Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Plerixafor is a safe, effective, rapid mobilizing agent when administered intravenously.
Lower rates of GVHD and CMV viremia with plerixafor-mobilized grafts may be related to a unique cellular composition of the graft.
Abstract
A single subcutaneous (SC) injection of plerixafor results in rapid mobilization of hematopoietic progenitors, but fails to mobilize 33% of normal allogeneic sibling donors in 1 apheresis. We hypothesized that changing the route of administration of plerixafor from SC to IV may overcome the low stem cell yields and allow collection in 1 day. A phase 1 trial followed by a phase 2 efficacy trial was conducted in allogeneic sibling donors. The optimal dose of IV plerixafor was determined to be 0.32 mg/kg. The primary outcome of reducing the failure to collect ≥2 × 106 CD34+/kg recipient weight in 1 apheresis collection to ≤10% was not reached. The failure rate was 34%. Studies evaluating the stem cell phenotype and gene expression revealed a novel plasmacytoid dendritic cell precursor preferentially mobilized by plerixafor with high interferon-α producing ability. The observed cytomegalovirus (CMV) viremia rate for patients at risk was low (15%), as were the rates of acute grade 2-4 graft-versus-host disease (GVHD) (21%). Day 100 treatment related mortality was low (3%). In conclusion, plerixafor results in rapid stem cell mobilization regardless of route of administration and resulted in novel cellular composition of the graft and favorable recipient outcomes. These trials were registered at clinicaltrials.gov as #NCT00241358 and #NCT00914849.
Introduction
Successful hematopoietic stem cell transplantation (HSCT) has increasingly relied on collection of hematopoietic stem and progenitor cells (HSPCs) from peripheral blood. Granulocyte colony stimulating factor (G-CSF) remains the standard for peripheral blood stem cell mobilization. Improved engraftment and only slightly increased rates of graft-versus-host disease (GVHD) when compared with bone marrow–harvested cell sources in the sibling allogeneic transplant setting is responsible for this trend,1,2 but this may not hold true in all circumstances, such as in the setting of reduced intensity conditioning (RIC).3
Plerixafor is a bicyclam reversible small molecule inhibitor of the chemokine receptor CXCR4, which prevents binding of its ligand CXCL12 and induces mobilization of cells expressing this receptor, including HSPCs.4,5 Plerixafor is approved for subcutaneous (SC) administration for the mobilization of autologous stem cells in combination with G-CSF. The theoretical advantages of plerixafor to G-CSF mobilization are decreasing time to mobilize and reduced morbidity. A single SC injection of plerixafor at doses ranging from 0.08 to 0.24 mg/kg resulted in up to a 14-fold increase in the number of circulating hematopoietic progenitor cells within 4 hours in healthy volunteers with no grade 3 or 4 adverse reactions. Only 66% of donors mobilized with plerixafor collected an adequate product after 1 dose.6 Furthermore, the cells engrafted normally and grades 2-4 acute GVHD (aGVHD) occurred in 35%, with only 1 subject developing grade 4 aGVHD.6
This study was designed to evaluate the safety and efficacy of IV plerixafor for HSPC mobilization and transplantation. We hypothesized that, compared with SC administration, allogeneic donors mobilized with IV plerixafor would require fewer apheresis collections to obtain an adequate stem cell product; mobilization kinetics would be rapid and result in higher maximum (peak) CD34 stem cells; and recipients would have prompt, durable, and stable multilineage hematopoietic engraftment. Two trials were conducted to test these hypotheses. Initially, a dose escalation phase 1 study to evaluate the safety and maximal effective dose (MED) of IV plerixafor for the mobilization of HLA-matched sibling donors was performed. Subsequently, a phase 2 trial to evaluate the MED of IV plerixafor on reducing day 1 collection failure rates from 33% to 11%, similar to those published for G-CSF alone,7 was conducted.
Methods
Donors and recipients
The Washington University School of Medicine institutional review board approved this research. Donors and recipients were HLA-identical siblings ages 18 to 70 years and gave written informed consent. Recipients were required to have adequate organ function, as previously described,6 and advanced hematologic malignancies suitable for transplant. Donors were required to be HIV and HTLV 1 and 2 negative with adequate organ function and European Cooperative Oncology Group performance status 0-1.
Phase 1
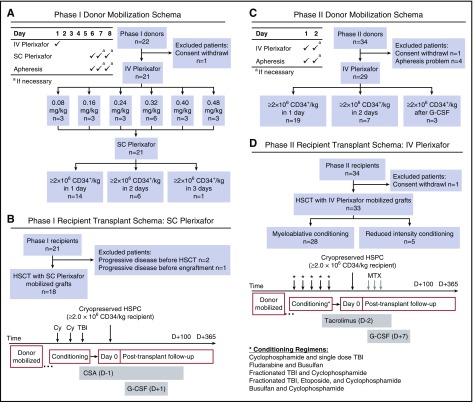
A phase 1 trial of IV plerixafor was performed using a standard 3+3 design with 6 dose-escalation cohorts (Figure 1A). Plerixafor was administered as a 30-minute infusion in the morning and electrocardiographs were performed at preinfusion, 1 hour, and 4 hours postinfusion to document any cardiac toxicity. Donors were allowed a 4-day washout, then were mobilized and collected with 0.24 mg/kg SC plerixafor on day 6 followed 4 hours later by a 20-L apheresis using a COBE Spectra pheresis machine. SC plerixafor dose and apheresis was repeated on days 7 and 8 if needed. Recipients received SC plerixafor-mobilized cells if ≥2 × 106 CD34+/kg recipient weight were collected (Figure 1B). If donors failed to mobilize an adequate product after 2 apheresis procedures with SC plerixafor, they were remobilized with G-CSF and recipients received G-CSF–mobilized products.
Figure 1.
Clinical trial schemas and STARD diagrams of flow of participants through the study. An open-label phase 1/2 trial was conducted to evaluate CD34+ HSPC mobilization with IV plerixafor. (A) Phase 1 donor mobilization schema and STARD flow diagram. HLA-identical sibling donors (≥18 years old) received IV plerixafor the morning of day 1 over 30 minutes with blood sampling obtained before and throughout the course of mobilization. After a 4-day washout period, SC plerixafor (0.24 mg/kg) was administered on day 6, approximately 4 hours before initiation of a 20-L apheresis. Products were cryopreserved and if the desired target of ≥2 × 106 CD34+ HSPCs/kg recipient weight was not achieved, the donor was remobilized using 0.24 mg/kg SC plerixafor. (B) Phase 1 recipient transplant schema and STARD flow diagram. Similar to our prior SC plerixafor trial, recipient conditioning used myeloablative cyclophosphamide (60 mg/kg per day on days −3 and −2) and single-dose total body irradiation (TBI; 550 cGy, day −1). Cyclosporine A (CSA) was used as GVHD prophylaxis and G-CSF was administered starting on day 1 until neutrophil engraftment. (C) Phase 2 donor mobilization schema and STARD flow diagram. HLA-identical sibling donors (≥18 years old) received IV plerixafor the morning of day 1 over 30 minutes with blood sampling obtained before initiation of a 20-L apheresis. Products were cryopreserved, and if the desired target of ≥2 × 106 CD34+ HSPCs/kg recipient weight was not achieved, the donor was remobilized the following day using 0.32 mg/kg IV plerixafor. If ≥2 × 106 CD34+ HSPCs/kg recipient weight was not achieved after 2 days of IV plerixafor, the donor was remobilized using G-CSF. (D) Phase 2 recipient transplant schema and STARD flow diagram. In contrast to our prior study, recipients in phase 2 of this study were conditioned with myeloablative or reduced intensity regimens (as outlined in the schema), followed by infusion of the entire collected product on day 0 and short-course methotrexate (MTX) and tacrolimus GVHD prophylaxis. In addition, G-CSF was administered starting on day 7 until neutrophil engraftment. STARD, Standards for Reporting of Diagnostic Accuracy.
Phase 2
After determining the MED of IV plerixafor based on pharmacodynamics of CD34 cell mobilization and pharmacokinetics (PKs) of IV plerixafor, a phase 2 open-label trial was conducted in which healthy 6/6 HLA-matched sibling donors age 18 to 70 were mobilized with 0.32 mg/kg IV plerixafor (>30 minutes), followed by a 20-L apheresis using a COBE Spectra pheresis machine starting 4 hours after injection (Figure 1C). Apheresis could be repeated on day 2 if ≥2 × 106 CD34+/kg actual recipient weight was not obtained on day 1. Donors failing to collect sufficient CD34+ cells after 2 apheresis procedures were remobilized with standard dose G-CSF. Definitions of engraftment, graft failure, relapse, and GVHD have previously been described.6 The conditioning regimen was left to the discretion of the treating physician, but could not include antithymocyte globulin. G-CSF was administered to recipients starting on day 1 until neutrophil engraftment. GVHD prophylaxis consisted of IV methotrexate (10 mg/m2 on day +1; 7.5 mg/m2 on days +3 and +6) and tacrolimus. Standard supportive care and prophylactic medications were administered after transplant, as previously described (Figure 1D).6
Correlative studies
PK of plerixafor, CXC12 genotyping, CD34 immunophenotyping, gene expression profiling, interferon-α (IFN-α) production, transmigration assays, and colony-forming cell assays were performed, as previously described and detailed in the supplemental Methods available on the Blood Web site.6,8
Statistical analysis
For the phase 2 portion of the trial, a sample of 27 was calculated a priori to provide 81% power to detect a difference in failure rates of 33% vs 11%. Comparator group data of SC plerixafor (0.24 mg/kg) failure rates have previously been reported.6 Linear repeated measures mixed models were used to evaluate mobilization of CD34 and other cell types, pharmacodynamics and PKs, and longitudinal analyses. As noted, some were carried out on a transformed scale to improve the fit of models. Results, including model-derived means and 95% confidence intervals (CIs), are presented on the original scale for ease of interpretation. Fisher exact test was used to test for difference in mobilization success rates. Kaplan-Meier and Cox proportional hazard models were used to estimate median overall survival, relapse-free interval, and times to neutrophil and platelet engraftment. Cumulative incidence models of aGVHD and chronic GVHD (cGVHD), engraftment, survival, and relapse were generated in Graph Pad Prism 5, version 5.0a, using the Kaplan-Meier method.
Results
Phase 1
Phase 1 enrolled 22 donor/recipient pairs, but 1 donor was removed and replaced in the 0.32 mg/kg cohort after withdrawal of consent. Three donor/recipient pairs were enrolled in each of the 6 cohorts, and 3 additional subjects were enrolled in the recommended phase 2 dosing cohort of 0.32 mg/kg. No dose-limiting toxicities were observed with IV plerixafor and none of the 21 donors administered varying doses of IV plerixafor and then remobilized with SC plerixafor failed to collect an adequate number of CD34/kg (≥2 × 106 CD34/kg) for HSCT. Six of 21 donors required a second collection, and 1 donor, who was anemic at baseline, required a third collection (Figure 1A).
Pharmacodynamics and PKs of IV plerixafor
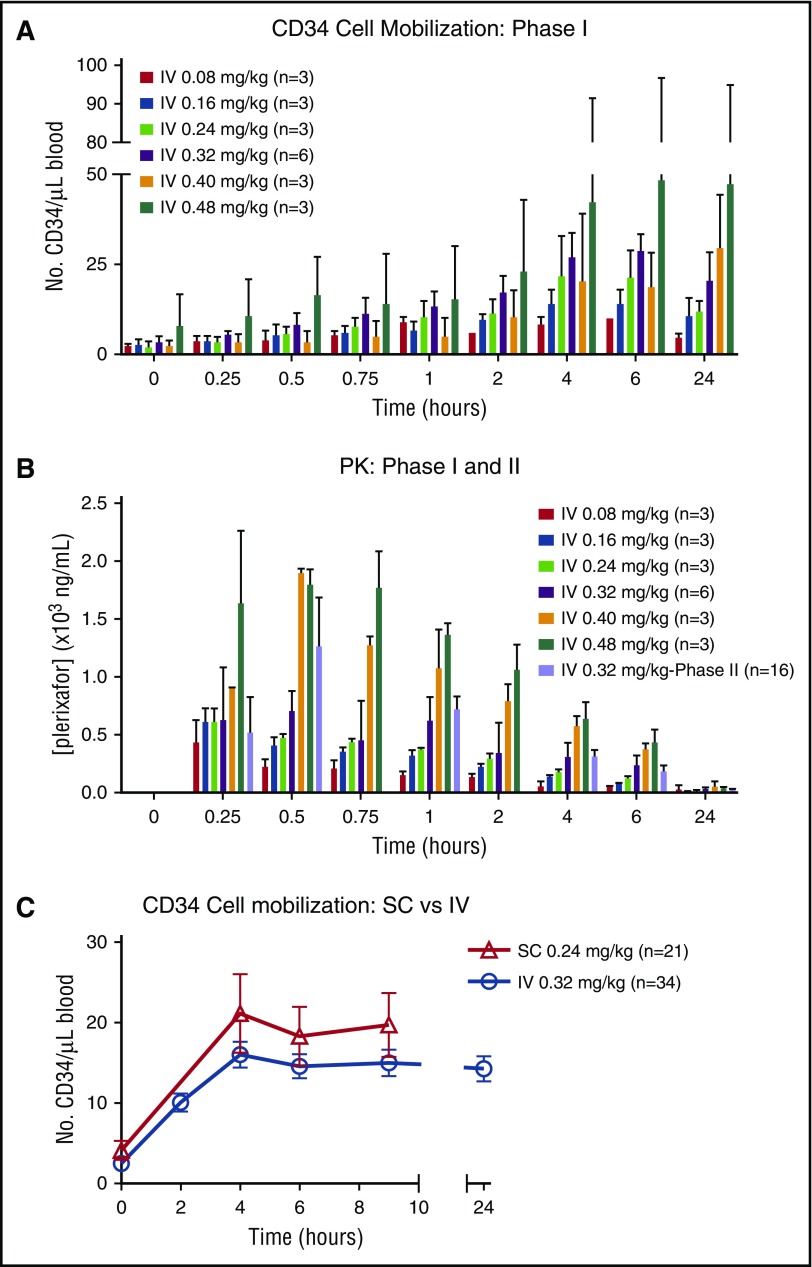
CD34 mobilization peaked at 4 to 6 hours after IV plerixafor administration in all cohorts (Figure 2A). There was significant variability in mobilization observed in the higher dose cohorts of 0.4 and 0.48 mg/kg. The peak plasma concentrations of plerixafor were measured between 15 and 30 minutes after initiating the IV infusion (Figure 2B). Elimination was consistent with a 2-compartment model with a half-life (t1/2) of ∼6 hours, similar to SC dosing. The maximum serum concentration (Cmax) in the highest dosing cohort (0.48 mg/kg) approached 2000 ng/mL, just below the 2500 ng/mL seen in HIV patients, who developed ventricular arrhythmias after continuous IV dosing.9 At a dose of 0.32 mg/kg, the estimated Cmax of IV plerixafor ranged between 633 and 1060 ng/mL (mean, 829 ± 176). The PKs for IV plerixafor was similar to what has been described previously.9-11 Based on the significant donor-to-donor variability as well as the marginal and inconsistent dose-response relationship with doses of IV plerixafor >0.32 mg/kg, the 0.32 mg/kg dose was determined to be the maximally effective dose for study in the phase 2 trial. At the phase 2 dose of 0.32 mg/kg IV, the mean Cmax was 1391 ng/mL, and this was detected toward the end of infusion (Figure 2B). Mean area under the curve (AUC) from time 0 to infinity was 5049 hours × ng/mL, with the extrapolated portion <30%. The median terminal t1/2 was 5.42 hours.
Figure 2.
CD34+HSPC mobilization and plerixafor PKs. (A) Mobilization of CD34+ cells to the peripheral blood over time from donors in phase 1 portion of the trial. (B) PK levels of plerixafor measured in blood over time in phases 1 and 2. (C) Summary of mobilization data of CD34+ cells over time in donors treated with IV and SC plerixafor. SC mobilization results were obtained from phase 1 of this study and IV data from phase 1 and 2 trials.
Phase 2
Phase 2 enrolled 34 donors. Thirty donor/recipient pairs were initially enrolled and 4 additional pairs of subjects were enrolled to replace donors who failed to collect goal apheresis volume resulting from technical problems primarily related to adequate access and blood flow at the time of the initial apheresis collections. One recipient was not transplanted because of donor withdrawal of consent to collect a second day after a failed first-day apheresis attempt. Table 1 summarizes patient characteristics for phase 2 of this trial. Comparison transplant schemas for recipients are shown in Figure 1B,D. Median follow-up of recipients is 278 days (range, 37-2368 days).
Table 1.
Patient characteristics, phase 2
| Patient characteristics | Donors | Recipients |
|---|---|---|
| Number | 34 | 33 |
| Median age, d (range) | 50 (28-65) | 54 (26-68) |
| Male, % | 53 | 65 |
| Mean weight, kg (range) | 95 (54-172) | 92 (61-183) |
| Mean apheresis volume, L (range) | 17.9 (3.7-20) | NA |
| Diagnosis (n) | NA | AML (14) |
| NHL (9) | ||
| CLL (3) | ||
| MDS (3) | ||
| ALL (3) | ||
| CML (1) | ||
| HD (1) | ||
| Refractory/persistent disease, % (n) | NA | 48.5 (16) |
| Conditioning regimen, % (n) | NA | Myeloablative: 85 (28) |
| Fludarabine RIC: 15 (5) | ||
| Median follow up, d (range) | 27-35 | 248 (37-695) |
| CMV at risk, % | 62 | |
CMV at risk is defined as CMV seropositive donor, recipient, or both.
AML, acute myeloid lymphoma; CLL, chronic lymphocytic leukemia; CML, chronic myeloid lymphoma; HD, Hodgkin disease; MDS, myelodysplastic syndrome; NA, nonapplicable; NHL, non-Hodgkin lymphoma.
The primary outcome of failure to reach ≥2 × 106 CD34+/kg recipient weight was analyzed on a per-protocol basis. Twenty-nine of 34 donors were evaluable for the primary outcome (Table 1). Thirty-four percent (10/29) of donors failed to reach the day 1 collection goal of ≥2 × 106 CD34/kg recipient weight (Figure 1C). Ten percent (3/29) failed to reach goal after 2 apheresis procedures. There was no appreciable difference in CD34 mobilization kinetics or failure rates when comparing IV vs SC dosing (Figure 2C; Table 2). Of those donors failing to mobilize an adequate number of CD34 cells (n = 3), none failed to collect after G-CSF mobilization. In addition to CD34 mobilization, peripheral blood cellular composition after plerixafor was evaluated and showed pan mobilization of neutrophils, monocytes, and T and B lymphocytes (supplemental Figure 1).
Table 2.
Characteristics of IV plerixafor-mobilized apheresis products, phase 2
| Characteristic | Mean (range) |
|---|---|
| Viability of product, % | 93 (87-99) |
| Length of product storage, d | 24 (6-241) |
| Peak mobilized CD34/μL, phase 1 | 28.7 (7-157) |
| Peak mobilized CD34/μL, phase 2 | 18.6 (4-46) |
| Total no. CD34 collected day 1 × 108 | 2.1 (0.5-6.6) |
| Pheresis volume day 1, L | 17.91 (3.7-20) |
| No. CD3 × 108/kg recipient weight | 5.25 (1.98-10) |
| No. CD34 × 106/kg recipient weight | 2.9 × 106 (2.0-9.7 × 106) |
Donor toxicity
IV plerixafor was well tolerated by donors in both phases 1 and 2 of the trial (supplemental Tables 1 and 2). There were no grade 3-4 toxicities. The most common reported side effects were grade 1 gastrointestinal toxicity (abdominal bloating [30%]) and grade 1 bradycardia (30%). All recorded toxicities have been previously reported for SC plerixafor.6
Predictors of CD34+ cell mobilization
Younger donor age, higher baseline circulating CD34, and higher baseline platelets were associated with higher CD34 collection (supplemental Figure 2; Table 3). There was also an association of male sex (P = .052) (supplemental Figure 2B). A change in peripheral blood CXCL12 levels from baseline to 30 minutes was associated with improved CD34 yield (supplemental Figure 2C-D; P = .0085). However, there was no association between CD34 and CXCL12 concentration at baseline or after 30 minutes. Evaluation of cellular subsets collected in the product demonstrated an inverse association of plasmacytoid dendritic cell (pDC) progenitor cells (pro-DC2) isolated from the CD34+ fraction of the apheresis product and CD34 yield (Table 3 and 4). Clinical factors implicated in mobilization were not found to be significant in this small cohort of subjects, including statin use, diabetes, tobacco use, and CXCL12 rs1801157 polymorphism (Tables 3 and 4; supplemental Figure 3).12 In the univariate analysis, the PKs of plerixafor, AUC, and Cmax in the phase 2 portion did not predict mobilization (P = .064 and 0.084, respectively). In the multivariate analysis, the strongest predictors of CD34/L collected on day 1 were age, baseline CD34, %pro-DC2 precursors in the product, baseline platelets, and AUC of plerixafor (Table 4).
Table 3.
Univariate analysis of factors affecting CD34 mobilization yields
| Covariate | F value | P value |
|---|---|---|
| Weight of donor | 1.50 | .23 |
| Age of donor (younger) | 6.02 | .020 |
| Sex of donor (male) | 4.07 | .052 |
| Baseline CD34 (high) | 14.94 | .0005 |
| Statin use by donor | 3.01 | .093 |
| % pDC in product | 14.24 | .0007 |
| CXCL12 at baseline | 1.15 | .29 |
| Change CXCL12 baseline to 30 min | t = 3.05 | .0048 |
| CXCL12 SNP genotype | 1.37 | .27 |
| Diabetes in donor | 0.87 | .36 |
| Hypertension in donor | 0.02 | .89 |
| Baseline platelets | 5.85 | .022 |
| Baseline WBC | 0.05 | .83 |
| Tobacco use by donor | 0.18 | .84 |
| Cmax of plerixafor | 3.19 | .084 |
| AUC of plerixafor | 3.70 | .064 |
A repeated measures regression model of CD34/L undergoing apheresis day 1 transformed to log10 by demographic and clinical variables was performed. Cellular mobilization over time was analyzed by mixed models (transformation shown in parentheses) specifying patient as a random effect and modeling the outcome of CD34/L day 1 on a log10 scale.
SNP, single-nucleotide polymorphism; WBC, white blood cell.
Table 4.
Multivariate analysis of factors affecting CD34 mobilization yields
| Covariate | F value | P value |
|---|---|---|
| Age of donor | 9.44 | .0051 |
| Baseline CD34 | 27.35 | <.0001 |
| % pDC in product | 5.81 | .024 |
| Baseline platelets | 11.64 | .0022 |
| AUC of plerixafor | 5.63 | .026 |
A repeated measures regression model of CD34/L undergoing apheresis day 1 transformed to log10 by demographic and clinical variables was performed. Cellular mobilization over time was analyzed by mixed models (transformation shown in parentheses) specifying patient as a random effect and modeling the outcome of CD34/L day 1 on a log10 scale. Factors reaching significance at the P < .1 level were carried forward to the multivariate analysis.
Plerixafor and G-CSF mobilize phenotypically different CD34+ subsets
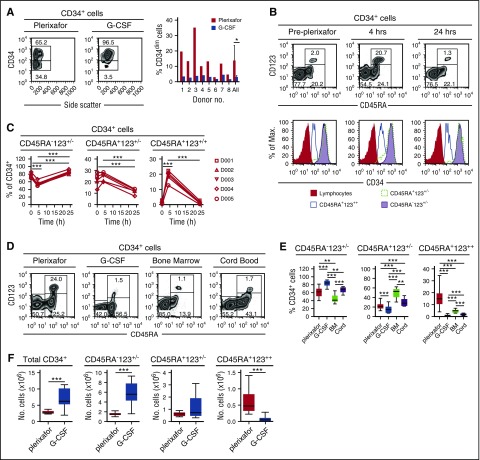
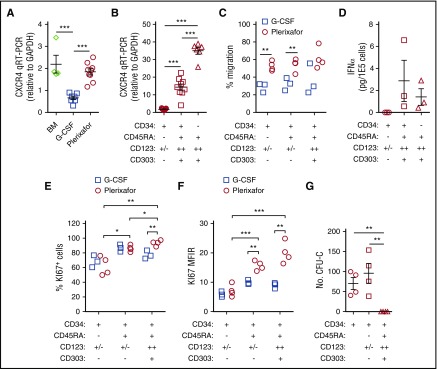
CD34+ cells obtained from 8 donors mobilized sequentially with plerixafor and G-CSF6 showed that plerixafor mobilized a population of CD34dim cells (Figure 3A). Human CD34+ cells can be divided into 3 distinct subsets based on their expression of CD45RA and CD123: (1) CD45RA−CD123+/− cells containing common myeloid progenitors, megakaryocyte/erythrocyte progenitors, and more primitive HSPCs; (2) CD45RA+CD123+/− cells containing more committed granulocyte/macrophage and lymphoid progenitors; and (3) CD45RA+CD123++ cells.13,14 Plerixafor preferentially mobilized CD34+CD45RA+CD123++ cells from 2% at baseline to nearly 18% at 4 hours (Figure 3B-C). Furthermore, consistent with the mobilization of CD34dim (Figure 3A), the CD45RA+CD123++ cells expressed lower levels of CD34 compared with the CD45RA−CD123+/− and CD45RA+CD123+/− subsets (Figure 3B). We observed variability of CD34+ cell subsets in leukapheresis products obtained from donors mobilized with G-CSF or plerixafor as well as in bone marrow and cord blood units (Figure 3D-F), G-CSF mobilized more CD45RA− primitive hematopoietic stem cells, bone marrow was enriched with CD45RA+CD123+/− committed progenitors, and plerixafor mobilized more CD45RA+CD123++ cells.
Figure 3.
Preferential mobilization CD34dimCD45RA+CD123++cells by plerixafor. (A) Eight healthy donors were mobilized sequentially with plerixafor (0.24 mg/kg SC), then 10 days later with G-CSF (10 μg/kg per day × 5 days). Plerixafor mobilized a population of CD34dim cells that were present, on average, in nearly fivefold higher numbers compared with the G-CSF–mobilized CD34+ cells (P = .02). (B) Coexpression of CD45RA and CD123 on CD34+ cells identifies the CD34dim subset preferentially mobilized by plerixafor. CD45RA and CD123 expression on purified CD34+ cells was evaluated by flow cytometry before and after treatment with plerixafor. (C) Relative contributions of CD34 subsets after plerixafor. Healthy donors were treated with a single injection of plerixafor (n = 5). CD34+ cells in the peripheral blood of donors before and after plerixafor were purified by CD34 immunomagnetic selection; expression of CD45RA and CD123 was evaluated by flow cytometry. The relative contribution of each CD34+ subset is shown as a function of time. (D-F) Healthy donors were treated with a single injection of plerixafor (n = 31) or given 10 μg/kg per day G-CSF (n = 17) for 5 days. CD34+ cells from the plerixafor- or G-CSF–mobilized leukapheresis products, normal healthy bone marrow (n = 5), or cord blood (n = 9) were purified by CD34 immunomagnetic selection; the expression of CD45RA and CD123 was evaluated by flow cytometry. (D) Representative flow cytometry profiles. (E) Relative contribution of each CD34+ subset. (F) Absolute number of different CD34+ cells subsets in leukapheresis products. Statistical comparisons were performed using an unpaired 2-tailed Student t test. *P < .05, **P < .01, ***P < .001. Data are mean ± standard deviation.
Preferential mobilization of a pDC precursor by plerixafor
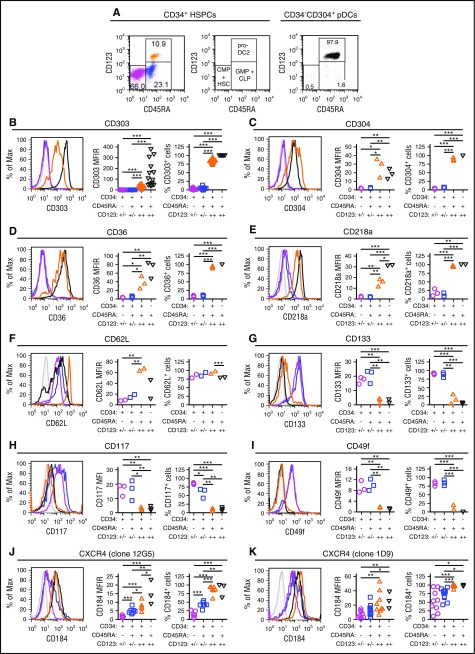
Blom et al previously characterized a CD34dimCD45RA+CD123++ pro-DC2 (for progenitor of pre-DC2) subset that differentiates into pDCs.15 Phenotyping studies comparing CD34+ stem cell subsets to mature CD304+ pDCs demonstrated that the CD34+CD45RA+CD123++ cells preferentially mobilized by plerixafor are pro-DC2 cells that express the pDC-specific antigen CD303 (Figures 4A-B). Additionally, these pro-DC2 cells expressed significantly more CD304, CD36, CD218a, CD62L, CD197, CD4, and CD2 than the CD34+CD45RA−CD123+/− and CD34+CD45RA+CD123+/− stem cell subsets (Figure 4C-F; supplemental Figure 4). In contrast, the pro-DC2 cells lacked expression of more primitive stem cell antigens such as CD133, CD117, CD49f, and CD90 (Figure 4G-I; supplemental Figure 4).
Figure 4.
Immune phenotyping of CD34 subsets. CD34+ HSPCs and CD304+ pDCs from plerixafor-mobilized leukapheresis products were purified by immunomagnetic selection and evaluated by flow cytometry. (A) Representative analysis of CD45RA and CD123 expression on purified CD34+ HSPCs and CD304+ pDCs. The percentages of CD34+CD45RA−CD123+/− common myeloid progenitors CMPs and hematopoietic stem cells (magenta), CD34+CD45RA+CD123+/− granulocyte/macrophage progenitors (GMPs) + common lymphoid progenitors (CLPs; blue), CD34+CD45RA+CD123++ pro-DC2s (orange), and CD34-CD45RA+CD123++CD304+ pDCs (black) cells are shown. (B-K) Expression of CD303 (B; n = 16), CD304 (C; n = 3), CD36 (D; n = 3), CD218a (E; n = 3), CD62L (F; n = 2), CD133 (G; n = 3), CD117 (H; n = 3), CD49f (I; n = 3), clone 12G5 of CD184 (J; n = 7 for CD34+ HSPCs and n = 3 for pDCs) and clone 1D9 of CD184 (K; n = 9 for CD34+ HSPCs and n = 3 for pDCs) on the different CD34+ HSPC subsets and CD304+ pDCs. Representative histograms as well as plots of MFIRs and percentage of positive cells are shown for each antigen. The CD34+CD45RA+CD123++ pro-DC2 cells preferentially mobilized by plerixafor express high levels of CD303, CD304, CD36, CD218a, CD62L, and CD184. MFIR, mean fluorescence intensity ratio. Statistical comparisons were performed using an unpaired 2-tailed Student t test. *P < .05, **P < .01, ***P < .001.
G-CSF mobilization is associated with decreased expression of CXCR4 on CD34+ HSPCs and receptor expression is increased upon the addition of plerixafor to G-CSF.16-21 We found that CD34+ HSPCs mobilized by G-CSF exhibited lower levels of CXCR4 messenger RNA (mRNA) compared with bone marrow and plerixafor-mobilized CD34+ cells (Figure 5A). Furthermore, the CD34+CD45RA+CD123++ pro-DC2 cells preferentially mobilized by plerixafor expressed high levels of CXCR4 (Figures 4J-K and 5B) and efficiently migrated toward SDF-1 (Figure 5C).
Figure 5.
Functional characterization of CD34 subsets. (A-B) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of CXCR4 mRNA. (A) Expression of CXCR4 mRNA in CD34+ HSPCs. Total CD34+ HSPCs were purified by immunomagnetic selection from normal healthy bone marrow (n = 4), G-CSF (10 μg/kg per day × 5 days) mobilized leukapheresis products (n = 7), or plerixafor-mobilized leukapheresis products (n = 9). qRT-PCR of CXCR4 mRNA in the different cell subsets was normalized to GAPDH. (B) Expression of CXCR4 mRNA in CD34+ HSPC subsets. CD34+ HSPCs (n = 7) from plerixafor-mobilized leukapheresis products were purified by magnetic-activated cell sorting and CD34+CD45RA−CD123+/− HSPCs (n = 9); CD34dimCD45RA+CD123++ pro-DC2 cells (n = 9) were isolated by flow cytometry. (C) CD34+ HSPCs from plerixafor (n = 4) or G-CSF (n = 3) mobilized leukapheresis products were purified by immunomagnetic selection and added into the upper chamber of a 5-μm pore size transwell. After 4 hours, migrated cells recovered from the lower chamber were counted and CD34+CD45RA−CD123+/−, CD34+CD45RA+CD123+/−, and CD34+CD45RA+CD123+ were enumerated by flow cytometry (D) after treatment with ODN2216. Flow cytometry–purified CD34+CD45RA+CD123++CD303+ pro-DC2 cells were treated with vehicle alone or ODN2216 (a CpG TLR9 agonist) in the presence of IL-3 and CD40 ligand-transfected L cells. After 48 hours, the amount of IFN-α in the supernatant was determined using an enzyme-linked immunosorbent assay. Purified CD34+CD45RA− HSPCs and CD34−CD45RA+CD123++CD303+ pDCs were included in these studies as negative and positive controls, respectively. (E) CD34+ HSPCs from plerixafor-mobilized (n = 4) or G-CSF–mobilized (n = 3) leukapheresis products were purified by immunomagnetic selection; KI67 expression was evaluated by flow cytometry. The percentage of KI67 positive cells (E) and KI67 MFIR (F) is shown. (G) MethoCult colony-forming unit assay of CD34 subsets. Statistical comparisons were performed using an unpaired 2-tailed Student t test. *P < .05, **P < .01, ***P < .001.
The pro-DC2 cells mobilized by plerixafor have the ability to produce IFN-α after exposure to a CpG TLR9 agonist, ODN2216, similar to mature pDCs (Figure 5D). Using KI-67 as a marker of cell proliferation, we observed that the plerixafor-mobilized pro-DC2 cells are more actively dividing than the primitive CD34+CD45RA−CD123+/− HSPCs (Figure 5E-F). Further, plerixafor-mobilized CD34+CD45RA+CD123+/− and CD34+CD45RA+CD123++ cells expressed more Ki67 than the G-CSF–mobilized cells (Figure 5E-F). Finally, the CD34+CD45RA+CD123++ pro-DC2 cells do not form colonies in a methylcellulose colony-forming unit assay (Figure 5G). These results suggest that the pro-DC2 cells mobilized by plerixafor lack the potential to differentiate into myeloid and erythroid cells.
Comparison of gene expression in plerixafor and G-CSF–mobilized CD34+ cells
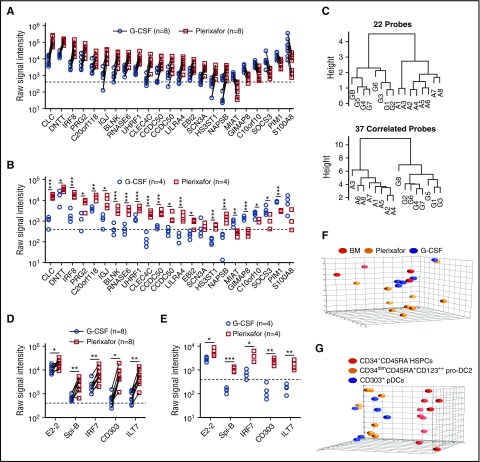
Using a dual-threshold gene expression analysis, we found that 16 genes had higher RNA expression levels in plerixafor-mobilized bulk CD34+ cells, whereas 6 genes were more abundantly expressed in G-CSF–mobilized CD34+ cells (Figure 6A). To confirm differential expression, we repeated the RNA profiling using purified CD34+ cells obtained from 8 unpaired donors mobilized with either plerixafor or G-CSF. All but 2 of the 22 genes (SCN3A and S100A8) identified in the paired analysis remained significantly different and exhibited the same trend in expression level between the plerixafor and G-CSF–mobilized CD34+ cells (Figure 6B). A genetic algorithm (GA)-Mantel analysis identified 37 additional genes that produced a distance matrix that correlated with the distance matrix produced by the 22 genes found by the threshold method (supplemental Table 3). Dendrograms based on the 22 genes identified by threshold method and 37 additional genes found by GA-Mantel analysis were constructed and show distinct separation between G-CSF and plerixafor CD34+ cells (Figure 6C). Plerixafor-mobilized CD34+ cells expressed significantly more of the specific transcriptional regulator of pDC development, E2-2, as well as additional transcriptional factors (SpiB, IRF7, IRF8) and cell surface markers (BDCA-2, ILT7) that are specific to the pDC lineage (Figure 6D-E).
Figure 6.
Gene expression profiling of plerixafor and G-CSF–mobilized donor CD34+cells from leukapheresis products. (A,D) mRNA profiling was performed using Affymetrix U133+2 arrays on purified CD34+ stem cells obtained from 8 individual normal donors mobilized sequentially with plerixafor and G-CSF. (A) Shown are 22 genes with a raw signal intensity ≥400 (dotted line) in all the microarrays from 1 of the mobilizing agents; the ratio of plerixafor to G-CSF within each pair for the gene had to be at least a twofold difference all in the same direction. (B,E) mRNA profiling was performed using Affymetrix U133+2 arrays on purified CD34+ stem cells obtained from 4 individual normal donors mobilized with plerixafor or G-CSF. Statistical comparisons were performed using an unpaired 2-tailed Student t test. (C) GA-Mantel algorithm distance matrix of 22 original probes and 37 correlated probes. The dendrogram on the left is based on the 22 genes identified from the dual-threshold analysis approach; the dendrogram on the right is based on 37 genes found with a high Mantel correlation. (D) Expression of pDC lineage-associated genes in purified CD34+ stem cells obtained from 8 individual normal donors mobilized sequentially with plerixafor and G-CSF. Statistical comparisons were performed using a paired parametric Student t test. (E) Expression of pDC lineage-associated genes in purified CD34+ stem cells obtained from 4 individual normal donors mobilized with plerixafor or G-CSF. Statistical comparisons were performed using an unpaired 2-tailed Student t test. (F) Principal component analysis comparing CD34+ cells isolated from plerixafor or G-CSF–mobilized donors or normal bone marrow, which shows that the majority of CD34+ cells isolated from plerixafor-mobilized donors were distinct from the G-CSF–mobilized and normal bone marrow CD34+ cells. (G) Principal component analysis showing evident separation of CD34+CD45RA− HSPCs, CD34dimCD45RA+CD123++ pro-DC2, and CD304+ pDCs isolated from plerixafor-mobilized donors. Statistical comparisons were performed using an unpaired 2-tailed Student t test. *P < .05, **P < .01, ***P < .001.
Principal component analyses on flow cytometry–purified subsets showed that the plerixafor-mobilized CD34+CD45RA− primitive HSPCs were a distinctive population compared with the G-CSF or unmobilized bone marrow (Figure 6F). In an analysis of variance relative to unmobilized bone marrow CD34+CD45RA− HSPCs, the expression of only 9 probes was significantly (P < .01; q < 0.05) altered at least threefold in response to G-CSF, whereas plerixafor treatment resulted in 311 differentially (at least threefold) regulated probes (supplemental Table 3). Comparison of plerixafor- and G-CSF–mobilized CD34+CD45RA− HSPCs in this analysis yielded 426 differentially expressed probes (supplemental Tables 4 and 5; supplemental Figure 5A). Pathway enrichment was done in 2 ways: (1) R package GAGE and (2) GeneGO pathway analysis using the 426 genes differentially expressed at least threefold between plerixafor and G-CSF HSPCs. The top 5 pathways distinguishing plerixafor and G-CSF–mobilized CD34+CD45RA− HSPCs by gene enrichment analysis were: (1) regulation of tissue factor signaling in cancer; (2) immune response interleukin-5 (IL-5) signaling via JAK/STAT; (3) immune response macrophage migration inhibitory factor–mediated glucocorticoid regulation; (4) immune response macrophage migration inhibitory factor–induced cell adhesion, migration, and angiogenesis; and (5) immune response IL-2 activation and signaling pathway (supplemental Table 6). Kyoto Encyclopedia of Genes and Genomes pathway analysis using GAGE identified several disease and signaling and metabolism pathways that distinguish plerixafor and G-CSF–mobilized CD34+CD45RA− HSPCs (supplemental Tables 7 and 8). The disease pathway analysis showed that genes associated with GVHD and allograft rejection were increased in plerixafor-mobilized CD34+CD45RA− HSPCs relative to G-CSF–mobilized HSPCs (supplemental Table 6; supplemental Figure 5B). The primary driver of this difference was increased expression of HLA genes on plerixafor HSPCs compared with G-CSF and bone marrow and decreased expression of FAS, FASLG, CD40LG, tumor necrosis factor, and CD86 (supplemental Figure 5B). Finally, the pro-DC2 and pDCs clustered together and separately from the CD34+CD45RA− HSPCs in the principal component analysis (Figure 6G).
Engraftment
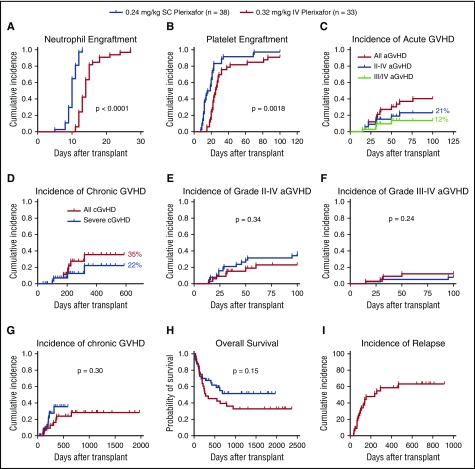
Phase 1 recipients received grafts mobilized by SC plerixafor, and outcomes were similar to those previously reported.6 All patients engrafted neutrophils and platelets promptly. The median time to absolute neutrophil count ≥0.5 × 109/L was 10 days (range, 8-32 days) and to platelet >50 × 109/L was 19 days (range, 12-33 days). For the 21 patients in which HSPCs were collected with SC plerixafor after initial exposure to IV plerixafor, 2 of 21 patients did not get transplanted because of progressive disease before HSCT (Figure 1B). One of 21 patients died on day 13 before engraftment because of progressive disease. The remaining 18 evaluable patients all promptly engrafted neutrophils and platelets (median absolute neutrophil count engraftment, 10 days [range, 9-12]; median platelet engraftment, 33 days [range, 19−70]).
All phase 2 recipients receiving products mobilized by IV plerixafor engrafted. The mean CD34 cell dose/kg recipient weight infused was 2.9 × 106 (2-9.7 × 106) (Table 2). The median time to neutrophil engraftment was 14 days (compared with 10 days for SC plerixafor), with 31/33 (94%) engrafting by day 21 (Figure 7A; Table 5). The median time to platelet engraftment was 25 days, with 23/33 (70%) engrafting by day 30 (compared with 22 days and 95% observed for SC plerixafor) (Figure 7B; Table 5). When comparing these results to those previously reported for SC plerixafor (Table 5; Figure 7A-B), there was a prolonged time to neutrophil and platelet engraftment in the IV plerixafor group attributable to the use of methotrexate in the GVHD prophylaxis. A total of 31/33 (94%) recipients receiving myeloablative conditioning had 100% donor chimerism by days 30 and 100. The 2 myeloablative patients who lost engraftment developed relapsed disease. In contrast, only 2/5 (40%) of recipients receiving RIC had 100% donor chimerism by day 100. Of these patients conditioned with RIC, 3 developed mixed chimerism after transplant by day 100. Two patients had relapse, and 1 had 50% mixed chimerism and subsequently died of cytomegalovirus (CMV) disease.
Figure 7.
Recipient outcomes. Recipients were transplanted with grafts mobilized with either SC (0.24 mg/kg; n = 38) or IV (0.32 mg/kg; n = 33) plerixafor. The time to neutrophil and platelet engraftment was defined as the first of 2 consecutive days with an absolute neutrophil count ≥0.5 × 109/L or a platelet count ≥50 × 109/L. (A) Cumulative incidence of neutrophil engraftment. (B) Cumulative incidence of platelet engraftment. (C) Cumulative incidence of aGVHD and (D) cGVHD in phase 2 recipients. (E) Incidence of grade 2-4 aGVHD in recipients of IV and SC plerixafor grafts. (F) Grade 3-4 aGVHD (G) and cGVHD in recipients surviving to 100 days. (H) Overall survival of recipients receiving plerixafor-mobilized allografts comparing IV with SC products. (I) Relapse-free survival of recipients receiving IV plerixafor grafts. Log-rank test was used to compare groups. Statistical comparisons of SC and IV plerixafor was performed by the log-rank (Mantel-Cox) test.
Table 5.
Engraftment SC vs IV plerixafor
| SC plerixafor 0.24 mg/kg (n = 38) | IV plerixafor 0.32 mg/kg (n = 33) | |
|---|---|---|
| Infused CD34+ cell dose/kg, mean (range) | 3.5 × 106 (1.9-19.7 × 106) | 2.9 × 106 (2.0-9.7 × 106) |
| Patients achieving neutrophil engraftment by day 21, n (%) | 37 (97%) | 31 (94%) |
| Median time to neutrophil engraftment, d (range) | 10 (5-13) | 14 (11-27)*,† |
| Patients achieving platelet engraftment by day 30, n (%) | 36 (95%) | 23 (70%)† |
| Median time to platelet engraftment, d (range) | 22 (8-102) | 25 (15-219)† |
| 100% donor chimerism by day 100 | MA: 100% | MA: 94% (31/33) |
| RIC: N/A | RIC: 40% (2/5) |
MA, myeloablative.
P < .05.
GVHD prophylaxis included methotrexate, which was not included in the SC plerixafor group.
GVHD
The cumulative incidence of grade 2-4 aGVHD was 7/33 (21%; Figure 7C). The incidence of grade 3-4 aGVHD was 4/33 (12%; Figure 7C). The cumulative incidence of cGVHD was 35%, and 22% had severe disease (Figure 7D). The incidence of aGVHD and cGVHD was similar in this study to what has been reported previously with SC plerixafor (Figure 7E-G). Compared with an institutional contemporary control cohort of recipients transplanted with G-CSF–mobilized peripheral blood stem cells from 2009 to 2011, with similar conditioning and GVHD prophylaxis, the incidence of grade 2-4 aGVHD was 48/70 (68%) and grade 3/4 was 12/70 (17%) (M.F., M.A.S., M.P.R., and J.F.D., unpublished data from the Center for International Blood and Marrow Transplant Research). The cumulative incidence of cGVHD in those living beyond 100 days was 7/25 (28%), and 4/25 (16%) had severe disease. This analysis contains 3 recipients that received G-CSF–mobilized grafts, and 1 of these recipients developed grade 3 skin GVHD and the other 2 developed grade 1 skin and grade 2 gut GVHD.
CMV reactivation after transplant
In a historical cohort of 98 recipients from our center, the incidence of CMV viremia (>10 000 copies/mL) and CMV disease were 62% and 6%, respectively, among those at risk (defined as serology positive in donor or recipient).22 In comparison, our current study found a low incidence of CMV viremia in at-risk recipients receiving plerixafor-mobilized products (15% [3/20]) (Table 6). The incidence of CMV disease in at-risk individuals was 5% (1/20). The relative risk for CMV viremia in the plerixafor group compared with our historical control is 0.2407 (95% CI, 0.0834-0.6943, P = .0084). There was no significant difference in CMV disease.
Table 6.
Incidence of CMV viremia and disease
| G-CSF | IV plerixafor | Risk difference (95% CI) | |
|---|---|---|---|
| CMV viremia ≥10 000 copies/mL | 62% (43/69) | 15% (3/20) | 47% (23%, 69%) |
| CMV disease with preemptive treatment | 6% (4/69) | 5% (1/20) | 0.8% (−24%, 25%) |
Relapse and survival
Median overall survival was 278 days with 45% 1-year survival (Figure 7H). Median relapse-free survival was 238 days (Figure 7I). The day 100 treatment-related mortality was 1/33 (3%). Of the 5 recipients conditioned with RIC, mortality was high (100%) related to relapse and infection. Ultimately, 4/5 relapsed within 150 days, and 1 recipient died of sepsis at day 52.
Discussion
Plerixafor has been shown to be a rapid mobilizing agent, but its use as a single agent has been hindered by lower stem cell yields compared with G-CSF. Thirty-three percent of SC plerixafor-mobilized donors fail to reach goal CD34 collection on day 1.6 We confirmed this failure rate in phase 1 of this study, in which 7/21 (33%) donors mobilized with SC plerixafor required additional collections to reach the minimum number of CD34/kg (≥2 × 106). In addition, we determined the maximum effective dose of IV plerixafor to be 0.32 mg/kg. We tested this dose in normal donors and found there was no improvement in the percentage of patients who successfully collected after a single IV infusion of 0.32 mg/kg plerixafor compared with 0.24 mg/kg SC plerixafor (66% vs 72%, respectively). These rates are in contrast to success rates using G-CSF in normal donors of >90% (7% first-day failure rate in 234 donors from 2009 to 2015 at Washington University in Saint Louis [M.F., M.A.S., M.P.R., and J.F.D., unpublished data]). We saw no significant difference in mobilization kinetics of CD34 cells/μL peripheral blood when comparing SC with IV dosing. One potential explanation may be prolonged exposure to low doses of drug by the SC route. We did not analyze the PK of SC plerixafor. SC and IV plerixafor in healthy volunteers has shown similar half-lives (M.F., M.A.S., M.P.R., and J.F.D., unpublished data), but Cmax and AUC from time 0 to infinity were higher following IV administration compared with SC dosing. In both routes of administration, the drug is cleared by 24 hours after administration. We conclude that failure to reach collection goal in 1 apheresis procedure remains high with single-agent plerixafor regardless of the route of administration.
Although the primary outcome was not reached, we made several additional observations. First, IV administration of plerixafor was well tolerated by donors. Second, we observed a dramatic donor-to-donor variability with either SC or IV dosing (range of peak mobilization, 4-157 CD34/μL). Third, rates of aGVHD and cGVHD were low compared with historical results for peripheral blood transplant from G-CSF–mobilized donors. Finally, the incidence of viral reactivation of CMV was lower than historic controls.
To examine the variation in mobilization in response to IV plerixafor, we looked for associations of known predictors for mobilization with G-CSF and clinical factors associated with higher circulating CD34 cell numbers.12,23-28 In this small cohort, the strongest predictors of higher CD34 cell mobilization were younger age, higher baseline platelet count, higher baseline circulating CD34, and higher AUC of plerixafor. Other factors associated with CD34 mobilization were CXCL12 change from baseline to 30 minutes, male gender, and a lower graft content of pDCs. We did not find an association of genetic polymorphisms in the CXCL12 3′UTR G801A and mobilization yield, as previously reported for G-CSF.29
We were able to characterize the difference between stem cell grafts mobilized with plerixafor and those mobilized by G-CSF. We found a preferential mobilization of a CD34+CD45RA+CD123++ population of cells by plerixafor that produce INF-α and are characteristic of a precursor pDC (pro-DC2). These cells express genes similar to classical pDCs and transcription factors critical for the development of pDCs from HSPCs. Importantly, we observed that recipients of plerixafor-mobilized grafts had a low incidence of CMV viremia and hypothesize this may be due to the pro-DC2 cells in the graft and the ability of these cells to express IFN-α. Genotyping of plerixafor and G-CSF–mobilized primitive stem cells (CD34+CD45RA−) further confirmed a unique gene expression profile in primitive stem cells mobilized by plerixafor compared with G-CSF. Although we did not observe any appreciable hematopoiesis differences either in vitro, as measured in a colony-forming cell assay, or in vivo as measured by engraftment in recipients, of plerixafor-mobilized grafts compared with G-CSF–mobilized grafts, further follow-up is warranted.
Recipient outcomes after transplant with IV plerixafor-mobilized products were remarkable for low GVHD and low rates of CMV viremia. Although CD34 numbers infused are lower than products mobilized by G-CSF, engraftment was rapid and durable. Our data suggest that plerixafor increased CXCR4 expression on CD34 precursors, possibly promoting their rapid and stable engraftment. These findings are in agreement with previous studies showing CXCR4 upregulation resulting from inhibition of CXCL12-induced receptor internalization by plerixafor.8,30,31 The observed delay in neutrophil and platelet engraftment compared with recipients of SC plerixafor-mobilized grafts is likely related to the inclusion of methotrexate as GVHD prophylaxis, a higher portion of active disease patients, and the inclusion of RIC. The reason for the low rates of CMV viremia observed in those recipients at risk may have to do with the cellular content of the graft, but is also likely confounded by lower rates of GVHD and less immunosuppression used in recipients. It is clear that the cellular composition of the graft changes with the mobilization regimen chosen. Current data on the role of T-cell subsets and pDC content of the graft are limited, with conflicting reports on their potential benefit in both the unrelated and sibling allogeneic transplant setting.31-35
Recipient outcomes after RIC conditioning were poor; however, the limited number of subjects prevents us from drawing any definitive conclusions. All of these recipients died: 4 relapsed within 150 days and 1 died of sepsis at day 52. Further study is needed before using this mobilization regimen for sibling allogeneic donors of RIC transplants, and these outcomes may be affected by marginal stem cell numbers and graft cellular composition.
In conclusion, plerixafor is a safe, effective, rapid mobilizing agent when administered IV; but, the PKs and pharmacodynamics are similar to SC plerixafor. A high single apheresis failure rate can be overcome with repeated collection, but 10% will not reach goal after 2 collections. These results are inferior to historic filgrastim data. Recently completed studies evaluating efficacy and outcomes of single agent SC plerixafor (Center for International Blood and Marrow Transplant Research protocol 09-PLEX, #NCT01696461) and the combination of plerixafor and GM-CSF (#NCT01158118) will soon be reported. These studies may help to confirm lower rates of GVHD and infections with plerixafor peripheral blood grafts and to better define the unique cellular makeup of these products.
Acknowledgments
The authors thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of their core facilities. The Siteman Cancer Center is supported in part by the National Cancer Institute at the National Institutes of Health (Cancer Center Support Grant P30 CA91842). The authors also thank J. Evan Sadler, Monica Bessler, and Matthew Walter for critical feedback and mentoring; Timothy A. Graubert for CXCL12 single-nucleotide polymorphism analysis, critical feedback, and mentoring; Darja Karpova for critical review of the manuscript and feedback; and Jackie Wu (Sanofi Genzyme) and Yaming Su (Sanofi Genzyme) for their statistical review/contributions to this publication surround pharmacokinetic data analysis (both are supported by funding from Sanofi Genzyme).
This research was supported Genzyme (J.F.D.); the National Institutes of Health (NIH) National Cancer Institute (grants R01 CA152329, R21 CA141523, and R21 CA132269) (J.F.D.); NIH, National Heart, Lung, and Blood Institute (grant 5K12HL08710703) (M.A.S.); the Washington University Institute of Clinical and Translational Sciences from the NIH National Center for Advancing Translational Sciences (grant UL1 TR000448); and the Cancer and Leukemia Group B and Alliance (M.A.S.).
The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A.S., M.P.R., J.S., K.T., and J.F.D. designed and performed the research, analyzed and interpreted data, and drafted the manuscript. K.T. performed statistical analyses. M.F., W.E., E.D., W.S., J.Y., R.V., K.S.-G., A.F.C., G.L.U., C.N.A., and P.W. analyzed and interpreted data and edited the manuscript. M.F., S.L., S.C., K.M., and F.A.M. collected and helped analyze data. All authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: M.P.R. and G.L.U. received honoraria from Sanofi Genzyme. The remaining authors declare no competing financial interests.
Correspondence: John F. DiPersio, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007, Saint Louis, MO 63110; e-mail: jdipersi@dom.wustl.edu.
References
- 1.Stem Cell Trialists’ Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23(22):5074-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang YJ, Weng CL, Sun LX, Zhao YT. Allogeneic bone marrow transplantation compared to peripheral blood stem cell transplantation for the treatment of hematologic malignancies: a meta-analysis based on time-to-event data from randomized controlled trials. Ann Hematol. 2012;91(3):427-437. [DOI] [PubMed] [Google Scholar]
- 3.Nagler A, Labopin M, Shimoni A, et al. Mobilized peripheral blood stem cells compared with bone marrow as the stem cell source for unrelated donor allogeneic transplantation with reduced-intensity conditioning in patients with acute myeloid leukemia in complete remission: an analysis from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(9):1422-1429. [DOI] [PubMed] [Google Scholar]
- 4.Varmavuo V, Mantymaa P, Nousiainen T, Valonen P, Kuittinen T, Jantunen E. Blood graft composition after plerixafor injection in patients with NHL. Eur J Haematol. 2012;89(2):128-135. [DOI] [PubMed] [Google Scholar]
- 5.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26(1):34-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112(4):990-998. [DOI] [PubMed] [Google Scholar]
- 7.Holig K. G-CSF in healthy allogeneic stem cell donors. Transfus Med Hemother. 2013;40(4):225-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uy GL, Rettig MP, Motabi IH, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119(17):3917-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrix CW, Collier AC, Lederman MM, et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37(2):1253-1262. [DOI] [PubMed] [Google Scholar]
- 10.Genzyme. Investigator's Brochure for Plerixafor Injection (AMD3100, Mozobil). http://www.mozobil.com/healthcare. Accessed 26 March 2017.
- 11.Hendrix CW, Flexner C, MacFarland RT, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44(6):1667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen KS, Cheng S, Larson MG, et al. Circulating CD34(+) progenitor cell frequency is associated with clinical and genetic factors. Blood. 2013;121(8):e50-e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA. 2002;99(18):11872-11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112(9):3543-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blom B, Ho S, Antonenko S, Liu YJ. Generation of interferon alpha-producing predendritic cell (Pre-DC)2 from human CD34(+) hematopoietic stem cells. J Exp Med. 2000;192(12):1785-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dlubek D, Drabczak-Skrzypek D, Lange A. Low CXCR4 membrane expression on CD34(+) cells characterizes cells mobilized to blood. Bone Marrow Transplant. 2006;37(1):19-23. [DOI] [PubMed] [Google Scholar]
- 17.Fruehauf S, Seeger T, Maier P, et al. The CXCR4 antagonist AMD3100 releases a subset of G-CSF-primed peripheral blood progenitor cells with specific gene expression characteristics. Exp Hematol. 2006;34(8):1052-1059. [DOI] [PubMed] [Google Scholar]
- 18.Fruehauf S, Veldwijk MR, Seeger T, et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009;11(8):992-1001. [DOI] [PubMed] [Google Scholar]
- 19.Larochelle A, Krouse A, Metzger M, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107(9):3772-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31(2):109-117. [DOI] [PubMed] [Google Scholar]
- 21.Oelschlaegel U, Bornhauser M, Boxberger S, et al. Kinetics of CXCR-4 and adhesion molecule expression during autologous stem cell mobilisation with G-CSF plus AMD3100 in patients with multiple myeloma. Ann Hematol. 2007;86(8):569-573. [DOI] [PubMed] [Google Scholar]
- 22.Verkruyse LA, Storch GA, Devine SM, Dipersio JF, Vij R. Once daily ganciclovir as initial pre-emptive therapy delayed until threshold CMV load > or =10000 copies/ml: a safe and effective strategy for allogeneic stem cell transplant patients. Bone Marrow Transplant. 2006;37(1):51-56. [DOI] [PubMed] [Google Scholar]
- 23.Bertani G, Santoleri L, Martino M, et al. Identification of hematopoietic progenitor cell donor characteristics predicting successful mobilization: results of an Italian multicenter study. Transfusion. 2014;54(8):2028-2033. [DOI] [PubMed] [Google Scholar]
- 24.Billen A, Madrigal JA, Szydlo RM, Shaw BE. Female donors and donors who are lighter than their recipient are less likely to meet the CD34+ cell dose requested for peripheral blood stem cell transplantation. Transfusion. 2014;54(11):2953-2960. [DOI] [PubMed] [Google Scholar]
- 25.DiPersio JF. Diabetic stem-cell “mobilopathy.” N Engl J Med. 2011;365(26):2536-2538. [DOI] [PubMed] [Google Scholar]
- 26.Ferraro F, Lymperi S, Mendez-Ferrer S, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3(104):104ra101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pusic I, Jiang SY, Landua S, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045-1056. [DOI] [PubMed] [Google Scholar]
- 28.Suzuya H, Watanabe T, Nakagawa R, et al. Factors associated with granulocyte colony-stimulating factor-induced peripheral blood stem cell yield in healthy donors. Vox Sang. 2005;89(4):229-235. [DOI] [PubMed] [Google Scholar]
- 29.Benboubker L, Watier H, Carion A, et al. Association between the SDF1-3′A allele and high levels of CD34(+) progenitor cells mobilized into peripheral blood in humans. Br J Haematol. 2001;113(1):247-250. [DOI] [PubMed] [Google Scholar]
- 30.Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527(1-3):255-262. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi A, Orba Y, Kimura T, et al. Inhibition of the SDF-1alpha-CXCR4 axis by the CXCR4 antagonist AMD3100 suppresses the migration of cultured cells from ATL patients and murine lymphoblastoid cells from HTLV-I Tax transgenic mice. Blood. 2009;114(14):2961-2968. [DOI] [PubMed] [Google Scholar]
- 32.Kean LS, Sen S, Onabajo O, et al. Significant mobilization of both conventional and regulatory T cells with AMD3100. Blood. 2011;118(25):6580-6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundqvist A, Smith AL, Takahashi Y, et al. Differences in the phenotype, cytokine gene expression profiles, and in vivo alloreactivity of T cells mobilized with plerixafor compared with G-CSF. J Immunol. 2013;191(12):6241-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lonial S, Akhtari M, Kaufman J, et al. Mobilization of hematopoietic progenitors from normal donors using the combination of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor results in fewer plasmacytoid dendritic cells in the graft and enhanced donor T cell engraftment with Th1 polarization: results from a randomized clinical trial. Biol Blood Marrow Transplant. 2013;19(3):460-467. [DOI] [PubMed] [Google Scholar]
- 35.Waller EK, Logan BR, Harris WA, et al. Improved survival after transplantation of more donor plasmacytoid dendritic or naive T cells from unrelated-donor marrow grafts: results from BMTCTN 0201. J Clin Oncol. 2014;32(22):2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]