Abstract
Separation of CO2 from other gasses offers environmental benefits since CO2 gas is the main contributor to global warming. Recently, graphene oxide (GO) based gas separation membranes are of interest due to their selective barrier properties. However, maintaining selectivity without sacrificing permeance is still challenging. Herein, we described the preparation and characterization of nanoscale GO membranes for CO2 separation with both high selectivity and permeance. The internal structure and thickness of the GO membranes were controlled by layer-by-layer (LbL) self-assembly. Polyelectrolyte layers are used as the supporting matrix and for facilitating CO2 transport. Enhanced gas separation was achieved by adjusting pH of the GO solutions and by varying the number of GO layers to provide a pathway for CO2 molecules. Separation performance strongly depends on the number of GO bilayers. The surfaces of the multilayered GO and polyelectrolyte films are characterized by atomic force microscopy and scanning electron microscopy. The (poly (diallyldimethylammonium chloride) (PDAC)/polystyrene sulfonate (PSS)) (GO/GO) multilayer membranes show a maximum CO2/N2 selectivity of 15.3 and a CO2 permeance of 1175.0 GPU. LbL-assembled GO membranes are shown to be effective candidates for CO2 separation based on their excellent CO2/N2 separation performance.
Introduction
Separation of CO2 from gas mixtures is of interest due to its corrosive nature in industrial environments, such as pipelines, and as a means of reducing greenhouse gasses that contribute to global warming1, 2. Among the separation method, membrane-based separation is widely used because of simple operation and low cost3. Research on CO2 separation has included investigation of a variety of polymeric membranes4, 5, inorganic membranes6, carbon membranes7, alumina membranes7, zeolite membranes8 and hybrid membranes and inorganic membranes9. Polymeric membrane was studied by various groups; Illing et al. reported polyaniline based membranes and the highest selectivity for CO2/N2: 17.05 and Iqbal et al. reported asymmetric polycarbonate membranes with maximum ideal selectivity for CO2/CH4: 173.8810. Many researches on hybrid membranes and inorganic membranes were studied such as, polyimide-carbon molecular sieve mixed membrane11, asymmetric nafion/(zirconium oxide) hybrid membrane12 and Mixed matrix hollow fiber membranes made with modified HSSZ-13 zeolite in polyetherimide polymer matrix13.
Among the various separation methods, membrane techniques utilizing graphene oxide (GO) show great potential in CO2 separation. GO is a well-known two-dimensional chemical compound based on a partially allotropic form of carbon. Although GO is one-atom thick, it has unique electrical properties and can serve as a perfect barrier to ions and molecules. The electron cloud of the GO π-orbitals blocks even the smallest gas molecules14. Recent research has revealed that GO can function as a gas separation membrane when its pore and stacking structures are controlled15. Park et al. reported selective gas transport through a few-layer GO membrane by adjusting centrifugal force and electrostatic repulsion during the adsorption process of GO sheets16. Also, Xu et al. have reported selective membranes with gas transport channels formed from laminar GO17.
Until now, GO membranes have been prepared mostly in aqueous solution and processed using spin coating, drop casting, and filtration methods. However, these GO membranes present several challenges. For example, the previously reported coating methods make difficulties in precise control of thickness and stacking density. Furthermore, GO membranes without a supporting layer are brittle, which is a limitation in industrial applications. In addition, electrostatic repulsion arising from the carboxyl groups in GO causes undesirable cracks that lead to low selectivity.
Herein, we propose a spray-assisted layer-by-layer (LbL) self-assembly method for precisely controlling the preparation of GO sheets to be used as CO2 separation membranes. LbL assembly is a well-developed multilayer deposition process. The most important advantage of the method is that it offers precise control of film thickness and internal structure without limitation by substrates and materials through complementary interactions (i.e., electrostatic interactions, covalent interactions, and hydrogen bonding)18–24.
To take full advantage of LbL assembly, we fabricated GO membranes that enhance two components of the CO2 separation process. The first component involves the strong CO2 affinity of the numerous polar groups on GO and the molecular sieving effect of aligned GO layers. The second component is the facilitation of CO2 transport by amine groups in the polyelectrolyte layers. However, conventional dipping LbL assembly is not suitable for preparation of membrane substrates, because the flexibility of polysulfone (PSf) leads to destruction of deposited nanoscale films. Therefore, we adopted a spray method to deposit a stable nanofilm on the flexible substrate25.
Results and Discussion
According to previous research, the CO2 separation performance of GO membranes results from the interlayer spaces in stacked GO sheets26–28. Defective pores were generated during both the oxidation and ultrasonication processes29. Ultrasonication is used to promote the dispersion of the GO sheet in aqueous solution. Porous support membrane can be covered with an increasing number of GO layers, which increases the CO2 selectivity. Conversely, CO2 permeance declines as the thickness of the GO layers increases because of the longer diffusion pathway that CO2 molecules must traverse to pass through the membrane. The balance between selectivity and permeance is extremely important in CO2 separation membranes, and it can be achieved by the precise control of the stacking structure of GO sheets. Therefore, we designed a GO membrane as shown in Fig. 1. The supporting matrix of the repeating (PDAC/PSS)n layers was first deposited onto the porous PSf surface, and the (GO/GO)n layers were then introduced selectively. The LbL film notation used here is (PDAC/PSS)n, representing a multilayer film consisting of PDAC and PSS, where n is the number of bilayers. For example, (PDAC/PSS)25 represents a 25-bilayer multilayer film consisting of repeated PDAC/PSS layers. Although the intrinsic barrier properties of GO layers block N2 molecules, a certain number permeate through non-selective cracks. The role of LbL-assembled polyelectrolyte multilayer is preventing the undesirable permeation of N2 molecules through cracks and wrinkles in the GO layers, and to enhance selectivity for CO2 over N2. Furthermore, a (PDAC/PSS)n layer covers the pores of the PSf membrane and prevents disordered GO aggregation in the membrane pores.
Figure 1.
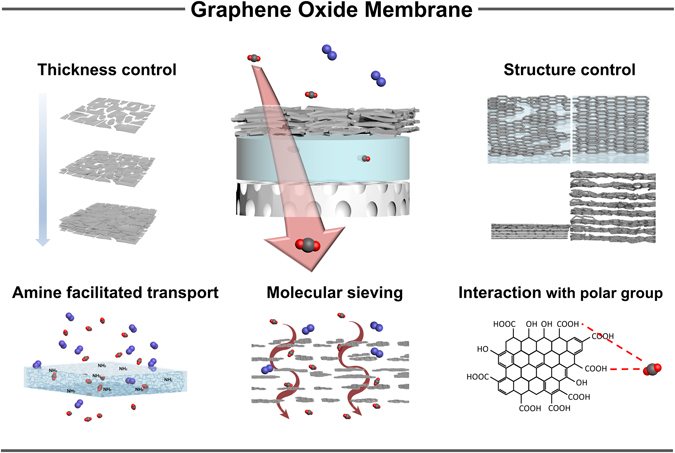
Schematic illustration of LbL-assembled polyelectrolyte/GO membranes for CO2 separation.
The thickness of the (PDAC/PSS) layer was controlled by means of the number of LbL assembly cycles and the salt (NaCl) concentration (Fig. 2 and Fig. S1). The appropriate salt concentration and PDAC/PSS layer thickness were determined by gas permeance analysis. Too much salt exerts an unfavorable effect on the base layer and can lead to gas leakage. At a low salt concentration (0.1 M NaCl), the CO2 and N2 permeabilities are 105.75 GPU and 3.17 GPU, respectively, with a layer thickness of 60 nm. Conversely, at a high salt concentration (1.0 M NaCl), the CO2 and N2 permeabilities are 423 GPU and 282 GPU, respectively, even though the layer thickness is over 1 μm. In order to shorten the CO2 molecule pathway, the salt concentration was fixed at 0.1 M. Furthermore, the PDAC/PSS layer is not only a defect barrier but also plays an important role in CO2 selectivity. The amine group of PDAC facilitates the CO2 transport by reversible reaction with CO2 30. The reaction between CO2 and the amine group can be explained by the zwitterion reaction mechanism that is proposed by Caplow (1968) and Danckwerts (1979) as shown below31, 32:
| 1 |
| 2 |
| 3 |
| 4 |
R: Functional group, B: Base (H2O or amine).
Figure 2.
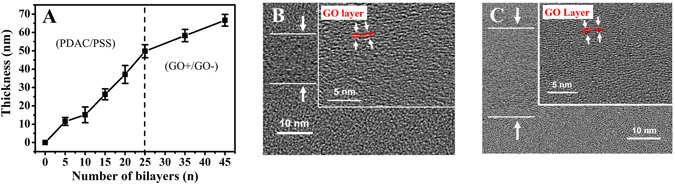
Thickness growth profile and TEM cross-sectional images. (A) Thickness growth profile of a spray-assisted LbL-assembled (PDAC/PSS)n(GO+/GO−)n film. (B) TEM cross-sectional images of (PDAC/PSS)25(GO/GO)20.5 and (C) (PDAC/PSS)25(GO/GO)40.5. A higher magnification image is shown in the inset, which represents LbL-assembled GO sheets.
CO2 reacts with the primary or secondary amine group to form an intermediate zwitterion. The zwitterion is deprotonated by base (H2O or the amine itself) to form a carbamate ion. The carbamate then reacts with H2O to form bicarbonate if it is not stable. Under mild pressure, carbamate and bicarbonate diffuse down the membrane pathway33.
The thickness of the GO layer was also controlled by repeating cycles of LbL assembly and by controlling the pH (Fig. 2 and Fig. S1). Carboxyl-functionalized GO-COOH solutions are negatively charged over the pH range 3–12. The GO-COOH functionalities are fully ionized at pH 12, and the degree of ionization decreases with decreasing pH. Conversely, the amine groups (GO-NH2) are fully protonated at pH 3 and begin to loose protons above pH 6.034. To ensure the CO2 pathway, we aligned the GO sheets by adjusting the pH value of both the GO-COOH and GO-NH2 solutions. The pH of GO-NH2 was adjusted to 4.3 to provide sufficient electrostatic interaction with the GO-COOH layers. The pH of the GO-COOH solution was also adjusted to 4.3. At this pH, the carboxylic groups of GO-COOH show limited electrostatic repulsion, which allows sufficient charge density for LbL assembly and the formation of a densely stacked selective GO layer.
The GO layer is the most important selective layer of our membrane. The polar groups such as amine, carboxyl, and hydroxyl groups on the surface of GO layers enhance the permeance of CO2 because of their affinity for CO2 molecules. Although CO2 is a nonpolar gas, it interacts with polar groups through its quadrupole moment35. However, gas molecules cannot permeate the GO sheets directly because of its inherent barrier properties. Therefore, gas molecules must pass through the aligned internal spaces of the GO layer, which leads to an enhanced molecular sieving effect. The kinetic diameters of CO2 and N2 are 0.33 and 0.36 nm, respectively. Thus, the interlayer spacing between aligned GO sheets should be less than 0.36 nm, and this precondition can be achieved by tuning the interactions between the GO sheets17.
The thickness growth curve on a Si wafer is shown in Fig. 2A. The smaller quantity of the adsorbed polyelectrolytes than those produced by the dipping method is a result of spray-assisted LbL deposition. The kinetics of this method are such that the adsorption duration is limited to a few seconds, leading to insufficient interdiffusion during the adsorption and rinsing step. A 30-bilayer (PDAC/PSS) film is only 60.4 nm thick, and a 50-bilayer (GO/GO) film is only 60.9 nm thick (Fig. S1), which demonstrates the advantage of our process in terms of the precise control of thickness and short preparation time.
The FT-IR analysis reveals the presence of polar functionalities, including hydroxyl and amine groups, on the GO sheets (Fig. S2). Furthermore, from the increase in the intensity of the OH peak as the number of GO layer increase, it is confirmed that the (GO/GO) layers are successively adsorbed onto the (PDAC/PSS)25 supporting layer. We investigated four different kinds of membranes to determine the roles of the polyelectrolyte and GO layers. The surface morphologies of films prepared by spray-assisted LbL deposition were analyzed by using top-view SEM (Fig. S3). The (PDAC/PSS)25 film (Fig. S3B) has a flatter surface than the other films (Fig. S3A,C and D), because the NaCl concentration is only 0.1 M. PDAC and PSS are strong polyelectrolytes and are almost fully charged in 0.1 M NaCl, which results in the flat surface. Furthermore, the flat surface ensures that the subsequent (GO/GO) layer will adsorb as we designed. If the GO solution is applied without the (PDAC/PSS) layer, it penetrates through the pores of the PSf membrane, forming an aggregated structure or a stacking conformation on the rough surfaces of the PSf membrane with a disordered structure. (PDAC/PSS)25(GO/GO)10.5, (PDAC/PSS)25(GO/GO)20.5, and (PDAC/PSS)25(GO/GO)40.5 films exhibit the structure of LbL-assembled GO sheets. As the number of GO layers increases, the relative thickness of GO layers with respect to polyelectrolytes layers also increases, which results in a rough surface with wrinkled GO sheets. The wrinkles in the aggregated GO result from the mechanism of spray-assisted LbL deposition. Spray-assisted LbL deposition limits the adsorption time of GO sheet so that interdiffusion doesn’t sufficiently occur compare to conventional LbL method. Furthermore, rinsing step is also limited in a few seconds leading to aggregated structure36.
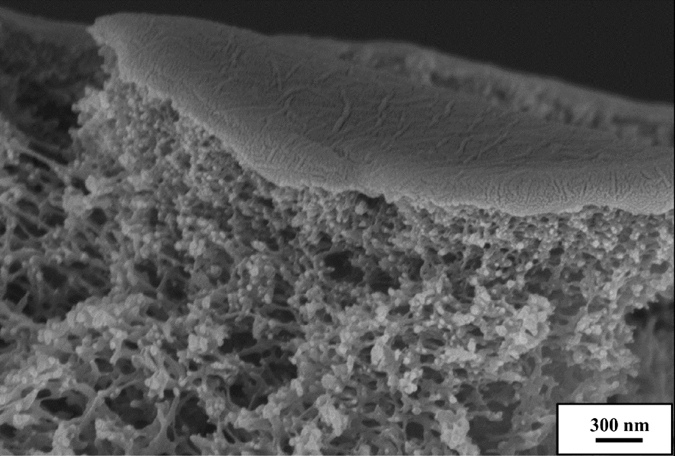
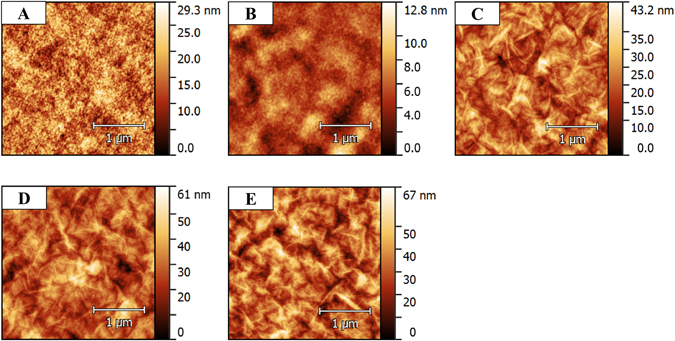
The surface morphologies of films were also analyzed by noncontact mode AFM (Fig. 3 and Fig. S4). Figure 3A shows the surface morphology and pore size of a PSf membrane. The roughness of PSf membrane is 4.0 nm RMS (root-mean-square). After deposition of (PDAC/PSS)25, the surface becomes smoother (RMS: 1.4 nm), because the membrane surface is fully covered by the polyelectrolyte layer (Fig. 3B). The subsequently adsorbed GO layers are shown in (Fig. 3C–E). The roughness of the layers increases (RMS: 5.4 nm for (PDAC/PSS)25(GO/GO)10.5, 7.6 nm for (PDAC/PSS)25(GO/GO)20.5, and 8.3 nm for (PDAC/PSS)25(GO/GO)40.5), because of the wrinkled structure of the GO sheets. The edges of the graphene oxide sheet are overlapped by other graphene oxide sheets, forming an interlocking jigsaw-type structure with fewer surfaces cracks, and thus ensuring the N2 barrier effect of the GO layers (Fig. 4). This interlocking GO sheet-like surface structure may result from the limited electrostatic repulsion of GO sheets. The protonated or deprotonated functional groups (COO− and NH3 +) at the edge of GO sheet (GO-COOH and GO-Amine) have repulsion. However, since pH value of GO solution was adjusted (GO-COOH: 4.3, GO-Amine: 4.3), the repulsion force was limited maintaining enough charge for successful LbL layer formation.
Figure 3.
AFM height images of LbL multilayer films deposited on membranes. (A) Bare membrane, (B) (PDAC/PSS)25.5, (C) (PDAC/PSS)25(GO + GO−)10.5, (D) (PDAC/PSS)25(GO + GO−)20.5, and (E) (PDAC/PSS)25.5(GO/GO)40.5.
Figure 4.
Tilted cross-sectional SEM image. Tilted cross-sectional SEM image of a (PDAC/PSS)25(GO/GO)20.5 membrane.
In the cross-sectional TEM images of (PDAC/PSS)25(GO/GO)20.5 and (PDAC/PSS)25(GO/GO)40.5 (Fig. 2B,C), the amorphous region at the bottom represents the (PDAC/PSS) layer and the upper laminar region indicates the GO/GO layer. The GO sheets are deposited on the polyelectrolyte layer with a LbL-assembled GO structure, as shown in the inset images. The inset TEM image shows that the LbL-assembled GO sheets are not uniformly stacked. A possible explanation for this structure is that, compared to the pristine regularly stacked graphene sheet, GO is not a perfect two-dimensional material and has many functional groups, which lead to an irregular structure. However, even though the GO sheets are irregularly stacked, there are no significant defects or aggregates in the internal space of the film. The inset image also indicates that the maximum d-spacing of the GO sheets is approximately 0.78 nm. Since d-spacing includes the thickness of the GO, interlayer spacing can be calculated as ca. 0.43 nm. Although the maximum size of the internal space is significantly larger than the size of gas molecules such as CO2 (0.33 nm) and N2 (0.36 nm), the multilayer structure of the GO/GO layer enables the separation of CO2 molecules.
The permeance and CO2/N2 selectivity of each film is shown in Table 1. The (PDAC/PSS)25 membrane was investigated to confirm that CO2 transport is facilitated by amine groups in the PDAC layer. The CO2/N2 selectivity is 3.7, and the CO2 permeance is 193.9 GPU. The increased solubility of CO2 resulting from its quadrupole moment also enhances selectivity. It was anticipated that the (PDAC/PSS)25(GO/GO)10.5 membrane would exhibit increased CO2/N2 selectivity. However, the CO2 and N2 permeance increase while the CO2/N2 selectivity decreases slightly. This unexpected result can be explained by the surface coverage and increased surface area of the GO layer. Although GO appears to fully cover the surface (Fig. S3), 10.5 GO bilayers do not block N2 sufficiently; this increases N2 permeance due to increased surface area. When the GO thickness exceeds 20 bilayers, it effectively blocks N2 permeation while retaining permeance to CO2 and results in increased CO2/N2 selectivity. (PDAC/PSS)25(GO/GO)20.5 and (PDAC/PSS)25(GO/GO)40.5 achieve selectivities of 11.3 and 15.3, respectively, and CO2 permeances of 1269.0 and 1175.0 GPU, respectively. Furthermore, both CO2 and N2 permeance of membranes increased after GO layer deposition onto the polyelectrolytes layer. This phenomenon can be explained by the change of support polyelectrolytes layer. When GO solution is sprayed onto the polyelectrolytes layer, polyelectrolytes layer is slightly swollen by GO solution. Polyelectrolytes films are sensitive to water and swollen in humid condition37. However, since the adsorption time of spray assisted LbL method is limited in a few seconds, the amount of swelling was also limited so that permeance of both CO2 and N2 gas was increased slightly maintaining selectivity.
Table 1.
Selectivity and gas permeance of (PDAC/PSS)25, (PDAC/PSS)25(GO/GO)10.5, (PDAC/PSS)25(GO/GO)20.5, and (PDAC/PSS)25(GO/GO)40.5 membranes.
| Membranes | Permeance (GPU) | Selectivity | |
|---|---|---|---|
| CO2 | N2 | CO2/N2 | |
| (PDAC/PSS)25 | 193.88 | 52.10 | 3.72 |
| (PDAC/PSS)25(GO/GO)10.5 | 1005.01 | 275.38 | 3.64 |
| (PDAC/PSS)25(GO/GO)20.5 | 1269.00 | 112.25 | 11.31 |
| (PDAC/PSS)25(GO/GO)40.5 | 1175.03 | 76.63 | 15.33 |
For CO2 separation from a mixed gas, a CO2/N2 selectivity of greater than 70 and a minimum CO2 permeance of 100 Barrels for a membrane thickness of 0.1 μm (a permeance of 1000 GPU) are required for industrial applications38. Our (PDAC/PSS)25(GO/GO)20.5 and (PDAC/PSS)25(GO/GO)40.5 membranes satisfy this commercial demand (Table 1 and Fig. 5). Our membrane measurements were conducted under dry conditions; however, humidity increases CO2/N2 selectivity due to facilitated CO2 transport.
Figure 5.
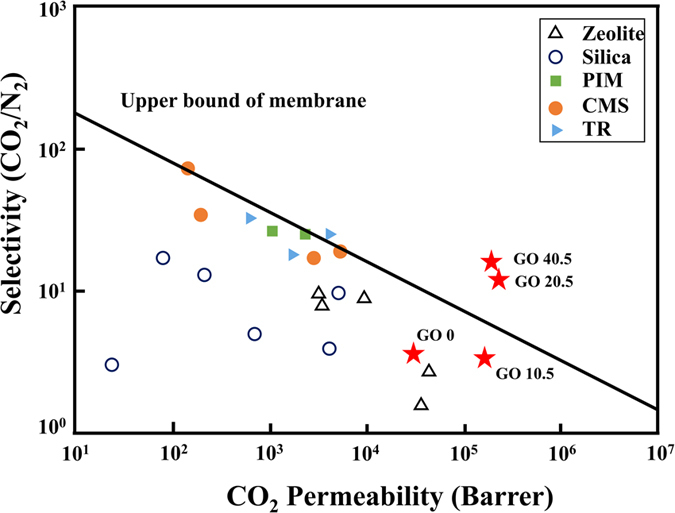
Relationship between CO2 permeability and CO2/N2 selectivity for GO membranes in the dry state. GO 0, GO 10.5, GO 20.5 and GO 40.5 represent (PDAC/PSS)25.5, (PDAC/PSS)25(GO + GO−)10.5, (PDAC/PSS)25(GO + GO−)20.5, and (PDAC/PSS)25.5(GO/GO)40.5 respectively.
The CO2 permeabilities of the (PDAC/PSS)25 and (PDAC/PSS)25(GO/GO)10.5 membranes are 29403.8 and 152419.8 Barrer, respectively, and the CO2/N2 selectivities of both are ca. 3.7. This separation efficiency is similar to that of other polymeric membranes, such as carbon molecular sieves (CMS) and polymers of intrinsic microporosity (PIMs). However, the CO2 separation performance increases markedly for (PDAC/PSS)25(GO/GO)20.5 and (PDAC/PSS)25(GO/GO)40.5, which exhibit CO2 permeabilities of 192456.5 and 178205.1 Barrels, respectively, and CO2/N2 selectivities of 11.3 and 15.3, respectively (Fig. 5). These performances exceed those of other polymeric membranes, including PIMs39, thermally rearranged (TR) polymer membranes40, silica membranes, and zeolite membranes41. The results indicate that spray-assisted LbL-assembled nanofilms have the ability to separate CO2, although their thicknesses are less than 100 nm. In addition, the (PDAC/PSS) polyelectrolyte layer possesses CO2/N2 selectivity because of its amine groups, and the CO2 selectivity increases with the number of GO layers on the polyelectrolyte layer.
Conclusion
In conclusion, we have investigated the ability of structure- and thickness-controlled polyelectrolyte/GO membranes prepared by spray-assisted LbL assembly to separate CO2. The molecular sieving effect of aligned GO layers produced by pH control of GO solutions and a high CO2 affinity resulting from polar functional groups on GO sheets lead to excellent performance for multilayer films less than 100 nm in thickness. Further research on methods for aligning GO layers in a highly ordered form should enhance membrane performance.
Materials and Methods
Materials
Graphite (20 microns), potassium permanganate, potassium persulfate, phosphorus pentoxide, ethylenediamine, poly(diallyldimethylammonium chloride) (MW 20–35 k, PDAC), and poly(sodium-4-styrenesulfonate) (MW 70 k, PSS) were purchased from Sigma Aldrich. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide methiodide (EDC) was purchased from Alfa Aesar. Macroporous polysulfone support having average 0.1 μm pore was purchased from Toray Inc., Ltd., Tokyo, Japan.
Synthesis of GO
GO was synthesized from graphite by a modification of Hummer's method42, 43. Negatively charged GO-COOH was prepared by oxidation with acid treatment to introduce carboxylic acid groups. Graphite powder (1 g) was added to a mixture of concentrated H2SO4 (4 mL), K2S2O8 (0.8 g), and P2O5 (0.8 g) at 80 °C. The dark mixture was stirred for 4.5 h. Distilled water was slowly added to the mixture, which was filtered and washed until the rinse water reached pH 6.0. After drying overnight at room temperature, the pre-oxidized powder was added to concentrated H2SO4 (26 mL) at 0 °C. KMnO4 was added slowly to maintain the solution temperature below 30 °C. The solution was stirred for 2 h at 36 °C, and distilled water (46 mL) was added gradually to maintain the solution temperature below 60 °C. The solution was stirred for 2 h at 36 °C. The reaction was terminated by addition of distilled water (140 mL) and 30% H2O2 solution (2.5 mL). The solution was filtered and rinsed with 10% HCl solution (350 mL). Dialysis was performed to remove the residual ions and HCl solution.
Preparation of polyelectrolyte and GO solutions
PDAC and PSS were dissolved in aqueous 0.1 M NaCl at 1 mg/mL each. Negatively charged GO-COOH solution was prepared by dissolving GO powder in pH 4.3 DI water at a concentration of 0.5 mg/mL with ultrasonication. The average flake size of GO was about 850 nm with wide size distribution from hundreds of nanometers to a few micron meters. We used as synthesized without further size separation or purification of GO solution. A positively charged GO-NH2 solution was prepared by functionalizing amine groups on the edge of negatively charged GO-COOH by an EDC-mediated reaction between excess ethylenediamine and carboxylic acid groups44. Excess reactants and byproducts were removed by dialysis for one week. The pH values of the GO-COOH and GO-NH2 solutions were adjusted to 4.3 to provide an ionic strength of more than 50%. Zeta-potential of GO-COOH solution was −53.83 mV and GO-NH2 was +57.77 mV.
Spray-assisted LbL film deposition on a silicon wafer and PSf membrane
Multilayer films were constructed on a 2.5 cm × 2.5 cm Si wafer and a 1.5 cm diameter circle of PSf membrane. Substrates were thoroughly cleaned in piranha solution (sulfuric acid/hydrogen peroxide 75/25 v/v) for 5 min following O2 plasma treatment (Femto Science) to produce a negatively charged surface. The substrates were then fixed on a film holder for spray-assisted LbL deposition. PDAC solutions (0.1 M NaCl) were sprayed four times to cover the entire substrate surfaces. Next, distilled water was sprayed four times to remove weakly attached polyelectrolytes. This half step was repeated with the PSS solutions (0.1 M NaCl), and the complete step was repeated to obtain the desired number of PDAC/PSS bilayers. GO-NH2/GO-COOH layers were fabricated by the same procedure.
Characterization
The thicknesses of the multilayer thin films on the Si wafers were measured by profilometry (Dektak 150, Veeco) and field-emission scanning electron microscopy (FE-SEM, SIGMA, Carl Zeiss). RMS roughness values and surface morphologies of membranes were obtained by atomic force microscopy (AFM, NX-10, Park Systems). The presence of functional groups in multilayer films was verified by Fourier-transform infrared spectroscopy (FT-IR-4700). The permeance of GO membranes was measured with a bubble meter to determine the value at a given membrane area and pressure. Permeance, defined as pressure-normalized mass flux, is expressed mathematically as J = Q × Δp, where Q is the permeance, J is the mass flux, and Δp is the pressure difference across the GO membrane. The volume of the permeating gas determines the permeance. Selectivity is defined as the ratio of the CO2 and N2 permeabilities. Gas permeance is expressed in terms of gas permeation units (GPU), where 1 GPU = 1 × 10−6 cm3 (STP)/(cm2 s cm-Hg).
Electronic supplementary material
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation of Korea (NRF) funded by the Korean Government (Grant 2012M3A9C6050104, 2016M3A9C6917405). Additionally, this research was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant HI14C-3266, HI15C-1653).
Author Contributions
J.H. (Jinkee Hong) & S.W.K. developed the idea, supervised the overall experiments. J.H. (Jiwoong Heo) carried out the major experiments. M.C., J.C. & D.J. prepared the experiment and characterization. All authors discussed the results, reviewed the manuscript and have given approval to the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00433-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sang Wook Kang, Email: swkang@smu.ac.kr.
Jinkee Hong, Email: jkhong@cau.ac.kr.
References
- 1.Chou CT, Chen CY. Carbon dioxide recovery by vacuum swing adsorption. Sep. Purif. Technol. 2004;39:51–65. doi: 10.1016/j.seppur.2003.12.009. [DOI] [Google Scholar]
- 2.Ebner AD, Ritter JA. State-of-the-art adsorption and membrane separation processes for carbon dioxide production from carbon dioxide emitting industries. Sep. Sci. Technol. 2009;44:1273–1421. doi: 10.1080/01496390902733314. [DOI] [Google Scholar]
- 3.Khan AL, Li X, Vankelecom IF. Mixed-gas CO2/CH4 and CO2/N2 separation with sulfonated PEEK membranes. J. Membr. Sci. 2011;372:87–96. doi: 10.1016/j.memsci.2011.01.056. [DOI] [Google Scholar]
- 4.Stern SA. Polymers for gas separations: the next decade. J. Membr. Sci. 1994;94:1–65. doi: 10.1016/0376-7388(94)00141-3. [DOI] [Google Scholar]
- 5.Illing G, Hellgardt K, Wakeman RJ, Jungbauer A. Preparation and characterisation of polyaniline based membranes for gas separation. J. Membr. Sci. 2001;184:69–78. doi: 10.1016/S0376-7388(00)00606-2. [DOI] [Google Scholar]
- 6.Luebke D, Myers C, Pennline H. Hybrid membranes for selective carbon dioxide separation from fuel gas. Energy Fuels. 2006;20:1906–1913. doi: 10.1021/ef060060b. [DOI] [Google Scholar]
- 7.Shekhawat, D., Luebke, D. R. & Pennline, H. W. A review of carbon dioxide selective membranes. US department of energy 9–11 (2003).
- 8.Kusakabe K, Yoneshige S, Murata A, Morooka S. Morphology and gas permeance of ZSM-5-type zeolite membrane formed on a porous α-alumina support tube. J. Membr. Sci. 1996;116:39–46. doi: 10.1016/0376-7388(96)00010-5. [DOI] [Google Scholar]
- 9.Yang H, et al. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008;20:14–27. doi: 10.1016/S1001-0742(08)60002-9. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal M, Man Z, Mukhtar H, Dutta BK. Solvent effect on morphology and CO2/CH4 separation performance of asymmetric polycarbonate membranes. J. Membr. Sci. 2008;318:167–175. doi: 10.1016/j.memsci.2008.02.040. [DOI] [Google Scholar]
- 11.Vu DQ, Koros WJ, Miller SJ. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J. Membr. Sci. 2003;211:311–334. doi: 10.1016/S0376-7388(02)00429-5. [DOI] [Google Scholar]
- 12.Apichatachutapan W, Moore RB, Mauritz KA. Asymmetric nafion/(zirconium oxide) hybrid membranes via in situ sol‐gel chemistry. J. Appl. Polym. Sci. 1996;62:417–426. doi: 10.1002/(SICI)1097-4628(19961010)62:2<417::AID-APP16>3.0.CO;2-Y. [DOI] [Google Scholar]
- 13.Husain S, Koros WJ. Mixed matrix hollow fiber membranes made with modified HSSZ-13 zeolite in polyetherimide polymer matrix for gas separation. J. Membr. Sci. 2007;288:195–207. doi: 10.1016/j.memsci.2006.11.016. [DOI] [Google Scholar]
- 14.Bunch JS, et al. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008;8:2458–2462. doi: 10.1021/nl801457b. [DOI] [PubMed] [Google Scholar]
- 15.Chae IS, Lee JH, Hong J, Kang YS, Kang SW. The platform effect of graphene oxide on CO2 transport on copper nanocomposites in ionic liquids. Chem. Eng. J. 2014;251:343–347. doi: 10.1016/j.cej.2014.04.017. [DOI] [Google Scholar]
- 16.Kim HW, et al. Selective gas transport through few-layered graphene and graphene oxide membranes. Sci. 2013;342:91–95. doi: 10.1126/science.1236098. [DOI] [PubMed] [Google Scholar]
- 17.Shen J, et al. Membranes with fast and selective gas‐transport channels of laminar graphene oxide for efficient CO2 capture. Angew. Chem. 2015;127:588–592. doi: 10.1002/ange.201409563. [DOI] [PubMed] [Google Scholar]
- 18.Hattori H. Anti‐reflection surface with particle coating deposited by electrostatic attraction. Adv. Mater. 2001;13:51–54. doi: 10.1002/1521-4095(200101)13:1<51::AID-ADMA51>3.0.CO;2-F. [DOI] [Google Scholar]
- 19.Kim HS, et al. Multifunctional layer-by-layer self-assembly of conducting polymers and magnetic nanoparticles. Thin Solid Films. 2002;419:173–177. doi: 10.1016/S0040-6090(02)00779-4. [DOI] [Google Scholar]
- 20.Zhai L, Nolte AJ, Cohen RE, Rubner MF. pH-gated porosity transitions of polyelectrolyte multilayers in confined geometries and their application as tunable bragg reflectors. Macromolecules. 2004;37:6113–6123. doi: 10.1021/ma049593e. [DOI] [Google Scholar]
- 21.Quinn A, Tjipto E, Yu A, Gengenbach TR, Caruso F. Polyelectrolyte blend multilayer films: surface morphology, wettability, and protein adsorption characteristics. Langmuir. 2007;23:4944–4949. doi: 10.1021/la0634746. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt DJ, Hammond PT. Electrochemically erasable hydrogen-bonded thin films. Chem. Commun. 2010;46:7358–7360. doi: 10.1039/c0cc02346a. [DOI] [PubMed] [Google Scholar]
- 23.DeRocher JP, Mao P, Han J, Rubner MF, Cohen RE. Layer-by-layer assembly of polyelectrolytes in nanofluidic devices. Macromolecules. 2010;43:2430–2437. doi: 10.1021/ma902451s. [DOI] [Google Scholar]
- 24.Choi D, Son B, Park TH, Hong J. Controlled surface functionality of magnetic nanoparticles by layer-by-layer assembled nano-films. Nanoscale. 2015;7:6703–6711. doi: 10.1039/C4NR07373H. [DOI] [PubMed] [Google Scholar]
- 25.Hong J, Kang SW. Carbon decorative coatings by dip-, spin-, and spray-assisted layer-by-layer assembly deposition. J. Nanosci. Nanotechnol. 2011;11:7771–7776. doi: 10.1166/jnn.2011.4737. [DOI] [PubMed] [Google Scholar]
- 26.Huang L, Zhang M, Li C, Shi G. Graphene-based membranes for molecular separation. J. Phys. Chem. Lett. 2015;6:2806–2815. doi: 10.1021/acs.jpclett.5b00914. [DOI] [PubMed] [Google Scholar]
- 27.Jiang DE, Cooper VR, Dai S. Porous graphene as the ultimate membrane for gas separation. Nano Lett. 2009;9:4019–4024. doi: 10.1021/nl9021946. [DOI] [PubMed] [Google Scholar]
- 28.Schrier J. Carbon dioxide separation with a two-dimensional polymer membrane. ACS Appl. Mater. Interfaces. 2012;4:3745–3752. doi: 10.1021/am300867d. [DOI] [PubMed] [Google Scholar]
- 29.Yoo, B. M., Shin, H. J., Yoon, H. W. & Park, H. B. Graphene and graphene oxide and their uses in barrier polymers. Journal of Applied Polymer Science131, doi:10.1002/app.39628 (2014).
- 30.Teramoto M, et al. Facilitated transport of CO2 through supported liquid membranes of various amine solutions-effects of rate and equilibrium of reaction between CO2 and amine- J. Chem. Eng. Jpn. 1997;30:328–335. doi: 10.1252/jcej.30.328. [DOI] [Google Scholar]
- 31.Caplow M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968;90:6795–6803. doi: 10.1021/ja01026a041. [DOI] [Google Scholar]
- 32.Danckwerts PV. The reaction of CO2 with ethanolamines. Chem. Eng. Sci. 1979;34:443–446. doi: 10.1016/0009-2509(79)85087-3. [DOI] [Google Scholar]
- 33.Huang J, Zou J, Ho WW. Carbon dioxide capture using a CO2-selective facilitated transport membrane. Ind. Eng. Chem. Res. 2008;47:1261–1267. doi: 10.1021/ie070794r. [DOI] [Google Scholar]
- 34.Park JS, Cho SM, Kim WJ, Park J, Yoo PJ. Fabrication of graphene thin films based on layer-by-layer self-assembly of functionalized graphene nanosheets. ACS Appl. Mater. Interfaces. 2011;3:360–368. doi: 10.1021/am100977p. [DOI] [PubMed] [Google Scholar]
- 35.Rallapalli P, et al. Sorption studies of CO2, CH4, N2, CO, O2 and Ar on nanoporous aluminum terephthalate [MIL-53 (Al)] J. Porous Mater. 2011;18:205–210. doi: 10.1007/s10934-010-9371-7. [DOI] [Google Scholar]
- 36.Hong J, Kang SW. Carbon Decorative Coatings by Dip-, Spin-, and Spray-Assisted Layer-by-Layer Assembly Deposition. J. Nanosci. Nanotechnol. 2011;11:7771–7776. doi: 10.1166/jnn.2011.4737. [DOI] [PubMed] [Google Scholar]
- 37.Kügler R, Schmitt J, Knoll W. The swelling behavior of polyelectrolyte multilayers in air of different relative humidity and in water. Macromol. Chem. Phys. 2002;203:413–419. doi: 10.1002/1521-3935(20020101)203:2<413::AID-MACP413>3.0.CO;2-R. [DOI] [Google Scholar]
- 38.Hirayama Y, et al. Permeation properties to CO2 and N2 of poly (ethylene oxide)-containing and crosslinked polymer films. J. Membr. Sci. 1999;160:87–99. doi: 10.1016/S0376-7388(99)00080-0. [DOI] [Google Scholar]
- 39.Du N, et al. Polymer nanosieve membranes for CO2-capture applications. Nat. Mater. 2011;10:372–375. doi: 10.1038/nmat2989. [DOI] [PubMed] [Google Scholar]
- 40.Park HB, et al. Polymers with cavities tuned for fast selective transport of small molecules and ions. Science. 2007;318:254–258. doi: 10.1126/science.1146744. [DOI] [PubMed] [Google Scholar]
- 41.Shekhawat, D., Luebke, D. R. & Pennline, H. W. A review of carbon dioxide selective membranes. US department of energy (2003).
- 42.Kovtyukhova NI, et al. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999;11:771–778. doi: 10.1021/cm981085u. [DOI] [Google Scholar]
- 43.Xu Y, Bai H, Lu G, Li C, Shi G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008;130:5856–5857. doi: 10.1021/ja800745y. [DOI] [PubMed] [Google Scholar]
- 44.Hong J, Char K, Kim BS. Hollow capsules of reduced graphene oxide nanosheets assembled on a sacrificial colloidal particle. J. Phys. Chem. Lett. 2010;1:3442–3445. doi: 10.1021/jz101441a. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


