Abstract
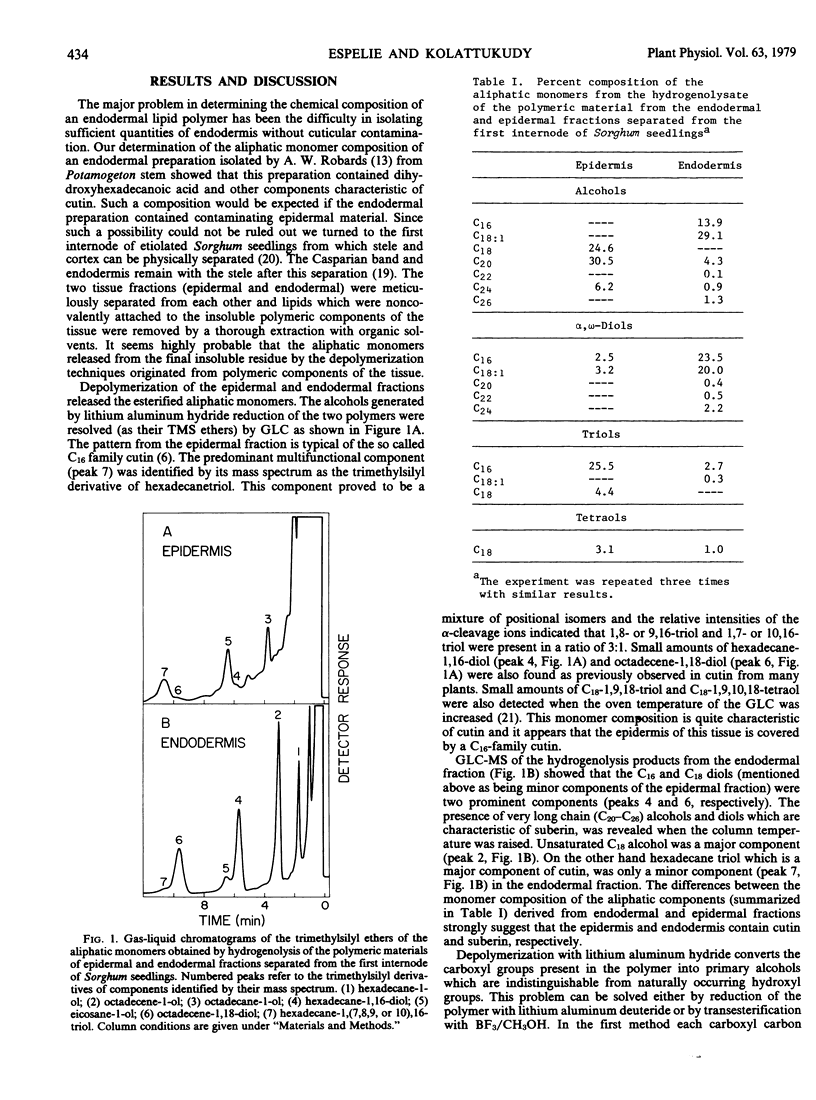
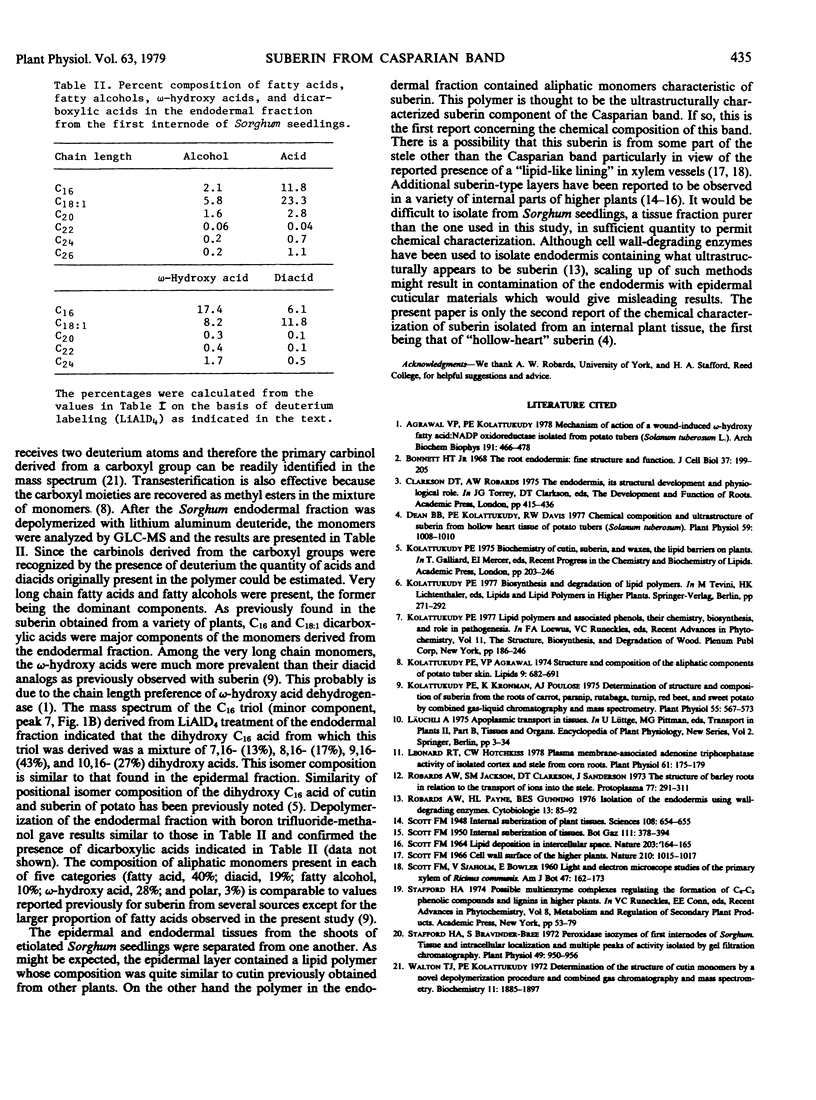
The stems from etiolated seedlings of Sorghum bicolor were separated into epidermal and endodermal fractions by manually removing the stele from the cortex. The epidermal fraction was shown to contain a lipid polymer whose monomeric composition was characteristic of cutin with dihydroxyhexadecanoic acid as a major component (25%). The endodermal fraction contained a lipid polymer whose monomeric composition was characteristic of suberin: hexadecanoic acid 12%, octadecenol 6%, octadecenoic acid 23%, ω-hydroxyhexadecanoic acid 17%, hexadecanedioc acid 8%, ω-hydroxyoctadecenoic acid 8%, and octadecenedioic acid 12%. This endodermal polymer is thought to be the suberin component of the Casparian band.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal V. P., Kolattukudy P. E. Mechanism of action of a wound-induced omega-hydroxyfatty acid:NADP oxidoreductase isolated from potato tubers (Solanum tuberosum L). Arch Biochem Biophys. 1978 Dec;191(2):466–478. doi: 10.1016/0003-9861(78)90385-5. [DOI] [PubMed] [Google Scholar]
- Dean B. B., Kolattukudy P. E., Davis R. W. Chemical Composition and Ultrastructure of Suberin from Hollow Heart Tissue of Potato Tubers (Solanum tuberosum). Plant Physiol. 1977 May;59(5):1008–1010. doi: 10.1104/pp.59.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E., Kronman K., Poulose A. J. Determination of structure and composition of suberin from the roots of carrot, parsnip, rutabaga, turnip, red beet, and sweet potato by combined gas-liquid chromatography and mass spectrometry. Plant Physiol. 1975 Mar;55(3):567–573. doi: 10.1104/pp.55.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Plasma Membrane-associated Adenosine Triphosphatase Activity of Isolated Cortex and Stele from Corn Roots. Plant Physiol. 1978 Feb;61(2):175–179. doi: 10.1104/pp.61.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott F. M. Internal Suberization of Plant Tissues. Science. 1948 Dec 10;108(2815):654–655. doi: 10.1126/science.108.2815.654. [DOI] [PubMed] [Google Scholar]
- Stafford H. A., Bravinder-Bree S. Peroxidase isozymes of first internodes of sorghum: tissue and intracellular localization and multiple peaks of activity isolated by gel filtration chromatography. Plant Physiol. 1972 Jun;49(6):950–956. doi: 10.1104/pp.49.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton T. J., Kolattukudy P. E. Determination of the structures of cutin monomers by a novel depolymerization procedure and combined gas chromatography and mass spectrometry. Biochemistry. 1972 May 9;11(10):1885–1896. doi: 10.1021/bi00760a025. [DOI] [PubMed] [Google Scholar]


