clin Oncol. 2013;31:4179–4187. doi: 10.1200/JCO.2013.49.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM, Meyskens FL, Jr, Goodman GE, Minasian LM, Parnes HL, et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J Natl Cancer Inst. 2014;106:djt456. doi: 10.1093/jnci/djt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albanes D, Till C, Klein EA, Goodman PJ, Mondul AM, Weinstein SJ, Taylor PR, Parnes HL, Gaziano JM, Song X, et al. Plasma tocopherols and risk of prostate cancer in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Cancer Prev Res (Phila) 2014;7:886–895. doi: 10.1158/1940-6207.CAPR-14-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez EE, Darke AK, Tangen CM, Goodman PJ, Fowke JH, Klein EA, Abdulkadir SA. A functional variant in NKX3.1 associated with prostate cancer risk in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Cancer Prev Res (Phila) 2014;7:950–957. doi: 10.1158/1940-6207.CAPR-14-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duffield-Lillico AJ, Slate EH, Reid ME, Turnbull BW, Wilkins PA, Combs GF, Jr, Park HK, Gross EG, Graham GF, Stratton MS, et al. Nutritional Prevention of Cancer Study Group: Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477–1481. doi: 10.1093/jnci/djg061. [DOI] [PubMed] [Google Scholar]
- 50.Dréno B, Euvrard S, Frances C, Moyse D, Nandeuil A. Effect of selenium intake on the prevention of cutaneous epithelial lesions in organ transplant recipients. Eur J Dermatol. 2007;17:140–145. doi: 10.1684/ejd.2007.0127. [DOI] [PubMed] [Google Scholar]
- 51.Lubinski J, Jaworska K, Durda K, Jakubowska A, Huzarski T, Byrski T, Stawicka M, Gronwald J, Górski B, Wasowicz W, et al. Selenium and the risk of cancer in BRCA1 carriers. Hered Cancer Clin Pract. 2011;9:A5. doi: 10.1186/1897-4287-9-S2-A5. (Suppl 2) [DOI] [Google Scholar]
- 52.Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GFJ, Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: A summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 53.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET, Marshall JR, Clark LC. Nutritional Prevention of Cancer Study Group: Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–612. doi: 10.1046/j.1464-410X.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 54.Vinceti M, Maraldi T, Bergomi M, Malagoli C. Risk of chronic low-dose selenium overexposure in humans: Insights from epidemiology and biochemistry. Rev Environ Health. 2009;24:231–248. doi: 10.1515/REVEH.2009.24.3.231. [DOI] [PubMed] [Google Scholar]
- 55.Yang GQ, Wang SZ, Zhou RH, Sun SZ. Endemic selenium intoxication of humans in China. Am J Clin Nutr. 1983;37:872–881. doi: 10.1093/ajcn/37.5.872. [DOI] [PubMed] [Google Scholar]
- 56.Barron E, Migeot V, Rabouan S, Potin-Gautier M, Séby F, Hartemann P, Lévi Y, Legube B. The case for re-evaluating the upper limit value for selenium in drinking water in Europe. J Water Health. 2009;7:630–641. doi: 10.2166/wh.2009.097. [DOI] [PubMed] [Google Scholar]
- 57.Frisbie SH, Mitchell EJ, Sarkar B. Urgent need to reevaluate the latest World Health Organization guidelines for toxic inorganic substances in drinking water. Environ Health. 2015;14:63. doi: 10.1186/s12940-015-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.European Commission. Brussels: 2000. Scientific Committee on Food: Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Selenium. [Google Scholar]
- 59.Tsubota-Utsugi M, Imai E, Nakade M, Tsuboyama-Kasaoka N, Morita A, Tokudome S. National Institute of Health and Nutrition. Japan: 2012. Dietary Reference Intakes for Japanese - 2010. The summary report from the Scientific Committee of ‘Dietary Reference intakes for Japanese’. [Google Scholar]
- 60.Nordic Nutrition Recommendations 2012. 5th. Nordic Council of Ministers; Copenhagen: 2014. doi: org/10.6027/Nord2014-002. [Google Scholar]
- 61.SICS. Milan: 2014. Società Italiana di Nutrizione Umana: LARN - Livelli di assunzione di riferimento di nutrienti e energia per la popolazione italiana - IV revisione. (In Italian) [Google Scholar]
- 62.Marschall TA, Bornhorst J, Kuehnelt D, Schwerdtle T. Differing cytotoxicity and bioavailability of selenite, methylselenocysteine, selenomethionine, selenosugar 1 and trimethylselenonium ion and their underlying metabolic transformations in human cells. Mol Nutr Food Res. 2016;60:2622–2632. doi: 10.1002/mnfr.201600422. [DOI] [PubMed] [Google Scholar]
- 63.Lazard M, Dauplais M, Blanquet S, Plateau P. Trans-sulfuration pathway seleno-amino acids are mediators of selenomethionine toxicity in Saccharomyces cerevisiae. J Biol Chem. 2015;290:10741–10750. doi: 10.1074/jbc.M115.640375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raine JC, Lallemand L, Pettem CM, Janz DM. Effects of chronic dietary selenomethionine exposure on the visual system of adult and F1 generation zebrafish (Danio rerio) Bull Environ Contam Toxicol. 2016;97:331–336. doi: 10.1007/s00128-016-1849-9. [DOI] [PubMed] [Google Scholar]
- 65.Dolgova NV, Hackett MJ, MacDonald TC, Nehzati S, James AK, Krone PH, George GN, Pickering IJ. Distribution of selenium in zebrafish larvae after exposure to organic and inorganic selenium forms. Metallomics. 2016;8:305–312. doi: 10.1039/C5MT00279F. [DOI] [PubMed] [Google Scholar]
- 66.Göen T, Schaller B, Jäger T, Bräu-Dümler Ch, Schaller KH, Drexler H. Biological monitoring of exposure and effects in workers employed in a selenium-processing plant. Int Arch Occup Environ Health. 2015;88:623–630. doi: 10.1007/s00420-014-0989-7. [DOI] [PubMed] [Google Scholar]
- 67.Jäger T, Drexler H, Göen T. Human metabolism and renal excretion of selenium compounds after oral ingestion of sodium selenate dependent on trimethylselenium ion (TMSe) status. Arch Toxicol. 2016;90:149–158. doi: 10.1007/s00204-014-1380-x. [DOI] [PubMed] [Google Scholar]
- 68.Ito K, Mathes R, Ross Z, Nádas A, Thurston G, Matte T. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect. 2011;119:467–473. doi: 10.1289/ehp.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heck JE, Park AS, Qiu J, Cockburn M, Ritz B. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int J Hyg Environ Health. 2014;217:662–668. doi: 10.1016/j.ijheh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burk RF., Jr Selenium deficiency in search of a disease. Hepatology. 1988;8:421–423. doi: 10.1002/hep.1840080240. [DOI] [PubMed] [Google Scholar]
- 71.Yu FF, Liu H, Guo X. Integrative multivariate logistic regression analysis of risk factors for Kashin-Beck disease. Biol Trace Elem Res. 2016;174:274–279. doi: 10.1007/s12011-016-0712-5. [DOI] [PubMed] [Google Scholar]
- 72.Yu FF, Zhang YX, Zhang LH, Li WR, Guo X, Lammi MJ. Identified molecular mechanism of interaction between environmental risk factors and differential expression genes in cartilage of Kashin-Beck disease. Medicine (Baltimore) 2016;95:e5669. doi: 10.1097/MD.0000000000005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133:1463S–1467S. doi: 10.1093/jn/133.5.1463S. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 74.Sheridan PA, Zhong N, Carlson BA, Perella CM, Hatfield DL, Beck MA. Decreased selenoprotein expression alters the immune response during influenza virus infection in mice. J Nutr. 2007;137:1466–1471. doi: 10.1093/jn/137.6.1466. [DOI] [PubMed] [Google Scholar]
- 75.Gu BQ. Pathology of Keshan disease. A comprehensive review. Chin Med J (Engl) 1983;96:251–261. [PubMed] [Google Scholar]
- 76.Levander OA, Beck MA. Interacting nutritional and infectious etiologies of Keshan disease. Insights from coxsackie virus B-induced myocarditis in mice deficient in selenium or vitamin E. Biol Trace Elem Res. 1997;56:5–21. doi: 10.1007/BF02778980. [DOI] [PubMed] [Google Scholar]
- 77.Xu GL, Wang SC, Gu BQ, Yang YX, Song HB, Xue WL, Liang WS, Zhang PY. Further investigation on the role of selenium deficiency in the aetiology and pathogenesis of Keshan disease. Biomed Environ Sci. 1997;10:316–326. [PubMed] [Google Scholar]
- 78.Chen J. An original discovery: Selenium deficiency and Keshan disease (an endemic heart disease) Asia Pac J Clin Nutr. 2012;21:320–326. [PubMed] [Google Scholar]
- 79.Guanqing H. On the etiology of Keshan disease: Two hypotheses. Chin Med J (Engl) 1979;92:416–422. [PubMed] [Google Scholar]
- 80.Cermelli C, Vinceti M, Scaltriti E, Bazzani E, Beretti F, Vivoli G, Portolani M. Selenite inhibition of Coxsackie virus B5 replication: Implications on the etiology of Keshan disease. J Trace Elem Med Biol. 2002;16:41–46. doi: 10.1016/S0946-672X(02)80007-4. [DOI] [PubMed] [Google Scholar]
- 81.Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, Padidela R, Ceron-Gutierrez L, Doffinger R, Prevosto C, et al. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest. 2010;120:4220–4235. doi: 10.1172/JCI43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scoto M, Cirak S, Mein R, Feng L, Manzur AY, Robb S, Childs AM, Quinlivan RM, Roper H, Jones DH, et al. SEPN1-related myopathies: Clinical course in a large cohort of patients. Neurology. 2011;76:2073–2078. doi: 10.1212/WNL.0b013e31821f467c. [DOI] [PubMed] [Google Scholar]
- 83.Ardissone A, Bragato C, Blasevich F, Maccagnano E, Salerno F, Gandioli C, Morandi L, Mora M, Moroni I. SEPN1-related myopathy in three patients: Novel mutations and diagnostic clues. Eur J Pediatr. 2016;175:1113–1118. doi: 10.1007/s00431-015-2685-3. [DOI] [PubMed] [Google Scholar]
- 84.Schomburg L, Dumitrescu AM, Liao XH, Bin-Abbas B, Hoeflich J, Köhrle J, Refetoff S. Selenium supplementation fails to correct the selenoprotein synthesis defect in subjects with SBP2 gene mutations. Thyroid. 2009;19:277–281. doi: 10.1089/thy.2008.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.EFSA NDA Panel: Scientific opinion on dietary reference values for selenium. EFSA J. 2014;12:3846. doi: 10.2903/j.efsa.2014.3846. [DOI] [Google Scholar]
- 86.Ge KY, Wang SQ, Bai J, Xue AN, Deng XJ, Su CQ, Wu SQ. The protective effect of selenium against viral myocarditis in mice. In: Part B, Combs GF, Spallholz JE, Levander OA, Oldfield JE, editors. Selenium in biology and medicine. Avi Book, Van Nostrand Reinhold Co.; New York: 1987. pp. 761–768. [Google Scholar]
- 87.Spallholz JE. Free radical generation by selenium compounds and their prooxidant toxicity. Biomed Environ Sci. 1997;10:260–270. [PubMed] [Google Scholar]
- 88.Stewart MS, Spallholz JE, Neldner KH, Pence BC. Selenium compounds have disparate abilities to impose oxidative stress and induce apoptosis. Free Radic Biol Med. 1999;26:42–48. doi: 10.1016/S0891-5849(98)00147-6. [DOI] [PubMed] [Google Scholar]
- 89.Letavayová L, Vlasáková D, Spallholz JE, Brozmanová J, Chovanec M. Toxicity and mutagenicity of selenium compounds in Saccharomyces cerevisiae. Mutat Res. 2008;638:1–10. doi: 10.1016/j.mrfmmm.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 90.Misra S, Boylan M, Selvam A, Spallholz JE, Björnstedt M. Redox-active selenium compounds - from toxicity and cell death to cancer treatment. Nutrients. 2015;7:3536–3556. doi: 10.3390/nu7053536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee KH, Jeong D. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox (Review) Mol Med Rep. 2012;5:299–304. doi: 10.3892/mmr.2011.651. [DOI] [PubMed] [Google Scholar]
- 92.Brozmanová J, Mániková D, Vlčková V, Chovanec M. Selenium: A double-edged sword for defense and offence in cancer. Arch Toxicol. 2010;84:919–938. doi: 10.1007/s00204-010-0595-8. [DOI] [PubMed] [Google Scholar]
- 93.Schiar VP, Dos Santos DB, Paixão MW, Nogueira CW, Rocha JB, Zeni G. Human erythrocyte hemolysis induced by selenium and tellurium compounds increased by GSH or glucose: A possible involvement of reactive oxygen species. Chem Biol Interact. 2009;177:28–33. doi: 10.1016/j.cbi.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 94.Guo CH, Hsia S, Hsiung DY, Chen PC. Supplementation with selenium yeast on the prooxidant-antioxidant activities and anti-tumor effects in breast tumor xenograft-bearing mice. J Nutr Biochem. 2015;26:1568–1579. doi: 10.1016/j.jnutbio.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 95.Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974;104:580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 96.Oh SH, Sunde RA, Pope AL, Hoekstra WG. Glutathione peroxidase response to selenium intake in lambs fed a Torula yeast-based, artificial milk. J Anim Sci. 1976;42:977–983. doi: 10.2527/jas1976.424977x. [DOI] [PubMed] [Google Scholar]
- 97.Kramer GF, Ames BN. Mechanisms of mutagenicity and toxicity of sodium selenite (Na2SeO3) in Salmonella typhimurium. Mutat Res. 1988;201:169–180. doi: 10.1016/0027-5107(88)90123-6. [DOI] [PubMed] [Google Scholar]
- 98.Macallan DC, Sedgwick P. Selenium supplementation and selenoenzyme activity. Clin Sci (Lond) 2000;99:579–581. doi: 10.1042/cs0990579. [DOI] [PubMed] [Google Scholar]
- 99.Tsongas TA, Ferguson SW. Selenium concentrations in human urine and drinking water. In: Kirchgessner N, editor. Proceedings of ‘Trace Elements in Man and Animal-3’. Institute for Nursing Physiology Technical University Munchen; Freising: 1978. pp. 320–321. [Google Scholar]
- 100.Tsongas TA, Ferguson SW. Human health effects of selenium in a rural Colorado drinking water supply. In: Proceedings of ‘Trace substances in environmental health-XI. In: Hemphill DD, editor. A symposium’. University of Missouri; Columbia: 1977. pp. 30–35. [Google Scholar]
- 101.Valentine JL. Environmental occurrence of selenium in waters and related health significance. Biomed Environ Sci. 1997;10:292–299. [PubMed] [Google Scholar]
- 102.Valentine JL, Kang HK, Dang PM, Schluchter M. Selenium concentrations and glutathione peroxidase activities in a population exposed to selenium via drinking water. J Toxicol Environ Health. 1980;6:731–736. doi: 10.1080/15287398009529892. [DOI] [PubMed] [Google Scholar]
- 103.Valentine JL, Faraji B, Kang HK. Human glutathione peroxidase activity in cases of high selenium exposures. Environ Res. 1988;45:16–27. doi: 10.1016/S0013-9351(88)80003-3. [DOI] [PubMed] [Google Scholar]
- 104.Valentine JL, Reisbord LS, Kang HK, Schluchter M. Effects on human health of exposure to selenium in drinking water. In: Combs GF, Levander OA, Spallholz JE, Oldfield JE, editors. Proceedings of ‘Selenium in Biology and Medicine - Part B’. Van Nostrand Reihold Co.; New York: 1987. pp. 675–687. [Google Scholar]
- 105.Longnecker MP, Taylor PR, Levander OA, Howe M, Veillon C, McAdam PA, Patterson KY, Holden JM, Stampfer MJ, Morris JS, et al. Selenium in diet, blood, and toenails in relation to human health in a seleniferous area. Am J Clin Nutr. 1991;53:1288–1294. doi: 10.1093/ajcn/53.5.1288. [DOI] [PubMed] [Google Scholar]
- 106.Brätter P, de Negretti Brätter VE. Influence of high dietary selenium intake on the thyroid hormone level in human serum. J Trace Elem Med Biol. 1996;10:163–166. doi: 10.1016/S0946-672X(96)80027-7. [DOI] [PubMed] [Google Scholar]
- 107.Fordyce FM, Zhan G, Green K, Liu X. Soil, grain and water chemistry in relation to human selenium-responsive diseases in Enshi District, China. Applied Geochemistry. 2000;15:117–132. doi: 10.1016/S0883-2927(99)00035-9. doi: 10.1016/S0883-2927(99)00035-9. [DOI] [Google Scholar]
- 108.Zhu J, Wang N, Li S, Li L, Su H, Liu C. Distribution and transport of selenium in Yutangba, China: Impact of human activities. Sci Total Environ. 2008;392:252–261. doi: 10.1016/j.scitotenv.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 109.Hira CK, Partal K, Dhillon KS. Dietary selenium intake by men and women in high and low selenium areas of Punjab. Public Health Nutr. 2004;7:39–43. doi: 10.1079/PHN2003513. [DOI] [PubMed] [Google Scholar]
- 110.Lemire M, Fillion M, Frenette B, Passos CJ, Guimarães JR, Barbosa F, Jr, Mergler D. Selenium from dietary sources and motor functions in the Brazilian Amazon. Neurotoxicology. 2011;32:944–953. doi: 10.1016/j.neuro.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 111.Lemire M, Philibert A, Fillion M, Passos CJ, Guimarães JR, Barbosa F, Jr, Mergler D. No evidence of selenosis from a selenium-rich diet in the Brazilian Amazon. Environ Int. 2012;40:128–136. doi: 10.1016/j.envint.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 112.Qin HB, Zhu JM, Liang L, Wang MS, Su H. The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ Int. 2013;52:66–74. doi: 10.1016/j.envint.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 113.Martens IB, Cardoso BR, Hare DJ, Niedzwiecki MM, Lajolo FM, Martens A, Cozzolino SM. Selenium status in preschool children receiving a Brazil nut-enriched diet. Nutrition. 2015;31:1339–1343. doi: 10.1016/j.nut.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 114.Chawla R, Loomba R, Chaudhary RJ, Singh S, Dhillon KS. Global advance in selenium research from theory to application. CRC Press; Sao Paulo: 2015. Impact of high selenium exposure on organ function and biochemical profile of the rural population living in seleniferous soils in Punjab, India; pp. 93–94. [DOI] [Google Scholar]
- 115.Yu SY, Zhu YJ, Li WG. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol Trace Elem Res. 1997;56:117–124. doi: 10.1007/BF02778987. [DOI] [PubMed] [Google Scholar]
- 116.Yu SY, Zhu YJ, Li WG, Huang QS, Huang CZ, Zhang QN, Hou C. A preliminary report on the intervention trials of primary liver cancer in high-risk populations with nutritional supplementation of selenium in China. Biol Trace Elem Res. 1991;29:289–294. doi: 10.1007/BF03032685. [DOI] [PubMed] [Google Scholar]
- 117.Li WG. Preliminary observations on effect of selenium yeast on high risk populations with primary liver cancer. Zhonghua Yu Fang Yi Xue Za Zhi. 1992;26:268–271. (In Chinese) [PubMed] [Google Scholar]
- 118.Li W, Zhu Y, Yan X, Zhang Q, Li X, Ni Z, Shen Z, Yao H, Zhu J. The prevention of primary liver cancer by selenium in high risk populations. Zhonghua Yu Fang Yi Xue Za Zhi. 2000;34:336–338. (In Chinese) [PubMed] [Google Scholar]
- 119.Lotan Y, Goodman PJ, Youssef RF, Svatek RS, Shariat SF, Tangen CM, Thompson IM, Jr, Klein EA. Evaluation of vitamin E and selenium supplementation for the prevention of bladder cancer in SWOG coordinated SELECT. J Urol. 2012;187:2005–2010. doi: 10.1016/j.juro.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marshall JR, Tangen CM, Sakr WA, Wood DP, Jr, Berry DL, Klein EA, Lippman SM, Parnes HL, Alberts DS, Jarrard DF, et al. Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev Res (Phila) 2011;4:1761–1769. doi: 10.1158/1940-6207.CAPR-10-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]